Abstract
Objective:
To determine if higher exposures measured in early childhood to environmental phenols, phthalates, pesticides, and/or trace elements, are associated with increased odds of having a diagnosis of Autism Spectrum Disorder (ASD), Developmental Delay (DD), or Other Early Concerns (OEC) compared to typically developing children (TD).
Methods:
This study included 627 children between the ages of 2–5 who participated in the Childhood Autism Risks from Genetics and Environment (CHARGE) study. Urine samples were collected at the same study visit where diagnostic assessments to confirm diagnosis indicated during the recruitment process were performed. Adjusted multinomial regression models of each chemical with diagnosis as the outcome were conducted. Additionally, two methods were used to analyze mixtures: repeated holdout multinomial weighted quantile sum (WQS) regression for each chemical class; and a total urinary mixture effect was assessed with repeated holdout random subset WQS.
Results:
Many urinary chemicals were associated with increased odds of ASD, DD or OEC compared to TD; however, most did not remain significant after false discovery rate adjustment. Repeated holdout WQS indices provided evidence for associations of both a phenol/paraben mixture effect and a trace element mixture effect on DD independently. In analyses adjusted for confounders and other exposures, results suggested an association of a pesticide mixture effect with increased risk for ASD. Results also suggested associations of a total urinary mixture with greater odds of both ASD and DD separately..
Conclusion:
Higher concentrations of urinary biomarkers were associated with ASD, DD, and OEC compared to TD, with consistency of the results comparing single chemical analyses and mixture analyses. Given that the biospecimens used for chemical analysis were generally collected many months after diagnoses were made, the direction of any causal association is unknown. Hence findings may reflect higher exposures among children with non-typical development than TD children due to differences in behaviors, metabolism, or toxicokinetics.
Keywords: autism, mixtures, environmental phenols, weighted quantile sum, paraben, pesticide
Introduction
As rates of autism spectrum disorder (ASD) diagnosis have risen in the last quarter century, the burden on our communities has become a major public health concern. ASD affects about 1 in 54 children (Maenner, Shaw et al. 2020). Associations of ASD with environmental exposures such as air pollution (Lam, Sutton et al. 2016, Gong, Dalman et al. 2017, Raz, Levine et al. 2018, Ritz, Liew et al. 2018, Pagalan, Bickford et al. 2019) and nutrition (Schmidt, Hansen et al. 2011, Schmidt, Tancredi et al. 2012, Surén, Roth et al. 2013, Schmidt, Kogan et al. 2017, Goodrich, Volk et al. 2018) have been emerging rapidly, leading to increased recognition that environmental exposures likely contribute, either alone or in combination with genetic factors, to ASD risk (National Research Council 2000).
There is a growing body of epidemiologic evidence considering exposure to various classes of pesticides and ASD. Studies have found higher prenatal organophosphate, pyrethroid, and/or organochlorine exposures (Roberts, English et al. 2007, Shelton, Geraghty et al. 2014, von Ehrenstein, Ling et al. 2019) for children with ASD, when prenatal residences were linked to a curated database of nearby agricultural applications that has been validated in relation to measurements from concurrent air samples (Wofford, Segawa et al. 2014). Additionally, a study of pesticide metabolite levels in multiple urine samples collected during pregnancy in a high-risk ASD birth cohort, found that pyrethroid metabolite 3-phenoxy-benzoic acid (3-PBA) had a suggestive association with ASD (adjusted OR 1.5, 95% CI: 0.9, 2.5) (Barkoski, Philippat et al. 2020). In the same study population OP metabolite levels were associated with ASD only among girls (Philippat, Barkoski et al. 2018).
For phthalates, robust research demonstrates associations with neurodevelopmental outcomes generally, including cognitive impairments, internalizing and externalizing behaviors, and attention deficit-hyperactivity disorders, but only limited evaluations of ASD (Engel, Patisaul et al. 2021) (Zhang, Chen et al. 2019). In studies with laboratory animals, phthalates have been found to have neuro-developmental toxicities that mirror those found in humans (Engel, Patisaul et al. 2021). Sex differences in neurodevelopmental outcomes are commonly observed in relation to phthalate exposures (Engel, Patisaul et al. 2021). A Canadian study found that boys with higher in utero phthalate exposures, as measured by prenatal urinary phthalate concentrations, had more autistic behaviors, especially if mothers had inadequate folic acid supplementation (Oulhote, Lanphear et al. 2020). However, results in a study conducted in high risk populations did not show a relationship (Shin, Schmidt et al. 2018).
Less studied are the environmental phenols, such as BPA and parabens, which are also potential risk factors for child behavioral outcomes (Harley, Gunier et al. 2013, Braun, Muckle et al. 2017, Philippat, Nakiwala et al. 2017) and altered sex thyroid levels (Aker, Johns et al. 2018, Berger, Gunier et al. 2018). Recent work in a high-risk ASD birth cohort using weighted quantile sum regression (WQSR) showed that a mixture of prenatal urine metabolites of environmental phenols was suggestively associated with ASD, and significantly associated with children who were non-TD (but not ASD) compared to TD (Barkoski, Busgang et al. 2019). Another recent study suggested that associations between BPA and ASD have sex differences in dysregulated genes related to the hippocampus (Thongkorn, Kanlayaprasit et al. 2019).
Metals such as lead (Bellinger 2008), and mercury (Ruth and Zota 2019), have been well studied and an abundant literature of many decades has established adverse neurological effects of clinical relevance. The metalloid arsenic is associated with both ASD (Wang, Houssain et al. 2019) and adverse neurological effects more genrally (Tyler and Allan 2014). In contrast, other trace elements have been less studied. Few studies have addressed elements such as cadmium or molybdenum and adverse neurological or neurodevelopmental outcomes (Ciesielski, Weuve et al. 2012, Vázquez-Salas, López-Carrillo et al. 2014, Sanders, Henn et al. 2015).
The goal of this project was to evaluate if exposures to pesticides, phthalates, phenols and trace elements at 2–5 years of age are associated with neurodevelopmental outcomes. For diagnostic outcomes, three main comparisons are made, based on distinct neurodevelopmental case groups: ASD vs TD (typical development), DD (developmental delays other than ASD) vs TD, and Other Early Concerns (OEC) vs TD.
Methods
This investigation was conducted within the CHARGE study (Hertz-Picciotto, Croen et al. 2006), a case control study that recruits participants from three groups: children with an autism spectrum disorder (ASD), children with developmental delay but not ASD, and general population controls. The first two groups are primarily identified from the California Department of Developmental Services, which coordinates services for persons with developmental disabilities, and is inclusive of all residents of California regardless of place of birth, religion, or financial resources. General population controls are sampled from California birth files, with frequency-matching to ASD cases on age, sex and broad geographic regions encompassing up to 10 counties. Children from all three groups must be: a) aged 24–60 months at recruitment; b) living with a biologic parent who speaks English or Spanish; c) born in California; and d) residing in the study catchment area. Participants that were recruited to the study between 2006 and 2017 and had at least 16 ml of urine collected at their assessment and available for chemical analyses, constituted our study sample, comprising a total of 627 participants.
Developmental Assessment:
We assessed for autism diagnosis to confirm diagnosis indicated through the recruitment process using two gold standard psychometric instruments, both of which are widely accepted for research; the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter et al. 1994, Lord, Pickles et al. 1997, Le Couteur, Lord et al. 2003) and the Autism Diagnostic Observation Schedules (ADOS) (Lord, Risi et al. 2000). The ADI-R is a semi-structured interview for the primary caregiver that reviews the child’s development. The ADOS is a semi-structured assessment in which the researcher observes the social interaction, communication, play and imaginative use of materials by children suspected of having ASD (Lord, Risi et al. 2000). To assign final diagnoses of ASD, we used DSM-5, and followed established algorithms utilizing both the ADOS and ADI-R (Risi, Lord et al. 2006). Children from all three groups were administered the Mullen Scales of Early Learning (MSEL) and the Vineland Adaptive Behaviors Scores (VABS). Children who did not meet criteria for an ASD diagnosis and have scores on either the MSEL or VABS that fell below 1.5 SD lower than the mean, and had scores on the other instrument less than 2.0 SD lower than the mean were classified as DD. Children in both the developmental delay and general population groups were screened for ASD using the Social Communications Questionnaire to confirm that they do not have ASD (Rutter, Bailey et al. 2003). For those who screened positive, the ADI-R and ADOS was then administered to determine whether or not they have ASD. All other children who were enrolled because of a community diagnosis of ASD or DD, but were not confirmed for either of these two diagnoses, were grouped together as Other Early Concerns (OEC). Children enrolled as general population controls who did not meet criteria for either ASD or DD were classified as TD. All classification groups are mutually exclusive. All clinicians who psychometrically evaluated children achieved research reliability on the instruments they administered. English and Spanish speaking clinicians were used in this study.
