Abstract
The cranial base is formed by endochondral ossification and functions as a driver of anteroposterior cranial elongation and overall craniofacial growth. The cranial base contains the synchondroses that are composed of opposite-facing layers of resting, proliferating and hypertrophic chondrocytes with unique developmental origins, both in the neural crest and mesoderm. In humans, premature ossification of the synchondroses causes midfacial hypoplasia, which commonly presents in patients with syndromic craniosynostoses and skeletal Class III malocclusion. Major signaling pathways and transcription factors that regulate the long bone growth plate—PTHrP–Ihh, FGF, Wnt, BMP signaling and Runx2—are also involved in the cranial base synchondrosis. Here, we provide an updated overview of the cranial base synchondrosis and the cell population within, as well as its molecular regulation, and further discuss future research opportunities to understand the unique function of this craniofacial skeletal structure.
Keywords: cranial base, synchondrosis, chondrocyte(s), osteoblast, endochondral ossification, craniofacial development, regeneration, skeleton
1. Introduction
The skull is an important bone structure for human survival—it protects the central nervous system, forms part of the respiratory airway, and enables mastication. Craniofacial skeletal tissues develop, grow, and maintain their functionality throughout life through a series of highly coordinated molecular processes that regulate relevant cell populations. Of the craniofacial bone structures, the cranial base serves as an important component of the skull, containing the cartilaginous synchondroses with autonomous growth potential development. What was previously thought to serve as simply a structural platform for the central nervous system, the cranial base is now regarded as an important autonomous growth center of the craniofacial complex [1]. Unlike the extensively characterized growth plate in long bones [2], little is known about the molecular regulation of the cranial base and its cartilaginous growth center synchondroses. Here, we provide an overview of the unique morphology of the cranial base synchondrosis and the cell population within, as well as its molecular regulation, and discuss future research opportunities to understand the unique function of this craniofacial skeletal structure.
2. Overview of the Cranial Base Morphology
2.1. Developmental Origins of the Chondrocranium
The development origins of the chondrocranium—the cranial base and its cartilaginous synchondroses—are distinct from those of other endochondral bones in the axial and appendicular skeleton. In mice, neural crest-derived cells migrate ventrally under the forebrain and populate the presumptive cranial base rostral to Rathke’s pouch at E10.5 [3]. These neural crest cells contribute to the most anterior portion of the cranial base, while the more posterior portion of the cranial base is derived from the mesoderm, with the exception of hypochiasmatic cartilages in the most anterior position of the cranial base that are derived from the cephalic paraxial mesoderm [4]. Importantly, paraxial mesoderm remains the principal contributor to both the neurocranium and chondrocranium. Somitomeres, which differentiate from paraxial mesoderm during early embryonic development, undergo divergent morphogenesis to contribute to approximately 80% of the head mesenchyme in mice [5]. These structures, representing the cellular precursors to somites, are critically important for forming the segmental pattern of the cranial mesoderm of later stage embryos. Somitomeres in the cranial region undergo morphogenesis in unison with neural plate development and neuromeric morphogenesis, eventually differentiating into somites [6]. Aberrant somitomere formation may lead to craniofacial skeletal abnormalities.
2.2. Development and Growth of the Cranial Base and Its Synchondroses
The neurocranium of the skull contains structures enclosing the central nervous system and its supporting vasculature. These structures include the cranial base (formed by endochondral ossification) and the cranial vault (formed by intramembranous ossification). In mice, the cranial base is composed of several segmented bones including ethmoid, pre-sphenoid, basisphenoid and basioccipital bones. Like the long bones, the cranial base first appears as a sheet of undifferentiated mesenchymal cells in the form of a cartilaginous anlage in early skeletal development. Over time, these cells undergo chondrogenesis to form the chondrocranium, which is a solid cartilaginous structure spanning the entire length of the skull formed by the fusion of several individual cartilages that appear at distinct locations. The cartilages of the chondrocranium then undergo endochondral ossification to form the bones of the cranial base [3]. In humans, the chondrocranium is formed between the 6th–8th week of gestation [7]. The chondrocranium rapidly grows between the 11th–23rd week of gestation, elongating the anterior cranial base antero-posteriorly and enlarging the posterior cranial base transversely [8]. Importantly, in primates, the cranial base flexion directly correlates with brain growth [9]. Thus, there is an intimate relationship between the central nervous system and the chondrocranium.
Separating the bones of the cranial base are the synchondroses, cartilaginous tissues that drive anterior–posterior growth of the skull. Positioned between the pre-sphenoid and basisphenoid bones is the inter-sphenoid synchondrosis (ISS), whereas the more posterior positioned spheno-occipital synchondrosis (SOS) lies between the basisphenoid and basioccipital bones. Similar to the long bone growth plate, the synchondroses are composed of distinct layers of round, proliferating and hypertrophic chondrocytes [10]. However, unlike the unidirectional organization of chondrocytes observed in long bones [11], the synchondroses are bidirectionally organized in a mirror image manner sharing a central round layer (Figure 1). The anisotropic growth mechanism used by these chondrocytes to facilitate anteroposterior growth is not currently understood [12]. The cranial base synchondrosis is composed of a combination of hyaline and fibrous cartilages [13]. During skeletal maturation, chondrocytes proliferate, differentiate into pre-hypertrophic chondrocytes and are eventually replaced by bones at the chondro-osseous junction. Although not examined in the synchondroses, there is evidence in growth plates that chondrocytes can undergo ‘trans-differentiation’ to form osteoblasts [14].
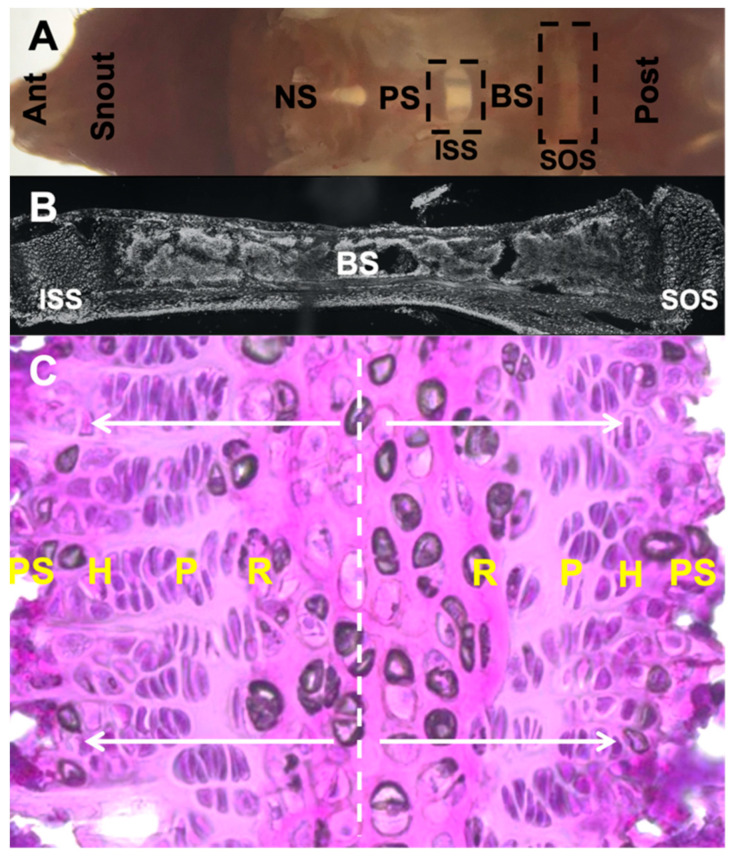
Figure 1.
Morphology of cranial base and SOS harvested at postnatal day 28 from C57BL/6 mouse. (A). Gross morphology of dissected cranial base positioned in an anteroposterior manner. (B). Sagittal section of ISS, basi-sphenoid bone and SOS. (C). Magnified image highlighting bidirectional arrangement of chondrocyte layers in SOS stained with hematoxylin and eosin and presence of presumptive central hypertrophic chondrocytes. Ant: anterior, NS: nasal septum, PS: pre-sphenoid, BS: basi-sphenoid, Post: posterior, ISS: inter-sphenoid synchondrosis, SOS: spheno-occipital synchondrosis, R: resting zone, P: proliferating zone, H: hypertrophic zone, PS: primary spongiosa.
Significantly, in humans, the ISS ossifies at 2–3 years of age, whereas the SOS ossifies much later between 16–18 years in humans [15,16]. While 60% of cranial base growth occurs embryonically, 40% of additional growth occurs postnatally, extending well into adolescence [17]. This emphasizes the importance of the SOS as a potential target for pharmacological intervention in patients with prematurely ossified synchondroses. The anterior positioned ISS is neural crest-derived, while the SOS is comprised of both neural crest cells and paraxial mesoderm in mice [3]. These differences in embryonic origins of the ISS and SOS may contribute to their differential ossification rates and roles in facilitating the elongation of the cranial base. Unlike humans, the synchondroses of mice remain patent throughout adulthood. The basis for these interspecies differences has not been fully elucidated.
2.3. Craniofacial Anomalies Associated with Cranial Base Malformation
Growth of the postnatal cranial base guides overall anteroposterior elongation of the craniofacial complex. Defective cranial base growth in humans leads to midfacial hypoplasia [18], skeletal Class III malocclusion [19], and various forms of syndromic craniosynostoses [20,21]. These patients have significant challenges associated with breathing, speaking, and chewing and often feel socially stigmatized. Invasive surgical intervention, such as Le Fort III osteotomy, is currently the primary treatment option to correct these structural deficiencies.
Malformations of the cranial base are commonly found in craniofacial and skeletal genetic anomalies, including cleidocranial dysplasia and achondroplasia. In cleidocranial dysplasia, which is caused by heterozygous loss-of-function mutations in RUNX2, patients present with delayed ossification of the cranial base, leading to midfacial hypoplasia and ‘dish-face like’ appearance, in addition to presenting generalized skeletal malformations [22]. Achondroplasia is associated with premature fusion of the long bone growth plates in addition to premature fusion of the SOS, leading to shortening of the posterior cranial base and midfacial hypoplasia [23]. Additionally, patients presenting with skeletal Class III malocclusion often have significant midfacial hypoplasia, due to precocious ossification of the SOS [24]. In more recent years, several studies have established a relationship between premature ossification rates of the ISS and SOS and various forms of syndromic craniofacial deformities, such as Apert, Pfeiffer and Crouzon [20,21,25], which often present with craniosynostosis. In these studies, closure rates of the synchondroses were assessed radiographically by measuring of ‘open’, ‘partial’ and ‘closed’ synchondroses. The authors determined that the ossification rates of the synchondroses in affected patients were accelerated compared to healthy individuals. These results are potentially important, as there is still debate in the field as to whether the premature fusion of the cranial suture is secondary to the fusion of the synchondrosis fusion or vice versa [26]. Lastly, assessment of cranial base synchondrosis closure rates in patients with Down and Klinefelter syndromes also show premature fusion of the synchondroses, resulting in shortened cranial base length and midfacial hypoplasia [27,28,29]. Collectively, the activity and differentiation trajectories of cranial base chondrocytes, coupled with the fusion rates of the synchondroses, are critical factors in determining anteroposterior elongation of the skull, both in humans and other vertebrate species. Importantly, coupled with the fusion rates of the cranial vault sutures, the ossification rates of the cranial base synchondroses represent unique physiological phenomena that are required for proper organization of the entire craniofacial complex.
