Abstract
AIM
To describe the clinical characteristics and treatments associated with antibody positive optic neuropathies including anti-myelin oligodendrocyte glycoprotein (MOG) and anti-aquaporin 4 (AQP4), alongside diagnostic modalities, investigations, and outcomes.
METHODS
A cross-sectional single-centre retrospective case series consisting of 16 patients including 12 anti-MOG positive patients and 4 anti-AQP4 positive patients. Each of these patients had clinical signs and symptoms of optic neuritis and consisted of all patients who had a positive blood antibody result in our centre. Clinical findings including presence of a relative afferent pupillary defect, colour vision and disc assessment were recorded. Structured clinical exam and multimodal imaging was undertaken sequentially on each. Optical coherence tomography (OCT) scanning was preformed to examine the correlation between ganglion cell layer (GCL) thickness and visual acuity (VA) at presentation and as a determinant of final visual outcome in both groups. Initial and long-term treatment is also summarised.
RESULTS
A total of 16 patients were included in the study consisting of 12 anti-MOG and 4 anti-AQP4 positive patients. Nine of the 16 patients were female and the average age of onset was 29.2y in the MOG group and 42y in the AQP4 group. There was no statistically significant correlation (Pearson correlation) between GCL thickness and presenting and final VA [r(10)=0.081, P=0.08 and r(10)=0.089, P=0.34 respectively]. The same statistical analysis was performed for the correlation between retinal nerve fibre layer (RNFL) and VA and similar outcomes were observed [r(10)=0.04, P=0.22 and r(10)=0.09, P=0.04]. No correlation was seen for initial RNFL thickness and final visual outcome in this group either [r(2)=0.19, P=0.38]. Visual field testing and radiological findings for each group are described.
CONCLUSION
No correlation between initial VA or RNFL and final visual outcome is identified. A broad range of visual field and radiographic findings are identified, a consensus on treatment of neuromyelitis optica spectrum disorders and anti-MOG positive optic neuropathies has yet to be accepted but initial high dose immunosuppression followed by low dose maintenance therapy is favoured.
Keywords: neuromyelitis, anti-myelin oligodendrocyte glycoprotein, antibody, anti-aquaporin 4
INTRODUCTION
Neuromyelitis optica (NMO; previously known as Devic's disease) and anti-myelin oligodendrocyte glycoprotein (MOG) optic neuropathy are antibody mediated demyelinating diseases that primarily affect the optic nerves and spinal column and therefore can lead to profound vision and mobility impairment[1]. They are distinct clinical entities from other demyelinating diseases such as “typical optic neuritis (ON)” and multiple sclerosis (MS; the most common demyelinating disease) and this distinction is important, as these antibody mediated optic neuropathies tend to have more severe presentations, atypical signs and symptoms, recurrent attacks of disease (including simultaneously bilateral or sequential optic neuritis), and they tend to respond well to immunosuppressive therapy and may require long term treatment[2]–[3]. These diseases have a predilection for the optic nerves and visual loss due to ON is often the presenting feature of the disease.
As the name of the disease suggest, NMO is defined by both a myelitis and an ON[4]. The disease has a predilection for both the optic nerves and the spinal cord, and the lesions commonly spare the brain in the early stages[5]. Patients who have an ON associated with NMO tend to have an acute profound vision loss associated with disc swelling[6]. However, they may also develop optic nerve atrophy and cavitation akin to glaucomatous optic nerve change in severe cases[7]. The cavitation is postulated to be secondary to a demyelination associated necrosis of the optic nerve head. The episodes of ON may be recurrent and simultaneously bilateral[8] causing progressive optic atrophy with cumulative inflammatory damage.
ON seen in MOG positive cases tend to have atypical findings. The 80% patients of those with MOG positive ON tend to have moderate to severe disc oedema[9]. Patients tend to have recurrent and bilateral attacks of ON[10]. These patients tend to be highly steroid responsive and can prove to be steroid dependant[11].
In contrast to typical ON, high dose intravenous glucocorticoids are recommended are at initial presentation in MOG and anti-Aquaporin 4 (AQP4) positive cases of ON[1]. Guidelines for treatment have proved difficult to establish due to the low number of patients and therefore the absence of randomised controlled clinical trials.
To describe all cases of anti-MOG antibody positive and AQP4 antibody positive ON that have presented to a specialist ophthalmology quaternary referral centre. This will consist of demographic data, clinical characteristics, ancillary tests [optical coherence tomography (OCT), perimetry and neuroimaging] and treatment/outcome.
SUBJECTS AND METHODS
Ethical Approval
This study was a retrospective review and was conducted in accordance with the Declaration of Helsinki and the Irish Data Protection Act. The protocol of the study adhered to the tenets of the Declaration of Helsinki. This study adheres to the legal requirements of the General Data Protection Regulation (GDPR, articles 6 and 9).
A cross-sectional single-centre retrospective case series consisting of 16 patients including 12 anti-MOG positive patients, and 4 anti-AQP4 positive patients. Inclusion criteria consisted of all patients who had a positive anti-MOG or positive anti-AQP4 blood result for investigation of a unilateral or bilateral ON who attended the Royal Victoria Eye and Ear Hospital, Dublin, Ireland. Exclusion criteria consisted of patients with negative blood markers for the autoantibodies. There was no exclusion based on age or pre-existing comorbidity.
At the initial and each follow up consultation the following parameters were recorded; visual acuity (VA) in logarithm of the minimum angle of resolution (logMAR), colour vision (Ishihara pseudoisochromatic testing), presence/absence of a relative afferent pupillary defect (RAPD), slit lamp biomicroscopy with optic nerve assessment, kinetic visual fields (Octopus Visual Field, Haag-Streit, Switzerland), OCT (Cirrus 5000, Carl Zeiss, Meditec, Dublin, CA, USA) analysis of the macula, ganglion cell layer (GCL) and the retinal nerve fibre layer (RNFL). Each of the patients had magnetic resonance imaging (MRI) scanning of the brain and optic nerves with gadolinium contrast. Colour vision was assessed using 14 of the 38 Ishihara plates and graded as mild (10-14/14), moderate (6-10/14) or severe (0-5/14) depending on number of plates correctly identified as outlined in parentheses. RAPD was assessed for its presence or absence and graded from 0 to 3 in terms of increasing severity[12]. Red desaturation was assessed using a red hat pin on a white background and participants were asked to compare colour intensity between each eye[13].
Statistical Analysis
Data was collated and analysed using the statistical package STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). A statistically significant result was defined as P<0.05. Best corrected VA was recorded in the patient's medical notes in Snellen format in metres which was subsequently converted to the logMAR for the purpose of statistical analysis. A VA of count fingers (CF), hand motion (HM), perception of light (PL) and no perception of light (NPL) were denoted 2.1, 2.4, 2.7, and 3.0 on the logMAR scale, respectively.
RESULTS
Baseline Characteristics
A total of 16 patients were include in the study. This consisted of 12 (75%) anti-MOG positive ON patients and 4 (25%) anti-AQP4 positive patients. These 16 patients comprised all recorded cases of antibody positive ON that had attended the Royal Victoria Ear and Ear Hospital. Clinical characteristics of the anti-MOG cohort are seen in Table 1 and the anti-AQP4 cohort are seen in Table 2. The average length of the follow up was 16.5mo (range 2 to 41mo) and 47mo (range 2 to 131mo) for the MOG and AQP4 groups, respectively. Female to male ratio was 7:5 in the MOG group and 1:1 in the AQP4 group. Average age of onset in the MOG group was 29.2y with a range of 10 to 61y, while in the AQP4 group the average age was 42y (range 18-55y). Presenting complaint was blurring of vision in 10 of 12 of the MOG group (83.33%). The remaining 2 patients had pain as their presenting symptom (16.67%). In the AQP4 group, 3 out of the 4 patients (75%) experienced a decrease in vision while the remaining one patient had horizontal diplopia. Pain was a feature of all but one of the anti-MOG cohort (91.67%), either retrobulbar (27.27%) or more commonly on eye movement (72.73%). Interestingly, all the patients in the AQP4 cohort (100%) reported an absence of pain.
