Abstract
AIM
To investigate the effect of 0.01% atropine sulphate eye gel on myopia progression and axial elongation in a 6-month treatment in children.
METHODS
Totally 185 children aged 6-12y with binocular myopia of 3.0 D or less in both eyes were enrolled in this prospective cohort study. The atropine group (n=125) received one drop of 0.01% atropine sulphate eye gel in each eye before bedtime daily. The control group included 60 matched children without drug intervention during the same period. The spherical equivalent and axial length was recorded at baseline and the sixth month of treatment. The efficacy was evaluated by the change of the spherical equivalent and axial length. Adverse events were also recorded.
RESULTS
The average spherical equivalent and axial length at baseline were not statistically significant between the atropine group (-1.64±0.80 D, 24.13±0.76 mm) and the control group (-1.59±0.94 D, 24.06±0.77 mm, P>0.05). After 6mo, there was significantly difference in the spherical equivalent progression between the atropine and the control group (-0.27±0.33 vs -0.60±0.35 D, P<0.001), with a relative reduction of 55.0% in myopia progression. The increase in axial elongation in the atropine group was significantly less than control group (0.19±0.14 vs 0.26±0.14 mm, P<0.001), with a relative reduction of 26.9% in axial length. The 84.4% and 38.4% of the eyes progressed by less than 0.50 D and remained stable in the atropine group, compared with 51.7% and 4.2% in the control group. No adverse events were observed.
CONCLUSION
Atropine sulphate eye gel 0.01% can slow down myopia progression and axial elongation in children with a 6-month treatment.
Keywords: atropine sulphate eye gel, myopia, spherical equivalent, axial length
INTRODUCTION
Myopia is considered a serious public health concern globally. Holden et al[1] predicted that 51% of Europeans would be myopic in the year 2050 by using simple regression analysis. In another study the author foretold that an average of -2.0 D would develop and the predicted prevalence of myopia is 63% in 2050[2]. Many population-based studies in children have shown that the prevalence of myopia is obviously higher in urbanized East Asian countries[3]. During the past decade, the prevalence of myopia and vision impairment was highly prevalent among Chinese school students, and increased with grade in a nonlinear manner; vision impairment was high in high school students who had a high prevalence of high myopia[4]–[6]. The rising prevalence of myopia is also accompanied by earlier onset, which in turn leads to an increased risk of high myopia. However, high myopia is a known risk factor for sight-threatening conditions such as retinal detachment, myopic macular degeneration and glaucoma later in life. Each additional 1 D of myopia progression associated with a 58%, 20%, 21%, and 30% increase in the risk of myopic maculopathy, open-angle glaucoma, posterior subcapsular cataract, and retinal detachment, respectively. The predicted years of visual impairment ranges from 4.42 in a person with myopia of -3.0 D to 9.56 in a person with myopia of -8.0 D, and 1.0 D reduction would lower these by 0.74 and 1.21y, respectively[7]. Therefore, myopia is a serious public health problem for the next few decades especially in China, and high myopia becomes a leading cause of blindness worldwide due to associated ocular illness[8].
Several treatments to slow myopia progression have been investigated, including increase outdoor activity time, orthokeratology, peripheral defocus modifying contact lenses, and the topical use of low-concentration atropine eye drops. Previous review and Meta-analyses suggested that atropine eye drops conferred the best efficacy among all myopia prevention methods[9]. There have been many controlled clinical trials which indicate that low-concentration atropine eye drops can delay the occurrence and development of myopia. A review of 41 studies[10] and a meta-analysis of 16 interventions[11] for myopia showed that topical antimuscarinic drugs, such as 0.01%-1% atropine eye drops, were effective in slowing myopia progression and axial elongation in children. Moreover, 1% atropine effectively inhibited myopia progression and promoted myopic rebound, although it was associated with adverse effects such as blurred near vision and hypersensitivity reactions[12]–[13]. Low-concentration (0.01%-0.5%) atropine eye drops can effectively prevent myopia progression[14]–[15]. Based on available evidence, 0.01% atropine eye drops are widely used for myopia control, with good efficacy and few side effects[16]–[21].
Eye-drops are the most conventional dosage form of currently accessible ophthalmic formulations. Despite the excellent acceptance by patients, the major problems encountered are having low bioavailability profiles, rapid removal from the administration site, and thus ineffective delivery of drugs. However, eye gel has been investigated as a delivery system which is able to overcome some of these challenges[22]. Therefore, we developed a 0.01% atropine sulphate eye gel and evaluate its efficacy and safety on myopia progression.
