Abstract
Previous studies have consistently revealed that both local and systemic inflammations are the key to the onset and progression of osteoarthritis (OA). Thus, anti-inflammatory biologic agents could potentially attenuate the progression of OA. We conducted this meta-analysis to examine the efficacy and safety of ant-inflammatory biologic agents among OA patients. Methods: Five databases were searched for randomized controlled trials (RCTs) comparing biologics with placebo or each other in OA patients. Data of pain, physical function, stiffness, and adverse events (AEs) were extracted for a conventional and a Bayesian network meta-analysis. Results: 15 studies with data for 1566 patients were analyzed. In the conventional meta-analysis, etanercept (SMD −0.47; 95% CI −0.89, −0.05) and infliximab (SMD −2.04; CI −2.56, −1.52) were superior to placebo for knee pain. In the network meta-analysis, infliximab was superior to all the other biologic agents in improving pain (vs. hyaluronic acid (SMD −22.95; CI −34.21, −10.43), vs. adalimumab (SMD −21.71; CI −32.65, −11.00), vs. anakinra (SMD −24.63; CI −38.79, −10.05), vs. canakinumab (SMD −32.83; CI −44.45, −20.68), vs. etanercept (SMD −18.40; CI −29.93, −5.73), vs. lutikizumab (SMD −25.11; CI −36.47, −14.78), vs. naproxen (SMD −30.16; CI −41.78, −17.38), vs. tocilizumab (SMD −24.02; CI −35.63, −11.86) and vs. placebo (SMD −25.88; CI −34.87, −16.60)). No significant differences were observed between biologics and placebo regarding physical function, stiffness, and risk of AEs. Conclusions: The findings suggest that infliximab may relieve pain more than other biological agents in OA patients. No significant differences were observed between biologics and placebo regarding physical function, stiffness, and risk of AEs. The results must be interpreted cautiously; therefore, further randomized controlled trials are warranted.
Keywords: osteoarthritis, biological therapy, inflammation, infliximab, meta-analysis
1. Introduction
Osteoarthritis (OA) has become a major health challenge around the world due to its rising prevalence and enormous burden caused individually and socially. There are no approved drugs with disease-modifying effects, let alone the number of risk considerations for the available medications that could relieve symptoms [1,2,3]. Thus, developing new drugs to address unmet medical needs is crucial.
Although OA used to be considered as a noninflammatory disease, it is now well recognized that chronic and low-grade inflammation is involved in OA progression. Inflammatory factors and chemokines have been reported to contribute to inflammation in both synovial cells and chondrocytes [4]. Anti-inflammatory biologic agents, including but not limited to TNF-α inhibitors (e.g., adalimumab), interleukin-1 (IL-1) inhibitors (e.g., canakinumab), and IL-6 inhibitors (e.g., tocilizumab), have been used in treating rheumatoid arthritis (RA) and other inflammatory diseases. They can suppress specific components of the immune system and thereby inhibit the activation of inflammatory pathways mediated by inflammatory factors [5,6,7]. For this reason, anti-inflammatory agents may be promising agents to attenuate disease progression of OA. However, randomized controlled trials (RCTs) examining the efficacy and safety of biologics in OA patients have shown inconclusive results. For example, Fleischmann et al. [8] suggested that lutikizumab use significantly relieved pain compared with placebo, while Kloppenburh et al. [9] reported that lutikizumab did not alleviate pain or imaging outcomes in comparison to placebo. Moreover, previous systematic reviews also indicated the inconsistent efficacy of biologic agents [10,11,12]. Therefore, we performed an up-to-date network meta-analysis to compare the efficacy and safety of biologics targeting inflammation among OA patients. By using network meta-analysis, we can estimate the efficacy and safety between all possible pairs of treatments and then rank them in order of the size of effects.
2. Methods
2.1. Protocol and Registration
This network meta-analysis was performed according to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) extension statement for network meta-analysis (Supplementary Table S1) [13], and registered in PROSPERO (CRD42020196343).
2.2. Search Strategies and Selection Criteria
A systematic search was conducted using electronic databases of Medline (PubMed), Embase, Web of Science, Cochrane Library Central Register of Controlled Trials (CENTRAL), and www.ClinicalTrials.gov, from inception to 1 February 2022. The language was limited to English. The search procedures and strategies are shown in Supplementary Table S2. Eligible studies met the following criteria:
RCTs.
Patients with clinically or radiographically diagnosed primary OA at any joints.
Interventions or exposures included adalimumab, lutikizumab (ABT981), canakinumab, anakinra, etanercept, infliximab, and any other TNF-α, IL-1, IL-6 or IL-17 inhibitors alone or in combination.
Studies met the following criteria were excluded:
Retrospective research, review, or meta-analysis.
Studies that only published as abstract or without extractable data.
Follow-up duration <1 week.
Studies that did not report pain, physical function, stiffness, or adverse events (AEs) as outcomes.
All retrieved articles were imported into EndNote X9 software. After excluding repeated ones, two investigators (YL and YM) screened the titles and abstracts independently according to the inclusion criteria. Any disagreements between them over the eligibility of particular studies were resolved through discussion with a third investigator (ZZ).
3. Outcomes and Data Extraction
The primary outcomes were mean changes from baseline in pain and physical function score. Secondary outcomes were AEs and mean change in stiffness score. For pain, physical function, and stiffness, the time point was at or nearest to 12 weeks, and for Aes, the time point was at the end of the study. When pain, physical function, and stiffness were measured using different scales in one study, we referred to a previously described hierarchy of relative outcomes and extracted data that was highest on the list (Supplementary Table S3) [14,15].
Two investigators (YL and YM) extracted data independently with standardized forms. The data were checked by a third investigator (ZZ). For pain, physical function, and stiffness, the changes from baseline at or nearest to 12 weeks were extracted and calculated as the arithmetic differences between baseline and follow-up. If standard deviations (SD) were not provided, we calculated or imputed them using methods reported in Cochrane Handbook for Systematic Reviews of Interventions [16]. For AEs, the number of patients who experienced any AEs and withdrawal due to AEs was calculated. For graphical information, numerical data was extracted using Engauge Digitizer 12.1 software (Mark Mitchell, Palos Verdes Peninsula, CA, USA). If a study involved multiple treatment groups with different doses or administration of the same drugs, the data were combined into one treatment group.
For crossover studies, if the data were given based on the order in which the participants received the treatments, the data from each period were extracted and analysed separately. Other extracted data included first author, year of publication, study design, details of interventions, sample size, demographic characteristics [age, sex, and body mass index (BMI)], follow-up duration, study joint, and outcome assessment.
4. Quality Assessments
Two investigators (YL and YM) assessed the risk of bias of included studies independently using the Cochrane Risk of Bias Tool for RCT and the Newcastle-Ottawa scale (NOS) for prospective cohort study [17,18]. The Cochran Risk of Bias Tool for RCT assessed five aspects: random sequence generation, allocation concealment, blinding method, outcome assessment, and reporting of result. Each aspect was judged to be low, unclear, or high risk of bias. For NOS, selection of the study groups, comparability among different groups, and ascertainment of either the interested exposure or outcome were evaluated. A score less than 4 indicates a high risk of bias; a score between 4 and 6 indicates a moderate risk of bias; and a score equal to or higher than 7 indicates a low risk of bias.