Exposure Assessment:
Urine samples were collected from the participating child at the time of the visit and immediately frozen at −20°C. Samples remained frozen until analysis (mean 7.4 years, 5–95% range 2.2 – 11.7 years). They were then shipped on dry ice to the New York State Department of Health’s (NYSDOH) Wadsworth Center’s Human Health Exposure Analysis Resource (HHEAR) Targeted Analysis Laboratoryfor analysis. The target phenolic compounds were analyzed in urine samples after enzymatic deconjugation, followed by liquid-liquid extraction, as previously described (Asimakopoulos, Thomaidis et al. 2014, Li, Xue et al. 2018). Briefly, 2.5 ng of isotopically labeled internal standards were spiked into 250 μL of urine. The samples were buffered with 400 μL of 1 M ammonium acetate containing 200 unit/mL of β-glucuronidase (MP Biomedicals, LLC) and incubated at 37 °C for 12 h. Ethyl acetate (10 mL) was added, shaken for 60 min and centrifuged at 4500 rpm for 5 min. The supernatants were washed with water before being concentrated under a nitrogen stream. The extract was reconstituted with 250 μL of methanol, and analyzed by HPLC-MS/MS. HPLC-MS/MS parameters are described in detail elsewhere (Rocha, Asimakopoulos et al. 2018).
The method for the analysis of urinary phthalate metabolites (PhMs) entailed enzymatic deconjugation, followed by solid-phase extraction (SPE) and an isotope diluted method of quantification (Li, Martinez-Moral et al. 2019). Briefly, 250 μL of urine sample with 2.5 ng of labeled internal standard mixture was buffered with 300 μL of 1 M ammonium acetate containing 100 unit/mL of β-glucuronidase and was incubated at 37 °C for 12 h. The samples were passed through ABS Elut-NEXUS SPE cartridges (60 mg 3 mL, Agilent, Santa Clara, CA) that were conditioned with acetonitrile (1.5 mL) and phosphate buffer (1.2 mL). Eluates of acetonitrile (1.2 mL) and ethyl acetate (1.2 mL) were evaporated to near-dryness under nitrogen and re-dissolved in 250 μL of acetonitrile/water (10:90 v/v) for further analysis by HPLC-MS/MS. Details of the instrumental methods are described earlier (Rocha, Asimakopoulos et al. 2017).
The six dialkylphosphate metabolites (DAPs) of organophosphates were extracted from urine samples by a SPE method. Briefly, urine samples (250 μL) were spiked with 2.5 ng of isotopically labelled internal standard mixture and mixed with 2% formic acid (750 μL). The samples were then passed through WAX cartridges (60 mg/3 mL; Biotage, Salem, NH, USA) that were conditioned with methanol (2 mL) and water (2 mL). The eluates (2 mL of 5% ammonia in methanol) were dried under a gentle stream of nitrogen, reconstituted with 250 μL of acetonitrile/20 mM ammonium acetate (90:10, v/v), and filtered (0.22 μm nylon, Spin-X, Costar; Corning, NY, USA) before analysis by HPLC-MS/MS, as described in Li et al. (2020) (Li, Banjabi et al. 2020).
Urine specimens were analyzed for trace elements in the Laboratory of Inorganic and Nuclear Chemistry at the Wadsworth Center using a well-established biomonitoring method based on ICP–MS (Minnich, Miller et al. 2008).
In brief, urine samples, and 4 different levels of urine quality control materials were diluted 1+19 with a reagent containing nitric acid, Triton X-100, and appropriate internal standards. Diluted samples were analyzed for As, Be, Cd, Mo, Tl, and U on a PerkinElmer® NexION® 300D ICP-MS instrument calibrated with NIST-traceable standards. Method accuracy was assured via analysis of NIST SRM 2668 Toxic Elements in Frozen Human Urine. Ongoing laboratory performance was monitored via satisfactory participation in numerous proficiency testing (PT) programs for trace elements in urine, including those operated by Le Centre de Toxicologie du Québec, the UK Trace Elements External Quality Assessment Scheme, and the NYSDOH Biomonitoring PT program for Trace Elements.
Urinary biomarkers were specific gravity (SG) corrected using the following formula: Pc = P × [(SGp − 1)/(SG − 1)](Hauser, Meeker et al. 2004), where Pc is the SG-corrected metabolite concentration (ng/mL), P is the measured metabolite concentration in ng/mL, SG is the specific gravity of the urine sample, and SGp (1.0223) is the median specific gravity across CHARGE participants providing urine for this study with the full set of covariates. Specific gravity correction factors [(SGp − 1)/(SG − 1)] greater than 2 were assigned a value of 2 and for values below 0.5 were assigned 0.5.
Data Analysis –
Since all instrument software-generated values were provided, negative values can arise legitimately at or below the limit of detection (LOD) (as a result of random error). The calculation of the LOD represents a theoretical relative standard deviation (RSD) of ±33% of the signal, and this is equivalent to a relative uncertainty of ±94% in the concentration number reported, resulting in some negative values. For each urinary chemical with a minimum SG-corrected concentration of 0 or less (i.e. negative), the minimum concentration and a value of 0.01 were added to all values such that concentrations were shifted to a positive nonzero value. All chemicals were measured in ng/ml. To account for right skewedness of biomarker data, natural log transformed values were used.
Two regression approaches were used for assessing the chemicals of interest in relation to child diagnoses: single chemical and mixture models. Additionally, the mixture models were applied in two ways: to combine individual compounds within a chemical class, and to combine across all classes of chemicals. Chemicals used in regression analyses had at least 60% of measured concentrations above the study wide LOD prior to SG correction.
All models were adjusted for covariates selected a priori or that were related to the exposure and outcome (p<0.20). These included child’s sex, year of birth, age in months at time of diagnosis, and race, as well as parental homeowner status, and maternal metabolic conditions during pregnancy. We strove to use the most parsimonious model that still adjusted for important confounders, and thus selected a single variable, parental homeowner status, to represent SES. Year of birth was centered by subtracting the mean birth year.
Preliminarily, single chemical multinomial logistic regression models were used for each individual chemical, to simultaneously estimate three regression coefficients, representing the strength of associations with ASD, DD, or OEC versus TD. This approach has been used to analyze chemical exposures in a high-risk autism cohort, for example comparing ASD to TD (Barkoski, Busgang et al. 2019). A false discovery rate (FDR) correction was applied to p-values per outcome and chemical class to account for multiple comparisons.
Weighted quantile sum (WQS) regression was used to test for a mixture effect of chemicals on the outcome while accommodating for a complex correlation pattern among the chemical components of that mixture. Analyses were conducted by focusing the inference in a positive direction (i.e., increased risk) using one sided confidence limits. The 5th percentile (PCT) was used to define the lower limit. An advantage of WQS regression is that estimation of the weights can be conducted while focusing the inference in a specified direction with a powerful 1 degree of freedom test, thereby improving the stability in estimation due to the complex exposure correlation pattern. Forty percent of the participants were used to estimate the weights associated with each chemical; next the WQS index, using these estimated weights, was calculated for the remaining 60% of participants and the association between the index and diagnosis group was tested in this validation group. When the WQS index was significant, the weight contribution of each chemical was displayed and important chemicals in the mixture were identified if they contribute a weight greater than 1/c where c is the number of chemicals in the mixture (Carrico, Gennings et al. 2015). We refer to 1/c as the chemical of concern threshold moving forward. We implemented this approach using multinomial WQS for individual chemical classes. To increase robustness of our estimates, we applied repeated holdout validation, randomly splitting the participants 100 times, to examine the distribution of effect estimates and associated weights (Tanner, Bornehag et al. 2019). When at least 95% of the holdouts resulted in increased odds, per diagnosis group, chemical weight distributions were displayed. Chemicals with 90% of repetitions above the chemical of concern threshold were defined as being probable contributors to the mixture effect and those with fifty to less than 90% of repetitions above threshold were defined as possible contributors. Chemicals with more than 90% of repetitions below the threshold were defined as probably not contributors and those with fifty to less than 90% below the threshold were defined as possibly not contributors.