In this study, 0.01% atropine sulphate ophthalmic gel was used to treat myopia in adolescent patients with a 6-month clinical application, and its effects on myopia control were evaluated by changes in spherical equivalent (SE) and axial length (AL).
SUBJECTS AND METHODS
Ethical Approval
This prospective cohort study followed the tenets of the Helsinki Declaration on ethical principles for medical research involving human subjects and was approved by Xi'an No.1 Hospital Ethics Committee. Informed consent of the children and their guardians was obtained. The protocol of this study is registered in the ChiCTR Database (ChiCTR2000038218).
Population
The study recruited 185 children who had been diagnosed with myopia in Xi'an No.1 Hospital and Shaanxi Provincial Institute of Ophthalmology between February 2008 and October 2019. The inclusion criteria were as follows: age 6-12y, male or female, binocular myopia of 3.0 D or less, near visual acuity equal or greater than 1.0 in both eyes, and astigmatism of 2.0 D or less in both eyes. The exclusion criteria were as follows: other eye diseases such as strabismus, amblyopia, high intraocular pressure (intraocular pressure is higher than 21 mm Hg) or glaucoma, cataracts, fundus abnormalities, or corneal ocular surface diseases; allergy to atropine; systemic diseases such as heart or lung disease; contact lens use, or failure to provide signed written informed consent. Subjects who were lost to follow-up or took other drugs that affected the efficacy evaluation were also excluded. The termination criteria were as follows: allergy or a severe adverse reaction to the test drug or the appearance of inappropriate changes.
Study Design
This study comprised an atropine group and a control group. Patients in the atropine group received one drop of 0.01% atropine sulphate eye gel (prepared by Xi'an No.1 Hospital, which obtained approval for the clinical study of nosocomial preparations from the Shaanxi Medical Products Administration) in each eye before bedtime daily for 6mo. The control group comprised matched children without drug intervention during the same period.
Outcome Measures
Efficacy in controlling myopia progression was evaluated by changes in the SE and AL in the eye. If the values in the atropine group were significantly less than those in the control group, the treatment was considered effective. Local toxicity, irritation, allergic reaction or other clinical adverse reactions were noted during the study period.
Statistical Analysis
All the data were analysed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). The Chi-square test was used for categorical data, and the independent t-test was used for measurement data. The efficacy of 0.01% atropine sulphate eye gel was statistically analysed by the number of eyes.
RESULTS
Demographics
There were 125 effective cases in the atropine group, and 30 cases were excluded due to delayed drug review. The control group included 60 cases in accordance with the criteria during the same period. The demographics of the two groups are shown in Table 1.
Table 1. Demographics of participants.
| Variable | Control group (n=60) | Atropine group (n=125) | P |
| Age, y, mean±SD | 9.52±1.36 | 9.47±1.42 | 0.842 |
| 6 to <7 | 0 | 3 (2.4) | |
| 7 to <8 | 4 (6.7) | 10 (8.0) | |
| 8 to <9 | 12 (20.0) | 17 (13.6) | |
| 9 to <10 | 12 (20.0) | 34 (27.2) | |
| 10 to <11 | 17 (28.3) | 23 (18.4) | |
| 11 to <12 | 11 (18.3) | 32 (25.6) | |
| 12 to <13a | 4 (6.7) | 6 (4.8) | |
| Male/female | 30/30 | 58/67 | 0.648 |
aThese patients were younger than 12 years old at the time of enrolment and 10 of them were between 12 and 13 years old after the 6-month observation period.
n (%)
Efficacy
The initial SE and AL were not significantly different between the control group (-1.59±0.94 D, 24.06±0.77 mm) and the atropine group (-1.64±0.80 D, 24.13±0.76 mm; P>0.05). After 6mo, the SE in the control group (-2.19±0.92 D) was significantly different than that in the atropine group (-1.91±0.87 D, P<0.05), and the change in the SE was also significantly different (-0.60±0.35 and -0.27±0.33 D, respectively, P<0.001). The relative reduction in myopia progression in this study was 55.0%. The increase in axial elongation was also significantly different (0.26±0.14 vs 0.19±0.14 mm, P<0.001). The relative reduction in AL in this study was 26.9% (Table 2).