5. Statistical Analyses
To estimate the pooled odds ratios (OR) for dichotomous outcomes and standardized mean differences (SMD) for continuous variables, we first performed a conventional meta-analysis with RevMan 5.4 software (Cochrane Collaborating, Copenhagen, Denmark). Heterogeneity in each direct comparison was assessed using the I2 test (I2 ≥ 50% was considered heterogeneous and a random-effect model was used, otherwise a fixed-effect model was used). Sensitivity analyses were conducted to test the robustness of the results under the fixed and random models. Subgroup analyses were also conducted if applicable.
A Bayesian network meta-analysis was then performed using ADDIS 1.16.5 software (Drug Information Systems, Groningen, The Netherlands) [19]. Based on the Markov chain Monte Carlo (MCMC) simulation method, the Bayesian network meta-analysis method can integrate all direct and indirect comparisons and estimate the probability of each intervention becoming the best one. The consistency between direct and indirect comparisons was tested by node-splitting analysis and inconsistency standard deviation (ISD). When node-splitting analysis determined a p value > 0.05 and 95% CI of ISD included 1, the consistency model was used for pooled analysis; otherwise, the inconsistency model was used [20]. The model convergence was assessed using a potential scale reduction factor (PSRF) of the Brooks-Gelman-Rubin (BGR) diagnostic [21]. PSRF closer to 1 indicated better convergence, and it was acceptable if PSRF < 1.2. Finally, the ranking probability of agents for each outcome was calculated.
Stata 15.1 software (Stata Corp, College Station, TX, USA) was used to draw the network plot and assess the publication bias by examining funnel plot asymmetry and Egger’s test. A roughly symmetrical funnel plot and an Egger’s test p value over 0.05 indicates no evidence of publication bias.
6. Results
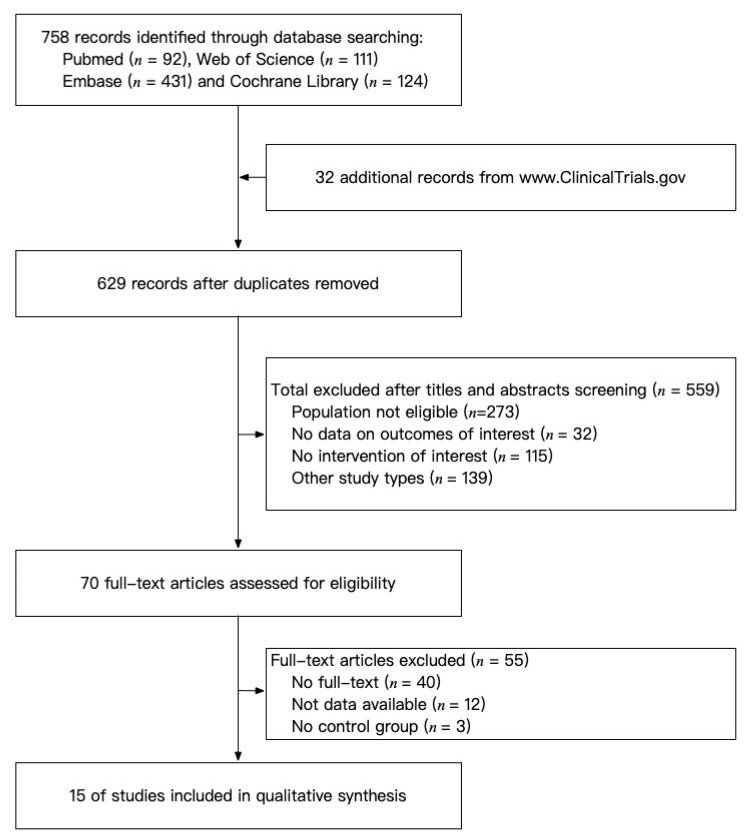
A total of 758 records were retrieved, of which 15 RCTs met the predefined criteria, including 1566 patients (Figure 1) [8,9,22,23,24,25,26,27,28,29,30,31,32,33,34]. No observational study was included. Table 1 showed the baseline demographic characteristics of included studies. Eight studies included patients with knee OA and seven studies included patients with hand OA. The mean age of included patients ranged from 54.3 to 66.0 years. All the patients were categorized into 12 intervention groups according to different treatments they had received: placebo, adalimumab, lutikizumab (ABT981), canakinumab, naproxen, hyaluronic acid (HA), anakinra, etanercept, infliximab, AMG108, tocilizumab, and standard care. Naproxen and HA were the control groups in some of the studies and were therefore included in the analysis. AEs were reported in all the studies and 12 studies reported outcome measures for pain, nine for physical function, and six for stiffness. Baseline characteristics of patients were generally comparable regarding age, sex composition, BMI, OA severity, and disease duration within studies.
Figure 1.
Detailed study selection process.
Table 1.
Characteristics of included studies.
