Abstract
AIM
To evaluate the incidence and severity of ocular graft versus host disease (oGVHD) in patients who underwent allogeneic stem cell transplant (SCT) in King Abdul-Aziz Medical City, Saudi Arabia.
METHODS
This is a retrospective cohort study conducted in King Abdul Aziz Medical City on patients who underwent allogeneic hematopoietic cell transplant (allo-HCT) from 2010 to 2017. The ocular examination findings including visual acuity, meibomian gland dysfunction, corneal and conjunctival staining with severity, corneal scarring, tear film meniscus and breakup time, anterior and posterior segment examination findings, intraocular pressure, treatment given, punctual plugs used or not, and follow up response were collected.
RESULTS
The five years cumulative incidence of oGVHD among post-transplant patients was 56.98% (95%CI 38.6%-71.7%). The potential risk factors assessed for developing ocular manifestation were age, gender, donor's age, donor gender mismatch CD3 and CD34 infusion, while none of the correlates were identified as statistically significant risk factors of developing ocular manifestation. However, the incidence was statistically significantly different between patients diagnosed with acute myelocytic leukemia and acute lymphocytic leukemia (P=0.038). The mean latent period to develop ocular symptoms was 20.5mo. All patients had variable degree of dry eyes. None of the patients developed any posterior segment complication.
CONCLUSION
The incidence of oGVHD is low in King Abdul-Aziz Medical City. This can be attributed to the preconditioning and immunosuppressive regime.
Keywords: graft versus host disease, allogenic stem cell transplant, dry eye
INTRODUCTION
Allogenic hematopoietic cell transplantation (allo-HCT) is the routine treatment and a potential cure with proven efficacy for wide variety of life threatening hematological diseases[1]. According to World Wide Network of Blood and Bone Marrow Transplantation, more than 90 000 hematopoietic stem cell transplants (HSCTs; 53% autologous and 47% allogenic) are performed every year worldwide[2]. Ever since the first successful allo-HCT in 1968, it has a significant contribution towards survival of patients suffering from these diseases. The new developments in this field like better immunosuppressive regimens, improved preconditioning protocols and human leukocyte antigen (HLA) typing have led to substantial increase in the survival rates after transplants[3]. But, like any other successful therapy, allo-HCT is also associated with its side effects. One of the major complications of allo-HCT is graft versus host disease (GVHD). The incidence of GVHD was reported to be 70.5% in 1974[4]. Even today, with all the advances, the incidence remains high (25%-70%), making it a worrisome cause of morbidity and mortality[5].
The common sites involved in chronic GVHD in order of frequency are skin (75%), mouth (51%-63%) and liver (29%-51%)[6]–[7]. Literature shows that 50%-90% of transplant recipients with systemic GVHD have ocular complication[8]–[9]. The 40%-60% of patients receiving allo-HCT get ocular chronic GVHD[10]–[11]. The mean latency from the transplant to the development of ocular GVHD (oGVHD) has been reported to be 16.4mo[12]. It affects almost all structures of the eye (lids, lacrimal glands, conjunctiva, cornea, uvea, vitreous, and choroids), but typically affects anterior segment. Posterior segment complications are less common as compared to the ocular surface disease[13]–[15].
Ocular involvement can be of variable severity. It can even restrict the daily life activities of the patient thus effecting the quality of life. Timely recognition of the problem, diagnosis and aggressive treatment can improve the quality of life and save the vision[16]. Effective and appropriate preventive therapies have yet to be developed for oGVHD. Once diagnosed, ocular treatment includes intense lubrication and support of the ocular surface, stabilization of existing tear film, inflammation control and surgical intervention in form of punctal plugs, punctal cautery and limbal stem cell transplants[17]. We aim to evaluate the incidence and severity of oGVHD in patients who underwent allogenic stem cell transplants in this institution between 2010 till 2017. After assessing the disease burden and intensity, we also aim to design a protocol for the comprehensive assessment, timely diagnosis and ophthalmologic management of oGVHD.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the King Abdullah International Medical Research Center Institutional Review Board (No.RC18/165/R). The data was collected from patients' electronic medical record. The patients' medical record number were identified through HCT database.
Study Design and Participants
This retrospective cohort study was conducted in patients who underwent allo-HCT from 2010 to 2017 in King Abdul-Aziz Medical City, Riyadh, Saudi Arabia. In this study, we defined oGVHD as dry eyes occurring after HSCT in patients who developed systemic GVHD and were not known to have previous history dry eyes. The criteria set for acute oGVHD was new onset eye discomfort with classic systemic acute GVHD, and that for chronic oGVHD was newly documented keratoconjunctivitis sicca (KCS) signs detected on slit lamp examination.