Table 1. Initial patient characteristics anti-MOG cohort.
| Patient | Onset age (gender) | Symptoms; duration (d) | Clinical findings | VA (RE, LE; logMAR) | RAPD (RE, LE) | Colour VA (Ishihara test; RE, LE) | Red desaturation (present, absent) | Average GCL; RE, LE (µm) | Average RNFL; RE, LE (µm) | Pain (retrobulbar or on movement) | MRI brain and orbits demyelination protocol | Initial treatment |
| 1 | 41 (F) | Decrease in right VA; pain (7) | Normal ONH bilaterally | 1.0, 0.6 | +3, 0 | 0/14, 14/14 | Present RE | 65, 78 | 80, 104 | On movement | High signal in retrobulbar optic nerves bilaterally that does not extend to chiasm | Admitted for 3/7 IVMP with oral taper |
| 2 | 33 (F) | Blurring of vision BE; pain on eye movements (2) | Bilateral swollen ONH | 1.0, 1.0 | 0, 0 | 0/14, 0/14 | Present BE | 84, 91 | 175, 205 | On movement BE | High signal in optic nerves bilaterally. | Admitted for 3/7 IVMP with oral taper |
| 3 | 36 (F) | Left orbital pain (11) | Normal RE ONH; LE ONH swollen inferiorly | 0, 0.1 | 0, 0 | 14/14, 14/14 | Absent BE | 87, 85 | 104, 114 | Retrobulbar | Irregular enhancement of left optic nerve. MRI spine NAD | Admitted for 3/7 IVMP with oral taper |
| 4 | 37 (M) | Decrease in right VA; pain on eye movements (14) | Right swollen ONH | 1.0, 0 | +3, 0 | 0/14, 14/14 | Present RE | 83, 83 | 139, 91 | On movement of RE only | Low grade thickening and enhancement of right optic nerve | Admitted for 3/7 IVMP with oral taper |
| 5 | 23 (M) | Decrease in left VA; pain (7) | Pale left ONH | 0, 2.1 | 0, +1 | 14/14, 1/14 | Present LE | 61, 61 | 59, 55 | Retrobulbar RE | High signal in both retrobulbar optic nerves, more marked on left with enhancement | Admitted for 3/7 IVMP with oral taper |
| 6 | 24 (M) | Decrease in left VA; pain (7) | Normal ONH bilaterally | 0, 0.3 | 0, +2 | 14/14, 14/14 | Present LE | 87, 76 | 98, 94 | Pain on eye movements LE | Abnormal T2 hyperintensity of left optic nerve which appears markedly atrophied | Admitted for 3/7 IVMP with oral taper |
| 7 | 10 (M) | Decrease in left VA, followed by right VA and pain (10) | Swollen ONH bilaterally | 1.8, 2.1 | 1, +3 | 1/14, 1/14 | Present BE | 32, 34 | 254, 379 | Retrobulbar pain BE | Numerous bilateral asymmetric areas of abnormal signal intensity within the white matter sparing the corpus callosum | Admitted for 3/7 IVMP with oral taper |
| 8 | 14 (M) | Decrease in left VA with pain (14) | Swollen left ONH | 0.1, 1 | 0, +3 | 14/14. 3/14 | Present LE | 68, 73 | 92, 235 | Pain on eye movement LE | Signal abnormality in the retrobulbar left optic nerve extending into the canalicular component. The nerve is expanded and demonstrates enhancement | Admitted for 3/7 IVMP with oral taper |
| 9 | 21 (F) | Decrease left VA and eye pain (14) | Swollen left ONH | 0, 0.2 | 0, +2 | 14/14, 7/14 | Present LE | 79, 80 | 93, 203 | Pain on eye movement LE | T2 hyperintensity within the anterior aspect of the intraorbital segment of the left optic nerve | Admitted for 3/7 IVMP with oral taper |
| 10 | 61 (F) | Decrease in right VA (3) | Swollen right ONH | 0.5, 0.1 | +2, 0 | 6/14, 12/14 | Present RE | 54, 49 | 58, 54 | No pain | Increased signal from the retrobulbar and canalicular optic nerve | Presumed NAION, normal ESR, follow up OPD |
| 11 | 19 (F) | Decrease in right VA and eye pain (7) | Swollen right ONH | 0.4, 0 | +3, 0 | 4/14, 14/14 | Present RE | 79, 83 | 143, 95 | Pain on eye movements RE | Abnormal signal within the orbital segment of the right optic nerve which appears expanded | Admitted for 3/7 IVMP with oral taper |
| 12 | 31 (F) | Pain on movement in RE (3) | Swollen right ONH | 0, 0 | +2, 0 | 13/14, 14/14 | Present RE | 33, 34 | 254, 379 | Pain on eye movements RE | High signal within the intraorbital segment of the right optic nerve measuring 8 mm | Admitted for 3/7 IVMP with oral taper |
F: Female; M: Male; VA: Visual acuity; ONH: Optic nerve heads; RAPD: Relative afferent pupillary defect; RE: Right eye; LE: Left eye; BE: Both eyes; GCL: Ganglion cell layer; RNFL: Retinal nerve fibre layer; MRI: Magnetic resonance imaging; NAD: No abnormality detected; IVMP: Intravenous methylprednisolone; NAION: Nonarteritic anterior ischemic optic neuropathy; ESR: Erythrocyte sedimentation rate; OPD: Outpatient department.
Table 2. Initial patient characteristics anti-AQP4 cohort.
| Patient | Onset (gender) | Symptoms duration (d) | Clinical findings | VA (RE, LE; (logMAR) | RAPD (RE, LE) | Colour VA (Ishihara plates, RE, LE) | Red desaturation (present, absent) | Average GCL; RE, LE (µm) | Average RNFL; RE, LE (µm) | Pain (retrobulbar or on movement) | MRI brain and orbits demyelination protocol | Initial treatment |
| 1 | 18 (M) | Decrease in left VA (3) | Pale left ONH | 0, 3 | 0, +3 | 14/14, 0/14 | NA | 54, 55 | 51, 50 | Absent | Marked high signal in the left retrobulbar optic nerve | Admitted for 3/7 IVMP with oral taper |
| 2 | 55 (F) | Decrease in left VA (4) | Normal ONH bilaterally | 0, 2.7 | 0, +2 | 14/14, 0/14 | Present | 68, 68 | 81, 87 | Absent | No evidence of optic neuritis | Admitted for 3/7 IVMP with oral taper |
| 3 | 33 (F) | Diplopia worse when looking to right (6) | Normal ONH, right INO | 0, 0 | 0, 0 | 14/14, 14/14 | Absent | 75, 74 | 99, 94 | Absent | High signal in the canalicular components of both optic nerves extending back to chiasm with expansion of the chiasm | Admitted for 3/7 IVMP with oral taper |
| 4 | 51 (M) | Decrease in vision in BE, particular night vision (63) | Pale ONH bilaterally | 0.2, 0.2 | 0, 0 | 3/14, 3/14 | Present BE | 60, 61 | 67, 63 | Absent | Evidence of chiasmitis with T2 weighted chiasmal abnormalities | Admitted for 5/7 IVMP with oral taper |
F: Female; M: Male; VA: Visual acuity; ONH: Optic nerve heads; RAPD: Relative afferent pupillary defect; RE: Right eye; LE: Left eye; BE: Both eyes; GCL: Ganglion cell layer; RNFL: Retinal nerve fibre layer; MRI: Magnetic resonance imaging; NA: Not applicable; IVMP: Intravenous methylprednisolone; INO: Intranuclear ophthalmoplegia.