| First Author, Publication Year | Study Design | Intervention | Sample Size | Female, n (%) | Age (Year) | BMI | Duration of Complaints (Year) | Follow-Up | Joint | Outcome Assessment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D. Aitken, 2018 [22] | Crossover RCT | Placebo | 25 | 18 (72) | 61.2 ± 8.4 | 28.8 ± 4.5 | NA | 12 weeks | hand | pain, function, stiffness and adverse events. | |
| Adalimumab (40 mg) | 18 | 15 (83) | 63.1 ± 8.4 | 29.2 ± 3.8 | NA | ||||||
| Chevalier, 2009 [23] | RCT | Placebo | 69 | 44 (64) | 62.2 ± 10 | NA | 6 ± 6.2 | 12 weeks | knee | pain, function, stiffness and adverse events. | |
| Anakinra (50 mg) | 34 | 17 (50) | 63.3 ± 9.8 | NA | 8.1 ± 9.8 | ||||||
| Anakinra (150 mg) | 67 | 46 (69) | 62.6 ± 9.4 | NA | 5.2 ± 5.7 | ||||||
| Chevalier, 2014 [24] | RCT | Placebo | 42 | 35 (83.3) | 62.2 ± 7 | 24.7 ± 3.5 | 13.5 ± 9.1 | 26 weeks | hand | function and adverse events. | |
| Adalimumab (40 mg) | 41 | 36 (87.8) | 62.8 ± 6.9 | 25.2 ± 4.6 | 13.5 ± 9.8 | ||||||
| Verbruggen, 2014 [29] | RCT | Placebo | 30 | (83.3) | 60.7 ± 6.9 | NA | 14.4 ± 8.8 | 52 weeks | hand | pain, stiffness, function and adverse events. | |
| Adalimumab (40 mg) | 30 | (86.7) | 61.9 ± 6.1 | NA | 9.6 ± 6.1 | ||||||
| Fleischmann, 2019 [8] | RCT | Placebo | 85 | 52 (61.2) | 59.5 ± 8.9 | 28.6 ± 3.6 | 7.9 ± 8 | 52 weeks | knee | pain and adverse events. | |
| Lutikizumab (25 mg) | 89 | 63 (70.8) | 61.6 ± 7.5 | 28.7 ± 3.8 | 7.6 ± 9 | ||||||
| Lutikizumab (100 mg) | 85 | 53 (62.4) | 60.2 ± 8.2 | 29 ± 3.5 | 7.9 ± 8.7 | ||||||
| Lutikizumab (200 mg) | 88 | 57 (64.8) | 59.1 ± 10.3 | 28.7 ± 3.5 | 8.7 ± 8.6 | ||||||
| Kloppenburg, 2018 [9] | RCT | Placebo | 67 | 58 (87) | 66 ± 7 | 28 ± 5 | 11 ± 8 | 26 weeks | hand | function; adverse events. | |
| Lutikizumab (200 mg) | 64 | 53 (83) | 66 ± 8 | 27 ± 5 | 11 ± 9 | ||||||
| Wang S.X., 2017 [30] | RCT | Part A | Placebo | 6 | 5 (83.3) | 60 ± 5.9 | 28.4 ± 2.3 | NA | 127 days | knee | adverse events. |
| ABT981 (0.3 mg/kg) | 7 | 5 (71.4) | 61.3 ± 5.1 | 27.6 ± 4.4 | NA | ||||||
| ABT981 (1 mg/kg) | 7 | 5 (71.4) | 62.6 ± 3.6 | 26.4 ± 1.1 | NA | ||||||
| ABT981 (3 mg/kg) | 7 | 7 (100) | 61.4 ± 5 | 27.3 ± 2.9 | NA | ||||||
| Part B | Placebo | 2 | 2 (100) | 55 ± 1.4 | 28.7 ± 0.5 | NA | |||||
| ABT981 (3 mg/kg) | 7 | 7 (100) | 60 ± 6.1 | 29.3 ± 3 | NA | ||||||
| Kloppenburg, 2018a [27] | RCT | Placebo | 45 | 36 (80) | 60.1 ± 8.7 | 25.5 ± 3.8 | 10.7 ± 8 | 1 year | hand | pain and adverse events. | |
| Etanercept (25–50 mg) | 45 | 37 (82) | 59.4 ± 6.5 | 26.3 ± 3.8 | 8.8 ± 6 | ||||||
| NCT01144143, 2018 [33] | RCT | Placebo | 4 | 4 (100) | NA | NA | NA | 2 months | knee | adverse events. | |
| Standard care (Methylprednisolone acetate) | 4 | 4 (100) | NA | NA | NA | ||||||
| Infliximab | 8 | 5 (62.5) | NA | NA | NA | ||||||
| NCT01160822, 2012 [32] | RCT | Part A | Placebo | 5 | 2 (40) | 57.8 ± 7.8 | NA | NA | 126 days | knee | pain, stiffness, function and adverse events. |
| Canakinumab (150 mg) | 6 | 3 (50) | 58.3 ± 12.8 | NA | NA | ||||||
| Canakinumab (300 mg) | 7 | 4 (57.1) | 61 ± 9.6 | NA | NA | ||||||
| Canakinumab (600 mg) | 6 | 2 (33.3) | 64.2 ± 10.7 | NA | NA | ||||||
| Part B | Placebo | 47 | 31 (66) | 60.3 ± 9.7 | NA | NA | |||||
| Canakinumab (600 mg) | 45 | 31 (68.9) | 61.4 ± 9.0 | NA | NA | ||||||
| Naproxen (500 mg) | 53 | 34 (64.2) | 62.2 ± 8.1 | NA | NA | ||||||
| Cohen, 2011 [25] | RCT | Part A | placebo | 16 | 10 (63) | 60.8 | 30.4 | 9.6 | 140 days | knee | Part A: adverse events; Part B: pain, function, stiffness and adverse events. |
| AMG108 (100 mg) | 12 | 11 (92) | 61.1 | 30.8 | 6.9 | ||||||
| AMG108 (300 mg) | 12 | 7 (58) | 62.8 | 31.9 | 10.2 | ||||||
| AMG108 (300 mg) | 12 | 5 (42) | 59.6 | 29.8 | 6.6 | ||||||
| AMG108 (75 mg) | 12 | 9 (75) | 62.3 | 30.9 | 10 | ||||||
| Part B | Placebo | 80 | 54 (68) | 60.1 | 31.9 | 6.1 | 12 weeks | ||||
| AMG108 (300 mg) | 80 | 54 (68) | 61.3 | 32 | 6.1 | ||||||
| Wang J., 2018 [31] | Open label RCT | HA (25 mg) | 28 | 21 (75) | 56.9 ± 9.1 | 24.7 ± 3.3 | NA | 4 weeks | knee | pain, function, stiffness and adverse events. | |
| Adalimumab (10 mg) | 28 | 19 (68) | 54.3 ± 8.7 | 25.3 ± 3.2 | NA | ||||||
| Ohtori, 2015 [28] | RCT | HA (25 mg) | 20 | 13 (65) | 64.3 ± 5.6 | NA | NA | 4 weeks | knee | pain, function, stiffness and adverse events. | |
| Etanercept (10 mg) | 19 | 13 (68) | 63.3 ± 7.2 | NA | NA | ||||||
| Fioravanti, 2009 [26] | RCT | Placebo | 10 * | 10 (100) | 60.7 ± 6.2 | NA | 7.5 ± 3.5 | 1 year | hand | pain and adverse events. | |
| Infliximab (0.2 mg) | NA | ||||||||||
| Richette, 2020 [34] | RCT | Placebo | 41 | 34 (82.9) | 64.7 ± 8.6 | 25.7 ± 4.9 | 10.7 ± 9.8 | 12 weeks | hand | pain, function and adverse events. | |
| Tocilizumab (8 mg/kg) | 42 | 34 (81) | 64.1 ± 8.9 | 23.1 ± 3.9 | 9.1 ± 6.3 | ||||||
* 10 participants were enrolled in the study. For each patient, the most affected hand was identified and treated with infliximab, while the contralateral hand was treated with placebo. Treatment consisted in injection of infliximab or placebo in each affected proximal interphalangeal and distal interphalangeal joint. The total number of joints treated with infliximab and placebo was 56 and 34, respectively. The number of treated joints was used for analysis. RCT, randomized controlled clinical trial. BMI, body mass index. HA, hyaluronic acid. NA, not avaliable. ABT981, the alias of lutikizumab.