Study Procedure
After receiving ethical approval, the ocular data was picked from the ophthalmology history and examination charts. It included the date of referral to ophthalmology, ocular examination findings (visual acuity, meibomian gland dysfunction, corneal and conjunctival staining with severity, corneal scarring, tear film meniscus and breakup time, anterior and posterior segment examination findings, intraocular pressure, treatment given, punctual plugs used or not, and follow up response). Visual symptoms, ocular disturbances and ocular signs was graded according to the DEWS 2007 classification. This was determined by measuring the parameters which include symptoms of dry eyes, tear film breakup time (TBUT), and other abnormalities noted in the conjunctiva, cornea, tear film, lid, and meibomian glands[18]. We did not take Schirmer's test in our evaluation as the test was not being done during study period in our center.
Statistical Analysis
Quantitative variables, patient and donor's age, were summarized and reported in terms of median. Categorical variables gender, pre-transplant and post-transplant characteristics and patients' outcomes were reported in terms of frequency tables and percentages. The association between ocular manifestation and chronic GVHD was analyzed using the Fisher exact test. Statistical tests were declared significant if P-value was less than 0.05. The incidence was reported in terms of incidence rate and corresponding Wilson 95% confidence intervals (95%CI).
Logistic regression was used to explore the risk factors for developing ocular manifestation among patients diagnosed with post-transplant GVHD. Dependent variable was ocular manifestation (yes/no). The independent variables were patient's age, donor's age, CD3 and CD34 infused, donor's gender mismatch, use of total body irradiation (TBI) and anti-thymocyte globulin (ATG). The results were reported as odds ratios (OR), 95%CI, and P-values. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline Characteristics
A total of 330 patients had allogeneic HSCT in King Abdul Aziz Medical City during year 2010-2017. Out of these 61 patients who developed GVHD (acute, overlap, and chronic) and documented in patients' medical charts were selected. Of the total sample of 61, there were 33 (54.1%) males and 28 females (45.9%). The mean age at diagnosis was 27y and the donor's mean age was 23y. The demographic characteristics are summarized in Table 1.
Table 1. Baseline characteristics of the study cohort.
| Variables | n=61 |
| Age at diagnosis, median (range), y | 27 (22-38) |
| Donor's age, median (range), y | 23 (18-31) |
| Gender, n (%) | |
| Male | 33 (54.1) |
| Female | 28 (45.9) |
| Primary diagnosis, n (%) | |
| Acute lymphoblastic leukemia | 32 (52.46) |
| Acute myeloid leukemia | 21 (34.43) |
| Langer cell histiocytosis | 3 (4.92) |
| Hodgkin lymphoma | 2 (3.28) |
| Aplastic anemia | 1 (1.64) |
| Chronic lymphocytic leukemia | 1 (1.64) |
| Chronic myeloid leukemia | 1 (1.64) |
| Previous transplant (yes), n (%) | 7 (11.48) |
| Source of stem cells, n (%) | |
| Peripheral stem cells | 60 (98.36) |
| Bone marrow | 1 (1.64) |
| Type of donor, n (%) | |
| Matched related | 55 (90.16) |
| Matched unrelated | 2 (3.28) |
| Haploidentical | 3 (4.92) |
| Matched other relatives | 1 (1.64) |
| ABO, n (%) | |
| Compatible | 36 (59.02) |
| Major | 12 (19.67) |
| Minor | 13 (21.31) |
| Donor-recipient gender mismatch (yes), n (%) | 28 (45.90) |
| Number of CD3 cells infused (107 cell/kg), median (range) | 10.44 (7.2-15) |
| Number of CD34 cells infused (106 cell/kg), median (range) | 5.6 (4.56-7) |
| Type of conditioning, n (%) | |
| Myeloablative (yes) | 47 (77.05) |
| Reduced intensity (yes) | 14 (22.95) |
| Use of total body irradiation (yes) | 30 (49.18) |
| Antithymocyte globulin (yes) | 6 (9.84) |
| GVHD prophylaxis, n (%) | |
| CNI (CsA or Tacrolimus)+MTX | 55 (90.16) |
| CNI (CsA or Tacrolimus)+MMF | 4 (6.56) |
| PTCy+Tacrolimus+MMF | 2 (3.28) |
| Cyclophosphamide | 3 (4.92) |
GVHD: Graft versus host disease; CNI: Calcineurin inhibitor; CsA: Cyclosporine A; MTX: Methotrexate; MMF: Mycophenolate mofetil; PTCy: Post-transplant cyclophosphamide.
