Abstract
Actinomycetes has large habitats and can be isolated from terrestrial soil, rhizospheres of plant roots, and marine sediments. Actinomycetes produce several bioactive secondary metabolites with antibacterial, antifungal, and antiviral properties. In this study, some Actinomycetes strains were isolated from the rhizosphere zone of four different plant species: rosemary, acacia, strawberry, and olive. The antagonistic activity of all isolates was screened in vitro against Escherichia coli and Bacillus megaterium. Isolates with the strongest bioactivity potential were selected and molecularly identified as Streptomyces sp., Streptomyces atratus, and Arthrobacter humicola. The growth-promoting activity of the selected Actinomycetes isolates was in vivo evaluated on tomato plants and for disease control against Sclerotinia sclerotiorum. The results demonstrated that all bacterized plants with the studied Actinomycetes isolates were able to promote the tomato seedlings’ growth, showing high values of ecophysiological parameters. In particular, the bacterized seedlings with Streptomyces sp. and A. humicola showed low disease incidence of S. sclerotiorum infection (0.3% and 0.2%, respectively), whereas those bacterized with S. atratus showed a moderate disease incidence (7.6%) compared with the positive control (36.8%). In addition, the ability of the studied Actinomycetes to produce extracellular hydrolytic enzymes was verified. The results showed that A. humicola was able to produce chitinase, glucanase, and protease, whereas Streptomyces sp. and S. atratus produced amylase and pectinase at high and moderate levels, respectively. This study highlights the value of the studied isolates in providing bioactive metabolites and extracellular hydrolytic enzymes, indicating their potential application as fungal-biocontrol agents.
Keywords: biocontrol, phytopathogens, bioactive substances, microbial biostimulants, antagonistic activity, Actinobacteria
1. Introduction
Recently, new agrochemical drugs have been registered in agriculture field, but they can have different negative effects on plants, the environment, and humans. Furthermore, several phytopathogenic microorganisms have become resistant to some agrochemicals, which requires the development of new antimicrobial agents to avoid this serious phenomenon [1,2]. Currently, many scientists all over the world are trying to discover new natural drugs of plant or microbial origin [3,4,5,6,7]. Many plant and microorganisms produce different bioactive secondary metabolites that can potentially be used in the agro-pharmaceutical industry as efficient alternatives for several chemical pesticides [3,8,9,10].
The soil is a rich matrix of living microorganisms and is a valuable resource of biological control agents [11,12,13]. The rhizosphere, which is made up of aggregates containing accumulated organic matter, is a repository of microbial activity in the soil. The rhizosphere has great importance because it can support large populations of active microorganisms [14]. Furthermore, soil microorganisms provide an excellent source for important bioactive products [15]. There is growing interest in using bacteria for medicinal and agricultural purposes due to their ability to produce a wide range of biologically active substances with antibiotic, fungicidal, herbicidal, hydrolytic enzymatic, antitumor, antivirals, and immune-suppressant activities [16,17,18]. Recently, pathogen resistance has necessitated the discovery of new antimicrobial agents effective against bacteria and fungi. There is strong interest in screening new microorganisms from different habitats for antimicrobial activity in order to discover new and promising antibiotics in the treatment against multi-drug resistant pathogens (MDRPs).
Actinomycetes, a type of unicellular Gram-positive bacteria, are widely distributed in nature from different habitats and are well-known and important producers of several bioactive secondary metabolites, antibiotics, and growth-promoting factors [19]. Actinomycetes are very similar to fungi, though they form hyphae much smaller than fungi [19,20]. The phylum Actinobacteria is considered one of the important groups of Actinomycetes [21,22]. Girão et al. [23] reported that many thousands of bioactive substances have been identified from Actinobacteria, especially those from terrestrial sources. The produced bioactive metabolites from Actinomycetes, especially those from terrestrial sources, represent about the 45% of known microbial bioactive metabolites [23,24]. In addition, Girão et al. [23] studied the antimicrobial activity of the organic extracts from some Actinobacteria isolated from Laminaria ochroleucahe and concluded that several isolates were able to inhibit the growth of Candida albicans and Staphylococcus aureus. Streptomyces, among the Actinobacteria, is considered an important genus able to produce the majority of the identified bioactive compounds, as reported by Berdy [25].
The isolation and biochemical characterization of Actinomycetes may allow finding new bioactive substances for pharmaceutical and agricultural purposes. The main objectives of the current study were to: (i) isolate and identify new strains of Actinomycetes from different soil habitats; (ii) evaluate the in vitro antagonistic effect of the tested isolates against some common phytopathogens; and (iii) evaluate the in vivo growth-promoting effect of the most bioactive isolates and their antifungal activity against Sclerotinia sclerotiorum on tomato seedlings.
2. Results
2.1. Isolation and Preliminary Screening
The isolation from the soil samples allowed obtaining ten pure Actinomycetes isolates (Table 1). All isolates were preliminarily evaluated for their antagonistic activity against the two target microorganisms (Escherichia coli and Bacillus megaterium). The isolates AC1 and RS3 showed the highest biological activity against both tested microorganisms, whereas FG1 showed moderate activity against both tested microorganisms (Table 1). The OL2 isolate showed the highest activity against E. coli and the most promising activity against B. megaterium (Table 1). Based on the obtained results, the isolates AC1, RS3, and OL2 were selected for molecular identification and further biological assays.