Significant pain reductions were found in the following comparisons of conventional meta-analysis (Supplementary Figure S1): etanercept vs. placebo (SMD −0.47; −0.89 to −0.05), infliximab vs. placebo (SMD −2.04; −2.56 to −1.52), tocilizumab vs. placebo (SMD −0.60; −1.05 to −0.15), and adalimumab vs. HA (SMD −0.62; −1.16 to −0.09). In addition, canakinumab showed a weaker analgesic effect compared with both placebo (SMD −1.69; −1.2 to −2.19) and naproxen (SMD −0.66; −0.24 to −1.08). According to the network meta-analysis (Table 2 and Figure 2), Infliximab was associated with significantly more pain reduction than all the other drugs: infliximab vs. HA (SMD −22.95; −34.21 to −10.43), infliximab vs. adalimumab (SMD −21.71; −32.65 to −11.00), infliximab vs. anakinra (SMD −24.63; −38.79 to −10.05), infliximab vs. canakinumab (SMD −32.83; −44.45 to −20.68), infliximab vs. etanercept (SMD −18.40; −29.97 to −5.73), infliximab vs. lutikizumab (SMD −25.11; −36.47 to −14.78), infliximab vs. naproxen (SMD −30.16; −41.78 to −17.38), infliximab vs. placebo (SMD −25.88; −34.87 to −16.60), infliximab vs. tocilizumab (SMD −24.02; −35.63 to −11.86). Adalimumab (SMD −11.11; −20.16 to −1.26) and etanercept (SMD −14.40; −26.10 to −3.24) were significantly better than canakinumab. And etanercept have a stronger analgesic effect than naproxen (SMD −11.71; −23.06 to −0.52). But adalimumab, naproxen, and etanercept were both not superior to placebo. All other comparisons did not show significant differences. Similar result was found in probability ranking (Supplementary Table S4), which indicated that infliximab was the best drug (98% chance) for analgesia, while canakinumab (79% chance) was the worst.
Table 2.
Network meta-analysis of pain for different interventions.
| HA | −1.29 (−7.19, 6.82) |
1.53 (−11.33, 15.78) |
9.86 (−0.86, 21.70) |
−4.34 (−12.06, 3.83) |
−22.95 (−34.21, −10.43) |
2.10 (−6.40, 13.15) |
7.07 (−3.31, 18.93) |
2.82 (−4.28, 11.63) |
0.94 (−9.31, 12.69) |
| 1.29 (−6.82, 7.19) |
adalimumab | 2.61 (−10.00, 15.01) |
11.11 (1.26, 20.16) |
−3.20 (−12.21, 4.56) |
−21.71 (−32.65, −11.00) |
3.36 (−4.59, 11.75) |
8.32 (−2.04, 17.30) |
4.05 (−1.85, 9.67) |
2.13 (−7.16, 11.53) |
| −1.53 (−15.78, 11.33) |
−2.61 (−15.01, 10.00) |
anakinra | 8.42 (−5.25, 21.59) |
−6.23 (−20.18, 7.22) |
−24.63 (−38.79, −10.05) |
0.57 (−12.07, 13.76) |
5.61 (−7.82, 18.79) |
1.37 (−10.19, 12.35) |
−0.55 (−13.71, 12.67) |
| −9.86 (−21.70, 0.86) |
−11.11 (−20.16, −1.26) |
−8.42 (−21.59, 5.25) |
canakinumab | −14.40 (−26.10, −3.24) |
−32.83 (−44.45, −20.68) |
−7.88 (−16.56, 2.73) |
−2.76 (−10.55, 4.47) |
−7.04 (−14.91, 0.88) |
−8.92 (−19.54, 2.60) |
| 4.34 (−3.83, 12.06) |
3.20 (−4.56, 12.21) |
6.23 (−7.22, 20.18) |
14.40 (3.24, 26.10) |
etanercept | −18.40 (−29.97, −5.73) |
6.78 (−2.66, 17.44) |
11.71 (0.52, 23.06) |
7.49 (−0.57, 15.93) |
5.59 (−5.45, 17.28) |
| 22.95 (10.43, 34.21) |
21.71 (11.00, 32.65) |
24.63 (10.05, 38.79) |
32.83 (20.68, 44.45) |
18.40 (5.73, 29.97) |
infliximab | 25.11 (14.78, 36.47) |
30.16 (17.38, 41.78) |
25.88 (16.60, 34.87) |
24.02 (11.86, 35.63) |
| −2.10 (−13.15, 6.40) |
−3.36 (−11.75, 4.59) |
−0.57 (−13.76, 12.07) |
7.88 (−2.73, 16.56) |
−6.78 (−17.44, 2.66) |
−25.11 (−36.47, −14.78) |
lutikizumab | 5.13 (−6.00, 13.83) |
0.79 (−5.86, 6.12) |
−1.05 (−11.31, 8.10) |
| −7.07 (−18.93, 3.31) |
−8.32 (−17.30, 2.04) |
−5.61 (−18.79, 7.82) |
2.76 (−4.47, 10.55) |
−11.71 (−23.06, −0.52) |
−30.16 (−41.78, −17.38) |
−5.13 (−13.83, 6.00) |
naproxen | −4.34 (−11.64, 3.87) |
−6.17 (−16.78, 5.46) |
| −2.82 (−11.63, 4.28) |
−4.05 (−9.67, 1.85) |
−1.37 (−12.35, 10.19) |
7.04 (−0.88, 14.91) |
−7.49 (−15.93, 0.57) |
−25.88 (−34.87, −16.60) |
−0.79 (−6.12, 5.86) |
4.34 (−3.87, 11.64) |
placebo | −1.86 (−9.55, 5.93) |
| −0.94 (−12.69, 9.31) |
−2.13 (−11.53, 7.16) |
0.55 (−12.67, 13.71) |
8.92 (−2.60, 19.54) |
−5.59 (−17.28, 5.45) |
−24.02 (−35.63, −11.86) |
1.05 (−8.10, 11.31) |
6.17 (−5.46, 16.78) |
1.86 (−5.93, 9.55) |
tocilizumab |
HA, hyaluronic acid.
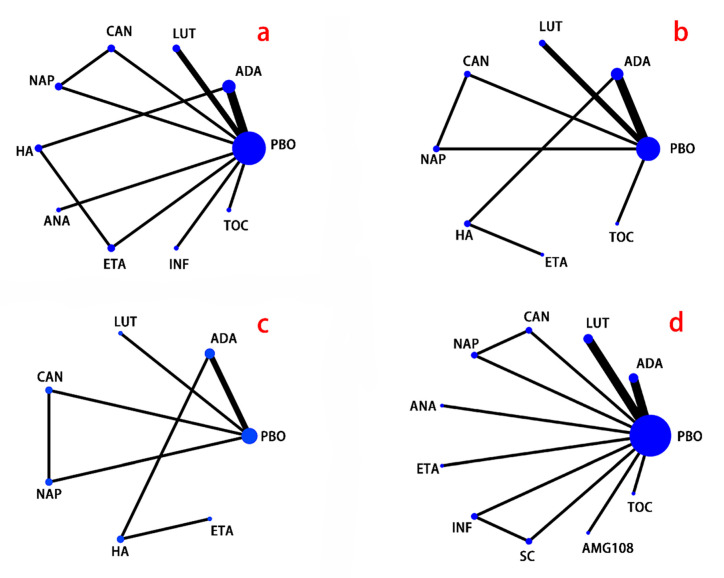
Figure 2.
Network plot. (a), pain; (b), physical function; (c), stiffness; (d), adverse events; PBO, placebo; ADA, adalimumab; LUT, lutikizumab (ABT981); CAN, canakinumab; NAP, naproxen; HA, hyaluronic acid; ANA, anakinra; ETA, etanercept; INF, infliximab; SC, standard care; TOC, tocilizumab. The size of the circle represents the number of participants, and the thickness of the line represents the number of studies.
