Abstract
Lipid peroxidation is associated with the development of some pathologies, such as cardiovascular diseases. Reduction in oxidative stress by antioxidants, such as Arthrospira (formely Spirulina), helps improving this redox imbalance. The aim of the study was to evaluate the effect of the Arthrospira liquid extract “Spirulysat®” on oxidative markers—in particular, oxidized LDL (oxLDL)/total LDL cholesterol—and isoprostanes and to investigate its impact on lipid and glucose metabolism in the metabolic syndrome subject. A controlled, randomised, double-blind design was conducted in 40 subjects aged 18 to 65 years with metabolic syndrome after a daily intake of Spirulysat® or placebo for twelve weeks. Blood and urinary samples were collected at three visits (V1, V2, V3) in the two groups for parameters determination. Although the Spirulysat® group showed a decrease at all visits of the oxLDL/total cholesterol ratio, there was no significant difference compared to the placebo (p = 0.36). The urinary isoprostanes concentration in the Spirulysat® group was reduced (p = 0.014) at V3. Plasma triglycerides decreased at V3 (p = 0.003) and HDL-cholesterol increased (p = 0.031) at all visits with Spirulysat®. In conclusion, Spirulysat® did not change the oxidized LDL (oxLDL)/LDL ratio but decreased the urinary isoprostanes, plasma triglycerides and increased HDL cholesterol, suggesting a beneficial effect on metabolic syndrome.
Keywords: metabolic syndrome, dyslipidemia, oxLDL/LDL cholesterol, isoprostanes, Arthrospira liquid extract
1. Introduction
Metabolic syndrome is related to cardio metabolic risk factors and lipid disorders [1]. Worldwide, cardiovascular diseases (CVD) are the leading causes of mortality and morbidity. It is expected that by 2030, mortality from CVD will reach 22.5 million people, compared with 17.5 million deaths in 2012 [2].
It has been reported that patients with metabolic syndrome have a decreased efficiency of their antioxidant defences and an increased level of protein and lipid oxidation. They also suffer from hyperglycaemia and elevated triglycerides, as well as reduced concentrations of HDL-C [3,4] and so, a higher risk of cardiovascular disease [5]. As lipids are a target of oxidative stress [6], the resulted modification of plasma low-density lipoproteins (LDL) to the oxidized form (ox-LDL) is strongly involved in the development of atherosclerosis [7]. Isoprostanes are also considered as reliable markers of oxidative stress [6,8], while high-density lipoprotein (HDL or HDL-c) are known to exert an antiatherogenic role, including an important antioxidative activity [9]. Many studies have reported the importance of the dietary antioxidant supplementation as a strategy to boost antioxidant defences [10,11].
A new area of research in pharmacy and bromatology on the beneficial effects of microorganisms have been reported by researchers due to various chemical, biological and nutritional properties, this is the case of Arthrospira (formely Spirulina) [12,13].
Arthrospira platensis of the phylum Cyanobacteria grows in aquatic ecosystems [14], and species of Arthrospira have been isolated from tropical waters to the North Sea [15]. It has been consumed by humans for centuries in different parts of the world [16]. Several Arthrospira varieties have been studied, the most studied being Arthrospira platensis, Arthrospira maxima and Arthrospira fusiformis [14]. Arthrospira platensis, which is the species studied in our study, is identified to have a low concentration of heavy metals [17] and could play a useful role in respecting the environment for bioremediation, nitrification and carbon dioxide (CO2) fixation [18].
Arthrospira through its various beneficial properties on health, including its antioxidant activity could be an effective remedy in the prevention of the metabolic syndrome [18,19,20]. Although the number of microalgae species in nature is estimated between 200,000 and 800,000, only a few are used in food applications [21]. Microalgae exploitation as a source of protein and other bioactive products in human nutrition still presents some drawbacks, mainly due to the poor development of targeted technologies and processes for microalgae processing. Spirulysat® obtained from Arthrospira microalgae, is liquid extract concentrated in phycocyanin, a powerful antioxidant, and contained polysaccharides. In recent studies, we showed that a Arthrospira liquid extract (Spirulysat®) concentrated in phycocyanin, a powerful antioxidant, and polysaccharides, increases antioxidant defences in mice and hamsters submitted to a hypercaloric diet [22,23]. In high fat high sucrose fed mice, Spirulysat® supplementation protects against non-alcoholic steatohepatitis [22]. We also reported that Spirulysat® supplementation prevent metabolic syndrome-associated metabolic disturbances in hamsters [23] and atherosclerosis development in apolipoprotein E-deficient mice [24].
The aim of the present study was to evaluate the antioxidant efficacy of Spirulysat® on plasma oxidized LDL levels and urinary isoprostanes concentration and then investigate lipid and glucose metabolism in human subjects with metabolic syndrome using a controlled, randomised, double-blind design.