Table 1.
Antagonistic activity of the Actinomycetes isolates.
| Isolates | Antagonistic Activity | |
|---|---|---|
| E. coli | B. megaterium | |
| AC1 * | +++ | +++ |
| AC2 | - | - |
| AC3 | + | + |
| RS1 | + | - |
| RS2 | - | - |
| RS3 * | +++ | +++ |
| FG1 | + | ++ |
| FG2 | + | - |
| OL1 | + | - |
| OL2 * | +++ | ++ |
Note: +++, very high activity; ++, high activity; +, moderate activity; -, no activity. AC1, AC2, and AC3 were isolated from acacia rhizosphere; RS1, RS2, and RS3 were isolated from rosemary rhizosphere; FG1 and FG2 were isolated from strawberry rhizosphere; OL1 and OL2 were isolated from olive rhizosphere. *, isolates that showed the highest antagonistic effect.
2.2. Molecular Identification
The amplification with the primers Y1/Y2 produced amplicons with molecular weight of about 434 bp. No amplicons were observed in the negative control. The amplified DNA were sequenced (BMR Genomics, Padova, Italy), and the obtained sequences were compared with those available in GenBank nucleotide archive using Basic Local Alignment Search Tool software (BLAST) (RKV, MD, USA). The results of sequences analysis of AC1, RS3, and OL2 showed high similarity percentages to the sequences of Streptomyces sp., Streptomyces atratus, and Arthrobacter humicola, respectively, present in GenBank with the following accession numbers: ON241810, ON241816, and ON241806, respectively.
2.3. Extracellular Hydrolytic Enzymes
The results showed that all studied isolates were able to produce some extracellular hydrolytic enzymes (Table 2). In particular, the highest significant hydrolytic activity of chitinase (chitin azure), glucanase, and protease was observed in the case of A. humicola, where the diameter of the hydrolysis zones was 31.5, 36.0, and 21.5 mm, respectively. On the other hand, Streptomyces sp. and S. atratus showed the highest significant activity of amylase with a diameter of hydrolysis area of 37.5 and 42.0 mm, respectively, whereas the same two isolates showed moderate activity for pectinase with a diameter of hydrolysis area of 14.0 and 10.5 mm, respectively. However, S. atratus and A. humicola did not show either glucanase or pectinase activity, respectively. None of the three tested isolates showed hydrolytic activity for chitinase (chitin from crab shells) and polygalacturanase.
Table 2.
Extracellular hydrolytic enzymes produced by the tested Actinomycetes isolates.
| Enzyme | Substrates | Staining | Diameter of Hydrolysis Area (mm) | ||
|---|---|---|---|---|---|
| AC1 Streptomyces sp. |
RS3 S. atratus |
OL2 A. humicola |
|||
| Chitinase | Chitin azure (1%) | Congo red (0.03%) | 23.0 ± 2.3 b | 0.0 ± 0.0 c | 31.5 ± 1.7 a |
| Chitin crab shells (1%) | Congo red (0.03%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Amylase | Soluble starch (1%) | Lugol solution (a) | 37.5 ± 2.9 a | 42.0 ± 1.2 a | 28.0 ± 3.5 b |
| Glucanase | Lichenan (0.2%) | Congo red (0.03%) | 22.0 ± 2.3 b | 0.0 ± 0.0 c | 36.0 ± 1.2 a |
| Pectinase | Pectin (0.5%) | CTAB (b) (2%) | 14.0 ± 1.2 a | 10.5 ± 1.7 a | 0.0 ± 0.00 b |
| Protease | Skim milk (1%) | - | 14.5 ± 2.9 b | 12.5 ± 2.9 b | 21.5 ± 1.7 a |
| Polygalacturanase | Polygalacturonic acid (1%) | Ruthenium red (0.1%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
(a) Lugol solution was prepared as follows: 0.35 g iodide + 0.66 g potassium iodide KI in 100 mL dis. H2O; (b) CTAB: hexadecyltrimethylammonium bromide; values followed by different letters in each row for each tested enzyme were significantly different according to Tukey’s B multiple comparison test post hoc test at p < 0.05.
2.4. In Vivo Growth Promoting and Disease Control
2.4.1. Eco-Physiological Parameters
The results revealed that all studied Actinomycetes isolates were able to stimulate the growth of bacterized tomato seedlings, which showed higher values of eco-physiological parameters in comparison with the negative control (non-bacterized plants), as represented in Table 3. In particular, seedlings inoculated with Streptomyces sp. and A. humicola showed the highest significant values (p < 0.05) of number of leaves, shoot length, shoot fresh weight, and shoot dry weight. The eco-physiological parameters of bacterized tomato seedlings artificially infected with S. sclerotiorum are reported in Table 4. In particular, seedlings inoculated with Streptomyces sp. and A. humicola demonstrated high values (p < 0.05) of number of leaves, twigs, shoot fresh weight, and shoot dry weight. However, S. atratus showed a moderate growth-promoting effect on tomato seedlings, especially in terms of the number of twigs, shoot length, and total shoot dry weight.