Additionally, a total chemical mixture was tested, combining across all urinary chemical classes, using random subset WQS (rsWQS). This approach randomly selects a subset of a selected number of predictors and estimates weight parameters across multiple ensemble steps with varying predictor subsets and averages the weight contributions across 1000 subsets (Curtin, Kellogg et al. 2019). For the current study, six chemicals were included in each subset such that each chemical’s weight was estimated approximately 140 times across all subsets. Similar to the bootstrap WQS, we used repeated holdout validation with 100 random splits of the data where 60% of subjects were selected into the training dataset and the remaining 40% were used for validation.
An alpha of 0.05 was the criterion for statistical significance. For mixture analyses, WQS models were constrained in the positive direction to assess the hypothesized harmful effects of chemical mixtures and significance was assessed using a one-sided test. The interpretation of the odds ratio (X) for a WQS index is that for every 1 decile increase in the weighted index there is an X increase in odds of ASD compared to TD. All statistical analyses were conducted with SAS statistical analysis software version 9.4 (SAS, Cary, NC, USA) and an independent programmer validated the results in this report.
Results
The population included 237 TD, 224 ASD, 81 DD, and 85 OEC participants. The population had a greater fraction of male participants, as ASD is more prevalent in males. The population was 49% non-Hispanic White, followed by 30% Hispanic, and 21% non-White, non-Hispanic. Sample characteristics are stratified by diagnosis in Table 1 as well as the association of each covariate with log transformed methyl paraben (MEPB), selected as a representative compound as it was widely detected and had an association with all three outcomes to help select model covariates. Supplementary Table S1 provides the chemical names and abbreviations of all the chemicals analyzed, stratified by chemical class. Supplementary Table S2 provides the % above LOD for each chemical to indicate which chemicals met our threshold of above 60% to be included in the remaining analyses as described above. Based on this threshold, urinary concentrations of 30 phenols, 20 phthalate metabolites, 6 pesticide metabolites, and 6 trace elements, per outcome, are displayed in Supplemetary Table S3. Matrixes of correlation coefficients between compounds are presented in Supplemetary Figures S1 and S2.
Table 1:
Characteristics of mothers and children included in the analysis, by 3-year clinical outcome, from the CHARGE cohort (N=627)
| Typical Development N=237 |
Autism Spectrum Disorder N= 224 |
Developmental Delay N=81 |
Other early concerns or high risk N=85 |
Mean MEPB (ng/ml) | Association with ln MEPB p-valuea | Outcome p-valueb | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Freq (%) | Freq (%) | Freq (%) | Freq (%) | ||||
|
| |||||||
| Sex | 0.680 | <0.001 | |||||
| Male | 193 (81.43) | 181 (80.80) | 60 (74.07) | 50 (58.82) | 568.0 | ||
| Female | 44 (18.57) | 43 (19.20) | 21 (25.93) | 35 (41.18) | 389.9 | ||
|
| |||||||
| Race | 0.010 | 0.140 | |||||
| White (non-Hispanic) | 128 (54.01) | 106 (47.32) | 32 (39.51) | 41 (48.24) | 416.6 | ||
| Non-White (non-Hispanic) | 46 (19.41) | 55 (24.55) | 16 (19.75) | 15 (17.65) | 904.7 | ||
| Hispanic (any race) | 63 (26.58) | 63 (28.13) | 33 (40.74) | 29 (34.12) | 443.5 | ||
|
| |||||||
| Highest education in household | 0.756 | <0.001 | |||||
| High school diploma/GED or less | 6 (2.53) | 28 (12.50) | 18 (22.22) | 10 (11.76) | 760.2 | ||
| Some college (inc. vocational, 2 yr degree) | 82 (34.60) | 62 (27.68) | 28 (34.57) | 31 (36.47) | 606.5 | ||
| Bachelor’s degree | 90 (37.97) | 81 (36.16) | 26 (32.10) | 28 (32.94) | 440.3 | ||
| Graduate or professional degree | 59 (24.89) | 53 (23.66) | 9 (6.57) | 16 (18.82) | 448.0 | ||
|
| |||||||
| Homeowner | 0.154 | <0.001 | |||||
| No | 57 (24.05) | 73 (32.59) | 36 (44.44) | 38 (44.71) | 706.4 | ||
| Yes | 180 (75.95) | 151 (67.41) | 45 (55.56) | 47 (55.29) | 441.1 | ||
|
| |||||||
| Prenatal Vitamin Use in 3 months before or during 1st month of pregnancy (31 Missing) | 0.473 | 0.190 | |||||
| No | 67 (29.13) | 74 (34.58) | 32 (41.56) | 22 (29.33) | 431.7 | ||
| Yes | 163 (70.87) | 140 (65.42) | 45 (58.44) | 53 (70.67) | 537.7 | ||
|
| |||||||
| Maternal metabolic conditions | 0.074 | 0.006 | |||||
| Healthy weight and no metabolic conditions | 119 (50.21) | 101 (45.09) | 31 (38.27) | 39 (45.88) | 508.9 | ||
| Overweight and no metabolic conditions | 58 (24.47) | 43 (19.20) | 18 (22.22) | 10 (11.76) | 519.7 | ||
| Obese and no other metabolic conditions | 34 (14.35) | 30 (13.39) | 15 (18.52) | 10 (11.76) | 566.8 | ||
| Any hypertensive disorder or diabetes at any BMI | 26 (10.97) | 50 (22.32) | 17 (20.99) | 26 (30.59) | 551.4 | ||
|
| |||||||
| Mother’s birthplace | 0.557 | 0.024 | |||||
| USA | 202 (85.23) | 164 (73.21) | 64 (79.01) | 68 (80.00) | 565.4 | ||
| Mexico | 10 (4.22) | 18 (8.04) | 9 (11.11) | 8 (9.41) | 239.5 | ||
| Outside the US and Mexico | 25 (10.55) | 42 (18.75) | 8 (9.88) | 9 (10.59) | 456.6 | ||
|
| |||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
|
| |||||||
| Child age at assessment (months) | 46.02 (9.04) | 48.67 (8.70) | 47.88 (7.99) | 48.00 (9.25) | 0.015 | 0.013 | |
|
| |||||||
| Child’s year of birth | 2006.5 (2.90) | 2007.3(3.22) | 2006.1 (2.73) | 2006.4 (2.87) | 0.172 | 0.006 | |
|
| |||||||
| Mother’s age at time of child’s birth (years) | 30.23 (5.27) | 30.67 (5.74) | 30.11 (6.41) | 31.00 (5.79) | 0.160 | 0.617 | |
|
| |||||||
| Pre-pregnancy BMI | 25.71 (5.46) | 26.40 (6.17) | 26.64 (6.23) | 27.35 (7.83) | 0.569 | 0.183 | |
For categorical variables with only two levels, p-values are shown from t-tests; for categorical characteristics with more than two levels, p-values are shown from ANOVA tests; for continuous characteristics, p-value are shown from univariate linear models.
For categorical charactertics, p-values are shown from Chi square test; for continuous charactertics, p-values are shown from univariate multinomial logistic models.
For each chemical class, we present results from the repeated holdout WQS as well as the single chemical analysis. We highlight chemicals that were probably or possibly strong contributers in the WQS. Due to the large number of results of the single chemical analysis, we focused our discussion on results that were statistically significant at p<0.05, or had sizable (non-null) effect size (OR>1.1 or <0.9), noting that significance should not be the only measured considered.
Phenols and parabens:
The repeated holdout WQS indicated a significant association between the phenol/paraben index and DD diagnosis [average OR=2.40, (5th PCT=1.05); Table 1, Figure S3]. A weaker but still significant mixture effect was also found for diagnosis of ASD with the phenol/paraben index [average OR=1.50, (5th PCT=1.04). There was a positive trend with the phenol index and OEC diagnosis, however the signal of that association was relatively weak [average OR=1.65, (5th PCT=0.94)]. The compounds contributing to these associations can be seen in Figures 1A and 1B, which presents how often a chemical exceeded the concern threshold, and Table 3, which indicates compounds with either a probable or possible contribution in the mixures analysis.
