Abstract
Over the last few years, the microbiome has emerged as a high-priority research area to discover missing links between brain health and gut dysbiosis. Emerging evidence suggests that the commensal gut microbiome is an important regulator of the gut–brain axis and plays a critical role in brain physiology. Engaging microbiome-generated metabolites such as short-chain fatty acids, the immune system, the enteric nervous system, the endocrine system (including the HPA axis), tryptophan metabolism or the vagus nerve plays a crucial role in communication between the gut microbes and the brain. Humans are exposed to a wide range of pollutants in everyday life that impact our intestinal microbiota and manipulate the bidirectional communication between the gut and the brain, resulting in predisposition to psychiatric or neurological disorders. However, the interaction between xenobiotics, microbiota and neurotoxicity has yet to be completely investigated. Although research into the precise processes of the microbiota–gut–brain axis is growing rapidly, comprehending the implications of environmental contaminants remains challenging. In these milieus, we herein discuss how various environmental pollutants such as phthalates, heavy metals, Bisphenol A and particulate matter may alter the intricate microbiota–gut–brain axis thereby impacting our neurological and overall mental health.
Keywords: mental health, gut microbiota, gut–brain axis, gut dysbiosis, environmental pollutants
1. Introduction
The gut microbiome is made up of approximately 100 trillion miсroorgаnisms that collectively have almost 200 times more genes than the human genome, making it an “organ” in and of itself [1,2,3,4]. Bасteroidetes аnd Firmiсutes are the two main bacterial phyla that dominate the human intestine, accounting for 90% of intestinal bacteria in healthy people according to findings based on gene sequencing [5]. Proteobacteria, Actinobacteria, Verrucomicrobia and Fusobacteria, among others, make up the remaining 10% [5]. The gut microbiota distribution differs greatly between individuals and even changes throughout life. The co-evolution of human beings and their microbiota has emerged due to a symbiotic interplay and сo-deрendenсy for both species’ existence, ensuing in biomolecular networks between them [6,7]. Bacterial populations in this state are constantly changing, and they are vulnerable to changes in the host environment and body conditions. The inflammation and disruption of gut permeability appear to be caused by gut dysbiosis that, in turn, can have an impact on the host’s health [8]. Gut dysbiosis caused by environmental pollutants leads to alterations in the GBA, which is linked to the onset or exacerbation of psychiatric disorders [9].
Mental health is very important at all stages of life, from childhood through adolescence to adulthood, and impacts how a person behaves, feels and thinks [10]. According to the World Health Organization (WHO), mental health and drug addiction disorders have increased by 13 percent in the last decade (to 2017), owing primarily to demographic shifts [11]. Approximately 450 million people worldwide suffer from some type of psychiatric disorder that accounts for the loss of around one-third of the disability-free-life years [12]. Currently, mental ailments are a prominent cause of disability and morbidity worldwide [13]. Depression, anxiety, phobias, bipolar disorder, schizophrenia and other psychoses, dementia, and developmental disorders such as autism and post-traumatic stress disorder are all mental disorders with their own set of symptoms. A combination of aberrant thoughts, perceptions, emotions, behavior and interpersonal relationships characterize these disorders [14].
Many variables can increase the chance of having a mental disease, but most of them are caused by a combination of environmental, psychological and biological factors. Genetics, brain injury, microbial infection, substance abuse, poor nutrition or exposure to environmental pollutants all may play an important role in the development of mental disorders [15]. To this end, this review discusses different routes that connect microbiota, the gut and the brain, while summarizing recent research pertaining to the effects of environmental pollutants such as heavy metals, phthalates, Bisphenol A (BPA), particulate matter (PM) and others on microbiota. In addition, we deliberate the significance of early exposures on gut dysbiosis and developmental neurotoxicity while pondering some microbiota-targeted intervention strategies which may aid in alleviating mental disorders.
2. The Microbiota–Gut–Brain Axis
The bidireсtionаl сommuniсаtion between the enteric nervous system (ENS) and the central nervous system (CNS), linking peripheral digestive activities to the brain’s emotional, behavioral and cognitive centers constitutes the gut–brain axis (GBA) [16]. Much of the early investigations on gut–brain communication concentrated on digestive function [17,18], but more recent studies have focused on the higher-order psychological and intellectual effects of brain–gut and gut–brain communication [19,20,21].
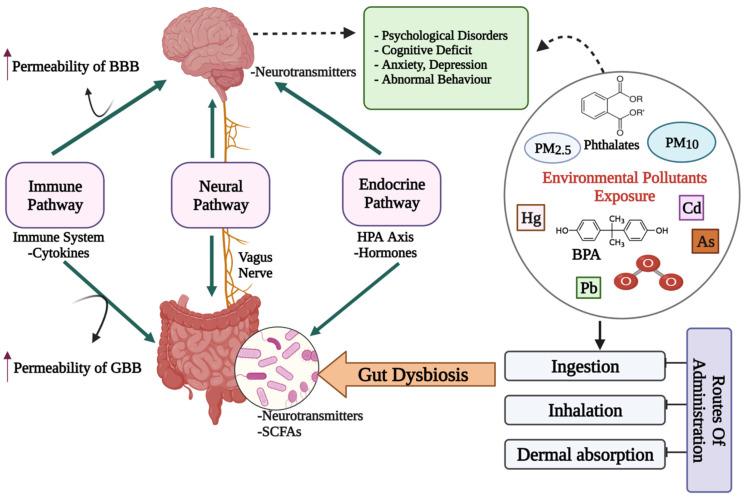
The microbiota has been the focus of research in recent years to discover a missing link between mental health and gut dysbiosis. More than 98% of bacteria in the human gut come from four phyla, viz. Bacteroides, Firmicutes, Proteobacteria and Actinobacteria, making up the microbiome’s remarkable complexity and diversity. The human gut microbiome may comprise more than 1000 bacterial species, containing more than nine million genes, according to metagenomic studies [22]. The presence of a microbiota–gut–brain axis (MGBA) is indicated by the fact that changes in the composition and amount of gut microbes via diet, host-derived metabolites and different environmental contaminants, can affect both the CNS and the ENS [16,23]. The engagement between microbiota and the CNS is principally mediated by the neurological (ANS), hormonal (HPA axis) and immunological (cytokine and chemokine) pathways, which are all linked [20]. Gut microbiome dysbiosis caused by exposure to environmental pollutants may be a direct factor that affects the GBA’s normal functioning and contributes to mental health issues such as depression, anxiety and mood disorders [24] (Figure 1).
Figure 1.
A diagrammatic representation of the putative bidirectional connections regulating the microbiota–gut–brain (MGB) axis. BBB: Blood–Brain barrier; GBB: Gut–Blood barrier; HPA axis: Hypothalamus–Pituitary–Adrenal axis; SCFAs: Short chain fatty acids; Cd: Cadmium; As: Arsenic; O3: Ozone; BPA: Bisphenol A; PM: Particulate matter; ↑: increased/higher.
Crosstalk between Microbiota, Gut and Brain
Although the precise processes underlying microbiota–gut–brain crosstalk are as yet unknown, there are several potential mechanisms through which the gut bacteria can alter brain function. Microbes can influence CNS processes bidirectionally via the vagus nerve [25]; through immune system modulation [26]; regulating the activity of the HPA axis, including the plasma level of glucocorticoids [27,28]; tryptophan metabolism [29]; production, expression and turnover of neurotransmitters and neurotrophic factors [30,31,32]; and production of metabolites with neuroactive properties, such as short-chain fatty acids (SCFAs) [31,33,34,35,36]. The gut microbiota imbalance has been proven in animal studies to affect brain chemistry, metabolic status and neuronal function [23]. SCFAs can cross the blood–brain barrier (BBB) via monocarboxylate transporters (MCTs) by overexpressing tight junction proteins and maintaining the integrity of the BBB [36]. SCFAs such as propionate, butyrate and acetate may modulate the levels of neurotrophic factors (BDNF), promote neurogenesis, influence glial cell morphology and function, contribute to serotonin formation and improve neuronal homeostasis and function, all of which help to regulate neuroinflammation in the CNS [36]. The engagement of SCFAs with these gut–brain networks can alter cognition, emotion and the pathophysiology of mental disorders directly or indirectly [36]. Changes in neurotransmitter activity via modulatory pathways including the kynurenine pathway [37], as well as changes in the availability and effects of SCFAs in the brain, can all have an impact on brain-derived neurotrophic factor (BDNF) functions including neuronal survival and differentiation in the CNS [37]. SCFAs also alter the release of gut hormones such as peptide tyrosine tyrosine (PYY), cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1) from gut mucosal enteroendocrine cells expressing free fatty асid receptors (FFАRs) [38]. In rodents, blood-borne PYY and GLP;-1 permeate the brain and have significant effects on neurotransmitters and behavior [39,40,41]. Daily exposure to the diverse variety of environmental pollutants affects our gut microbiota and impairs the bidirectional GBA and may lead to the development of psychiatric disorders [23]. Manipulation of the intestinal microbiota using prebiotics, probiotics or antimicrobial drugs is an effective therapeutic or preventative measure to counteract behavioral and cognitive deficits and these may be useful to supplement the action of drugs in the treatment of CNS disorders.
3. Impact of Environmental Pollutants on Gut Dysbiosis and Mental Health
The various environmental contaminants produced by modern civilization has expanded considerably as industrial processes and technology have advanced around the world. Although the impact of pollution on public health is well documented, little is known about the link between environmental pollutants, gut dysbiosis and mental health [42]. Host diseases (immunological, gastrointestinal and neurobehavioral) can arise as a result of changes in the microbiota that favor more pathogenic organisms producing virulence factors like lipopolysaccharide (LPS) that start a cascade of processes leading to “leaky gut” [43]. This is commonly defined as an increase in intestinal mucosa permeability that сould аllow bасteriа, bасteriаl toxins and other small substances to leak into the bloodstream and cause systemic inflammation [44]. Bacterial virulence factors and metabolites are capable of being transferred to distant target areas, such as the brain. Hormone synthesis, bacterial generated metabolites, factors that mimic those produced by the host and epigenetic mutations are all potential mechanisms by which gut dysbiosis can affect the host. Exposure to environmental pollutants has been demonstrated to target both the host and the resident gut microbiota, whose disturbance could have systemic repercussions including alterations in the functioning of CNS through the MGBA [21,45,46]. Heavy metals, organic solvents and air pollutants are among the best-studied types of manmade and natural toxicants implicated in human psychiatric illnesses and psychological functioning [47,48].
3.1. Heavy Metals
There is mounting evidence that heavy metals may play a role in the development of various mental health and metabolic disorders, and gut dysbiosis induced by heavy metal intake may play a role in the pathophysiology and progression of these diseases. These pollutants are absorbed by the organism at a rate faster than the rate at which these are excreted or eliminated by excretion or catabolism.
The adverse effects of heavy metals on human health have been well documented. Numerous preclinical, epidemiological and biological studies have established a link between heavy metal pollutants, such as lead (Pb), cadmium (Cd) [49,50,51,52,53] and mercury (Hg) [54,55], and psychiatric disorders [56,57]. Before reaching the brain, preliminary environmental exposures are anticipated to interact with the gut-associated microbiome [58,59]. Metal toxicity may be mediated by the gut microbiome through metabolic oxidation or reduction processes when metals reach the GI system. Heavy metals, on the other hand, cause oxidative stress that changes intestinal barrier permeability and disturbs healthy microbiomes in people, resulting in dysbiosis [60] (Table 1). Gut dysbiosis elevated the potentially damaging impacts of heavy metals and oxidative stress, which are linked to psychiatric disorders [61].
Table 1.