Table 3.
Effect of Actinomycetes isolates on eco-physiological parameters of tomatoes (health control).
| Actinomycetes Isolates | Eco-Physiological Parameters | ||||
|---|---|---|---|---|---|
| TN (n) | SL (cm) | LN (n) | SFW (g) | SDW (g) | |
| Cont. -ve | 8 ± 1.4 a | 36.05 ± 3.2 ab | 116 ± 6.9 b | 150.02 ± 4.1 b | 15.33 ± 1.4 b |
| AC1: Streptomyces sp. | 8 ± 0.9 a | 39.01 ± 4.1 a | 195 ± 11.8 a | 204.00 ± 13.4 a | 33.02 ± 4.5 a |
| RS3: Streptomyces atratus | 6 ± 1.2 a | 38.25 ± 7.1 a | 123 ± 13.6 b | 119.33 ± 8.0 c | 15.78 ± 1.9 b |
| OL2: Arthrobacter humicola | 7 ± 1.0 a | 46.00 ± 3.2 a | 151 ± 7.2 a | 184.01 ± 7.9 a | 24.76 ± 2.7 a |
Note: TN: twig number; SL: shoot length; LN: leaf number: SFW and SDW: fresh and dry weight of shoot systems, respectively. Values followed by different letters in each vertical column for each measured parameter were significantly different according to Tukey’s B multiple comparison test post hoc test at p < 0.05.
Table 4.
Effect of Actinomycetes isolates on eco-physiological parameters of tomatoes (artificially infected with S. sclerotiorum).
| Actinomycetes Isolates | Eco-Physiological Parameters | ||||
|---|---|---|---|---|---|
| TW (n) | SL (cm) | LN (n) | SFW (g) | SDW (g) | |
| Cont. -ve | 5 ± 0.2 b | 42.32 ± 0.3 a | 161 ± 1.0 bc | 142.33 ± 1.0 c | 24.04 ± 0.2 ab |
| AC1: Streptomyces sp. | 8 ± 0.1 a | 54.31 ± 0.4 a | 333 ± 2.3 a | 240.12 ± 2.5 a | 30.67 ± 0.3 a |
| RS3: Streptomyces atratus | 5 ± 0.1 b | 44.34 ± 0.8 a | 210 ± 1.8 b | 214.67 ± 1.9 ab | 23.00 ± 1.0 ab |
| OL2: Arthrobacter humicola | 8 ± 0.1 a | 51.76 ± 0.2 a | 477 ± 3.7 a | 304.65 ± 0.8 a | 29.67 ± 0.6 a |
Note: TN: twig number; SL: shoot length; LN: leaf number: SFW and SDW: fresh and dry weight of shoot systems, respectively. Values followed by different letters in each vertical column for each measured parameter were significantly different according to Tukey’s B multiple comparison test post hoc test at p < 0.05.
2.4.2. Disease Control
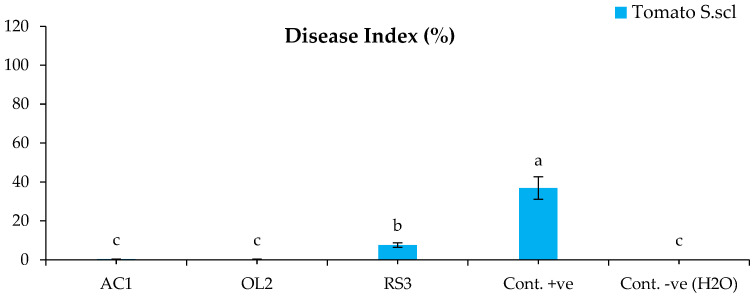
The bacterized plants with Streptomyces sp. and A. humicola did not show any symptoms on their leaves and roots after the infection with S. sclerotiorum. The disease indexes of the plants bacterized with Streptomyces sp. and A. humicola were 0.3% and 0.2% (Figure 1), whereas the control effects were 99.2% and 99.5%, respectively (Figure 2). The seedlings bacterized with S. atratus showed a moderate disease index of 7.6% (Figure 1) and a control effect of 79.5% (Figure 2). Regarding the positive control (plants inoculated only with S. sclerotiorum), the results showed the development of leaf yellowing and chlorosis at 20 DAI, where the leaf chlorotic zone of infected tomato plants became necrotic. Moreover, complete leaf wilting and root necrosis was also observed at 35 DAI. In particular, a significantly higher symptomatic leaves percentage (p < 0.05) was observed in the positive control, where the disease index was 36.8% compared with the negative control and bacterized plants with Actinomycetes isolates (Figure 1). S. sclerotiorum was always re-isolated from the inoculated plants.
Figure 1.
Disease index of tomato inoculated with S. sclerotiorum. Bars with different letters indicate mean values significantly different at p < 0.05 according to Tukey’s B test. Data are expressed as mean of 3 replicates ± SDs. DI (%) = [Σ (Scale × No. of SL)/(HS × TL)] × 100 (Equation (1)). AC1, OL2, and RS3 are Streptomyces sp., Arthrobacter humicola, and Streptomyces atratus, respectively.
Figure 2.