Figure 1:
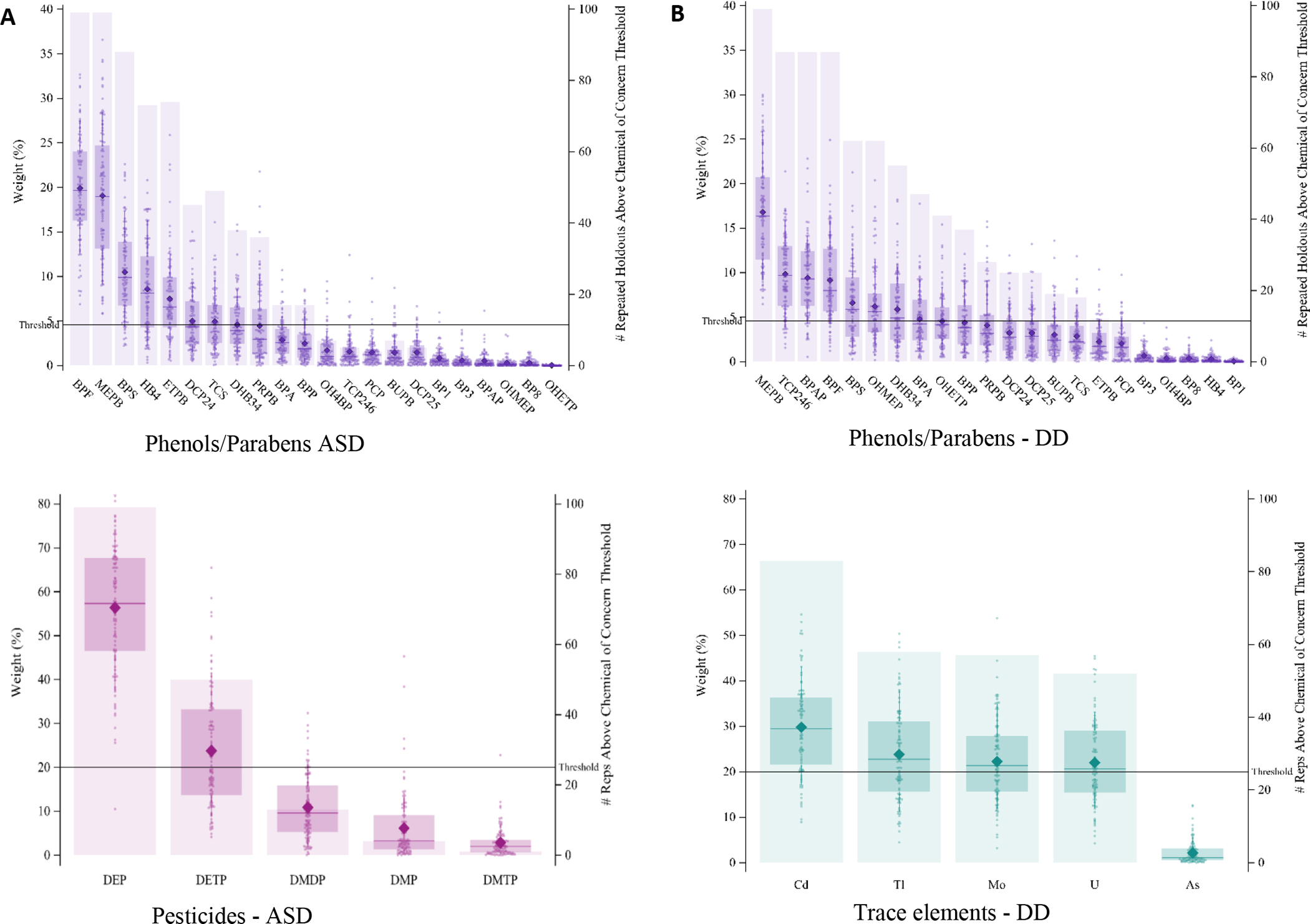
Weight distributions of each chemical from each 100 repetitions (left axis) and the number of repetitions in which the chemical exceeded the 1/c threshold contribution of concern for class mixture multinomial WQS models with more than 90% repititions above the null, where weights associated with ASD for the phenol/paraben mixture are shown in A, DD for the phenol/paraben mixture are shown in B, ASD for the pesticides mixture are shown in C, DD for the trace elements mixture shown in D.
Table 3:
Chemicals included in the analysis with either a significant association or sizable effect size with either ASD, DD or OEC, or that were a contributor to a mixture that was associated with either ASD, DD, or OEC.
| ASD | DD | OECƗ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Class | Chemical Abbreviation | Linear | Quartile | Class Mixture | Overall Mixture | Linear | Quartile | Class Mixture | Overall Mixture | Linear | Quartile | Overall Mixture |
| Phenols/Parabens | BP1 | −(*) | − | |||||||||
| BP3 | − | − | −(*) | |||||||||
| BP8 | −(*) | − | − | − | − | |||||||
| BPA | + | possible | + | |||||||||
| BPAP | + | + | possible | possible | + | |||||||
| BPF | + | + | probable | possible | possible | possible | −(*) | |||||
| BPP | + | + | possible | + | + | possible | ||||||
| BPS | + | possible | possible | * | + | possible | possible | |||||
| BUPB | + | possible | * | + | possible | |||||||
| DCP24 | + | + | possible | * | + | possible | + | + | possible | |||
| DHB34 | * | + | * | * | possible | possible | + | + | possible | |||
| ETPB | * | possible | probable | + | + | + | + | possible | ||||
| HB4 | + | * | possible | possible | + | + | + | * | possible | |||
| MEPB | ** | ** | probable | probable | ** | ** | probable | probable | * | * | probable | |
| OH4BP | + | + | + | |||||||||
| OHETP | −(**) | −(**) | ||||||||||
| OHMEP | * | + | possible | possible | + | + | possible | |||||
| PCP | + | |||||||||||
| PRPB | * | * | probable | * | * | probable | * | * | possible | |||
| TCP246 | + | * | possible | probable | ||||||||
| TCS | + | + | possible | |||||||||
| Phthalates | MCINP | * | probable | + | + | possible | + | + | possible | |||
| MCIOP | + | + | + | * | probable | |||||||
| MCPP | * | * | probable | + | ||||||||
| MECPP | + | + | + | possible | + | + | possible | |||||
| MEHHP | + | + | * | probable | + | + | ||||||
| MEOHP | + | possible | + | * | possible | + | + | |||||
| MHPP | + | |||||||||||
| MIBP | + | + | possible | + | + | possible | ||||||
| Pesticides | DEP | * | ** | probable | probable | + | * | probable | ||||
| DETP | * | * | possible | possible | + | + | ||||||
| DMP | + | possible | + | + | + | possible | ||||||
| DMTP | + | + | possible | |||||||||
| Trace Elements | As | + | + | possible | + | |||||||
| Cd | − | + | + | possible | possible | − | ||||||
| Mo | + | + | possible | possible | + | |||||||
| Tl | + | * | possible | probable | + | + | possible | |||||
| U | * | * | probable | * | * | possible | possible | + | + | possible | ||
= OR>1.10, but not significant
OR<0.90, significance indicated
= OR increased and value significant
= OR increased, FDR correction value also significant
gray fill = indicates there was no relationship between the class mixture and the outcome
= For the OEC group, there was no relationship between any class mixture and the outcome
MEPB was a probable contributor to the mixture effect for the relationship between the phenol/paraben index and ASD diagnosis. Additionally, the single chemical multinomial regression indicated higher concentrations of MEPB in urine were associated with increased odds of ASD to TD [OR=1.19, 95% CI=(1.08–1.32)] (See Table S4), and remained significant after correcting for false discovery rate (FDR) (FDR-corrected p-value 0.014). Natural log-transformed single chemical results are summarized in Figure 2 using a volcano plot to indicate directionality and magnitude of the association and chemicals are labeled if it passes the 0.05 significant threshold, with similar plots for all other compound classes available in the Supplementary Information, Figure S4. The quartile analysis used quartiles ordinally such that the results can be interpreted as an increase odds per quartile increase. Single chemical analysis with quartiles supported associations with MEPB [OR=1.40 (1.18–1.68), FDR-corrected p-value 0.004] (see Table S5). The alignment of the measures for MEPB can be seen in Table 3, which includes all compounds for which there are any single compound odds ratios that are significant, or greater or equal to 1.10 or less than or equal to 0.90, or for which there are probable or possible contribution in any mixture analysis.
Figure 2:

Volcano plot of raw p-values of log transformed phenols and parabens in association with ASD (blue), DD (red), and OEC (green) compared to TD. The x-axis shows the direction and magnitude of the OR, while the y-axis shows raw p-values.
