Abstract
We have investigated the transformation of chlorinated hydroxybiphenyls by laccase produced by Pycnoporus cinnabarinus. The compounds used were transformed to sparingly water-soluble colored precipitates which were identified by gas chromatography-mass spectrometry as oligomerization products of the chlorinated hydroxybiphenyls. During oligomerization of 2-hydroxy-5-chlorobiphenyl and 3-chloro-4-hydroxybiphenyl, dechlorinated C—C-linked dimers were formed, demonstrating the dehalogenation ability of laccase. In addition to these nonhalogenated dimers, both monohalogenated and dihalogenated dimers were identified.
Because of their low water solubility and low chemical reactivity, polychlorinated biphenyls (PCBs) are used mainly as solvent extenders, heat transfer fluids, plastilizers, organic solvents, flame retardants, and hydraulic fluids. Due to their hydrophobicity and stability, PCBs persist for a long time in the environment. For a long time, the search for microbes capable of degrading these hazardous pollutants has been focused on bacteria. Aerobic bacteria have been found which can degrade the less-chlorinated congeners (mono- to hexachlorobiphenyls) by using a biphenyl-induced dioxygenase enzyme system (1, 15). More highly chlorinated biphenyls have only been found to be attacked reductively by bacteria from river sediments under anaerobic conditions by a mechanism involving the removal of meta and para chlorines (2, 25). Recent papers have also reported ortho dechlorination besides meta and para dechlorination of PCBs under anaerobic (3, 30) and even aerobic (28) conditions.
However, aerobic degradation of PCBs has also been described for white rot fungi, e.g., Phanerochaete chrysosporium, Pleurotus ostreatus, Lentinus edodes, and Trametes versicolor (11, 12, 13, 27, 31). Fungi are also known to dechlorinate or mineralize several monoaromatic compounds, like pentachlorophenol and methoxychlor by P. chrysosporium (16, 22) or monochlorophenols by the yeast Candida maltosa (24).
Laccase, one of the ligninolytic enzymes produced by many of white rot fungi, is able to dechlorinate chlorophenols, accompanied by oligomerization of the substrate (26). Dec and Bollag (9, 10) proposed coupling reactions between free radicals generated during the catalytic oxidation of 2,4-dichlorophenol and 4-chloroanilines, respectively. The resulting products turn out to be dechlorinated dimers.
There are no reports of dehalogenation of chlorinated biarylic compounds by laccases. Our study shows that laccase of the white rot fungus Pycnoporus cinnabarinus can dechlorinate and detoxify chlorinated hydroxybiphenyls. The three derivatives used (Promochem, Wesel, Germany) represent chloro substitutions at different positions relative to the hydroxyl group: the ortho position (3-chloro-4-hydroxybiphenyl), the para position (2-hydroxy-5-chlorobiphenyl), and the para position at the nonhydroxylated ring (4-hydroxy-4′-chlorobiphenyl). The study of the reaction allows us to estimate the importance of such processes for detoxification and binding of biphenyls to the humus fraction of soils.
The fungus P. cinnabarinus SBUG-M 1044 was initially cultivated on malt agar plates for 7 days at 30°C. Laccase-producing cultures were prepared by inoculating a nitrogen-rich medium (6) as described by Jonas et al. (20). After 6 days of incubation, the cultures were harvested and the supernatant was separated from mycelia by filtration through glass fiber filters (GF6; Schleicher & Schuell, Dassel, Germany). The extracellular enzyme was bound to DEAE-Sephacel (Sigma, Steinheim, Germany) as described by Jonas et al. (20) and eluted with a high-salt buffer (20 mM histidine buffer, 700 mM sodium chloride, pH 6.0). The desalted enzyme fraction (HiPrep 26/10 Desalting; Pharmacia Biotech, Freiburg, Germany) containing no other ligninolytic enzymes (18) was then concentrated by ultrafiltration using 30-kDa cutoff membranes (Centriprep, 30 kDa; Amicon, Witten, Germany). The laccase activity of the concentrated supernatant was adjusted to 450 nmol ml−1 min−1 by addition of 20 mM histidine buffer adjusted with acetic acid to pH 6.0 rather than with HCl to avoid the formation of artificial chlorinated products.
