Abstract
Background:
Cyclosporine is a rescue treatment alternative to avoid colectomy in corticosteroid refractory acute severe ulcerative colitis. In this study, we aimed to evaluate the long-term efficacy and safety of cyclosporine therapy in acute severe ulcerative colitis patients.
Methods:
Acute severe ulcerative colitis (basal Lichtiger score > 10) patients who did not respond to 40 mg intravenous methylprednisolone therapy after 3-5 days were included in the study. The presence of clinical response and remission was assessed at 1st week, 1st, 6th, and 12th month according to the Lichtiger index.
Results:
In this study, 40 patients, whose steroid refractory acute severe ulcerative colitis and basal Lichtiger score > 10 points were enrolled. The median disease duration was 49.3 months (2-204). All patients received cyclosporine for 132 ± 78 days (7-270). Clinical response was obtained on seventh day in 82.5%. The clinical response rates of the first and sixth months were 72.5% and 62.5%, respectively. A total of 17/40 (42.5%) patients underwent colectomy within 1 year. In the patients who underwent colectomy, the basal LS (14.2 ± 1.9 vs 12.3 ± 1.7) (P = .002) was higher and the basal hemoglobin value (11.8 ± 2.3 vs 10.1 ± 1.5) (P = .037) was lower than those who did not undergo colectomy.
Conclusion:
Our findings suggest that cyclosporine treatment may be successfully and safely used in steroid refractory acute severe ulcerative colitis patients. Cyclosporine is a drug that has recently started to come up again with the introduction of new maintenance treatments. Especially in patients who develop a loss of response to infliximab therapy, or where infliximab therapy is contraindicated, or who have azathioprine intolerance, or are unresponsive.
Keywords: Cyclosporine, rescue treatment, steroid refractory, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a disease characterized by chronic mucosal inflammation of the colon. UC patients have a lifetime risk of between 20% and 25% acute severe exacerbation.1 Intravenous (IV) steroid therapy is recommended in acute severe ulcerative colitis (ASUC) patients. Unresponsiveness to IV steroid therapy in this patient group is not rare, which is about 40%.2 If there is an inadequate response to IV steroid therapy by day 3, salvage therapy such as infliximab (IFX) or calcineurin inhibitors (cyclosporine (Cyc) or tacrolimus) should be initiated, and early colectomy should be considered if there is no response to 7-day rescue therapy or worsening of the condition.3,4
Cyclosporine acts by directly inhibiting calcineurin—an important component of cytokine gene transcription. This terminates T-lymphocyte activity by down-regulating interleukin (IL)-2, IL-3, IL-4, tumor necrosis factor (TNF)-alpha, granulocyte–macrophage colony-stimulating factor, and interferon-gamma and has many studies on ASUC. First, Lichtiger et al5 reported that Cyc can be used in cases of steroid refractory ulcerative colitis. However, later studies support its effectiveness and showed short-term response rates of about between 65% and 85%. However, retrospective studies have questioned its long-term effectiveness and increasing rates of colectomy over time.6-8 There are studies showing that Cyc can be used not only as a rescue therapy but also in remission induction instead of steroid in the first step.9 Studies comparing the effectiveness of IFX and Cyc in steroid refractory UC showed that these 2 drugs were not superior to each other in the early period. Also, in studies comparing the late effects, no differences were found in terms of the rate of colectomy and its safety profile.10,11,12 Conflicting results are encountered in studies comparing the effectiveness of IFX and Cyc. For example, in the metanalysis study of Narula et al13 it was found that there was no difference between the colectomy rates between the 2 drugs in randomized controlled studies, and in non-randomized studies, it was reported that the colectomy rates were statistically less in those receiving IFX.13 The efficacy and safety of IFX in this setting are considered equivalent to Cyc following 2 randomized studies demonstrating short-term response rates with avoidance of colectomy in between 80% and 85% of patients for both drugs.14,15 Because of its short half-life, Cyc may be a safe alternative compared to IFX. In addition, there is limited evidence of the association between drug blood levels, postoperative complications, and drug-related complications.16 However, there are studies showing that Cyc and IFX treatments do not pose a significant risk for postoperative complications in IBD patients.12,17, In other studies, the rate of colectomy was found to be high in thiopurine-experienced patients who had remission with Cyc.6 One of the reasons for this is the lack of effective drugs in maintenance treatment at that time. Therefore, it is said that it is necessary to start IFX as a rescue treatment in patients with azathioprine (AZA) experienced. Today this perception is about to change.