We had 28 patients with oGVHD, out of which only 1 had acute ocular GVHD with only eye manifestation. Rest 27 patients with oGVHD had chronic GVHD (including overlap). All patients with oGVHD among chronic GVHD patients had liver and skin involvement in our study. All patients received corticosteroids as a systemic treatment (Table 2).
Table 2. Post-transplant characteristics of the study cohort.
| Variables | n (%) |
| Acute GVHD | n=30/61 |
| Grade 1 | 5 (8.20) |
| Grade 2 | 16 (26.23) |
| Grade 3 | 9 (14.75) |
| Chronic GVHD | n=60/61 |
| Mild | 12 (19.67) |
| Moderate | 26 (42.62) |
| Severe | 22 (36.07) |
| Overlap GVHD | 23 (37.70) |
| Systemic involvement | n=61 |
| Liver | 48 (78.69) |
| Skin | 36 (59.02) |
| Eyes | 28 (45.90) |
| Gastrointestinal tract | 19 (31.15) |
| Mouth | 15 (24.59) |
| Genitals | 3 (4.92) |
| Others | 2 (3.28) |
| Systemic treatment (yes) | n=61 |
| Cyclosporine | 59 (96.72) |
| Corticosteroid | 61 (100) |
| MMF | 5 (8.20) |
| Rituximab | 2 (3.28) |
| ATG | 2 (3.28) |
| Etnercept | 1 (1.64) |
GVHD: Graft versus host disease; MMF: Mycophenolate mofetil; ATG: Antithymocyte globulin.
Ocular Findings
The five years cumulative incidence of oGVHD among post-transplant patients was 56.98% (Figure 1) with 95%CI (38.6%-71.7%). The incidence of developing oGVHD was not statistically different between gender (P=0.418; Figure 2). However, the incidence oGVHD was statistically significant in patients diagnosed with acute myelocytic leukemia (AML) as compared to acute lymphocytic leukemia (ALL; P=0.038; Figure 3). The mean latent period to develop ocular symptoms was 20.51±17.80mo (669.2±626.68d). Ocular signs and severity of ocular involvement was showed in Table 3. All patients with oGVHD received lubricant drops and 42.86% patients received cyclosporine 0.5% eye drops (Table 4). Local treatment for patients with eye involvement were showed in Table 4.
Figure 1. Five years cumulative incidence of ocular manifestations in GVHD patients.
GVHD: Graft versus host disease.
Figure 2. Incidence of ocular manifestations in GVHD patients by gender.

GVHD: Graft versus host disease.
Figure 3. Incidence of ocular manifestations in GVHD patients by primary diagnosis (AML vs ALL).
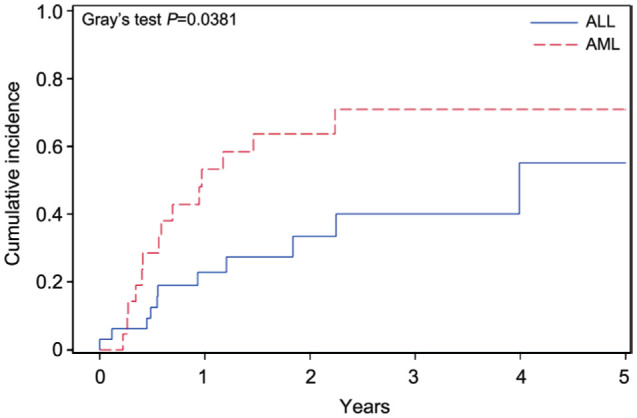
GVHD: Graft versus host disease; AML: Acute myelocytic leukemia; ALL: Acute lymphocytic leukemia.