Table 2. Changes of SE and AL at the 6-month follow-up in the atropine and control group.
| Variables | Control group (n=120 eyes) | Atropine group (n=250 eyes) | Difference | 95%CI | t | P |
| SE, D | ||||||
| Initial | -1.59±0.94 | -1.64±0.80 | 0.05±0.09 | -0.14 to 0.23 | 0.514 | 0.608 |
| After 6mo | -2.19±0.92 | -1.91±0.87 | -0.28±0.10 | -0.48 to -0.09 | -2.895 | 0.004 |
| Changes at 6mo | -0.60±0.35 | -0.27±0.33 | -0.33±0.04 | -0.41 to -0.26 | -8.891 | 0.000 |
| AL, mm | ||||||
| Initial | 24.06±0.77 | 24.13±0.76 | -0.08±0.08 | -0.24 to 0.09 | -0.902 | 0.367 |
| After 6mo | 24.31±0.76 | 24.32±0.76 | -0.01±0.09 | -0.17 to 0.16 | -0.105 | 0.916 |
| Change at 6mo | 0.26±0.14 | 0.19±0.14 | 0.07±0.02 | 0.04 to 0.10 | 4.306 | 0.000 |
SE: Spherical equivalent; AL: Axial length; CI: Confidence interval.
mean±SD
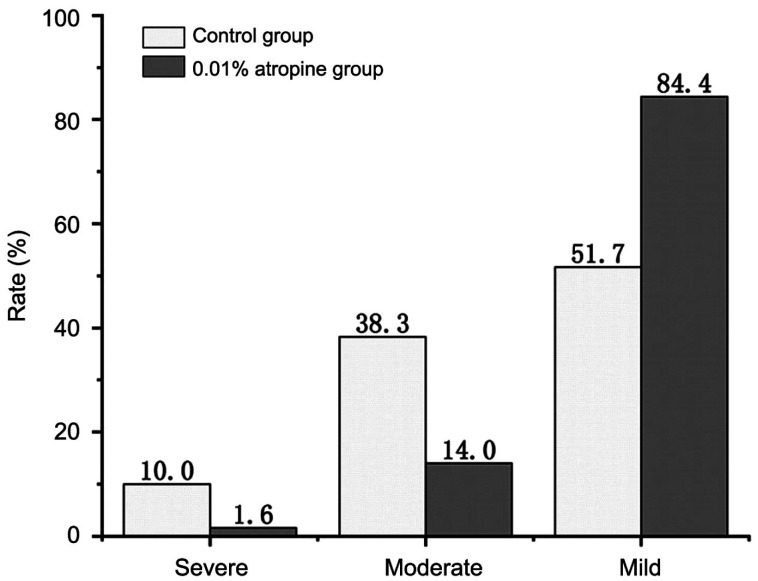
Among the 120 eyes in the control group, 51.7% had myopic progression of less than or equal to 0.50 D, and the maximum degree of myopic progression was 1.75 D; 5 eyes had no change in the SE, accounting for 4.2%. Among the 250 eyes in the atropine group, 84.4% had myopic progression of less than or equal to 0.50 D, and the maximum degree of myopic progression was 1.50 D. The SE in 96 eyes remained unchanged, at 38.4%. Ten eyes (4%) recovered by 0.12-0.38 D in the atropine group (Figure 1).
Figure 1. Myopia progression of less than 0.50 D (mild), between 0.50 and 1.00 D (moderate), and more than 1.00 D (severe) from baseline over 6mo in the atropine and the control group.
Safety
In this study, the 0.01% atropine sulphate eye gel was generally well tolerated. None of the participants developed dilated pupils, local toxicity reactions, irritation, allergic reactions or other clinical adverse reactions.
DISCUSSION
In this study, we explored short-term effect of 0.01% atropine sulphate eye gel on myopia progression in children in China. Our result revealed that a once-nightly dose of 0.01% atropine sulphate eye gel resulted in significant reductions in myopia progression and axial elongation. To our knowledge, this study is the first study to show the efficacy of 0.01% atropine sulphate eye gel on myopia progression in mainland of China. Eye gel, a viscous formulation, is more likely to adhere to the cornea and conjunctival surface after blinking, which can make the action time of the drug more durable, higher local bioavailability. When used as a myopia control drug, atropine is used in one drop per eye every night, so eye gel is a more suitable dosage form than eye drops for night application.