2. Result
2.1. Demographics and Anthropometric Measurements of Patients with Metabolic Syndrome
The average age in the Spirulysat® group was 51.8 ± 11.01 and in the placebo group 48.1 ± 8.52 with a male predominance of 55%. At inclusion at visit 0 (V0), no difference between groups was observed neither for waist circumference, nor BMI (Table 1), heart rate (bpm) or systolic (mmHg) and diastolic (mmHg) blood pressure (data not shown). Spirulysat® supplementation did not induce any change in all these parameters.
Table 1.
Characteristics of study population at the inclusion visit.
| Variables | Placebo (n = 20) | Spirulysat® (n = 20) |
|---|---|---|
| Age (years) | 48.1 ± 11.01 | 51.8 ± 8.52 |
| Gender: | ||
| Male (n, %) | 11 (55.0) | 11 (55.0) |
| Female (n, %) | 9 (45.0) | 9 (45.0) |
| BMI (kg/m2) | 29.73 ± 2.74 | 29.65 ± 2.72 |
| Waist circumference (cm) | 100 ± 7 | 100 ± 6 |
| Triglycerides (g/L) | 1.83 ± 0.82 | 1.35 ± 0.46 |
| Total cholesterol (g/L) | 2.36 ± 0.46 | 2.33 ± 0.36 |
| LDL cholesterol (g/L) | 1.53 ± 0.36 | 1.52 ± 0.33 |
| HDL cholesterol (g/L) | 0.47 ± 0.13 | 0.54 ± 0.09 |
2.2. Effects of Spirulysat® on Oxidative Stress
Mean oxidized LDL cholesterol (oxLDL)/total LDL concentrations between Spirulysat® and placebo group are presented in Table 2. No significant difference in the oxLDL/total LDL cholesterol ratio was found between the supplemented and placebo groups at any visits (p = 0.36 at V3). Spirulysat® supplementation decreased urinary isoprostane at V3 compared to placebo (2.31 ± 0.91 vs. 3.51 ± 2.11, p = 0.014).
Table 2.
Effect of Spirulysat® supplementation on different clinical parameters in studied subjects.
| Measured Variables | V1 | V2 | V3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo n = 20 | Spirulysat® n = 20 | p-Value | Placebo n = 20 | Spirulysat® n = 20 | p-Value | Placebo n = 20 | Spirulysat® n = 20 | p-Value | |
| oxLDL/LDL cholesterol ratio (U/g) | 67.44 ± 23.04 | 61.15 ± 20.53 | 0.58 | 72.22 ± 17.76 | 67.16 ±14.37 | 0.41 | 74.06 ± 16.08 | 69.08 ± 12.72 | 0.36 |
| Triglycerides (g/L) | 1.83 ± 0.82 | 1.35 ± 0.46 | 0.11 | 2.02 ± 0.89 | 1.58 ± 0.80 | 0.13 | 1.97 ± 0.80 | 1.23 ± 0.57 | 0.003 |
| LDL cholesterol (g/L) | 1.53 ± 0.36 | 1.52 ± 0.33 | 0.88 | 1.51 ±0.44 | 1.49 ± 0.32 | 0.96 | 1.43 ± 0.38 | 1.49 ± 0.33 | 0.22 |
| HDL cholesterol (g/L) | 0.47 ± 0.13 | 0.54 ± 0.09 | 0.004 | 0.47 ± 0.16 | 0.54 ± 0.11 | 0.012 | 0.48 ± 0.18 | 0.55 ± 0.14 | 0.031 |
| Total cholesterol (g/L) | 2.36 ± 0.46 | 2.33 ± 0.36 | 0.96 | 2.38 ± 0.50 | 2.34 ± 0.35 | 0.77 | 2.31 ± 0.43 | 2.30 ± 0.34 | 0.57 |
| Fatty Liver Index ( FLI) | 72.95 ± 15.39 | 68.90 ± 19.89 | 0.71 | 74.25 ± 16.46 | 69.18 ± 20.36 | 0.48 | 75.32 ± 17.53 | 65.63 ± 22.16 | 0.19 |
| Glycemia (mmol/L) | 5.26 ± 0.64 | 5.56 ± 0.56 | 0.072 | 5.48 ± 0.76 | 5.55 ± 0.65 | 0.84 | 5.38 ± 0.76 | 5.90 ± 0.67 | 0.056 |
| Insulinemia (mU/L) | 10.06 ± 4.78 | 14.17 ± 8.11 | 0.044 | 11.39 ± 3.41 | 13.89 ± 6.60 | 0.33 | 11.95 ± 4.82 | 16.13 ± 10.88 | 0.28 |
| Urinary isoprostane (µg/24 h) | 2.86 ± 1.96 | 2.61 ±1.44 | 0.82 | 2.98 ± 1.56 | 2.20 ± 1.09 | 0.057 | 3.51 ± 2.11 | 2.31 ± 0.91 | 0.014 |
| ALAT (µkat/L) | 0.48 ± 0.31 | 0.59 ± 0.35 | 0.27 | 0.45 ± 0.23 | 0.57 ± 0.33 | 0.16 | 0.62 ± 0.58 | 0.57 ± 0.48 | 0.7 |
| ASAT (µkat/L) | 0.41 ± 0.14 | 0.43 ± 0.14 | 0.63 | 0.42 ± 0.13 | 0.42 ± 0.15 | 0.85 | 0.49 ± 0.30 | 0.43 ± 0.16 | 0.64 |
| GGT (U/L) | 50.22 ± 50.07 | 46.84 ± 34.97 | >0.99 | 45.67 ± 37.75 | 45.23 ± 28.84 | 0.69 | 54.64 ± 59.04 | 49.07 ± 36.69 | 0.54 |
Missing data for fatty liver index (FLI) (n = 4; placebo = 1, Spirulysat® = 3); glycemia (n = 1); insulinemia (n = 1); urinary isoprostane (Spirulysat®, n = 2); urinary isoprostane (Spirulysat, n = 2). Results are presented as Mean (SD); Wilcoxon rank sum test. oxLDL: oxidized low-density lipoprotein, LDL: low density lipoprotein, HDL: high density lipoprotein, ALAT: alanine aminotransferase, ASAT: aspartate aminotransferase, GGT (gamma-glutamyltransferases).