Table 3. Ocular signs and severity of ocular involvement.
| Ocular signs | n (%), n=28 |
| Ocular involvement (yes) | 28 (100) |
| Visual symptoms | |
| None/episodic | 11 (39.29) |
| Annoying | 7 (25.0) |
| Chronic constant | 6 (21.43) |
| Constant/disabling | 4 (14.29) |
| Corneal/conjunctival staining | |
| None to mild | 13 (46.43) |
| Variable | 5 (17.86) |
| Moderate to marked | 6 (21.43) |
| Marked | 4 (14.29) |
| Conjunctival hyperemia | |
| None to mild | 16 (57.14) |
| Mild to moderate | 3 (10.71) |
| Severe | 9 (32.14) |
| Corneal sign | |
| None to mild | 6 (21.43) |
| Mild debris | 12 (42.86) |
| Filamentary keratitis, clumps | 9 (32.14) |
| Ulceration | 1 (3.57) |
| Corneal stain | |
| Mild | 11 (39.29) |
| Moderate | 7 (25.0) |
| Severe | 5 (17.86) |
| SPE | 5 (17.86) |
| Ocular discomfort | |
| Mild/episodic | 9 (32.14) |
| Moderate/episodic | 7 (25.0) |
| Severe frequent constant | 8 (28.57) |
| Severe disabling | 4 (14.29) |
| Tear film breakup time | |
| Variable | 13 (46.43) |
| Less than 10s | 5 (17.86) |
| Less than 5s | 6 (21.43) |
| Immediate | 4 (14.29) |
| Dry eye syndrome | |
| Mild | 8 (28.57) |
| Moderate | 6 (21.43) |
| Severe | 9 (32.14) |
| Very severe | 5 (17.86) |
| Periocular skin (yes) | 3 (10.71) |
| Blepharitis (yes) | 14 (50.0) |
| Meibomian gland dysfunction (yes) | 16 (57.14) |
| Corneal ulcer (yes) | 2 (7.14) |
| Cataract (yes) | 3 (10.71) |
| Posterior segment (yes) | 1 (3.57) |
| Visual acuity right eye | |
| Normal | 14 (50.0) |
| Impaired | 14 (50.0) |
| Visual acuity left eye | |
| Normal | 11 (39.29) |
| Impaired | 17 (60.71) |
SPE: Severe punctate erosion.
Table 4. Local treatment for patients with eye involvement.
| Variables | n (%), n=28 |
| Lubricants | 28 (100) |
| Acetylcysteine | 8 (28.57) |
| Cyclosporine 0.5% | 12 (42.86) |
| Topical steroid | 7 (25.0) |
| Punctal plug | 5 (17.86) |
| Cyclosporine 1% | 4 (14.29) |
| Cautery | 1 (3.57) |
Risk Factors of Developing Ocular Manifestations
The potential risk factors we assessed for developing ocular manifestation were age, gender, donor gender mismatch, CD3 and CD4 infusion, systemic skin involvement, chronic GVHD severity, reduced intensity conditioning (RIC) versus myeloablative (MYLO), TBI (no versus yes), prophylactic ATG (no versus yes), prophylactic cyclophosphamide (no versus yes). Recipient age was the only statistically significant predictor of developing ocular manifestation among patients with GVHD. None of the other correlates were identified as statistically significant risk factors of developing ocular manifestation (Table 5).
Table 5. Predictors of developing ocular manifestation among patients diagnosed with GVHD.
| Variables | OR | 95%CI | P |
| Gender (females vs males) | 2.31 | 0.70-7.654 | 0.169 |
| Recipient's age | 1.05 | 1.003-1.104 | 0.038 |
| CD3 infused | 0.90 | 0.816-1.004 | 0.058 |
| CD4 infused | 1.04 | 0.787-1.391 | 0.755 |
| Donor gender mismatch (no vs yes) | 1.57 | 0.464-5.361 | 0.465 |
| Systemic skin infection (no vs yes) | 1.68 | 0.478-5.911 | 0.418 |
| Conditioning (RIC vs MYLO) | 1.61 | 0.371-7.049 | 0.521 |
| Conditioning TBI (no vs yes) | 1.77 | 0.553-5.689 | 0.335 |
| Prophylaxis ATG (no vs yes) | 0.79 | 0.112-5.629 | 0.817 |
| Prophylaxis cyclophosphamide (no vs yes) | 6.34 | 0.166-243.26 | 0.320 |
| Chronic GVHD (mild vs moderate/severe) | 0.66 | 0.179-2.439 | 0.534 |
OR: Odds ratio; 95%CI: 95% confidence interval; RIC: Reduced intensity conditioning; MYLO: Myeloablative; TBI: Total body irradiation; ATG: Anti-thymocyte globulin; GVHD: Graft versus host disease.
We studied the outcome of patients with oGVHD and Chi-square test was used to calculate the significance of survival rate between the ocular GVHD and non-ocular cases which was not found to be significant with P value of 0.4928 (Table 6). Major cause of death among oGVHD patients was primary disease itself. In the study group 82.14% of oGVHD patients had follow up more than 1y (Table 6).