In our study, we retrospectively examined the clinical response, clinical remission, and long-term colectomy rates of patients who received Cyc therapy as rescue therapy in steroid refractory ASUC patients. Side effect profile was also evaluated.
MATERIALS AND METHODS
Patients: According to Lichtiger score (LS), ASUC (activity index > 10 points), patients who did not respond to IV 40 mg methylprednisolone treatment (despite using steroid for 3-5 days) were included in the study. Minimum follow-up was 12 months (maximum 96 months). After Cys treatment, clinical response was accepted as LS of lesser than 10 points with a decrease of at least 3 points compared with baseline scores. Partial response in patients was considered as a decrease in LS < 10, but a decrease in LS of 2 or less compared to the baseline. Relapse was defined as an increase of at least 3 points in the LS from the value of 3 consecutive days before, and this situation leads to a change in treatment. Patients who started AZA treatment within 2 months before ASUC attack (considering that AZA effect had not started yet) were included in the study. All primary non-responders to Cyc treatment or some of the relapsed patients were referred to surgery for colectomy. Thus, triple therapy required the administration of trimethoprim-sulphametoxazol, as recommended by current guidelines, to avoid a potentially lethal opportunistic infection by Pneumocystis jiroveccii. All patients were examined for Cytomegalovirus (CMV) infection and difficile infection. Chest x-ray was performed at least once a week, and blood tests were done 2 or 3 times a week for all patients.
Treatment
Cyc was given as 2 mg/kg/day as IV infusion for 7-10 days. If the clinical response was achieved on day 7, then oral Cyc (2 × 2 mg/kg/day) maintenance treatment was started. C0 level was aimed to be between 150 and 250 ng/mL (2 mg/kg/day). This treatment was continued for 6 months. LS was evaluated at the time of admission, in the 1st week, 1st, 6th, and 12th month. The minimum follow-up period was 12 months (maximum 96 months) in patients in remission. The steroid dose was gradually reduced and discontinued within 4-6 weeks. On the seventh day of Cyc therapy, treatment was started with 50 mg/day in patients who were not treated with AZA, and the dose was gradually increased. Since thiopurine methyltransferase levels were not measured before the treatment of AZA, blood values were closely monitored and increased to the appropriate dose (2-2.5 mg/kg). AZA was continued with the same dose in patients who were already taking the drug. Cholesterol and magnesium were checked for all patients at baseline and 48 hours after starting Cyc. Because of drug-related neurotoxicity, Cyc was avoided in patients with a cholesterol level < 1.15 mg/L or in those with magnesium serum levels < 1.4 mEq/L.18 Cyc blood levels were measured every other day starting at 48 hours after initiating Cyc treatment. Patients’ blood Cyc levels were measured every week for the first month and then biweekly over the second month and then at least once every month (target C0 level: 300-400 ng/mL). Hypertension and nephrotoxicity, other important side effects of Cyc use, patients were closely monitored for these aspects, and therefore, treatment was discontinued in those with an increase in serum creatinine levels of more than 25%. Seizures, paresthesia, hypertension, hyperkalemia, and gum swelling were also monitored and recorded.
Our study was performed in accordance with the Helsinki recommendations. Approval of the local ethical committee was acquired (project number: 831-577).
The primary endpoints were to assess the efficacy of Cyc in terms of the rate of short- and long-term colectomy together with predictive factors of colectomy.
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 22.0 for Windows (IBM Corp.; Armonk, NY, USA) was used for the statistical analysis of data. Descriptive data are given as the number of participants and frequency. Categorical variables are expressed as the number of cases and percentage value. The comparison of categorical variables was performed using chi-square and Fisher’s exact tests. Continuous variables are given as median and minimum–maximum. For continuous variables, the Mann–Whitney U-test was used according to the situation of variables. A P value of <.05 was considered statistically significant.