Control effect of the tomato inoculated with S. sclerotiorum. Bars with different letters indicate mean values significantly different at p < 0.05 according to Tukey’s test. Data are expressed as mean of 3 replicates ± SDs. CE (%) = 100 × (DI-P-DI-B)/DI-P (Equation (2)).
3. Discussion
The obtained results proved that the studied Actinomycetes isolates were able to promote the growth of tomato plants by improving the eco-physiological characteristics and also are promising for the biocontrol of S. sclerotiorum on tomato seedlings. In particular, the biological activity and growth-promoting effect of the studied Actinomycetes strains may be due to their ability to produce some bioactive metabolites, such as growth hormones, which enhance the tomato seedlings’ growth [26,27,28,29]. The application of microbial plant stimulants is considered an important strategy for sustainable agriculture systems for enhancing plant growth and increasing production, especially under abiotic stress [30].
Sousa and Olivares [31] concluded that plant-growth-promoting Streptomyces (PGPS) was able to biostimulate plant growth by direct and indirect pathways such as phytohormones production, phosphate solubilization, and alleviation of various abiotic stresses. In particular, endophytic Actinobacteria can biostimulate the secretion of plant growth hormones such as indole acetic acid (IAA), as reported by Manulis et al. [32] and Dochhil et al. [33].
The treatments with Streptomyces sp. and Arthrobacter humicola showed high reduction in disease symptoms on tomato seedlings against the tested pathogenic fungi. Furthermore, the bacterization treatments induced a significant disease protection of tomato seedlings compared with non-bacterized plants against fungal infection with S. sclerotiorum. These results are in agreement with those of several researchers who reported that many soil-borne Actinomycetes are able to reduce the growth of some pathogenic fungi such as Colletotrichum gloeosporioides, C. capsici, and Fusarium solani f. sp. pisi [34,35,36,37].
The production of hydrolytic enzymes can also play an important role in controlling phytopathogenic fungi [38,39]. The cell wall lytic enzymes glucanase and protease can contribute to the degradation of fungal cell wall (skeletal) components through embedment in its protein matrix [40,41]. In addition, Ordentlich et al. [40] reported that chitinase and other lytic enzymes produced by Serratia marcescens were able to control the pathogenic fungus Sclerotium rolfsii, causing the release of β-glucanase, which can increase the chitinolytic activity in hyphal degradation [42].
Chaudhary et al. [43] studied the antagonistic activity of some Actinomycetes strains isolated from different niche habitats of Sheopur (India) and observed that some strains were highly active against Bacillus cereus, Enterococcus faecalis, Shigella dysenteriae, Streptococcus pyogenes, Staphylococcus saprophyticus, S. epidermidis, methicillin-resistant Staphylococcus, and S. xylosus. The same authors also reported that all studied isolates were able to inhibit the extracellular growth of tested microorganisms, whereas they were not able to inhibit intracellular growth of mycelium. The latter phenomena may be due to the production of some bioactive secondary metabolites that may not reach to the intracellular cells of the tested bacteria and hence were not able to denature their cell walls [43].
In a recent study conducted by Odumosu et al. [42], it was reported that some species of Streptomyces sp. showed promising antibacterial activity against some food and human pathogens such as S. aureus, E. coli, Klebsiella pneumonia, and Salmonella typhi. The same authors also chemically analyzed the secondary metabolites produced by the studied species using GC-MS and verified their antibiotic properties may be used in novel antimicrobials. The antifungal activity of Streptomyces strains may also be due to their ability to produce some bioactive secondary metabolites such as isoikarugamycin, a novel polycyclic tetramic acid macrolactam produced by Streptomyces zhaozhouensis active against C. albicans, as reported by Lacret et al. [44].
4. Materials and Methods
4.1. Soil Sampling and Isolation
For isolation of Actinomycetes, 200 g subsamples were collected from the rhizosphere zone of four different plant species: rosemary (3 samples), acacia (3 samples), strawberry (2 samples), and olive (2 samples) from Potenza (Basilicata region, southern Italy). Each soil sample was air-dried on the benches for one week and sieved through a 250 μm pore sieve (Glenammer, Scotland, UK). The samples were further held in a hot-air oven at 121 °C for 1 h to prevent the growth of other microorganisms. The isolation was carried out following the membrane filter technique using DifcoTM Actinomycetes Isolation Agar (Sparks, MD, USA) [45] with some minor modifications. The cultivated plates were incubated for 4 days at 28 °C until the Actinomycetes become visible. The prepared nutrient media was supplemented with 100 μg/mL cycloheximide to suppress eventual growth of fungi. All obtained isolates were cultured in triplicates and further purified for obtaining the pure cultures, which were conserved on slant agar nutrient glycerol (ANG) tubes at 4 °C for further biological assays. The obtained isolates were initially examined based on their microscopic morphological features with a light microscope. For exact identification, the obtained isolates were further analyzed by the molecular method.