Laccase activity in the cell-free culture fluid was determined by monitoring the oxidation of 2,2′-azino-bis(3-ethyl-benzthiazoline-sulfonic acid) (ABTS; Boehringer, Mannheim, Germany) buffered with sodium acetate, pH 5.0 (5, 26). Peroxidase activity was determined by using phenol red as described by Pick and Keisary (23). For manganese peroxidase activity determination, 10 μl of 10 mM manganese sulfate was also added. Controls with catalase were used to correct for the influence of biogenic H2O2. Lignin peroxidase activity was assayed by monitoring the change in A310 corresponding to the oxidation of veratryl alcohol (21).
All biotransformation experiments were performed in 20- ml reaction tubes. Two milliliters of laccase (450 nmol ml−1 min−1) per tube was mixed with the test substance dissolved in N,N-dimethylformamide to a final concentration of 2 mM. Reaction tubes were tightly closed with plastic caps and incubated on a horizontal shaker at 200 rpm at room temperature for 24 h. The samples were extracted with an equal volume of ethyl acetate, and the organic phases were evaporated to dryness (20) and redissolved in 1 ml of methanol.
Reaction products were separated by high-performance liquid chromatography (HPLC; Merck, Darmstadt, Germany) on a C18 reversed-phase column (LiChrocart, 125-4 endcapped; Merck) using a nonlinear gradient (20). Analysis of metabolites was performed by gas chromatography-mass spectrometry (GC-MS) after their conversion into methyl derivatives by diazomethane (7). The conditions used included a temperature program of 100 to 320°C at 12°C min−1 and 5 min at 320°C, an injection mode of 1 μl split-splitless, He carrier gas, and a scan range of 45 to 600 Da. The release of chloride was measured in a colorimetric assay (Spectroquant; Merck).
The fungus P. cinnabarinus secreted extracellular laccase in nitrogen-rich medium containing 10 mM 3,4-dimethoxybenzyl alcohol. The maximum enzyme activity, reached at day 9, was 500 nmol ml−1 min−1. Neither manganese peroxidase nor lignin peroxidase was detected under these conditions. The enriched laccase preparation was then used to transform the hydroxylated monochlorobiphenyls 3-chloro-4-hydroxybiphenyl, 4-hydroxy-4′-chlorobiphenyl, and 2-hydroxy-5-chlorobiphenyl. After 30 min, colored precipitates became visible in the reaction mixture. Extraction of the whole assay after 24 h and analysis of the extract by HPLC showed that 97% of the 3-chloro-4-hydroxybiphenyl, 89% of the 4-hydroxy-4′-chlorobiphenyl, and 92% of the 2-hydroxy-5-chlorobiphenyl used were transformed. Furthermore, the organic extracts of all assays seemed to contain many products more hydrophobic than the parent compounds since they have longer retention times (tRs) on a reverse-phase column.
After transformation of 1 mM 2-hydroxy-5-chlorobiphenyl by laccase, the solution turned red. HPLC analysis revealed one product at a tR of 10.45 min. Other products separated sufficiently were obtained at tRs of 23.0 and 23.56 min, and a pool of nonseparated products were found from tRs of 24 to 28 min. The whole assay was extracted and measured by GC-MS after methylation, which marks free hydroxyl groups by increasing the molecular mass of 14 and gives further information on binding type and the number of free hydroxyl groups.
The main product was a compound with a molecular weight of 366 (Table 1) without the isotopic pattern of the fragment ion typical for a chlorinated substance. The GC retention time and the mass spectrum were identical to those of the separated dimer, 5,5′-di-(2-hydroxybiphenyl), formed during the transformation of 2-hydroxybiphenyl with laccase as the main product (19). Obviously, one coupling of 2-hydroxy-5-chlorobiphenyl occurred at position C-5 in the para position with respect to the hydroxyl group; consequently, both chlorine atoms were split off. Further products obtained by GC-MS analysis showed molecular weights of 420 and 434 after methylation, and each occurred once (Table 1). They may represent dimers with no dechlorination in which monomers had been bonded in the C—O and C—C positions, respectively. The C—O-bonded dimer showed a molecular ion peak at m/z 420 and displayed fragment ions at m/z 370 (M+ − CH3/Cl), 168 (M+ − C12H7OCH3Cl/Cl), 152 (M+ − C12H7O2CH3Cl/Cl), and 139. The C—C-coupled dimer, with a molecular weight of 434, displayed fragment ions at m/z 384 (M+ − CH3/Cl), 334, 276, 167 (M+ − C12H7OCH3Cl/CH3/Cl), and 138. Dimers with only one chlorine atom would have molecular weights of 386 (C—O) and 400 (C—C), but none were found (Table 1).