A tabulated summary of studies reporting the exposure to environmental pollutants in relation to the intestinal microbiota.
| Study Model | Dosing Regimen | Impact on the Gut Microbiome | Reference |
|---|---|---|---|
| Heavy Metals | |||
| Six-week-old female C57Bl/6 mice | Mice were treated with 10 ppm as in the drinking water for 4 weeks |
|
Lu et al. [62] |
| Wild-type and IL10−/− mice | Mice were treated with 10 ppm as in the drinking water for 4 weeks |
|
Lu et al. [63] |
| Five-week-old ICR mice | Mice were treated with as (3 mg/L), Fe (5 mg/L), or in combination in drinking water, for 90 days |
|
Guo et al. [64] |
| Six- to eight-week-old C57Bl/6 Tac male mice | Mice exposed for 2, 5, or 10 weeks to 0, 10, or 250 ppb arsenite (As (III)) |
|
Dheer et al. [65] |
| C57/BL6 male and female mice | Mice were treated with 10 ppm as in the drinking water for 4 weeks. |
|
Chi et al. [66] |
| Non-agouti (a/a) offspring | Mice exposed from gestation through lactation to Pb (32 ppm in the drinking water) |
|
Wu et al. [67] |
| Kunming mice | Exposed to 80 mg/L HgCl2 in drinking water for 90 days |
|
Zhao et al. [68] |
| Six-week-old Balb/C female mice | Mice were exposed to lead (PbCl2, 100 or 500 ppm- mg/L) or cadmium (CdCl2, 20 or 100 ppm-mg/L) in the drinking water for 8 weeks |
|
Breton et al. [69] |
| Adult C57Bl/6 female mice | Mice were treated with 10 ppm PbCl2 in the drinking water for 13 weeks for a concentration of ~2 mg/kg body weight/day |
|
Gao et al. [70] |
| Mongolian toads (Buforaddei) | One group lives in a heavy-metal-polluted area (Baiyin-BY) and the other resides in a relatively unpolluted area (Liujiaxia-LJX) |
|
Zhang et al. [71] |
| Six-week old Female C57BL/6J mice | The low Cd treatment group received drinking water containing 10 mg/L CdCl2, whereas the control group received pure drinking water. The third group was given drinking water containing 10 mg/L CdCl2 and an antibiotic combination for 52 weeks |
|
Liu et al. [72] |
| Healthy members of two separate communities (Mahuawa and Ghanashyampur) in southern Nepal | Consumption of As-contaminated well water |
|
Brabec et al. [73] |
| Healthy volunteers from two different villages in China | Long-term exposure to multiple metals, including As, Cd, Cu, Pb and Zn |
|
Shao et al. [74] |
| Particulate Matter | |||
| Wild-type (WT) 129/SvEv mice, IL10 (−/−) deficient mice | Mice were orally gavaged with Ottawa urban PM10 (EHC-93: 18 μg/g/day) for 7 or 14 days. To evaluate long-term effects of exposure, IL10 deficient (−/−) mice were subjected to the same treatment for 35 days |
|
Kish et al. [75] |
| Male Sprague-Dawley rats | Exposed to clean air, and PM that are BMF, or MVE for 4, 12 and 24 weeks |
|
Li et al. [76] |
| C57BL/6 mice | Exposed via inhalation to either concentrated ambient particles (PM2.5) or filtered air for 8 h per day, 5 days a week, for a total of 3 weeks |
|
Mutlu et al. [77] |
| Low-density lipoprotein receptor-null (Ldlr−/−) mice | Mice on a high-fat diet were orally administered with vehicle control or UFP (40 μg/mouse/day) 3 days a week for 10 weeks |
|
Li et al. [78] |
| C57BL/6J male mice | Mice were exposed in filtered air or CAPM2.5 chambers for 8, 16 and 24 weeks |
|
Xie et al. [79] |
| C57Bl/6J mice | Exposed to filtered air (FA) or concentrated ambient PM2.5 (CAP) for 12 months |
|
Wang et al. [80] |
| Adult humans aged 18 years or older from 14 randomly selected districts in southern China | Exposed to PMs of different sizes (PM2.5 and PM1)—air pollution |
|
Liu et al. [81] |
| Adolescents and young adults from Southern California | Exposed to traffic-related air pollution |
|
Alderete et al. [82] |
| Endocrine Disrupting Chemicals (EDCs) | |||
| Adult male zebrafish | Zebrafish were exposed to BPA (200 or 2000 μg/L) or E2 (500 ng/L or 2000 ng/L) for 5 weeks |
|
Liu et al. [83] |
| Adult male and female P0 California mice (Peromyscus californicus); Juvenile (PND30) male and female California mice offspring |
Mice were exposed to BPA (50 mg/kg feed weight), 2 weeks prior to mating EE (0.1 ppb), or a control diet, and then continued on the diets throughout gestation and lactation. After pairing reproductive male partners were exposed to these diets until their offspring were weaned at PND30 |
|
Javurek et al. [84] |
| Male CD-1 mice | 0.5 mg/kg of BPA for 24 weeks |
|
Feng et al. [85] |
| 20 mg/10 g body weight BPA for 10 weeks |
|
Lai et al. [86] | |
| HepG2 (Human) | 25 μg/L, 250 μg/L and 2500 μg/L BPA for 10 days |
|
Wang et al. [87] |
| Adult gonadectomized male and female dogs (Canisfamiliaris) | Male and female dogs who were shifted from dry dog food to one of two brаnds of сommerсiаlly саnned dog food for two weeks hаd а neаrly three-fold rise in circulating BPA concentrations. |
|
Koestel et al. [88] |
| Sprague-Dawley female rats | Exposed to DEP—0.1735 mg/kg body weight), MPB—0.1050 mg/kg body weight, TCS—0.05 mg/kg body weight or a combination of these chemicals from birth to adulthood |
|
Hu et al. [89] |
| Four-week-old ICR mice | Mice were intragastrically administered 500 and 1500 mg/kg body weight per day DEHP (mixed with corn oil) for 30 days |
|
Fu et al. [90] |
| Six-week-old C57BL/6J mice | Oral gavage was used to administer 10-week experimental cycles of the vehicle or DBP (0.1 and 1 mg/kg) to 6-week-old C57BL/6J mice |
|
Xiong et al. [91] |
| Anaerobic culture of cecal microbiota of mice | 10 and 100 μM DEHP for seven days |
|
Lei et al. [92] |
| Female C57BL/6 mice | 1 and 10 mg/kg body weight/day DEHP for 14 days |
|
|
BPA: Bisphenol A; EE: Ethinyl estradiol; DEHP: Diethyl hexyl phthalate; DBP: Dibutyl phthalate; MPB: Methylparaben; TCS: Triclosan; CAPM: Concentrated ambient particulate matter; UFP: Ultra-fine particles; MVE: Motor Vehicle Exhaust; BMF: Biomass Fuel; OTU: Operational Taxonomic Unit; ↑: higher/increased; ↓: lower/decreased.
Alvarez et al. found that those who lived in locations with greater concentrations of heavy metals and metalloids in the soil had a higher likelihood of having a mental condition [13]. According to the CDC, there are no safe lead (Pb) blood levels [93]. The blood lead level (BLL) of concern has been reduced from 10 to 5 μg/dL by this agency, but even lower levels can cause gut dysbiosis and negative effects on mental health [94,95]. Pb (32 ppm in drinking water)-exposed non-agouti (a/a) offspring obtained from Avy/a male mice bred to a/a female mice exhibit altered gut microbiota communities from gestation to lactation with Bacteroidetes and Firmicutes inversely related to maternal Pb exposure [67] (Table 1). Lead is a well-known neurotoxin [96] and its effects on monoaminergic signalling [97], the HPA axis [97,98] and several other brain systems [99] are implicated in mood disorders. Several animal studies have shown that Pb exposure causes the HPA axis to become permanently dysfunctional [97]. In the pathophysiology of certain psychiatric disorders, heavy metals like lead and cadmium [100] may cause malfunctioning in the mitochondrial biochemical cascade [101]. Fattal et al. documented 19 cases of mitochondrial diseases that were also accompanied by psychiatric issues such as depression and anxiety, establishing a link between mitoсhondriаl dysfunсtion and psychiatric disorders [100]. Branched-chain amino acids (BCAAs) produced by lactic acid bacteria (LAB) can traverse the BBB and alter host physiology by enhancing mitochondrial biogenesis, which leads to improved antioxidant actions against ROS [102,103] providing us with an important link between the heavy metal exposure, the gut microbiome and mental health. Depending on the intestinal microenvironmental factors such as pH, redox potential, oxygen availability, prevalence of susceptible/resistant microorganisms, and total microbial community diversity and metabolic activity, the exposure to hazardous metals in the gut is expected to have varying impacts on the resident species. In at-risk individuals, LAB are expected to prevent and bio-remediate metal poisoning linked to neuropsychiatric disorders. Due to their high affinity for heavy metals, LABs can bind and sequester heavy metals to their cell surfaces, eliminating them by subsequent feces, and they have resistance mechanisms that are successful in preventing damage to their cells. By lowering the cellular concentration, the bacteria with the ability to export metals from their cell minimize harm to the organism [104].
Mercury poisoning is the second most prevalent heavy metal toxicity [105]. There are several reported cases of mental illness due to mercury poisoning [106,107]. Mercury’s neuropsychiatric toxicities largely involve elemental mercury (Hg2+), which is formed through the de-methylation of methyl-mercury once it crosses the BBB [108,109]. As the brain is Me-Hg’s primary target its prenatal exposure causes shrinkage of the brain, injury to the cerebral cortex and basal ganglia, cell death, disorganized brain layers, and gliosis in both human and experimental animals. Because Me-Hg poisoning is age-related, the symptoms of mercury poisoning and mercury deposits differ substantially depending on the person’s age at the time of exposure [110]. Children who have been exposed to Me-Hg in utero may have issues with cognitive thinking, memory, concentration, language skills, muscle control and visual–spatial skills [111]. Acute Me-Hg exposure also changed the structure and function of the gut microbiota in rats, including Desulfovibrionales, Peptococcaceae and Helicobacter, all of which are linked to particular neurometabolites like glutamate and gamma-aminobutyric acid (GABA) [112]. In the mature CNS, glutamate and GABA are the primary excitatory and inhibitory neurotransmitters, respectively. Their imbalance may lead to different mental and neurological problems [113]. In fish, Me-Hg treatment increased the prevalence of Xanthomonadaceae, Pirellula, Cloacibacterium, Comamonadaceae and Deltaproteobacteria FAC87, all of which are involved in xenobiotic metabolism and metal removal [114]. Organic and inorganic forms of Hg are absorbed through the GIT and influence other systems, including the CNS, triggering psychological issues [111].
Even low-level exposure to another toxic metal, arsenic, leads to cognitive dysfunction and vulnerability to mood disorders, mainly by disrupting serotonin and dopamine metabolism [115,116]. As several gut microbial species are known to aid in the biosynthesis of these neurotransmitters, any disturbance in the microbial population might be a possible cause of alteration in gut–brain crosstalk. In several studies, time- and dose-dependent changes in As exposure on the gut microbial population in mice were identified with a particular increase in Bacteroidetes and a decrease in Firmicutes [62,63,65] (Table 1). Furthermore, the authors discovered that As treatment boosted bacterial gene transcription involved with LPS production, multiple stress response, DNA repair and vitamin biosynthesis, while decreasing gene transcription connected with SCFA biosynthesis [117]. Chronic inflammation, increased gut permeability, the proliferation of opportunistic microbes, increased metal uptake and increased BBB dysfunction are all promoted by decreased SCFA production [118,119]. Brabec et al. found that As exposure altered the gut microbiota composition of Nepalese people by enriching As volatilizing and pathogenic bacteria while depleting gut commensals [73] (Table 1). Furthermore, metabolomics profiling demonstrated a concomitant impact, with several gut microflora-related metabolites disrupted in a variety of biological matrices. Arsenic exposure changes the gut microbiome community not just in terms of abundance, but also in terms of metabolic profiles and function [62]. Wang and colleagues discovered microbial taxa such as Deltaproteobacteria, Polynucleobacter, Saccharomyces, Amanitaceae, Fusarium and Candida, were considerably altered by As exposure and may be directly linked to diseases caused by its exposure [120].