Ocular Findings
With regards the affected eye in the MOG group, 5 were right eyes, 5 were left eyes and 2 of the patients had bilateral involvement giving us a total of 14 eyes available for analysis. There were 6 affected eyes in the AQP4 group comprising of 2 left eyes and 2 bilateral. The 11 of 14 (78.57%) eyes in the MOG group had visible swollen optic nerve heads (ONH), 2 (14.29%) had normal appearing ONH and the remaining 1 (7.14%) had a pale ONH on presentation. In the AQP4 group, 3 out of the 6 (50%) eyes had either normal or pale appearing ONH and none had a swollen ONH. The presence or absence of an RAPD was also assessed at presentation to the ophthalmic emergency department. Of the MOG group, 11 of the 14 eyes (78.57%) had a RAPD. The presence of an RAPD in the AQP4 group was less with only 2 of the 6 (33.33%) eyes displaying one. The presence of a colour vision defect was also assessed. This was categorised as mild, moderate, or severe as outlined in the methods section. Of the 14 eyes in the MOG cohort, 12 (85.71%) had a colour vision defect. Of these, 9 (75%) were severe, 2 (16.67%) were moderate and 1 (8.33%) was mild. In the AQP4 group, a colour deficiency was observed in 4 of the 6 eyes (66.67%), all 4 being severe in nature. The presence of red desaturation was also assessed in a binary fashion. It was present in all but one eye in the MOG group (92.86%). Only 5 eyes in the AQP4 group were examinable as one eye was NPL, and 3 of the 5 eyes (60%) had this subjective finding.
Visual Acuity and Optical Coherence Tomography Findings
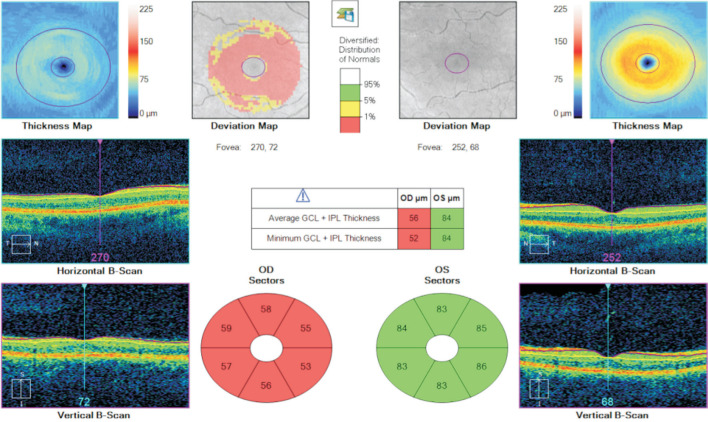
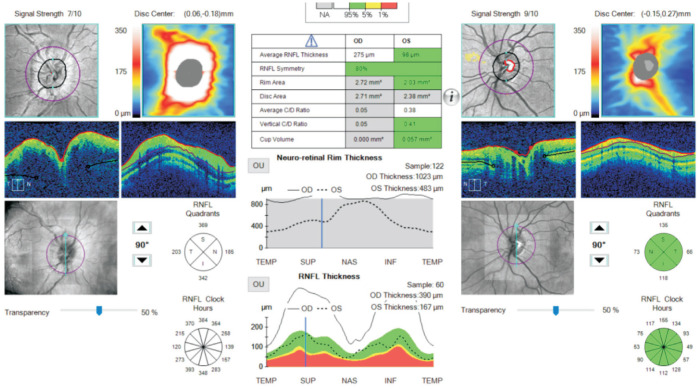
Tables 3 and 4 outline initial and final VA in logMAR units, initial and final average GCL and RNFL thickness and the respective change in each for both groups. Only the records for the involved eye were taken for analysis and if both eyes were affected then the data for the more affected eye was utilised. The average presenting logMAR VA in the MOG group was 0.82 (range 0-2.1) while in the AQP4 group it was 1.475 (range 0-3). At last review, the average improvement in VA in the MOG group was -0.66 (range -2.1 to +0.1) and in the AQP4 group was -0.05 (range -0.3 to +0.1). With regards GCL analysis (Figure 1), a modest decrease in thickness was seen in both groups with an average reduction of 2.92 and 7.75 µm in the MOG and AQP4 respectively. The retinal nerve fibre thickness analysis (Figure 2) displayed a greater reduction in thickness, especially in the MOG cohort with an average reduction of 58.75 µm in this group as opposed to only 10.5 µm in the AQP4 group. We examined the correlation between GCL thickness and VA at presentation and as a determinant of final visual outcome in the MOG group. There was no statistically significant correlation (Pearson correlation) between GCL thickness and presenting and final VA [r(10)=0.081, P=0.08 and r(10)=0.089, P=0.34 respectively]. The same statistical analysis was performed for the correlation between RNFL and VA and similar outcomes were observed [r(10)=0.04, P=0.22 and r(10)=0.09, P=0.04]. In the AQP4 group, moderate correlation was observed between GCL thickness and presenting VA [r(2)=0.61, P=0.42], but this result was not statistically significant. No correlation was seen for initial RNFL thickness and final visual outcome in this group either [r(2)=0.19, P=0.38].
Table 3. Initial, final, and relative change in GCL, RNFL and VA for anti-MOG cohort.
| Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Average |
| GCL | 65, 62, -3 | 84, 62, -12 | 85, 82, -3 | 83, 56, -26 | 61, 58, -3 | 76, 81, +5 | 34, 35, +1 | 73, 50, -23 | 80, 72, -8 | 54, 52, -2 | 79, 78, -1 | 33, 73, +40 | 67.23, 63.42, -2.92 |
| RNFL | 104, 60, -44 | 175, 88, -87 | 114, 91, -23 | 139, 57, -82 | 55, 55, 0 | 94, 92, -2 | 379, 86, -293 | 235, 47, -188 | 203, 80, -123 | 58, 58, 0 | 143, 110, -33 | 254, 84, 170 | 162.75, 75.67, -58.75 |
| VA | 1, 0, -1 | 1, 0.2, -0.8 | 0.1, 0.1, 0 | 1, 0.5, -0.5 | 2.1, 0.1, -2 | 0.3, 0.2, -0.1 | 2.1, 0, -2.1 | 1, 0.1, -0.9 | 0.2, 0, -0.2 | 0.5, 0.2, -0.3 | 0.5, 0, -0.5 | 0, 0.1, +0.1 | 0.82, 0.13, -0.66 |
GCL: Ganglion cell layer; RNFL: Retinal nerve fiber layer; VA: Visual acuity.
initial, final, change; µm
Table 4. Initial, final, and relative change in GCL, RNFL and VA for anti-AQP4 cohort.
| Parameters | 1 | 2 | 3 | 4 | Average |
| GCL | 55, 48, -7 | 68, 43, -25 | 74, 76, +2 | 61, 60, -1 | 64.5, 56.75, -7.75 |
| RNFL | 50, 49, -1 | 87, 51, -36 | 94, 91, -3 | 63, 61, -2 | 73.5, 63, -10.5 |
| VA | 3, 3, 0 | 2.7, 2.4, -0.3 | 0 | 0.2, 0.3, +0.1 | 1.475, 1.425, -0.05 |
GCL: Ganglion cell layer; RNFL: Retinal nerve fiber layer; VA: Visual acuity.
initial, final, change; µm
Figure 1. An example of ganglion cell layer analysis of a patient with right anti-MOG positive optic neuritis.
Marked thinning of the GCL in his right eye is noted in comparison to his left.
Figure 2. An example of a retinal nerve fibre layer analysis in a right anti-MOG positive optic neuritis with a swollen disc.
