Abstract
Background: The clinical presentation of viral respiratory infections is unspecific. We assessed the performances of two new RT-PCR, the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV, and two isothermal amplification assays, the ID NOW COVID and the ID NOW influenza A & B 2. Methods: The study was conducted in two parts: (i) the Idylla™ assays were assessed using a collection of nasopharyngeal swabs which were positive for various respiratory viruses. (ii) The performances of the four assays were assessed prospectively: all of the symptomatic patients admitted to the emergency department from 10 to 21 December were enrolled. Results: (i) All of the SARS-CoV-2 false negatives with the Idylla™ assays had a Ct value greater than 30 with the reference RT-PCR. No cross-reactivity was identified. (ii) Overall, 218 patients were enrolled. The respective prevalences of SARS-CoV-2, influenza A, and RSV were 19.8%, 4.8%, and 3.2%. All of the assays were 100% specific. The sensitivity of SARS-CoV-2 detection was 97.7%, 82.5%, and 86.3% for the Idylla™ SARS-CoV2, the Idylla™ SARS-CoV2/Flu/RSV, and the ID NOW COVID-19, respectively. For influenza A, it was 90.0% for the Idylla™ SARS-CoV2/Flu/RSV and 80.0% for the ID NOW Influenza. Discussion. All of the assays are suitable for testing patients with respiratory symptoms. False negatives should be considered, and the test should be repeated regarding the context.
Keywords: Idylla, COVID-19, ID NOW, Flu, influenza, SARS-CoV-2, respiratory syncytial virus, Abbott, Biocartis, RSV
1. Introduction
The burden of respiratory viruses such as SARS-CoV-2 and influenza is high in elderly and immunocompromised patients. The specific curative and prophylactic treatments available are more efficient if they are administered rapidly after the onset of the symptoms or the infectious contact [1,2]. Furthermore, the identification of infected patients is required, especially in hospital settings and community living spaces, in order to implement infection prevention and control measures to prevent outbreaks [3]. However, the SARS-CoV-2 pandemic has changed the epidemiology of respiratory viruses. After the almost-disappearance of “common” respiratory viruses, the activity of seasonal viruses has resumed [4,5,6]. Indeed, while some viruses were sparsely detected, the RSV 2020–2021 season was delayed by about 10 weeks. It is therefore likely patients with respiratory symptoms might be infected by SARS-CoV-2, influenza, or another respiratory virus such as RSV.
The clinical presentation of respiratory viral infection is unspecific, and their etiological diagnostics require biological tests. Several assays based on antigen detection or molecular amplification, which is considered the gold standard for routine tests, are available, but their performances are heterogeneous. The Idylla™ SARS-CoV-2 (Biocartis, Mechelen, Belgium) and the Idylla™ SARS-CoV2/Flu/RSV (Biocartis) are fully integrated RT-PCR methods that allow the detection of SARS-CoV-2, and SARS-CoV-2, influenzas A and B, and RSV, respectively. While the performance of the Idylla™ SARS-CoV-2 was previously assessed, that of the Idylla™ SARS-CoV2/Flu/RSV had never been assessed to date [7,8]. The ID NOW COVID-19 (Abbott Rapid Diagnostic, Scarborough, ME, USA) and ID NOW Influenza A & B 2 (Abbott Rapid Diagnostic) are isothermal amplification methods that detect SARS-CoV-2 and Influenza A and B, respectively. All of the assays are user-friendly and provide results within 90 min and 15 min of processing for the Idylla™ and the ID NOW instruments, respectively. None of them were assessed in the epidemiological context of the co-circulation of SARS-CoV-2, influenza and RSV.
The aim of this study is to assess the performances of four commercial molecular assays for the detection of respiratory viruses in comparison to a reference multiplex RT-PCR.
2. Materials and Methods
2.1. Study Design
Four commercial fully integrated molecular assays—the ID NOW COVID-19, ID NOW Influenza A & B 2, The Idylla™ SARS-CoV-2, and the Idylla™ SARS-CoV2/Flu/RSV—were evaluated in comparison to a reference multiplex RT-PCR which is routinely used in our laboratory: the Alinity M RESP-4-Plex. The assays were performed on their respective and specific instruments: Alinity M (Abbott molecular, Des Plaines, IL, USA), ID NOW (Abbott Rapid Diagnostic), and Idylla (Biocartis, Mechelen, Belgium). The software of all of the instruments automatically interpreted amplification curves as positive, negative or uninterpretable. The respiratory viruses and the viral targets detected by each assay, as well as the volume of the sample required for each assay, are described in Table 1.
Table 1.