Bisphenol F (BPF) was both a probable contributor to the mixture effect on ASD and was borderline significant in the single chemical linear model [OR=1.14 (0.99–1.31)]. Probable contributor ethyl paraben (ETPB) was not significant in the linear model [OR=1.07 (0.95–1.20)] but was significant in the quartile analysis [OR=1.19 (1.01–1.42)]. This relationship was not significant after FDR correction. Henceforth, we only include FDR correction values in the main text if they are significant or boarderline significant. Bisphenol S (BPS) was a possible contributor to the mixture effect but was not significant in linear or quartile single chemical analysis[linear OR=1.07 (0.90–1.28)]. There were compounds that were possible contributors to the mixture effect and singifcnat in quartile but not significant in linear single chemical analyses, specifically ethyl paraben (ETPB) [quartile OR=1.19 (1.01, 1.42)] and 4-hydroxybenzoic acid (HB4) [quartile OR=1.19 (1.00–1.41)] There were additional compounds found to be significant in the single chemical analysis, specifically, 3,4-dihydroxy benzoic acid (DHB34) [linear OR=1.28 (1.02–1.61)]and propyl paraben (PRPB) [linear OR=1.13 (1.02–1.24), quartile OR=1.22 (1.03–1.45)], that were not significant in the mixtures model. The compounds 2,2’-dihydroxy-methoxybenzophenone (BP8) and protocatechuic acid ethyl ester (OHETP) were associated with decreased odds of ASD in both the linear and quartile single compound analysis, with OHETP remaining significant after FDR-correction, but there was no evidence of a mixture of compounds associated with decreased odds.
For the DD diagnosis, MEPB, BPS, 3,4-dihydroxy-benzoic acid methyl ester (DHB34), and 2,4,6-trichlorophenol (TCP246) had probable, or possible contributions in the mixture analysis (Figure 1B) and had significant results in the single chemical analysis [MEPB linear OR=1.31 (1.14–1.50), FDR corrected p-value 0.002, quartile OR=1.63 (1.27–2.10), FDR corrected p-value 0.003], [linear BPS OR=1.27 (1.01–1.60)], [linear DHB34 OR=1.37 (1.01, 1.87), quantile OR=1.27 (1.00, 1.60)] and [quantile TCP246 OR=1.32 (1.03–1.67)] respectively. There were additional compounds that were “possible contributors” in the mixtures analysis, but not significant in either of the single compound analysis, specifically 4,4’-(1-phenylethylidene) bisphenol (BPAP) [linear OR=1.17 (0.82–1.67)] BPF [linear OR=1.06 (0.88–1.27)]. Compounds with significant findings in the single compound analysis but were not likely contributors in the mixtures analysis include DCP24 [linear OR=1.49 (1.02–2.19)], OHMEP [linear OR=1.22 (1.00–1.47)], and PRPB [linear OR=1.20 (1.06–1.37), quantile OR=1.37 (1.08–1.74)].
For OEC, BUPB [linear OR=1.26 (1.07–1.49)], MEPB [linear OR=1.20 (1.04–1.37)], and PRPB [linear OR=1.20 (1.05–1.38)] all having a borderline significant effect after FDR correction in the linear model, with MEPB and PRPB also having significant results in the quartile model.
Phthalates:
The WQS analysis resulted in no significant mixture effect for phthalate metabolites on any of the outcomes (Table 2). All effect estimates were positive, on average, with at least 75% of the repetitions greater than 1; however, the signals were noisy, decreasing our confidence in a significant association (Figure S3). Quartiled results show that higher levels of mono-carboxy isononyl phthalate (MCINP) were associated with increased odds of ASD [OR=1.19 (1.00–1.41)]. There were multiple single chemical analyses associated with increased odds of DD compared to TD, including mono (3-carboxypropopyl phthalate (MCPP) [linear OR=1.46 (1.07–1.97), quartile OR=1.29 (1.01–1.65)], mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) [quartile: OR=1.35 (1.05–1.73)], and mono (2-ethyl-5-oxohexyl) phthalate (MEOHP) [quartile OR=1.31 (1.01–1.69)]. For the OEC group, the only significant finding in the single chemical analysis was for the quantile analysis for MCIOP [OR=1.31 (1.03–1.67).
Table 2:
Results from the multinomial WQS for each of the 4 classes of compounds, as well as for all compounds in a discrete full mixture rsWQS models, comparing relative odds of ASD, DD, and OEC with TD, with positively constrained betas in estimating the weights, adjusted for covariates (sex, year of birth, homeowner status, child’s race, child’s age in months, and maternal metabolic conditions). In the discreet full mixture rsWQS models, weights were estimated by randomly selecting 6 chemicals across 1000 subsets. The table provides the distribution of OR estimates across 100 splits for each chemical class, and for the mixture of all chemical classes combined, in relation to each of the developmental outcomes, and for the mixture of all chemical classes combined.
| ASD vs TD | DD vs TD | OEC vs TD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Class Mixture | Mean | Median | 5th PCT | 95th PCT | Mean | Median | 5th PCT | 95th PCT | Mean | Median | 5th PCT | 95th PCT | |
| PCT | PCT | PCT | PCT | PCT | PCT | ||||||||
| Phenols/Parabens | OR | 1.50 | 1.46 | 1.04 | 2.10 | 2.40 | 2.25 | 1.05 | 4.59 | 1.65 | 1.58 | 0.94 | 2.50 |
| Phthalates | OR | 1.12 | 1.08 | 0.88 | 1.42 | 1.33 | 1.29 | 0.89 | 1.93 | 1.30 | 1.26 | 0.90 | 1.74 |
| Pesticides | OR | 1.10 | 1.11 | 1.02 | 1.19 | 1.05 | 1.05 | 0.93 | 1.19 | 1.11 | 1.11 | 0.99 | 1.25 |
| Trace Elements | OR | 1.17 | 1.16 | 0.88 | 1.46 | 1.75 | 1.68 | 1.19 | 2.51 | 1.22 | 1.18 | 0.80 | 1.77 |
| Total Mixture | OR | 1.84 | 1.86 | 1.08 | 2.70 | 3.44 | 3.09 | 1.43 | 7.04 | 2.20 | 2.07 | 1.07 | 3.93 |
Pesticides:
Mixture analyses resulted in a statistically significant association between the DAP pesticide metabolites of organophosphate pesticides mixture and ASD [(average OR=1.10, 5th PCT=1.02); Table 3, Figure 1]. The pesticide mixture was positive, on average, for DD but the confidence interval overlaps with 1, likewise for OEC. In the mixture associated with ASD, diethylphosphate (DEP) was a contributor in all but one hold-out set (Figure 1C), and was also significant in the single chemical analysis [linear: OR=1.20 (1.02–1.42), quartile: OR=1.37 (1.15–1.64), 0.002 FDR corrected P-value]. Diethylthiophosphate (DETP) is a possible contributor to the mixture effect and was also significant in the single chemical analysis [linear: OR=1.16 (1.00–1.35), quartile: OR=1.19 (1.00–1.42)]. There were no significant single chemical results for the DD group, and only one for the OEC group, specifically DEP [quartile OR=1.33 (1.04–1.64)].
Trace Elements:
The trace element mixture was significantly associated with increased odds of DD compared to TD [(average OR=1.75, 5th PCT=1.19); Table 2, Figure 1]. No one chemical showed strong evidence of contribution, such that Cd, Mo, Tl and U are possibly important (Figure 1D). Thus, it appears that four different individual elements each contribute only modestly, but together the impact is noticeable. Of these, U was significant in single element models [linear: OR=2.25 (1.17–4.35) 0.077 FDR-corrected p-value, quartile models: OR=1.30 (1.02–1.65) 0.084 FDR-corrected p-value], while Tl was only significant in quartile models [OR=1.32 (1.04–1.67) 0.084 FDR-corrected p-value].
Although the mixture effect was not significant for ASD, U was associated with increased odds of ASD compared to TD and was borderline significant after FDR correction in the linear model [linear: OR=1.96 (1.16–3.32) 0.060 FDR-corrected p-value, quartile models: OR=1.22 (1.03–1.45)]. There were no significant findings for the OEC group.