Results
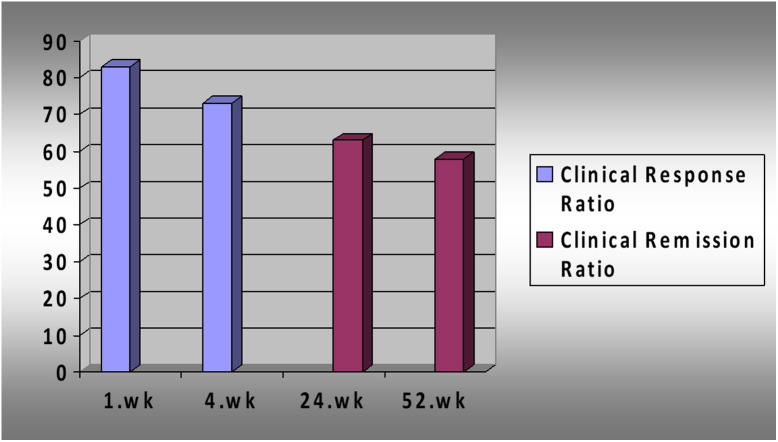
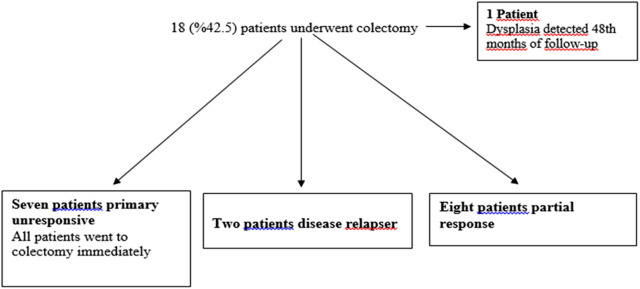
Forty patients unresponsive to CS therapy were included in the study. Thirty-two percent of the patients were women. The median age of the patients was 29.5 years (18-68), and the median duration of disease was 34.5 months (2-204). The median Lichtiger score was 12.5 (10-18) before treatment (Table 1). All patients who responded to Cyc treatment received an average of 180 days (7-330) of Cyc treatment. The median blood level of Cyc was 218.5 ng/mL (155-321). The clinical response rate was 82.5% (n = 33) in 1st week, and 72.5% in 1st month. Clinical remission was 62.5% in 6th month and 57.5% in the 1st year (Figure 1). Seventy percent of patients who had a clinical response at first week were still in remission after 1 year. At the end of 1 year, 42.5% (n = 17) of the patients underwent colectomy. Seven of these patients had primary nonresponse to Cyc, 8 had partial response, and 2 had relapsed disease. In addition to these cases, 1 patient had a colectomy in the 48th month of his follow-up, although dysplasia was detected on control colonoscopy (Figure 2). Recurrence occurred in 5 patients who responded to Cyc treatment. Two of these patients underwent surgery. IFX was administered to the remaining 3 patients as sequential salvage therapy and remained in remission during follow-up. The age of the disease was higher in patients who did not respond to Cyc treatment and underwent colectomy (P = .013).
Table 1.
Demographic Characteristics of Patients
| All Patients | Clinical Responders at First Week (n = 33) | Clinically Non-responders at First Week (n = 7) | P (Responders vs. Non-responders) | |
|---|---|---|---|---|
| Age (years) | 34.7 ± 12.8 | 34.9 ± 12.6 | 33.4 ± 14.3 | .78 |
| Gender (W/M) (N/%) | 13/27 (32.5/67.5) | 11/22 (27.5/55) | 2/5 (5/12.5) | .8 |
| Disease duration (months) | 49.3 ± 50.6 | 52.2 ± 54.5 | 35.5 ± 22.2 | .2 |
| Involvement of ulcerative colitis (diffuse/left colon) (n, %) | 35/5 (87.5/12.5) | 29 (72.5)/4(10) | 6 (15)/1(2.5) | .83 |
| Mayo endoscopic score 2 (N/%) | 18/40 (45) | 18 (54.5) | 0 | .011 |
| Mayo endoscopic score 3 (N/%) | 22/40 (55) | 15 (45.5) | 7 (100) | |
| Basal Lichtiger score | 13.1 ± 2 | 12.9 ± 2.2 | 13.6 ± 1.3 | .48 |
| AZA usage (experienced /naive) (N/%) | 20/20 (50/50) | 16/17 (40/42.5) | 4/3 (10/ 7.5) | .68 |
| Leukocyte | 12 600 ± 11 800 | 13 000 ± 12 400 | 9100 ± 4800 | .6 |
| Hemoglobulin | 10.9 ± 2 | 11 ± 1.8 | 9.4 ± 3.5 | .19 |
| CRP | 43.7 ± 45.3 | 44 ± 48 | 39 ± 2 | .89 |
| Statistical significance was accepted as p<0.05. | ||||
Figure 1.