3. Molecular Regulation of the Cranial Base
To date, limited numbers of studies have directly assessed the molecular regulation of cranial base development. This may be due in part to an underlying assumption that the synchondroses are structurally similar to the long bone growth plate, therefore controlled by the same developmental signals regulating the endochondral pathway. However, the synchondrosis differs from the growth plate in several key aspects. First, unlike long bone growth plates that undergo unidirectional growth, cranial base synchondroses grow in a bidirectional manner with a mirror-image arrangement of the proliferating layers. Second, synchondroses lack an overlying articular synovial layer, which is critical for long bones to withstand mechanical loads. The cranial base is not subjected to direct mechanical loading, due to its proximity to the central nervous system. Third, the cranial base lacks a secondary ossification center (SOC). This may be a critical difference governing behavior, as development of the SOC has been associated with formation of a resident stem cell niche within the resting zone of the postnatal epiphysis [30,31,32]. Presence of a similar microenvironment has not yet been detected in the cranial base. Thus, growth plates and synchondroses may not necessarily share the same molecular regulations. In this review we will compare what is known about the regulation of these two structures with the goal of highlighting areas for future research.
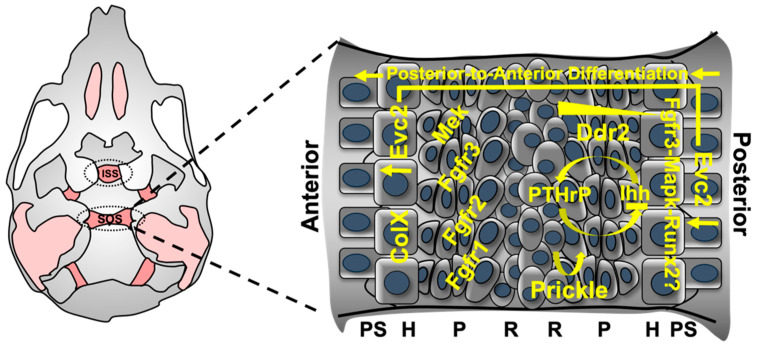
The following molecular regulators are discussed in this review to highlight their contribution to cranial base development: parathyroid hormone–related protein (PTHrP), Indian hedgehog (Ihh) and EvC ciliary complex subunit 1/2 (Evc1/2, encoding Limbin), fibroblast growth factor receptors 1/2/3 (FGFR1/2/3), discoidin domain receptors 1/2 (DDR1/2), Wnt/β-catenin and runt-related transcription factor 2 (Runx2) (Figure 2). This article will complement previous reviews on cranial base development [10,33,34,35].
Figure 2.
Molecular regulation of the postnatal cranial base synchondrosis. PTHrP-Ihh feedback regulates activity of proliferating chondrocytes until their differentiation into pre-hypertrophic cells. Prickle, a component of the Wnt/PCP pathway, directs asymmetric division of resting cells into adjacent columnar chondrocytes. Fgfr1, Fgfr2 and Fgfr3 expression in proliferating chondrocytes directly controls cranial base elongation. Activating mutations in each receptor are associated with various forms of syndromic craniosynostoses, which commonly present midfacial hypoplasia. Evc2 expression is localized in a gradient-like fashion, wherein which levels increase in the anterior most portions of the SOS and ISS are indirectly regulated through Hedgehog signaling. This relationship instructs posterior-to-anterior differentiation and eventual mineralization of cranial base chondrocytes in addition to formation of the segmented cranial base bones. Ddr2 expression is found in a gradient fashion, with highest levels localized to resting cells. Currently established in the long bone growth plate, Fgfr3-Mapk-Runx2 signaling is a critical component regulating both chondrocyte proliferation and hypertrophy. Whether this relationship exists in the cranial base synchondrosis remains to be elucidated. ISS: inter-sphenoid synchondrosis, SOS: spheno-occipital synchondrosis, R: resting zone, P: proliferating zone, H: hypertrophic zone, PS: primary spongiosa.
3.1. Role of Parathyroid Hormone Related Protein in Synchondrosis Organization
PTHrP was initially discovered and cloned in 1987 as a factor in the serum of patients with humoral hypercalcemia of malignancy, a skeletal disease associated with bone metastatic cancers [36]. In long bones, a PTHrP-Ihh negative feedback loops are essential for maintaining the mitotic capability of proliferating chondrocytes and initiating the differentiation of these cells into pre-hypertrophic zone chondrocytes [11,37,38]. Pthrp is also expressed in resting and proliferating zone chondrocytes in mouse synchondroses [39]. Further, it may have direct functions in this tissue since PTHrP-deficient mice display cranial base abnormalities [40,41]. Specifically, in PTHrP-deficient newborns, chondrocytes in the ISS undergo premature hypertrophy in all layers. Hypertrophy in the SOS of these mice is even more extensive with loss of resting and proliferative layers and only a small hypertrophic chondrocyte layer remaining within the central part of the SOS, surrounded by thick layers of bone-like matrix on both sides. Thus, PTHrP-deficient mice have defective synchondroses, with the SOS undergoing more rapid precocious ossification. This accelerated hypertrophy/ossification of synchondroses is similar to what is seen in long bone growth plates of Pthrp-deficient mice [41]. Together, these studies suggest that PTHrP may have similar functions in the synchondroses and long bone growth plate, although further studies will be required to confirm this.
Recently, a subset of resting zone chondrocytes expressing Pthrp were identified in long bone growth plates where they function as skeletal stem cells [31], giving rise to columnar chondrocytes in the proliferating zone, hypertrophic chondrocytes as well as osteoblasts and bone marrow stromal cells in the metaphysis. These cells acquire their ‘stem-like’ qualities following the formation of the SOC. Thus, the SOC may have the ability to instruct cells within the resting zone to acquire self-renewal and multi-lineage differentiation potential. Importantly, the cranial base lacks a SOC and, thus, may not possess similar stem cell-inductive capacity. However, the exact relationship between the SOC and this growth plate stem cell population remains to be elucidated. In our recent study, we examined the presence of a Pthrp-expressing stem cell population in synchondroses [42]. Surprisingly, Pthrp expressing cells were found predominantly within a wedge-shaped structure on the lateral borders of the SOS. PTHrP+ cells were not detected in the central resting zone chondrocyte layers of the SOS at any of the early postnatal time points examined. This is in marked contrast to the long bone growth plate where Pthrp-expressing cells populate the entire central resting zone. Moreover, lineage-tracing experiments, using a tamoxifen-inducible Pthrp-creER line combined with a tdTomato reporter allele, revealed that PTHrP+ chondrocytes sparsely occupied the SOS and their progeny failed to establish columnar chondrocytes, whereas in the femur growth plate, these cells robustly labeled the resting zone and differentiated into proliferating and hypertrophic chondrocytes. These recent findings highlight more passive roles of PTHrP+ chondrocytes in the postnatal cranial base synchondroses. Importantly, PTHrP+ chondrocytes in the cranial base synchondrosis lack the innate capacity to acquire stem cell properties, potentially due to the absence of instructive signals from the SOC.
3.2. Indian Hedgehog Is a Critical Regulator of Cranial Base Development
The Hedgehog family of proteins contains three separate members: sonic hedgehog (Shh), desert hedgehog (Dhh) and Indian hedgehog (Ihh). In the long bone growth plate, Ihh is expressed by pre-hypertrophic chondrocytes where it facilitates the onset of chondrocyte hypertrophy. Concurrently, Ihh promotes Pthrp expression from chondrocytes in the round cell layer, which in turn inhibits Ihh expression from pre-hypertrophic chondrocytes, establishing a negative feedback loop with PTHrP [38]. Ihh also induces osteoblast differentiation from progenitor cells in the adjacent perichondrium in conjunction with BMPs [43].
The synchondroses of Ihh-deficient mice display significant abnormalities in early skeletal development. More specifically, the SOS and ISS are disorganized and chondrocyte proliferation is markedly suppressed. Pthrp expression is downregulated, and chondrocyte maturation is initially delayed, but is subsequently accelerated, as exemplified by the presence of type X collagen-positive chondrocytes within the SOS [39,44]. This suggests that Ihh may directly or indirectly dictate the topographical distribution of chondrocytes in the synchondroses and growth plate. Consequently, the overall anteroposterior elongation of Ihh-deficient mice is markedly decreased compared to wild type littermates. Interestingly, in one study [45], the authors highlight the importance of the overlying perichondrium in establishing the polarity of adjacent chondrocyte zones through the sequestration of extracellular matrix genes [45,46,47]. The authors also postulate that loss of Ihh leads to aberrant function of primary cilia. This is of particular importance, as primary cilia have been shown to regulate chondrocyte polarity [48] and are the sites of Ihh signaling [49].
3.3. Primary Cilia EVC/EVC2 as a Regulator of Cranial Base through Hedgehog Modulation
There is now considerable evidence that primary cilia have important functions in organizing the cranial base synchondroses. Evc/Evc2 (encoding Limbin protein) are N-terminal anchored transmembrane proteins that exist as a complex within the primary cilia where they bind Smoothened, a key Hedgehog signaling effector protein [50,51]. Pathogenic mutations in Evc/Evc2 (Limbin) in humans cause Ellis-van Creveld syndrome, a disorder associated with a wide range of craniofacial abnormalities, including an enlarged skull, skeletal Class III skeletal malocclusion, skeletal open bite and midfacial hypoplasia [52]. Evc2 expression is found in nearly all layers of the synchondroses [53]. Further, global knockout of Evc2, as well as neural crest cell-specific conditional knockouts (P0-cre; Evc2fl/fl and Wnt1-cre; Evc2fl/fl) in mice display midface hypoplasia, resulting from the anterior shortening of the cranial base due to premature fusion of the ISS [54,55]. Moreover, cephalometric analysis of Evc2-deficient mice shows significant reductions in overall cranial base length as well as lengths of all segmented cranial base bones [55,56]. These phenotypic observations were also observed in a patient with Ellis-van Creveld syndrome, appearing as pronounced Class III malocclusion [57]. Thus, the primary cilia proteins, EVC/EVC2, play prominent roles in dictating proper cranial base growth and anteroposterior elongation of the midface in both humans and mice.
