Abstract
Colorectal cancer (CRC) is the third most common cause of cancer mortality worldwide and the most prevalent cancer in Taiwan. The matrix metalloproteinase (MMP)-11 is a proteolytic enzyme of the MMP family which is involved in extracellular matrix degradation and tissue remodeling. In this study, we focused on the associations of MMP-11 single-nucleotide polymorphisms (SNPs) with CRC susceptibility and clinicopathological characteristics. The MMP-11 SNPs rs131451, rs738791, rs2267029, rs738792, and rs28382575 in 479 controls and 479 patients with CRC were analyzed with real-time polymerase chain reaction. We found that the MMP-11 SNP rs738792 “TC + CC” genotype was significantly associated with perineural invasion in colon cancer patients after controlling for clinical parameters [OR (95% CI) = 1.783 (1.074–2.960); p = 0.025]. The MMP-11 rs131451 “TC + CC” genotypic variants were correlated with greater tumor T status [OR (95% CI):1.254 (1.025–1.534); p = 0.028] and perineural invasion [OR (95% CI):1.773 (1.027–3.062); p = 0.040) in male CRC patients. Furthermore, analyses of The Cancer Genome Atlas (TCGA) revealed that MMP-11 levels were upregulated in colorectal carcinoma tissue compared with normal tissues and were correlated with advanced stage, larger tumor sizes, and lymph node metastasis. Moreover, the data from the Genotype-Tissue Expression (GTEx) database exhibited that the MMP-11 rs738792 “CC” and “CT” genotypic variants have higher MMP-11 expression than the “TT” genotype. In conclusion, our results have demonstrated that the MMP-11 SNPs rs738792 and rs131451 may have potential to provide biomarkers to evaluate CRC disease progression, and the MMP-11 rs131451 polymorphism may shed light on sex discrepancy in CRC development and prognosis.
Keywords: colorectal cancer, MMP-11, polymorphism
1. Introduction
Colorectal cancer (CRC), which is the third leading cause of cancer death worldwide, ranks as the third most common adult cancer in men the second most common in women [1,2,3,4]. In Taiwan, the incidence of CRC is the highest among all cancers, and CRC is responsible for the third highest cancer mortality [5]. Risk factors such as age, male sex, family history of colorectal cancer, race and ethnicity, cigarette smoking, excessive alcohol consumption, high consumption of red and processed meat, diabetes, obesity, and inflammatory bowel disease were suggested to be associated with CRC carcinogenesis [6,7,8,9,10,11,12,13,14,15,16,17].
The matrix metalloproteinases (MMPs) are a zinc-dependent endopeptidases family which is involved in extracellular matrix (ECM) degradation and tissue remodeling [18,19,20,21]. MMP-11, or stromelysin 3, is a proteolytic enzyme which belongs to the MMP family [22,23,24]. Previous studies have suggested that the MMP-11 may play a regulatory role in cancer cell growth, tumor migration, invasion, and metastasis in various cancers [22,24,25,26,27,28]. In colorectal cancer, it was suggested that MMP-11 is highly expressed in colonic carcinoma [29], and the elevated serum levels and mRNA expression of MMP-11 were correlated with poor prognosis in colon cancer patients [30,31]. Single-nucleotide polymorphisms (SNPs) are the most common type of genetic variations which may lead to amino acid substitution and alteration of protein function [32,33]. The MMP-11 SNPs were found to be associated with increased risk of cancer progression and development, metastasis, recurrence, or poor prognosis in many cancers including oral squamous cell carcinoma (OSCC) [34], hepatocellular carcinoma (HCC) [35,36], prostate cancer [37], and urothelial cell carcinoma (UCC) [38]. However, the associations and influences of MMP-11 polymorphisms regarding CRC tumor progression and clinicopathologic characteristics remained uninvestigated. In this study, we focused on five SNPs of MMP-11 rs131451, rs738791, rs2267029, rs738792, and rs28382575, and try to unveil their correlations to CRC susceptibility and clinicopathologic characteristics.
2. Materials and Methods
2.1. Subjects
In this study, we enrolled 479 CRC patients and 479 cancer-free controls. All participants were recruited from 2016 to 2020 at Chung Shan Medical University Hospital in Taichung, Taiwan. According to the American Joint Committee on Cancer (AJCC) [39], the TNM staging of the CRC patients who enrolled in our study were staged clinically at the time of diagnosis. The tumor differentiation was examined and rated under the AJCC classification by a pathologist. The demographic data of age and gender were reported by each participant and recorded. Individuals with neither a history of cancer of any sites nor any self-reported diseases, such as asthma, diabetes, or cardiovascular, autoimmune, and neurological diseases, were enrolled in the control group. This project was approved by the institutional review board of Chung Shan Medical University Hospital (IRB number CS1-20111), and informed written consent was provided by each participant at enrollment.
2.2. Sample Preparation and DNA Extraction
For genomic DNA extraction, the peripheral blood specimens from normal controls and CRC patients who enrolled in our study were collected. The EDTA containing tubes were used to preserve the samples of peripheral whole blood. The blood samples were centrifuged with the settings of 3000 rpm, 10 min, and the buffy coats from centrifuged whole blood specimens were extracted and further used for the DNA extraction [40,41]. The genomic DNA extraction assay was performed with QIAamp DNA blood mini kits following the protocols of the manufacturer’s manual to collect the DNA. The DNA elution was completed with the Tris-EDTA (TE) buffer, which was used to dissolve the DNA. Extracted DNA was further used as a DNA template in the real-time polymerase chain reactions (PCRs).
2.3. Selection of MMP-11 SNPs
In the current study, a total of five SNPs of MMP-11 rs131451, rs738791, rs2267029, rs738792, and rs28382575 were selected based on the International HapMap Project database [33]. The MMP-11 rs131451 SNP was selected because the MMP-11 rs131451 polymorphisms were suggested to be associated with late-stage tumors and high-risk D’Amico classification in prostate cancer patients with biochemical recurrence [37]. The MMP-11 rs738791 was selected because patients with the rs738791 polymorphic variant were observed to have greater risk of HCC compared with the wild-type (CC) carriers [35]. The MMP-11 SNP rs738792 was selected because the OSCC patients who carried at least one polymorphic C allele of MMP-11 rs738792 showed an increased incidence of lymph node metastasis compared with those patients with homozygous T/T [34], and carriers who have at least one C allele of the MMP-11 SNP rs738792 are likely to progress to Child–Pugh B or C grade in HCC patients [35]. The MMP-11 SNP rs28382575 was selected because the HCC patients with at least one C allele of the MMP-11 SNP rs28382575 are suggested to have a higher risk of developing stage III/IV disease, large tumors, or lymph node metastasis [35].