In the conventional meta-analysis for physical function (Supplementary Figure S2), adalimumab was associated with a greater physical function improvement compared with HA (SMD −0.88; −1.44 to −0.33), and tocilizumab can significantly improve function compared with placebo (SMD −1.48; −2.00 to −0.97). However, canakinumab showed a weaker physical function improvement compared to placebo (SMD −1.55; −1.07 to −2.03) and naproxen (SMD −0.52; −0.10 to −0.94). No significant difference was found in other comparisons. None of the drugs showed significant differences compared with placebo in network meta-analysis (Table 3), while probability ranking provided the hierarchy of physical function-improving effect and indicated that etanercept (28% chance) could be the best option for function improvement (Supplementary Table S5).
Table 3.
Network meta-analysis of physical function for different interventions.
| HA | −11.20 (−27.02, 5.06) |
−7.42 (−31.21, 18.42) |
−5.56 (−36.82, 26.75) |
−12.09 (−33.96, 12.03) |
−8.30 (−32.06, 17.23) |
−10.49 (−28.85, 9.75) |
−11.42 (−34.67, 14.45) |
| 11.20 (−5.06, 27.02) |
adalimumab | 3.79 (−14.76, 23.57) |
5.74 (−29.24, 41.42) |
−0.71 (−16.17, 16.80) |
2.88 (−15.57, 22.17) |
0.84 (−9.75, 12.78) |
−0.18 (−18.07, 19.53) |
| 7.42 (−18.42, 31.21) |
−3.79 (−23.57, 14.76) |
canakinumab | 1.97 (−36.87, 40.63) |
−4.61 (−22.84, 14.81) |
−0.94 (−15.52, 14.22) |
−2.98 (−18.17, 12.22) |
−3.96 (−25.31, 17.87) |
| 5.56 (−26.75, 36.82) |
−5.74 (−41.42, 29.24) |
−1.97 (−40.63, 36.87) |
etanercept | −6.65 (−44.41, 30.86) |
−2.80 (−41.62, 35.74) |
−4.73 (−41.97, 31.34) |
−5.69 (−45.02, 32.45) |
| 12.09 (−12.03, 33.96) |
0.71 (−16.80, 16.17) |
4.61 (−14.81, 22.84) |
6.65 (−30.86, 44.41) |
lutikizumab | 3.72 (−15.12, 21.86) |
1.57 (−10.56, 12.82) |
0.68 (−19.27, 19.82) |
| 8.30 (−17.23, 32.06) |
−2.88 (−22.17, 15.57) |
0.94 (−14.22, 15.52) |
2.80 (−35.74, 41.62) |
−3.72 (−21.86, 15.12) |
naproxen | −2.12 (−16.69, 12.69) |
−3.03 (−24.39, 18.37) |
| 10.49 (−9.75, 28.85) |
−0.84 (−12.78, 9.75) |
2.98 (−12.22, 18.17) |
4.73 (−31.34, 41.97) |
−1.57 (−12.82, 10.56) |
2.12 (−12.69, 16.69) |
placebo | −0.91 (−15.59, 14.26) |
| 11.42 (−14.45, 34.67) |
0.18 (−19.53, 18.07) |
3.96 (−17.87, 25.31) |
5.69 (−32.45, 45.02) |
−0.68 (−19.82, 19.27) |
3.03 (−18.37, 24.39) |
0.91 (−14.26, 15.59) |
tocilizumab |
HA, hyaluronic acid.
In terms of stiffness, the conventional meta-analysis demonstrated that canakinumab was associated with a weaker stiffness improvement compared to placebo (SMD −1.61; −1.12 to −2.09) and naproxen (SMD −0.83; −0.40 to −1.25). The remaining interventions were not associated with significant improvement in stiffness (Supplementary Figure S3). Network meta-analysis demonstrated no significant differences in all comparisons (Supplementary Table S6). Based on the probability ranking, lutikizumab (37% chance) was the best option for stiffness, while etanercept (30% chance) was the worst (Supplementary Table S7).
All studies reported outcomes of AEs. No significant difference was reported regarding incidence rates of AEs between treatment and control groups (Supplementary Figure S4). AEs were common in most studies except for that two studies [26,28] did not record any AEs and one study [31] recorded only one patient developed AEs. Reported AEs included fall, headache, infections, sinusitis, vertigo, eczema, rash, or itching, injection site reaction, neutropenia, malignancies, and death. The most frequently reported AEs were infections, injection site reaction, and arthralgia. Yet, serious AEs were rare. Dose-dependent increases in AEs were found in anakinra and lutikizumab [9,23].
Three studies [26,28,31] were excluded from the network meta-analysis of AEs to prevent a widely pooled confidence interval and inaccurate results because their number of AEs in the treatment group and/or the control group were zero. An inconsistency model was used for network meta-analysis because the calculated 95% CI of ISD (ISD 0.43; 0.03 to 0.82) did not include 1. No significant results were found in the conventional and network meta-analysis (Supplementary Figure S4 and Table S8), suggesting that anti-inflammatory biologics did not increase AEs. Rank probability was not available in the inconsistency model.
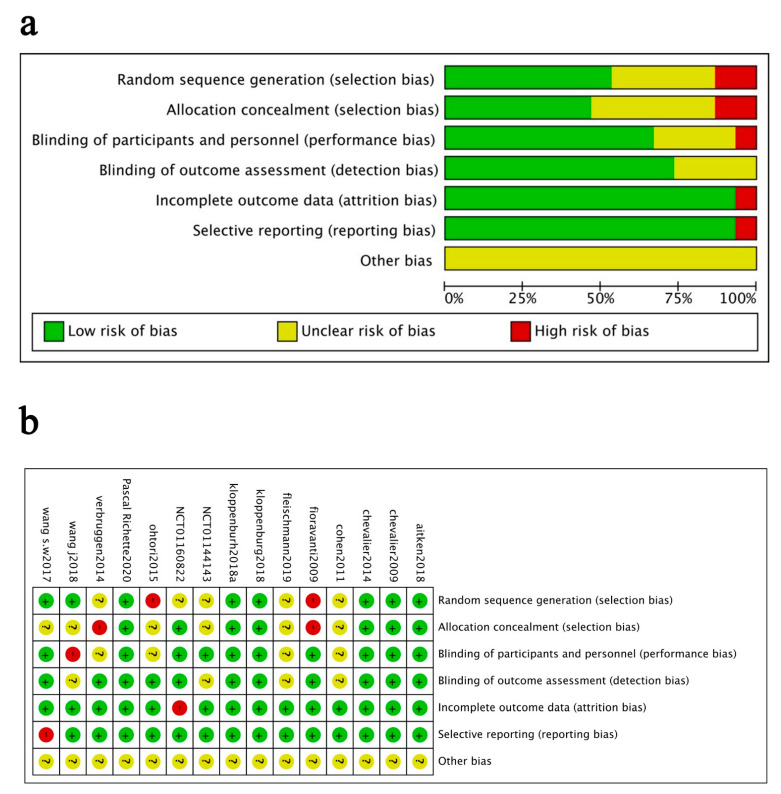
The quality assessments for pain, physical function, stiffness and adverse events indicated no serious risk of bias (data not shown). Figure 3 shows the quality assessment for adverse events. 53% of the studies were judged to have a low risk of bias for random sequence generation, 47% for allocation concealment, 93% for incomplete outcome data, 60% for blinding of participants, 93% for selective reporting, and 73% for blinding of outcome assessment. Two studies (13%) were judged to have a high risk of bias for random sequence generation since they did not mention randomization; two (13%) for allocation concealment since their allocation results could be predicted; one (7%) for incomplete data since it only analysed the completers’ data; one (7%) for blinding of participants since it was an open-label design, and one (7%) for selective reporting since it did not fully report the outcomes. All studies had unclear risks of other bias because they could not be judged clearly.