Characteristics of the reference RT-PCR and evaluated assays.
| Method | Volume of Sample ** | Viral Genes Amplified (Number of Fluorphore) | ||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 | Influenza A | Influenza B | RSV | |||
| Alinity M RESP-4-Plex | RT-PCR | 500 µL | ORF1b and N (1) | Matrix (1) | NS1 (1) | Matrix (1) |
| Idylla™ SARS-CoV-2 | RT-PCR | 200 µL | ORF1b (3) N (2) |
|||
| Idylla™ SARS-CoV2/Flu/RSV | RT-PCR | 400 µL | ORF1b (1) N (2) |
- * | - | - |
| ID NOW COVID-19 | Isothermal amplification | 200 µL | ORF1b (1) | |||
| ID NOW influenza A & B 2 | Isothermal amplification | 200 µL | Matrix (1) | Matrix (1) | Fusion and Nucleocapsid (1) | |
* Pathogen detection not included in the assay. ** Recommended by the manufacturer.
The study was performed in two parts. As the Idylla assays have never been evaluated previously, we first assessed their analytical performances using a collection of clinical samples. Then, the performances of the four assays were assessed in a prospective study. All of the nasopharyngeal swabs (NPS) were sampled on universal transport media (UTM) using a Yocon virus sampling kit (Yocon biology technology company, Beijing, China) from symptomatic patients suspected of viral respiratory infections. All of the tests were performed on fresh NPS stored for a maximum of 16 h at +5 °C, except for those included for the assessment of cross-reactivity in the evaluation of the analytical performances of the Idylla assays (Table 2). Those samples were stored at −70 °C before testing. The screening of mutations E484K, E484Q, and L452R was performed using the IDTM SARS-CoV-2/VOC evolution Pentaplex (ID solutions, Grabels, France) for all of the SARS-CoV-2-positive samples. Statistical analyses were performed using R software [9].
Table 2.
Number of NPS and tests performed in the analytical and the prospective studies.
| Conservation | Idylla™ SARS-CoV-2 | Idylla™ SARS-CoV2/Flu/RSV | ID NOW COVID |
ID NOW Influenza A & B 2 | |
|---|---|---|---|---|---|
| Analytical | |||||
| 15 SARS-CoV-2 | Fresh | X * | X | ||
| 11 influenza A | Fresh | X | |||
| 1 influenza B | Fresh | X | |||
| 1 RSV | Fresh | X | |||
| 20 Others viruses | Frozen | X | X | ||
| Prospective | |||||
| 218 NPS | Fresh | X | X | X | X |
* The tests performed are marqued with an X.
2.2. Analytical Performances of the Idylla Assays
The performances of the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV were evaluated using a selection of NPS collected in December 2021: 15 SARS-CoV-2-positive fresh NPS (Ct range 15.1–37.3) tested with both assays, 11 influenza-positive (Ct range 15.4–38.1), and 11 RSV-positive (Ct range 17.2–36.4) fresh NPS tested with the Idylla COVID-Flu-RSV. As the Idylla assays amplified several viral targets of the same genes, the median Ct for each viral target was calculated.
In order to assess the cross-reactivity, twenty frozen NPS which were negative for SARS-CoV-2 but positive for another respiratory virus with the AllPlex RP1, RP2, and RP3 (Seegene, Seoul, South Korea) were selected. They were collected between April and October 2021. The number of NPS which were positive for each viral pathogen are listed in Table 3: four influenza (Idylla™ SARS-CoV-2 only), 3 parainfluenza, 1 metapneumovirus, 6 coronavirus, 3 human respiratory syncytial virus (RSV) (Idylla™ SARS-CoV-2 only), 1 human rhinovirus, 1 adenovirus, and 1 human enterovirus.
Table 3.
Panel of commonly found respiratory viruses in respiratory infections used for the evaluation of cross-reactivity.
| Clinical NPS * with Known Viruses | Number Tested | |
|---|---|---|
| Idylla™ SARS-CoV2/Flu/RSV | Idylla™ SARS-CoV-2 | |
| Influenza A | - | 3 |
| Influenza B | - | 1 |
| RSV | - | 3 |
| Parainfluenza 1 | 1 | 1 |
| Parainfluenza 3 | 1 | 1 |
| Parainfluenza 4 | 1 | 1 |
| Coronavirus OC43 | 2 | 2 |
| Coronavirus NL63 | 2 | 2 |
| Coronavirus 229E | 2 | 2 |
| Human Metapneumovirus | 1 | 1 |
| Adenovirus | 1 | 1 |
| Enterovirus | 1 | 1 |
| Rhinovirus | 1 | 1 |
* NPS: nasopharyngeal swab.
The results of the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV were compared with those of the Alinity M RESP-4-Plex assay using the overall percentage of agreement, the positive percentage agreement, and a Kappa test.