Response rates of patients to cyclosporine treatment over time.
Figure 2.
Flowchart of patients treated with Cyc who finally underwent colectomy. Cyc, cyclosporine.
There was no statistically significant difference between those who used AZA and those who did not while staying in remission at the first year of Cyc treatment (P = .39). Eighty percent of the patients with partial response, whether in the early or late period, underwent colectomy.
There was a statistical difference between the group that underwent colectomy and the group that remained in remission in terms of basal LS and hemoglobin values (Table 2). It was found that the basal LS (14.2 ± 1.9 vs 12.3 ± 1.7) (P = .002) of the patients undergoing colectomy was higher and the hemoglobin value (11.8 ± 2.3 vs 10.1 ± 1.5) (P = .037) was lower. Cyc concentration showed no significant differences between colectomy and responder groups. Endoscopic Mayo score 3 was found in all patients who did not respond clinically in the first week. Similarly, endoscopic Mayo score 3 was found to be statistically significant in patients who underwent colectomy (P = .001).
Table 2.
Comparison Between Patients with and Without Colectomy
| Colectomy (n = 17) | No Colectomy (n = 23) | P | |
|---|---|---|---|
| Age (years) | 38.1 ± 14 | 32.2 ± 11 | .148 |
| Gender (F/M) (N/%) | 6/11 (35.3/64.7) | 7/16 (30.4/69.6) | .746 |
| Disease duration (months) | 72 ± 65 | 33 ± 28 | .013 |
| Involvement of ulcerative colitis (diffuse/left colon) (n, %) | 15/2 (88.2/11.8) | 20/3 (87/13) | .659 |
| Mayo endoscopic score 2 (N/%) | 1 (5.9) | 17 (73.9) | <.001 |
| Mayo endoscopic score 3 (N/%) | 16 (94.1) | 6 (26.1) | |
| Basal Lichtiger score | 14.2 ± 1.9 | 12.3 ± 1.7 | .002 |
| AZA usage (experienced/naive) (N/ %) | 10/7 (58.8/41.2) | 10/13 (56.5/ 43.5) | .523 |
| Hemoglobin | 11.8 ± 2.3 | 10.1 ± 1.5 | .037 |
| CRP | 26 ± 18 | 53 ± 52 | .214 |
| Statistical significance was accepted as p<0.05. | |||
Side effects developed in 17.5% (n = 7) of the patients (Table 3). These were hypertension, Herpes simplex virus (HSV) conjunctivitis, gingival hyperplasia, hypertrichosis, disseminated Varicella-zoster virus (VZV) infection, urticaria, and convusion. Cyc discontinuation was required in only 1 patient with disseminated VZV.
Discussion
Cyclosporine is an effective drug in providing remission in steroid refractory ASUC cases. In our study, the clinical response rate was found to be 82.5% in the early period, similar to other studies.6,7 Therefore, most of the patients were saved from colectomy in the early period. However, this effect gradually decreases, and close to half of the patients undergo colectomy in the long term. In our study, similar to other studies, colectomy is generally performed within the first 18 months after discontinuation of Cyc.6,7,19,20 Our rates of staying in remission or having colectomy at the end of 1 year with Cyc in steroid refractory ASUC patients were similar to the rates in other studies.5-8 In the study of Cohen et al6 the rate of patients going to surgery during the follow-up was 38%, while Moskovitz et al7 determined the rate of patients going to surgery as 45%.6,7 In our study, this rate was found to be 42.5% similarly.
Reinisch et al21 found a relationship between low basal hemoglobin value, poor prognosis, and non-response to treatment. In our study, patients with low basal hemoglobin levels were found to be more likely to undergo colectomy. In addition, a statistically significant relationship was found between high basal LS and going to colectomy (P = .002). Salameh et al22 as shown in their study, those with high basal LS were found to have a higher rate of going to colectomy (P = .03).
In our study, unlike other studies,6,8 no association was found between response to Cyc therapy and duration of the disease, similar to the Perragi et al23 study, where an association was found between the duration of the disease and the possibility of colectomy (P = .013).