Several previous studies indicated that 0.01% atropine eye drops exhibited a varied relative reduction on myopia progression. A randomized, double-masked, placebo-controlled trial in Beijing evaluating the efficacy of atropine 0.01% eye drops revealed that the eye drops had 44.4% reduction at 6mo and 34.2% reduction at 1y[23]. In another study conducted in Europe, the relative reduction on myopia progression by 0.01% atropine eye drops was 50.5% at 1y[16], and that was 24.1% at 1y in the LAMP study conducted in Hong Kong[15]; moreover, the relative reduction in a study in Japan was 14.86% at 2y[24]. In our study, the relative reduction in myopia progression, at 55.0%, was slightly greater than that in the European study and greater than that in case-control studies in Beijing, Hong Kong and Japan. This may be related to the use of a placebo as well as shortened periods of observation and differences in ethnicities, regions and learning and living habits among the observed subjects.
In this study, 84.4% of the eyes in the 0.01% atropine group had myopic progression of less than or equal to 0.50 D at 6mo compared with 51.7% of the eyes in the control group. It should be noted that the spherical degree remained unchanged in 38.4% of the eyes in the atropine group at 6mo, while the spherical degree in the control group remained unchanged in only 4.2% of the eyes. Ten eyes (4%) recovered by 0.12-0.38 D in the atropine group. The LAMP study found that 43.8% of patients had myopia progression of less than 0.50 D in the 0.01% atropine group at 1y, and that in the study at Beijing Tongren Hospital was 48.7%[23].
Atropine has a dose-related effect on myopia progression, with greater effects associated with more obvious side effects. Li et al[25] reported that younger age was associated with poor treatment response to low-concentration atropine. A 2-year clinical trial in the LAMP study showed that the efficacy of 0.05% atropine was double that of 0.01% atropine[26]. Fu et al[27] reported that 0.02% atropine eye drops had a better effect on myopia progression than 0.01% atropine eye drops, but 0.02% and 0.01% atropine showed similar effects on pupil diameter and accommodative amplitude after 12mo of treatment. In children for whom myopia is poorly controlled with 0.01% atropine, a slightly higher concentration of atropine in preparations can be considered.
Axial elongation is the preferred endpoint for assessing myopic progression[18]. In our study, we found that the mean axial elongation values were 0.26±0.14 and 0.19±0.14 mm in the control and atropine groups at 6mo, respectively, with a reduction of 26.9% in axial elongation. The LAMP study in Hong Kong showed that the mean axial-length changes were 0.36±0.29 and 0.41±0.22 mm in the 0.01% atropine and placebo groups at 1y, respectively, with a reduction of 12% in axial elongation[28]. The changes in the Beijing Tongren Hospital study were 0.32±0.19 and 0.41±0.19 mm at 1y, with a reduction of 22.0% in axial elongation[23].
Various studies have shown that scleral extracellular matrix (ECM) remodelling plays a critical role in myopia development[29]. Hypoxia is a key modulator for scleral ECM remodelling during myopia development. It is plausible that changes in choroidal thickness in myopia might result from reduced choroidal blood perfusion, which in turn may lead to scleral hypoxia. This condition could in turn trigger downstream receptor-linked signalling pathways events that induce responses promoting myopia progression. Atropine is an antimuscarinic agent that causes pupil dilation and loss of accommodation, even in concentrations as low as 0.01%[7]. In addition, atropine also has the effect of dilating blood vessels. It is suggest that the choroid may be the target site for atropine[30]–[34]. Although the above studies have provided some evidence for the mechanism of atropine, the detailed pharmacological mechanism, by which atropine can slow myopia progression and axial elongation, need further research.
The advantage of this experiment was the use of a novel atropine eye gel rather than eye drops and proved its efficacy on controlling myopia progression and axial elongation. Eye gel has several advantages over eye drops in dosage form. Atropine is used as a prevention and control drug to slow the myopia progression. It does not mean that myopia does not increase after atropine application but delays the increase of myopia degree. In this study, we also noted that 38.4% of the eyes in the atropine group remained unchanged at 6mo, while only 4.2% of those in the control group remained unchanged.