2.3. Prospective Analysis
All of the adult patients suspected of respiratory viral infections admitted to the emergency department from 9 December 2021 to 21 December 2021 were enrolled. Socio-demographic and clinical data were prospectively recorded: date of birth, sex, date of first symptoms, nature of the symptoms, and SARS-CoV-2 vaccine status. Fresh NPS were processed using the Alinity M RESP-4-Plex (Abbott Molecular) as the reference RT-PCR in comparison to the ID Now COVID-19, the ID NOW Influenza A & B 2, Idylla™ SARS-CoV-2, the Idylla™ SARS-CoV2/Flu/RSV. The Alinity M RESP-4-Plex was repeated for all of the discrepant results, and performances were assessed after discrepant resolution. The sensitivity and specificity of each test was calculated for each assay and pathogen.
3. Results
3.1. Analytical Performances of the Idylla Assays
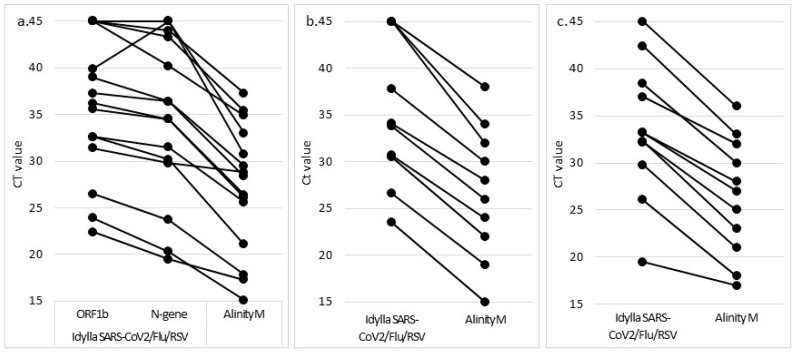
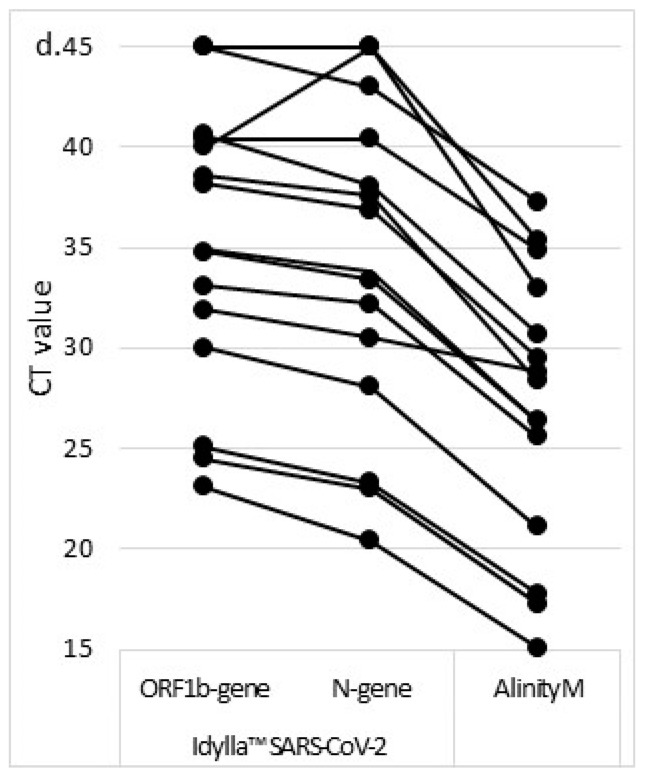
Of the 15 SARS-CoV-2-positive NPS, 13 (86.7%) and 12 (80.0%) were found positive with the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV, respectively (Figure 1). All of the samples which were negative with the Idylla assays had a Ct value greater than 30 with the Alinity M. Sample No. 925301 (Alinity M Ct value of 35.4) was found positive with the Idylla™ SARS-CoV-2 (one of five viral targets amplified with a Ct value of 39.9) but negative with the Idylla™ SARS-CoV2/Flu/RSV, although one of the three SARS-CoV-2 viral targets was amplified with a Ct value of 43.3. Sample No. 820201 (Alinity M Ct value of 37.3) was found negative with both assays, although a single target of the N gene was amplified in each assay with Ct values of 43.0 and 44.0 for the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV, respectively. No viral targets were amplified with both the Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV for sample No. 641201, which displayed a Ct value of 33.0 with the Alinity M RESP-4-Plex. In comparison to the Alinity M SARS-CoV-2 target, the median Ct values of the N-gene and the ORF1b-gene were 2.5 and 6.1 cycles higher with the Idylla™ SARS-CoV-2, respectively. The Ct values were respectively 2.8 et 8.4 cycles higher with the Idylla™ SARS-CoV2/Flu/RSV.
Figure 1.
CT values of the viral targets obtained with the Idylla and the Alinity M assays during the study of the analytical performance. Idylla SARS-CoV2/Flu/RSV assay for SARS-CoV-2 (a), influenza A (b), and RSV (c) targets. Idylla SARS-CoV2 assay (d).