2.4. MMP-11 SNPs Genotyping
Assessment of allelic discrimination for the MMP-11 rs131451 (assay IDs: C___2213679_30), rs738791 (assay IDs: C___2448099_30), rs2267029 (assay IDs: C__15871447_20), rs738792 (assay IDs: C___2213764_20), and rs28382575 (assay IDs: C__61238655_10) SNPs was performed with an ABI StepOne Software v2.3 Real-Time PCR System. The TaqMan assay was used for the analysis of genotyping. The analysis and calculation of the final data of genotyping was processed with the SDS 7000 series software (Applied Biosystems, Foster City, CA, USA).
2.5. Statistical Analysis
To compare the age (years), gender, tumor location, tumor stage, tumor T status, lymph node status, metastasis, lymphovascular invasion, perineural invasion, and pathologic grading, the Chi-squared test or Student’s t test was performed between the patients with CRC and the controls. A statistical significance was suggested if p < 0.05. To compare the odds ratio (ORs) with their 95% confidence intervals (CIs) of the association between the genotypic frequencies and CRC risk, and the clinical pathological characteristics, the data was analyzed and assessed by the logistic regression models. All the data analysis in the current study was calculated and evaluated with SAS statistical software (Version 9.1, 2005; SAS Institute, Cary, NC, USA).
3. Results
3.1. Demographic and Clinical Characteristics of Study Cohorts
The distribution of demographical characteristics in 479 controls and 479 patients with CRC is demonstrated in Table 1. In our current study, we observed that the distribution of age (years) < 65 was 278 (58.0%) in controls and 251 (52.4%) in CRC patients, and the age ≧ 65 in controls and CRC patients was 201 (42.0%) and 228 (47.6%), respectively. For the distributions of gender, the male controls and CRC patients were 294 (61.4%) and 282 (58.9%), whereas the female controls and patients were 185 (38.6%) and 197 (41.1%), respectively. However, no statistically significant differences were found for age and gender between the CRC patients and the controls (Table 1).
Table 1.
The distributions of demographical and clinical characteristics in 479 controls and 479 patients with CRC.
| Variable | Controls (N = 479) n (%) |
Patients (N = 479) n (%) |
p Value |
|---|---|---|---|
| Age (yrs) | |||
| <65 | 278 (58.0%) | 251 (52.4%) | 0.079 |
| ≥65 | 201 (42.0%) | 228 (47.6%) | |
| Gender | |||
| Male | 294 (61.4%) | 282 (58.9%) | 0.428 |
| Female | 185 (38.6%) | 197 (41.1%) | |
| Tumor location | |||
| Rectum | 110 (23.0%) | ||
| Left colon | 222 (46.3%) | ||
| Right colon | 147 (30.7%) | ||
| Stage | |||
| I + II | 229 (47.8%) | ||
| III + IV | 250 (52.2%) | ||
| Tumor T status | |||
| T1–T2 | 116 (24.2%) | ||
| T3–T4 | 363 (75.8%) | ||
| Lymph node status | |||
| N0 | 239 (49.9%) | ||
| N1 + N2 | 240(50.1%) | ||
| Metastasis | |||
| M0 | 402 (83.9%) | ||
| M1 | 77 (16.1%) | ||
| Lymphovascular invasion | |||
| No | 267 (55.7%) | ||
| Yes | 212 (44.3%) | ||
| Perineural invasion | |||
| No | 272 (56.8%) | ||
| Yes | 207 (43.2%) | ||
| Pathologic grading | |||
| Well | 6 (1.3%) | ||
| Moderately | 437 (91.2%) | ||
| Poorly | 36 (7.5%) |
3.2. MMP-11 Gene Polymorphisms were Associated with the Clinicopathological Characteristics of CRC
The genotype distributions of MMP-11 gene polymorphisms in 479 controls and 479 patients with CRC are listed in Table 2. The highest distribution frequencies in patients with CRC of MMP-11 genetic polymorphisms rs131451, rs738791, rs2267029, rs738792, and rs28382575 were heterozygous for TC, homozygous for CC, homozygous for GG, homozygous for TT, and homozygous for TT, respectively. Logistic regression models were adopted to estimate the odds ratios (ORs) and their 95% confidence intervals (CIs). After adjustment for the effects of age and gender, no significant associations were found between the CRC patients and the controls (Table 2).
Table 2.
Genotype distributions of MMP-11 gene polymorphisms in 479 controls and 479 patients with CRC.