However, this study has some limitations. First, this study evaluated the efficacy of this preparation for the control of myopia (change of less than -3.0 D) only in the children aged 6-12y with binocular myopia. Second, a follow-up period of 6mo may not be long enough for evaluating the efficacy of an atropine treatment regime. Third, 19% of subjects in the experimental group were lost to follow-up, which may affect the deviation of the experimental result. Fourth, although all parents are told to administrate the drug before bed every night, it is difficult to guarantee that they will fully adhere to the doctor's advice. Fifth, risk factors for myopia, such as outdoor activities time, near work time, and hereditary factor are not considered in this study. Importantly, as adolescents are a special study population, a placebo control group is a recognized potential weakness in studies on the efficacy and safety of atropine at low concentrations.
In this study, no dilated pupils, local toxicity reactions, irritation, allergic reactions or other clinical adverse reactions were observed during the study. Similar to other studies with low concentration of atropine eye drops, our study suggest that 0.01% atropine sulphate eye gel can slow myopia progression and axial elongation in children during a short-term administration. Therefore, low concentration atropine eye drops may be a novel choice that can be used in clinical practice to retard myopia. However, long-term prevention effects and adverse events of 0.01% atropine sulphate eye gel on myopia progression, as well as its detailed pharmacological mechanism need further investigation.
Acknowledgments
Foundations: Supported by Shaanxi Province Social Development and Technology of Research Project (No. 2020SF-274; No.2014K11-03-07-05); Xi'an Science and Technology Project (No.20YXYJ0008-6).
Conflicts of Interest: Pan SY, None; Wang YZ, None; Li J, None; Zhang XH, None; Wang J, None; Zhu XP, None; Xiao XH, None; Liu JT, None.
REFERENCES
- 1.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Medina A. The cause of myopia development and progression: Theory, evidence, and treatment. Surv Ophthalmol. 2022;67(2):488–509. doi: 10.1016/j.survophthal.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura S, Ching-Yu C, Saw SM. Global epidemiology of myopia. Updates on Myopia. Singapore: Springer Singapore; 2019. pp. 27–51. [Google Scholar]
- 4.Wang J, Ying GS, Fu X, Zhang R, Meng J, Gu F, Li J. Prevalence of myopia and vision impairment in school students in Eastern China. BMC Ophthalmol. 2020;20(1):2. doi: 10.1186/s12886-019-1281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Wu A, Zhang L, Wang W, Chen X, Yu X, Wang K. The increasing prevalence of myopia and high myopia among high school students in Fenghua City, Eastern China: a 15-year population-based survey. BMC Ophthalmol. 2018;18(1):159. doi: 10.1186/s12886-018-0829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Liu J, Qi P. The increasing prevalence of myopia in junior high school students in the Haidian District of Beijing, China: a 10-year population-based survey. BMC Ophthalmol. 2017;17(1):88. doi: 10.1186/s12886-017-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561–1579. doi: 10.1016/j.ophtha.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Mérida S, Villar VM, Navea A, Desco C, Sancho-Tello M, Peris C, Bosch-Morell F. Imbalance between oxidative stress and growth factors in human high myopia. Front Physiol. 2020;11:463. doi: 10.3389/fphys.2020.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, Saw SM, Chen H, Bao F, Zhao Y, Hu L, Li X, Gao R, Lu W, Du Y, Jinag Z, Yu A, Lian H, Qu J. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020;1:CD004916. doi: 10.1002/14651858.CD004916.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, Liu L. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135(6):624–630. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Q, Tang Y, Guo L, Tighe S, Zhou Y, Zhang X, Zhang J, Zhu Y, Hu M. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci. 2020;17(2):176–181. doi: 10.7150/ijms.39365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prousali E, Haidich AB, Fontalis A, Ziakas N, Brazitikos P, Mataftsi A. Efficacy and safety of interventions to control myopia progression in children: an overview of systematic reviews and meta-analyses. BMC Ophthalmol. 2019;19(1):106. doi: 10.1186/s12886-019-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–457.e1. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Sacchi M, Serafino M, Villani E, Tagliabue E, Luccarelli S, Bonsignore F, Nucci P. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 2019;97(8):e1136–e1140. doi: 10.1111/aos.14166. [DOI] [PubMed] [Google Scholar]