One of 11 (9.1%) RSV-positive NPS and three of 10 (30.0%) influenza A-positive NPS were not detected with the Idylla™ SARS-CoV2/Flu/RSV (Figure 1). The RSV-positive sample (No. 569901) displayed a Ct value of 36, while the three influenza A-positive NPS (No. 672701, 629501, and 008901) had Ct values of 32, 34, and 38 with the Alinity M RESP-4-Plex. In comparison to the Alinity M RESP-4-Plex, the median Ct value for positive samples was 6.7 cycles higher with the Idylla™ SARS-CoV2/Flu/RSV for both influenza A and RSV. The single influenza B sample was tested positive with the Idylla COVID-Flu-RSV. No cross-reactivity was identified with the NPS positive for another respiratory virus (Table 3).
The overall percentage agreements between the Idylla™ SARS-CoV2/Flu/RSV and the Alinity RESP-4-Plex were respectively 80.6%, 86.9%, and 95.8% for SARS-CoV-2, influenza A, and RSV. The positive percent agreements were 80.0%, 66.7%, and 90.9%, respectively, and the Kappa values were 0.88, 0.75, and 0.92. For the Idylla™ SARS-CoV-2, the overall percentage agreement and the positive percentage agreement were 94.3% and 86.7%, respectively, and the Kappa value was 0.88.
3.2. Prospective Analysis
Overall, 218 patients were enrolled in the prospective analysis. Their median age was 56.7 [interquartile range 40.4–77.3], and the male/female ratio was 0.93. The respective prevalence of SARS-CoV-2, influenza A, and RSV were 19.8% (n = 43), 4.8% (n = 10), and 3.2% (n = 7). No patient tested positive for influenza B. A single virus was detected in all of the patients, except for a SARS-CoV-2/RSV co-infection (patient No. 632901). Overall, the median delay from the onset of symptoms to NPS sampling was 3 days (interquartile range 2–7 days) for the patients infected with a respiratory virus. This timeframe was shorter for influenza A (2 days [1.3–3]), than RSV (3 days [3,4,5]) and SARS-CoV-2 (4 days [2–8.5])-positive patients. Among the SARS-CoV-2 positive patients, 28 (65.1%) were discharged on the day of admission, 10 (23.3%) were hospitalized in medical departments, and 5 (11.6%) were admitted to the intensive care unit. The median Ct of the RNAseP gene, assessed with the Idylla™ SARS-CoV2/Flu/RSV, was 30.8 (29.4–33.6). Eighteen (8.3%) patients had an RNAseP Ct value greater than 35.0, suggesting a suboptimal sampling according to the manufacturer’s instruction.
The numbers of tests providing no results due to processing error were one (0.5%) for the Idylla™ SARS-CoV-2 and the ID NOW influenza A & B 2, two (0.9%) for the Idylla™ SARS-CoV2/Flu/RSV, and three (1.4%) for the ID NOW COVID-19 assays. The Idylla™ SARS-CoV-2 and the Idylla™ SARS-CoV2/Flu/RSV were repeated using the same conditions: two of them provided a valid result, while the third provided no result with the Idylla™ SARS-CoV2/Flu/RSV, and was consequently considered invalid. Due to insufficient sample quantity, the ID NOW COVID-19 and the ID NOW Influenza A & B 2 could not be repeated.
Eighteen NPS (8.3%) were considered invalid using the Idylla™ SARS-CoV2/Flu/RSV due to the low amplification of the human RNAseP gene (Ct value > 35.0). Of these, four (22.2%) were positive for a respiratory virus with the Alinity M RESP-4-Plex (Table 4): one RSV (No. 345101) and three SARS-CoV-2 (No. 959702, 811101, and 879801). At least one viral target was amplified for three of these samples with the Idylla™ SARS-CoV2/Flu/RSV: one RSV (No. 345101) and two SARS-CoV2 (No. 959702 and 879801). No viral target was amplified for the remaining one (No. 811101), while it tested positive with the ID NOW COVID-19, Idylla™ SARS-CoV-2 (the amplification of all viral targets with a Ct value > 39.8), and the Alinity M RESP-4-Plex (a Ct value of 31.5).
Table 4.