| Variable | Controls (N = 479) n (%) |
Patients (N = 479) n (%) |
OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| rs131451 | ||||
| TT | 162 (33.8%) | 161 (33.6%) | 1.000 (reference) | 1.000 (reference) |
| TC | 234 (48.9%) | 246 (51.4%) | 1.058 (0.798–1.403) | 1.065 (0.802–1.413) |
| CC | 83 (17.3%) | 72 (15.0%) | 0.873 (0.595–1.281) | 0.889 (0.605–1.306) |
| TC + CC | 317 (66.2%) | 318 (66.4%) | 1.009 (0.772–1.319) | 1.019 (0.779–1.333) |
| rs738791 | ||||
| CC | 234 (48.9%) | 213 (44.5%) | 1.000 (reference) | 1.000 (reference) |
| CT | 196 (40.9%) | 214 (44.7%) | 1.199 (0.917–1.569) | 1.204 (0.920–1.575) |
| TT | 49 (10.2%) | 52 (10.9%) | 1.166 (0.757–1.796) | 1.202 (0.778–1.856) |
| CT + TT | 245 (51.1%) | 266 (55.5%) | 1.193 (0.925–1.538) | 1.203 (0.933–1.553) |
| rs2267029 | ||||
| GG | 266 (55.5%) | 263 (54.9%) | 1.000 (reference) | 1.000 (reference) |
| GA | 185 (38.6%) | 188 (39.2%) | 1.028 (0.789–1.340) | 1.022 (0.784–1.333) |
| AA | 28 (5.9%) | 28 (5.9%) | 1.011 (0.583–1.755) | 1.033 (0.594–1.794) |
| GA + AA | 213 (44.5%) | 216 (45.1%) | 1.026 (0.795–1.323) | 1.024 (0.793–1.321) |
| rs738792 | ||||
| TT | 246 (51.4%) | 241 (50.3%) | 1.000 (reference) | 1.000 (reference) |
| TC | 195 (40.7%) | 203 (42.4%) | 1.063 (0.815–1.385) | 1.070 (0.820–1.396) |
| CC | 38 (7.9%) | 35 (7.3%) | 0.940 (0.575–1.538) | 0.955 (0.583–1.564) |
| TC + CC | 233 (48.6%) | 238 (49.7%) | 1.043 (0.809–1.343) | 1.051 (0.815–1.355) |
| rs28382575 | ||||
| TT | 457 (95.4%) | 446 (93.1%) | 1.000 (reference) | 1.000 (reference) |
| TC | 22 (4.6%) | 33 (6.9%) | 1.537 (0.882–2.677) | 1.596 (0.914–2.787) |
| CC | 0 (0%) | 0 (0.0%) | --- | --- |
| TC + CC | 22 (4.6%) | 33 (6.9%) | 1.537 (0.882–2.677) | 1.596 (0.914–2.787) |
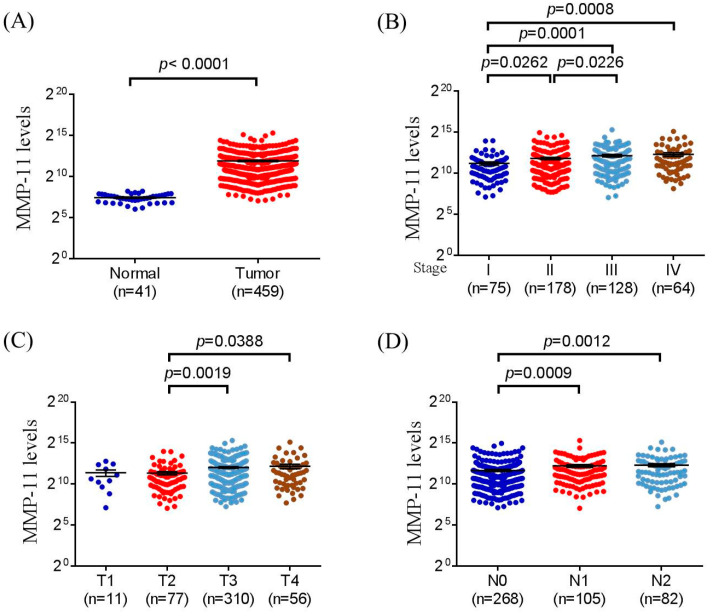
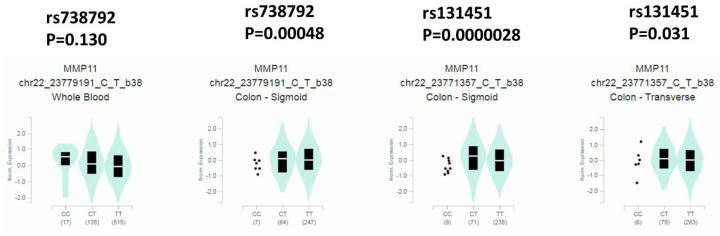
We further analyzed the distribution frequency of the clinical status and MMP-11 genotype frequencies. In 369 of a total 479 CRC patients, a significant association was found in those individuals who carried the MMP-11 rs738792 “TC + CC” genotypic variants, with a higher risk of perineural invasion of colon cancer patients after controlling for stages, tumor T status, lymph node status, metastasis, lymphovascular invasion, and cell differentiation (p = 0.025) (Table 3). We further analyzed the MMP-11 polymorphisms of the clinical status in male and female CRC patients. The results demonstrated that the male CRC patients who carried the MMP-11 rs131451 “TC + CC” genotype were associated with greater tumor T status (p = 0.028) and perineural invasion (p = 0.040) (Table 4). We further analyzed correlations of MMP-11 levels and their clinical parameters in CRC from The Cancer Genome Atlas (TCGA) dataset. We observed that MMP-11 expression was prone to be upregulated in colorectal carcinoma tissue compared with normal tissues (Figure 1A). Moreover, MMP-11 levels were also correlated with late stage, larger tumor sizes, and lymph node metastasis (Figure 1B–D). We further used the Genotype-Tissue Expression (GTEx) database to evaluate the correlations of MMP-11 rs738792 and rs131451 SNPs to MMP-11 expression. The results of the GTEx database exhibited that individuals with the “C” allele of the MMP-11 rs738792 (CC and CT) genotype were associated with higher MMP-11 expression in the sigmoid colon compared with the “TT” carriers (p = 0.00048) (Figure 1). For MMP-11 rs131451 SNPs, individuals with the rs131451 polymorphisms “CC” and “CT” genotype were associated with higher MMP-11 expression in the sigmoid colon (p = 0.0000028) and transverse colon (p = 0.031), respectively, compared with the wild-type “TT” carriers (Figure 2).
Table 3.
Distribution frequency of the clinical status and MMP-11 rs738792 genotype frequencies in 479 CRC patients.