4.2. Antagonistic Activity
The studied isolates were verified for their biological activity against E. coli and B. megaterium using the cross-streak method as reported by Odumosu et al. [42]. Briefly, a single, small mass from a fresh culture (24 h) of each studied isolate was streaked in the center of a Petri dish containing KingʹB (KB) nutrient media [46] and then incubated at 37 °C for 48 h. Successively, the plates were inoculated with the tested microorganisms by a single streak at a perpendicular close to the initial inoculum of each studied Actinomycetes isolate. All plates were incubated at 37 °C and the antagonistic activity was evaluated after 24 h. The bacterial antagonistic activity was recorded as follows: very high activity (+++); high activity (++); moderate activity (+); no activity (-). The most bioactive isolates were selected for molecular identification and further in vitro and in vivo biological assays.
4.3. Molecular Identification
The bacterial isolates that demonstrated potentially antagonistic effects against the tested target microorganisms were previously morphologically identified under a light microscope (60×) and then by the molecular method based on the analysis of genomic DNA (gDNA) sequences. The gDNA of each studied isolate was extracted using a Qiagen Genomic DNA Kit (Qiagen, Heidelberg, Germany). The extracted gDNA was amplified using the universal primers for bacteria Y1/Y2 (Table 5). The PCR reaction was carried out in a final volume of 25 μL containing: 200 ng DNA, 0.2 µL of 1 U Taq DNA polymerase, 2.5 µL Taq buffer (20 mM MgCl2), 5 µL of each primer (2.5 µM), 5 μL of dNTPs (4 mM) and ultrapure dH2O for a final volume of 25 μL. Both the concentration and purity of the total DNA extracted from each sample were measured using a Nano-drop (Thermo Fisher Scientific, Waltham, MA USA). Each DNA sample was subjected to PCR amplification following the cycling profile: 94 °C for 5 min (initial denaturation), followed by 34 cycles of 94 °C for 30 c (denaturation), 57 °C for 30 s (annealing), and 72 °C for 1 min (extension), with a final extension step of 5 min at 72 °C. The amplified DNA, stained by Bromophenol blue (3 µL/10 µL), was applied for agarose gel electrophoresis (1.2%) stained by SYBR green dye (4 µL/100 gel). The obtained amplicons were directly sequenced and compared with those available in the GenBank nucleotide archive using BLAST software [47].
Table 5.
Primers used in this study.
| Primers | Sequences | Target | Amplified Fragment (kb) | Gene | Reference |
|---|---|---|---|---|---|
| Y1 | 5′-TGGCTCAGAACGAACGCTGGCGGC-3′ | Bacteria | 0.43 | 16S rDNA | Darrasse et al. [53] |
| Y2 | 5′-CCCACTGCTGCCTCCCGTAGGAGT-3′ |
4.4. Extracellular Hydrolytic Enzymes
The enzymatic activity of the studied Actinomycetes isolates was screened by carrying out an assay of extracellular hydrolytic enzymes on KB media supplemented with the below specific substrates for each enzyme: chitin azure (1%) or chitin from crab shells (1%) for chitinase [48]; skim milk (1%) for protease [48]; and lichenan (0.2%) for glucanase [49]. In addition, soluble starch (1%), pectin (0.5%), and polygalacturonic acid (1%) were used for amylase, pectinase, and polygalacturanase, respectively [50,51]. All plates were incubated at 30 °C for 96 h and then flooded with specific staining solutions as follows: Congo red (0.03%) for chitinase and glucanase; lugol solution for amylase; CTAB: hexadecyltrimethylammonium bromide (2%) for pectinase and ruthenium red (0.1%) for polygalacturanase. The enzymatic activity was taken as evidence of the appearance of hydrolysis clear zones around the colonies, and their diameters were measured in millimeters.
4.5. In Vivo Growth Promoting Effect and Disease Control
An in vivo pot experiment was carried out in a greenhouse (School of Agricultural, Forestry, Food and Environmental Sciences-SAFE, University of Basilicata, Potenza, Italy) to evaluate the growth-promoting effect (GPE) of the tested Actinomycetes isolates on tomato plants, and the disease control (DC) of the most bioactive isolates was studied against S. sclerotiorum.
The pot experiment was carried out in a glass greenhouse at 25 °C for a 15-h photoperiod. Each pot was 20 cm high and 25 cm wide, and previously sterilized with 1.2% sodium hypochlorite for 5 min, rinsed twice with distilled water, and filled with a growing medium mixture (compost/peat moss, 1:1). Seeds of Solanum lycopersicum L. cv. cerasiforme were surface sterilized by ethanol (70%) and sowed in a cell tray. The temperature and relative humidity in the greenhouse remained stable at 25 ± 2 °C and 70–80%, respectively, for the duration of the experiment.
For the Actinomycetes treatment, an initial nutrient culture of peptone yeast calcium agar (PY-Ca) was prepared for the tested isolates and incubated for 5 days at 28 ± 2 °C. A suspension of each studied isolate was prepared by inoculating 106 CFU/mL from the original culture into minimal mineral (MM) media prepared as follows: (g/L) 10.5 K2HPO4, 4.5 KH2PO4, 1.0 (NH4)2SO4, 0.5 Na3C6H5O7 × 2H2O, 0.2 MgSO4 and 5.0 dextrose. The pH value was adjusted at 7.0. The suspensions were then incubated for 7 days at 28 ± 2 °C. The broth cultures were poured into the rhizosphere zone of tomato seedlings (100 mL/pot) 15 days after germination (DAG).