Results for the four samples displaying a Ct value > 35.0 for the RNAseP gene but positive for a respiratory virus with the Alinity M.
| Sample ID | Idylla™ SARS-CoV2/Flu/RSV (Ct Value) | Alinity M Target (Ct Value) |
ID NOW COVID-19 | ID NOW Influenza A & B 2 |
Idylla™ SARS-CoV-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF1b | N | N | Flu A | VRS | Flu B | RNAseP | |||||
| 959702 | 29.1 | 27.2 | 27.3 | n.d. * | n.d. | n.d. | 37.2 | COVID (17.6) | Positive | Negative | Positive |
| 811101 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 37.6 | COVID (31.5) | Positive | Negative | Positive |
| 879801 | 31.1 | 28.9 | 29.1 | n.d. | n.d. | n.d. | 36.6 | COVID (19.1) | Positive | Negative | Positive |
| 345101 | n.d. | n.d. | n.d. | n.d. | 37.1 | n.d. | 36.5 | RSV (24.0) | Negative | Negative | Negative |
* n.d. not detected.
No false positive was found with all of the assays for any of the respiratory viruses. The discrepancies between the reference test and the evaluated assays are listed in Table 5. The Ct value of a SARS-CoV-2 false-negative ranges from 31.2 to 36.4. All of the patients were aged less than 50 years. They had mild symptoms for 3 to 14 days, with three of them being symptomatic for at least 10 days before testing. All of the patients were discharged on the day of their admission. The single SARS-CoV-2 false negative of the Idylla™ SARS-CoV-2 was also found to be negative with the Idylla™ SARS-CoV2/Flu/RSV and the ID NOW COVID-19. Three other SARS-CoV-2-positive NPS were found negative with both the ID NOW COVID-19 and the Idylla COVID-Flu-RSV. Of note, despite the Idylla™ SARS-CoV2/Flu/RSV providing a negative result, at least one viral target was amplified for three NPS (No. 832301, 909701, and 909301) with a Ct value greater than 40.0. Except for sample No. 909301, the Ct of the RNAseP gene was greater than 33.0 for all of the samples. All of the patients hospitalized or admitted to the intensive care unit were identified with all three assays. The overall sensitivities for SARS-CoV-2 detection were 97.7%, 82.5%, and 86.3% for the Idylla™ SARS-CoV-2, the Idylla™ SARS-CoV2/Flu/RSV, and the ID NOW COVID-19, respectively.
Table 5.
Discrepancies between the Alinity M RESP-4-Plex and the evaluated assay for SARS-CoV-2 (a), influenza A (b), and RSV (c).
| (a) | |||||||||||||
| Sample ID | Sex | Age |
Alinity M
(Ct) |
ID NOW COVID |
Idylla™
SARS-CoV-2 |
Idylla™ SARS-CoV2/Flu/RSV | Delay since Symptoms Onset | COVID-19 Vaccine | Symptoms | Oxygen Therapy | Outcome | ||
| Qualitative Result | Targets Ct | RNAseP (Ct) | |||||||||||
| 832301 | F | 21 | 31.2 | Negative | Positive | Negative | ORF1b a = nd N b = 44.3 N c = 44.3 |
34,5 | 3 | No | Abdominal pain | No | Discharge |
| 930001 | M | 46 | 31.8 | Negative | Negative | Negative | ORF1b a = nd N b = nd N c = nd |
34.3 | 14 | No | Flu-like syndrome | No | Discharge |
| 882501 | F | 31 | 32.3 | Negative | Positive | Negative | ORF1b a = nd N b = nd N c = nd |
35.3 | 5 | No | Flu-like syndrome | No | Discharge |
| 909701 | M | 29 | 33.9 | Negative | Positive | Negative | ORF1b a = nd N b = nd N c = 43.13 |
33.4 | 10 | n.a. | Headhache hypoaesthesia of the right side |
No | Discharge |
| 909301 | M | 22 | 36.4 | Negative | Positive | Negative | ORF1b a = nd N b = 41.15 N c = 43.29 |
30.2 | 14 | No | Flu-like syndrome | No | Discharge |
| (b) | |||||||||||||
| Sample ID | Sex | Age |
Alinity M
(Ct) |
ID NOW Indluenza A & B 2 | Idylla™ SARS-CoV2/Flu/RSV |
Delay since
Symptoms Onset |
Symptoms | Oxygen Therapy | Outcome | ||||
| Flu A (Ct) | RNAseP | ||||||||||||
| 501001 | M | 68 | 31 | Negative | Positive (39.7) | 33.6 | 7 | Dyspnea | 4 L | Hospitalization | |||
| 661701 | M | 39 | 36 | Negative | Negative | 33.6 | 1 | Flu-like syndrome | No | Discharge | |||
| (c) | |||||||||||||
| Sample ID | Sex | Age |
Alinity M
(Ct) |
Idylla™ SARS-CoV2/Flu/RSV |
Delay since
Symptoms Onset |
Symptoms | Oxygen Therapy | Outcome | |||||
| RSV | RNAseP E | ||||||||||||
| 842101 | F | 81 | 31 | Negative | 33.4 | n.a. | Dyspnea | 6 L | Hospitalization | ||||
There were two and one false negatives on the ID NOW Influenza A & B 2 and the Idylla™ SARS-CoV2/Flu/RSV for influenza A, respectively (Table 5). Sample No. 501001, which had a Ct value of 31 with the Alinity RESP-4-Plex, was negative with the ID NOW Influenza A & B 2 only. The patient was sampled 7 days after the onset of dyspnea. Sample No. 661701 (Alinity M Ct value of 36.0) was negative with both the ID NOW Influenza A & B 2 and the Idylla™ SARS-CoV2/Flu/RSV assays. All of the false negatives had a Ct value greater than 33.0 for the RNAseP gene. The sensitivity of the Idylla™ SARS-CoV2/Flu/RSV and the ID NOW Influenza were 90.0% and 80.0%, respectively, for influenza A. The single RSV false negative of the Idylla™ SARS-CoV2/Flu/RSV had a Ct value of 31.0 with the Alinity M. The sensivity of the Idylla™ SARS-CoV2/Flu/RSV was 83.3% for RSV.