In a more recent study, the cranial base phenotype of Evc2-deficient mice was described in greater detail [58]. Two key findings were reported: first, chondrocytes in the ISS all became hypertrophic as early as E18.5 in Evc2-deficient mice, whereas a majority of the chondrocytes in the SOS remained undifferentiated. Second, as a consequence of premature ISS hypertrophy, the anterior cranial base was more severely shortened in Evc2-deficient mice after E18.5, while the length of the posterior part of the skull floor (controlled by the SOS) was less affected. Thus, targeted Evc2 deletion more profoundly affects cells of neural crest origin [3]. Given the function of Evc2 in Hedgehog signaling, the authors postulate that premature fusion of the ISS is due to a decreased gradient of Ihh expression, which preferentially affects the anterior portion of the ISS relative to the posterior ISS and SOS. Further, resting chondrocytes in Evc2-deficient mice undergo premature differentiation into pre-hypertrophic chondrocytes, leading to precocious ossification. The authors hypothesize that during embryonic development, chondrocytes located at the most posterior end of the cranial base cartilage primordia begin differentiating to form the hypertrophic zone, due to an activity of the future basi-occipital bone. Hypertrophic chondrocytes in this zone induce differentiation of chondrocytes at the middle of the cranial base cartilage primordia to form the second hypertrophic zone, which is the future basi-sphenoid bone, and, finally, hypertrophy of the chondrocyte zone for the future pre-sphenoid bone occurs at the anterior end of the braincase floor cartilage primordia. These hypertrophic zones mineralize from posterior to anterior as embryogenesis progresses, and the remaining cartilage will form the intervening ISS and SOS. Evc2-deficiency therefore causes aberrant formation of the SOS hypertrophic zone, which is subsequently amplified in the ISS, leading to reduced Ihh expression and precocious ossification. Future analyses should seek to examine the molecular regulation controlling the differential ossification rates of the ISS versus SOS, particularly in the context of the regulation of Ihh activation through Evc2 signaling. This is particularly important, as precocious ossification of the anterior portion of the cranial base manifests in several syndromic craniosynostoses, such as Apert, Pfeiffer and Crouzon.
3.4. Fibroblast Growth Factor Receptor 3 Regulates Proliferation of Synchondrosis Chondrocytes
Evc2 and Ihh signaling functions to regulate the growth and fusion of the SOS and ISS; however, there are additional pathways that mediate this process. For example, in long bones, FGFR3 is recognized as a target of Ihh signaling that negatively regulates Ihh signaling and inhibits chondrocyte hypertrophy [59]. Therefore, Ihh-Fgfr3 signaling is critical in the context of growth plate development. Yet, whether a similar relationship exists in the synchondroses is currently unknown.
The mammalian fibroblast growth factor (FGF) family contains eighteen secreted proteins that interact with four tyrosine kinase FGF receptors (FGFRs), performing important functions in embryonic and postnatal development by maintaining progenitor cells and their proliferation [60]. During early skeletal development, FGFs regulate chondrogenesis, osteogenesis, and bone mineral homeostasis [61]. Of the FGF ligands, FGF18 regulates cell proliferation and differentiation during chondrogenesis and osteogenesis [62]. Fgf18-deficient mice display shortened long bones and reductions in anteroposterior elongation of the skull, associated with reduced mineralization of possibly aberrant postnatal growth of the cranial base synchondroses. Several other FGF ligands also have significant roles in skeleto-genesis. Ectopic expression of Fgf2 causes enlarged occipital bones, skeletal dwarfism and coronal suture synostosis in mice [63]. Conversely, Fgf2-deficient mice display premature closure of the growth plate [64]. Targeted overexpression of Fgf9 in cartilage causes achondroplasia-like phenotypes in mice, characterized by premature closure of the growth plate, associated with significant reduction in the anteroposterior dimension of the skull [65]. Importantly, FGF9 has high affinity for its receptor, FGFR3, and, as discussed later in this review, over-activating mutations in FGFR3 in humans and mice cause achondroplasia and midfacial hypoplasia. Lastly, overexpression of Fgf3/4 in mice causes a Crouzon syndrome-like phenotype, characterized by premature fusion of the cranial vault sutures and reduced anteroposterior dimension of the skull [66]. The precise roles of the FGF ligands in regulating the cranial base synchondrosis need to be investigated in future studies.
Additionally, in the growth plate, FGFRs 1/2/3 play critical roles in maintaining chondrocyte proliferation and differentiation. For example, FGFR3 signaling diverges to regulate either chondrocyte proliferation via STAT1-p21 [67,68] or hypertrophy via MEK-MAPK pathways [69]. Both signaling cascades are mediated in part by Snail1, which is induced by FGFR3 and plays a role in the manifestation of FGFR-related skeletal dysmorphisms [70]. It is not known if similar signaling cascades exist in the cranial base. Nevertheless, disruption of FGF signaling in a variety of genetic disorders has profound effects on craniofacial development, as is described below.
Of the FGF receptors, FGFR3 mutations have the most profound impact on the skeleton. An activating point mutation in FGFR3 (FGFR3G308R) is associated with the most common genetic form of human dwarfism, achondroplasia [71]. Patients present with shortened long bones resulting from premature fusion of the growth plates, due to overactive FGF signaling [72]. In addition, Fgfr3G308R patients show craniofacial phenotypes including a shorter anteroposterior cranial base, due to premature fusion of the synchondroses leading to midfacial hypoplasia. Conversely, conditional deletion of Fgfr3 in chondrocytes (Col2a1-cre; Fgfr3fl/fl) causes aberrant upregulation of cell proliferation in the growth plate, leading to the formation of cartilaginous tumors, including osteochondromas and enchondromas [73]. Col2a1-cre; Fgfr3fl/fl mice displayed slightly elongated skulls. However, the authors did not examine the cranial base in this study. In addition, overactive FGF signaling due to FGFR3K650E gain of function mutations, causes the most common embryonically lethal skeletal dysplasia, thanatophoric dysplasia type II (TDII) [74,75]. TDII fetuses have shortened limbs, straight femurs and cloverleaf-shaped skulls leading to a midfacial hypoplastic phenotype [76]. Although not clinically observed, due to the early lethality of the mutation, these craniofacial manifestations are likely due to congenital malformation of the cranial base synchondroses.
Premature fusion of the synchondroses was shown in a patient with homozygous achondroplasia mutations [77]. In the same study, the authors showed that a mouse model harboring an activating mutation in FGFR3 (e.g., Fgfr3G374R) [78], displayed similar premature fusion of the ISS and SOS [77]. The authors showed that constitutive activation of the downstream Fgfr3 effector, MEK1, in chondrocytes (Col2a1-cre; MEKS218/222E, Δ32-51) [79] caused premature fusion of the synchondroses, recapitulating the phenotype observed in FGFR3 gain-of-function mice. Additional FGFR3 gain-of-function mouse models that display premature ossification of the synchondroses include Fgfr3369/369 [80], Fgfr3P244R [81] and Fgfr3365/+ [82]. Interestingly, over-activation of FGFR3 in several of these mutants results in decreased expression of Ihh in the pre-hypertrophic zone and Pthrp in the resting zone of the synchondroses. Disruption of PTHrP-Ihh feedback may be an etiology of the premature ossification phenotype observed in the synchondroses of these Fgfr3 mutant mice.
FGFR1 and 2 also exert significant roles in regulating postnatal cranial base growth and ossification. For example, FGFR1-activating mutations cause Pfeiffer syndrome [83]. This genetic condition is linked to a pericentromic region of chromosome 10 in relation to FGFR1 [84]. Patients with Pfeiffer syndrome commonly present with abnormal fusion of the synchondroses leading to bulging and wide-set eyes, high and prominent forehead and underdeveloped upper jaw leading to midfacial hypoplasia [85]. In a recent study assessing single nucleotide polymorphisms (SNP) in a large patient cohort, two SNPs found in FGFR1 were correlated with overall head size and midfacial development [85]. The authors discovered that modified FGFR1 alleles harboring SNPs had a small midface and hypertelorism, which are hallmark craniofacial features of Pfeiffer Syndrome. Thus, although FGFR1’s role in craniofacial development is largely unknown, more recent observations point to its role in the cranial base synchondrosis.
Apert syndrome is one of the most common syndromic craniosynostoses [86]. Apert syndrome is caused by activating mutations in FGFR 2 (S252W or P253R) and clinically manifests as premature fusion of the cranial vault sutures and cranial base synchondroses, leading to midfacial hypoplasia and frontal bossing [87]. Fgfr2P253R mice display abnormalities in both osteogenesis and chondrogenesis, including premature closure of the coronal suture and delayed growth of the growth plate and cranial base synchondroses leading to shortened midfaces [88]. Blockade of downstream FGFR2 signaling via in vitro inhibition of Erk1/2 with PD98059 partially rescued the calvarial and long bone defects in Fgfr2P253R mice. More specifically, in the growth plate, treatment caused the partial elongation of the proliferating, hypertrophic and calcifying zones. However, the cranial base synchondroses were not evaluated. In an additional study, Fgfr2P253R mice displayed premature fusion of the SOS and ISS due to accelerated differentiation of proliferating and hypertrophic chondrocytes, resulting in midfacial hypoplasia [89]. FGFR2S252W mutations in humans and mice have also been associated with Apert syndrome [90,91]. Similar to the Fgfr2P253R mutant phenotype, patients harboring the FGFR2S252W mutation present several craniofacial phenotypes, including hypertelorism, frontal bossing and midfacial hypoplasia [90]. In Apert patients harboring this mutation, the rate at which the SOS fuses is accelerated by 1–3 years when compared to unaffected individuals [92]. Fgfr2S252W mutant mice present a similar midfacial hypoplasia phenotype as humans, in addition to long bone defects due to delayed formation of the SOC [91]. Although the authors did not assess the cranial base, these animals present with significant shortening of all growth plate zones. Thus, the midfacial hypoplastic phenotype observed in Fgfr2 mutant mice may be the result of aberrant development of the cranial base synchondroses. In an additional study, mesoderm-specific or neural crest-specific over-activation of FGFR2 signaling (Mesp1-cre; Fgfr2S252W or Wnt1-cre; Fgfr2S252W) in mice causes midfacial shortening and significant curvature of the cranial base [93]. In summary, FGFR2 over-activation is a significant contributor to the etiology of Apert syndrome. How it directly impacts on the cranial base synchondrosis requires further investigation.
Methods to reverse the pathogenicity of activating mutant FGFRs are in development [94]. Currently, the only treatment modality available for patients is multiple rounds of surgery. Efforts to revert the pathogenicity of overactive FGFR3 using FGFR3 inhibitors, statins, meclozine and C-type natriuretic peptide are also being explored. In a recent study, Fgfr3G380R mice treated with Recifercept, a soluble FGFR3 protein inhibitor that mimics the extracellular domain of FGFR3, and, thus, competes with FGFR3 for ligand binding, resulted in the stabilization of endogenous downstream signaling of the protein [95]. Fgfr3G30R mice treated with Recifercept restored normal body weight and prevented premature ossification of the SOS. In these animals, skull length, length/width skull ratio and foramen magnum area were all improved after 3-week treatment with Recifercept [96]. In another study, statins were found to ameliorate aberrant chondrocyte differentiation in a human induced pluripotent stem (iPS) cell model of thanatophoric dysplasia type I, characterized by overactivated FGFR3 signaling [97]. Rosuvastatin stimulates bone elongation in Fgfr3G380R mice, associated with increased expression of Sox9, Col2a1, Acan, Runx2 and Col10a1, and a rescue of the achondroplasia phenotype. Furthermore, meclozine, an anti-histamine used to treat motion sickness, inhibits FGFR3 activity in chondrocyte cell lines and in a mouse model of achondroplasia, where it rescued the skeletal defects [98,99,100]. Lastly, C-type natriuretic peptide inhibits aberrantly activated FGFR3-mediated MAPK signaling in achondroplasia, thereby restoring longitudinal growth of endochondral bones. Further, overexpression of Nppc, which regulates C-type natriuretic peptide production, ameliorates the skeletal and craniofacial abnormalities associated with the achondroplasia phenotype in mice [101,102]. Thus, approaches aimed at reversing the pathogenic effects of FGFR3 overactivation and other FGFRs appear to be effective.