Mixtures:
A total mixture effect, combining all urinary chemicals (44 chemicals in total) was tested with each outcome discretely using random subset WQS with repeated holdout samples. The mixture effect was positively associated with ASD (average OR=1.84, 5th PCT=1.08), DD (average OR=3.44, 5th PCT=1.43), and OEC (average OR=2.20, 5th PCT=1.07) diagnoses (Table 2, Figure 3). Figure 4 displays chemical contributions per outcome and are classified by color based on the chemical class. DEP, MEPB, PRPB, ETPB, MCINP, and U are probably important contributors to the total mixture effect on ASD diagnosis (Table 2). MEPB, TCP246, DHB34, MCPP, MEHHP, and Tl are probably important contributors to the total mixture effect on DD diagnosis. DEP, MEPB, and MCIOP are probably important contributors to the total mixture effect on OEC diagnosis. These compounds all had indications of being important contributors, either because they were identified as contributors in the single class mixtures, or because they were significant in single chemical analysis. There were many other chemicals that were possible contributors.
Figure 3:

Visual representation of OR distributions from discrete full mixture rsWQS comparing odds of ASD, DD, and OEC to TD with positively constrained betas in estimating the weights, adjusted for covariates (sex, year of birth, homeowner status, child’s race, child’s age in months, and maternal metabolic conditions). The box represents 25th and 75th percentiles, the line represents the median, the closed diamond represents the mean, and the whiskers show the 5th and 95th percentiles.
Figure 4:
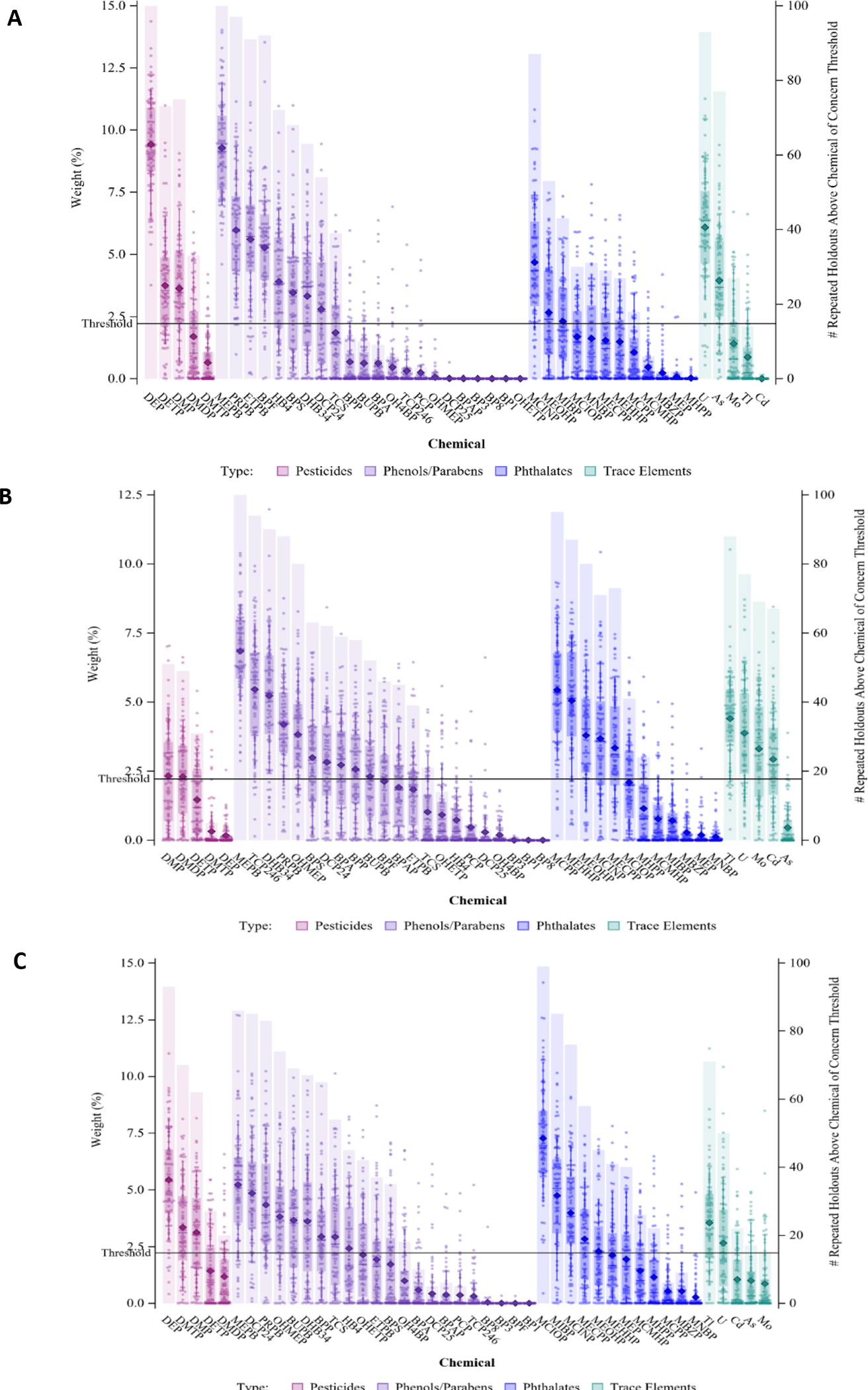
Weight distributions of each chemical from each 100 repetitions (left axis) and the number of repetitions in which the chemical exceeded the 1/c threshold contribution of concern (4.54%; right axis) for total mixture multinomial WQS models with more than 90% repititions above the null, where weights associated with ASD are shown in A, DD are shown in B, and OEC are shown in C.
Discussion
This mixture analysis demonstrates associations of phenols/parabens, pesticide metabolites, and trace metals as chemical classes associated with increased odds of ASD and/or DD. When all 44 individual chemicals were analyzed many of the same individual chemicals that were prominent in their contribution to their respective chemical class mixture’s association with outcome also appeared to be the probable important contributors to the total mixture associations, and were often significant in either the linear or quartile single chemical analysis. The same chemicals were often associated with all three outcomes, although the strength of the association often varied between the three outcomes. Still, this is indicative of the compounds impacting overall neurodevelopment, and not necessarily limited to one particular outcome. Findings were weaker for the OEC group, which is somewhat expected as this group was closer to the typically developing group.
The compound with the most consistent results was MEPB, with associations with both ASD and DD that were significant after FDR-correction for both linear and quartile models, and probable significance in both the phenol and overall mixture. ETPB, a structuraly similar compound, was also a probable contributer to ASD. Interestingly, these compounds were also noted as contributors to increased risk of either ASD or non-typical development as compared to those developing typically in a high risk ASD cohort (Barkoski, Busgang et al. 2019).
For other compounds, the evidence was not as clear. For example, neither the linear or quartile models reached statistical significance for BPF in association with ASD, yet this compound was a probable contributor in the class and a possible overall mixture. In this case, as well as most other cases, the OR was positive. The opposite example can also be found, for example, PRPB was significant in both the linear and quartile single chemical models in association with ASD, and was not even a probable contributor in the class mixture.
In interpreting these results, first and foremost is the fact that measurements were made after the child initially received the diagnoses, or in a rare number of instances, concurrently with the timing of the diagnosis. Additionally, urinary measurements of the organic compounds examined here represent recent exposures due to a half-lives on the order of days in the human body, and should ideally be accessed with multiple repeated urine samples (Hoppin et al. 2002, Barr et al. 2005, Perrier et al 2016,). For trace elements, the half-lives range from days to years (Barbosa et al. 2005). Therefore, the study does not provide evidence as to whether these chemicals contributed to the diagnoses of concern. Additionally, for some of these chemicals there can be considerable day-to-day variation in metabolite levels (Barkoski et al. 2018, Shin et al. 2019). The higher urinary levels may, however, indicate differences in recent external exposures. For example, if children with ASD, DD or OEC spend more time indoors, they may have higher exposures to certain chemicals used in household furnishings or products (phenols, parabens, phthalates). Similarly, food-borne chemicals such as pesticides in fruits or fruit juices or phthalates in food packaging could be higher in children with non-typical development if they consume more than TD children of certain foods. Also, some children with ASD or DD have other conditions as well and may be perscribed one or more medications, including medications to address behavioral concerns, and phthalates are used in enteric coatings of medications.
Confounders were selected for the analysis based on them being confounders for only one chemical (specifically MEPB). This combination was selected as it had one of the strongest associations in the uncorrected model. However, there could be other confounders that were important in other associations, but it was beyond the scope of this study to select a different set of confounders for each model. Therefore, there is the possibility of residual confounding due to unmeasured confounders.