TABLE 1.
Dechlorinated and chlorinated dimer products formed after transformation of 3-chloro-4-hydroxybiphenyl, 2-hydroxy-5-chlorobiphenyl, and 4-hydroxy-4′-chlorobiphenyl by culture medium containing laccase of the white rot fungus P. cinnabarinus
| Mol wt of structure after methylation | No. of chlorines bound to molecule | No. of hydroxyl groups bound to molecule | Occurrence of products after transformation ofa:
|
||
|---|---|---|---|---|---|
| 3-Cl-4-OH-BP | 2-OH-5-Cl-BP | 4-OH-4′-Cl-BP | |||
| 366 | 0 | 2 | + | + | − |
| 386 | 1 | 1 | + | − | − |
| 400 | 1 | 2 | + | − | + |
| 420 | 2 | 1 | + | + | − |
| 434 | 2 | 2 | + | + | + |
3-Cl-4-OH-BP, 3-chloro-4-hydroxybiphenyl; 2-OH-5-Cl-BP, 2-hydroxy-5-chlorobiphenyl; 4-OH-4′-Cl-BP, 4-hydroxy-4′-chlorobiphenyl.
Further chlorinated 4-hydroxybiphenyls were chosen for transformation with laccase. The compounds were 3-chloro-4-hydroxybiphenyl and 4-hydroxy-4′-chlorobiphenyl. The transformation of 3-chloro-4-hydroxybiphenyl was accompanied by the formation of white precipitates. During the transformation of 4-hydroxy-4′-chlorobiphenyl, slightly violet precipitates were formed. HPLC analysis of the extracts of the incubation assays of both compounds showed elution patterns very similar to that obtained with 4-hydroxybiphenyl as a substrate. Major products well separated from each other were found at tRs of 22 to 24.5 min, and a pool of nonseparated products with longer tRs was observed.
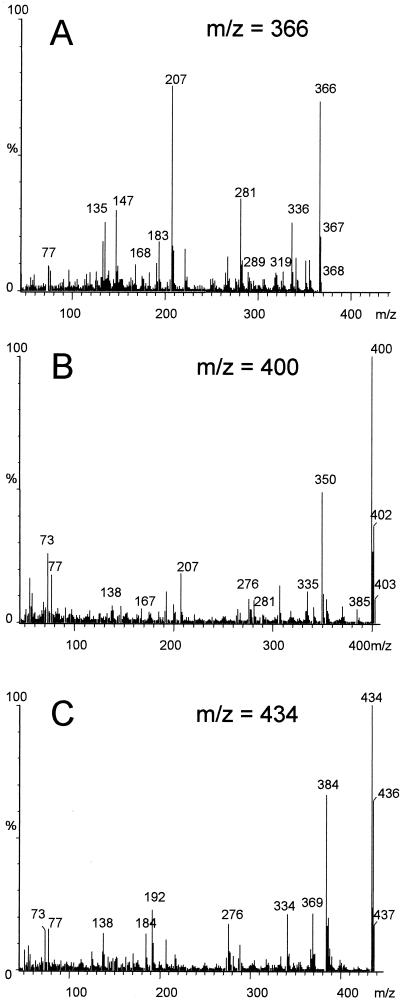
GC-MS analysis of the whole methylated extract of the assay with 3-chloro-4-hydroxybiphenyl also revealed dimers with different molecular weights (366, 386, 400, 420, and 434; Table 1). Three dimers with C—C bonds and two with C—O bonds were produced with a different number of chlorines. Formation of a completely dechlorinated product and therefore coupled at carbon atom 3 was represented by a compound with a molecular weight of 366 (Fig. 1A). The C—C bond dimer with only one chlorine (representing the loss of one chlorine) was the product with an m/z of 400 (Fig. 1B) which could not be found after the transformation of 2-hydroxy-5-chlorobiphenyl. A C—C bond dimer with both chlorine atoms in the molecule showed an m/z of 434 (Fig. 1C). The formation of a C—O-coupled dimer which retained both chlorine substituents was indicated by a product with a molecular weight of 420, accompanied by fragment ions at m/z 370 (M+ − CH3/Cl), 352, 334, 168 (M+ − C12H7OCH3Cl/Cl), 152 (M+ − C12H7O2CH3Cl/Cl), and 139. A C—O bond dimer with one chlorine (m/z 386), consequently coupled at the oxygen and the C-3, contained ion peaks at 386 (M+), 336 (M+ − Cl/CH3), 168 (M+ − C12H7OCH3Cl), 152 (M+ − C12H7O2CH3Cl), and 139. The formation of dechlorinated compounds was confirmed by measurement of the chloride released during the coupling reaction. The chloride release amounted to 22% of the whole chlorine content of the parent compound. Since the measurement of the HPLC peaks revealed a proportion of 18% for the fully dechlorinated product, about 4% can be attributed to the partially dechlorinated products.