Table 6. Outcome of the study cohort and patients with oGVHD.
| Variables | Study cohort | oGVHD |
| Follow up | n=61 | n=28 |
| Less than one year | 18 (29.51) | 5 (17.86) |
| More than one year | 43 (70.49) | 23 (82.14) |
| Outcome | n=61 | n=28 |
| Dead | 17 (27.87) | 9 (32.14) |
| Alive | 44 (72.13) | 19 (67.86) |
| Cause of death | n=17 | n=9 |
| GVHD | 4 (23.53) | 2 (22.22) |
| Primary disease | 11 (64.71) | 7 (77.78) |
| Others | 2 (11.76) | 0 |
GVHD: Graft versus host disease; oGVHD: Ocular graft versus host disease.
DISCUSSION
A variety of life-threatening hematologic malignancies like lymphomas, leukemias, aplastic anemia, severe combined immunodeficiency, certain metabolic diseases such as lysosomal storage disorders and mucopolysaccharidosis are treated by HSCT[12],[19]–[20]. HSCT includes bone marrow transplantation (BMT), peripheral blood stem cell transplantation and cord blood transplantation. It can be autologous (when the cells are harvested from the patient), syngeneic (when taken from an identical twin), and allogenic (when the donor cells are from either a related or an unrelated individual). Despite of the revolutionary advances in the management strategies, GVHD still remains one of the major complications of allogenic stem cell transplantation. Though post-transplant survival rates are increasing, GVHD remains a major cause of non-relapse morbidity and mortality in these patients[4],[7],[21].
Historically, GVHD had been classified into two broad categories (acute and chronic) by the National Institutes of Health (NIH). This classification was based on the appearance of symptoms before or after 100d of transplant, even if the clinical manifestations were indistinguishable from acute GVHD. In 2005, the NIH sponsored a consensus conference that proposed new criteria for diagnosis and classification of chronic GVHD for clinical trials[22]. Two new terms named persistent, recurrent and late onset were added for cases of acute GVHD which persisted for >3mo and term overlap syndrome for those with features of chronic and acute GVHD appear together without any consideration to time limit. According to the consensus criteria, clinical manifestations rather than time after transplantation should be used to distinguish chronic GVHD from late acute GVHD, as shown in Table 7.
Table 7. National Institutes of Health classification of GVHD.
| Category | Time interval between SCT & onset of GVHD | Presence of acute GVHD features | Presence of chronic GVHD features |
| Acute GVHD | |||
| Classic | <100d | Yes | No |
| Late-onset | >100d | Yes | No |
| Chronic GVHD | |||
| Classic | No time limit | No | Yes |
| Overlap syndrome | No time limit | Yes | Yes |
SCT: Stem cell transplantation; GVHD: Graft versus host disease.
Out of 330 patients who underwent HSCT between years 2010-2017, incidence of systemic GVHD has been 18.48% which is very low compared to other studies with reported incidence of acute and chronic GVHD to be around 40% and 30%-70% respectively among the HLA-matched patients[7],[23]. In literature, oGVHD develops in about 10% of acute GVHD patients and is poor prognostic sign[24]–[26]. Among 28 (44.26%) patients who developed ocular manifestation in our study only one patient had acute oGVHD.
The reported risk factors for the development of GVHD are multiple[12],[15]–[16],[25],[27] and these mainly include disparity in HLA and incomplete HLA matching. The conditioning regimens which used high dose of irradiations for the whole body has also been described as a risk factor. Other studied factors include a history of prior acute GVHD and lack of prophylaxis for acute GVHD, cyclosporine-based prophylaxis with higher incidence of acute GVHD as compared to Tacrolimus-based prophylaxis, ABO incompatibility, the primary diagnosis of chronic myeloid leukemia or aplastic anemia, peripheral blood as a source of stem cells and lack of T-cell depletion. The donor associated risk factors include unrelated donor, old age of recipient and donor, female donor to male recipient and female donor with history of pregnancies and transfusions. In this study, we tried to find the risk factors for development of oGVHD in patients with chronic GVHD after allo-HCT. We reviewed a number of publications in this regard. The comparison has not been very simple due to differences in study designs, sample sizes, and the diagnostic criteria. The referrals based on ocular signs and symptoms and then follow up durations especially in ophthalmology department has also been quiet variable[10],[28]–[29]. One of our observations has been that oGVHD was more common among the patients who suffered from AML and underwent HSCT. Most of our patients who developed the ocular manifestations had moderate to severe systemic chronic GVHD which showed patients with moderate and severe systemic GVHD are at increased risk of developing oGVHD as compared to mild disease. Previous studies have also linked increased risk of oGVHD with moderate-severe systemic disease[30]. The median age at diagnosis in our study was 27 and recipient age was a predictor for developing oGVHD which was statistically significant. Researchers have reported that male patients who received transplants from female donors were at increased risk for oGVHD, similar results have been published by Kamoi et al[30] and Jacobs et al[31]. This observation didn't replicate in our study. The mean onset time of oGVHD in our patients has been estimated to be 669.2±626.68d (20.51mo). This is significantly longer than reported previously. The 171d (5.7mo, in a group of 53 patients) was observed by Ogawa et al[32]. Tichelli et al[33] reported a median onset time of 13.8mo in a group of 48 patients, whereas Shikari et al[34] reported 293d between the transplant and appearance of ocular signs which is approximately 9.8mo, in a group of 200 patients. The difference can be attributed due to the difference in pre-conditioning regime and immunosuppressant therapy that our patients had received.