3.5. Discoidin Domain Receptors Play Critical Roles in the Organization and Maintenance of Cranial Base Synchondrosis Chondrocytes
The discoidin domain receptors (DDRs) are a class of receptor tyrosine kinases (RTKs) that, together with β1 integrins, mediate interactions between cells and the extracellular matrix by specifically binding triple helical collagens [103,104]. As described below, disruption of DDR signaling can have profound effects on cranial base function.
DDR1 was identified in a screen for tyrosine kinase proteins expressed in human malignancies, but has more recently been shown to play roles in mediating tumor progression of soft tissue carcinomas [105,106,107]. In long bones, conditional ablation of DDR1 (Col2a1-cre; Ddr1fl/fl) in mice causes hind limb abnormalities, including delayed formation of the SOC, decreased body length and weight, decreased chondrocyte proliferation and apoptosis and delayed chondrocyte hypertrophy [108]. Although the skulls of these animals were not analyzed, the anteroposterior length of the skull appeared to be reduced, potentially due to aberrant development of the cranial base synchondroses. Thus, although there is only limited evidence available, DDR1 may play an important role in cranial base development; thus, further studies are needed to examine its putative role in the synchondroses.
DDR2 mutations in humans cause spondylo-meta-epiphyseal dysplasia (SMED), classified by shortened limbs, abnormal calcifications and craniofacial phenotypes including prominent forehead, open fontanelles, hypertelorism and midfacial hypoplasia [109,110,111,112]. In mice, Ddr2 is expressed in a gradient fashion in the long bone growth plate, with highest levels in the resting zone and lowest in the hypertrophic zone [113]. In the cranial base synchondroses, a similar expression gradient is observed [114]. Lineage tracing using a tamoxifen-inducible Ddr2-creER line showed that Ddr2-labeled cells are present in the cranial sutures and the resting and proliferating zones of the ISS and SOS, which could then differentiate into hypertrophic chondrocytes, osteoblasts and osteocytes, supporting the concept that Ddr2 is expressed by skeletal progenitors. Ddr2-deficient mice display craniofacial abnormalities, including significant reductions in the lengths of the anterior and posterior cranial base and reduced mineralization of the frontal suture. Defects in cranial base length were related to reduced proliferation in both ISS and SOS chondrocytes in the absence of altered apoptosis or reduced osteoblast differentiation [114]. Interestingly, the reduced cranial base growth was associated with widening of synchondroses, loss of chondrocyte polarity and abnormal distribution of ColX and Col2, suggesting a role for Ddr2 in regulating fibrillogenesis and extracellular matrix distribution.
In the same study, the authors observed partial colocalization of Ddr2 with the putative skeletal stem cell marker, Gli1 [115]. Conditional deletion of Ddr2 in Gli1+ skeletal progenitors (Gli1-creER; Ddr2fl/fl) caused a similar cranial base phenotype to that seen in globally deficient mice, including widening of the ISS and SOS and significant reductions in the lengths of the segmented cranial base bones, leading to midfacial hypoplasia. Additionally, conditional ablation of Ddr2 in chondrocytes (Col2a1-creER; Ddr2fl/fl) caused a similar cranial base phenotype as observed in the other two mutants. The authors postulate that reductions in cranial base endochondral growth underlying midfacial hypoplasia are likely due to the following: (1) inhibition of resting-to-proliferating chondrocyte differentiation leading to inhibited chondrocyte proliferation and (2) misalignment of synchondrosis chondrocytes with loss of cell polarity, causing aberrant chondrocyte orientation and thus improper local differentiation gradients.
In summary, DDR2 functions in skeletal progenitors to control synchondrosis extracellular matrix organization, chondrocyte proliferation and polarity and formation of the cranial base bones. It is unknown if DDR2 has a direct role in regulating activity of major regulatory pathways in the synchondroses, such as PTHrP-Ihh, Fgfr3 or Wnt signaling. Yet, chondrocyte abnormalities and cranial base growth defects presented in Ddr2-deficient and conditional knockout mice would suggest a functional role for DDR2 in these pathways. In the future, DDR2 function should be examined in the context of other signaling cascades that are pertinent to cranial base development, in addition to other (non)-syndromic diseases manifesting abnormalities in the cranial base synchondrosis.
3.6. Bone Morphogenic Proteins Are Expressed in the Cranial Base Synchondroses
Unlike the DDR proteins, which function to establish and maintain the extracellular matrix of the growth plate and synchondroses, the Bone Morphogenetic Proteins (BMPs) play important roles in regulating the formation of bone and cartilage and overall skeletal development [116]. BMP ligands induce bone formation in physiological, pathological and therapeutic contexts. However, this has primarily been shown in long bone fracture healing and periodontal regeneration [117,118]. In the cranial base, BMP function remains largely uninvestigated. Several studies have shown that BMP receptors and ligands are expressed in the embryonic and postnatal cranial base synchondroses. Bmp2, Bmp5, and Bmp6 are expressed in the early mesenchymal condensations [119]. During early postnatal development, Bmp2, Bm3, Bmp4, and Bmp5 were detected in the perichondrium and in the adjacent mesenchyme. Subsequently, Bmp2 and Bmp6 expression was confined to hypertrophic chondrocytes, while Bmp3, Bmp4, and Bmp5 were expressed in osteoblasts of the trabecular bone and bone collar. Interestingly, Bmp3 is expressed in the presumptive central hypertrophic zone of the SOS [119]. These expression data suggest that BMPs may play critical roles in mediating chondrocyte proliferation and differentiation, and possibly, regulating chondrocyte-to-osteoblast transition in the synchondroses. BMPs have been shown to possess similar functions in long bones [116]. As would be expected if BMPs function in the cranial base, the Bmp receptors, Alk6 and Bmpr1b are expressed in the embryonic cranial base anlagen [120,121]. In a more recent study, constitutive activation of Bmpr1a in neural crest cells (P0-Cre; caBmpr1a) caused precocious ISS ossification during postnatal development. More specifically, P0-Cre; caBmpr1a mice displayed significant reductions in the width of the ISS proliferating zone, decreased cell proliferation and increased apoptosis in the overlying perichondrium [122]. Thus, proper regulation of Bmpr1a levels in neural crest cells is critically important for the formation of the ISS. Given the major roles of BMPs in endochondral bone growth, the functions of the BMP ligands and their receptors should be further examined in the context of cranial base formation, maintenance and ossification.
3.7. Canonical and Non-Canonical Wnt/β-Catenin Signaling in Synchondrosis Formation
The Wnt/β-catenin pathway functions, in mesenchymal progenitor cells and chondrocytes, to control endochondral bone osteoblasts [123]. In long bones, canonical Wnt signaling dampens chondrogenic differentiation, but promotes chondrocyte maturation and hypertrophy [124,125,126]. In the cranial base, conditional deletion of β-catenin in undifferentiated mesenchymal cells using Dermo1-cre (Dermo1-cre; Ctnnb1fl/f/) increased synchondrosis cartilage formation and decreased osteoblast formation [127]. Additionally, conditional deletion of β-catenin in chondrocytes (Col2a1-cre; β-cateninfl/fl) caused aberrant replacement of osteoblasts with chondrocytes during embryonic development, resulting in abnormal retention of chondrocytes [128]. More specifically, mutant mice had less-defined chondrocyte layers associated with ectopic ColX and Mmp13 expression outside the synchondrosis hypertrophic zones. The authors also noted abnormal distribution of Ihh and Pthrp in mutant synchondroses, suggesting that loss of β-catenin in synchondrosis chondrocytes leads to mis-regulation of PTHrP-Ihh feedback. Additionally, these mice had significant reductions in the anteroposterior elongation of the skull, due to reductions in cranial base bone lengths. In the same study, the authors also showed that constitutive activation of canonical Wnt/β-catenin signaling via constitutive activation of Lef1 (CA-Lef1) in chondrocytes caused accelerated differentiation of all chondrocyte layers, increased expression of Col10a1 and the post-hypertrophic/bone marker, Opn, in the synchondrosis. Thus, canonical Wnt/β-catenin signaling is important for regulating chondrocyte differentiation in the cranial base synchondrosis.
In long bones, non-canonical Wnt/planar cell polarity (PCP) signaling plays a key role in facilitating asymmetric divisions of resting chondrocytes [129]. The hallmark characteristics of growth plate chondrocytes are oriented division, rearrangement and intercalation of chondrocyte clones in the resting zone, and their subsequent asymmetric divisions into their daughter cells aligned with the axis of growth [130]. Few studies to date have examined the role of the Wnt/PCP pathway in the cranial base synchondroses. Prickle1 is a critical regulator of Wnt/PCP signaling that interacts intracellularly with PCP proteins, including Dishevelled (Dsh), resulting in the modulation of Wnt/PCP and canonical Wnt/β-catenin signaling pathways [131,132]. Prickle1-deficient mice display skeletal and craniofacial abnormalities, including misalignment of growth plate chondrocytes leading to shortened bones and midfacial hypoplasia, respectively [133]. PrickleBj/Bj mice harboring a single nucleotide non-synonymous mutation (c.G482T:p.C161F) have defects in endochondral ossification, resulting in shortened long bones and significant abnormalities in the cranial base synchondroses [134]. More specifically, PrickleBj/Bj mice displayed shortened primary ossification centers in the cranial base and absence of well-defined chondrocyte layers, including replacement of proliferating chondrocytes with resting cells. Additionally, mutant synchondroses displayed expanded Sox9, Col2 and Ihh expression domains. ColX and Alp expression patterns extended across the entire hypertrophic zone, versus being restricted to a defined layer of cells in controls. These expression patterns suggest that mutant chondrocytes remain immature, thereby delaying ossification of the synchondroses. The authors postulate that the abnormalities observed in all chondrocyte layers of PrickleBj/Bj mice are potentially due to impaired binding of PrickleBj to Dsh2/3, leading to the loss of chondrocyte polarity, random distribution of core PCP proteins in synchondrosis chondrocytes and altering of the plane of cell division of chondrocyte layers. Thus, the Wnt/PCP pathway plays critical roles in the orientation, alignment and differentiation trajectories of synchondrosis chondrocytes through early cranial base development.