2.3. Effects of Spirulysat® on Lipid and Glucose Metabolism
Mean triglycerides levels were significantly decreased in the Spirulysat® group compared to placebo at V3 (1.23 ± 0.57 vs. 1.97 ± 0.80 g/L, p = 0.003). At V3, HDL cholesterol was significantly increased in the Spirulysat® group compared to the placebo (0.55 ± 0.14 vs. 0.48 ± 0.18 g/L, p = 0.031). This increase was also observed at visit 2. Although glycemia showed an increase at all visits in Spirulysat® group, the values were not significantly different. Insulinemia tended to be higher in Spirulysat® group than in the placebo group at inclusion (p = 0.044); this difference was no longer found at the following visits (Table 2). Fatty liver index (FLI) did not show significant difference between the two groups. Nevertheless, the variation between visits 3 and visit 1 (V3–V1) (−3.48 ± 7.94 vs. 1.56 (13.47), p = 0.036) and between visit 3 and visit 2 (−3.35 (7.18) vs. −0.09 (12.38), p = 0.016) in Spirulysat® is significantly decreased compared to placebo group. No significant change between visits was found for liver enzymes (data not shown).
3. Discussion
This controlled, randomised, double-blind clinical trial has evaluated the antioxidant efficacy of 12 weeks of supplementation with a liquid extract of Arthrospira (Spirulysat®) rich in C-Phycocyanin (C-PC) and polysaccharides compared to a placebo on the level of oxidized LDL, glucose, lipid metabolism and 24-h urinary isoprostanes in subjects with metabolic syndrome. This trial did not show a significant effect of Spirulysat® supplementation on the oxidized LDL (oxLDL)/LDL cholesterol ratio. Nevertheless, there was a significant decrease in the 24-h urinary isoprostanes concentration and the mean plasma triglycerides concentration and an increase in HDL cholesterol at all visits in the Spirulysat® group compared to the placebo. We also measured a decrease in variation between visit 3 and visit 1 and between visit 3 and visit 2 of the fatty liver index in the Spirulysat® group compared to placebo.
Our study is the first to investigate the effect of liquid Arthrospira extract on oxidative stress, using a reliable marker, such as oxLDL and urinary isoprostanes, in subject with metabolic syndrome. Spirulysat is composed of several bioactive molecules, but the molecule with the highest concentration is C-PC followed, at a much lower level, by polysaccharides.