4. Discussion
The main strength of our study was the prospective assessment of four molecular assays during the co-circulation of three respiratory viruses: SARS-CoV-2, influenza, and RSV. All of the assays were in agreement with the reference RT-PCR for NPS displaying a high viral load, i.e., a CT value lower than 30.0, whatever the viral target. The sensitivity of the Idylla™ SARS-CoV2 Test was the highest for SARS-CoV2 detection among the assays evaluated. No cross-reactivities or false positives were identified, confirming the high specificity of all of the assays.
Influenza and RSV are seasonal viruses, with RSV mainly circulating between October and December, and Influenza mainly circulating between December and January. Since its emergence, SARS-CoV-2 has spread in a multiwave dynamic. Furthermore, Influenza and RSV activity strongly decreased after the beginning of the COVID-19 pandemic. All of these findings make the co-circulation of these viruses unlikely. Our study took place in December 2021, which was marked by the increased activity of SARS-CoV-2 and influenza and the end of the seasonal outbreak of RSV. This allowed us to assess four molecular assays in the singular context of viral co-circulation.
The evaluation of the Idylla™ assays using a collection of fresh NPS displaying various CT values shows that those displaying a CT value greater than 30.0 are likely not detected. Similar findings were previously reported for the ID NOW assays [10,11,12]. They reflect that the patients displaying a low viral load might be not detected using these assays. Notably, viable virus is rarely cultured at Ct values > 30.0 on or after 14 days of illness, suggesting that the probability of infectivity decreases with increasing Ct values [13,14,15,16]. Therefore, the CT value has been suggested as a parameter to take into account for the decision to discontinue isolation in hospitalized patients with COVID-19 [15,17,18]. Most of the SARS-CoV-2-positive patients included in our prospective study had a Ct-value below 30.0. These results suggest that a diagnostic assay should be evaluated both using a collection of clinical samples displaying various viral loads and prospectively on fresh clinical samples. However, a low CT value could be reported at the early or late stages of the disease, or in the case of suboptimal sampling. Therefore, while all of the evaluated tests appeared acceptable for routine use, a negative result should be interpreted with caution considering the context and clinical symptoms, and re-testing should be recommended in case of the persistence of symptoms.
The sensitivity of a RT-PCR depends on pre-analytical issues such as the sampling, mainly for those displaying a Ct-value greater than 33.0, with a suboptimal sampling being associated with lower performances [19]. The Idylla™ SARS-CoV2/Flu/RSV amplified the RNAseP gene, a human housekeeping gene, allowing us to assess the quality of sampling. The main strategy of this assay is to avoid a false negative due to suboptimal sampling by providing an invalid result. In the present study, a significant number of NPS (8.3%) had low cellularity assessed with the Idylla™ SARS-CoV2/Flu/RSV. However, a viral target was amplified with a Ct value < 40.0 for three NPS, and they were found to be positive with the reference RT-PCR, as did the other evaluated assays. An inhibition during the analytical process of the Idylla COVID-Flu-RSV test can be excluded because the internal controls were amplified in the expected range. Therefore, we suggest that the algorithm of the Idylla™ SARS-CoV2/Flu/RSV be improved to provide a valid result for NPS reaching criteria of positivity whatever the amplification of the RNAseP gene.
The time-to-result is challenging for clinical laboratories mainly for COVID-19 diagnosis, as infection prevention and control measures should be implemented rapidly and hospital departments are overwhelmed during the successive waves of the pandemic. The analytic process of the Idylla assays is 90 min regardless of the result. We suggest that it could be shortened for positive results by ending the analytic process immediately after the detection of positivity, as for the ID NOW [20,21]. This change would enhance the instrument’s throughput. However, the Idylla instrument can include up to eight modules performing as many simultaneous tests, which compensated for the longer time-to-result.