Visual Field Testing
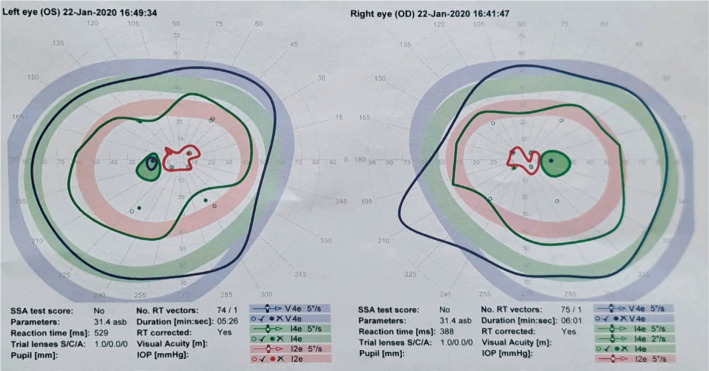
Table 5 depicts the visual fields findings associated with each patient in both cohorts and the change in each. Of the MOG cohort, 5 of the 12 patients (41.67%) had generalised visual field depression or an enlarged blind spot (Figure 3). Five of the 12 (41.67%) fields demonstrated an improvement, 5 fields were unchanged from the baseline and 2 (16.67%) demonstrating deterioration in the follow up period. One field in the AQP4 (25%) group showed an improvement from baseline to final while the others remained unchanged.
Table 5. Initial and final visual field findings.
| Patient | Initial visual field defect (MOG) | Final visual field defect (MOG) | Initial visual field defect (AQP4) | Initial visual field defect (AQP4) |
| 1 | General depression | Full fields | Unreadable | Unreadable |
| 2 | General depression | Enlarged blindspot | General depression | General depression |
| 3 | Full fields | Full fields | Full fields | Full fields |
| 4 | General depression | Enlarged blindspot | Unreadable | General depression |
| 5 | Temporal defect | Full fields | - | - |
| 6 | Inferior defect | Inferior defect | - | - |
| 7 | Unreadable | Unreadable | - | - |
| 8 | Superior defect | General depression | - | - |
| 9 | Enlarged blindspot | Full fields | - | - |
| 10 | Inferior defect | Temporal defect and enlarged blindspot | - | - |
| 11 | Full fields | Full fields | - | - |
| 12 | Enlarged blindspot | Enlarged blindspot | - | - |
Figure 3. Octopus visual fields of a 35-year-old female with bilateral anti-MOG positive optic neuritis which displays constriction of inner field and enlarged blind spots bilaterally.
Radiological Findings
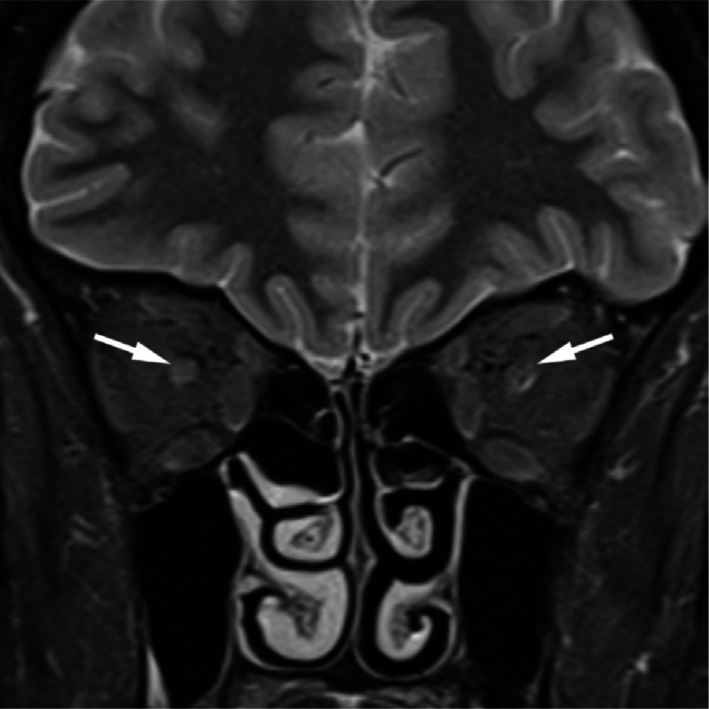
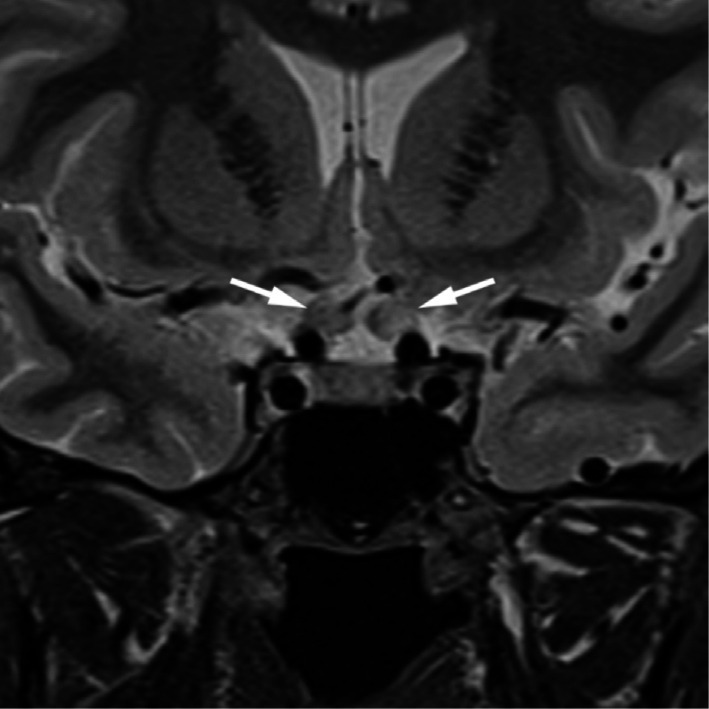
All but one of the patients in the anti-MOG cohort had sparing of brain involvement (91.67%). All had optic nerve abnormalities observed on MRI imaging (Figure 4). Two (16.67%) of the patients had enhancement in the affected nerve and in the contralateral clinically unaffected nerve. In the AQP4 group, 2 (50%) of the patients had optic nerve enhancement on MRI, one had no evidence of optic neuritis and the final patient had evidence of chiasmitis (Figure 5; Signal abnormality on T2 weighted imaging and uptake with gadolinium).
Figure 4. A coronal MRI showing bilateral enhancement of both optic nerves in female with bilateral anti-MOG positive optic neuritis.
Figure 5. Coronal MRI demonstrating high signal in canalicular components of both optic nerves with prechiasmal expansion in a patient with bilateral anti-AQP4 optic neuritis.
Treatment and Maintenance Therapy
All patients were treated as inpatients for 3 to 5d of 500 mg twice daily dosing of intravenous methylprednisolone at presentation. Current immunosuppressant therapy, if any, is outlined in Table 6. In the MOG group, azathioprine was the maintenance medication of choice with 5 of the 12 (41.67%) patients receiving it. Two patients were on methotrexate (16.67%), 1 was taking mycophenolate mofetil (8.33%) and 3 of the remaining 4 (33.33%; 1 of which was pregnant) were on no maintenance therapy. The final patient was placed on rituximab, underwent plasma exchange and intravenous immunoglobulins (IVIG) and maintenance low dose steroid. In the AQP4 group, two of the patients (50%) were on low dose prednisolone and of the other two, one was taking azathioprine and the other methotrexate to control disease relapse.
Table 6. Long term treatment.
| Patient | Treatment for anti-MOG cohort | Treatment for anti-AQP4 cohort |
| 1 | Methotrexate 10 mg and folic acid 5 mg weekly | Prednisolone 10 mg once daily |
| 2 | Azathioprine 150 mg once daily | Prednisolone 10 mg once daily |
| 3 | None | Azathioprine 150 mg once daily |
| 4 | Azathioprine 150 mg once daily | Methotrexate 22.5 mg weekly |
| 5 | Azathioprine 150 mg once daily | - |
| 6 | None | - |
| 7 | Rituximab, previous IVIG and prednisolone 10 mg | - |
| 8 | Azathioprine 150 mg once daily | - |
| 9 | Mycophenolate mofetil 1500 mg once daily | - |
| 10 | Methotrexate 25 mg and folic acid once weekly | - |
| 11 | Azathioprine 150 mg once daily | - |
| 12 | None (currently pregnant) | - |
IVIG: Intravenous immunoglobulins.