The pathophysiology and clinical features of acute oGVHD and chronic oGVHD varies. The acute oGVHD is mainly T cell mediated process in the conjunctival tissue which causes pseudomembranous and hemorrhagic conjunctivitis. We had only one patient with acute oGVHD who presented with severe conjunctivitis. Chronic oGVHD is due to increase in number of stromal CD34 β fibroblasts as well as infiltration of T cell causing inflammatory destruction of conjunctiva and lacrimal gland with fibrosis. KCS or dry eyes is the most common ocular manifestation of chronic oGVHD along with inflammatory signs like conjunctival edema, chemosis, membrane formation and Meibomian gland dysfunction (MGD)[6],[25],[35]. As mentioned above, all patients who were diagnosed with oGVHD had dry eye disease irrespective of differences in conditioning regimen. We modified DEWS 2007 classification for grading the severity of the dry eye disease and didn't include Schirmer's test. Severe dry eyes usually end up with filamentary keratitis, corneal ulceration, corneal neovascularization, and ultimately corneal perforation if not treated. In our study group only 2 patients developed corneal ulceration and treated promptly with topical antibiotics, heavy lubrication and punctal plugs along with topical cyclosporine 0.05%. Ogawa et al[35] showed that MGD was severely damaged in patients with severe dry eye and chronic GVHD and in their study 47% of patients developed MGD. In the present study 57.1% of patients had MGD on presentation which suggests that MGD may allow us to diagnose severe dry eye with GVHD early in the course of the disease. The only posterior segment complication in our study is papilledema in a patient, which in our observation was due to systemic cyclosporine rather than direct GVHD complication.
All patients were treated with preservative free lubricating eye drops and ointments. Seven (25.0%) patients needed steroids to control inflammation whereas 12 (42.86%) needed additional cyclosporine eye drops and 8 (28.57%) patient needed acetylcysteine. Five (17.86%) were inserted with punctal plugs whereas one patient needed punctal cautery. Seventeen (62.96%) improved whereas 10 remained stable during follow up period. None of the patient worsened after initial referral.
One of the limitations of our study is its retrospective nature. There was lack of pretransplant baseline ocular examination. Lack of complete ocular data in few patients. As Schirmer test was not done in any of the patient, we were, therefore unable to meet NIH criteria for grading the dry eyes. Initiation of dry eye treatment for some symptomatic patients with mild disease by the primary team with no ophthalmology referrals might be a reason for relatively lower incidence. Moreover, we have taken mean time of onset of oGVHD from transplantation date to presentation of patient in ophthalmology clinic as an urgent referral from hematology department which in turn has caused wide range in mean time.
In conclusion, the incidence of systemic and oGVDH has been low in patients who underwent HSCT in the current study. The incidence of oGVHD was higher in AML compared to ALL patients. The latent period for appearance of ocular GVHD was longer than prior reported numbers in the literature. The variation can be attributed to the differences in pre-conditioning regime and immunosuppressant therapies across patients. Baseline ophthalmology assessment is needed in order to diagnose oGVHD earlier and increase awareness of vision threatening complications of oGVHD. In order to do this, we suggest designing an agreed referral pathway, and setting up oGVHD clinics with new diagnostic modalities in collaboration with Hematology Department. A mutually agreed protocol needs to be tailored for pre transplant baseline assessment and post-transplant follow up. This will help in picking up the cases early along the course and prompt treatment. A multipronged treatment approach (lubrication and tear preservation, prevention of tear evaporation, reducing ocular inflammatory process and surgical interventions) will decrease the long-term morbidity.