- 17.Joachimsen L, Böhringer D, Gross NJ, Reich M, Stifter J, Reinhard T, Lagrèze WA. A pilot study on the efficacy and safety of 0.01% atropine in German schoolchildren with progressive myopia. Ophthalmol Ther. 2019;8(3):427–433. doi: 10.1007/s40123-019-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. doi: 10.1016/j.preteyeres.2020.100923. [DOI] [PubMed] [Google Scholar]
- 19.Larkin GL, Tahir A, Epley KD, Beauchamp CL, Tong JT, Clark RA. Atropine 0.01% eye drops for myopia control in American children: a multiethnic sample across three US sites. Ophthalmol Ther. 2019;8(4):589–598. doi: 10.1007/s40123-019-00217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Feng K, Liu RB, Pan JH, Zhang LL, Xu ZP, Lu XJ. Atropine 0.01% eye drops slow myopia progression: a systematic review and Meta-analysis. Int J Ophthalmol. 2019;12(8):1337–1343. doi: 10.18240/ijo.2019.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Fan L, Tao J, Hua R, Yang Q, Gu H, Yu S, Li L, Zhao X. Use of topical 0.01% atropine for controlling near work-induced transient myopia: a randomized, double-masked, placebo-controlled study. J Ocul Pharmacol Ther. 2020;36(2):97–101. doi: 10.1089/jop.2019.0062. [DOI] [PubMed] [Google Scholar]
- 22.Lynch CR, Kondiah PPD, Choonara YE, du Toit LC, Ally N, Pillay V. Hydrogel biomaterials for application in ocular drug delivery. Front Bioeng Biotechnol. 2020;8:228. doi: 10.3389/fbioe.2020.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei S, Li SM, An W, Du J, Liang X, Sun Y, Zhang D, Tian J, Wang N. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178–1184. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hieda O, Hiraoka T, Fujikado T, Ishiko S, Hasebe S, Torii H, Takahashi H, Nakamura Y, Sotozono C, Oshika T, Morimoto T, Nishida K, Nishikawa N, Song YS, Tokutake T, Nishi Y, Shigeno Y, Kurihara T, Negishi K, Tsubota K, Ono M, Nakai T, Tan D, Tanaka S, Kinoshita S, Group AJS Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315–325. doi: 10.1007/s10384-021-00822-y. [DOI] [PubMed] [Google Scholar]
- 25.Li FF, Zhang Y, Zhang X, Yip BHK, Tang SM, Kam KW, Young AL, Chen LJ, Tham CC, Pang CP, Yam JC. Age effect on treatment responses to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2021;128(8):1180–1187. doi: 10.1016/j.ophtha.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Yam JC, Li FF, Zhang X, Tang SM, Yip BHK, Kam KW, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127(7):910–919. doi: 10.1016/j.ophtha.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Fu A, Stapleton F, Wei L, Wang W, Zhao B, Watt K, Ji N, Lyu Y. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104(11):1535–1541. doi: 10.1136/bjophthalmol-2019-315440. [DOI] [PubMed] [Google Scholar]
- 28.Li FF, Kam KW, Zhang Y, Tang SM, Young AL, Chen LJ, Tham CC, Pang CP, Yam JC. Differential effects on ocular biometrics by 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2020;127(12):1603–1611. doi: 10.1016/j.ophtha.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 29.McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp Eye Res. 2013;114:128–140. doi: 10.1016/j.exer.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhao W, Li Z, Hu Y, Jiang J, Long W, Cui D, Chen W, Yang X. Short-term effects of atropine combined with orthokeratology (ACO) on choroidal thickness. Contact Lens Anterior Eye. 2021;44(3):101348. doi: 10.1016/j.clae.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Zhang S, Zhang G, Chen Y, Lei Y, Xiang J, Xu R, Qu J, Zhou X. Increased choroidal blood perfusion can inhibit form deprivation myopia in Guinea pigs. Invest Ophthalmol Vis Sci. 2020;61(13):25. doi: 10.1167/iovs.61.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye L, Li S, Shi Y, Yin Y, He J, Zhu J, Xu X. Comparisons of atropine versus cyclopentolate cycloplegia in myopic children. Clin Exp Optom. 2021;104(2):143–150. doi: 10.1111/cxo.13128. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Zhang Z, Wu Z, Sun S, Fu Y, Ke B. Change and recovery of choroid thickness after short-term application of 1% atropine gel and its influencing factors in 6-7-year-old children. Curr Eye Res. 2021;46(8):1171–1177. doi: 10.1080/02713683.2020.1863431. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Ye C, Wang X, Zhou W, Reinach P, Qu J. Choroidal blood perfusion as a potential “rapid predictive index” for myopia development and progression. Eye Vis (Lond) 2021;8(1):1. doi: 10.1186/s40662-020-00224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]