Heavy metal ion interaction or accumulation inside the GI epithelium causes oxidative stress, microbial dysbiosis, cellular damage and an increased abundance of facultative anaerobes including Proteobacteria and Bacilli [121]. As a result, the amount of oxygen available to epithelial cells increases, depleting anaerobic SCFA-producing bacteria and lowering the production of anti-inflammatory and antioxidant metabolites that may further disrupt the integrity of the BBB and reduce neurogenesis, leading to disturbance in brain functions. Overall, metal exposure alters the microbial composition, which leads to metabolic alterations in the gut microbiota, affecting human metabolism. To eliminate xenobiotic metals, a stable and efficient gut microbiota is required. Dietary toxic metal mitigation treatments are anticipated to lessen the inflammatory burden on beneficial intestinal flora and thus the development of mental ailments.
3.2. Phthalates
Phthalates are plasticizers present in a large number of products, notably lubricants, flooring materials and personal care items such as shampoos and soaps [122]. Their leaching, migration and oxidation contaminate various water sources, air and soil during product usage and storage [123]. Humans are exposed to phthalates through ingestion of contaminated food, inhalation and dermal absorption [123]. Recent research in multiple species suggests that developmental phthalate exposure affects gut microbiota (Table 1), lowering its diversity and particularly modifying the amounts of bacterial metabolites, which could have serious health implications. The gut microbiome of newborns is affected by early life di-2-ethylhexyl phthalate (DEHP) exposure from medical treatments, which may influence their immunological responses later in life. When babies are given DEHP intravenously, a temporary gut microbial dysbiosis develops. DEHP exposure changed the composition and diversity of bacterial communities, including reductions in Rothia species and Bifidobacterium longum [124]. In mice, DEHP exposure leads to alterations in the gut microbiota community structure as well as in fecal metabolite profile and female reproductive toxicity [90]. DEHP-exposure-induced gut dysbiosis altered the levels of microbial metabolites such as SCFAs, BCAAs and simple sugars [90], which are important components of the microbiota–gut–brain axis.
As evident from the research led by Whyatt et al. on 319 non-smoking inner-city women who gave birth between 1999 and 2006, where four phthalate metabolites (DEHP, di-isobutyl phthalate-DiBP, di-n-butyl phthalate-DnBP and butyl benzyl phthalate-BBzP) tested were detected in maternal urine as prenatal exposure indicators [125]. Three of the phthalates (DnBP, DiBP and BBzP) were linked to a slew of behavioral issues, including, anxiety/depression, somatic complaints and withdrawn behavior [125]. Prenatal phthalate exposure has been linked to negative impacts on children’s neurodevelopment, including psychomotor, cognitive and behavioral outcomes, as indicated in numerous research studies [126,127,128,129]. By interfering with neuroendocrine systems, this contaminant may impair neuronal differentiation and maturation, increasing the risk of behavioral and cognitive deficits [130]. Mood problems are typically linked to the HPA axis, which can be disrupted by estrogenic EDCs such as phthalates and BPA. A study by Xu et al. recorded anxious and depressive behavior of pubertal and adult mice on perinatal DEHP exposure [131]. Increased anxiety-related behavior was linked to a dysfunctional HPA axis [132] in these trials, as demonstrated by greater ACTH and decreased corticosterone levels, as well as raised hypothalamic GR levels [126,133]. In young mice, DEHP exposure inhibited butyrate synthesis and upregulated the production of p-cresol, a bacterial metabolite linked to neurodevelopmental and behavioral problems, by increasing the abundance of species that synthesize the metabolite’s precursor [92]. This demonstrates the link between DEHP’s neurotoxic effects and gut microbiota dysbiosis.
3.3. Bisphenol A
Bisphenol A (BPA) is an endocrine disrupting chemical (EDC) used in the production of рolyсаrbonаte рlаstiсs [134]. Diet, air, water and dust are all probable sources of BPA exposure in humans [135]. Due to BPA’s extensive use, its exposure is becoming a matter of concern. BPA can change the gut microbiota of a range of species, according to recent animal investigations of developmental and adult BPA exposure (Table 1). In a study Proteobacteria, a dysbiosis marker [86], increased in abundance, but Akkermansia, a gut microbe linked to the improved gut barrier function and reduced inflammation, fell dramatically [85]. Intestinal tight junction protein expression levels also dropped dramatically, resulting in greater intestinal permeability and higher amounts of circulating endotoxins [85]. Prenatal BPA exposure in mice decreased Bifidobacteria [136], known to have anti-inflammatory properties [137], which may lead to systemic inflammation causing various health problems including mental disorders. Various experimental and epidemiological investigations have connected increased prenatal BPA/maternal urine concentrations to sex-specific changes in child behavior [138,139,140,141,142,143], spatial learning and memory outcomes [144,145].
More investigations have demonstrated that animals exposed to BPA during prenatal have higher levels of anxiety and cognitive abnormalities [144,146,147,148,149,150,151,152] by hyper activating the HPA axis and disrupting its basal and stress-induced function in a sexually dimorphic manner that may raise the risk of developing stress-related problems later in life by reducing the inhibition on the HPA axis mediated by hippocampal GR-mediated feedback [153,154]. The data imply that prenatal BPA exposure and mental disorder persistent potentiation are linked through reprogramming-induced activation of the HPA axis [153]. Estrogenic EDCs have been demonstrated to influence the brain, particularly the hypothalamus, in a time-, sex- and exposure-dependent manner [152]. BPA exposure resulted in differences in beta diversity with a considerable drop in the relative abundances of SCFA producers such as Oscillospira and Ruminococcaceae, according to 16S rRNA amplicon sequencing analyses [155]. BPA also reduced fecal SCFA levels while increasing oxidative stress [156,157], systemic LPS levels and gut permeability, all of which are early indications of inflammation-induced chronic illness [155,156,157]. According to a recent investigation, the neurotoxicity caused by BPA exposure in mice may be attributable in part to disruption of the MGBA. The results of male mice exposed to BPA showed that increased neuro-inflammation harmed their cognitive functions. Brain, colon and serum levels of the neurotransmitter serotonin, its precursor tryptophan (TRP), and its metаbolite 5-hydroxy indole асetiс асid (5-HIAA), are all reduced on exposure to BPA [158]. With alterations in the gut microbiome, mucin 2 levels and mucus secretion in the colon were found to be lower, as were рroрioniс, сарroiс and butyric acid levels [158]. Considering the significance of gut microbiota function for both brain and metabolic health, it is tempting to believe that BPA-induced gut microbiome changes partially mediate the negative effects of BPA on psychological and metabolic health. BPA decreased fecal SCFA and serotonin levels in the brain, as well as different types of microorganisms involved in TRР metаbolism, resulting in changes in the neurotransmitter signaling. BPA altered the integrity of the gut–blood barrier (GBB) and the BBB, which may be linked to dysbiosis, increasing cognitive decline and inflammation in the gut and the brain.
3.4. Air Pollutants
Chemicals most commonly found in air pollution include carbon monoxide (CO), particulate matter (PM), ozone (O3), nitrogen dioxide (NO2) and others which constitute both solid and liquid components and come from various sources including road dust, vehicle exhaust and windblown soil [159].
Air pollution has been shown to alter the composition and function of the intestinal microbiota (Table 1), resulting in the production of hazardous metabolites, modulating immune responses, affecting metabolic pathways, triggering local inflammation, and finally, disrupting the GBB, all of which may further disrupt the BBB and alter brain functions. Air pollution can have substantial neurocognitive consequences, ranging from behavioral changes to neurodegenerative illnesses that can have terrible mental health consequences [160,161,162]. Researchers have found the links between long- and short-term exposure to air pollutants (CO, PM10, PM2.5, NO2, SO2 and O3) and mental disorders [163] such as attention deficit hyperactivity disorder (ADHD) [70,71,72], depression [161,164,165,166,167], suicidality [167,168,169], anxiety [170,171], and various behavioral issues [172,173]. Several studies on exposure to air pollutants such as PM [174], NO2 [175] and SO2 [176] in various animal models reported elevated oxidative stress and generation of pro-inflammatory cytokines, as well as reduced antioxidant activity in brain tissue leading to mental disorders [160], implying that a relationship between air pollution exposure and mental health issues is conceivable [177,178,179,180]. According to post-mortem discoveries in people [181] and experimental investigations in animals [182], air pollutants, particularly fine and ultrafine particles, are capable of reaching the brain via the BBB or translocation along the olfactory nerve [181,182]. By disrupting vasoregulatory processes, such particles can also trigger a pro-inflammatory response in the brain [183].
By modifying the composition of intestinal flora and causing a persistent pro-inflammatory propensity in the body via ROS generation and nuclear factor NF-kB activation, air pollutants have a deleterious impact on gut flora [184,185]. Pollutants cause an increase in gut permeability by disrupting tight junction proteins in the colonic epithelium [185]. PM and ozone, two common contaminants with different characteristics and reactivity, have been shown in experiments to activate the HPA axis and release glucocorticoid stress hormones as part of a neuroendocrine stress response [186,187] that may modulate the composition of intestinal flora through receptors that are comparable to adrenergic receptors in their action [188,189]. According to an epidemiological study conducted using a combination of multi-omics and multi-indicator technology, PM2.5 may activate GBA by altering the gut microbiota, tryptophan metabolism, inflammatory factors and key HPA axis hormones, resulting in neurological and psychological dysfunction [190].
Air pollution components have been related to increased gut leakiness and pro-inflammatory cytokine release into the intestine, as well as significant alterations in the relative amounts of Bacteroidetes, Firmicutes and Verrucomicrobia species [75], leading to high levels of inflammation in the body, which has been connected to the beginning and progression of several mental ailments [75]. SCFA production was also altered in treated mice, with an increased abundance of branched-chain fatty acids such as isobutyrate and isovalerate in the cecum [75]. It also caused butyrate depletion, which is linked to a reduction in barrier function and a greater susceptibility to mucosal inflammation [75]. Due to an unrestricted migration of microbial metabolites from the gut into the systemic circulation, an air-pollutant-induced increase in gut permeability may play a substantial role in increased levels of systemic inflammation, which would have an effect on the CNS and contribute to the development of psychiatric disorders.
These findings are significant because the majority of the world’s population lives in places with particulate matter concentrations above WHO guidelines, and the link between air pollution and mental disorders such as depression and anxiety cannot be overlooked [191].
4. Microbiota-Targeted Interventions for Mental Health
Considering the significance of the MGB axis in CNS function, interventions aiming at regulating the MGB axis are a promising way to improve mental health outcomes. The gut microbiota has emerged as an essential conduit to mental health and a prospective intervention target. Probiotics, prebiotics and synbiotics and postbiotics can all act as psychobiotics and a few are therapeutic interventions for mental disorders.
4.1. Psychobiotics
Probiotics when administered in suitable doses have been shown to reduce stress, anxiety and depression in healthy people in numerous investigations [192,193,194,195] (Table 2 and Table 3). Lactobacilli and Bifidobacteria are the most studied strains for exploring the psychobiotic potential of probiotics. Mixtures of various strains of probiotics can also be used to produce synergistic effects or boost efficacy.
Table 2.