Furthermore, prospective studies with larger study population are needed to assess the disease burden and intensity more accurately.
Acknowledgments
Conflicts of Interest: Aldebasi T, None; Bashir R, None; Gangadharan S, None; Shaheen N, None; Alhussain B, None; Almudhaiyan T, None; Alahmari B, None.
REFERENCES
- 1.Niederwieser D, Baldomero H, Atsuta Y, et al. One and half million hematopoietic stem cell transplants (HSCT). Dissemination, trends and potential to improve activity by telemedicine from the worldwide network for blood and marrow transplantation (WBMT) Blood. 2019;134(Supplement_1):2035. [Google Scholar]
- 2.Pavletic SZ, Martin PJ, Schultz KR, Lee SJ. The future of chronic graft-versus-host disease: introduction to the 2020 national institutes of health consensus development project reports. Transplant Cell Ther. 2021;27(6):448–451. doi: 10.1016/j.jtct.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Hahn T, McCarthy PL, Jr, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, Isola LM, Lazarus HM, Lee SJ, LeMaistre CF, Loberiza F, Maziarz RT, Rizzo JD, Joffe S, Parsons S, Majhail NS. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini M, Bernabei F, Barbato F, Arpinati M, Giannaccare G, Versura P, Bonifazi F. Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Am J Ophthalmol. 2021;227:25–34. doi: 10.1016/j.ajo.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Vanathi M, Mahapatra M, Seth T, Kaur J, Velpandian T, Ravi A, Titiyal JS, Tandon R. Tear inflammatory mediators and protein in eyes of post allogenic hematopoeitic stem cell transplant patients. Ocul Surf. 2018;16(3):352–367. doi: 10.1016/j.jtos.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Murillo SE, Soifer M, Perez VL. Ocular graft versus host disease: just a severe form of dry eye or something more? Curr Ophthalmol Rep. 2022;10(2):53–61. [Google Scholar]
- 7.Holtan SG, Majhail NS, Weisdorf DJ. Hematology: Basic Principles and Practice. Elsevier Inc; 2018. Complications after hematopoietic cell transplantation; pp. 1669–1684.e2. [Google Scholar]
- 8.Nassiri N, Eslani M, Panahi N, Mehravaran S, Ziaei A, Djalilian AR. Ocular graft versus host disease following allogeneic stem cell transplantation: a review of current knowledge and recommendations. J Ophthalmic Vis Res. 2013;8(4):351–358. [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, Eberwein P, Reinhard T, Bertz H, Nepp J, Lawitschka A, Heiligenhaus A, Seitz B, Messmer EM, Meyer-ter-Vehn T, Basara N, Greinix H, Datiles MB, Lee SJ, Pavletic SZ, Wolff D. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea. 2012;31(3):299–310. doi: 10.1097/ICO.0b013e318226bf97. [DOI] [PubMed] [Google Scholar]
- 10.Kezic JM, Wiffen S, Degli-Esposti M. Keeping an ‘eye’ on ocular GVHD. Clin Exp Optom. 2022;105(2):135–142. doi: 10.1080/08164622.2021.1971047. [DOI] [PubMed] [Google Scholar]
- 11.Giannaccare G, Pellegrini M, Bernabei F, Scorcia V, Campos E. Ocular surface system alterations in ocular graft-versus-host disease: all the pieces of the complex puzzle. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1341–1351. doi: 10.1007/s00417-019-04301-6. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Cavanagh HD. Ocular manifestations of graft-versus-host disease: 10 years' experience. Clin Ophthalmol. 2015;9:1209–1213. doi: 10.2147/OPTH.S84704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamoto Y, Petriček I, Burns L, et al. Non-GVHD ocular complications after hematopoietic cell transplantation: expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54(5):648–661. doi: 10.1038/s41409-018-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butt F, McKibbin M. Rare retinal complications of bone marrow transplantation (BMT): a case report. BMC Ophthalmol. 2018;18(Suppl 1):225. doi: 10.1186/s12886-018-0855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol. 2012;12(5):540–547. doi: 10.1097/ACI.0b013e328357b4b9. [DOI] [PubMed] [Google Scholar]