3.8. Runt Related Transcription Factor 2 Is a Novel Regulator of Cranial Base Development
We have described the functional roles of various signaling cascades in the context of the development of the cranial base synchondrosis. Importantly, Runt-related Transcription Factor 2 (Runx2) has been previously shown to be a putative downstream target of several key regulatory pathways in endochondral bone formation. However, its function in the context of cranial base development remains largely unknown. In the following, we provide an update to our current understanding on the Runx2’s role in the formation, maintenance and eventual fusion of the cranial base synchondroses in an attempt to highlight this promising target.
Runx2 is a master regulator of osteoblast and hypertrophic chondrocyte differentiation in long bones [135,136]. Runx2-deficient mice display deficient skeleto-genesis due to aberrant chondrocyte and osteoblast function. Runx2 expression is also found in chondrocytes of the cranial base synchondroses and osteoblasts in the adjacent region [135]. In humans, RUNX2 haploinsufficiency causes cleidocranial dysplasia (CCD), associated with hypoplastic clavicles, patent sutures and fontanelles, supernumerary teeth and short stature [137]. CCD patients display midfacial hypoplasia, due to persistent cartilage formation in the cranial base synchondroses. However these results are based on radiographic interpretation alone [138]. Constitutive activation of Runx2 in Col2a1+ chondrocytes partially rescued the aberrant skeleto-genesis phenotype caused by global Runx2 ablation in [139]. Moreover, these mice displayed accelerated hypertrophic chondrocyte differentiation leading to premature ossification of long bone growth plates.
Current knowledge of Runx2 function in cranial base development is somewhat limited, yet, in a recent study, T-box transcription factor 1 (Tbx1) was discovered as a negative regulator of Runx2 during cranial base development [140]. Pathogenic mutations in TBX1 in humans are associated with DiGorge and velocardiofacial syndromes, which present with microcephaly, low-set ears, and cleft palate [141]. Tbx1-deficient mice display precocious ossification of the SOS, but not the ISS, leading to premature fusion of the basisphenoid and basioccipital bones and midfacial hypoplasia [140]. Mesoderm-specific deletion of Tbx1 (Mesp1-cre; Tbx1fl/fl) recapitulated this phenotype, suggesting a functional role for Tbx1 in cells of the mesoderm lineage. Analysis of signaling cascades involved in Tbx1-mediated downstream gene regulation, using a proximity ligation assay, provided evidence for the involvement of Runx2. Tbx1 was discovered to directly inhibit Runx2 transcriptional activity through its Runt domain. The authors postulate that Tbx1 ablation causes the upregulation of Runx2 and, subsequently, the upregulation of its downstream targets, Sp7, Col1a1, Vegfa, and other factors involved in vascular invasion into the hypertrophic chondrocyte and osteogenic layers [142]. This indirect upregulation of osteoblastic genes may be involved in the precocious SOS ossification phenotype observed in Tbx1-deficient mice. Additionally, histone deacetylase 4 (HDAC4) has been shown to inhibit Runx2 activity [143]. HDAC4 is activated by PTHrP-promoted dephosphorylation, resulting in the initiation of HDAC4’s translocation into the nucleus [144]. Hdac4-deficient mice exhibited premature ossification of the synchondroses in [143]. Thus, PTHrP-HDAC4-Runx2 signaling may represent a critical axis that controls postnatal cranial base development and ossification.
Previous studies have begun to shed light on a novel signaling cascade involving Runx2 that is critical for maintaining postnatal cranial base organization. For example, several studies have described a Fgfr3-Mapk-Runx2 signaling axis that controls osteoblast and chondrocyte differentiation in long bones and calvarial osteoblasts [145,146]. As described earlier, FGFR3-MAPK signaling is required for proper postnatal growth of the cranial base. Further investigation into its overlapping functions with PTHrP-HDAC4 and FGFR3-MAPK signaling pathways may unravel a novel biological paradigm regulating cranial base synchondrosis development. However, whether this relationship exists in the context of cranial base development remains unknown. Collectively, Runx2 may possess both direct and indirect functions in regulating the cranial base synchondrosis.
4. Conclusions
The cranial base, once believed to function simply as a structural platform for the central nervous system, is now recognized to have unique functions in shaping the vertebrate craniofacial complex. Although the cranial base and long bone growth plate have similar endochondral origins and contain comparable cell types, the molecular regulation of these two tissues may be vastly different (Figure 2). Using recent technological advances, such as in vivo lineage-tracing, single-cell and spatial transcriptomics and CRISPR screening assays, more detailed analyses of the cellular and molecular properties of the cranial base synchondroses are now possible [147]. Importantly, while these technologies offer significant benefits, they may also present inherent challenges. For example, use of a transgenic line expressing Aggrecan enhancer-driven, Tetracycline-inducible cre that is sufficient to activate the Col2a1 promoter caused unexpected precocious ossification of the ISS and SOS likely because of cre-mediated toxicity [148,149]. Thus, further optimization of these techniques is required to discern unique biological phenomena.
This article summarized our current understanding of the molecular regulation of cranial base formation. However, additional investigation is required to elucidate the molecular etiology of cranial base malformations, as well as to identify potential targets for clinical intervention. In doing so, we become one step closer to bridging the gap between basic knowledge and clinical applications related to the development of novel regenerative and pharmacological therapies for patients suffering from congenital cranial base malformations and traumatic injuries to the craniofacial skeleton.
Acknowledgments
We would like to thank Yuji Mishina for providing detailed information on the P0-cre; caBmpr1a synchondrosis phenotype.
Author Contributions
S.A.H. performed the literature search and drafted the literature review. S.A.H., W.O., R.T.F. and N.O. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by NIH/NIDCR F30DE030675 (to S.A.H.), NIH/NIDCR R01DE029181 (to W.O.), NIH/NIDCR R01DE029465 and DOD W81XWH-19-PRMRP-IIRA (to R.T.F.) and NIH/NIDCR R01DE026666 and R01DE030630 (to N.O.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koski K. Cranial growth centers: Facts or fallacies? Am. J. Orthod. 1968;54:566–583. doi: 10.1016/0002-9416(68)90177-2. [DOI] [PubMed] [Google Scholar]
- 2.Hallett S.A., Ono W., Ono N. Growth plate chondrocytes: Skeletal development, growth and beyond. Int. J. Mol. Sci. 2019;20:6009. doi: 10.3390/ijms20236009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBratney-Owen B., Iseki S., Bamforth S.D., Olsen B.R., Morriss-Kay G.M. Development and tissue origins of the mammalian cranial base. Dev. Biol. 2008;322:121–132. doi: 10.1016/j.ydbio.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dierbach A.R. Morphogenesis of the cranium of Cavia porcellus L. II: Comparative part and literature. Gegenbaurs Morphol. Jahrb. 1985;131:617–642. [PubMed] [Google Scholar]
- 5.Tam P.P., Meier S. The establishment of a somitomeric pattern in the mesoderm of the gastrulating mouse embryo. Am. J. Anat. 1982;164:209–225. doi: 10.1002/aja.1001640303. [DOI] [PubMed] [Google Scholar]
- 6.Tam P.P.L., Meier S., Jacobson A.G. Differentiation of the Metameric Pattern in the Embryonic Axis of the Mouse: II. Somitomeric Organization of the Presomitic Mesoderm. Differentiation. 1982;21:109–122. doi: 10.1111/j.1432-0436.1982.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 7.Müller F., O’Rahilly R. The human chondrocranium at the end of the embryonic period, proper, with particular reference to the nervous system. Am. J. Anat. 1980;159:33–58. doi: 10.1002/aja.1001590105. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery N. A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology. 2002;44:358–366. doi: 10.1007/s00234-001-0753-z. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman D.E., Ross C.F., Ravosa M.J. The primate cranial base: Ontogeny, function, and integration. Am. J. Phys. Anthropol. 2000;113((Suppl. S31)):117–169. doi: 10.1002/1096-8644(2000)43:31+<117::AID-AJPA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Wei X., Hu M., Mishina Y., Liu F. Developmental regulation of the growth plate and cranial synchondrosis. J. Dent. Res. 2016;95:1221–1229. doi: 10.1177/0022034516651823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 12.Kaucka M., Zikmund T., Tesarova M., Gyllborg D., Hellander A., Jaros J., Kaiser J., Petersen J., Szarowska B., Newton P.T., et al. Oriented clonal cell dynamics enables accurate growth and shaping of vertebrate cartilage. Elife. 2017;6:e25902. doi: 10.7554/eLife.25902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thilander B., Ingervall B. The human spheno-occipital synchondrosis II. A histological and microradiographic study of its growth. Acta Odontol. Scand. 1973;31:323–334. doi: 10.3109/00016357309002520. [DOI] [PubMed] [Google Scholar]
- 14.Hallett S.A., Ono W., Ono N. The hypertrophic chondrocyte: To be or not to be. Histol. Histopathol. 2021;36:1021–1036. doi: 10.14670/HH-18-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhazmi A., Vargas E., Palomo J.M., Hans M., Latimer B., Simpson S. Timing and rate of spheno-occipital synchondrosis closure and its relationship to puberty. PLoS ONE. 2017;12:e0183305. doi: 10.1371/journal.pone.0183305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madeline L.A., Elster A.D. Postnatal development of the central skull base: Normal variants. Radiology. 1995;196:757–763. doi: 10.1148/radiology.196.3.7644640. [DOI] [PubMed] [Google Scholar]
- 17.Scott J.H. The cranial base. Am. J. Phys. Anthropol. 1958;16:319–348. doi: 10.1002/ajpa.1330160305. [DOI] [PubMed] [Google Scholar]
- 18.Twigg S.R.F., Wilkie A.O.M. New insights into craniofacial malformations. Hum. Mol. Genet. 2015;24:R50–R59. doi: 10.1093/hmg/ddv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J.H., Cha B.K., Choi D.S., Park J.H., Jang I. Time and pattern of the fusion of the spheno-occipital synchondrosis in patients with skeletal Class i and Class III malocclusion. Angle Orthod. 2019;89:470–479. doi: 10.2319/052218-386.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahiri Y., Paliga J.T., Vossough A., Bartlett S.P., Taylor J.A. The Spheno-occipital synchondrosis fuses prematurely in patients with crouzon syndrome and midface hypoplasia compared with age- and gender-matched controls. J. Oral Maxillofac. Surg. 2014;72:1173–1179. doi: 10.1016/j.joms.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Paliga J.T., Goldstein J.A., Vossough A., Bartlett S.P., Taylor J.A. Premature closure of the spheno-occipital synchondrosis in pfeiffer syndrome: A link to midface hypoplasia. J. Craniofac. Surg. 2014;25:202–205. doi: 10.1097/SCS.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 22.Jensen B.L., Kreiborg S. Craniofacial growth in cleidocranial dysplasia--a roentgencephalometric study. J. Craniofac. Genet. Dev. Biol. 1995;15:35–43. [PubMed] [Google Scholar]