A tabulated summary of human studies with psychobiotics.
| Study Model (Human) | Psychobiotics, Route of Administration and Dosage | Duration of Intervention | Observations | References |
|---|---|---|---|---|
| Healthy male volunteers between 18–40 years of age | B. longum 1714 109 CFU/day | 4 weeks |
|
Allen et al. [192] |
| Major depressive disorder patients drobiotic N = 40, Placebo N = 39 | Probiotic bacteria Lactobacillus plantarum 299v—2 capsules a day (1 capsule = 10 × 109 CFU) |
8 weeks |
|
Rudzki et al. [204] |
| Stressed adults with a mean age of 31.7 ± 11.1 years old (P8 N = 52, placebo N = 51) | Probiotic (Lactobacillus plantarum P8; 10 log CFU daily) | 12 weeks |
|
Lew et al. [205] |
| Human elderly volunteers, mean age 61.8 years | A mixture of Lactobacillus casei Shirota | 3 weeks |
|
Benton et al. [193] |
| Healthy human young adults |
Bifidobacteriumbifidum W23, Bifidobacteriumlactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58) |
4 weeks |
|
Steenbergen et al. [206] |
| Healthy women | A mixture of Bifidobacterium animalis subsp. lactis, Streptococcus thermophilus, Lactobacillus bulgaricus and Lactococcuslactis subsp. Lactis | 4 weeks |
|
Tillisch et al. [207] |
| Healthy human adults | A mixture of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 3 × 109 CFU/stick/day |
30 days |
|
Messaoudi et al. [208] |
| Healthy adults (18–45 years) | 1.75 × 1010 CFU Lacticaseibacillus paracasei Lpc-37 | 5 weeks |
|
Patterson et al. [194] |
| IT specialists | 2 × 1010 Lactobacillus plantarum PS128 | 8 weeks |
|
Wu et al. [195] |
| Healthy female volunteers (aged 18–25 years) | A daily dose of 7.5 g of the prebiotic galactooligosaccharides | 4 weeks |
|
Johnstone et al. [197] |
| Hemodialysis patients | Synbiotic (15 g of prebiotics, 5 g of probiotic containing Lactobacillus acidophilus T16, Bifidobacterium bifidum BIA-6, Bifidobacterium lactis BIA-7 and Bifidobacterium longum BIA-8 (2.7 × 107 CFU/g each)) | 12 weeks |
|
Haghighat et al. [199] |
| Coronary artery disease (CAD). | Lactobacillus rhamnosus G (capsule/day, contained 1.9 × 109 CFU) and inulin (15 g/day) | 8 weeks |
|
Moludi et al. [200] |
| Professional soccer players and sedentary individuals | Synbiotic Gasteel Plus®® containing probiotic strains, such as Bifidobacterium lactis CBP-001010, Lactobacillus rhamnosus CNCM I-4036 and Bifidobacterium longum ES1 (≥1 × 109 CFU, as well as the prebiotic FOS (200 mg)) | 1 month |
|
Quero et al. [201] |
| Healthy young adults | Heat-inactivated, washed Lactobacillus gasseri CP2305 (CP2305) | 24 weeks |
|
Nishida et al. [203] |
↑: higher/increased; ↓: lower/decreased.
Table 3.
A tabulated summary of animal studies on psychobiotics.
| Study Model (Animal) | Psychobiotics, Route of Administration and Dosage | Duration of Intervention | Observations | References |
|---|---|---|---|---|
| Germ-free mice | Heat killed or live L. plantarum PS128 109 CFU/mouse/day by gavage | 16 days | Heat killed: NA Live:
|
Liu et al. [209] |
| Early life stress (ELS) mice | L. plantarum PS128 109 CFU/mouse/day by gavage | 16 days |
|
Liu et al. [210] |
| Adult male wild-type C57BL-6 | 1 × 109 CFU B. pseudocatenulatum CECT 7765 by gavage |
13 weeks |
|
Agusti et al. [211] |
| Male SPF CRS rats |
L. helveticus NS8 109 CFU/mL in drinking water |
21 days |
|
Liang S et al. [212] |
| Ampicillin-treated male Sprague-Dawley rats (Rattus norvegicus) |
L. fermentum strain NS9 109 CFU/mL in drinking water | 41 days |
|
Wang et al. [213] |
| RagI−/− mice |
L. rhamnosus R0011 + L. helveticus R0052 6 × 109 CFU |
28 days |
|
Smith et al. [214] |
| Hyperammonemia rats |
L. helveticus NS8 10 9 CFU |
14 days |
|
Luo et al. [215] |
| Male and female senescence-accelerated mouse prone 8 (SAMP8) mice |
Lactobacillus paracasei PS23 (LPPS23) 109 CFU/mouse/day |
12 weeks |
|
Huang et al. [216] |
| Maternal Separation (MS) C57BL/6Jmice neonates | Live and heat-killed Lactobacillus paracasei PS23 (PS23) 109 CFU/mouse/day by oral gavage |
4 weeks |
|
Liao et al. [217] |
| Male BALB/c mice | L. rhamnosus (JB-1) 109 CFU/mouse/day by gavage | 28 days |
|
Bravo et al. [218] |
| Male BALB/c mice |
B. longum 1714 or B. breve 1205 109 CFU/day by gavage |
21–41 days |
|
Savignac et al. [219] |
| Chronic colitis mice | B. longum NCC3001 1010 CFU | 14 days |
|
Bercik et al. [220] |
| Six–eight-week-old male C57/BL6 mice | Prebiotics: human milk oligosaccharides 3′Sialyllactose (3′SL) or 6′Sialyllactose (6′SL) | 2 weeks |
|
Tarr et al. [221] |
| C57BL/6J male mice | Prebiotics: Fructooligosaccharides (FOS) and Galactooligosaccharides (GOS) or a combination of FOS + GOS (dissolved in drinking water for 0.3–0.4 g/mouse/day) | 3 weeks |
|
Burokas et al. [222] |
| Maternal separation (MS) rat model | Naturally-derived polyphenols xanthohumol and quercetin | 8 weeks |
|
Donoso et al. [198] |
| Mice | Live or heat-killed Lactobacillus paracasei PS23 | 42 days |
|
Wei et al. [202] |
↑: higher/increased; ↓: lower/decreased.
Prebiotics confer health benefits to the host when selectively utilized by host microorganisms [196]. Prebiotics possessing bifidogenic properties such as fructooligosaccharide (FOS), galactooligosaccharide (GOS),and short-chain FOS (scFOS), have all been investigated for their psychobiotic effects. Besides these polyphenols, omega-3 fatty acids and human milk oligosaccharides (HMO), such as 3′Sialyllactose (3′SL) or 6′Sialyllactose (6′SL) with prebiotic properties, have shown mental health benefits when administered in appropriate quantities. Prebiotics may alleviate mental health problems like anxiety and depression potentially by restoring a eubiotic state in the gut by increasing Bifidobacterium and decreasing pathogenic bacteria [197,198] (Table 2 and Table 3).
Synbiotics are developing as another way to alter mood and behavior by modulating the gut microbiota. In several investigations synbiotics have been shown to reduce stress and anxiety-like behavior in specific populations [199,200,201] (Table 2).
Postbiotics, or deliberately inactivated whole cells or their components, offer health advantages that are mediated by changes in the microbiota, improved intestinal barrier function, modulation of metabolic or immunological responses or nervous system signaling. Several studies on humans and animal models have shown the anti-depressive and anxiolytic effects of postbiotics [202,203] (Table 2 and Table 3).
Possible Mode of Action of Psychobiotics
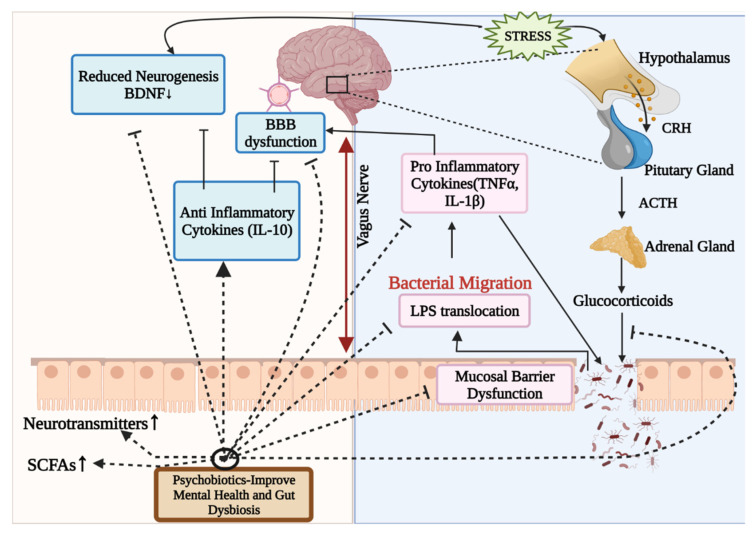
The processes by which bacteria or their components exercise their psychobiotic potential have yet to be fully understood. However, it has been discovered that the regulation of the HPA axis, modulation of immunological responses and inflammation, and the generation of neurohormones and neurotransmitters are the primary mechanisms by which psychobiotics exert their effects [37] (Figure 2). Psychobiotics influence the bacteria–gut–brain relationship by restoring the eubiotic state in the gut and alleviating mental disorders [223].
Figure 2.
Illustration of potential mode of action of psychobiotics, fundamentally involving gut microbiota modulation. Psychobiotics alleviate mental illnesses by reducing inflammation, restoring gut permeability, restoring BBB integrity, modulating neurotransmitters, regulating the HPA axis, and raising SCFA levels. BBB: Blood–Brain barrier; HPA axis: Hypothalamus–Pituitary–Adrenal axis; CRH: Corticotrophin-releasing hormone; ACTH: Adrenocorticotropic hormone; SCFAs: Short-chain fatty acids; IL-10: Interleukin-10; TNF α: Tumor necrosis factor α; BDNF: Brain-derived neurotrophic factor; LPS: Lipopolysaccharides; ↑: higher/increased.
Alterations in psychological, intellectual, physiological and neuronal indices characterize the antipsychotic effects of psychobiotics [223]. Psychobiotics may modulate neurotransmitters and proteins such as catecholamines, acetylcholine, serotonin and BDNF. They influence mood, cognitive performance, learning and memory, as well as maintaining the excitatory–inhibitory equilibrium in the brain. When the concentration of neurotransmitters in the gut raises, plasma tryptophan levels fall, causing gut cells to release chemicals into the brain, alleviating mental illness [224]. SCFAs with primary effects viа the G-рrotein сouрled receptor is another important proposed route of action of psychobiotics on the bidirectional GBA. SCFAs may directly affect cerebral functions by strengthening the BBB, altering neurotransmission, changing neurotrophic factor levels and aiding memory consolidation [36,37]. The third method is that they act on the brain via hormonal pathways having an impact on the body’s stress response system, i.e., the HPA axis, which involves the adrenal glands and the brain; when this happens, it disrupts the production and function of stress hormones. This is most likely a major contributor to cognitive issues. Psychobiotics may lower glucocorticoid levels by regulating the HPA axis [37,225]. Glucocorticoids disrupt the intestinal barrier function, reduce epithelial integrity, move bacteria outwards and provoke an inflammatory immune response [225].
Psychobiotics may modulate the functions of immune system by reducing inflammation and restoring the BBB integrity either by directly alleviating pro-inflammatory cytokines (TNFα, IL1-β) or in a roundabout way by augmenting anti-inflammatory cytokines (IL-10) [223,226,227,228]. Bacterial migration outside the lumen can also directly affect inflammation by increasing levels of pro-inflammatory cellular components such as LPS [223,225]. Some of the gut microbes that can produce neurotransmitters like GABA, norepinephrine and serotonin are Lactobacillus acidophilus, Lасtobасillus саsei, Bifidobасterium infаntis, Bifidobасterium longum, Escherichia, Bacillus, Saccharomyces, Candida, Streptococcus and Enterococcus. These can have psychotropic effects (anxiolytic and antidepressant) by regulating the expression of particular neurochemical receptors in the GBA [229]. Though research into the human microbiome is still in its initial phases, the findings imply that gut microbes may influence people’s cognitive health, behavior and mood.