- 16.Sun YC, Chai X, Inamoto Y, Pidala J, Martin PJ, Flowers ME, Shen TT, Lee SJ, Jagasia M. Impact of ocular chronic graft-versus-host disease on quality of life. Biol Blood Marrow Transplant. 2015;21(9):1687–1691. doi: 10.1016/j.bbmt.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathak M, Diep PP, Lai X, Brinch L, Ruud E, Drolsum L. Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplant. 2018;53(7):863–872. doi: 10.1038/s41409-018-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 19.Abud TB, Amparo F, Saboo US, Di Zazzo A, Dohlman TH, Ciolino JB, Hamrah P, Dana R. A clinical trial comparing the safety and efficacy of topical tacrolimus versus methylprednisolone in ocular graft-versus-host disease. Ophthalmology. 2016;123(7):1449–1457. doi: 10.1016/j.ophtha.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramaniam SC, Raja H, Nau CB, Shen JF, Schornack MM. Ocular graft-versus-host disease: a review. Eye Contact Lens. 2015;41(5):256–261. doi: 10.1097/ICL.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton BK. Updates in chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2021;2021(1):648–654. doi: 10.1182/hematology.2021000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair S, Vanathi M, Mukhija R, Tandon R, Jain S, Ogawa Y. Update on ocular graft-versus-host disease. Indian J Ophthalmol. 2021;69(5):1038–1050. doi: 10.4103/ijo.IJO_2016_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinner MA, Fernández-Viña M, Creary LE, Quinn O, Elder L, Arai S, Johnston LJ, Meyer EH, Miklos DB, Muffly LS, Negrin RS, Shizuru JA, Weng WK, Laport GG, Strober S, Lowsky R, Rezvani AR. HLA-mismatched unrelated donor transplantation using TLI-ATG conditioning has a low risk of GVHD and potent antitumor activity. Blood Adv. 2017;1(17):1347–1357. doi: 10.1182/bloodadvances.2017007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Shinagawa K, Takenaka K, Matsuo K, Yoshino T, Kiura K, Niiya K, Harada M. Ocular manifestation of acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2002;75(3):332–334. doi: 10.1007/BF02982052. [DOI] [PubMed] [Google Scholar]
- 25.Nair S, Vanathi M, Ganger A, Tandon R. Ocular graft versus host disease: a review of clinical manifestations, diagnostic approaches and treatment. Open Journal of Ophthalmology. 2016;6(1):20–33. [Google Scholar]
- 26.Holler E. Risk assessment in haematopoietic stem cell transplantation: GvHD prevention and treatment. Best Pract Res Clin Haematol. 2007;20(2):281–294. doi: 10.1016/j.beha.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Wang JCC, Teichman JC, Mustafa M, O'Donnell H, Broady R, Yeung SN. Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. 2015;99(11):1514–1518. doi: 10.1136/bjophthalmol-2014-306438. [DOI] [PubMed] [Google Scholar]
- 28.Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, Al-Zahrani H, Mohammed SY, Nassar A, Aljurf M. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009;116(9):1624–1629. doi: 10.1016/j.ophtha.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–37. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamoi M, Ogawa Y, Uchino M, Tatematsu Y, Mori T, Okamoto S, Tsubota K. Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye (Lond) 2011;25(7):860–865. doi: 10.1038/eye.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs R, Tran U, Chen H, Kassim A, Engelhardt BG, Greer JP, Goodman SG, Clifton C, Lucid C, Vaughan LA, Savani BN, Jagasia M. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. 2012;47(11):1470–1473. doi: 10.1038/bmt.2012.56. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa Y, Okamoto S, Wakui M, Watanabe R, Yamada M, Yoshino M, Ono M, Yang HY, Mashima Y, Oguchi Y, Ikeda Y, Tsubota K. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999;83(10):1125–1130. doi: 10.1136/bjo.83.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tichelli A, Duell T, Weiss M, Socié G, Ljungman P, Cohen A, van Lint M, Gratwohl A, Kolb HJ. Late-onset keratoconjunctivitis sicca syndrome after bone marrow transplantation: incidence and risk factors. European Group or Blood and Marrow Transplantation (EBMT) Working Party on Late Effects. Bone Marrow Transplant. 1996;17(6):1105–1111. [PubMed] [Google Scholar]
- 34.Shikari H, Amparo F, Saboo U, Dana R. Onset of ocular graft-versus-host disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea. 2015;34(3):243–247. doi: 10.1097/ICO.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa Y, Yamazaki K, Kuwana M, Mashima Y, Nakamura Y, Ishida S, Toda I, Oguchi Y, Tsubota K, Okamoto S, Kawakami Y. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42(1):111–119. [PubMed] [Google Scholar]