Disease Course
As previously stated, 2 of the 12 patients (16.67%) in the MOG cohort and 2 of the 4 patients (50%) in AQP4 cohort the had bilateral involvement at presentation. With regards number of relapses, 5 patients in the MOG cohort had a relapsing course (average of 1.6 relapses, range 1-3) while the remining 7 patients had a single attack (monophasic course) at the time of writing. Two of the patients (60%) who had a recurrent course had sequential progression to the contralateral eye. In the AQP4 group, 2 of the 4 patients (50%) had bilateral involvement at presentation. Three of the 4 patients (75%) had a recurrent course (average of 3 relapses, range 1-7) and 1 of these patients had sequential progression with multiple relapses. The remaining one patient had a monophasic course.
DISCUSSION
Antibody positive optic neuropathies are uncommon. The limited number of seropositive patients available for analysis in our quaternary centre cohort is indicative of this. The female predominance usually seen with these diseases was evident in our sample with an overall female to male ratio of 9:7.
Visual Acuity and Optical Coherence Tomography Findings
Reduction in VA is a hallmark feature in optic nerve disease and the main reason why these patients attend the ophthalmic emergency department as their primary referral source. 81.25% of all eyes (13/16) had a reduction in their VA at presentation. The average presenting logMAR VA in the MOG group was 0.82 as opposed to the AQP4 group whose average VA was 0.655 units worse at 1.475. The average VA in the AQP4 group is compounded by the fact that 1 patient (25%) had a perfect presenting VA of 0 logMAR units. Interestingly, even though the AQP4 group had the greater scope for improvement in vision, it was the MOG group that achieved this (improvement of 0.66 units for the MOG group as opposed to just 0.05 units for the AQP4). If we define long term visual disability as 0.3 logMAR or greater (threshold for driving), all but one of the eyes in the MOG group achieved this (92.86%) as opposed to just 1 eye (25%) in the AQP4 group. These findings are in keeping with what is in the literature where anti-AQP4 positive patients tend to have more severe visual disability[6],[14].
The authors found that presenting and final VA was not be correlated with RNFL or GCL. With regards to GCL, an average reduction of 2.92 and 7.75 µm was seen in the MOG and AQP4 group respectively. These are extremely modest differences and negligible when the standard error of the OCT machine is taken in account. The RNFL analysis showed a reduction of 58.75 µm in the MOG group as opposed to only 10.5 µm in the AQP4 group. However, this is not an indication of worsening disease but is in keeping with the predominance of OHN swelling in the MOG cohort. This swelling resides with time and is in keeping with the observed reduction in RNFL thickness. Therefore, the authors suggest that analysis of the GCL layer is a more accurate representation of nerve damage as opposed to RNFL, especially in the acute phase of the disease. Waiting 3mo to accurately interpret RNFL would be more appropriate[15]. RNFL may be used to assess subtle swelling of the nerve and to assess change in chronic disease. OCT is unquestionably finding its foothold in the monitoring of optic nerve disease, where it offers an excellent objective numerical interpretation of inner retinal layers. Loss of these unmyelinated axons is postulated to be secondary to an antegrade process[16].
Differentiating from Ischaemic Optic Neuropathies
The average age of onset of disease was 29.2y in the MOG group and 42y in the AQP4 group. It is known that that these antibody positive diseases can occur at any age[14] whereas multiple sclerosis and typical optic neuritis tend to have a younger age of onset usually between 18 and 50y with an average of 32[17]. One patient in our MOG cohort and two in our AQP4 court were over the age of 50 at their initial presentation. Of these 3 patients, 2 were assumed to have ischaemic optic neuritis (ION) because of their age. In this age group it is imperative to out rule an arteritic cause, as this is a preventable potentially bilaterally blinding condition, and therefore any patient over 50 should have an erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) undertaken in the ophthalmic emergency department. It can be difficult to differentiate ION from atypical ON in this age bracket. Both anti-MOG positive ON and non arteritic ischemic optic neuropathy (NAION) can present with disc oedema and peripapillary haemorrhage[18]. However, absence of a history of vasculopathy would warrant further investigation in these patients. Usually, NAION is associated with an absence of pain, however, all the patients in our study over the age of 50 had a subjective absence of pain, again clouding the distinction between the two entities. NAION is usually linked with altitudinal defects on visual field testing[19], nevertheless when the presenting VA is too poor to undertake formal visual field testing, then the defect cannot be elucidated. NAION is usually associated with a small or crowded ONH, with an absent cup[20], and a consolidating clinical feature would be to examine the optic disc in the contralateral eye to assess if it has a diminished size. Atypical features, involvement of the other eye and a relapsing and remitting should warrant further investigation.
Pain
Pain is typically a feature of demyelinating optic neuropathies. The literature states that it is very frequently associated with ON from MS and anti-MOG. It is frequently associated with AQP4 positive ON[21]. However, an absence of pain does not out rule a demyelinating cause. The 91.67% of our MOG cohort had pain as one of their presenting symptoms while none of our AQP4 cohort reported pain, either periorbital or retrobulbar. Absence of pain in a patient with other signs and symptoms indicative of an ON should be investigated for other cause such as a compressive or space occupying lesion compromising the optic nerve. The vision loss in these patients is usually gradual and progressive[22] as opposed to the acute or subacute presentations of those with demyelinating lesions.
Optic Nerve Head Assessment
Optic nerve head assessment is a fundamental aspect of every ocular exam and it is crucial when it comes to the assessment of optic neuropathies as the appearance of the nerve can give crucial hints with respect to aetiology of the disease. Similar topographic changes can appear in chronic or late-stage disease[23] and therefore it is essential that the disc is assessed at presentation with baseline fundal disc photos taken for comparison with its appearance in follow up visits.
78.57% in the affected eye of our MOG cohort had a swollen disc at presentation whereas none of our AQP4 cohort had a swollen disc with 50% of the AQP4 cohort having either a pale disc or normal appearing ONHs. A pale optic disc is usually indicative of a chronic optic nerve condition such as compressive, hereditary, toxic/nutritional optic neuropathies however it can also occur as a sequela of an acute inflammatory or ischemic optic neuropathy[22]. If the nerve becomes pale secondary to a previous attack of ON, the nerve may not become swollen on repeat attacks highlighting the importance of optic nerve assessment at initial presentation[24]. One prospective cohort study of patients with ON showed that by testing all cases of ON with bilateral ON, recurrent ON, or optic disc swelling on fundoscopy for MOG antibody, all cases of MOG ON would be detected and only 50% of ON cases in the cohort would be tested overall[9].
Relative Afferent Pupillary Defect
The presence of a RAPD is a pathognomonic indication of organic disease. In conjunction with a normal retinal examination, it is highly suggestive of optic nerve dysfunction[25]. However, if pupillary function is brisk and reactive, it does not equate to an absence of an optic nerve disorder. 78.57% of our ant-MOG cohort had a RAPD at presentation. In contrast, only 33.33% of analysed eyes in the anti-AQP4 had an RAPD recorded at presentation. However, these findings need to be taken in conjunction with the clinical context. Two of the eyes which had an absence of an RAPD in the MOG group were a simultaneous bilateral ON with a logMAR VA of 1.0. In this instance, one can assume that both optic nerves are equally dysfunctional and therefore the absolute presence of an RAPD in the MOG cohort could be as high as 92.86%. In the AQP4 group, 2 patients had bilateral simultaneous involvement, but one patient had symptoms of an intranuclear ophthalmoplegia (INO) and brisk pupillary reactions whilst the other had symptoms of consensual optic nerve compromise and therefore the examiner may have had difficulty elucidating an RAPD. Extrapolating from this we can assume that the RAPD incidence at presentation in the AQP4 cohort to be 66.67%.
Subtle RAPDs can be difficult to measure[26] and so it is essential that if a patient presents with signs and/or symptoms of optic nerve dysfunction and there is a query over the presence of an RAPD that a senior clinician examines the pupillary responses. A patient should never be dilated in the ophthalmic emergency department or the neuro-ophthalmology clinic without the prior confirmation of the presence or absence of this essential ocular finding. If there is optic nerve dysfunction there should be an RAPD. The verification of this sign will lead the ophthalmologist down specific diagnostic pathways. The presence of an RAPD is nearly always of clinical significance and should always prompt red flags especially in the absence of other ocular or systemic comorbidities[27].