| Variable | All (N = 479) | Rectum (N = 110) | Colon (N = 369) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TT (N = 241) |
TC + CC (N = 238) |
p Value | TT (N = 60) |
TC + CC (N = 50) |
p Value | TT (N = 181) |
TC + CC (N = 188) |
p Value | |
| Stages | |||||||||
| I + II | 115 (47.7%) | 114 (47.9%) | p = 0.892 | 31 (51.7%) | 29 (58.0%) | p = 0.482 | 84 (46.4%) | 85 (45.2%) | p = 0.945 |
| III + IV | 126 (52.3%) | 124 (52.1%) | 29 (48.3%) | 21 (42.0%) | 97 (53.6%) | 103 (54.8%) | |||
| Tumor T status | |||||||||
| T1 + T2 | 62 (25.7%) | 54 (22.7%) | p = 0.805 | 20 (33.3%) | 16 (32.0%) | p = 0.569 | 42 (23.2%) | 38 (20.2%) | p = 0.885 |
| T3 + T4 | 179 (74.3%) | 184 (77.3%) | 40 (66.7%) | 34 (68.0%) | 139 (76.8%) | 150 (79.8%) | |||
| Lymph node status | |||||||||
| Negative | 119 (49.4%) | 120 (50.4%) | p = 0.630 | 32 (53.3%) | 30 (60.0%) | p = 0.411 | 87 (48.1%) | 90 (47.9%) | p = 0.643 |
| Positive | 122 (50.6%) | 118 (49.6%) | 28 (46.7%) | 20 (40.0%) | 94 (51.9%) | 98 (52.1%) | |||
| Metastasis | |||||||||
| Negative | 204 (84.6%) | 198 (83.2%) | p = 0.955 | 46 (76.7%) | 45 (90.0%) | p = 0.090 | 158 (87.3%) | 153 (81.4%) | p = 0.212 |
| Positive | 37 (15.4%) | 40 (16.8%) | 14 (23.3%) | 5 (10.0%) | 23 (12.7%) | 35 (18.6%) | |||
| Lymphovascular invasion | |||||||||
| No | 136 (56.4%) | 131 (55.0%) | p = 0.457 | 38 (63.3%) | 33 (66.0%) | p = 0.830 | 98 (54.1%) | 98 (52.1%) | p = 0.426 |
| Yes | 105 (43.6%) | 107 (45.0%) | 22 (36.7%) | 17 (34.0%) | 83 (45.9%) | 90 (47.9%) | |||
| Perineural invasion | |||||||||
| No | 147 (61.0%) | 125 (52.5%) | p = 0.051 | 39 (65.0%) | 34 (68.0%) | p = 0.998 | 108 (59.7%) | 91 (48.4%) | p = 0.025 a |
| Yes | 94 (39.0%) | 113 (47.5%) | 21 (35.0%) | 16 (32.0%) | 73 (40.3%) | 97 (51.6%) | |||
| Cell differentiation | |||||||||
| Well/Moderately | 227 (94.2%) | 216 (90.8%) | p = 0.164 | 60 (100%) | 49 (98.0%) | ----- | 167 (92.3%) | 167 (88.8%) | p = 0.323 |
| Poorly | 14 (5.8%) | 22 (9.2%) | 0 (0.0%) | 1 (2.0%) | 14 (7.7%) | 21 (11.2%) | |||
a AOR (95% CI):1.783 (1.074–2.960). The adjusted odds ratios (AORs) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for stages, tumor T status, lymph node status, metastasis, lymphovascular invasion, perineural invasion, and cell differentiation.
Table 4.
Distribution frequency of the clinical status and MMP-11 rs131451 genotype frequencies in 479 CRC patients with different genders.
| Variable | All (N = 479) | Male (N = 282) | Female (N = 197) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TT (N = 161) |
TC + CC (N = 318) |
p Value | TT (N = 96) |
TC + CC (N = 186) |
p value | TT (N = 65) |
TC + CC (N = 132) |
p Value | |
| Stages | |||||||||
| I + II | 80 (49.7%) | 149 (46.9%) | p = 0.317 | 51 (53.1%) | 94 (50.5%) | p = 0.812 | 29 (44.6%) | 55 (41.7%) | p = 0.134 |
| III + IV | 81 (50.3%) | 169 (53.1%) | 45 (46.9%) | 92 (49.5%) | 36 (55.4%) | 77 (58.3%) | |||
| Tumor T status | |||||||||
| T1 + T2 | 47 (29.2%) | 69 (21.7%) | p = 0.216 | 34 (35.4%) | 43 (23.1%) | p = 0.028 a | 13 (20.0%) | 26 (19.7%) | p = 0.999 |
| T3 + T4 | 114 (70.8%) | 249 (78.3%) | 62 (64.6%) | 143 (76.9%) | 52 (80.0%) | 106 (80.3%) | |||
| Lymph node status | |||||||||
| Negative | 81 (50.3%) | 158 (49.7%) | p = 0.238 | 52 (54.2%) | 99 (53.2%) | p = 0.545 | 29 (44.6%) | 59 (44.7%) | p = 0.172 |
| Positive | 80 (49.7%) | 160 (50.3%) | 44 (45.8%) | 87 (46.8%) | 36 (55.4%) | 73 (55.3%) | |||
| Metastasis | |||||||||
| Negative | 135 (83.9%) | 267 (84.0%) | p = 0.380 | 84 (87.5%) | 157 (84.4%) | p = 0.663 | 51 (78.5%) | 110 (83.3%) | p = 0.152 |
| Positive | 26 (16.1%) | 51 (16.0%) | 12 (12.5%) | 29 (15.6%) | 14 (21.5%) | 22 (16.7%) | |||
| Lymphovascular invasion | |||||||||
| No | 95 (59.0%) | 172 (54.1%) | p = 0.942 | 59 (61.5%) | 104 (55.9%) | p = 0.697 | 36 (55.4%) | 68 (51.5%) | p = 0.554 |
| Yes | 66 (41.0%) | 146 (45.9%) | 37 (38.5%) | 82 (44.1%) | 29 (44.6%) | 64 (48.5%) | |||
| Perineural invasion | |||||||||
| No | 99 (61.5%) | 173 (54.4%) | p = 0.341 | 66 (68.8%) | 104 (55.9%) | p = 0.040 b | 33 (50.8%) | 69 (52.3%) | p = 0.849 |
| Yes | 62 (38.5%) | 145 (45.6%) | 30 (31.2%) | 82 (44.1%) | 32 (49.2%) | 63 (47.7%) | |||
| Cell differentiation | |||||||||
| Well/Moderately | 154 (95.7%) | 289 (90.9%) | p = 0.096 | 91 (94.8%) | 165 (88.7%) | p = 0.129 | 63 (96.9%) | 124 (93.9%) | p = 0.371 |
| Poorly | 7 (4.3%) | 29 (9.1%) | 5 (5.2%) | 21 (11.3%) | 2 (3.1%) | 8 (6.1%) | |||
a AOR (95% CI):1.254 (1.025–1.534); b AOR (95% CI):1.773 (1.027–3.062). The adjusted odds ratios (AORs) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for stages, tumor T status, lymph node status, metastasis, lymphovascular invasion, perineural invasion, and cell differentiation.