Phycocyanin composed of an apoprotein and a phycocyanobilin is light-sensitive and must be kept in darkness [25,26], and the participating subjects in the present trial were advised to use the ampoule-containing Spirulysat® as soon as it is removed from its box. Oral administration of Spirulysat® exposes phycocyanin to gastrointestinal proteolysis. Based on in vitro studies [27], C-PC is rapidly digested by pepsin in simulated gastric fluid. There are scarce literature data about the bioactivities of peptides obtained after phycocyanin digestion but we can suppose from this study [27] that the effects we observed in this trial are due to phycocyanobilin. Indeed it has also been reported that phycocyanin at a dose of 300 mg/kg administered orally for 10 weeks showed the same protective effect as oral administration of phycocyanobilin at a dose of 15 mg/kg for 2 weeks [28]. Spirulysat® is a liquid extract of fresh Arthrospira platensis titrated in C-PC. This extract contains, in addition to phycocyanin C-PC, other water-soluble molecules, proteins, amino acids, enzymes, sugars, water-soluble vitamins and mineral salts. The observed results in the present trial are probably related to a synergic effect of these molecules and essentially to a predominant antioxidant component, the phycocyanin C-PC. It is well established that oxidative stress and inflammation are involved in the development of cardiovascular disease [7,29]. Phycocyanin, the main component of Spirulysat®, has antioxidant activity as it is able to scavenge various radicals and inhibit lipid peroxidation [30,31] and has anti-inflammatory properties [32,33]. We have shown that supplementation with Spirulysat®, which contains a high amount of C-PC, significantly decreases the 24-h urinary isoprostanes concentration, suggesting a better redox balance. Isoprostanes are considered to be specific markers of oxidative lipid damage in the body, and an overproduction of isoprostanes is linked to oxidative stress [8,34]. The decrease in isoprostanes in the present study is consistent with our previous study in mice, which reported a decrease in oxidative stress [22]. In the mice study, we observed a strong association between Spirulysat® supplementation and antioxidant parameters, bile acid modification, impact on food intake and modulation of gene expression. Furthermore, numerous studies on human and animal models have reported the antioxidant effect of Arthrospira [22,35,36], and it was proved to be related essentially to C-PC [32,37]. It also appears that the antioxidant effect can also be linked to the presence of polysaccharides in the liquid extract isolated from Arthrospira platensis [38] and that these sulphated polysaccharides prevent many potential health risks, including cerebrovascular disease, cancer and chronic inflammation [39]. The effect of Arthrospira on urinary isoprostanes has not been reported in the literature in patients with metabolic syndrome but data from some studies [35,40] have shown a favourable effect of Arthrospira on oxidative stress and inflammation. [24]. A review from Spahis et al. enumerated several types of connection between metabolic disturbances and oxidative stress in metabolic syndrome [41]. Indeed, many studies showed that supplementation with antioxidant reverse metabolic disturbances, such as dyslipidaemia and insulin resistance [42,43]. In our study, the supplementation with Spirulysat® induces a significant change on the lipid profile. The mean plasma triglycerides level decreased significantly in the Spirulysat® group compared to the placebo, while HDL-cholesterol levels increased at all visits in this group. Thus, these results suggest a protective effect of Spirulysat® against cardiovascular disease and are consistent with our data obtained in mice [24] and hamsters [23], fed hyperenergetic diets. Indeed, Spirulysat® supplementation of apolipoprotein E-deficient mice, a model of a human atherosclerosis, during gestation and lactation, decrease atherosclerosis development in adult offspring [24]. Similarly, we showed in hamsters fed with a high-fat diet, that Spirulysat® supplementation improves sphingolipids profile and protects from lipid accumulation in aorta, suggesting a protective effect against cardiovascular disease [23]. Using whole Arthrospira, several studies in humans [44,45] support the beneficial effects of Arthrospira in improving hyperlipidaemia and reducing risk factors for cardiovascular disease. Similarly, a meta-analysis, including twelve trials with a dose of Arthrospira ranging from 1 to 19 g/d over an intervention period of 2 to 48 weeks, reports a significant reduction in LDL-cholesterol, triglycerides and total cholesterol levels.
In this study, we were also interested in the effect of Spirulysat® on glucose homeostasis. Spirulysat® did not change glucose homeostasis, as assessed by fasting glucose and insulin concentration. In our previous studies in mice and hamsters, a higher dose of Spirulysat® has improved glucose homeostasis [22,23]. It will be interesting to examine the effect of higher doses of Spirulysat® in metabolic syndrome subjects exhibiting a lower glucose tolerance. Meta-analysis of eight trials reports a favourable effect of Arthrospira supplementation on fasting blood glucose and lipid profile [46]. Another meta-analysis of 12 trials reported a decrease in fasting glucose [47].
Finally, in the present study, we determined the effect of Spirulysat® supplementation on fatty liver index. Although in the supplemented group we observed a significant difference between V3 and V1 and between V3 and V2 (data not shown), we measured no difference between the two groups (Spirulysat® vs. placebo). This apparent contradiction is probably related to the small number of studied subjects and/or the small amount of Spirulysat® consumed during the clinical trial. Indeed, using the same Spirulysat® liquid extract but 10 times concentrated in C-PC, we have reported a significant protective effect on non-alcoholic steatohepatitis in mice submitted to a high-fat high-sucrose diet [22]. We also observed a lower lipid accumulation in the liver of hamsters fed a hyper-energetic diet supplemented with more concentrated Spirulysat® [23]. It has been shown that the addition of whole Arthrospira to the diet plays a role in the decrease in liver fat and also a significant change in ALAT and ASAT [48]. In addition, animal studies have reported that the hepatoprotective activities of Arthrospira are associated with its antioxidant and anti-inflammatory components (C-phycocyanin, β-carotene and vitamin E) and with the reduction in the liver lipid profile [40,49].