Colour Vision and Red Desaturation
Many authors argue that colour vision testing is an integral part of assessment of optic nerve functionality[28]. Multiple difference methods of colour vision testing are available including the Ishihara pseudoisochromic plates, the Hardy-Rand-Rittler test and Farnsworth-Munsell 100 hue test. The most practical and widely used of these is the Ishihara test[29] which contains 38 plates for testing, but a condensed version of either 10, 14 or 24 plates can be used. Ideally these tests are used to screen, classify, and grade severity of colour deficency[30] but for optic nerve disorders they are used to measure baseline, assess extent, and elicit change in follow up. The results of the tests themselves are dependent on a multitude of patient and environmental factors including lighting, refractive correction, literacy, pre-existing pathology, undiagnosed colour deficiency, cognition etc. In our cohort, 85.71% and 66.67% in the MOG and AQP4 group respectively had a deficiency on interpreting the Ishihara plates at presentation. We performed a Pearson correlation between presenting VA and number of plates correctly tested which revealed a moderate positive correlation between the two variables [r(14)=0.5, P=0.002). One can assume that presenting VA is a surrogate marker of colour vision i.e. the worst the presenting VA the fewer plates correctly identified. Some reviews now exclude colour vision testing as a requisite in the neuro ophthalmology exam[31] unless the examiner is looking to reduce subtle colour defects. In our cohort is the actual VA was 1.0 logMAR units or greater, the number of plates tested correctly was ubiquitously 3 or less and therefore the authors would question the rational of undertaking colour vision testing at this level of vision or worse.
Red desaturation is a subjective finding and was assessed in our cohort in a binary fashion. It was present in all but one eye in our MOG cohort (92.86%) and in all eyes that displayed signs of ON in our AQP4 cohort. Red desaturation is a simple, quick, and most importantly a sensitive test for patients with optic nerve disease[32]. It can be used a surrogate marker of an optic neuropathy as opposed to cumbersome colour vision testing, especially when the presenting VA is poor. However, it is difficult to quantify as it is a subjective test. One study revealed that almost 25% of healthy volunteers, with no documented evidence or ocular or optic nerve disease, stated that they experienced some degree of red desaturation[33].
Visual Field Testing
41.67% of our anti-MOG cohort have either improved or unchanged visual field from baseline, while the remaining 16.67% demonstrated a dis-improvement. 75% of the patients in our AQP4 cohort showed no change in their visual fields. It has been proposed that anti-AQP4 ON can produce a higher incidence of non-central and altitudinal defects in comparison to MS related ON and that an ischaemic mechanism may underpin this disease[34], however these findings were not echoed in our cohort with no altitudinal defects observed. There was a higher incidence of altitudinal defects seen in our MOG cohort with 3 of the 12 eyes (25%) presenting with either a superior or inferior altitudinal defect. The visual field defects in anti-MOG ON tend to be more severe than those seen with MS related ON[35].
Visual field testing in ON is used to classify baseline deficit and to monitor change over time. Routinely it is performed at onset if possible and at 3, 6 and 12mo. Visual fields are useful to analyse progression or recovery but due to the wide range of visual fields defects that an ON can produce, it is exceedingly difficult to use pattern of loss to distinguish it from other optic nerve disease. Visual field data extrapolated from the ON treatment trial showed that the most common pattern of loss included diffuse visual field loss in 48%, altitudinal defects in 15% and central or cecocentral scotoma in 8.3%[36]. There is debate over which type of visual field analyser is the most appropriate for monitoring patients with optic nerve disease with kinetic perimetry, such as the octopus visual field analyser, able to record abnormalities in the far periphery better than the Humphrey visual field analyser (static perimerty). The point was made by Keltner et al[37] when they compared kinetic peripheral and central static field in 448 patients in the optic neuritis treatment trial (ONTT) that only 2.9% of affected eye peripheral defects were missed on static perimetry. The authors would argue that this equated to 13 patients that had they visual field results interpreted incorrectly and therefore due to the wide range of visual field defects that ON can produce, kinetic visual fields should be the testing modality of choice.
Magnetic Resonance Imaging
MRI can provide clues regarding the aetiology of the demyelination. ON associated with MS is usually displays unilaterality with respect to lesions in the optic nerve and enhancing lesions are frequently seen in the periventricular, subcortical and juxtacortical areas of the brain[38]. Those positive for anti-AQP4 tend to have to more posterior involvement of the optic nerve including chiasm and simultaneous bilateral disease. These lesions tend to be longer than those seen in MS-ON[39]. In contrast, the MRI findings in anti-MOG disease tend to have more anterior involvement of the optic nerve sparing the chiasm[40], with perineural enhancement of the optic nerve sheath[41], but again they tend to be more longitudinally extensive and bilateral in up to 25% of cases[42].
Only one patient in our MOG group had brain involvement (8.33%) while 100% of this cohort had involvement in their affected nerve on primary MRI. Two of the patients have simultaneous bilateral involvement of the optic nerves (18.67%). In contrast the AQP4 group had more diverse findings, with bilateral optic nerve enhancement extending to the chiasm in one patient, one patient had no evidence of ON and the final patient had nonspecific T2 hyperintensity.
MRI with gadolinium contrast is the gold standard of radiographic imaging for demyelinating disease. All patients should have an MRI brain and optic nerves with contrast preformed. MRI provides high soft tissue contrast and the ability to characterise tissue properties[43] without exposing the patient to the harmful effects of ionising radiation. The findings in our MOG group are in keeping with what is already in the literature and the findings in the AQP4 group were not in keeping with typical ON and so therefore would warrant further investigation for atypical disease.
Treatment
Initial treatment for anti-MOG ON and anti-AQP4 is 1 g per day of intravenous methylprednisolone for 3 to 5d and a slow steroid taper over a period of 1 to 2mo to prevent relapse with steroid withdrawal[11]. It has been postulated that early intervention with IVMP in these diseases would lead to better long term visual outcomes[44] however this has not been proven in any prospective randomised trial. There are no clear criteria on the acute treatment for these diseases however we do know that they can present with profound visual loss, tend to relapse and have the tendency to become steroid dependant and therefore high dose steroid emerges to be the initial treatment of choice. The clinician should be careful with the use of high dose steroid especially in the elderly and diabetics. There is no doubt that they have great efficiency in the acute setting however their multitude of side effects warrant careful supervision and deliberate decision making. IVMP has been associated with serious side effects in the acute setting including hepatic insufficiency, avascular necrosis of the femoral head, steroid induced psychosis and autoimmune encephalitis[45]. The role of plasma exchange remains unclear in Anti-MOG disease whereas there appears to be some benefit in those positive for anti-AQP4[46].
All our patients received IVMP 1 g per day for 3 to 5d with an oral prednisolone taper. One patient suffered from steroid induced psychosis and was admitted to a psychiatric hospital for treatment for a short period and subsequently recovered fully with no psychiatric sequalae. In terms of long-term immunosuppression in our cohort, azathioprine was the maintenance medication of choice with 5 of the 12 (41.67%) patients receiving it in the MOG group. Two patients were on methotrexate (16.67%), 1 was taking mycophenolate mofetil (8.33%) and 3 of the remaining 4 (25%; 1 of which was pregnant) were on no maintenance therapy. The final patient was placed on rituximab, underwent plasma exchange and IVIG and maintenance low dose steroid. The 50% of the patients in the AQP4 group either on low dose oral prednisolone or azathioprine. One of our patients in the AQP4 has been subsequently sent for IVIG therapy. Both IVIG and monoclonal antibodies use in these diseases is well documented, but a lack of conclusive evidence exists[47]–[48]. Some medications used in the treatment of multiple sclerosis have proven to be ineffective in treating these atypical cases, particularly anti-MOG related disease. These include interferon beta[48]–[49], glatiramer acetate[49] and natalizumab[48]. There are emerging therapies including the monoclonal antibodies tocilizumab and eculizumab which are both undergoing clinical trials in AQP4 positive patients[50]–[51]. There is a potential role for low dose steroid in conjunction with steroid sparing agents with an increase in the number of relapses observed when prednisolone doses were reduced below 10 mg per day[52].