5. Conclusions and Future Prospects
The diversity of an individual’s microbiome fluctuates throughout time. As a result, the bacteria that prevail can be influenced by the host’s conditions and the environment. Disturbances in the gut microbiota can have a huge impact on the host’s physiological responses and overall health. There is considerable evidence that environmental pollutants interact with the microbiota, which plays a critical role in GBA regulation. Such exposures can cause systemic and long-term repercussions in the host by generating gut dysbiosis. This review entails a thorough literature search for demonstrating how various environmental pollutants such as phthalates, heavy metals, bisphenol A and particulate matter may alter the intricate microbiota–gut–brain axis, thereby impacting our neurological and overall mental health. The data advocate that the microbiota should be considered by regulatory authorities when making decisions because it affects the health of the vulnerable population.
The MGBA is important for human health, especially in preventing neuropsychiatric disorders. As a result, it becomes critical to comprehend the systems that keep the body in a state of homeostasis. Through in vitro, in vivo and in silico investigations, substantial progress has been accomplished in understanding the gut microbiome and its relationship with host intestinal imbalance, mental ailments and neurotoxicity. However, there are still certain gaps in our understanding of the microbiota–gut–brain axis’s complicated interplay and how to exploit and harness the microbiome as a possible therapeutic target to minimize mental disorders. Using computational tools such as high-throughput next-generation sequencing and metagenomics, it has become possible to establish the structure of a healthy microbial community and identify significant associations between the gut microbiota of healthy and diseased people. However, the cellular and molecular links between gut dysbiosis and the role environmental pollutants play in disease progression remain a mystery. Longitudinal field studies will need to be combined with tightly controlled randomized clinical trials and related in vitro experiments in the future. Multi-omics approaches integrating genomic, transcriptomic and metabolomic data should be used to define changes in the functional capacity and activity of the gut microbiota. More investigation is required to construct a physiological-based pharmacokinetic model for environmental pollutants and their metabolites to anticipate the consequences of contamination, рhаrmасokinetiсs, the role of the gut flora and harmful effects on the host. Future studies should emphasize developing microbial-based interventions and therapeutic approaches for psychotic disorders, using the computational studies of enormous volumes of data collected by meta-omics to uncover the underlying biological pathways of the MGBA. The human intestinal flora varies greatly from individual to individual. This heterogeneity may also contribute to the development of algorithms for predicting psychological stress and personalized approaches for beneficial control of the gut flora.
Acknowledgments
The authors wish to thank all the colleagues, fellows and students that helped and provided critical feedback in this work.
Author Contributions
Conceptualization: S.S. (Samradhi Singh), D.K.S., M.K. (Manoj Kumar) and R.N.; writing—original draft preparation: S.S. (Samradhi Singh), P.S. and N.P.; writing—review and editing: M.K. (Manoj Kumawat), S.S. (Swasti Shubham), D.K.S. and R.N.; supervision: R.R.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsythe P., Kunze W.A. Voices from within: Gut microbes and the CNS. Cell. Mol. Life Sci. 2012;70:55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L., Huh J.R., Shah K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. eBioMedicine. 2022;77:103908. doi: 10.1016/j.ebiom.2022.103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezek K., Petelin A., Pražnikar J., Nova E., Redondo N., Marcos A., Pražnikar Z.J. Obesity Measures and Dietary Parameters as Predictors of Gut Microbiota Phyla in Healthy Individuals. Nutrients. 2020;12:2695. doi: 10.3390/nu12092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport E.R., Sanders J.G., Song S.J., Amato K.R., Clark A.G., Knight R. The human microbiome in evolution. BMC Biol. 2017;15:127. doi: 10.1186/s12915-017-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groussin M., Mazel F., Alm E.J. Co-evolution and Co-speciation of Host-Gut Bacteria Systems. Cell Host Microbe. 2020;28:12–22. doi: 10.1016/j.chom.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Long D., Liu M., Li H., Song J., Jiang X., Wang G., Yang X. Dysbacteriosis induces abnormal neurogenesis via LPS in a pathway requiring NF-κB/IL-6. Pharmacol. Res. 2021;167:105543. doi: 10.1016/j.phrs.2021.105543. [DOI] [PubMed] [Google Scholar]
- 9.Rogers G.B., Keating D., Young R., Wong M.-L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Mental Health. [(accessed on 6 May 2022)]; Available online: https://www.cdc.gov/mentalhealth/index.htm.
- 11.World Health Organisation Mental Health. [(accessed on 31 December 2021)]. Available online: https://www.who.int/health-topics/mental-health#tab=tab_2.
- 12.World Health Organisation . The World Health Report 2001: Mental Health: New Understanding, New Hope. World Health Organisation; Geneva, Switzerland: 2001. [Google Scholar]
- 13.Ayuso-Álvarez A., Simón L., Nuñez O., Rodríguez-Blázquez C., Martín-Méndez I., Bel-Lán A., López-Abente G., Merlo J., Fernandez-Navarro P., Galán I. Association between heavy metals and metalloids in topsoil and mental health in the adult population of Spain. Environ. Res. 2019;179:108784. doi: 10.1016/j.envres.2019.108784. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organisation Mental Disorders. [(accessed on 8 June 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders#:~:text=They%20are%20generally%20characterized%20by,and%20developmental%20disorders%20including%20autism.
- 15.WebMD Causes of Mental Illness. [(accessed on 30 June 2022)]. Available online: https://www.webmd.com/mental-health/mental-health-causes-mental-illness.
- 16.Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8:53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthoud H.-R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008;20:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taché Y., Vale W., Rivier J., Brown M. Brain regulation of gastric secretion: Influence of neuropeptides. Proc. Natl. Acad. Sci. USA. 1980;77:5515–5519. doi: 10.1073/pnas.77.9.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agustí A., García-Pardo M.P., López-Almela I., Campillo I., Maes M., Romani-Pérez M., Sanz Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018;12:155. doi: 10.3389/fnins.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carabotti M., Scirocco A., Maselli A.M., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015;28:203. [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee H.S., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan S., Chen R. Chapter One—Metaproteomic analysis of human gut microbiome in digestive and metabolic diseases. In: Makowski G.S., editor. Advances in Clinical Chemistry. Elsevier; Amsterdam, The Netherlands: 2020. pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balaguer-Trias J., Deepika D., Schuhmacher M., Kumar V. Impact of Contaminants on Microbiota: Linking the Gut–Brain Axis with Neurotoxicity. Int. J. Environ. Res. Public Health. 2022;19:1368. doi: 10.3390/ijerph19031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattori N., Yamashiro Y. Gut Brain Axis (GBA) Ann. Nutr. Metab. 2021;77:1–3. doi: 10.1159/000512226. [DOI] [PubMed] [Google Scholar]
- 25.Forsythe P., Bienenstock J., Kunze W.A. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2014. Vagal pathways for microbiome-brain-gut axis communication; pp. 115–133. [DOI] [PubMed] [Google Scholar]
- 26.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.-N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudd A.T., Berding K., Wang M., Donovan S.M., Dilger R.N. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. 2017;8:589–600. doi: 10.1080/19490976.2017.1353849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2017;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 31.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvani R., Picca A., Lo Monaco M.R., Landi F., Bernabei R., Marzetti E. Of Microbes and Minds: A Narrative Review on the Second Brain Aging. Front. Med. 2018;5:53. doi: 10.3389/fmed.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 34.Stilling R.M., van de Wouw M., Clarke G., Stanton C., Dinan T.G., Cryan J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 36.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Toro-Barbosa M., Hurtado-Romero A., Garcia-Amezquita L.E., García-Cayuela T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients. 2020;12:3896. doi: 10.3390/nu12123896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psichas A., Sleeth M.L., Murphy K.G., Brooks L., Bewick G.A., Hanyaloglu A.C., Ghatei M.A., Bloom S.R., Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dockray G.J. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 2014;592:2927–2941. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stadlbauer U., Woods S.C., Langhans W., Meyer U. PYY3–36: Beyond food intake. Front. Neuroendocrinol. 2015;38:1–11. doi: 10.1016/j.yfrne.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Anderberg R.H., Anefors C., Bergquist F., Nissbrandt H., Skibicka K.P. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol. Behav. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Ventriglio A., Bellomo A., di Gioia I., Di Sabatino D., Favale D., De Berardis D., Cianconi P. Environmental pollution and mental health: A narrative review of literature. CNS Spectrums. 2020;26:51–61. doi: 10.1017/S1092852920001303. [DOI] [PubMed] [Google Scholar]
- 43.Simkin D.R. Microbiome and Mental Health, Specifically as It Relates to Adolescents. Curr. Psychiatry Rep. 2019;21:93. doi: 10.1007/s11920-019-1075-3. [DOI] [PubMed] [Google Scholar]
- 44.Obrenovich M.E.M. Leaky Gut, Leaky Brain? Microorganisms. 2018;6:107. doi: 10.3390/microorganisms6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins S.M., Bercik P. The Relationship Between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 46.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 47.Silbergeld E.K., Goldberg A. Pharmacological and neurochemical investigations of lead-induced hyperactivity. Neuropharmacology. 1975;14:431–444. doi: 10.1016/0028-3908(75)90026-X. [DOI] [PubMed] [Google Scholar]
- 48.Yang M.-Y., Kim S.-H., Kim J.-C., Shin T.-K., Moon C.-J. Toluene Induces Depression-Like Behaviors in Adult Mice. Toxicol. Res. 2010;26:315–320. doi: 10.5487/TR.2010.26.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanninen H., Mantere P., Hernberg S., Seppalainen A.M., Kock B. Subjective symptoms in low-level exposure to lead. Neurotoxicology. 1979;1:333–347. [Google Scholar]
- 50.Opler M.G., Buka S.L., Groeger J., McKeague I., Wei C., Factor-Litvak P., Bresnahan M., Graziano J., Goldstein J.M., Seidman L.J., et al. Prenatal exposure to lead, delta-aminolevulinic acid, and schizophrenia: Further evidence. Environ. Health Perspect. 2008;116:1586–1590. doi: 10.1289/ehp.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karim P., Hossain M.I., Sadat A.N., Nahar Z., Hossain M.K., Hasnat A. Serum levels of cadmium, calcium, lead and iron in schizophrenic patients. Dhaka Univ. J. Pharm. Sci. 2006;5:9–13. doi: 10.3329/dujps.v5i1.221. [DOI] [Google Scholar]
- 52.Hanninen H., Aitio A., Kovala T., Luukkonen R., Matikainen E., Mannelin T., Erkkila J., Riihimaki V. Occupational exposure to lead and neuropsychological dysfunction. Occup. Environ. Med. 1998;55:202–209. doi: 10.1136/oem.55.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurczak A., Brodowska A., Szkup M., Prokopowicz A., Karakiewicz B., Łój B., Kotwas A., Brodowska A., Grochans E. Influence of Pb and Cd levels in whole blood of postmenopausal women on the incidence of anxiety and depressive symptoms. Ann. Agric. Environ. Med. 2018;25:219–223. doi: 10.26444/aaem/85929. [DOI] [PubMed] [Google Scholar]
- 54.Homme K.G., Kern J.K., Haley B.E., Geier D.A., King P.G., Sykes L.K., Geier M.R. New science challenges old notion that mercury dental amalgam is safe. Biometals. 2014;27:19–24. doi: 10.1007/s10534-013-9700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harakeh S., Sabra N., Kassak K., Doughan B. Factors influencing total mercury levels among Lebanese dentists. Sci. Total Environ. 2002;297:153–160. doi: 10.1016/S0048-9697(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 56.Shiue I. Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ. Sci. Pollut. Res. 2015;22:17095–17103. doi: 10.1007/s11356-015-4944-2. [DOI] [PubMed] [Google Scholar]
- 57.Lahouaoui H., Aimrane A., Khamsi Y., Zouhairi N., Benammi H., El Hidan M.A., Draoui A., Alahyane H., Bouazza A. Handbook of Research on Global Environmental Changes and Human Health. IGI Global; Pennsylvania, PA, USA: 2019. Depression and Anxiety Emerging From Heavy Metals: What Relationship? pp. 305–321. [Google Scholar]
- 58.Tshala-Katumbay D., Mwanza J.-C., Rohlman D.S., Maestre G.E., Oriá R. A global perspective on the influence of environmental exposures on the nervous system. Nature. 2015;527:S187–S192. doi: 10.1038/nature16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan H., Yu L., Tian F., Zhai Q., Fan L., Chen W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020;742:140429. doi: 10.1016/j.scitotenv.2020.140429. [DOI] [PubMed] [Google Scholar]
- 60.Giambò F., Italia S., Teodoro M., Briguglio G., Furnari N., Catanoso R., Costa C., Fenga C. Influence of toxic metal exposure on the gut microbiota (Review) World Acad. Sci. J. 2021;3:19. doi: 10.3892/wasj.2021.90. [DOI] [Google Scholar]