- 23.Cohen M.M.J., Walker G.F., Phillips C. A morphometric analysis of the craniofacial configuration in achondroplasia. J. Craniofac. Genet. Dev. Biol. Suppl. 1985;1:139–165. [PubMed] [Google Scholar]
- 24.Andria L.M., Leite L.P., Prevatte T.M., King L.B. Correlation of the cranial base angle and its components with other dental/skeletal variables and treatment time. Angle Orthod. 2004;74:361–366. doi: 10.1043/0003-3219(2004)0742.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Mullikcn J.B., Steinberger D., Kunze S., Müller U. Molecular diagnosis of bilateral coronal synostosis. Plast. Reconstr. Surg. 1999;104:1603–1615. doi: 10.1097/00006534-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg P., Arlis H.R., Haworth R.D., Heier L., Hoffman L., LaTrenta G. The role of the cranial base in facial growth: Experimental craniofacial synostosis in the rabbit. Plast. Reconstr. Surg. 1997;99:1396–1407. doi: 10.1097/00006534-199705000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Allareddy V., Ching N., Macklin E.A., Voelz L., Weintraub G., Davidson E., Prock L.A., Rosen D., Brunn R., Skotko B.G. Craniofacial features as assessed by lateral cephalometric measurements in children with Down syndrome. Prog. Orthod. 2016;17:35. doi: 10.1186/s40510-016-0148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintanilla J.S., Biedma B.M., Rodríguez M.Q., Mora M.T.J., Cunqueiro M.M.S., Pazos M.A. Cephalometrics in children with Down’s syndrome. Pediatr. Radiol. 2002;32:635–643. doi: 10.1007/s00247-002-0703-x. [DOI] [PubMed] [Google Scholar]
- 29.Brkic H., Kaic Z., Poje Z., Singer Z. Shape of the craniofacial complex in patients with Klinefelter syndrome. Angle Orthod. 1994;64:371–376. doi: 10.1043/0003-3219(1994)0642.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Newton P.T., Li L., Zhou B., Schweingruber C., Hovorakova M., Xie M., Sun X., Sandhow L., Artemov A.V., Ivashkin E., et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019;567:234–238. doi: 10.1038/s41586-019-0989-6. [DOI] [PubMed] [Google Scholar]
- 31.Mizuhashi K., Ono W., Matsushita Y., Sakagami N., Takahashi A., Saunders T.L., Nagasawa T., Kronenberg H.M., Ono N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563:254–258. doi: 10.1038/s41586-018-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muruganandan S., Pierce R., Teguh D.A., Perez R.F., Bell N., Nguyen B., Hohl K., Snyder B.D., Grinstaff M.W., Alberico H., et al. A FoxA2+ long-term stem cell population is necessary for growth plate cartilage regeneration after injury. Nat. Commun. 2022;13:2515. doi: 10.1038/s41467-022-30247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rengasamy Venugopalan S., Van Otterloo E. The Skull’s Girder: A Brief Review of the Cranial Base. J. Dev. Biol. 2021;9:3. doi: 10.3390/jdb9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funato N. New Insights Into Cranial Synchondrosis Development: A Mini Review. Front. Cell Dev. Biol. 2020;8:706. doi: 10.3389/fcell.2020.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie X. Cranial base in craniofacial development: Developmental features, influence on facial growth, anomaly, and molecular basis. Acta Odontol. Scand. 2005;63:127–135. doi: 10.1080/00016350510019847. [DOI] [PubMed] [Google Scholar]
- 36.Suva L.J., Winslow G.A., Wettenhall R.E.H., Hammonds R.G., Moseley J.M., Diefenbach-Jagger H., Rodda C.P., Kemp B.E., Rodriguez H., Chen E.Y., et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: Cloning and expression. Science. 1987;237:893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 37.Lanske B., Karaplis A.C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L.H.K., Ho C., Mulligan R.C., et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 38.Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., Tabin C.J. Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 39.Young B., Minugh-Purvis N., Shimo T., St-Jacques B., Iwamoto M., Enomoto-Iwamoto M., Koyama E., Pacifici M. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev. Biol. 2006;299:272–282. doi: 10.1016/j.ydbio.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Ishii-Suzuki M., Suda N., Yamazaki K., Kuroda T., Senior P.V., Beck F., Hammond V.E. Differential responses to parathyroid hormone-related protein (PTHrP) deficiency in the various craniofacial cartilages. Anat. Rec. 1999;255:452–457. doi: 10.1002/(SICI)1097-0185(19990801)255:4<452::AID-AR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Karaplis A.C., Luz A., Glowacki J., Bronson R.T., Tybulewicz V.L., Kronenberg H.M., Mulligan R.C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 42.Hallett S.A., Zhou A., Herzog C., Arbiv A., Ono W., Ono N. Cranial base synchondrosis lacks PTHrP-expressing col-umn-forming chondrocytes. Int. J. Mol. Sci. :2022. doi: 10.3390/ijms23147873. submitted . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long F., Chung U.I., Ohba S., McMahon J., Kronenberg H.M., McMahon A.P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 44.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez J., Horton J., Sohn P., Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001;221:311–321. doi: 10.1002/dvdy.1141. [DOI] [PubMed] [Google Scholar]
- 46.Serra R., Karaplis A., Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J. Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama E., Shimazu A., Leatherman J.L., Golden E.B., Nah H.D., Pacifici M. Expression of syndecan-3 and tenascin-C: Possible involvement in periosteum development. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1996;14:403–412. doi: 10.1002/jor.1100140310. [DOI] [PubMed] [Google Scholar]
- 48.Olsen B.R., Kolpakova E., McBratney-Owen B., Li X., Zhou J., Fukai N. Genetic and epigenetic determinants of skeletal morphogenesis–role of cellular polarity and ciliary function in skeletal development and growth. Oral Biosci. Med. 2005;2:57–65. [Google Scholar]
- 49.Huangfu D., Anderson K. V Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorn K.V., Hughes C.E., Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev. Cell. 2012;23:823–835. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caparrós-Martín J.A., Valencia M., Reytor E., Pacheco M., Fernandez M., Perez-Aytes A., Gean E., Lapunzina P., Peters H., Goodship J.A., et al. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum. Mol. Genet. 2013;22:124–139. doi: 10.1093/hmg/dds409. [DOI] [PubMed] [Google Scholar]
- 52.Ellis R.W., van Creveld S. A Syndrome Characterized by Ectodermal Dysplasia, Polydactyly, Chondro-Dysplasia and Congenital Morbus Cordis: Report of Three Cases. Arch. Dis. Child. 1940;15:65–84. doi: 10.1136/adc.15.82.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badri M.K., Zhang H., Ohyama Y., Venkitapathi S., Alamoudi A., Kamiya N., Takeda H., Ray M., Scott G., Tsuji T., et al. Expression of Evc2 in craniofacial tissues and craniofacial bone defects in Evc2 knockout mouse. Arch. Oral Biol. 2016;68:142–152. doi: 10.1016/j.archoralbio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulkarni A.K., Louie K.W., Yatabe M., de Ruellas A.C.O., Mochida Y., Cevidanes L.H.S., Mishina Y., Zhang H. A Ciliary Protein EVC2/LIMBIN Plays a Critical Role in the Skull Base for Mid-Facial Development. Front. Physiol. 2018;9:1484. doi: 10.3389/fphys.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon E.K., Louie K., Kulkarni A., Yatabe M., de Ruellas A.C.O., Snider T.N., Mochida Y., Cevidanes L.H.S., Mishina Y., Zhang H. The Role of Ellis-Van Creveld 2(EVC2) in Mice During Cranial Bone Development. Anat. Rec. (Hoboken) 2018;301:46–55. doi: 10.1002/ar.23692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badri M.K., Zhang H., Ohyama Y., Venkitapathi S., Kamiya N., Takeda H., Ray M., Scott G., Tsuji T., Kunieda T., et al. Ellis Van Creveld2 is Required for Postnatal Craniofacial Bone Development. Anat. Rec. (Hoboken) 2016;299:1110–1120. doi: 10.1002/ar.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Susami T., Kuroda T., Yoshimasu H., Suzuki R. Ellis-van Creveld syndrome: Craniofacial morphology and multidisciplinary treatment. Cleft Palate-Craniofac. J. 1999;36:345–352. doi: 10.1597/1545-1569_1999_036_0345_evcscm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Louie K.W., Kulkarni A.K., Zapien-Guerra K., Yang J., Mishina Y. The Posterior Part Influences the Anterior Part of the Mouse Cranial Base Development. JBMR Plus. 2022;6:e10589. doi: 10.1002/jbm4.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minina E., Kreschel C., Naski M.C., Ornitz D.M., Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP Signaling Integrates Chondrocyte Proliferation and Hypertrophic Differentiation. Dev. Cell. 2002;3:439–449. doi: 10.1016/S1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 60.Ornitz D.M., Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ornitz D.M. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–213. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohbayashi N., Shibayama M., Kurotaki Y., Imanishi M., Fujimori T., Itoh N., Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coffin J.D., Florkiewicz R.Z., Neumann J., Mort-Hopkins T., Dorn G.W., 2nd, Lightfoot P., German R., Howles P.N., Kier A., O’Toole B.A. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol. Biol. Cell. 1995;6:1861–1873. doi: 10.1091/mbc.6.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montero A., Okada Y., Tomita M., Ito M., Tsurukami H., Nakamura T., Doetschman T., Coffin J.D., Hurley M.M. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Investig. 2000;105:1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garofalo S., Kliger-Spatz M., Cooke J.L., Wolstin O., Lunstrum G.P., Moshkovitz S.M., Horton W.A., Yayon A. Skeletal Dysplasia and Defective Chondrocyte Differentiation by Targeted Overexpression of Fibroblast Growth Factor 9 in Transgenic Mice. J. Bone Miner. Res. 1999;14:1909–1915. doi: 10.1359/jbmr.1999.14.11.1909. [DOI] [PubMed] [Google Scholar]