Although Cyc treatment is more cost effective than IFX treatment,24 in recent years, IFX treatment has been preferred in many centers for steroid refractory ASUC patients due to the side effects of Cyc treatment and the need for strict monitoring of drug levels. Nowadays, induction of remission with Cyc treatment has become popular due to the availability of new drugs in maintenance therapies (vedolizumab, ustekinumab) in ASUC patients who develop loss of response to IFX treatment or for whom IFX treatment is contraindicated or whom is AZA intolerance. In these recent studies, 60% of patients who were initiated maintenance therapy with vedolizumab after induction of remission with Cyc were achieved colectomy-free survival at the end of 1 year in patients with steroid refractory ASUC who had anti-TNF insufficiency or contraindications previously.25-27 However, in a case recently reported by Ganzleben et al28 it has been reported that the patient with ASUC, who did not respond to thiopurine, anti-TNF, tofacitinib, and vedolizumab treatments before, was in remission with Cyc treatment, then maintenance treatment was started with ustekinumab and remained in remission clinically, laboratory (CRP, fecal calprotectin), and histologically after 195 days of follow-up.28
However, there are studies showing effectiveness as a second-line rescue therapy with Cyc treatment.27,29,30 In the study by Weisshof et al27, the largest patient cohort receive Cyc as second-line rescue therapy for ASUC. With this study, they aimed to investigate the efficacy and safety of Cyc in patients with steroid-resistant ASUC who failed first-line rescue therapy with IFX. They showed survival without colectomy at 1 month, 3 months, and 1 year as 65%, 59.4%, and 41.8%, respectively. Cyc may be offered to selected patients prior to referral for colectomy. Current guidelines recommend colectomy after initial salvage therapy fails,31,32 due to safety concerns and lack of data regarding sequential medical rescue treatment.
After the studies showing the efficacy of new maintenance treatments (such as vedolizumab, ustekinumab) in refractory patients before going to colectomy, remission induction as first-line and second-line salvage therapy with Cyc for refractory ASUC patients started to come to the fore again. The aim of this study was to present our knowledge about Cyc treatment experiences, administration method, and drug-related side effects in steroid-resistant ASUC patients as a tertiary center. In the future, randomized controlled prospective studies will be needed to report early long-term results comparing patients with IFX maintenance after IFX salvage therapy versus patients receiving maintenance therapy with novel biological agents after Cyc rescue therapy in steroid-resistant ASUC patients.
There is concern about the side effect profile during Cyc use. To better understand the safety profile of Cyc in IBD patients, in a study by Sternthal et al18, the results of 111 patients given IV Cyc followed by an oral dose were retrospectively reviewed and highlighted adverse effects including seizures, paresthesia, hypertension, hyperkalemia, and gingival swelling.18 As the side effects of Cyc are thought to be dose-dependent, attention should be paid to drug interactions. For example, drugs such as phenytoin, carbamazepine, and octreotide, which are potent metabolites of the cytochrome P450 3A pathway, reduce blood Cyc levels, while erythromycin and ketoconazole increase blood Cyc levels.33 Van Assche et al34 presented a double-blind, randomized controlled trial that supported treating patients at lower doses (2 mg/kg/day vs 4 mg/kg/day) and with similar therapeutic results while reducing the side effect profile.
However, in our study, side effects developed in 17.5% of the patients and those were hypertension, HSV conjunctivitis, gingival hyperplasia, hypertrichosis, disseminated Varicella-zoster virus (VZV) infection, urticaria, and convusion. Only 1 (2.5%) patient had serious side effects that required discontinuation of Cyc (disseminated VZV infection). In our study, less side effects were observed compared to other studies.18,35,36 This result may have been seen with close monitoring of patients for the occurrence of side effects and close drug level monitoring.
This study was conducted retrospectively, and the retrospective design does not allow for objective measures of severity of disease and response to therapy. Also, the main strength of this study is that all cases have been treated at a single tertiary center. All patients were treated using the same protocol by a team of IBD experts. The colectomy decision was made by the same team. In addition, objective criteria were used in patient selection such as LS. One of the limitations of this study is that it is retrospective and there is no long follow-up.
In conclusion, Cyc is still a valid option to treat refractory ASUC patients. There is a relationship between the risk of colectomy and long disease duration, basal hemoglobin levels, and high basal LS values. Even though novel therapies have been introduced in the past few years, the rate of need for colectomy remains high. The decision on which agent to use should be made on a case-to-case basis, based on factors such as previous exposure to medications, co-morbidities, costs, and feasibility of laboratory testing.