FIG. 1.
Mass spectra of the C—C bond dimers formed during transformation of 3-chloro-4-hydroxybiphenyl.
After transformation of 4-hydroxy-4′-chlorobiphenyl, only two products with C—C bonds and one (m/z 400) or two (m/z 434) chlorine atoms could be demonstrated. The mass spectra of these products showed the same fragment ions as the corresponding products obtained after transformation of 3-chloro-4-hydroxybiphenyl but in different intensities. Clearly, dimers completely dechlorinated or with C—O bond monomers were not produced (Table 1).
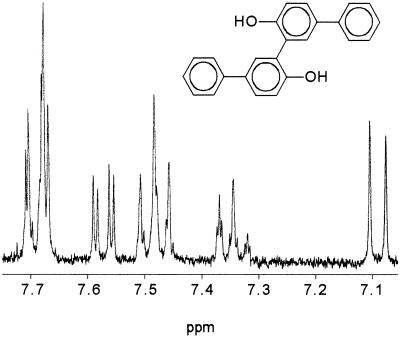
The use of nonchlorinated hydroxylated biphenyl is very useful in determining the preferred position for the entry of a new substituent. To get more information on the coupling mechanism of 3-chloro-4-hydroxybiphenyl, transformation experiments with 4-hydroxybiphenyl and laccase were carried out. After 30 min, white precipitates were formed and products more hydrophobic than the substrate could be shown by HPLC. GC-MS analysis of the whole methylated extract indicated the formation of C—C bond dimers with a molecular weight of 366. One of them showed the same mass spectrum as a product formed from 3-chloro-4-hydroxybiphenyl (Fig. 1A), thus confirming the dehalogenation of the parent compound due to the coupling process. The 1H NMR spectrum of a main dimer, purified by HPLC, showed only six signals, indicating a very symmetrical structure. The 1H NMR data were [δ 6.99 (d = 5-H), 7.26 (m = 4′-H), 7.37 (m = 3′,5′-H), 7.48 (dd = 6-H), 7.58 (s = 2-H), 7.59 (m = 2′,6′-H) ppm]. The occurrence of the singlet at 7.58 ppm and the lower intensity of the doublets at 6.99 and 7.48 ppm, in comparison with the 1H NMR data of the parent compound, showed that the binding of the two monomers took place at position C-3. These data point out that the preferred position for a coupling reaction between 4-hydroxybiphenyl and laccase is the ortho position with respect to the hydroxyl group (Fig. 2).
FIG. 2.
H NMR spectrum of the dimer 3,3′-di(4-hydroxybiphenyl) formed during transformation of 4-hydroxybiphenyl and 3-chloro-4-hydroxybiphenyl by P. cinnabarinus laccase (300 MHz; CD3OD). The dimer structure is shown in the inset.