Figure 3.
Risk of bias assessment. (a) judgements of each bias item presented as percentages across all included studies. (b) judgements of each bias item for each included study.
Sensitivity analyses were conducted to test the robustness of the results under the fixed model and random model, and no change was revealed (Supplementary Tables S9 and S10). Except for the AEs comparison, which showed a significant inconsistency, the homogeneity and consistency assumptions of the remaining outcomes comparisons were confirmed (Supplementary Tables S11 and S12). The funnel plot (Supplementary Figure S5) and the Egger’s test (Supplementary Table S13) found no publication bias. Subgroup analyses by OA locations (hand and knee) using conventional meta-analysis did not show statistically significant symptoms relief by comparing biologics with placebo (data not shown).
7. Discussion
We estimated the relative efficacy and safety of novel biologics targeting inflammations for the treatment of OA using network meta-analysis. Despite limited sample size, we found that infliximab was the most effective treatment compared with all other biologics regarding pain relief. Moreover, according to conventional meta-analysis, etanercept was associated with greater pain relief, and tocilizumab was associated with improvement in pain and physical function, compared with placebo. All the biologics did not increase AEs and were tolerable for OA patients.
The efficacy and safety of anti-inflammatory biologics have been widely studied in other inflammatory diseases. Multiple clinical trials found that infliximab, an anti-TNF-α biologics, was effective for ankylosing spondylitis (AS) and RA [35,36]. Sbidian et al. reported that biologics targeting IL-17, IL-12/23, and TNF-α were more effective than placebo while retaining a sound safety profile for the treatment of psoriasis [37]. A meta-analysis also confirmed the efficacy of anti-TNF-α biologics for inducing and maintaining mucosal healing in patients with Crohn’s disease and ulcerative colitis [38]. By using pooled analysis of the latest clinical trials based on the MCMC simulation method, we can retain direct effects of treatments in each trial and compare all the treatments across trials with a sound statistical precision at the same time. Our study found that infliximab achieved a greater pain relief than any other biologics or placebo, yet it did not increase AEs. Infliximab is a monoclonal IgG1 antibody against TNF-α. It exerts an anti-inflammatory effect by directly binding to TNF-α and blocking its affinity with the corresponding receptors [39]. Our finding suggests that targeting TNF-α could also be an effective therapeutic strategy for OA.
In contrast, other types of TNF-α inhibitors (e.g., adalimumab and etanercept) were not significantly associated with improved OA symptoms. Adalimumab and etanercept exert anti-inflammatory effects through the same mechanism as infliximab, but with a different antibody-protein composition [40]. One possible reason for the inconsistent efficacy of TNF-α inhibitors is the presence of anti-drug antibodies (ADAs) [41]. Since biologics are proteins, they can trigger the immune response and induce ADAs formation. ADAs can cause non-response to the treatment and increase the risk of AEs in RA, psoriasis and other inflammatory diseases [42,43,44]. Numerous factors such as molecular structure, dose, sex, and co-administration with other anti-inflammatory drugs, may have influenced the immunogenic of biologics. To the best of our knowledge, there is no study examining how ADAs affect biologic therapies for OA. Hence, more research are needed in the future to disentangle this, and it is vital to consider immunogenic when selecting biologics as the therapy.
Another reason may be related to the way of drug administration. It is reported that free drugs injected in the articular joint can be rapidly cleared, resulting in decreased retention time, low peak drug concentration, and limited therapeutic effect [45]. Given that nearly half of the included studies used intra-articular injection, it was not surprising that our meta-analysis and most of the clinical trials demonstrated negative results. Recently, several advanced drug delivery systems have been developed and proved to be effective in prolonging retention time and improving targeting specificity in animal models [46,47]. Combining novel drug delivery systems and investigational biologics could be an optimal strategy for the treatment of OA, albeit rational designed clinical trials are warranted to validate their efficacies.
Cytokines play important roles in OA progression [48]. However, our network meta-analysis indicated the remaining biologics did not result in symptoms relief compared to placebo. This may be due to the heterogeneity of OA phenotypes and the complexity of the interaction of pro-inflammatory signaling pathways [49,50]. Current meta-analyses found that although biologic agents were generally effective for OA pain relief, subgroup of IL-1 inhibitors or TNF-α inhibitors were not superior to placebo [10,11,12]. We demonstrated consistent results on the ineffectiveness of IL-1 inhibitors, but inconsistently we found infliximab could be effective. It may suggest that the efficacy of biologic agents varies according to mechanism of action, and pro-inflammatory cytokines are not the key drivers of OA symptoms. Meanwhile, only one to two RCTs were performed for each of the remaining agents, suggesting it is too early to jump to a definite conclusion. We notice that there are currently numerous RCTs in progress and it can be inferred that more studies on novel biological interventions targeting inflammation of OA will appear in the next few years.
We try our best to summarize three potential criteria to profile patients that could benefit the most from infliximab treatment. First, since women generally have more inflammation compared to men, infliximab could be more effective in female OA patients [51,52]. Second, a trial has shown that anti-TNFα could halt the progression in OA patients with swollen joint [29]. Thus, OA patients with inflammatory phenotypes such as synovitis and/or effusion could be more suitable for infliximab treatment. Third, when anti-TNFα therapy was applied to erosive hand OA patients who already have cartilage damage, limited improvement was observed for the structure [22], suggesting that infliximab may achieve better efficacy in the early stage of OA.
To verify our findings, we used different models for analysis which all provided consistent results. Moreover, the well-fitted network model and the low-level heterogeneity indicated the robustness and accuracy of the results. However, this meta-analysis is also subject to potential limitations. First, the number of pooled studies was relatively small, and some included studies had limited sample sizes. Our main finding of infliximab was based on only two trials with only 26 included patients, and there were few direct comparisons. Second, we combined groups of different doses and administration methods of the same intervention. We also combined data on hand and knee OA patients. Women could response more actively to anti-inflammatory agents since they have higher OA prevalence and more inflammation. Unfortunately, we were unable to perform further subgroup analysis at the gender level due to limited number of studies. Nevertheless, gender compositions were largely similar across the 12 intervention groups, ranging from 62.4% to 83.3%, suggesting the impact of gender position on efficacy was minimum. Third, estimated SD values and image data extracted by software were used for analyses, which may be inaccurate. However, the estimated SD values were calculated by official methods of Cochrane Handbook for Systematic Reviews of Intervention, and image data were extracted using Engauge Digitizer software, both of which were considered reliable [16,53]. Fourth, we only extracted data at or nearest to 12 weeks for analysis. The analyses may not be generally applicable to other time points. Last, six studies had high risk of methodological bias. But we could not assess the impact of high-risk studies through sensitive analysis, because most comparisons have only one study. Thus, our results must be interpreted with caution.