Footnotes
Ethics Committee Approval: The study was approved by the local medical ethics committee (No: 831-.577).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.E., S.K.; Design – S.E., S.K.; Supervision – S.E., S.K.; Resources – S.E., S.K.; Materials – S.E.; Data Collection and/or Processing – S.E.; Analysis and/or Interpretation – S.E., R.İ.; Literature Search – S.E.; Writing Manuscript – S.E.; Critical Review – S.E., S.K.
Declaration of Interest: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
The content of the manuscript has been presented as a poster in DDW 2015.
References
- 1. . Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299 315. 10.1136/gut.4.4.299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9(1):18 27. 10.5009/gnl14226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Moss AC, Peppercorn MA. Steroid-refractory severe ulcerative colitis: what are the available treatment options? Drugs. 2008;68(9):1157 1167. 10.2165/00003495-200868090-00001) [DOI] [PubMed] [Google Scholar]
- 4. . Weber A, Fein F, Koch S, et al. Treatment of ulcerative colitis refractory to steroid therapy by oral microemulsion cyclosporine (neoral). Inflamm Bowel Dis. 2006;12(12):1131 1135. 10.1097/01.mib.0000235096.78736.8e) [DOI] [PubMed] [Google Scholar]
- 5. . Järnerot G, Rolny P, Sandberg-Gertzén H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89(5):1005 1013. 10.1016/0016-5085(85)90201-x) [DOI] [PubMed] [Google Scholar]
- 6. . Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4(6):760 765. 10.1016/j.cgh.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 7. . Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94(6):1587 1592. 10.1111/j.1572-0241.1999.01149.x) [DOI] [PubMed] [Google Scholar]
- 8. . Bamba S, Andoh A, Imaeda H, et al. Prognostic factors for colectomy in refractory ulcerative colitis treated with calcineurin inhibitors. Exp Ther Med. 2012;4(1):99 104. 10.3892/etm.2012.545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . D’Haens G, Lemmens L, Geboes K, et al. Intravenous cyclosporine versus intravenous glucocorticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001;120(6):1323 1329. 10.1053/gast.2001.23983) [DOI] [PubMed] [Google Scholar]
- 10. . Chang KH, Burke JP, Coffey JC. Infliximab versus cyclosporine as rescue therapy in acute severe steroid-refractory ulcerative colitis: a systematic review and meta-analysis. Int J Colorectal Dis. 2013;28(3):287 293. 10.1007/s00384-012-1602-8) [DOI] [PubMed] [Google Scholar]
- 11. . Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380(9857):1909 1915. 10.1016/S0140-6736(12)61084-8) [DOI] [PubMed] [Google Scholar]
- 12. . Powar MP, Martin P, Croft AR, et al. Surgical outcomes in steroid refractory acute severe ulcerative colitis: the impact of rescue therapy. Colorectal Dis. 2013;15(3):374 379. 10.1111/j.1463-1318.2012.03188.x) [DOI] [PubMed] [Google Scholar]
- 13. . Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111(4):477 491. 10.1038/ajg.2016.7) [DOI] [PubMed] [Google Scholar]
- 14. . Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1(1):15 24. 10.1016/S2468-1253(16)30003-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Sjöberg M, Walch A, Meshkat M, et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis. 2012;18(2):212 218. 10.1002/ibd.21680) [DOI] [PubMed] [Google Scholar]
- 16. . Sobrado CW, Sobrado LF. Management of acute severe ulcerative colitis: a clinical update. Arq Bras Cir Dig. 2016;29(3):201 205. 10.1590/0102-6720201600030017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Hyde GM, Jewell DP, Kettlewell MG, Mortensen NJ. Cyclosporin for severe ulcerative colitis does not increase the rate of perioperative complications. Dis Colon Rectum. 2001;44(10):1436 1440. 10.1007/BF02234594) [DOI] [PubMed] [Google Scholar]
- 18. . Sternthal MB, Murphy SJ, George J, Kornbluth A, Lichtiger S, Present DH. Adverse events associated with the use of cyclosporine in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103(4):937 943. 10.1111/j.1572-0241.2007.01718.x) [DOI] [PubMed] [Google Scholar]