Finally, Spirulysat® supplementation for 12 weeks did not induce any side effects or increase in biological parameters measured, such as ALAT and ASAT, compared to the placebo group. None of the subjects stopped the study prematurely due to an adverse event. Hemodynamic measurements appeared stable throughout the study in both groups and in the entire study population. Using a higher dose of Spirulysat® in hamsters for 12 weeks and in mice for 25 weeks, we did not observe deleterious effects on biological parameters or mortality [22,24]. No adverse effects are reported in previous studies using higher doses of phycocyanin, notably in mice [32,50] and in rats [51]. Thus, the safety profile of the products during the study can be considered as good. However, it will be necessary to confirm the safety of Spirulysat® in humans over a longer period with studied dose or higher.
4. Materials and Methods
4.1. Preparation of Arthrospira Liquid Extract (Spirulysat®)
The Arthrospira extract used in the SPIROX study is a water extract obtained without any chemical solvents and using only mechanical devices. The extraction and formulation process are made at cold temperatures (15 °C) to preserve the active molecules of the extract, in particular phycocyanin. The main components are protein 2 g/L of which phycocyanin is 1 g/L (50%) and polysaccharide constitute 0.5 g/L. Spirulysat® contains, among other components, phycocyanin, polysaccharides and other molecules, such as proteins, amino acids, enzymes, water-soluble vitamins and mineral salts.
The product is standardized using spectrophotometry thanks to the blue colour of phycocyanin. The production process can be described as follows. First, Arthrospira is cultivated in controlled conditions in a greenhouse, using the Algosource based culture medium. Both pH and temperature are daily monitored. The Arthrospira strain cultivated is the PCC 8005 from the Pasteur Institute in France. Fresh water is taken from the French national network and is allowed for human consumption. All nutrients involved are quality controlled. This stage is the purpose of a patent “Production process for micro algae” (Patent FR 92-11877).
The quality control is performed using microscopic analysis and Spectrophotometry on a daily basis. Arthrospira is harvested by filtration; each batch is controlled, and the following parameters are recorded: production unit, harvesting date, dry weight. The Arthrospira biomass is then frozen. In order to perform the water-based extraction, the Arthrospira biomass has been thawed, and after de-freezing Arthrospira cells are broken using centrifugation and water as the solvent. Hydrophilic compounds are then separated from lipids and cell residues using membrane filtration. This stage is covered by the patent N°EP3601319. Finally, the liquid extract is sterilized by micro filtration at 0.2 microns (Sartorius filter) and controlled with Spectrophotometry. It goes through a sterilizing filter into the final packaging: ampoules or sterile bag. Analysis for heavy metal detection and measurement and various nutrients (Table 3) are performed by a certified laboratory, Eurofins (www.eurofins.fr, accessed on 27 June 2018). The batch used for this clinical trial was subject to the same quality requirements and procedures as the industrial batches. Active products were prepared by the company Alpha Biotech—La Frostidié—44410 Asserac—France, according to good manufacturing practices. This food supplement Spirulysat® has been marketed in France since 2012.
Table 3.
Composition of Spirulysat® for 100 mL.
| Amount in 100 mL | |
|---|---|
| Proteins | ˃0.2 g |
| Carbohydrate | 0.05 g |
| Lipids | Traces |
| Vitamin B12 | 0.3 µg |
| Iron | 0.2 mg |
| Magnesium | 30 mg |
| Calcium | 40 mg |
| Potassium | 8 mg |
| Sodium | 20 mg |
| Copper | 30 µg |
| Zinc | 20 µg |
| Phycocyanine | 110 mg |
4.2. Study Design
4.2.1. Pre-study Recommendations
Participants were given the following instructions: not to change lifestyle habits during the study (physical activity, smoking and alcohol consumption), not to do strenuous exercise during the 2 days preceding each visit, their eating habits during the study (no special diet, no taking of food supplements, no heavy meals or alcohol abuse), not to donate blood chronic drug treatments for those authorized (active and dosage) and not to take any new drugs during the study (having an impact on the study parameters), except in extreme cases.
4.2.2. Study Design
The study was designed as a randomized, double-blind, placebo-controlled, two-groups parallel. Participants were randomly assigned to one of two groups: Spirulysat® or placebo (Table S1). Spirulysat®, as well as placebo, are packaged in 10 mL vials. The comparative product (placebo) exhibits the same characteristics, appearance, packaging and composition as the Spirulysat®, except that the product is replaced by a classic blue food colouring (Rainbow Dust, Colour Flo, Preston, UK).