- 66.Carlton M.B., Colledge W.H., Evans M.J. Crouzon-like craniofacial dysmorphology in the mouse is caused by an insertional mutation at the Fgf3/Fgf4 locus. Dev. Dyn. 1998;212:242–249. doi: 10.1002/(SICI)1097-0177(199806)212:2<242::AID-AJA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 67.Legeai-Mallet L., Benoist-Lasselin C., Munnich A., Bonaventure J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 correlates with phenotypic severity and defective chondrocyte differentiation in FGFR3-related chondrodysplasias. Bone. 2004;34:26–36. doi: 10.1016/j.bone.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Su W.C.S., Kitagawa M., Xue N., Xie B., Garofalo S., Cho J., Deng C., Horton W.A., Fu X.Y. Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature. 1997;386:288–292. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- 69.Raucci A., Laplantine E., Mansukhani A., Basilico C. Activation of the ERK1/2 and p38 Mitogen-activated Protein Kinase Pathways Mediates Fibroblast Growth Factor-induced Growth Arrest of Chondrocytes. J. Biol. Chem. 2004;279:1747–1756. doi: 10.1074/jbc.M310384200. [DOI] [PubMed] [Google Scholar]
- 70.De Frutos C.A., Vega S., Manzanares M., Flores J.M., Huertas H., Martínez-Frías M.L., Nieto M.A. Snail1 Is a Transcriptional Effector of FGFR3 Signaling during Chondrogenesis and Achondroplasias. Dev. Cell. 2007;13:872–883. doi: 10.1016/j.devcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 71.Shiang R., Thompson L.M., Zhu Y.Z., Church D.M., Fielder T.J., Bocian M., Winokur S.T., Wasmuth J.J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 72.Bonaventure J., Rousseau F., Legeai-Mallet L., Le Merrer M., Munnich A., Maroteaux P. Common mutations in the gene encoding fibroblast growth factor receptor 3 account for achondroplasia, hypochondroplasia and thanatophoric dysplasia. Acta Paediatr. Suppl. 1996;417:33–38. doi: 10.1111/j.1651-2227.1996.tb14291.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhou S., Xie Y., Tang J., Huang J., Huang Q., Xu W., Wang Z., Luo F., Wang Q., Chen H., et al. FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling. PLoS Genet. 2015;11:e1005214. doi: 10.1371/journal.pgen.1005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Webster M.K., D’Avis P.Y., Robertson S.C., Donoghue D.J. Profound ligand-independent kinase activation of fibroblast growth factor receptor 3 by the activation loop mutation responsible for a lethal skeletal dysplasia, thanatophoric dysplasia type II. Mol. Cell. Biol. 1996;16:4081–4087. doi: 10.1128/MCB.16.8.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tavormina P.L., Shiang R., Thompson L.M., Zhu Y.Z., Wilkin D.J., Lachman R.S., Wilcox W.R., Rimoin D.L., Cohn D.H., Wasmuth J.J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat. Genet. 1995;9:321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- 76.Chen C.P., Chang T.Y., Lin M.H., Chern S.R., Su J.W., Wang W. Rapid detection of K650E mutation in FGFR3 using uncultured amniocytes in a pregnancy affected with fetal cloverleaf skull, occipital pseudoencephalocele, ventriculomegaly, straight short femurs, and thanatophoric dysplasia type II. Taiwan J. Obstet. Gynecol. 2013;52:420–425. doi: 10.1016/j.tjog.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Matsushita T., Wilcox W.R., Chan Y.Y., Kawanami A., Bükülmez H., Balmes G., Krejci P., Mekikian P.B., Otani K., Yamaura I., et al. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum. Mol. Genet. 2009;18:227–240. doi: 10.1093/hmg/ddn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y., Spatz M.K., Kannan K., Hayk H., Avivi A., Gorivodsky M., Pines M., Yayon A., Lonai P., Givol D. A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc. Natl. Acad. Sci. USA. 1999;96:4455–4460. doi: 10.1073/pnas.96.8.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami S., Balmes G., McKinney S., Zhang Z., Givol D., De Crombrugghe B. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 2004;18:290–305. doi: 10.1101/gad.1179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L., Adar R., Yang X., Monsonego E.O., Li C., Hauschka P.V., Yayon A., Deng C.X. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J. Clin. Investig. 1999;104:1517–1525. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laurita J., Koyama E., Chin B., Taylor J.A., Lakin G.E., Hankenson K.D., Bartlett S.P., Nah H.-D. The Muenke syndrome mutation (FgfR3P244R) causes cranial base shortening associated with growth plate dysfunction and premature perichondrial ossification in murine basicranial synchondroses. Dev. Dyn. 2011;240:2584–2596. doi: 10.1002/dvdy.22752. [DOI] [PubMed] [Google Scholar]
- 82.Chen L., Li C., Qiao W., Xu X., Deng C. A Ser365→Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum. Mol. Genet. 2001;10:457–466. doi: 10.1093/hmg/10.5.457. [DOI] [PubMed] [Google Scholar]
- 83.Schell U., Hehr A., Feldman G.J., Robin N.H., Zackai E.H., de Die-Smulders C., Viskochil D.H., Stewart J.M., Wolff G., Ohashi H. Mutations in FGFR1 and FGFR2 cause familial and sporadic Pfeiffer syndrome. Hum. Mol. Genet. 1995;4:323–328. doi: 10.1093/hmg/4.3.323. [DOI] [PubMed] [Google Scholar]
- 84.Muenke M., Schell U., Hehr A., Robin N.H., Losken H.W., Schinzel A., Pulleyn L.J., Rutland P., Reardon W., Malcolm S. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat. Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- 85.Adel M., Yamaguchi T., Tomita D., Nakawaki T., Kim Y.-I., Hikita Y., Haga S., Takahashi M., Nadim M.A., Kawaguchi A., et al. Contribution of FGFR1 Variants to Craniofacial Variations in East Asians. PLoS ONE. 2017;12:e0170645. doi: 10.1371/journal.pone.0170645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen M.M.J., Kreiborg S., Lammer E.J., Cordero J.F., Mastroiacovo P., Erickson J.D., Roeper P., Martínez-Frías M.L. Birth prevalence study of the Apert syndrome. Am. J. Med. Genet. 1992;42:655–659. doi: 10.1002/ajmg.1320420505. [DOI] [PubMed] [Google Scholar]
- 87.Cohen M.M., Jr., Kreiborg S. Skeletal abnormalities in the Apert syndrome. Am. J. Med. Genet. 1993;47:624–632. doi: 10.1002/ajmg.1320470509. [DOI] [PubMed] [Google Scholar]
- 88.Yin L., Du X., Li C., Xu X., Chen Z., Su N., Zhao L., Qi H., Li F., Xue J., et al. A Pro253Arg mutation in fibroblast growth factor receptor 2 (Fgfr2) causes skeleton malformation mimicking human Apert syndrome by affecting both chondrogenesis and osteogenesis. Bone. 2008;42:631–643. doi: 10.1016/j.bone.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 89.Nagata M., Nuckolls G.H., Wang X., Shum L., Seki Y., Kawase T., Takahashi K., Nonaka K., Takahashi I., Noman A.A., et al. The primary site of the acrocephalic feature in Apert syndrome is a dwarf cranial base with accelerated chondrocytic differentiation due to aberrant activation of the FGFR2 signaling. Bone. 2011;48:847–856. doi: 10.1016/j.bone.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Passos-Bueno M.R., Richieri-Costa A., Sertié A.L., Kneppers A. Presence of the Apert canonical S252W FGFR2 mutation in a patient without severe syndactyly. J. Med. Genet. 1998;35:677–679. doi: 10.1136/jmg.35.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen P., Zhang L., Weng T., Zhang S., Sun S., Chang M., Li Y., Zhang B., Zhang L. A Ser252Trp Mutation in Fibroblast Growth Factor Receptor 2 (FGFR2) Mimicking Human Apert Syndrome Reveals an Essential Role for FGF Signaling in the Regulation of Endochondral Bone Formation. PLoS ONE. 2014;9:e87311. doi: 10.1371/journal.pone.0087311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGrath J., Gerety P.A., Derderian C.A., Steinbacher D.M., Vossough A., Bartlett S.P., Nah H.-D., Taylor J.A. Differential Closure of the Spheno-occipital Synchondrosis in Syndromic Craniosynostosis. Plast. Reconstr. Surg. 2012;130:681e–689e. doi: 10.1097/PRS.0b013e318267d4c0. [DOI] [PubMed] [Google Scholar]
- 93.Holmes G., Basilico C. Mesodermal expression of Fgfr2S252W is necessary and sufficient to induce craniosynostosis in a mouse model of Apert syndrome. Dev. Biol. 2012;368:283–293. doi: 10.1016/j.ydbio.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ornitz D.M., Legeai-Mallet L. Achondroplasia: Development, pathogenesis, and therapy. Dev. Dyn. 2017;246:291–309. doi: 10.1002/dvdy.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonçalves D., Rignol G., Dellugat P., Hartmann G., Sarrazy Garcia S., Stavenhagen J., Santarelli L., Gouze E., Czech C. In vitro and in vivo characterization of Recifercept, a soluble fibroblast growth factor receptor 3, as treatment for achondroplasia. PLoS ONE. 2021;15:e0244368. doi: 10.1371/journal.pone.0244368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rignol G., Garcia S., Authier F., Smith K., Tosello L., Marsault R., Dellugat P., Goncalves D., Brouillard M., Stavenhagen J., et al. Longitudinal Imaging of the Skull Base Synchondroses Demonstrate Prevention of a Premature Ossification After Recifercept Treatment in Mouse Model of Achondroplasia. JBMR Plus. 2022;6:e10568. doi: 10.1002/jbm4.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamashita A., Morioka M., Kishi H., Kimura T., Yahara Y., Okada M., Fujita K., Sawai H., Ikegawa S., Tsumaki N. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513:507–511. doi: 10.1038/nature13775. [DOI] [PubMed] [Google Scholar]
- 98.Matsushita M., Kitoh H., Ohkawara B., Mishima K., Kaneko H., Ito M., Masuda A., Ishiguro N., Ohno K. Meclozine facilitates proliferation and differentiation of chondrocytes by attenuating abnormally activated FGFR3 signaling in achondroplasia. PLoS ONE. 2013;8:e81569. doi: 10.1371/journal.pone.0081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsushita M., Hasegawa S., Kitoh H., Mori K., Ohkawara B., Yasoda A., Masuda A., Ishiguro N., Ohno K. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology. 2015;156:548–554. doi: 10.1210/en.2014-1914. [DOI] [PubMed] [Google Scholar]
- 100.Matsushita M., Esaki R., Mishima K., Ishiguro N., Ohno K., Kitoh H. Clinical dosage of meclozine promotes longitudinal bone growth, bone volume, and trabecular bone quality in transgenic mice with achondroplasia. Sci. Rep. 2017;7:7371. doi: 10.1038/s41598-017-07044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yasoda A., Kitamura H., Fujii T., Kondo E., Murao N., Miura M., Kanamoto N., Komatsu Y., Arai H., Nakao K. Systemic Administration of C-Type Natriuretic Peptide as a Novel Therapeutic Strategy for Skeletal Dysplasias. Endocrinology. 2009;150:3138–3144. doi: 10.1210/en.2008-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yasoda A., Komatsu Y., Chusho H., Miyazawa T., Ozasa A., Miura M., Kurihara T., Rogi T., Tanaka S., Suda M., et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat. Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 103.Shrivastava A., Radziejewski C., Campbell E., Kovac L., McGlynn M., Ryan T.E., Davis S., Goldfarb M.P., Glass D.J., Lemke G., et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell. 1997;1:25–34. doi: 10.1016/S1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 104.Vogel W., Gish G.D., Alves F., Pawson T. The Discoidin Domain Receptor Tyrosine Kinases Are Activated by Collagen. Mol. Cell. 1997;1:13–23. doi: 10.1016/S1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 105.Foehr E.D., Tatavos A., Tanabe E., Raffioni S., Goetz S., Dimarco E., De Luca M., Bradshaw R.A. Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: Activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors. FASEB J. 2000;14:973–981. doi: 10.1096/fasebj.14.7.973. [DOI] [PubMed] [Google Scholar]