For the fungal artificial infection, Ø 5 mm agar discs from a pure fresh culture (96 h) of S. sclerotiorum were inoculated in a sterilized flask filled with potato dextrose broth (PDB) and incubated for 7 days at 22 ± 2 °C. After that, 50 mL of the incubated broth was inoculated in the rhizosphere zone of the seedlings 10 days after the Actinomycetes treatment. Ten seedlings were used as the negative health control. The whole experiment was repeated twice with five replicates per treatment. The experimental pots were distributed in a randomized block design in the greenhouse and watered once a day.
For the eco-physiological parameters, plant growth was monitored at the end of the experiment, about 40 DAG, by measuring stem length (SL) in centimeters, number of leaves (NL), number of twigs (NT), the total fresh weights of shoots (TFwS) in grams, and total dry weight of shoots (TDwS) in grams. Regarding the evaluation of the disease incidence, tomato plants were monitored daily, fifteen days after the infection (DAI), to observe the eventual appearance of disease symptoms. The disease incidence was assessed using the following scale (0 = no symptoms observed; 1 = 1 to 20% of leaf chlorosis; 2 = 21 to 50% of leaf chlorosis; 3 = 51 to 80% of leaf chlorosis; 4 ≥ 80% of leaf chlorosis), as reported by Elshafie et al. [4]. The infection percentage (IP %) was measured using Equation (1), whereas the disease index (DI %) and the control effect (CE %) were calculated using Equations (2) and (3), respectively, as described by Lee et al. [52].
| IP % = (SL/TL) × 100 | (1) |
| DI % = [∑ (Scale × No. of SL)/(HS × TL)] × 100 | (2) |
| CE % = 100 × (DI.P−DI.B)/DI.P | (3) |
where SL is symptomatic leaves; TL is total number of leaves; HS is highest scale; DI-P is disease index of infection; DI-B is disease index of control.
4.6. Statistical Analysis
The obtained results were subjected to one-way ANOVA for the statistical analysis. The significance level was checked by applying the Tukey’s B post hoc multiple comparison test with a probability of p < 0.05 using Statistical Package for the Social Sciences (SPSS) version 13.0, 2004 (Chicago, IL, USA).
5. Conclusions
The obtained results of the current research confirmed the promising biological activity of Actinomycetes, particularly of the genus Streptomyces. This study also underlined the usefulness of the new isolated strains for producing some important bioactive metabolites and extracellular hydrolytic enzymes; hence, they can be effectively used as biocontrol agents against S. sclerotiorum. Furthermore, the studied isolates also demonstrated an important plant-growth-promoting effect, which may be due to the production of phytohormones. Further studies remain necessary to identify and biochemically characterize the produced bioactive metabolites from the Actinomycetes isolates and evaluate their biological effects against other serious phytopathogens.
Author Contributions
Conceptualization, H.S.E. and I.C.; methodology, H.S.E.; formal analysis, H.S.E.; investigation, H.S.E. and I.C.; data curation, H.S.E. and I.C.; writing—original draft preparation, H.S.E.; writing—review and editing, H.S.E. and I.C. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Urban C., Segal-Maurer S., Rahal J.J. Considerations in control and treatment of nosocomial infections due to multidrug resistant Acinetobacter baumannii. Clin. Infect. Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 2.Paterson D.L., Ko W.C., Von Gottberg A., Mohapatra S., Casellas J.M., Goossens H., Mulazimoglu L., Trenholme G., Klugman K.P., Bonomo R.A., et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Inter. Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 3.Gerwick B.C., Sparks T.C. Natural products for pest control: An analysis of their role, value and future. Pest. Manag. Sci. 2014;70:1169–1185. doi: 10.1002/ps.3744. [DOI] [PubMed] [Google Scholar]
- 4.Elshafie H.S., Sakr S., Bufo S.A., Camele I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017;8:57–60. [Google Scholar]
- 5.Della Pepa T., Elshafie H.S., Capasso R., De Feo V., Camele I., Nazzaro F., Scognamiglio M.R., Caputo L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy) Molecules. 2019;24:2576. doi: 10.3390/molecules24142576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruľová D., Caputo L., Elshafie H.S., Baranová B., De Martino L., Sedlák V., Camele I., De Feo V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules. 2020;25:595. doi: 10.3390/molecules25030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elshafie H.S., Camele I., Sofo A., Mazzone G., Caivano M., Masi S., Caniani D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere. 2020;252:126597. doi: 10.1016/j.chemosphere.2020.126597. [DOI] [PubMed] [Google Scholar]
- 8.Elshafie H.S., Viggiani L., Mostafa M.S., El-Hashash M.A., Bufo S.A., Camele I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017;90:96–103. [Google Scholar]