8. Conclusions
The findings suggest that infliximab may relieve pain more than other biological agents in OA patients. No significant differences were observed between biologics and placebo regarding physical function, stiffness, and risk of AEs. The results must be interpreted cautiously; therefore, further randomized controlled trials are warranted.
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11143958/s1, Table S1: PRISMA extension statement for network meta-analysis; Table S2: Literature search procedures and strategies; Table S3: A hierarchy list of data extraction; Table S4: Rank probability of pain; Table S5: Rank probability of physical function; Table S6: Network meta-analysis of stiffness for different interventions; Table S7: Rank probability of stiffness; Table S8: Network meta-analysis of AEs for different interventions; Table S9: Sensitivity analysis in pain, stiffness and function; Table S10: Sensitivity analysis in adverse events; Table S11: Node-splitting analyses of pain; Table S12: ISD of inconsistency test; Table S13: Results of Egger’s test; Figure S1: Results of the conventional meta-analysis of pain; Figure S2: Results of the conventional meta-analysis of physical function; Figure S3: Results of the conventional meta-analysis of stiffness; Figure S4: Results of the conventional meta-analysis of adverse events; Figure S5: Publication bias examined by funnel plot.
Author Contributions
Conceptualization, Y.L., Y.M., P.C., X.W. (Xin Wen) and Z.Z.; data curation, Y.L., Y.M. and P.C.; formal analysis, Y.L., Y.M., P.C. and Z.Z.; project administration, Z.Z.; software, Y.L. and Y.M.; supervision, Z.Z.; validation, Y.M., P.C. and Z.Z.; writing—original draft, Y.L. and Y.M.; writing—review & editing, P.C., X.W. (Xin Wen), T.F., X.W. (Xiaoshuai Wang), G.R., S.T., C.D. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (grant number: 32000925), Science and Technology Program of Guangzhou, China (grant number: 202002030481) and Wu Jieping Medical Foundation Program (grant number: 320.6750.2020-03-12). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors thank all the participants and staffs who made this study possible.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.OARSI White Paper: OA as a Serious Disease. [(accessed on 10 August 2020)]. Available online: https://oarsi.org/education/oarsi-resources/oarsi-white-paper-oa-serious-disease.
- 2.Hawker G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019;37((Suppl. S120)):3–6. [PubMed] [Google Scholar]
- 3.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., Kraus V.B., Lohmander L.S., Abbott J.H., Bhandari M., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Shen J., Abu-Amer Y., O’Keefe R.J., McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017;58:49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathak N. Biologics for Treating Rheumatoid Arthritis 2020. [(accessed on 10 August 2020)]. Available online: https://www.webmd.com/rheumatoid-arthritis/biologics#1.
- 6.Jethwa H., Abraham S. Biologic agents in inflammatory arthritis. Br. J. Gen. Pract. 2018;68:204–205. doi: 10.3399/bjgp18X695705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Z., Li J., Ruan G., Wang G., Huang C., Ding C. Investigational drugs for the treatment of osteoarthritis, an update on recent developments. Expert Opin. Investig. Drugs. 2018;27:881–900. doi: 10.1080/13543784.2018.1539075. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann R.M., Bliddal H., Blanco F.J., Schnitzer T.J., Peterfy C., Chen S., Wang L., Feng S., Conaghan P.G., Berenbaum F., et al. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheumatol. 2019;71:1056–1069. doi: 10.1002/art.40840. [DOI] [PubMed] [Google Scholar]
- 9.Kloppenburg M., Peterfy C., Haugen I.K., Kroon F., Chen S., Wang L., Liu W., Levy G., Fleischmann R.M., Berenbaum F., et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 2019;78:413–420. doi: 10.1136/annrheumdis-2018-213336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson M.S.M., Sarmanova A., Doherty M., Zhang W. Conventional and biologic disease-modifying antirheumatic drugs for osteoarthritis: A meta-analysis of randomized controlled trials. Rheumatology. 2018;57:1830–1837. doi: 10.1093/rheumatology/key131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Z., Li Y., Wang W., Jie S., Hu X., Zhou J., Wu T., Aili D., Long Z., Li Y., et al. Is Lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, efficacious for osteoarthritis? Results from a Bayesian network meta-analysis. Biomed Res. Int. 2020;2020:9013283. doi: 10.1155/2020/9013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng F., Li H., Feng H., Long H., Yang Z., Li J., Wang Y., Xie D. Efficacy and safety of biologic agents for the treatment of osteoarthritis: A meta-analysis of randomized placebo-controlled trials. Ther. Adv. Musculoskelet. Dis. 2022;14:1–25. doi: 10.1177/1759720X221080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 14.Juhl C., Lund H., Roos E.M., Zhang W., Christensen R. A hierarchy of patient-reported outcomes for meta-analysis of knee osteoarthritis trials: Empirical evidence from a survey of high impact journals. Arthritis. 2012;2012:136245. doi: 10.1155/2012/136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jüni P., Reichenbach S., Dieppe P. Osteoarthritis: Rational approach to treating the individual. Best Pract. Res. Clin. Rheumatol. 2006;20:721–740. doi: 10.1016/j.berh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane2021. [(accessed on 10 August 2020)]. Available online: www.training.cochrane.org/handbook.