- 106.Johansson F.K., Göransson H., Westermark B. Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene. 2005;24:3896–3905. doi: 10.1038/sj.onc.1208553. [DOI] [PubMed] [Google Scholar]
- 107.Yamanaka R., Arao T., Yajima N., Tsuchiya N., Homma J., Tanaka R., Sano M., Oide A., Sekijima M., Nishio K. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene. 2006;25:5994–6002. doi: 10.1038/sj.onc.1209585. [DOI] [PubMed] [Google Scholar]
- 108.Chou L.-Y., Chen C.-H., Lin Y.-H., Chuang S.-C., Chou H.-C., Lin S.-Y., Fu Y.-C., Chang J.-K., Ho M.-L., Wang C.-Z. Discoidin domain receptor 1 regulates endochondral ossification through terminal differentiation of chondrocytes. FASEB J. 2020;34:5767–5781. doi: 10.1096/fj.201901852RR. [DOI] [PubMed] [Google Scholar]
- 109.Yilmaz Gulec E., Ali B.R., John A., Tuysuz B. Spondylometaepiphyseal Dysplasia Short Limb-Abnormal Calcification Type in Turkish Patients Reveals a Novel Mutation and New Features. Mol. Syndromol. 2022;13:23–37. doi: 10.1159/000517848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ürel-Demir G., Şimşek-Kiper P., Akgün-Doğan ., Göçmen R., Wang Z., Matsumoto N., Miyake N., Utine G.E., Nishimura G., Ikegawa S., et al. Further expansion of the mutational spectrum of spondylo-meta-epiphyseal dysplasia with abnormal calcification. J. Hum. Genet. 2018;63:1003–1007. doi: 10.1038/s10038-018-0473-4. [DOI] [PubMed] [Google Scholar]
- 111.Bargal R., Cormier-Daire V., Ben-Neriah Z., Le Merrer M., Sosna J., Melki J., Zangen D.H., Smithson S.F., Borochowitz Z., Belostotsky R., et al. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am. J. Hum. Genet. 2009;84:80–84. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Kindi A., Kizhakkedath P., Xu H., John A., Sayegh A.A., Ganesh A., Al-Awadi M., Al-Anbouri L., Al-Gazali L., Leitinger B., et al. A novel mutation in DDR2 causing spondylo-meta-epiphyseal dysplasia with short limbs and abnormal calcifications (SMED-SL) results in defective intra-cellular trafficking. BMC Med. Genet. 2014;15:42. doi: 10.1186/1471-2350-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohamed F.F., Ge C., Cowling R.T., Lucas D., Hallett S.A., Ono N., Binrayes A.-A., Greenberg B., Franceschi R.T. The collagen receptor, discoidin domain receptor 2, functions in Gli1-positive skeletal progenitors and chondrocytes to control bone development. Bone Res. 2022;10:11. doi: 10.1038/s41413-021-00182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohamed F.F., Ge C., Cowling R.T., Ono N., Binrayes A.-A., Greenberg B., Kaartinen V.M., Franceschi R.T. Control of Craniofacial Development by the Collagen Receptor, Discoidin Domain Receptor 2. bioRxiv. 2022 doi: 10.1101/2022.02.03.479017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao H., Feng J., Ho T.-V., Grimes W., Urata M., Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015;17:386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salazar V.S., Gamer L.W., Rosen V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016;12:203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 117.Rao S.M., Ugale G.M., Warad S.B. Bone morphogenetic proteins: Periodontal regeneration. N. Am. J. Med. Sci. 2013;5:161–168. doi: 10.4103/1947-2714.109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garrison K.R., Shemilt I., Donell S., Ryder J.J., Mugford M., Harvey I., Song F., Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010;2010:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kettunen P., Nie X., Kvinnsland I.H., Luukko K. Histological development and dynamic expression of Bmp2–6 mRNAs in the embryonic and postnatal mousecranial base. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006;288A:1250–1258. doi: 10.1002/ar.a.20402. [DOI] [PubMed] [Google Scholar]
- 120.Verschueren K., Dewulf N., Goumans M.-J., Lonnoy O., Feijen A., Grimsby S., Vande Spiegle K., ten Dijke P., Moren A., Vanscheeuwijck P., et al. Expression of type I and type IB receptors for activin in midgestation mouse embryos suggests distinct functions in organogenesis. Mech. Dev. 1995;52:109–123. doi: 10.1016/0925-4773(95)00395-H. [DOI] [PubMed] [Google Scholar]
- 121.Dewulf N., Verschueren K., Lonnoy O., Morén A., Grimsby S., Vande Spiegle K., Miyazono K., Huylebroeck D., Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- 122.Ueharu H., Pan H., Yang J., Mishina Y. Augmentation of BMP signaling in cranial neural crest cells deforms skull base due to premature fusion of intersphenoidal synchondrosis. Genesis. :2022. doi: 10.1002/dvg.23509. submitted . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Regard J.B., Zhong Z., Williams B.O., Yang Y. Wnt signaling in bone development and disease: Making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012;4:a007997. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Enomoto-Iwamoto M., Kitagaki J., Koyama E., Tamamura Y., Wu C., Kanatani N., Koike T., Okada H., Komori T., Yoneda T., et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev. Biol. 2002;251:142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 125.Day T.F., Guo X., Garrett-Beal L., Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 126.Mak K.K., Chen M.H., Day T.F., Chuang P.T., Yang Y. Wnt/β-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development. 2006;133:3695–3707. doi: 10.1242/dev.02546. [DOI] [PubMed] [Google Scholar]
- 127.Wu M., Li C., Zhu G., Wang Y., Jules J., Lu Y., McConnell M., Wang Y.J., Shao J.Z., Li Y.P., et al. Deletion of core-binding factor β (Cbfβ) in mesenchymal progenitor cells provides new insights into Cbfβ/Runxs complex function in cartilage and bone development. Bone. 2014;65:49–59. doi: 10.1016/j.bone.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagayama M., Iwamoto M., Hargett A., Kamiya N., Tamamura Y., Young B., Morrison T., Takeuchi H., Pacifici M., Enomoto-Iwamoto M., et al. Wnt/beta-catenin signaling regulates cranial base development and growth. J. Dent. Res. 2008;87:244–249. doi: 10.1177/154405910808700309. [DOI] [PubMed] [Google Scholar]
- 129.Hallett S.A., Matsushita Y., Ono W., Sakagami N., Mizuhashi K., Tokavanich N., Nagata M., Zhou A., Hirai T., Kronenberg H.M., et al. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-inhibitory environment. Elife. 2021;10:e64513. doi: 10.7554/eLife.64513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L., Newton P.T., Bouderlique T., Sejnohova M., Zikmund T., Kozhemyakina E., Xie M., Krivanek J., Kaiser J., Qian H., et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017;31:1067–1084. doi: 10.1096/fj.201600918R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao B., Yang Y. Planar cell polarity in vertebrate limb morphogenesis. Curr. Opin. Genet. Dev. 2013;23:438–444. doi: 10.1016/j.gde.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katoh M., Katoh M. Identification and characterization of human PRICKLE1 and PRICKLE2 genes as well as mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue polarity gene prickle. Int. J. Mol. Med. 2003;11:249–256. doi: 10.3892/ijmm.11.2.249. [DOI] [PubMed] [Google Scholar]
- 133.Gibbs B.C., Damerla R.R., Vladar E.K., Chatterjee B., Wan Y., Liu X., Cui C., Gabriel G.C., Zahid M., Yagi H., et al. Prickle1 mutation causes planar cell polarity and directional cell migration defects associated with cardiac outflow tract anomalies and other structural birth defects. Biol. Open. 2016;5:323–335. doi: 10.1242/bio.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wan Y., Szabo-Rogers H.L. Chondrocyte Polarity During Endochondral Ossification Requires Protein–Protein Interactions Between Prickle1 and Dishevelled2/3. J. Bone Miner. Res. 2021;36:2399–2412. doi: 10.1002/jbmr.4428. [DOI] [PubMed] [Google Scholar]
- 135.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.H., Inada M., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 136.Komori T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells. 2020;43:168–175. doi: 10.14348/molcells.2019.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pan C.Y., Tseng Y.C., Lan T.H., Chang H.P. Craniofacial features of cleidocranial dysplasia. J. Dent. Sci. 2017;12:313–318. doi: 10.1016/j.jds.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaissi A.A., Chehida F.B., Kenis V., Ganger R., Radler C., Hofstaetter J.G., Klaushofer K., Grill F. Broad Spectrum of Skeletal Malformation Complex in Patients with Cleidocranial Dysplasia Syndrome: Radiographic and Tomographic Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2013;6:45–55. doi: 10.4137/CMAMD.S11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takeda S., Bonnamy J.P., Owen M.J., Ducy P., Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Funato N., Srivastava D., Shibata S., Yanagisawa H. TBX1 Regulates Chondrocyte Maturation in the Spheno-occipital Synchondrosis. J. Dent. Res. 2020;99:1182–1191. doi: 10.1177/0022034520925080. [DOI] [PubMed] [Google Scholar]
- 141.McDonald-McGinn D.M., Sullivan K.E., Marino B., Philip N., Swillen A., Vorstman J.A.S., Zackai E.H., Emanuel B.S., Vermeesch J.R., Morrow B.E., et al. 22q11. 2 deletion syndrome. Nat. Rev. Dis. Prim. 2015;1:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N., et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 143.Vega R.B., Matsuda K., Oh J., Barbosa A.C., Yang X., Meadows E., McAnally J., Pomajzl C., Shelton J.M., Richardson J.A., et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 144.Kozhemyakina E., Cohen T., Yao T.-P., Lassar A.B. Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2A/histone deacetylase 4/MEF2 pathway. Mol. Cell. Biol. 2009;29:5751–5762. doi: 10.1128/MCB.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao G., Jiang D., Thomas P., Benson M.D., Guan K., Karsenty G., Franceschi R.T. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 146.Kawane T., Qin X., Jiang Q., Miyazaki T., Komori H., Yoshida C.A., dos Matsuura-Kawata V.K.S., Sakane C., Matsuo Y., Nagai K., et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci. Rep. 2018;8:1–17. doi: 10.1038/s41598-018-31853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matsushita Y., Ono W., Ono N. Synergy of single-cell sequencing analyses and in vivo lineage-tracing approaches: A new opportunity for stem cell biology. Biocell Off. J. Soc. Latinoam. Microsc. Electron. 2022;46:1157–1162. doi: 10.32604/biocell.2022.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dy P., Wang W., Bhattaram P., Wang Q., Wang L., Ballock R.T., Lefebvre V. Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes. Dev. Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qi S., Wang Y., Wei X., Xie D., Mohsen R., Hsieh Y.-L., Mishina Y., Liu F. Expression of Cre recombinase in chondrocytes causes abnormal craniofacial and skeletal development. Transgenic Res. 2022;31:399–411. doi: 10.1007/s11248-022-00308-8. [DOI] [PubMed] [Google Scholar]