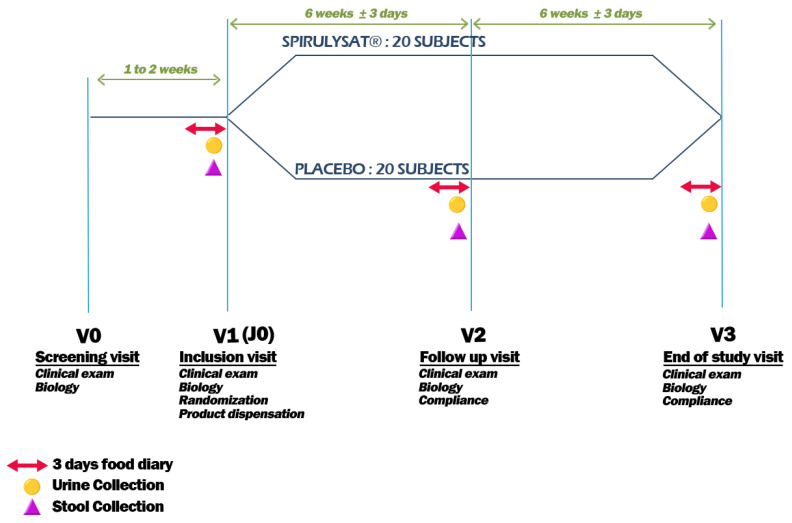
C-Phycocyanin is the main pigment of Arthrospira. The Food and Drug Administration (FDA) has granted Arthrospira the status of GRAS (Generally Recognized as Safe (GRAS Notice No. GRN 000391). Indeed, phycocyanin is used as blue dye in the formulation of food products, such as desserts and sweets. According to the EFSA’s Scientific Opinion, 2010, the daily dose recommended to produce a claim effect is 2 g daily of Arthrospira. This corresponds to an amount of 200–300 mg of Phycocyanin. Thus, in the present study we chose to investigate a low dose of about 20 mg per day per person (2 × 10 mg). Each randomized subject received 10 boxes of 20 vials with the prescription of 2 vials to be consumed per day (morning, just before breakfast) of Spirulysat® or placebo for 12 weeks. As this study is the first clinical trial whose aim is to investigate the effect of Spirulysat® on the oxidative status, we based the design of the present trial on the hamster data that showed a preventive effect of Spirulysat on metabolic syndrome. Indeed, hamster supplementation for 12 weeks prevents the perturbation of some metabolic parameters characteristic of the metabolic syndrome. Thus, we hypothesised that 12 weeks might be sufficient to see an effect on at least one component of the metabolic syndrome. Moreover, the choice of taking the Spirulysat at breakfast was done without any supporting scientific data, and only so that all participants take it at the same time to avoid any variability related to the time of intake. Subjects included in the study were scheduled for a screening visit (V0) followed by an inclusion visit (V1) that took place 1 to 2 weeks after V0. An intermediate follow-up visit (V2) was scheduled after 6 weeks of intervention and the end-of-study visit was conducted after 12 weeks of supplementation (V3) (Figure 1). This randomized clinical protocol was approved by the Ethics Committee protection (CPP) of Ouest of Rennes. It is registered under the number PEC15039 and NTC 02817620, respectively, in CCP and clinicaltrials.gov. A written informed consent was obtained from each subject.
Figure 1.
Study design.
4.3. Study Participants
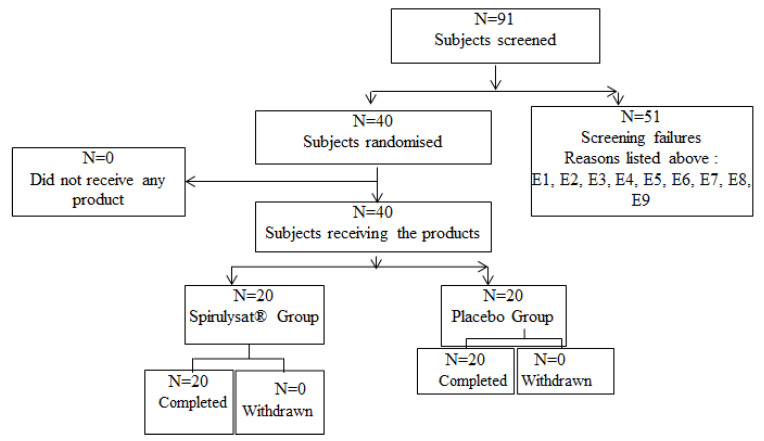
The clinical trial was conducted in 2016 by Biofortis Mérieux NutriSciences. The flowchart of the study is shown in Figure 2. A total of 91 participants with metabolic syndrome were registered and finally 40 participants (22 men, 18 women, 20 per group) were enrolled in the present study. Inclusion criteria were: age between 18 and 65 years, BMI between 25 and 35 kg/m2, with a metabolic syndrome defined as central obesity, waist circumference >94 cm for men and >80 cm for women associated with at least 2 observed criteria (fasting blood triglycerides >1.5 g/L, fasting HDL cholesterol <0.4 g/L for men and <0.5 g/L for women, fasting blood glucose >1 g/L, blood pressure >130/85 mmHg) or under antihypertensive treatment, non-smoker or with tobacco consumption <10 cigarettes/day, for non-menopausal women with the same reliable contraception for at least three months prior to the start of the study and committed to maintaining it for the duration of the study or menopausal women without or with hormone replacement therapy started at stable dose. Ninety-one subjects were identified for the study and fifty-one were eventually not selected since they did not meet one or more of the eligible criteria inclusion for the study (assessment based on medical examination at V0 with verification at V1). In the clinical trial, subjects belonging to these categories are not included: suffering from a metabolic disorder, such as diabetes or uncontrolled thyroid disorder (E1), suffering from a serious chronic disease (cancer, HIV, renal failure, ongoing liver or biliary disorders, chronic inflammatory digestive disorders) or gastrointestinal disorders (coeliac disease) (E2), with a history of ischemic cardiovascular event (E3), having undergone recent surgical procedure, less than 6 month (E4), suffering from uncontrolled hypertension (systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg) (E5), regular intake of dietary supplements or “functional foods” impacting on lipid metabolism or stopped less than 3 months prior to the V0 visit (E6), with a significant change in dietary habits or physical activity in the 3 months prior to the V0 visit or not agreeing to comply (E7), fasting blood triglycerides > 3.5 g/L (3.95 mmol/L) (E8) and blood hsCRP > 10 mg/L (E9).