- 17.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newscastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; Ottawa, ON, Canada: 2011. [(accessed on 10 August 2020)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 18.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillege H., Brock B.D., Valkenhoef G.V., Zhao J. ADDIS: An Automated Way to Do Network Meta-Analysis. University of Groningen, Research Institute SOM (Systems, Organisations and Management); Groningen, The Netherlands: 2012. Research Report. [Google Scholar]
- 20.Dias S., Welton N.J., Caldwell D.M., Ades A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 21.Brooks S., Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 1998;7:434–455. [Google Scholar]
- 22.Aitken D., Laslett L.L., Pan F., Haugen I.K., Otahal P., Bellamy N., Bird P., Jones G. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis—The HUMOR trial. Osteoarthr. Cartil. 2018;26:880–887. doi: 10.1016/j.joca.2018.02.899. [DOI] [PubMed] [Google Scholar]
- 23.Chevalier X., Goupille P., Beaulieu A.D., Burch F.X., Bensen W.G., Conrozier T., Loeuille D., Kivitz A.J., Silver D., Appleton B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier X., Ravaud P., Maheu E., Baron G., Rialland A., Vergnaud P., Roux C., Maugars Y., Mulleman D., Lukas C., et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015;74:1697–1705. doi: 10.1136/annrheumdis-2014-205348. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S.B., Proudman S., Kivitz A.J., Burch F.X., Donohue J.P., Burstein D., Sun Y.-N., Banfield C., Vincent M.S., Ni L., et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011;13:R125. doi: 10.1186/ar3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioravanti A., Fabbroni M., Cerase A., Galeazzi M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: A pilot study. Rheumatol. Int. 2009;29:961–965. doi: 10.1007/s00296-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 27.Kloppenburg M., Ramonda R., Bobacz K., Kwok W.-Y., Elewaut D., Huizinga T.W.J., Kroon F.P.B., Punzi L., Smolen J.S., Cruyssen B.V., et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): A multicentre, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018;77:1757–1764. doi: 10.1136/annrheumdis-2018-213202. [DOI] [PubMed] [Google Scholar]
- 28.Ohtori S., Orita S., Yamauchi K., Eguchi Y., Ochiai N., Kishida S., Kuniyoshi K., Aoki Y., Nakamura J., Ishikawa T., et al. Efficacy of Direct Injection of Etanercept into Knee Joints for Pain in Moderate and Severe Knee Osteoarthritis. Yonsei Med. J. 2015;56:1379–1383. doi: 10.3349/ymj.2015.56.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbruggen G., Wittoek R., Vander Cruyssen B., Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: A double blind, randomised trial on structure modification. Ann. Rheum. Dis. 2012;71:891–898. doi: 10.1136/ard.2011.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S.X., Abramson S.B., Attur M., Karsdal M.A., Preston R.A., Lozada C.J., Kosloski M.P., Hong F., Jiang P., Saltarelli M.J., et al. Safety, tolerability, and pharmacodynamics of an anti-interleukin-1α/β dual variable domain immunoglobulin in patients with osteoarthritis of the knee: A randomized phase 1 study. Osteoarthr. Cartil. 2017;25:1952–1961. doi: 10.1016/j.joca.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: An open-label randomized controlled trial. J. Int. Med. Res. 2018;46:326–334. doi: 10.1177/0300060517723182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To Determine the Safety, Tolerability, Pharmacokinetics and Effect on Pain of a Single Intra-Articular Administration of Canakinumab in Patients with Osteoarthritis in the Knee. [(accessed on 10 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01160822?term=01160822&draw=2&rank=1.
- 33.Treatment of Knee Osteoarthritis with Intra-Articular Infliximab. [(accessed on 10 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01144143?term=NCT01144143&draw=2&rank=1.
- 34.Richette P., Latourte A., Sellam J., Wendling D., Piperno M., Goupille P., Pers Y.-M., Eymard F., Ottaviani S., Ornetti P., et al. Efficacy of tocilizumab in patients with hand osteoarthritis: Double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. 2020;80:349–355. doi: 10.1136/annrheumdis-2020-218547. [DOI] [PubMed] [Google Scholar]
- 35.van der Heijde D., Dijkmans B., Geusens P., Sieper J., DeWoody K., Williamson P., Braun J. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–591. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 36.Maini R., Clair E.W.S., Breedveld F., Furst D., Kalden J., Weisman M., Smolen J., Emery P., Harriman G., Feldmann M., et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/S0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 37.Sbidian E., Chaimani A., Garcia-Doval I., Do G., Hua C., Mazaud C., Droitcourt C., Hughes C., Ingram J.R., Naldi L., et al. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2017;12:Cd011535. doi: 10.1002/14651858.CD011535.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cholapranee A., Hazlewood G.S., Kaplan G.G., Peyrin-Biroulet L., Ananthakrishnan A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017;45:1291–1302. doi: 10.1111/apt.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutgeerts P., Sandborn W.J., Feagan B.G., Reinisch W., Olson A., Johanns J., Travers S., Rachmilewitz D., Hanauer S.B., Lichtenstein G.R., et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 40.Wong M., Ziring D., Korin Y., Desai S., Kim S., Lin J., Gjertson D., Braun J., Reed E., Singh R.R. TNFalpha blockade in human diseases: Mechanisms and future directions. Clin. Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorovits B., Baltrukonis D.J., Bhattacharya I., Birchler M.A., Finco D., Sikkema D., Vincent M.S., Lula S., Marshall L., Hickling T.P. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin. Exp. Immunol. 2018;192:348–365. doi: 10.1111/cei.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Schouwenburg P.A., Rispens T., Wolbink G.J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2013;9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 43.Strand V., Balsa A., Al-Saleh J., Barile-Fabris L., Horiuchi T., Takeuchi T., Lula S., Hawes C., Kola B., Marshall L. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs. 2017;31:299–316. doi: 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcês S., Demengeot J., Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: A systematic review of the literature with a meta-analysis. Ann. Rheum. Dis. 2013;72:1947–1955. doi: 10.1136/annrheumdis-2012-202220. [DOI] [PubMed] [Google Scholar]
- 45.Brown T.J., Laurent U.B., Fraser J.R. Turnover of hyaluronan in synovial joints: Elimination of labelled hyaluronan from the knee joint of the rabbit. Exp. Physiol. 1991;76:125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- 46.Rahimi M., Charmi G., Matyjaszewski K., Banquy X., Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021;123:31–50. doi: 10.1016/j.actbio.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Mancipe Castro L.M., Sequeira A., García A.J., Guldberg R.E. Articular Cartilage- and Synoviocyte-Binding Poly(ethylene glycol) Nanocomposite Microgels as Intra-Articular Drug Delivery Vehicles for the Treatment of Osteoarthritis. ACS Biomater. Sci. Eng. 2020;6:5084–5095. doi: 10.1021/acsbiomaterials.0c00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelletier J.P., Roughley P.J., DiBattista J.A., McCollum R., Martel-Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin. Arthritis Rheum. 1991;20((Suppl. S2)):12–25. doi: 10.1016/0049-0172(91)90024-T. [DOI] [PubMed] [Google Scholar]
- 49.Wiegertjes R., van de Loo F.A.J., Blaney Davidson E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology. 2020;59:2681–2694. doi: 10.1093/rheumatology/keaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Agostino M.A., Conaghan P., Le Bars M., Baron G., Grassi W., Martinmola E., Wakefield R.J., Brasseur J.-L., So A., Backhaus M., et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: Prevalence of inflammation in osteoarthritis. Ann. Rheum. Dis. 2005;64:1703–1709. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschon M., Contartese D., Pagani S., Borsari V., Fini M. Gender and sex are key determinants in osteoarthritis not only confounding variables. A systematic review of clinical data. J. Clin. Med. 2021;10:3178. doi: 10.3390/jcm10143178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trivedi S., Fang W., Ayyalasomayajula I., Vangsness C.T. Pharmacotherapeutic considerations and options for the management of osteoarthritis in women. Expert Opin. Pharmacother. 2020;21:557–566. doi: 10.1080/14656566.2020.1718649. [DOI] [PubMed] [Google Scholar]
- 53.Wojtyniak J.G., Britz H., Selzer D., Schwab M., Lehr T. Data digitizing: Accurate and precise data extraction for quantitative systems pharmacology and physiologically-based pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2020;9:322–331. doi: 10.1002/psp4.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created in this study. Data sharing is not applicable to this article.