Figure 1.
MMP-11 level of colorectal cancer patients from TCGA database. (A) MMP-11 levels were compared between the colorectal cancer tumor tissues and normal tissue. (B) MMP-11 levels were compared between stage I + II and stage III + IV. (C) MMP-11 levels were compared between the T1 + T2 stage and T3 + T4 stage. (D) MMP-11 levels were compared between the N0 stage and N1 stage.
Figure 2.
Distribution of MMP-11 expression in whole blood and colon (sigmoid, transverse) of MMP-11 SNPs rs738792, rs131451 from Genotype-Tissue Expression (GTEx) database.
The odds ratios (ORs) with their 95% confidence intervals were estimated by logistic regression models.
The adjusted odds ratios (AORs) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age and gender.
4. Discussion
In this study, we demonstrated the associations between the MMP-11 SNPs and CRC. The incidence of CRC is typically low at ages younger than 50 years but strongly increases with age [6,42]. Although the incidence of early-onset CRC (EOCRC) patients, referring to those individuals younger than 50 years, has been rapidly rising over the last 20 years [43,44,45], the median age at diagnosis of CRC is about 70 years in developed countries [6,46]. Previous study has suggested that the MMP-11 polymorphisms and environmental carcinogens were associated with an increased risk for the development of OSCC [34]. Moreover, carriers of the CT + TT allele of the MMP-11 rs738791 variant were suggested to possess greater risk of HCC compared with the wild-type (CC) carriers [35], and those with the MMP-11 rs28382575 polymorphic “CT” genotype were found to be susceptible to UCC [38]. However, after we analyzed the genotype distributions of MMP-11 polymorphisms in 479 controls and 479 patients with CRC, no statistically significant association was found between these two groups (Table 2), suggesting a limited effect of MMP-11 polymorphisms for the susceptibility of CRC carcinogenesis.
We further analyzed the correlations between the MMP-11 SNPs and clinical status of CRC, and we found that in 369 of a total 479 CRC patients, individuals who carried the MMP-11 rs738792 “TC + CC” polymorphic variants were associated with higher risk of perineural invasion in colon cancer patients after controlling for clinical parameters (p = 0.025) (Table 3). Moreover, after we analyzed the MMP-11 polymorphisms of the clinical status in CRC patients with different genders, we found that the male CRC patients who carried the MMP-11 rs131451 “TC + CC” genotypic variants were correlated with greater tumor T status (p = 0.028) and perineural invasion (p = 0.040) (Table 4). Furthermore, numerous studies reported that perineurial invasion is associated with poor prognosis in CRC patients [47,48,49]. Therefore, the correlations among CRC prognosis and MMP-11 polymorphism will be further investigated in our future work.
Generally, the colorectal cancer screening guidelines do not distinguish females from males, and sex specificity was not considered for interpretation in CRC despite the differences in tumor location between women and men [8,50,51]. Consistent with these results, the sex specificity was not significant between the CRC patients and controls in our study (p = 0.428) (Table 1). However, for the clinical status, results of the MMP-11 rs131451 polymorphic variants showed discrepancy between male and female CRC patients (Table 4). Intriguingly, similar results of MMP-11 rs131451 expression with sex differences were observed in our previous studies. The MMP-11 SNPs, including the rs131451, have shown no impact on uterine cervical cancer in Taiwanese women [52], whereas the MMP-11 rs131451 “TC + CC” polymorphic variants were correlated with advanced clinical stage (T stage; p = 0.007) and high-risk D’Amico classification (p = 0.015) in prostate cancer patients with biochemical recurrence [37]. Of note, analyses of TCGA revealed that MMP-11 levels were correlated with larger tumor sizes (Figure 1). Moreover, according to the data of the GTEx database, it was suggested that both the MMP-11 rs131451 “CC + CT” genotypic variants expressed in the sigmoid colon or transverse colon were associated with higher MMP-11 expression compared with the wild-type “TT” carriers (Figure 2). However, in CRC, not only the male but also the female CRC patients with rs131451 “TC + CC” genotype were significantly associated with advanced tumor T status and perineural invasion (Table 4). In contrast, in the aspect of CRC prognostic biomarkers, it was suggested that there are clear sex differences in CRC characteristics, and sex-specific CRC prognostic biomarkers including ESM1, GUCA2A, and VWA2 for males and CLDN1 and FUT1 for female CRC patients were proposed [53]. One possible mechanism to explain this phenomenon was the interaction of sex hormones and their regulators in CRC. The sex hormones were suggested as contributors for gender disparity in incidence and mortality of CRC [54,55,56,57]. Notably, despite its ambiguous and contradictory role in CRC, testosterone was suggested to be involved in CRC development and prognosis [56,58], and the androgen was suggested to regulate MMPs (MMP-2) and the cellular processes of intimal hyperplasia [59]. Moreover, in the aspect of androgen receptor (AR), it was suggested that the expression of MMP-2 and MMP-9 was associated with the presence of AR in epithelial ovarian tumors [60], and the expressions of the MMP-11 and AR were significantly higher in cancer-associated fibroblasts (CAFs) from castration-resistant prostate tumors (CRPC) [61], suggesting a possible mechanism: that the higher MMP-11 expression resulting from MMP-11 rs131451 polymorphisms might be associated with the induction of AR presence in CRC (Table 4) (Figure 1 and Figure 2). Men have a 20- to 25-fold higher testosterone production when compared with women [62,63], and the testosterone levels in women prior to menopause decline approximately 50% compared with their third decade [62,64]. Therefore, although the correlations and interactions of sex hormones with MMP-11 expression have remained unclear to date, it can be proposed that it is the AR presence induced by higher levels of MMP-11 expression which result from MMP-11 rs131451 “CC + CT” polymorphisms, but also the direct interaction of testosterone and MMP-11, which is responsible for the discrepancy of sex specificity and poor prognosis in prostate cancer [61] and CRC [30,31] (Table 4) (Figure 2). In addition, for the application of MMPs as biomarkers in CRC detection, a previous study led by Koga et al. [65] demonstrated that the messenger RNA (mRNA) expression of MMP-7 in the colonocytes isolated from feces was significantly higher in CRC patients than in healthy volunteers [65,66]. Moreover, numerous studies reported that expressions of MMP-14, MMP-17, and MMP-19 may be used as prognostic markers in CRC [67,68,69]. Therefore, a multi-SNP analysis for MMP-11, MMP-14, MMP-17, and MMP-19 will be investigated in our future work. Taken together, although the exact mechanisms and regulations remained incompletely understood, the MMP-11 rs738792 and rs131451 SNPs may provide potential candidates for CRC biomarkers since these polymorphic variants were both linked with perineural invasion (Table 3 and Table 4) and higher expression of MMP-11 (Figure 1). Of note, the MMP-11 rs131451 “TC + CC” genotype was further associated with greater tumor T status (p = 0.028) and perineural invasion (p = 0.040) in male CRC patients (Table 4), thereby providing a possible more detailed mechanism to explain the reason why the sex discrepancy of MMP-11 expression in CRC exists, and result in CRC disease development and prognosis with sex differences [31]. However, future well-designed studies are required to elucidate the exact mechanisms of MMP-11 polymorphisms in CRC progression considering sex specificity, especially the detailed influences of sex hormones such as the decreasing levels of androgen and testosterone with age to MMP-11 expression in CRC disease progression and prognosis.