It has been shown that the use of oral and intravenous methylprednisolone to be bioequivalent for the treatment of acute ON[53]. This may be applicable for those unable to stay in hospital or who live in rural areas and it has shown to be a cost effective measure with a 4d inpatient stay costing a factor of 38 times more than treatment with oral steroid as an outpatient[54]. However, care should be taken especially with those who may suffer from the side effects of unsupervised high dose steroid administration e.g. frail, elderly, osteoporotic, immunocompromised etc.
The management of optic neuritis has been heavily influenced by the ONTT[55], a study which is currently 31 years old. Recent trials have rebutted the inferiority of oral steroid in comparison to intravenous[56]. This was not tailored towards patients with either anti-MOG or anti-AQP4 related disease and therefore care should be taken when extrapolating treatment and outcome measures from this trial. A recent opinion piece by Petzold et al[57] has made a compelling argument for a new corticosteroid treatment trial for acute ON due to vague definitions of symptom onset, delay in initiating treatment and crude outcome measures in the ONTT.
Limitations
Limitations of our study included reviewing these patients from an ophthalmic point of view. We only based our cohort of anti-AQP4 positive patients on those who were seropositive and had an episode of ON as opposed to those who met the definition based on the Wingerchuk et al's[58] criteria. We did not include contrast sensitivity as a measurement of visual dysfunction, which in retrospect would have been an appropriate parameter to record[59]. We did not include data on cerebrospinal fluid samples and non-ophthalmic morbidity as we believed it to be outside the scope of this article. There was also a wide array of length of follow up ranging from 2 to 131mo. Our patient numbers are relatively low, however due to the paucity of clinical trials on this patient cohort worldwide, we believe that characterising our cohort is important to further classifying these rare disease entities.
In conclusion, our study has shown the range of clinical characteristics that can be associated with both anti-MOG and anti-AQP4 ON. The prompt diagnosis and treatment of these disease is essential to prevent long term ocular sequalae. The role of MRI is essential to delineate disease radiographically. OCT data is proving to be the modality of choice to stratify damage at onset and monitor progression. The role of visual field testing and colour vision testing is equivocal but are adequate subjective indicators of evolution of disease. Further randomised control trials have yet to be carried out to fully clarify most appropriate long terms treatments for these diseases, but work is underway.
Acknowledgments
Conflicts of Interest: Murtagh P, None; Coman A, None; Stephenson K, None; Gaughan M, None; Ryan D, None; McNeill G, None; McGuigan C, None; Cassidy L, None.
REFERENCES
- 1.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, Nakashima I, Apostolos-Pereira SL, Talim N, Simm RF, Lino AMM, Misu T, Leite MI, Aoki M, Fujihara K. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89–102. doi: 10.1038/s41582-018-0112-x. [DOI] [PubMed] [Google Scholar]
- 3.Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 2013;23(6):661–683. doi: 10.1111/bpa.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma XY, Kermode AG, Hu XQ, Qiu W. NMOSD acute attack: Understanding, treatment, and innovative treatment prospect. J Neuroimmunol. 2020;348:577387. doi: 10.1016/j.jneuroim.2020.577387. [DOI] [PubMed] [Google Scholar]
- 5.He D, Li Y, Dai QQ, Zhang YF, Xu Z, Li Y, Cai G, Chu L. Myopathy associated with neuromyelitis optica spectrum disorders. Int J Neurosci. 2016;126(10):863–866. doi: 10.3109/00207454.2015.1113175. [DOI] [PubMed] [Google Scholar]
- 6.Patterson SL, Goglin SE. Neuromyelitis optica. Rheum Dis Clin North Am. 2017;43(4):579–591. doi: 10.1016/j.rdc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, du Pasquier RA, Polman CH, Sorensen PS, Hemmer B. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019–1032. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga AS, Fryer JP, Leavitt JA, Weinshenker BG, McKeon A, Tillema JM, Lennon VA, Tobin WO, Keegan BM, Lucchinetti CF, Kantarci OH, McClelland CM, Lee MS, Bennett JL, Pelak VS, Pittock SJ. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8–15. doi: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyun JW, Woodhall MR, Kim SH, Jeong IH, Kong B, Kim G, Kim Y, Park MS, Irani SR, Waters P, Kim HJ. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88(10):811–817. doi: 10.1136/jnnp-2017-315998. [DOI] [PubMed] [Google Scholar]
- 11.Tajfirouz DA, Bhatti MT, Chen JJ. Clinical characteristics and treatment of MOG-IgG-associated optic neuritis. Curr Neurol Neurosci Rep. 2019;19(12):100. doi: 10.1007/s11910-019-1014-z. [DOI] [PubMed] [Google Scholar]
- 12.Broadway DC. How to test for a relative afferent pupillary defect (RAPD) Community Eye Health. 2012;25(79-80):58–59. [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin JF, Wray SH. Acquired color vision defects in retrobulbar neuritis. Am J Ophthalmol. 1978;86(2):193–201. doi: 10.1016/s0002-9394(14)76811-4. [DOI] [PubMed] [Google Scholar]
- 14.Jarius S, Ruprecht K, Kleiter I, et al. in cooperation with the Neuromyelitis Optica Study Group (NEMOS) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. doi: 10.1186/s12974-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 16.Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, Bullich S, Sepulveda M, Falcon C, Berenguer J, Saiz A, Sanchez-Dalmau B, Villoslada P. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75(1):98–107. doi: 10.1002/ana.24030. [DOI] [PubMed] [Google Scholar]
- 17.Abel A, McClelland C, Lee MS. Critical review: typical and atypical optic neuritis. Surv Ophthalmol. 2019;64(6):770–779. doi: 10.1016/j.survophthal.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen JJ, Bhatti MT. Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol. 2020;33(1):47–54. doi: 10.1097/WCO.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Jung JJ, Kim US. Differences between non-arteritic anterior ischemic optic neuropathy and open angle glaucoma with altitudinal visual field defect. Korean J Ophthalmol. 2015;29(6):418–423. doi: 10.3341/kjo.2015.29.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doro S, Lessell S. Cup-disc ratio and ischemic optic neuropathy. Arch Ophthalmol. 1985;103(8):1143–1144. doi: 10.1001/archopht.1985.01050080055019. [DOI] [PubMed] [Google Scholar]
- 21.Marzoli SB, Criscuoli A. Pain in optic neuropathies. Neurol Sci. 2018;39(1):25–31. doi: 10.1007/s10072-018-3334-1. [DOI] [PubMed] [Google Scholar]
- 22.Behbehani R. Clinical approach to optic neuropathies. Clin Ophthalmol. 2007;1(3):233–246. [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill EC, Danesh-Meyer HV, Kong GXY, Hewitt AW, Coote MA, MacKey DA, Crowston JG, Group ONS Optic disc evaluation in optic neuropathies: the optic disc assessment project. Ophthalmology. 2011;118(5):964–970. doi: 10.1016/j.ophtha.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Ducloyer JB, Caignard A, Aidaoui R, Ollivier Y, Plubeau G, Santos-Moskalyk S, Porphyre L, le Jeune C, Bihl L, Alamine S, Marignier R, Bourcier R, Ducloyer M, Weber M, le Meur G, Wiertlewski S, Lebranchu P. MOG-Ab prevalence in optic neuritis and clinical predictive factors for diagnosis. Br J Ophthalmol. 2020;104(6):842–845. doi: 10.1136/bjophthalmol-2019-314845. [DOI] [PubMed] [Google Scholar]