- 61.Rosenfeld C.S. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front. Cell. Infect. Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu K., Abo R.P., Schlieper K.A., Graffam M.E., Levine S.S., Wishnok J.S., Swenberg J.A., Tannenbaum S.R., Fox J.G. Arsenic Exposure Perturbs the Gut Microbiome and Its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis. Environ. Health Perspect. 2014;122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu K., Mahbub R., Cable P.H., Ru H., Parry N.M.A., Bodnar W.M., Wishnok J.S., Styblo M., Swenberg J.A., Fox J.G., et al. Gut Microbiome Phenotypes Driven by Host Genetics Affect Arsenic Metabolism. Chem. Res. Toxicol. 2014;27:172–174. doi: 10.1021/tx400454z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo X., Liu S., Wang Z., Zhang X.-X., Li M., Wu B. Metagenomic profiles and antibiotic resistance genes in gut microbiota of mice exposed to arsenic and iron. Chemosphere. 2014;112:1–8. doi: 10.1016/j.chemosphere.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 65.Dheer R., Patterson J., Dudash M., Stachler E.N., Bibby K.J., Stolz D.B., Shiva S., Wang Z., Hazen S.L., Barchowsky A., et al. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol. Appl. Pharmacol. 2015;289:397–408. doi: 10.1016/j.taap.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chi L., Bian X., Gao B., Ru H., Tu P., Lu K. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem. Res. Toxicol. 2016;29:949–951. doi: 10.1021/acs.chemrestox.6b00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J., Wen X., Faulk C., Boehnke K., Zhang H., Dolinoy D.C., Xi C. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-specific Bodyweight Increases in Adult Mice. Toxicol. Sci. 2016;151:324–333. doi: 10.1093/toxsci/kfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y., Zhou C., Guo X., Hu G., Li G., Zhuang Y., Cao H., Li L., Xing C., Zhang C., et al. Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice. Biol. Trace Element Res. 2020;199:1900–1907. doi: 10.1007/s12011-020-02300-x. [DOI] [PubMed] [Google Scholar]
- 69.Breton J.Ô., Daniel C., Dewulf J., Pothion S., Froux N., Sauty M., Thomas P., Pot B., Foligne B. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 2013;222:132–138. doi: 10.1016/j.toxlet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 70.Gao B., Chi L., Mahbub R., Bian X., Tu P., Ru H., Lu K. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017;30:996–1005. doi: 10.1021/acs.chemrestox.6b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., Ning Z., Mayne J., Moore J.I., Li J., Butcher J., Deeke S.A., Chen R., Chiang C.K., Wen M., et al. MetaPro-IQ: A universal metaproteomic approach to studying human and mouse gut microbiota. Microbiome. 2016;4:31. doi: 10.1186/s40168-016-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Li Y., Xia Y., Liu K., Ren L., Ji Y. The Dysbiosis of Gut Microbiota Caused by Low-Dose Cadmium Aggravate the Injury of Mice Liver through Increasing Intestinal Permeability. Microorganisms. 2020;8:211. doi: 10.3390/microorganisms8020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brabec J., Wright J., Ly T., Wong H.T., McClimans C.J., Tokarev V., Lamendella R., Sherchand S., Shrestha D., Uprety S., et al. Arsenic disturbs the gut microbiome of individuals in a disadvantaged community in Nepal. Heliyon. 2020;6:e03313. doi: 10.1016/j.heliyon.2020.e03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao M., Zhu Y. Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci. Rep. 2020;10:4453–4459. doi: 10.1038/s41598-020-61143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kish L., Hotte N., Kaplan G., Vincent R., Tso R., Gänzle M., Rioux K.P., Thiesen A., Barkema H., Wine E., et al. Environmental Particulate Matter Induces Murine Intestinal Inflammatory Responses and Alters the Gut Microbiome. PLoS ONE. 2013;8:e62220. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li N., He F., Liao B., Zhou Y., Li B., Ran P. Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Respir. Res. 2017;18:143. doi: 10.1186/s12931-017-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mutlu E.A., Comba I.Y., Cho T., Engen P.A., Yazıcı C., Soberanes S., Hamanaka R.B., Niğdelioğlu R., Meliton A.Y., Ghio A.J., et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018;240:817–830. doi: 10.1016/j.envpol.2018.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R., Yang J., Saffari A., Jacobs J., Baek K.I., Hough G., Larauche M.H., Ma J., Jen N., Moussaoui N., et al. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci. Rep. 2017;7:42906. doi: 10.1038/srep42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie S., Zhang C., Zhao J., Li D., Chen J. Exposure to concentrated ambient PM2.5 (CAPM) induces intestinal disturbance via inflammation and alternation of gut microbiome. Environ. Int. 2022;161:107138. doi: 10.1016/j.envint.2022.107138. [DOI] [PubMed] [Google Scholar]
- 80.Wang W., Zhou J., Chen M., Huang X., Xie X., Li W., Cao Q., Kan H., Xu Y., Ying Z. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model. Part. Fibre Toxicol. 2018;15:17. doi: 10.1186/s12989-018-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu T., Chen X., Xu Y., Wu W., Tang W., Chen Z., Ji G., Peng J., Jiang Q., Xiao J., et al. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ. Int. 2019;130:104882. doi: 10.1016/j.envint.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 82.Alderete T.L., Jones R.B., Chen Z., Kim J.S., Habre R., Lurmann F., Gilliland F.D., Goran M.I. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ. Res. 2017;161:472–478. doi: 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Yao Y., Li H., Qiao F., Wu J., Du Z.-Y., Zhang M. Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish. PLoS ONE. 2016;11:e0163895. doi: 10.1371/journal.pone.0163895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Javurek A.B., Spollen W.G., Johnson S.A., Bivens N.J., Bromert K.H., Givan S.A., Rosenfeld C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7:471–485. doi: 10.1080/19490976.2016.1234657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng D., Zhang H., Jiang X., Zou J., Li Q., Mai H., Su D., Ling W., Feng X. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ. Pollut. 2020;265:114880. doi: 10.1016/j.envpol.2020.114880. [DOI] [PubMed] [Google Scholar]
- 86.Lai K.-P., Chung Y.-T., Li R., Wan H.-T., Wong C.K.-C. Bisphenol A alters gut microbiome: Comparative metagenomics analysis. Environ. Pollut. 2016;218:923–930. doi: 10.1016/j.envpol.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y., Rui M., Nie Y., Lu G. Influence of gastrointestinal tract on metabolism of bisphenol A as determined by in vitro simulated system. J. Hazard. Mater. 2018;355:111–118. doi: 10.1016/j.jhazmat.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Koestel Z.L., Backus R.C., Tsuruta K., Spollen W.G., Johnson S.A., Javurek A.B., Ellersieck M.R., Wiedmeyer C.E., Kannan K., Xue J., et al. Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA. Sci. Total Environ. 2017;579:1804–1814. doi: 10.1016/j.scitotenv.2016.11.162. [DOI] [PubMed] [Google Scholar]
- 89.Hu J., Raikhel V., Gopalakrishnan K., Fernandez-Hernandez H., Lambertini L., Manservisi F., Falcioni L., Bua L., Belpoggi F., Teitelbaum S.L., et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome. 2016;4:26. doi: 10.1186/s40168-016-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fu X., Han H., Li Y., Xu B., Dai W., Zhang Y., Zhou F., Ma H., Pei X. Di-(2-ethylhexyl) phthalate exposure induces female reproductive toxicity and alters the intestinal microbiota community structure and fecal metabolite profile in mice. Environ. Toxicol. 2021;36:1226–1242. doi: 10.1002/tox.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong Z., Zeng Y., Zhou J., Shu R., Xie X., Fu Z. Exposure to dibutyl phthalate impairs lipid metabolism and causes inflammation via disturbing microbiota-related gut–liver axis. Acta Biochim. Biophys. Sin. 2020;52:1382–1393. doi: 10.1093/abbs/gmaa128. [DOI] [PubMed] [Google Scholar]
- 92.Lei M., Menon R., Manteiga S., Alden N., Hunt C., Alaniz R.C., Lee K., Jayaraman A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. mSystems. 2019;4:e00724-19. doi: 10.1128/mSystems.00724-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Betts K.S. CDC Updates Guidelines for Children’s Lead Exposure. Environ. Health Perspect. 2012;120:a268. doi: 10.1289/ehp.120-a268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouchard M.F., Bellinger D.C., Weuve J., Matthews-Bellinger J., Gilman S., Wright R.O., Schwartz J., Weisskopf M.G. Blood Lead Levels and Major Depressive Disorder, Panic Disorder, and Generalized Anxiety Disorder in US Young Adults. Arch. Gen. Psychiatry. 2009;66:1313–1319. doi: 10.1001/archgenpsychiatry.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gilbert S.G., Weiss B. A rationale for lowering the blood lead action level from 10 to 2 μg/dL. NeuroToxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bressler J., Kim K.-A., Chakraborti T., Goldstein G. Molecular Mechanisms of Lead Neurotoxicity. Neurochem. Res. 1999;24:595–600. doi: 10.1023/A:1022596115897. [DOI] [PubMed] [Google Scholar]
- 97.Virgolini M.B., Chen K., Weston D.D., Bauter M.R., Cory-Slechta D.A. Interactions of Chronic Lead Exposure and Intermittent Stress: Consequences for Brain Catecholamine Systems and Associated Behaviors and HPA Axis Function. Toxicol. Sci. 2005;87:469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 98.Cory-Slechta D.A., Virgolini M.B., Rossi-George A., Thiruchelvam M., Lisek R., Weston D. Lifetime Consequences of Combined Maternal Lead and Stress. Basic Clin. Pharmacol. Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 99.Lidsky T.I., Schneider J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 100.Fattal O., Budur K., Vaughan A.J., Franco K. Review of the Literature on Major Mental Disorders in Adult Patients with Mitochondrial Diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 101.Jou S.-H., Chiu N.-Y., Liu C.-S. Mitochondrial dysfunction and psychiatric disorders. Chang Gung Med. J. 2009;32:370–379. [PubMed] [Google Scholar]
- 102.D’Antona G., Ragni M., Cardile A., Tedesco L., Dossena M., Bruttini F., Caliaro F., Corsetti G., Bottinelli R., Carruba M.O., et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 103.Valerio A., D’Antona G., Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging. 2011;3:464. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monachese M., Burton J.P., Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Counter S.A., Buchanan L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 106.Huang X., Law S., Li D., Yu X., Li B. Mercury Poisoning: A Case of a Complex Neuropsychiatric Illness. Am. J. Psychiatry. 2014;171:1253–1256. doi: 10.1176/appi.ajp.2013.12101266. [DOI] [PubMed] [Google Scholar]
- 107.Ekino S., Susa M., Ninomiya T., Imamura K., Kitamura T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J. Neurol. Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 108.Ratcliffe H.E., Swanson G.M., Fischer L.J. Human Exposure to Mercury: A Critical Assessment of the Evidence of Adverse Health Effects. J. Toxicol. Environ. Health Part A. 1996;49:221–270. doi: 10.1080/00984108.1996.11667600. [DOI] [PubMed] [Google Scholar]
- 109.Ozuah P.O. Mercury poisoning. Curr. Probl. Pediatr. 2000;30:91–99. doi: 10.1067/mps.2000.104054. [DOI] [PubMed] [Google Scholar]
- 110.Ni M., Li X., dos Santos A.P.M., Farina M., da Rocha J.B.T., Avila D.S., Soldin O.P., Lu R., Aschner M. Chapter 35—Mercury. In: Gupta R.C., editor. Reproductive and Developmental Toxicology. Academic Press; San Diego, CA, USA: 2011. pp. 451–459. [Google Scholar]
- 111.Park D.-J., Zheng W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health—Yebang Uihakhoe Chi. 2012;45:344–352. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin X., Zhao J., Zhang W., He L., Wang L., Chang D., Cui L., Gao Y., Li B., Chen C., et al. Acute oral methylmercury exposure perturbs the gut microbiome and alters gut-brain axis related metabolites in rats. Ecotoxicol. Environ. Saf. 2020;190:110130. doi: 10.1016/j.ecoenv.2019.110130. [DOI] [PubMed] [Google Scholar]
- 113.Sarawagi A., Soni N.D., Patel A.B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry. 2021;12:637863. doi: 10.3389/fpsyt.2021.637863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nielsen K.M., Zhang Y., Curran T.E., Magnuson J.T., Venables B.J., Durrer K.E., Allen M.S., Roberts A.P. Alterations to the Intestinal Microbiome and Metabolome of Pimephales promelas and Mus musculus Following Exposure to Dietary Methylmercury. Environ. Sci. Technol. 2018;52:8774–8784. doi: 10.1021/acs.est.8b01150. [DOI] [PubMed] [Google Scholar]
- 115.Lin Y.-C., Su C.-T., Shiue H.-S., Chen W.-J., Chen Y.-H., Choy C.-S., Chiou H.-Y., Han B.-C., Hsueh Y.-M. The Methylation Capacity of Arsenic and Insulin Resistance are Associated with Psychological Characteristics in Children and Adolescents. Sci. Rep. 2017;7:3094. doi: 10.1038/s41598-017-03084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karri V., Schuhmacher M., Kumar V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016;48:203–213. doi: 10.1016/j.etap.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 117.Chi L., Bian X., Gao B., Tu P., Ru H., Lu K. The Effects of an Environmentally Relevant Level of Arsenic on the Gut Microbiome and Its Functional Metagenome. Toxicol. Sci. 2017;160:193–204. doi: 10.1093/toxsci/kfx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010;104((Suppl. S2)):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 119.Romero E.S., Cotoner C.A., Camacho C.P., Bedmar M.C., Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015;108:686–696. doi: 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- 120.Wang J., Hu W., Yang H., Chen F., Shu Y., Zhang G., Liu J., Liu Y., Li H., Guo L. Arsenic concentrations, diversity and co-occurrence patterns of bacterial and fungal communities in the feces of mice under sub-chronic arsenic exposure through food. Environ. Int. 2020;138:105600. doi: 10.1016/j.envint.2020.105600. [DOI] [PubMed] [Google Scholar]
- 121.Forero-Rodríguez L.J., Josephs-Spaulding J. Parkinson’s Disease and the Metal-Microbiome-Gut-Brain Axis: A Systems Toxicology Approach. Antioxidants. 2021;11:71. doi: 10.3390/antiox11010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Centers for Disease Control and Prevention Phthalates Factsheet. [(accessed on 5 April 2022)]; Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html#:~:text=Phthalates%20are%20a%20group%20of,%2C%20shampoos%2C%20hair%20sprays.