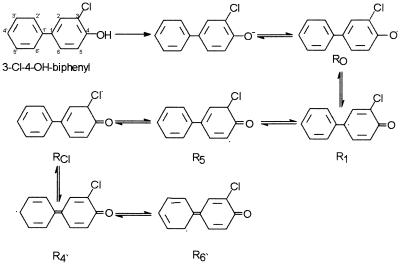
Our results present a total dechlorination of monochlorinated hydroxybiphenyls caused by a laccase preparation from the white rot fungus P. cinnabarinus. The replacement of one ring of the biphenyl with a hydroxyl group polarizes the ring, leads to an I-effect at this ring, and reduces the electron density. In consequence, the formation of radicals and the entry of a new substituent are preferred at the ortho or para positions whereas the meta position is nonreactive (29). In the cases of the three substances transformed by laccase in this study, dechlorination occurred only if the chlorine substituent was located in the ortho or para position with respect to the hydroxyl group of the same ring. Theoretically, the same number of radicals and products should be formed from each compound. However, our results reflect a different pattern of product formation which suggests that the radicals formed have different stabilities. Radical stability probably depends on the position of the chlorine with respect to the hydroxyl group. The declining number of the various dimers produced from 3-chloro-4-hydroxybiphenyl, 2-hydroxy-5-chlorobiphenyl, and 4-hydroxy-4′-chlorobiphenyl (Table 1) leads to the conclusion that the stability of the radical may weaken with an increase in the distance between the two substituents. The nearer the chlorine substituent is to the hydroxyl group (3-chloro-4-hydroxybiphenyl), the more stable radicals at the following positions are produced: a C radical at C-5, a chlorinated carbon atom at C-3, and an O radical in the hydroxyl group at C-4. Consequently, besides the totally dechlorinated dimer, the occurrence of C—O-linked single dechlorinated, as well as C—C-linked, dimers with both chlorines was found. In the case of 2-hydroxy-5-chlorobiphenyl, the two substituents pull the electrons of the ring in opposite directions, leading to less polarization of the aromatic ring. Subsequently, radicals with low stability are formed. Nevertheless, in both cases, this radical mechanism leads to the direct dechlorination of hydroxylated biphenyls (Fig. 3). In contrast to bacteria (13, 14), white rot fungi are able to attack the chlorinated ring first. In soil systems where a high hydroxylation potential due to microbial cytochrome P450 or other oxygenase systems exists, the oligomerization processes described here may be of environmental relevance. The removal of chlorines due to self-coupling of aromatic compounds or binding of the phenolic derivatives to the humic fraction by laccase may also be classified as detoxification (9) because such polymers show reduced acute toxicity (4, 17). On the other hand, they are more slowly degraded to carbon dioxide and water by soil microorganisms (8).
FIG. 3.
Radicalization of 3-chloro-4-hydroxybiphenyl by laccase.
Hydroxylation is a prerequisite for the dechlorination of PCBs under aerobic conditions, since laccase will not accept nonhydroxylated substrates.
Acknowledgments
This work was supported by a grant of the Kultusministerium des Landes Mecklenburg Vorpommern.
REFERENCES
- 1.Abramowicz D A. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–251. [Google Scholar]
- 2.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 127–216. [Google Scholar]
- 3.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollag J-M, Shuttleworth K L, Anderson D H. Laccase-mediated detoxification of phenolic compounds. Appl Environ Microbiol. 1988;54:3086–3091. doi: 10.1128/aem.54.12.3086-3091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbonnais R, Paice M G. Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 6.Braun-Lüllemann A, Majcherczyk A, Hüttermann A. Degradation of styrene by white rot fungi. Appl Microbiol Biotechnol. 1997;47:150–155. [Google Scholar]
- 7.De Boer T D, Backer H J. Diazomethane. In: Leonard N J, editor. Organic synthesis. New York, N.Y: Wiley; 1956. pp. 14–16. [Google Scholar]
- 8.Dec J, Bollag J-M. Microbial release and degradation of catechol and chlorophenols bound to synthetic humic acid. Soil Sci Soc Am J. 1988;52:1366–1371. [Google Scholar]
- 9.Dec J, Bollag J-M. Dehalogenation of chlorinated phenols during oxidative coupling. Environ Sci Technol. 1994;28:484–490. doi: 10.1021/es00052a022. [DOI] [PubMed] [Google Scholar]