- 9.Vitti A., Elshafie H.S., Logozzo G., Marzario S., Scopa A., Camele I., Nuzzaci M. Physico-chemical Characterization and biological activities of a digestate and a more stabilized digestate-derived compost from agro-waste. Plants. 2021;10:386. doi: 10.3390/plants10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elshafie H.S., Caputo L., De Martino L., Sakr S.H., De Feo V., Camele I. Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) Leathery Exocarp Extract. Plants. 2021;10:153. doi: 10.3390/plants10010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camele I., Elshafie H.S., Caputo L., Sakr S.H., De Feo V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019;92:39–45. doi: 10.4081/jbr.2019.8296. [DOI] [Google Scholar]
- 12.Sofo A., Elshafie H.S., Camele I. Structural and functional organization of the root system: A comparative study on five plant species. Plants. 2020;9:1338. doi: 10.3390/plants9101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Ippolito I., Mang S.M., Elshafie H.S., Camele I., Scillitani G., Mastrodonato M., Sofo A., Mininni A.N., Xylogiannis E. Morpho-anatomical and microbiological analysis of kiwifruit roots with KVDS symptoms. Acta Hortic. 2022;1332:131–136. doi: 10.17660/ActaHortic.2022.1332.18. [DOI] [Google Scholar]
- 14.El-Sayed W.S., Akhkha A., El-Naggar M.Y., Elbadry M. In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 2014;5:651. doi: 10.3389/fmicb.2014.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rani A., Saini K.C., Bast F., Mehariya S., Bhatia S.K., Lavecchia R., Zuorro A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules. 2021;26:1142. doi: 10.3390/molecules26041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffrey L.S.H., Norzaimawati A.N., Rosnah H. Prescreening of bioactivities from actinomycetes isolated from forest peat soil in Sarawak. J. Trp. Agric. Fd. Sci. 2011;39:245–254. [Google Scholar]
- 17.Manivasagan P., Kang K.H., Sivakumar K., Li-Chan E.C., Oh H.M., Kim S.K. Marine Actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014;38:172–188. doi: 10.1016/j.etap.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Vikineswary S., Nadaraj P., Wong W.H., Balabaskaran S. Actinomycetes from a tropical mangrove ecosystem—Antifungal activity of selected strains. Asia Pac. J. Mol. Biol. Biotechnol. 1997;5:81–86. [Google Scholar]
- 19.Pepper I.L., Gentry T.J. Environmental Microbiology. 3rd ed. Academic Press; Cambridge, MA, USA: 2015. Chapter 4—“Earth Environments”; pp. 59–88. [Google Scholar]
- 20.Holt J.G., Krieg N.R., Sneath P.H.A., Staley J.T., Williams S.T. Bergey’s Manual of Determinative Bacteriology. 9th ed. Williams and Wilkins; Baltimore, MD, USA: 1994. [Google Scholar]
- 21.Manivasagan P., Venkatesan J., Sivakumar K., Kim S.K. Pharmaceutically active secondary metabolites of marine Actinobacteria. Microbiol. Res. 2014;169:262–278. doi: 10.1016/j.micres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Marcolefas E., Leung T., Okshevsky M., McKay G., Hignett E., Hamel J., Aguirre G., Blenner-Hassett O., Boyle B., Lévesque R.C., et al. Culture-Dependent bioprospecting of bacterial isolates from the canadian high arctic displaying antibacterial activity. Front. Microbiol. 2019;10:1836. doi: 10.3389/fmicb.2019.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girão M., Ribeiro I., Ribeiro T., Azevedo I.C., Pereira F., Urbatzka R., Leão P.N., Carvalho M.F. Actinobacteria Isolated from Laminaria ochroleuca: A source of new bioactive compounds. Front. Microbiol. 2019;10:683. doi: 10.3389/fmicb.2019.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solecka J., Zajko J., Postek M., Rajnisz A. Biologically active secondary metabolites from Actinomycetes. Cent. Eur. J. Biol. 2012;7:373–390. doi: 10.2478/s11535-012-0036-1. [DOI] [Google Scholar]
- 25.Berdy J. Bioactive microbial metabolites. J. Antibiot. 2005;58:1. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 26.Vurukonda S.S., Giovanardi D., Stefani E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018;19:952. doi: 10.3390/ijms19040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan B.M., Kannabiran K. Extraction and identification of antibacterial secondary metabolites from marine Streptomyces sp. VITBRK2. Int. J. Mol. Cell Med. 2014;3:130–137. [PMC free article] [PubMed] [Google Scholar]
- 28.Khan A., Halo B.A., Elyassi A., Ali S., Al-Hosni K., Hussain J., Al-Harrasi A., Lee I. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016;21:58–64. doi: 10.1016/j.ejbt.2016.02.001. [DOI] [Google Scholar]
- 29.Moon Y.S., Ali S. Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol. 2022;67:523–533. doi: 10.1007/s12223-022-00959-4. [DOI] [PubMed] [Google Scholar]
- 30.Ali S., Moon Y.S., Hamayun M., Khan M.A., Bibi K., Lee I. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022;17:705–718. doi: 10.1080/17429145.2022.2091801. [DOI] [Google Scholar]
- 31.Sousa J.A.A., Olivares F.L. Plant growth promotion by Streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016;3:24. doi: 10.1186/s40538-016-0073-5. [DOI] [Google Scholar]