Figure 2.
Flowchart study.
After the screening period, each subject was randomly assigned to either Spirulysat® or a placebo. The random product attribution was performed after checking the subjects’ eligibility once the results were available for inclusion (after V0 screening visit), thus minimizing the selection bias. Products’ allocation depended only on the subjects’ inclusion sequence in the study.
4.4. Anthropometric and Hemodynamic Measurements
Body weight and waist circumference were measured at each visit from selection to the end of the study (4 visits: V0, V1, V2, and V3). Heart rate (bpm) and systolic (mmHg) and diastolic (mmHg) blood pressure were performed twice, separated by at least 2 min. The first measurement was performed after a minimum rest of 5 min.
4.5. Biological Parameters Measurements
All biological parameters were analysed in a central laboratory (Biofortis Mérieux Nutrisciences). Blood samples were taken after a 12-h fasting period. Urine was collected for 24-h and after mixing a sample was taken for analysis. The first one at visit 0 (screening), the second one at visit 1 (inclusion from 7 to 15 days after V0), the third one V2 (follow-up visit 15 ± 3 after V1), the fourth one V3 end of study visit (15 ± 3 days after V2). The blood is collected in different tubes depending on the dosage to be performed. For glucose determination the Fluor tubes were used and analysed by enzymatic Hexokinase/Roche diagnostic. Dry serum tubes were used for lipids determination. Concentration of total cholesterol and triglycerides were determined using enzymatic kit (Cobas integra 400+) and for HLD-c by photometry (Roche Diagnostic, Basel, Switzerland). The liver markers alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT) and gamma-glutamyltransferases (GGT) were analysed by UV test (Cobas integra 400+/Roche Diagnostic, Basel, Switzerland), and GGT by enzymatic (Roche Diagnostic, Basel, Switzerland). LDLc was calculated according to Friedewald formula, and LDLox by ELISA technique. For the measure of urinary isoprostane, samples were collected 24-h prior to each visit (V1, V2, V3) and the assay was performed only on the 24-h urine to take into account the difference in the dilution 328 of participant’s urine. The urine was homogenised before aliquoting, then each aliquot was treated with Butylhydroxytoluene (BHT) (1 mL of urine with 10 µL of 0.025 M BHT solution) before freezing for analysis. Urinary isoprostane (isoprostane F2 alpha) was analysed by ELISA (Oxford Biomedical Research). The raw results from the laboratory were expressed in ng/mL, and then converted to the concentration of the whole 24 h collected urine (µg/24 h).
The oxLDL/total LDLc ratio (U/g) was calculated using the following formula:
OxLDL/LDL ratio (U/g) = oxLDL (U/L) /LDL (g/L). To assess the effect of Spirulysat® on the liver lipids content, we calculated the fatty liver index (FLI) a validated parameter in diagnosing the presence of liver steatosis and its severity [52]. This index was calculated using the following formula:
FLI = (e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge, GGT (gamma-glutamyltransferase) + 0.053 × waist circumference − 15.745)/(1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference − 15.745) × 100, where triglycerides, GGT, wait circumference are expressed in mg/dL, U/L and Cm, respectively.
4.6. Statistical Analysis
The sample size was calculated according to the guidelines [53,54,55,56,57]. Statistical analysis was performed with R software version 4.1.2. Quantitative data are presented as mean ± SD (standard deviation of the mean). The comparison of continuous variables between groups at different visits was performed by independent sample Wilcoxon rank tests. To estimate the variation in fatty liver index (FLI) between visits (V3–V1 and V3–V2), Wilcoxon independent sample rank tests were performed. A p value of less than 0.05 was considered statistically significant.