5. Conclusions
In conclusion, our results have demonstrated that despite the fact that MMP-11 SNPs were not associated with CRC susceptibility, CRC patients who carried the MMP-11 rs738792 “TC + CC” polymorphic variants were associated with higher risk of perineural invasion of the colon, and the male CRC patients who carried the MMP-11 rs131451 “TC + CC” genotypic variants were associated with greater tumor T status and perineural invasion. The MMP-11 rs738792 and rs131451 polymorphisms were also associated with higher MMP-11 expression either in the sigmoid colon or transverse colon. The MMP-11 SNPs rs738792 and rs131451 may have potential to provide biomarkers to evaluate CRC disease progression, and the MMP-11 rs131451 polymorphisms may shed light on sex discrepancy in CRC development and prognosis.
Acknowledgments
We would like to thank the Human Biobank of Chung Shan Medical University Hospital for providing the biological specimens and related clinical data for our research.
Author Contributions
Conceptualization, H.-C.H., B.-H.S., S.-F.Y. and Y.-E.C.; methodology, Y.-E.C.; formal analysis, S.-C.S., L.-C.C. and S.-F.Y.; resources, B.-H.S., C.-C.H. and W.-C.T. writing—original draft preparation, H.-C.H., B.-H.S., S.-F.Y. and Y.-E.C.; writing—review and editing, H.-C.H., B.-H.S., S.-F.Y. and Y.-E.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the institutional review board of Chung Shan Medical University Hospital in Taichung, Taiwan (IRB number CS1-20111).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J., Ma X., Chakravarti D., Shalapour S., DePinho R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787–820. doi: 10.1101/gad.348226.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 4.Biller L.H., Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 5.Chuang J.P., Lee J.C., Leu T.H., Hidajah A.C., Chang Y.H., Li C.Y. Association of gout and colorectal cancer in Taiwan: A nationwide population-based cohort study. BMJ Open. 2019;9:e028892. doi: 10.1136/bmjopen-2019-028892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 7.Sninsky J.A., Shore B.M., Lupu G.V., Crockett S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022;32:195–213. doi: 10.1016/j.giec.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim S.E., Paik H.Y., Yoon H., Lee J.E., Kim N., Sung M.K. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 2015;21:5167–5175. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G., Law S.C., Ahn Y.O., Xiang Y.B., Yeole B.B., et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 10.Taylor D.P., Burt R.W., Williams M.S., Haug P.J., Cannon-Albright L.A. Population-based family history-specific risks for colorectal cancer: A constellation approach. Gastroenterology. 2010;138:877–885. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jess T., Rungoe C., Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Liang P.S., Chen T.Y., Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int. J. Cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 13.Fedirko V., Tramacere I., Bagnardi V., Rota M., Scotti L., Islami F., Negri E., Straif K., Romieu I., La Vecchia C., et al. Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. 2011;22:1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 14.Chan D.S., Lau R., Aune D., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y., Yang Y., Wang F., Zhang P., Shi C., Zou Y., Qin H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y., Ben Q., Shen H., Lu W., Zhang Y., Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 17.Keller D.S., Windsor A., Cohen R., Chand M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019;23:3–13. doi: 10.1007/s10151-019-1926-2. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao Y.H., Su S.C., Lin C.W., Chao Y.H., Yang W.E., Yang S.F. Pathological and therapeutic aspects of matrix metalloproteinases: Implications in childhood leukemia. Cancer Metastasis Rev. 2019;38:829–837. doi: 10.1007/s10555-019-09828-y. [DOI] [PubMed] [Google Scholar]
- 19.Yang J.S., Lin C.W., Su S.C., Yang S.F. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin. Drug Metab. Toxicol. 2016;12:191–200. doi: 10.1517/17425255.2016.1131820. [DOI] [PubMed] [Google Scholar]
- 20.Su S.C., Hsieh M.J., Yang W.E., Chung W.H., Reiter R.J., Yang S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017;62:e12370. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 21.Su C.W., Lin C.W., Yang W.E., Yang S.F. TIMP-3 as a therapeutic target for cancer. Ther. Adv. Med. Oncol. 2019;11:1758835919864247. doi: 10.1177/1758835919864247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Huang S., Guo J., Zhou L., You L., Zhang T., Zhao Y. Insights into the distinct roles of MMP-11 in tumor biology and future therapeutics (Review) Int. J. Oncol. 2016;48:1783–1793. doi: 10.3892/ijo.2016.3400. [DOI] [PubMed] [Google Scholar]
- 23.Matziari M., Dive V., Yiotakis A. Matrix metalloproteinase 11 (MMP-11; stromelysin-3) and synthetic inhibitors. Med. Res. Rev. 2007;27:528–552. doi: 10.1002/med.20066. [DOI] [PubMed] [Google Scholar]