Additionally, genetic differences by neurodevelopmental condition may result in differences in metabolism, distribution or excretion of certain chemicals. A number of studies have observed metabolomic differences between ASD and TD, suggesting that such pathways may explain some of the findings reported here (Orozco et al. 2019, Liu et al. 2019, Liang et al. 2020, Ming et al. 2012, and Glinton et al. 2019). Further work characterizing toxicokinetic differences by neurodevelopmental outcome would shed light on the mechanisms and directionality underlying the associations that emerged from our mixture analyses. As the children continue to mature, an additional future direction is to examine the chemicals measured in early childhood for potential influence on their long-term trajectories, which may diverge over time. Such analyses would provide appropriate temporality of measurements to support a known direction of potential causal interpretations.
Conclusions
Higher concentrations of urinary biomarkers increased the odds of ASD, DD, and OEC compared to TD for several compounds, with consistent results comparing single chemical analyses and mixture analyses. Findings were perticulalry consistent for MEPB for both ASD and DD, ETPB for ASD, and DEP for ASD. Biospecimens used for chemical analysis were collected many months after diagnoses were made the direction of any causal association is unknown.
Supplementary Material
Highlights.
Included 627 children between the ages of 2–5 from CHARGE, a case-control study.
-
Measured 44 environmental compounds and metabolites in urine.
Estimated risks of autism spectrum disorder (ASD) and developmental delay (DD).
Conducted a weighted quantile sum regression analysis and single chemical analyses.
Higher concentrations increased odds of ASD and DD compared to typical.
Acknowledgements
We would like to thank the CHARGE Study participants for making this research possible.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Number UG3OD023365, UH3OD023365 (Bennett, Schmidt, Hert-Picciotto, Schwizer), This research was also supported by NIEHS grants R01ES020392, R24ES028533, P30ES023513, P01ES11269, U2CES026555, U2CES026560, U.S EPA STAR grant 83543201, NICHD grant U54HD079125,; P50HD103526; U2CES026542-01 (Kannan, Parsons) and the UC Davis MIND Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Lab and epidemiological data are hosted at the HHEAR Data Center Repository (https://hheardatacenter.mssm.edu/) under the following DOI: 10.36043/1461_219 and 10.36043/1461_222.
List of abbreviations
- 3-PBA
3-phenoxy-benzoic acid
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS
Autism Diagnostic Observation Schedules
- As
Arsenic
- ASD
Autism Spectrum Disorder
- BP1
Benzophenone-1
- BP3
Benzophenone-3
- BP8
2,2’-dihydroxy-methoxybenzophenone
- BPA
Bisphenol A
- BPAP
4,4’-(1-phenylethylidene)bisphenol
- BPF
Bisphenol F
- BPP
4,4′-(1,4-Phenylenediisopropylidene) bisphenol
- BPS
Bisphenol S
- BUPB
Butyl Paraben
- Cd
Cadmium
- CHARGE
Childhood Autism Risks from Genetics and Environment
- DCP24
2,4-Dichlorophenol
- DD
Developmental Delay
- DEP
diethylphosphate
- DETP
diethylthiophosphate
- DHB34
3,4-Dihydroxy benzoic acid
- DMP
Dimethylphosphate
- DMTP
Dimethylthiophosphate
- ETPB
Ethyl paraben
- FDR
false discovery rate
- HB4
4-Hydroxybenzoic acid
- HHEAR
Human Health Exposure Analysis Resource
- LOD
Limit of Detection
- MCINP
mono-carboxy isononyl phthalate
- MCIOP
mono-carboxy isooctyl phthalate
- MCPP
mono (3-carboxypropyl) phthalate (multiple)
- MECPP
mono-(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono (2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono (2-ethyl-5-oxohexyl) phthalate
- MEPB
Methyl Paraben
- MHPP
mono-2-heptyl phthalate
- MIBP
mono-isobutyl phthalate (DiBP)
- Mo
Molybdenum
- MSEL
Mullen Scales of Early Learning
- OEC
Other Early Concerns
- OH4BP
4-hydroxybenzophenone
- OHETP
Protocatechuic acid ethyl ester
- OHMEP
3,4-Dihydroxy-benzoic acid methyl ester
- PCP
Pentachlorophenol
- PhMs
phthalate metabolites
- PRPB
Propyl paraben
- PT
proficiency testing
- rsWQS
random subset WQS
- SG
specific gravity
- SPE
solid-phase extraction
- TCP246
2,4,6-Trichlorophenol
- TCS
Triclosan
- TD
typically developing
- Tl
Thallium
- U
Uranium
- VABS
Vineland Adaptive Behaviors Scores
- WQS
weighted quantile sum
- WQSR
weighted quantile sum regression
Footnotes
Competing interests
Authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
The CHARGE study protocol and this study were approved by the institutional review boards for the State of California and the University of California-Davis (UC-Davis). Participants provided written informed consent before collection of any data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B and Meeker JD (2018). “Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study.” Environment international 113: 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulos AG, Thomaidis NS and Kannan K (2014). “Widespread occurrence of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters (parabens), benzophenone type-UV filters, triclosan, and triclocarban in human urine from Athens, Greece.” Sci Total Environ 470–471: 1243–1249. [DOI] [PubMed] [Google Scholar]
- Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons P, (2005) A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations and future needs, Environ Health Perspect, 113, pp. 1669–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoski JM, Busgang SA, Bixby M, Bennett D, Schmidt RJ, Barr DB, Panuwet P, Gennings C and Hertz-Picciotto I (2019). “Prenatal phenol and paraben exposures in relation to child neurodevelopment including autism spectrum disorders in the MARBLES study.” Environ Res 179(Pt A): 108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoski J, Bennett DH, Tancredi DJ, Boyd Barr D, Elms W, Hertz-Picciotto I. Variability of urinary pesticide metabolite concentrations during pregnancy in the MARBLES Study. Environmental Research. 2018; 165: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoski JM, Philippat C, Tancredi D, Schmidt RJ, Ozonoff S, Barr DB, Elms W, Bennett D and Hertz-Picciotto I (2020). “In utero pyrethroid pesticide exposure in relation to autism spectrum disorder (ASD) and other neurodevelopmental outcomes at 3 years in the MARBLES longitudinal cohort.” Environmental Research: 110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wang RY, Needham LL (2005) Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study, Environ Health Perspect, 113, pp. 1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC (2008). “Very low lead exposures and children’s neurodevelopment.” Current opinion in pediatrics 20(2): 172–177. [DOI] [PubMed] [Google Scholar]
- Berger K, Gunier RB, Chevrier J, Calafat AM, Ye X, Eskenazi B and Harley KG (2018). “Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels.” Environmental research 165: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Muckle G, Arbuckle T, Bouchard MF, Fraser WD, Ouellet E, Séguin JR, Oulhote Y, Webster GM and Lanphear BP (2017). “Associations of prenatal urinary bisphenol A concentrations with child behaviors and cognitive abilities.” Environmental health perspectives 125(6): 067008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC and Factor-Litvak P (2015). “Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting.” Journal of agricultural, biological, and environmental statistics 20(1): 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski T, Weuve J, Bellinger DC, Schwartz J, Lanphear B and Wright RO (2012). “Cadmium exposure and neurodevelopmental outcomes in US children.” Environmental health perspectives 120(5): 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin P, Kellogg J, Cech N and Gennings C (2019). “A random subset implementation of weighted quantile sum (WQSRS) regression for analysis of high-dimensional mixtures.” Communications in Statistics - Simulation and Computation: 1–16. [Google Scholar]
- Engel S, Patisaul H, Brody C, Hauser R, Zota A, Bennett D, Swanson M and Whyatt R (2021). “Neurotoxicity of ortho-phthalates: Recommendations for critical policy reforms to protect brain development in children.” American Journal of Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinton KE, Elsea SH (2019). “Untargeted Metabolomics for Autism Spectrum Disorders: Current Status and Future Directions.” Frontiers in Psychiatry, 10:647. DOI: 10.3389/fpsyt.2019.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Dalman C, Wicks S, Dal H, Magnusson C, Lundholm C, Almqvist C and Pershagen G (2017). “Perinatal exposure to traffic-related air pollution and autism spectrum disorders.” Environmental health perspectives 125(1): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL and Schmidt RJ (2018). “Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder.” Autism Research 11(1): 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM and Eskenazi B (2013). “Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children.” Environmental research 126: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ and Calafat AM (2004). “Temporal variability of urinary phthalate metabolite levels in men of reproductive age.” Environ Health Perspect 112(17): 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J and Pessah IN (2006). “The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism.” Environ Health Perspect 114(7): 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD (2002), Reproducibility of urinary phthalate metabolites in first morning urine samples, Environ. Health Perspect, 110, pp. 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, Lawler C, Davidson L, Daniels N and Newschaffer C (2016). “A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder.” PloS one 11(9): e0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Lord C and Rutter M (2003). Autism Diagnostic Interview - Revised (ADI-R),, Western Psychological Services. [Google Scholar]