- 25.Cox TA, Thompson HS, Corbett JJ. Relative afferent pupillary defects in optic neuritis. Am J Ophthalmol. 1981;92(5):685–690. doi: 10.1016/s0002-9394(14)74662-8. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki A, Moore P, Kardon RH. Variability of the relative afferent pupillary defect. Am J Ophthalmol. 1995;120(5):622–633. doi: 10.1016/s0002-9394(14)72209-3. [DOI] [PubMed] [Google Scholar]
- 27.Strachan K, Jamieson A. The relative afferent pupillary defect: its role in the diagnosis of metastatic malignancy. QJM. 2012;105(5):463–466. doi: 10.1093/qjmed/hcr053. [DOI] [PubMed] [Google Scholar]
- 28.Chan CKM, Jindahra P, Muñoz S, Robert MP, Pula JH, Vaphiades M. Neuro-ophthalmic literature review. Neuro-Ophthalmology. 2013;37(4):175–180. [Google Scholar]
- 29.Fanlo Zarazaga A, Gutiérrez Vásquez J, Pueyo Royo V. Review of the main colour vision clinical assessment tests. Arch Soc Esp Oftalmol (Engl Ed) 2019;94(1):25–32. doi: 10.1016/j.oftal.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Birch J. A practical guide for colour-vision examination: report of the Standardization Committee of the International Research Group on Colour-Vision Deficiencies. Ophthalmic Physiol Opt. 1985;5(3):265–285. [PubMed] [Google Scholar]
- 31.Petzold A, Wattjes MP, Costello F, Flores-Rivera J, Fraser CL, Fujihara K, Leavitt J, Marignier R, Paul F, Schippling S, Sindic C, Villoslada P, Weinshenker B, Plant GT. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447–458. doi: 10.1038/nrneurol.2014.108. [DOI] [PubMed] [Google Scholar]
- 32.Almog Y, Gepstein R, Nemet AY. A simple computer program to quantify red desaturation in patients with optic neuritis. Graefes Arch Clin Exp Ophthalmol. 2014;252(8):1305–1308. doi: 10.1007/s00417-014-2687-2. [DOI] [PubMed] [Google Scholar]
- 33.Mikolajczyk B, Ritter A, Larson C, Connett J, Olson J, McClelland C, Lee M. Red desaturation prevalence and severity in healthy patients. Neurol Clin Pract. 2020;10(1212) doi: 10.1212/CPJ.0000000000001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima H, Hosokawa T, Sugino M, Kimura F, Sugasawa J, Hanafusa T, Takahashi T. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010;10:45. doi: 10.1186/1471-2377-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vicini R, Brügger D, Abegg M, Salmen A, Grabe HM. Differences in morphology and visual function of myelin oligodendrocyte glycoprotein antibody and multiple sclerosis associated optic neuritis. J Neurol. 2021;268(1):276–284. doi: 10.1007/s00415-020-10097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keltner J, Johnson C, Spurr J, Beck R. Baseline visual field profile of optic neuritis. The experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Arch Ophthalmol. 1993;111(2):231–234. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 37.Keltner JL, Johnson CA, Spurr JO, Beck RW. Comparison of central and peripheral visual field properties in the optic neuritis treatment trial. Am J Ophthalmol. 1999;128(5):543–553. doi: 10.1016/s0002-9394(99)00304-9. [DOI] [PubMed] [Google Scholar]
- 38.Beck RW, Arrington J, Murtagh FR, Cleary PA, Kaufman DI. Brain magnetic resonance imaging in acute optic neuritis. Experience of the Optic Neuritis Study Group. Arch Neurol. 1993;50(8):841–846. doi: 10.1001/archneur.1993.00540080050013. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, Klawiter EC, Sato DK, de Seze J, Wuerfel J, Banwell BL, Villoslada P, Saiz A, Fujihara K, Kim SH, Guthy-Jackson Charitable Foundation NMO International Clinical Consortium & Biorepository MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biotti D, Bonneville F, Tournaire E, Ayrignac X, Dallière CC, Mahieu L, Vignal C, Dulau C, Brochet B, Ruet A, Ouallet JC, Gout O, Heran F, Menjot de Champfleur N, Tourdias T, Deneve M, Labauge P, Deschamps R. Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J Neurol. 2017;264(10):2173–2175. doi: 10.1007/s00415-017-8615-8. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–482. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 42.Denève M, Biotti D, Patsoura S, et al. MRI features of demyelinating disease associated with anti-MOG antibodies in adults. J Neuroradiol. 2019;46(5):312–318. doi: 10.1016/j.neurad.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Grover VPB, Tognarelli JM, Crossey MME, Cox IJ, Taylor-Robinson SD, McPhail MJW. Magnetic resonance imaging: principles and techniques: lessons for clinicians. J Clin Exp Hepatol. 2015;5(3):246–255. doi: 10.1016/j.jceh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiebel-Kalish H, Hellmann MA, Mimouni M, Paul F, Bialer O, Bach M, Lotan I. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e572. doi: 10.1212/NXI.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walasik-Szemplińska D, Kamiński G, Sudoł-Szopińska I. Life-threatening complications of high doses of intravenous methylprednisolone for treatment of Graves' orbitopathy. Thyroid Res. 2019;12:13. doi: 10.1186/s13044-019-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnan M, Valentino R, Debeugny S, Merle H, Fergé JL, Mehdaoui H, Cabre P. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89(4):346–351. doi: 10.1136/jnnp-2017-316286. [DOI] [PubMed] [Google Scholar]
- 47.Ramanathan S, Mohammad S, Tantsis E, et al. Australasian and New Zealand MOG Study Group Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarius S, Ruprecht K, Kleiter I, et al. in cooperation with the Neuromyelitis Optica Study Group (NEMOS) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75(4):478–487. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M, Miyake S, Aranami T, Yamamura T. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82(15):1302–1306. doi: 10.1212/WNL.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, O'Toole O, Wingerchuk DM. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12(6):554–562. doi: 10.1016/S1474-4422(13)70076-0. [DOI] [PubMed] [Google Scholar]
- 52.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, Tackley G, Hamid S, Sheard A, Reynolds G, Chandratre S, Hemingway C, Jacob A, Vincent A, Leite MI, Waters P, Palace J. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–3138. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 53.Morrow SA, Fraser JA, Day C, Bowman D, Rosehart H, Kremenchutzky M, Nicolle M. Effect of treating acute optic neuritis with bioequivalent oral vs intravenous corticosteroids: a randomized clinical trial. JAMA Neurol. 2018;75(6):690–696. doi: 10.1001/jamaneurol.2018.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chataway J, Porter B, Riazi A, Heaney D, Watt H, Hobart J, Thompson A. Home versus outpatient administration of intravenous steroids for multiple-sclerosis relapses: a randomised controlled trial. Lancet Neurol. 2006;5(7):565–571. doi: 10.1016/S1474-4422(06)70450-1. [DOI] [PubMed] [Google Scholar]
- 55.Beck R. The optic neuritis treatment trial. Arch Ophthalmol. 1988;106(8):1051–1053. doi: 10.1001/archopht.1988.01060140207023. [DOI] [PubMed] [Google Scholar]
- 56.le Page E, Veillard D, Laplaud DA, Hamonic S, Wardi R, Lebrun C, Zagnoli F, Wiertlewski S, Deburghgraeve V, Coustans M, Edan G, Investigators C, West Network for Excellence in Neuroscience Oral vs intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974–981. doi: 10.1016/S0140-6736(15)61137-0. [DOI] [PubMed] [Google Scholar]
- 57.Petzold A, Braithwaite T, van Oosten BW, Balk L, Martinez-Lapiscina EH, Wheeler R, Wiegerinck N, Waters C, Plant GT. Case for a new corticosteroid treatment trial in optic neuritis: review of updated evidence. J Neurol Neurosurg Psychiatry. 2020;91(1):9–14. doi: 10.1136/jnnp-2019-321653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 59.Owidzka M, Wilczynski M, Omulecki W. Evaluation of contrast sensitivity measurements after retrobulbar optic neuritis in Multiple Sclerosis. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):673–677. doi: 10.1007/s00417-014-2590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]