- 32.Manulis S., Epstein E., Shafrir H., Lichter A., Barash I. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology. 1994;140:1045–1050. doi: 10.1099/13500872-140-5-1045. [DOI] [PubMed] [Google Scholar]
- 33.Dochhil H., Dkhar M.S., Barman D. Seed germination enhancing activity of endophytic Streptomyces isolated from indigenous ethno-medicinal plant Centella asiatica. Int. J. Pharm. Biol. Sci. 2013;4:256–262. [Google Scholar]
- 34.Sacramento D.R., Coelho R.R., Wigg M.D., Linhares L.F., Santos M.G., Semedo L.T., Silca A.J. Antimicrobial and antiviral activities of an actinomycetes (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World J. Microbiol. Biotechnol. 2004;20:225–229. doi: 10.1023/B:WIBI.0000023824.20673.2f. [DOI] [Google Scholar]
- 35.Prapagdee B., Kuekulvong C., Mongkolsuk S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int. J. Biol. Sci. 2008;4:330–337. doi: 10.7150/ijbs.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suwan N., Boonying W., Nalumpang S. Antifungal activity of soil actinomycetes to control chilli anthracnose caused by Colletotrichum gloeosporioides. J. Agric. Technol. 2012;8:725–737. [Google Scholar]
- 37.Soltanzadeh M., Nejad M.S., Bonjar G.H. Application of soil-borne actinomycetes for biological control against fusarium wilt of chickpea (Cicer arietinum) caused by Fusarium solani f. sp. Pisi. J. Phytopathol. 2016;164:967–978. doi: 10.1111/jph.12517. [DOI] [Google Scholar]
- 38.Elshafie H.S., Camele I., Racioppi R., Scrano L., Iacobellis N.S., Bufo S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 2012;13:16291–16302. doi: 10.3390/ijms131216291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djebaili R., Pellegrini M., Bernardi M., Smati M., Kitouni M., Del Gallo M. Biocontrol activity of actinomycetes strains against fungal and bacterial pathogens of Solanum lycopersicum L. and Daucus carota L.: In vitro and in planta antagonistic activity. Biol. Life Sci. Forum. 2021;4:27. [Google Scholar]
- 40.Ordentlich A., Elad Y., Chet I. The role of chitinase of Serratia marcescens in biocontrol Sclerotium rolfsii. Phytopathology. 1988;78:84–87. [Google Scholar]
- 41.Saligkarias I.D., Gravanis F.T., Harry A.S. Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. A study on mode of action. Biol. Control. 2002;25:151–161. doi: 10.1016/S1049-9644(02)00052-X. [DOI] [Google Scholar]
- 42.Odumosu B.T., Buraimoh O.M., Okeke C.J., Ogah J.O., Michel F.C., Jr. Antimicrobial activities of the Streptomyces ceolicolor strain AOBKF977550 isolated from a tropical estuary. J. Taibah Uni. Sci. 2017;11:836–841. doi: 10.1016/j.jtusci.2017.01.006. [DOI] [Google Scholar]
- 43.Chaudhary H.S., Yadav J., Shrivastava A.R., Singh S., Singh A.K., Gopalan N. Antibacterial activity of actinomycetes isolated from different soil samples of Sheopur (A city of central India) J Adv. Pharm. Technol. Res. 2013;4:118–123. doi: 10.4103/2231-4040.111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacret R., Oves-Costales D., Gómez C., Díaz C., de la Cruz M., Pérez-Victoria I., Vicente F., Genilloud O., Reyes F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs. 2014;13:128–140. doi: 10.3390/md13010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman M.A., Islam M.Z., Islam M.A. Antibacterial activities of Actinomycete isolates collected from soils of Rajshahi, Bangladesh. Biotechnol. Res. Int. 2011;2011:857925. doi: 10.4061/2011/857925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King E.O., Ward M.K., Raney D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 47.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSIBLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tahtamouni M.E.W., Hameed K.M., Saadoun I.M. Biological control of Sclerotinia sclerotiorum using indigenous chitolytic actinomycetes in Jordan. J. Plant Pathol. 2006;22:107–114. doi: 10.5423/PPJ.2006.22.2.107. [DOI] [Google Scholar]
- 49.Teather R.M., Wood P.J. Use of Congo red-polysacchatide interaction in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1998;43:777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhardwaj V., Garg N. Exploitation of Micro-Organisms for Isolation and Screening of Pectinase from Environment; Proceedings of the 8th International Conference, Making Innovation Work for Society: Linking, Leveraging and Learning; Kuala Lumpur, Malaysia. 1–3 November 2010. [Google Scholar]
- 51.Evmert M.K., Gabriele B.G., Piechulla B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 2007;187:351–360. doi: 10.1007/s00203-006-0199-0. [DOI] [PubMed] [Google Scholar]
- 52.Lee K.J., Kamala-Kannan S., Sub H.S., Seong C.K., Lee G.W. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World J. Microb. Biotecnol. 2008;24:1139–1145. doi: 10.1007/s11274-007-9585-2. [DOI] [Google Scholar]
- 53.Darrasse A., Priou S., Kotoujansky A., Bertheau Y. PCR and Restriction Fragment Length Polymorphism of a pel Gene as a Tool to Identify Erwinia carotovora in Relation to Potato Diseases. Appl. Environ. Microbiol. 1994;60:1437–1443. doi: 10.1128/aem.60.5.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.