- Li AJ, Banjabi AA, Takazawa M, Kumosani TA, Yousef JM and Kannan K (2020). “Serum concentrations of pesticides including organophosphates, pyrethroids and neonicotinoids in a population with osteoarthritis in Saudi Arabia.” Science of The Total Environment 737: 139706. [DOI] [PubMed] [Google Scholar]
- Li AJ, Martinez-Moral M-P, Al-Malki AL, Al-Ghamdi MA, Al-Bazi MM, Kumosani TA and Kannan K (2019). “Mediation analysis for the relationship between urinary phthalate metabolites and type 2 diabetes via oxidative stress in a population in Jeddah, Saudi Arabia.” Environment International 126: 153–161. [DOI] [PubMed] [Google Scholar]
- Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA and Kannan K (2018). “Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia.” Environ Res 166: 544–552. [DOI] [PubMed] [Google Scholar]
- Liang Y, Ke X, Xiao Z, Zhang Y, Chen Y, Li Y, Wang Z, Lin L, Yao P, & Lu J (2020). “Untargeted Metabolomic Profiling Using UHPLC-QTOF/MS Reveals Metabolic Alterations Associated with Autism.” BioMed Research International 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Zhou W, Qu L, He F, Wang H, Wang Y, Cai C, Li X, Zhou W, Wang M (2019). “Altered Urinary Amino Acids in Children With Autism Spectrum Disorders.” Frontiers in Cellular Neuroscience 13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M and Minshew N (1997). “Diagnosing autism: analyses of data from the Autism Diagnostic Interview.” J Autism Dev Disord 27(5): 501–517. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, Pickles A and Rutter M (2000). “The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism.” J Autism Dev Disord 30(3): 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M and Le Couteur A (1994). “Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders.” J Autism Dev Disord 24(5): 659–685. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee L-C, Harrington RA, Huston M, Hewitt A, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS and Dietz PM (2020) “Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016.” Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002) 69, 1–12 DOI: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Stein TP, Barnes V, Rhodes N, Guo L (2012). “Metabolic perturbance in autism spectrum disorders: a metabolomics study.” Journal of Proteome Research.11(12): 5856–62. [DOI] [PubMed] [Google Scholar]
- Minnich MG, Miller DC and Parsons PJ (2008). “Determination of As, Cd, Pb, and Hg in urine using inductively coupled plasma mass spectrometry with the direct injection high efficiency nebulizer.” Spectrochimica Acta Part B: Atomic Spectroscopy 63(3): 389–395. [Google Scholar]
- National Research Council (2000). Scientific Frontiers in Developmental Toxicology and Risk Assessment. Washington, DC, National Academies Press. [PubMed] [Google Scholar]
- Orozco JS, Hertz-Picciotto I, Abbeduto L, Slupsky CM (2019). “Metabolomics analysis of children with autism, idiopathic-developmental delays, and Down syndrome.” Translational Psychiatry 9(1):243-. DOI: 10.1038/s41398-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Lanphear B, Braun JM, Webster GM, Arbuckle TE, Etzel T, Forget-Dubois N, Seguin JR, Bouchard MF, MacFarlane A, Ouellet E, Fraser W and Muckle G (2020). “Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children.” Environ Health Perspect 128(2): 27004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, Hanley GE, Oberlander TF and Winters M (2019). “Association of prenatal exposure to air pollution with autism spectrum disorder.” JAMA pediatrics 173(1): 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, and Philippat C (2016). “Within-subject pooling of biological samples to reduce exposure misclassification in biomarker based studies” Epidemiology 27(3):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Barkoski J, Tancredi DJ, Elms B, Barr DB, Ozonoff S, Bennett DH and Hertz-Picciotto I (2018). “Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort.” International journal of hygiene and environmental health 221(3): 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R and Group EMCS (2017). “Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years.” Environmental health perspectives 125(9): 097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Levine H, Pinto O, Broday DM and Weisskopf MG (2018). “Traffic-related air pollution and autism spectrum disorder: a population-based nested case-control study in Israel.” American journal of epidemiology 187(4): 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH Jr., Leventhal BL and Pickles A (2006). “Combining information from multiple sources in the diagnosis of autism spectrum disorders.” J Am Acad Child Adolesc Psychiatry 45(9): 1094–1103. [DOI] [PubMed] [Google Scholar]
- Ritz B, Liew Z, Yan Q, Cui X, Virk J, Ketzel M and Raaschou-Nielsen O (2018). “Air pollution and autism in Denmark.” Environmental epidemiology (Philadelphia, Pa.) 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L and Wolff C (2007). “Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley.” Environmental health perspectives 115(10): 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Barbosa F Jr. and Kannan K (2017). “Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage.” Sci Total Environ 586: 152–162. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Honda M, da Costa NL, Barbosa RM, Barbosa F Jr. and Kannan K (2018). “Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage.” Environ Int 116: 269–277. [DOI] [PubMed] [Google Scholar]
- Ruth S and Zota A (2019). “Prenatal and Postnatal Mercury Exposure on Neurodevelopment: A Systematic Review of Human Evidence.”
- Rutter M, Bailey A, Berument SK, Le Couteur A, Lord C and Pickles A (2003). Social Communication Questionnaire (SCQ), Western Psychological Services, Los Angeles, CA. [Google Scholar]
- Sanders AP, Henn BC and Wright RO (2015). “Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature.” Current environmental health reports 2(3): 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F and Hertz-Picciotto I (2011). “Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism.” Epidemiology (Cambridge, Mass.) 22(4): 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Kogan V, Shelton JF, Delwiche L, Hansen RL, Ozonoff S, Ma CC, McCanlies EC, Bennett DH and Hertz-Picciotto I (2017). “Combined prenatal pesticide exposure and folic acid intake in relation to autism spectrum disorder.” Environmental health perspectives 125(9): 097007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tassone F and Hertz-Picciotto I (2012). “Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study.” The American journal of clinical nutrition 96(1): 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL and Hertz-Picciotto I (2014). “Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study.” Environmental health perspectives 122(10): 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Schmidt RJ, Tancredi D, Barkoski J, Ozonoff S, Bennett DH and Hertz-Picciotto I (2018). “Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study.” Environ Health 17(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi DJ, Hertz-Picciotto I (2019). Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environment International 122: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, Lie KK, Lipkin WI, Magnus P and Reichborn-Kjennerud T (2013). “Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children.” Jama 309(6): 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM, Bornehag CG and Gennings C (2019). “Repeated holdout validation for weighted quantile sum regression.” MethodsX 6: 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongkorn S, Kanlayaprasit S, Jindatip D, Tencomnao T, Hu VW and Sarachana T (2019). “Sex Differences in the Effects of Prenatal Bisphenol A Exposure on Genes Associated with Autism Spectrum Disorder in the Hippocampus.” Scientific Reports 9(1): 3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR and Allan AM (2014). “The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review.” Current environmental health reports 1(2): 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Salas RA, López-Carrillo L, Menezes-Filho JA, Rothenberg SJ, Cebrián ME, Schnaas L, Freitas de Souza Viana G and Torres-Sánchez L (2014). “Prenatal molybdenum exposure and infant neurodevelopment in Mexican children.” Nutritional neuroscience 17(2): 72–80. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Ling C, Cui X, Cockburn M, Park AS, Yu F, Wu J and Ritz B (2019). “Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study.” Bmj 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hossain F, Sulaiman R and Ren X (2019). “Exposure to inorganic arsenic and Lead and autism Spectrum disorder in children: a systematic review and meta-analysis.” Chemical research in toxicology 32(10): 1904–1919. [DOI] [PubMed] [Google Scholar]
- Wofford P, Segawa R, Schreider J, Federighi V, Neal R and Brattesani M (2014). “Community air monitoring for pesticides. Part 3: using health-based screening levels to evaluate results collected for a year.” Environ Monit Assess 186(3): 1355–1370. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen X-Z, Huang X, Wang M and Wu J (2019). “The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts.” Neurotoxicology 73: 199–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