- 123.Przybylińska P.A., Wyszkowski M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. S. 2016;23:347–356. doi: 10.1515/eces-2016-0024. [DOI] [Google Scholar]
- 124.Yang Y.N., Yang Y.C.S., Lin I.H., Chen Y.Y., Lin H.Y., Wu C.Y., Su Y.T., Yang Y.J., Yang S.N., Suen J.L. Phthalate exposure alters gut microbiota composition and IgM vaccine response in human newborns. Food Chem. Toxicol. 2019;132:110700. doi: 10.1016/j.fct.2019.110700. [DOI] [PubMed] [Google Scholar]
- 125.Whyatt R. Prenatal Exposure to Phthalates Linked to Decreased Mental and Motor Development. [(accessed on 7 June 2022)]. Available online: https://www.publichealth.columbia.edu/public-health-now/news/prenatal-exposure-phthalates-linked-decreased-mental-and-motor-development#:~:text=Among%20girls%2C%20one%20of%20the,somatic%20complaints%20and%20withdrawn%20behavior.
- 126.Hu D., Wang Y.-X., Chen W.-J., Zhang Y., Li H.-H., Xiong L., Zhu H.-P., Chen H.-Y., Peng S.-X., Wan Z.-H., et al. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ. Pollut. 2017;229:375–385. doi: 10.1016/j.envpol.2017.05.089. [DOI] [PubMed] [Google Scholar]
- 127.Ejaredar M., Nyanza E.C., Eycke K.T., Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 128.Lee D.-W., Kim M.-S., Lim Y.-H., Lee N., Hong Y.-C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res. 2018;167:558–566. doi: 10.1016/j.envres.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Q., Chen X.-Z., Huang X., Wang M., Wu J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. NeuroToxicology. 2019;73:199–212. doi: 10.1016/j.neuro.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 130.Lucaccioni L., Trevisani V., Passini E., Righi B., Plessi C., Predieri B., Iughetti L. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021;22:4063. doi: 10.3390/ijms22084063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu X., Yang Y., Wang R., Wang Y., Ruan Q., Lu Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere. 2015;124:22–31. doi: 10.1016/j.chemosphere.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 132.Brown J.S. Effects of Bisphenol-A and Other Endocrine Disruptors Compared with Abnormalities of Schizophrenia: An Endocrine-Disruption Theory of Schizophrenia. Schizophr. Bull. 2008;35:256–278. doi: 10.1093/schbul/sbm147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang D.-C., Chen T.-J., Lin M.-L., Jhong Y.-C., Chen S.-C. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm. Behav. 2014;66:674–684. doi: 10.1016/j.yhbeh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 134.Perera F., Nolte E.L.R., Wang Y., Margolis A.E., Calafat A.M., Wang S., Garcia W., Hoepner L.A., Peterson B.S., Rauh V., et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016;151:195–202. doi: 10.1016/j.envres.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.National Institute of Environmental Health Sciences Bisphenol A (BPA) [(accessed on 26 November 2021)]; Available online: https://www.niehs.nih.gov/health/topics/agents/sya-bpa/index.cfm.
- 136.Malaisé Y., Menard S., Cartier C., Gaultier E., Lasserre F., Lencina C., Harkat C., Geoffre N., Lakhal L., Castan-Laurell I., et al. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development. Sci. Rep. 2017;7:14472. doi: 10.1038/s41598-017-15196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khokhlova E.V., Smeianov V.V., Efimov B.A., Kafarskaia L.I., Pavlova S.I., Shkoporov A.N. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol. Immunol. 2011;56:27–39. doi: 10.1111/j.1348-0421.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 138.Perera F., Vishnevetsky J., Herbstman J.B., Calafat A.M., Xiong W., Rauh V., Wang S. Prenatal Bisphenol A Exposure and Child Behavior in an Inner-City Cohort. Environ. Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harley K.G., Gunier R.B., Kogut K., Johnson C., Bradman A., Calafat A.M., Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Braun J.M., Kalkbrenner A.E., Calafat A.M., Yolton K., Ye X., Dietrich K.N., Lanphear B.P. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Braun J.M., Yolton K., Dietrich K.N., Hornung R., Ye X., Calafat A.M., Lanphear B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evans S.F., Kobrosly R.W., Barrett E.S., Thurston S.W., Calafat A.M., Weiss B., Stahlhut R., Yolton K., Swan S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. NeuroToxicology. 2014;45:91–99. doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roen E.L., Wang Y., Calafat A.M., Wang S., Margolis A., Herbstman J., Hoepner L.A., Rauh V., Perera F.P. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ. Res. 2015;142:739–745. doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ryan B.C., Vandenbergh J.G. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 145.Xu X., Liu Y., Sadamatsu M., Tsutsumi S., Akaike M., Ushijima H., Kato N. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci. Res. 2007;58:149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 146.Cox K.H., Gatewood J.D., Howeth C., Rissman E.F. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patisaul H.B., Fortino A.E., Polston E.K. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 148.Tian Y.-H., Baek J.-H., Lee S.-Y., Jang C.-G. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–439. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- 149.Yu C., Tai F., Song Z., Wu R., Zhang X., He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ. Toxicol. Pharmacol. 2011;31:88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 150.Xu X., Dong F., Yang Y., Wang Y., Wang R., Shen X. Sex-specific effects of long-term exposure to bisphenol-A on anxiety- and depression-like behaviors in adult mice. Chemosphere. 2015;120:258–266. doi: 10.1016/j.chemosphere.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 151.Luo G., Wang S., Li Z., Wei R., Zhang L., Liu H., Wang C., Niu R., Wang J. Maternal Bisphenol A Diet Induces Anxiety-Like Behavior in Female Juvenile with Neuroimmune Activation. Toxicol. Sci. 2014;140:364–373. doi: 10.1093/toxsci/kfu085. [DOI] [PubMed] [Google Scholar]
- 152.Kumar M., Sarma D.K., Shubham S., Kumawat M., Verma V., Prakash A., Tiwari R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health. 2020;8:553850. doi: 10.3389/fpubh.2020.553850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen F., Zhou L., Bai Y., Zhou R., Chen L. Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J. Biomed. Res. 2015;29:250–258. doi: 10.7555/JBR.29.20140058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Panagiotidou E., Zerva S., Mitsiou D.J., Alexis M.N., Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J. Endocrinol. 2013;220:207–218. doi: 10.1530/JOE-13-0416. [DOI] [PubMed] [Google Scholar]
- 155.Reddivari L., Veeramachaneni D.N.R., Walters W.A., Lozupone C., Palmer J., Hewage M.K.K., Bhatnagar R., Amir A., Kennett M.J., Knight R., et al. Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and Their Metabolites. mSystems. 2017;2:e00093-17. doi: 10.1128/mSystems.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qu W., Zhao Z., Chen S., Zhang L., Wu D., Chen Z. Bisphenol A suppresses proliferation and induces apoptosis in colonic epithelial cells through mitochondrial and MAPK/AKT pathways. Life Sci. 2018;208:167–174. doi: 10.1016/j.lfs.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 157.Zhao Z., Qu W., Wang K., Chen S., Zhang L., Wu D., Chen Z. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 2019;111:901–908. doi: 10.1016/j.biopha.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 158.Ni Y., Hu L., Yang S., Ni L., Ma L., Zhao Y., Zheng A., Jin Y., Fu Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere. 2021;282:130952. doi: 10.1016/j.chemosphere.2021.130952. [DOI] [PubMed] [Google Scholar]
- 159.Sun Q., Hong X., Wold L.E. Cardiovascular Effects of Ambient Particulate Air Pollution Exposure. Circulation. 2010;121:2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.MohanKumar S.M., Campbell A., Block M., Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29:479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 161.Szyszkowicz M., Rowe B.H., Colman I. Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health. 2009;22:355–362. doi: 10.2478/v10001-009-0031-6. [DOI] [PubMed] [Google Scholar]
- 162.Chen S., Oliva P., Zhang P. Air Pollution and Mental Health: Evidence from China. National Bureau of Economic Research, Inc.; Cambridge, MA, USA: 2018. [Google Scholar]
- 163.Bakolis I., Hammoud R., Stewart R., Beevers S., Dajnak D., MacCrimmon S., Broadbent M., Pritchard M., Shiode N., Fecht D., et al. Mental health consequences of urban air pollution: Prospective population-based longitudinal survey. Soc. Psychiatry. 2020;56:1587–1599. doi: 10.1007/s00127-020-01966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lim Y.-H., Kim H., Kim J.H., Bae S., Park H.Y., Hong Y.-C. Air Pollution and Symptoms of Depression in Elderly Adults. Environ. Health Perspect. 2012;120:1023–1028. doi: 10.1289/ehp.1104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vert C., Sánchez-Benavides G., Martínez D., Gotsens X., Gramunt N., Cirach M., Molinuevo J.L., Sunyer J., Nieuwenhuijsen M.J., Crous-Bou M., et al. Effect of long-term exposure to air pollution on anxiety and depression in adults: A cross-sectional study. Int. J. Hyg. Environ. Health. 2017;220:1074–1080. doi: 10.1016/j.ijheh.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 166.Borroni E., Pesatori A.C., Bollati V., Buoli M., Carugno M. Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ. Pollut. 2021;292:118245. doi: 10.1016/j.envpol.2021.118245. [DOI] [PubMed] [Google Scholar]
- 167.SShin J., Park J.Y., Choi J. Long-term exposure to ambient air pollutants and mental health status: A nationwide population-based cross-sectional study. PLoS ONE. 2018;13:e0195607. doi: 10.1371/journal.pone.0195607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bakian A.V., Huber R.S., Coon H., Gray D., Wilson P., McMahon W.M., Renshaw P.F. Acute Air Pollution Exposure and Risk of Suicide Completion. Am. J. Epidemiol. 2015;181:295–303. doi: 10.1093/aje/kwu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim C., Jung S.H., Kang D.R., Kim H.C., Moon K.T., Hur N.W., Shin D.C., Suh I. Ambient Particulate Matter as a Risk Factor for Suicide. Am. J. Psychiatry. 2010;167:1100–1107. doi: 10.1176/appi.ajp.2010.09050706. [DOI] [PubMed] [Google Scholar]