The rate of invalid results remained low with all of the assays, which furthermore are user-friendly. Indeed, invalids could reach up to 35.5% with some assays [22]. Using the ID NOW instrument, invalids ranged from 0% to 7% [23,24,25,26], with this rate being lower when performed by laboratory-trained operators [22,23,25] in comparison to point-of-care use [24,26]. Consequently, the invalid rate of the Idylla™ instrument remains to be assessed if it is performed at the point of care.
As the study was carried out in a single care center setting, the results might be specific to the population of this center and might not be applicable to another group of patients, such as children for example. A single influenza B-positive sample was included; the performances of the four assays, therefore, remain to be evaluated for this virus. All of the assays were performed on NPS sampled on UTM; using a fresh swab would avoid dilution and likely improve sensitivity.
5. Conclusions
In conclusion, we evaluated four molecular assays in the singular epidemiologic context of the co-circulation of SARS-CoV-2, influenza, and RSV. The sensitivity of the Idylla™ SARS-CoV-2 was higher than that of the Idylla™ SARS-CoV2/Flu/RSV and ID-NOW, but all of the assays were suitable for testing patients with respiratory symptoms. Nevertheless, due to lower performances for samples displaying a CT value higher than 30.0, a false negative should be considered and the test repeated regarding the context. We also suggest two improvements in the algorithm of the Idylla assays. First, while a main advantage of the Idylla™ SARS-CoV2/Flu/RSV is to assess the quality of the NPS, an NPS reaching the criteria of positivity but with low cellularity should be interpreted as positive rather than invalid. Then, in order to reduce the time-to-result, the analytic process could be ended after the detection of the positivity.
Acknowledgments
The authors are grateful to Biocartis for providing the Idylla™ reagents.
Author Contributions
Conceptualization, E.F. and M.V.; methodology E.F. and M.V.; formal analysis, E.F.; investigation, E.F. and T.Y.; writing—original draft preparation, E.F.; writing—review and editing, E.F., T.Y., R.B. and M.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and its later amendments.
Informed Consent Statement
All the procedures were in accordance with the 1964 Helsinki Declaration and its later amendments. According to French Health Public Law (CSPArticle L1121-1), this type of study does not require specific informed consent or ethics committee approval.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The Idylla™ SARS-CoV2/Flu/RSV and the Idylla™ SARS-CoV-2 reagents were provided free of charge by Biocartis.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M., Gravenstein S., Hayden F.G., Harper S.A., Hirshon J.M., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin. Infect. Dis. 2019;68:1–47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Center for Disease Prevention and Control Treatment and Pharmaceutical Prophylaxis of COVID-19 2021. [(accessed on 5 February 2022)]. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/treatment.
- 3.Farfour E., Ballester M.-C., Lecuru M., Verrat A., Imhaus E., Mellot F., Karnycheff F., Vasse M., Cerf C., Lesprit P. COVID-19: Before stopping specific infection prevention and control measures; be sure to exclude the diagnosis. J. Hosp. Infect. 2020;105:375–376. doi: 10.1016/j.jhin.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farfour E., Pascreau T., Jolly E., Zia-Chahabi S., Mazaux L., Vasse M. Spring is coming, where are the Respiratory Syncytial Virus and Influenza viruses? J. Clin. Virol. 2021;139:104824. doi: 10.1016/j.jcv.2021.104824. [DOI] [PubMed] [Google Scholar]
- 5.Casalegno J.S., Ploin D., Cantais A., Masson E., Bard E., Valette M., Fanget R., Targe S.C., Myar-Dury A.-F., Doret-Dion M., et al. Characteristics of the delayed respiratory syncytial virus epidemic, 2020/2021, Rhône Loire, France. Eurosurveillance. 2021;26:2100630. doi: 10.2807/1560-7917.ES.2021.26.29.2100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marriott D., Beresford R., Mirdad F., Stark D., Glanville A., Chapman S., Harkness J., Dore G.J., Andresen D., Matthews G.V. Concomitant Marked Decline in Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Other Respiratory Viruses Among Symptomatic Patients Following Public Health Interventions in Australia: Data from St Vincent’s Hospital and associated screening clinics, Sydney, NSW. Clin. Infect. Dis. 2021;72:649–651. doi: 10.1093/cid/ciaa1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Luca C., Gragnano G., Conticelli F., Cennamo M., Pisapia P., Terracciano D., Malapelle U., Montella E., Triassi M., Troncone G., et al. Evaluation of a fully closed real time PCR platform for the detection of SARS-CoV-2 in nasopharyngeal swabs: A pilot study. J. Clin. Pathol. 2021 doi: 10.1136/jclinpath-2021-207516. [DOI] [PubMed] [Google Scholar]