- 24.Pittayapruek P., Meephansan J., Prapapan O., Komine M., Ohtsuki M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kossakowska A.E., Huchcroft S.A., Urbanski S.J., Edwards D.R. Comparative analysis of the expression patterns of metalloproteinases and their inhibitors in breast neoplasia, sporadic colorectal neoplasia, pulmonary carcinomas and malignant non-Hodgkin’s lymphomas in humans. Br. J. Cancer. 1996;73:1401–1408. doi: 10.1038/bjc.1996.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheau C., Badarau I.A., Costache R., Caruntu C., Mihai G.L., Didilescu A.C., Constantin C., Neagu M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019;2019:9423907. doi: 10.1155/2019/9423907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco M., Arcidiacono B., Chiefari E., Vitagliano T., Ciriaco A.G., Brunetti F.S., Cuda G., Brunetti A. HMGA1 and MMP-11 Are Overexpressed in Human Non-melanoma Skin Cancer. Anticancer Res. 2018;38:771–778. doi: 10.21873/anticanres.12283. [DOI] [PubMed] [Google Scholar]
- 28.Motrescu E.R., Rio M.C. Cancer cells, adipocytes and matrix metalloproteinase 11: A vicious tumor progression cycle. Biol. Chem. 2008;389:1037–1041. doi: 10.1515/BC.2008.110. [DOI] [PubMed] [Google Scholar]
- 29.Johnson L.D., Hunt D.M., Kim K., Nachtigal M. Amplification of stromelysin-3 transcripts from carcinomas of the colon. Hum. Pathol. 1996;27:964–968. doi: 10.1016/S0046-8177(96)90225-7. [DOI] [PubMed] [Google Scholar]
- 30.Pang L., Wang D.W., Zhang N., Xu D.H., Meng X.W. Elevated serum levels of MMP-11 correlate with poor prognosis in colon cancer patients. Cancer Biomark. 2016;16:599–607. doi: 10.3233/CBM-160601. [DOI] [PubMed] [Google Scholar]
- 31.Morini S.R., Denadai M.V., Waisberg J., Lopes Filho G.J., Matos D., Saad S.S. Metalloproteinases and colorectal cancer. Correlation of gene expression and clinical-pathological parameters. Acta Cir. Bras. 2020;35:e202000707. doi: 10.1590/s0102-865020200070000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koberle B., Koch B., Fischer B.M., Hartwig A. Single nucleotide polymorphisms in DNA repair genes and putative cancer risk. Arch. Toxicol. 2016;90:2369–2388. doi: 10.1007/s00204-016-1771-2. [DOI] [PubMed] [Google Scholar]
- 33.International HapMap C. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 34.Lin C.W., Yang S.F., Chuang C.Y., Lin H.P., Hsin C.H. Association of matrix metalloproteinase-11 polymorphisms with susceptibility and clinicopathologic characteristics for oral squamous cell carcinoma. Head Neck. 2015;37:1425–1431. doi: 10.1002/hed.23771. [DOI] [PubMed] [Google Scholar]
- 35.Wang B., Hsu C.J., Lee H.L., Chou C.H., Su C.M., Yang S.F., Tang C.H. Impact of matrix metalloproteinase-11 gene polymorphisms upon the development and progression of hepatocellular carcinoma. Int. J. Med. Sci. 2018;15:653–658. doi: 10.7150/ijms.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saad H., Zahran M.A., Hendy O., Abdel-Samiee M., Bedair H.M., Abdelsameea E. Matrix Metalloproteinase-11 Gene Polymorphisms as a Risk for Hepatocellular Carcinoma Development in Egyptian Patients. Asian Pac. J. Cancer Prev. 2020;21:3725–3734. doi: 10.31557/APJCP.2020.21.12.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh C.Y., Chou Y.E., Lin C.Y., Wang S.S., Chien M.H., Tang C.H., Lin J.C., Wen Y.C., Yang S.F. Impact of Matrix Metalloproteinase-11 Gene Polymorphisms on Biochemical Recurrence and Clinicopathological Characteristics of Prostate Cancer. Int. J. Environ. Res. Public Health. 2020;17:8603. doi: 10.3390/ijerph17228603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C.C., Hsieh M.J., Wang S.S., Hung S.C., Lin C.Y., Kuo C.W., Yang S.F., Chou Y.E. Impact of Matrix Metalloproteinases 11 Gene Variants on Urothelial Cell Carcinoma Development and Clinical Characteristics. Int. J. Environ. Res. Public Health. 2020;17:475. doi: 10.3390/ijerph17020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edge S.B., Compton C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 40.Chung T.T., Pan M.S., Kuo C.L., Wong R.H., Lin C.W., Chen M.K., Yang S.F. Impact of RECK gene polymorphisms and environmental factors on oral cancer susceptibility and clinicopathologic characteristics in Taiwan. Carcinogenesis. 2011;32:1063–1068. doi: 10.1093/carcin/bgr083. [DOI] [PubMed] [Google Scholar]
- 41.Hsiao P.C., Chen M.K., Su S.C., Ueng K.C., Chen Y.C., Hsieh Y.H., Liu Y.F., Tsai H.T., Yang S.F. Hypoxia inducible factor-1alpha gene polymorphism G1790A and its interaction with tobacco and alcohol consumptions increase susceptibility to hepatocellular carcinoma. J. Surg. Oncol. 2010;102:163–169. doi: 10.1002/jso.21539. [DOI] [PubMed] [Google Scholar]
- 42.The Lancet Oncology Colorectal cancer: A disease of the young? Lancet Oncol. 2017;18:413. doi: 10.1016/S1470-2045(17)30202-4. [DOI] [PubMed] [Google Scholar]
- 43.Patel S.G., Ahnen D.J. Colorectal Cancer in the Young. Curr. Gastroenterol. Rep. 2018;20:15. doi: 10.1007/s11894-018-0618-9. [DOI] [PubMed] [Google Scholar]
- 44.Mauri G., Sartore-Bianchi A., Russo A.G., Marsoni S., Bardelli A., Siena S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019;13:109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel S.G., Murphy C.C., Lieu C.H., Hampel H. Early age onset colorectal cancer. Adv. Cancer Res. 2021;151:1–37. doi: 10.1016/bs.acr.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Siegel R., DeSantis C., Virgo K., Stein K., Mariotto A., Smith T., Cooper D., Gansler T., Lerro C., Fedewa S., et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 47.Baxter N.N., Kennedy E.B., Bergsland E., Berlin J., George T.J., Gill S., Gold P.J., Hantel A., Jones L., Lieu C., et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022;40:892–910. doi: 10.1200/JCO.21.02538. [DOI] [PubMed] [Google Scholar]