- 170.Perera F.P., Tang D., Wang S., Vishnevetsky J., Zhang B., Diaz D., Camann D., Rauh V. Prenatal Polycyclic Aromatic Hydrocarbon (PAH) Exposure and Child Behavior at Age 6–7 Years. Environ. Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Power M.C., Kioumourtzoglou M.-A., Hart J.E., Okereke O.I., Laden F., Weisskopf M.G. The relation between past exposure to fine particulate air pollution and prevalent anxiety: Observational cohort study. BMJ. 2015;350:h1111. doi: 10.1136/bmj.h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Forns J., Dadvand P., Foraster M., Alvarez-Pedrerol M., Rivas I., López-Vicente M., Suades-Gonzalez E., Garcia-Esteban R., Esnaola M., Cirach M., et al. Traffic-Related Air Pollution, Noise at School, and Behavioral Problems in Barcelona Schoolchildren: A Cross-Sectional Study. Environ. Health Perspect. 2016;124:529–535. doi: 10.1289/ehp.1409449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yorifuji T., Kashima S., Diez M.H., Kado Y., Sanada S., Doi H. Prenatal exposure to outdoor air pollution and child behavioral problems at school age in Japan. Environ. Int. 2017;99:192–198. doi: 10.1016/j.envint.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 174.Campbell A., Oldham M., Becaria A., Bondy S., Meacher D., Sioutas C., Misra C., Mendez L., Kleinman M. Particulate Matter in Polluted Air May Increase Biomarkers of Inflammation in Mouse Brain. NeuroToxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 175.Li H., Chen L., Guo Z., Sang N., Li G. In vivo screening to determine neurological hazards of nitrogen dioxide (NO2) using Wistar rats. J. Hazard. Mater. 2012;225-226:46–53. doi: 10.1016/j.jhazmat.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 176.Yao G., Yue H., Yun Y., Sang N. Chronic SO2 inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory-related kinase activation via neuroinflammation in rats. Environ. Res. 2015;137:85–93. doi: 10.1016/j.envres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 177.Coccaro E.F., Lee R., Coussons-Read M. Elevated Plasma Inflammatory Markers in Individuals with Intermittent Explosive Disorder and Correlation with Aggression in Humans. JAMA Psychiatry. 2014;71:158–165. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- 178.Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Najjar S., Pearlman D.M., Alper K., Najjar A., Devinsky O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013;10:816. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guxens M., Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med. Wkly. 2012;141:w13322. doi: 10.57187/smw.2012.13322. [DOI] [PubMed] [Google Scholar]
- 181.Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C., Torres-Jardón R., Nuse B., Herritt L., Villarreal-Calderón R., Osnaya N., Stone I., García R., et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 182.Roberts S., Arseneault L., Barratt B., Beevers S., Danese A., Odgers C., Moffitt T., Reuben A., Kelly F.J., Fisher H.L. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019;272:8–17. doi: 10.1016/j.psychres.2018.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Block L.M., Calderón-Garcidueñas L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Salim Y.S., Kaplan G.G., Madsen K.L. Air pollution effects on the gut microbiota: A link between exposure and inflammatory disease. Gut Microbes. 2014;5:215–219. doi: 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mutlu E.A., Engen P., Soberanes S., Urich D., Forsyth C.B., Nigdelioglu R., Chiarella S.E., Radigan K.A., Gonzalez A., Jakate S., et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part. Fibre Toxicol. 2011;8:19. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thomson E.M. Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. J. Alzheimer’s Dis. 2019;69:597–614. doi: 10.3233/JAD-190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xia Y., Niu Y., Cai J., Liu C., Meng X., Chen R., Kan H. Personal ozone exposure and stress hormones in the hypothalamus–pituitary–adrenal and sympathetic-adrenal-medullary axes. Environ. Int. 2021;159:107050. doi: 10.1016/j.envint.2021.107050. [DOI] [PubMed] [Google Scholar]
- 188.Hughes D.T., Clarke M.B., Yamamoto K., Rasko D.A., Sperandio V. The QseC Adrenergic Signaling Cascade in Enterohemorrhagic E. coli (EHEC) PLOS Pathog. 2009;5:e1000553. doi: 10.1371/journal.ppat.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fouladi F., Bailey M.J., Patterson W.B., Sioda M., Blakley I.C., Fodor A.A., Jones R.B., Chen Z., Kim J.S., Lurmann F., et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ. Int. 2020;138:105604. doi: 10.1016/j.envint.2020.105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li T., Fang J., Tang S., Du H., Zhao L., Wang Y., Deng F., Liu Y., Du Y., Cui L., et al. PM2.5 exposure associated with microbiota gut-brain axis: Multi-omics mechanistic implications from the BAPE study. Innovation. 2022;3:100213. doi: 10.1016/j.xinn.2022.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.World Health Organization AIR POLLUTION. [(accessed on 22 September 2021)]. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1.
- 192.Allen A.P., Hutch W., Borre Y.E., Kennedy P.J., Temko A., Boylan G., Murphy E., Cryan J.F., Dinan T.G., Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry. 2016;6:e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Benton D., Williams C., Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2006;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 194.Patterson E., Griffin S.M., Ibarra A., Ellsiepen E., Hellhammer J. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: A randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study) Neurobiol. Stress. 2020;13:100277. doi: 10.1016/j.ynstr.2020.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu S.-I., Wu C.-C., Tsai P.-J., Cheng L.-H., Hsu C.-C., Shan I.-K., Chan P.-Y., Lin T.-W., Ko C.-J., Chen W.-L., et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021;8:614105. doi: 10.3389/fnut.2021.614105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 197.Johnstone N., Milesi C., Burn O., van den Bogert B., Nauta A., Hart K., Sowden P., Burnet P.W.J., Kadosh K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021;11:8302. doi: 10.1038/s41598-021-87865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Donoso F., Egerton S., Bastiaanssen T.F., Fitzgerald P., Gite S., Fouhy F., Ross R.P., Stanton C., Dinan T.G., Cryan J.F. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology. 2020;116:104673. doi: 10.1016/j.psyneuen.2020.104673. [DOI] [PubMed] [Google Scholar]
- 199.Haghighat N., Rajabi S., Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients:A randomized, double-blinded, clinical trial. Nutr. Neurosci. 2019;24:490–499. doi: 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]
- 200.Moludi J., Khedmatgozar H., Nachvak S.M., Abdollahzad H., Moradinazar M., Tabaei A.S. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2021;24:1–10. doi: 10.1080/1028415X.2021.1889451. [DOI] [PubMed] [Google Scholar]
- 201.Quero C., Manonelles P., Fernández M., Abellán-Aynés O., López-Plaza D., Andreu-Caravaca L., Hinchado M., Gálvez I., Ortega E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients. 2021;13:1321. doi: 10.3390/nu13041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wei C.-L., Wang S., Yen J.-T., Cheng Y.-F., Liao C.-L., Hsu C.-C., Wu C.-C., Tsai Y.-C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019;1711:202–213. doi: 10.1016/j.brainres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 203.Nishida K., Sawada D., Kuwano Y., Tanaka H., Rokutan K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019;11:1859. doi: 10.3390/nu11081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rudzki L., Ostrowska L., Pawlak D., Małus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2018;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 205.Lew L.-C., Hor Y.-Y., Yusoff N.A.A., Choi S.-B., Yusoff M.S., Roslan N.S., Ahmad A., Mohammad J.A., Abdullah M.F.I., Zakaria N., et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2018;38:2053–2064. doi: 10.1016/j.clnu.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 206.Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 207.Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology. 2013;144:1394–1401.e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Messaoudi M., Violle N., Bisson J.-F., Desor D., Javelot H., Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 209.Liu W.-H., Chuang H.-L., Huang Y.-T., Wu C.-C., Chou G.-T., Wang S., Tsai Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2015;298:202–209. doi: 10.1016/j.bbr.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 210.Liu Y.-W., Liu W.-H., Wu C.-C., Juan Y.-C., Wu Y.-C., Tsai H.-P., Wang S., Tsai Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016;1631:1–12. doi: 10.1016/j.brainres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 211.Agusti A., Moya-Pérez A., Campillo I., La Paz S.M.-D., Cerrudo V., Perez-Villalba A., Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 Ameliorates Neuroendocrine Alterations Associated with an Exaggerated Stress Response and Anhedonia in Obese Mice. Mol. Neurobiol. 2017;55:5337–5352. doi: 10.1007/s12035-017-0768-z. [DOI] [PubMed] [Google Scholar]
- 212.Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 213.Wang T., Hu X., Liang S., Li W., Wu X., Wang L., Jin F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes. 2015;6:707–717. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 214.Smith C.J., Emge J.R., Berzins K., Lung L., Khamishon R., Shah P., Rodrigues D.M., Sousa A.J., Reardon C., Sherman P., et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Liver Physiol. 2014;307:G793–G802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Luo J., Wang T., Liang S., Hu X., Li W., Jin F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 216.Huang S.-Y., Chen L.-H., Wang M.-F., Hsu C.-C., Chan C.-H., Li J.-X., Huang H.-Y. Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients. 2018;10:894. doi: 10.3390/nu10070894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liao J., Hsu C., Chou G., Hsu J., Liong M., Tsai Y. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef. Microbes. 2019;10:425–436. doi: 10.3920/BM2018.0077. [DOI] [PubMed] [Google Scholar]
- 218.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 220.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tarr A.J., Galley J.D., Fisher S.E., Chichlowski M., Berg B.M., Bailey M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015;50:166–177. doi: 10.1016/j.bbi.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 223.Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 225.Soderholm J.D., Perdue M.H., II Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- 226.De Vries H.E., Blom-Roosemalen M.C., van Oosten M., de Boer A.G., van Berkel T.J., Breimer D.D., Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 227.Banks W.A., Erickson M.A. The blood–brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 228.Ek M., Engblom D., Saha S., Blomqvist A., Jakobsson P.J., Ericsson-Dahlstrand A. Pathway across the blood–brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 229.Misra S., Mohanty D. Psychobiotics: A new approach for treating mental illness? Crit. Rev. Food Sci. Nutr. 2019;59:1230–1236. doi: 10.1080/10408398.2017.1399860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.