- 8.Hofman P., Boutros J., Benchetrit D., Benzaquen J., Leroy S., Tanga V., Bordone O., Allégra M., Lespinet V., Fayada J., et al. A rapid near-patient RT-PCR test for suspected COVID-19: A study of the diagnostic accuracy. Ann. Transl. Med. 2021;9:921. doi: 10.21037/atm-21-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R Core Team . R: A Language and Environment for Statistical Computing. R Found Statistical Computing; Vienna, Austria: 2020. [(accessed on 7 June 2022)]. Available online: https://www.r-project.org. [Google Scholar]
- 10.Smithgall M.C., Scherberkova I., Whittier S., Green D.A. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the Rapid Detection of SARS-CoV-2. J. Clin. Virol. 2020;128:104428. doi: 10.1016/j.jcv.2020.104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell S.L., George K.S. Evaluation of the COVID-19 ID NOW EUA assay. J. Clin. Virol. 2020;128:104429. doi: 10.1016/j.jcv.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin R., Pettengill M.A., Hartnett N.L., Auerbach H.E., Peiper S.C., Wang Z. Commercial Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Molecular Assays: Superior Analytical Sensitivity of cobas SARS-CoV-2 Relative to NxTAG CoV Extended Panel and ID NOW COVID-19 Test. Arch. Pathol. Lab. Med. 2020;144:1303–1310. doi: 10.5858/arpa.2020-0283-SA. [DOI] [PubMed] [Google Scholar]
- 13.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.La Scola B., Le Bideau M., Andreani J., Hoang V.T., Grimaldier C., Colson P., Gautret P., Raoult D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin. Infect. Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singanayagam A., Patel M., Charlett A., Bernal J.L., Saliba V., Ellis J., Ladhani S., Zambon M., Gopal R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance. 2020;25:2001483. doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mowrer C., Creager H., Cawcutt K., Birge J., Lyden E., Van Schooneveld T.C., Rupp M.E., Hewlett A. Evaluation of cycle threshold values at deisolation. Infect. Control Hosp. Epidemiol. 2021;43:794–796. doi: 10.1017/ice.2021.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therese S.S., Julian D., Bianca J., Carmen S., Sarah G., Stephan K., Sturmbauer C., Posch W., Walder G. An in vitro model for assessment of SARS-CoV-2 infectivity by defining the correlation between virus isolation and quantitative PCR value: Isolation success of SARS-CoV-2 from oropharyngeal swabs correlates negatively with Cq value. Virol. J. 2021;18:71. doi: 10.1186/s12985-021-01542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basso D., Aita A., Navaglia F., Franchin E., Fioretto P., Moz S., Bozzato D., Zambon C.F., Martin B., Prà C.D., et al. SARS-CoV-2 RNA identification in nasopharyngeal swabs: Issues in pre-analytics. Clin. Chem. Lab. Med. 2020;58:1579–1586. doi: 10.1515/cclm-2020-0749. [DOI] [PubMed] [Google Scholar]
- 20.Cao X.J., Fang K.Y., Li Y.P., Zhou J., Guo X.G. The Diagnostic Accuracy of Xpert Xpress to SARS-CoV-2: A systematic review. J. Virol. Methods. 2022;301:114460. doi: 10.1016/j.jviromet.2022.114460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Song J.U. Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2021;93:4523–4531. doi: 10.1002/jmv.26994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud S.A., Ganesan S., Ibrahim E., Thakre B., Teddy J.G., Raheja P., Zaher W.A. Evaluation of six different rapid methods for nucleic acid detection of SARS-CoV-2 virus. J. Med. Virol. 2021;93:5538–5543. doi: 10.1002/jmv.27090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farfour E., Roux A., Ballester M., Gagneur L., Renaux C., Jolly E., Vasse M. Improved performances of the second generation of the ID NOW influenza A & B 2® and comparison with the GeneXpert®. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1681–1686. doi: 10.1007/s10096-020-03905-9. [DOI] [PubMed] [Google Scholar]
- 24.Trabattoni E., Le V., Pilmis B., de Ponfilly G.P., Caisso C., Couzigou C., Vidal B., Mizrahi A., Ganansia O., Le Monnier A., et al. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an ED. Am. J. Emerg. Med. 2018;36:916–921. doi: 10.1016/j.ajem.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 25.Kanwar N., Michael J., Doran K., Montgomery E., Selvarangan R. Comparison of the ID Now Influenza A & B 2, Cobas Influenza A/B, and Xpert Xpress Flu Point-of-Care Nucleic Acid Amplification Tests for Influenza A/B Virus Detection in Children. J. Clin. Microbiol. 2020;58:3. doi: 10.1128/JCM.01611-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NguyenVan J.C., Gerlier C., Pilmis B., Mizrahi A., Péan de Ponfilly G., Khaterchi A., Enouf V., Ganansia O., Monnier A. Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department. J. Clin. Virol. 2021;145:105021. doi: 10.1016/j.jcv.2021.105021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.