- 19. . Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol. 2005;17(1):79 84. 10.1097/00042737-200501000-00016) [DOI] [PubMed] [Google Scholar]
- 20. . Stack WA, Long RG, Hawkey CJ. Short- and long-term outcome of patients treated with cyclosporin for severe acute ulcerative colitis. Aliment Pharmacol Ther. 1998;12(10):973 978. 10.1046/j.1365-2036.1998.00396.x) [DOI] [PubMed] [Google Scholar]
- 21. . Reinisch W, Reinink AR, Higgins PDR. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(4):635 642. 10.1016/j.cgh.2014.03.037) [DOI] [PubMed] [Google Scholar]
- 22. . Salameh R, Kirchgesner J, Allez M, et al. Long-term outcome of patients with acute severe ulcerative colitis responding to intravenous steroids. Aliment Pharmacol Ther. 2020;51(11):1096 1104. 10.1111/apt.15751) [DOI] [PubMed] [Google Scholar]
- 23. . Parragi L, Fournier N, Zeitz J, et al. Colectomy rates in ulcerative colitis are low and decreasing: 10-year follow-up data from the Swiss IBD Cohort Study. J Crohns Colitis. 2018;12(7):811 818. 10.1093/ecco-jcc/jjy040) [DOI] [PubMed] [Google Scholar]
- 24. . Löwenberg M, Duijvis NW, Ponsioen C, van den Brink GR, Fockens P, D’Haens GR. Length of hospital stay and associated hospital costs with infliximab versus cyclosporine in severe ulcerative colitis. Eur J Gastroenterol Hepatol. 2014;26(11):1240 1246. 10.1097/MEG.0000000000000187) [DOI] [PubMed] [Google Scholar]
- 25. . Ollech JE, Dwadasi S, Rai V, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment Pharmacol Ther. 2020;51(6):637 643. 10.1111/apt.15616) [DOI] [PubMed] [Google Scholar]
- 26. . Pellet G, Stefanescu C, Carbonnel F, et al. Groupe d’Etude thérapeutique des affections inflammatoires du tube digestif. [Efficacy and safety of induction therapy with calcineurin inhibitors in combination with vedolizumab in patients with refractory ulcerative colitis]. Clin Gastroenterol Hepatol. 2019;17(3):494 501. 10.1016/j.cgh.2018.08.081) [DOI] [PubMed] [Google Scholar]
- 27. . Weisshof R, Ollech JE, El Jurdi K, et al. Ciclosporin therapy after infliximab failure in hospitalized patients with acute severe colitis is effective and safe. J Crohns Colitis. 2019;13(9):1105 1110. 10.1093/ecco-jcc/jjz032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. . Ganzleben I, Geppert C, Osaba L, et al. Successful cyclosporin and ustekinumab combination therapy in a patient with severe steroid-refractory ulcerative colitis. Therap Adv Gastroenterol. 2020;13:1756284820954112. 10.1177/1756284820954112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol. 2019;17(3):486 493. 10.1016/j.cgh.2018.04.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. . Protic M, Seibold F, Schoepfer A, et al. The effectiveness and safety of rescue treatments in 108 patients with steroid-refractory ulcerative colitis with sequential rescue therapies in a subgroup of patients. J Crohns Colitis. 2014;8(11):1427 1437. 10.1016/j.crohns.2014.05.004) [DOI] [PubMed] [Google Scholar]
- 31. . Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501 23; quiz 524. 10.1038/ajg.2009.727) [DOI] [PubMed] [Google Scholar]
- 32. . Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769 784. 10.1093/ecco-jcc/jjx009) [DOI] [PubMed] [Google Scholar]
- 33. . Garud S, Peppercorn MA. Ulcerative colitis: current treatment strategies and future prospects. Therap Adv Gastroenterol. 2009;2(2):99 108. 10.1177/1756283X09102329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. . Van Assche G, D’Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025 1031. 10.1016/s0016-5085(03)01214-9) [DOI] [PubMed] [Google Scholar]
- 35. . Van Gossum A, Schmit A, Adler M, et al. Short- and long-term efficacy of cyclosporin administration in patients with acute severe ulcerative colitis. Belgian IBD Group. Acta Gastroenterol Belg. 1997;60(3):197 200. [PubMed] [Google Scholar]
- 36. . Santos J, Baudet S, Casellas F, Guarner L, Vilaseca J, Malagelada JR. Efficacy of intravenous cyclosporine for steroid refractory attacks of ulcerative colitis. J Clin Gastroenterol. 1995;20(4):285 289. 10.1097/00004836-199506000-00005) [DOI] [PubMed] [Google Scholar]