- 10.Dec J, Bollag J-M. Effect of various factors on dehalogenation of chlorinated phenols and anilines during oxidative coupling. Environ Sci Technol. 1995;29:657–663. doi: 10.1021/es00003a012. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich D, Hickey W J, Lamar R. Degradation 4,4′-dichlorobiphenyl, 3,3′, 4,4′-tetrachlorobiphenyl, and 2,2′, 4,4′, 5,5′-hexachlorobiphenyl by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:3904–3909. doi: 10.1128/aem.61.11.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton D C. Mineralization of polychlorinated biphenyls by Phanerochaete chrysosporium: a ligninolytic fungus. Enzyme Microb Technol. 1985;7:194–196. [Google Scholar]
- 13.Furukawa K, Matsumura F, Tonomura K. Alcaligenes and Acinetobacter strains capable of degrading polychlorinated biphenyls. Agric Biol Chem. 1978;42:543–548. [Google Scholar]
- 14.Furukawa K, Tomizuka N, Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979;38:301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson D T, Cruden D L, Haddock J D, Zylstra G J, Brand J M. Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707. J Bacteriol. 1993;175:4561–4564. doi: 10.1128/jb.175.14.4561-4564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifoll M, Hammel K E. Initial steps in the degradation of methoxychlor by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1997;63:1175–1177. doi: 10.1128/aem.63.3.1175-1177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoff T, Liu S Y, Bollag J-M. Transformation of halogen-, alkyl-, and alkoxy-substituted anilines by a laccase of Trametes versicolor. Appl Environ Microbiol. 1985;49:1040–1045. doi: 10.1128/aem.49.5.1040-1045.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonas U. Biotransformation von Biarylverbindungen durch Weißfäulepilze unter besonderer Berücksichtigung des ligninolytischen Enzymsystems von Pycnoporus cinnabarinus und Trametes versicolor. Ph.D. thesis. Greifswald, Germany: Ernst Moritz Arndt University of Greifswald; 1998. [Google Scholar]
- 19.Jonas U, Hammer E, Haupt E T K, Schauer F. Characterisation of coupling products formed by biotransformation of biphenyl and diphenyl ether by the white rot fungus Pycnoporus cinnabarinus. Arch Microbiol. 2000;174:393–398. doi: 10.1007/s002030000220. [DOI] [PubMed] [Google Scholar]
- 20.Jonas U, Hammer E, Schauer F, Bollag J-M. Transformation of 2-hydroxydibenzofuran by laccases of the white rot fungi Trametes versicolor and Pycnoporus cinnabarinus and characterization of oligomerization products. Biodegradation. 1998;8:321–328. doi: 10.1023/a:1008220120431. [DOI] [PubMed] [Google Scholar]
- 21.Kirk T K, Tien M, Kersten P J, Kalyanaraman B, Hammel K E, Farrell R L. Lignin peroxidase from fungi: Phanerochaete chrysosporium. Methods Enzymol. 1990;188:159–169. [Google Scholar]
- 22.Lamar R T, Glase J A, Kirk T K. Fate of pentachlorphenol (PCP) in sterile soils inoculated with the white-rot basidiomycete Phanerochaete chrysosporium: mineralization, volatilization and depletion of PCP. Soil Biol Biochem. 1990;22:433–440. [Google Scholar]
- 23.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 24.Polnisch E, Kneifel H, Franzke H H. Degradation and dehalogenation of monochlorophenols by the phenol-assimilating yeast Candida maltosa. Biodegradation. 1992;2:193–199. doi: 10.1007/BF00124493. [DOI] [PubMed] [Google Scholar]
- 25.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anearobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy-Arcand L, Archibald F S J. Direct dechlorination of chlorophenolic compounds by laccases from Trametes (coriolus) versicolor. Enzyme Microbiol Technol. 1991;13:194–203. [Google Scholar]
- 27.Sasek V, Volfola O, Erbanova P, Vyas B R M, Matucha M. Degradation of PCBs by white rot fungi, methylotrophic and hydrocarbon utilizing yeasts and bacteria. Biotechnol Lett. 1993;15:521–526. [Google Scholar]
- 28.Seeger M, Zielinski M, Timmis K N, Hofer B. Regiospecificity of dioxygenation of di- to pentachlorobiphenyls and their degradation to chlorbenzoates by bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl Environ Microbiol. 1999;65:3614–3621. doi: 10.1128/aem.65.8.3614-3621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter W. Mechanismen der mehrfachen elektrophilen Substitution am Benzolkern. In: Beyer H, Walter W, editors. Lehrbuch der organischen Chemie. S. Stuttgart, Germany: Hirzel Verlag; 1991. pp. 485–487. [Google Scholar]
- 30.Wu Q, Sowers K R, May H D. Microbial reductive dechlorination of Aroclor 1260 in anaerobic slurries of estuarine sediments. Appl Environ Microbiol. 1998;64:1052–1058. doi: 10.1128/aem.64.3.1052-1058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeddel A, Majcherczyk A, Hüttermann A. Degradation of polychlorinated biphenyls by white-rot fungi Pleurotus ostreatus and Trametes versicolor in a solid state system. Toxicol Environ Chem. 1993;40:255–266. [Google Scholar]