- 48.Leijssen L.G.J., Dinaux A.M., Taylor M.S., Deshpande V., Kunitake H., Bordeianou L.G., Berger D.L. Perineural Invasion Is a Prognostic but not a Predictive Factor in Nonmetastatic Colon Cancer. Dis. Colon Rectum. 2019;62:1212–1221. doi: 10.1097/DCR.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 49.Skancke M., Arnott S.M., Amdur R.L., Siegel R.S., Obias V.J., Umapathi B.A. Lymphovascular Invasion and Perineural Invasion Negatively Impact Overall Survival for Stage II Adenocarcinoma of the Colon. Dis. Colon Rectum. 2019;62:181–188. doi: 10.1097/DCR.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 50.Hansen I.O., Jess P. Possible better long-term survival in left versus right-sided colon cancer-a systematic review. Dan. Med. J. 2012;59:A4444. [PubMed] [Google Scholar]
- 51.Pal S.K., Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J. Clin. Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 52.Ng S.C., Wang P.H., Lee Y.C., Lee C.Y., Yang S.F., Shen H.P., Hsiao Y.H. Impact of matrix metalloproteinase-11 gene polymorphisms on development and clinicopathologcial variables of uterine cervical cancer in Taiwanese women. Int. J. Med. Sci. 2019;16:774–782. doi: 10.7150/ijms.33195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hases L., Ibrahim A., Chen X., Liu Y., Hartman J., Williams C. The Importance of Sex in the Discovery of Colorectal Cancer Prognostic Biomarkers. Int. J. Mol. Sci. 2021;22:1354. doi: 10.3390/ijms22031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hang D., Shen H. Sex Hormone and Colorectal Cancer: The Knowns and Unknowns. Cancer Epidemiol. Biomark. Prev. 2021;30:1302–1304. doi: 10.1158/1055-9965.EPI-21-0472. [DOI] [PubMed] [Google Scholar]
- 55.Hang D., He X., Kvaerner A.S., Chan A.T., Wu K., Ogino S., Hu Z., Shen H., Giovannucci E.L., Song M. Plasma sex hormones and risk of conventional and serrated precursors of colorectal cancer in postmenopausal women. BMC Med. 2021;19:18. doi: 10.1186/s12916-020-01895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang W., Giovannucci E.L., Hankinson S.E., Chan A.T., Ma Y., Wu K., Fuchs C.S., Lee I.M., Sesso H.D., Lin J.H., et al. Endogenous sex hormones and colorectal cancer survival among men and women. Int. J. Cancer. 2020;147:920–930. doi: 10.1002/ijc.32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin J.H., Zhang S.M., Rexrode K.M., Manson J.E., Chan A.T., Wu K., Tworoger S.S., Hankinson S.E., Fuchs C., Gaziano J.M., et al. Association between sex hormones and colorectal cancer risk in men and women. Clin. Gastroenterol. Hepatol. 2013;11:419–424.e1. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouras E., Papandreou C., Tzoulaki I., Tsilidis K.K. Endogenous sex steroid hormones and colorectal cancer risk: A systematic review and meta-analysis. Discov. Oncol. 2021;12:8. doi: 10.1007/s12672-021-00402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mountain D.J., Freeman B.M., Kirkpatrick S.S., Beddies J.W., Arnold J.D., Freeman M.B., Goldman M.H., Stevens S.L., Klein F.A., Grandas O.H. Androgens regulate MMPs and the cellular processes of intimal hyperplasia. J. Surg. Res. 2013;184:619–627. doi: 10.1016/j.jss.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 60.Morales-Vasquez F., Castillo-Sanchez R., Gomora M.J., Almaraz M.A., Pedernera E., Perez-Montiel D., Rendon E., Lopez-Basave H.N., Roman-Basaure E., Cuevas-Covarrubias S., et al. Expression of metalloproteinases MMP-2 and MMP-9 is associated to the presence of androgen receptor in epithelial ovarian tumors. J. Ovarian Res. 2020;13:86. doi: 10.1186/s13048-020-00676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eiro N., Fernandez-Gomez J., Sacristan R., Fernandez-Garcia B., Lobo B., Gonzalez-Suarez J., Quintas A., Escaf S., Vizoso F.J. Stromal factors involved in human prostate cancer development, progression and castration resistance. J. Cancer Res. Clin. Oncol. 2017;143:351–359. doi: 10.1007/s00432-016-2284-3. [DOI] [PubMed] [Google Scholar]
- 62.Horstman A.M., Dillon E.L., Urban R.J., Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67:1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodman-Gruen D., Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–918. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 64.Zumoff B., Strain G.W., Miller L.K., Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J. Clin. Endocrinol. Metab. 1995;80:1429–1430. doi: 10.1210/jcem.80.4.7714119. [DOI] [PubMed] [Google Scholar]
- 65.Koga Y., Yasunaga M., Moriya Y., Akasu T., Fujita S., Yamamoto S., Kozu T., Baba H., Matsumura Y. Detection of colorectal cancer cells from feces using quantitative real-time RT-PCR for colorectal cancer diagnosis. Cancer Sci. 2008;99:1977–1983. doi: 10.1111/j.1349-7006.2008.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lech G., Slotwinski R., Slodkowski M., Krasnodebski I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016;22:1745–1755. doi: 10.3748/wjg.v22.i5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang B., Gao J., Rao Z., Shen Q. Clinicopathological and prognostic significance of α5β1-integrin and MMP-14 expressions in colorectal cancer. Neoplasma. 2013;60:254–261. doi: 10.4149/neo_2013_034. [DOI] [PubMed] [Google Scholar]
- 68.Chen Z., Wu G., Ye F., Chen G., Fan Q., Dong H., Zhu X., Wu C. High expression of MMP19 is associated with poor prognosis in patients with colorectal cancer. BMC Cancer. 2019;19:448. doi: 10.1186/s12885-019-5673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nimri L., Barak H., Graeve L., Schwartz B. Restoration of caveolin-1 expression suppresses growth, membrane-type-4 metalloproteinase expression and metastasis-associated activities in colon cancer cells. Mol. Carcinog. 2013;52:859–870. doi: 10.1002/mc.21927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.