5. Conclusions
In conclusion, Spirulysat® did not change the oxidized LDL (oxLDL)/LDL ratio but decreased urinary isoprostanes and plasma triglycerides and increased HDL cholesterol, suggesting a beneficial effect on metabolic syndrome. We did not measure significative differences in oxLDL, which is also a good marker of oxidative stress. This could be related to the small number of subjects, which is low given the high inter-individual variability. To reinforce these data, and in particular those showing a trend with Spirulysat® supplementation, it would be interesting, in a future study, to measure the oxLDL, urinary isoprostanes and 8-Hydroxyguanosine/8-oxo-2’-désoxyguanosine ratio in a larger number of subjects using Spirulysat® more concentrated in C-PC.
Acknowledgments
We are very grateful to Biofortis Mérieux NutriSciences company for its technical support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20070441/s1, Table S1: Composition Group.
Author Contributions
N.L.N.K.: Data curation and analysis, writing—review & editing; N.I.S.: statistic analysis; O.L.: review & editing; K.O.: Conceptualization, data curation and analysis, investigation, writing, review & editing; J.-M.B.: Conceptualization, review & editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee protection of OUEST OF RENNES (protocol Code PEC15039, 29 June 2016) and registered on clinicaltrials.gov (NTC 02817620, 14 June 2016).
Informed Consent Statement
The consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interests. This work was supported by AlgoSource that supplied the Arthrospira aqueous extract. This sponsor had no role in the design and conduct of the study, in the collection, analysis, or interpretation of the data until the submission of the manuscript for publication.
Funding Statement
This work was supported by AlgoSource.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farhangi M.A., Jahangiry L. Dietary Diversity Score Is Associated with Cardiovascular Risk Factors and Serum Adiponectin Concentrations in Patients with Metabolic Syndrome. BMC Cardiovasc. Disord. 2018;18:68. doi: 10.1186/s12872-018-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Status Report on Noncommunicable Diseases. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 3.Vona R., Gambardella L., Cittadini C., Straface E., Pietraforte D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le N.-A. Postprandial Triglycerides, Oxidative Stress, and Inflammation. IntechOpen; London, UK: 2020. [Google Scholar]
- 5.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.-C., James W.P.T., Loria C.M., Smith S.C., et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Milne G.L., Yin H., Morrow J.D. Human Biochemistry of the Isoprostane Pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jürgens G., Hoff H.F., Chisolm G.M., Esterbauer H. Modification of Human Serum Low Density Lipoprotein by Oxidation--Characterization and Pathophysiological Implications. Chem. Phys. Lipids. 1987;45:315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- 8.Bauer J., Ripperger A., Frantz S., Ergün S., Schwedhelm E., Benndorf R.A. Pathophysiology of Isoprostanes in the Cardiovascular System: Implications of Isoprostane-Mediated Thromboxane A2 Receptor Activation. Br. J. Pharmacol. 2014;171:3115–3131. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navab M., Ananthramaiah G.M., Reddy S.T., Van Lenten B.J., Ansell B.J., Fonarow G.C., Vahabzadeh K., Hama S., Hough G., Kamranpour N., et al. The Oxidation Hypothesis of Atherogenesis: The Role of Oxidized Phospholipids and HDL. J. Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Mangge H., Becker K., Fuchs D., Gostner J.M. Antioxidants, Inflammation and Cardiovascular Disease. World J. Cardiol. 2014;6:462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Tan H.-Y., Wang N., Zhang Z.-J., Lao L., Wong C.-W., Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay R.A. Microalgae as Food and Supplement. Crit. Rev. Food Sci. Nutr. 1991;30:555–573. doi: 10.1080/10408399109527556. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q., Huang Y., Zhang R., Cai T., Cai Y. Medical Application of Spirulina Platensis Derived C-Phycocyanin. Evid.-Based Complement Altern. Med. ECAM. 2016;2016:7803846. doi: 10.1155/2016/7803846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataraman L.V. Spirulina Platensis (Arthrospira): Physiology, Cell Biology and Biotechnologym, Edited by Avigad Vonshak. J. Appl. Phycol. 1997;9:295–296. doi: 10.1023/A:1007911009912. [DOI] [Google Scholar]
- 15.Humm H.J., Wicks S.B. Introduction and Guide to the Marine Bluegreen Algae. Very Good Hardcover. Smith Family Bookstore. 1980. [(accessed on 21 May 2022)]. Available online: https://www.abebooks.com/9780471052173/Introduction-Guide-Marine-Bluegreen-Algae-0471052175/plp.
- 16.Ciferri O. Spirulina, the Edible Microorganism. Microbiol. Rev. 1983;47:551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandgruber F., Gielsdorf A., Baur A.C., Schenz B., Müller S.M., Schwerdtle T., Stangl G.I., Griehl C., Lorkowski S., Dawczynski C. Variability in Macro- and Micronutrients of 15 Commercially Available Microalgae Powders. Mar. Drugs. 2021;19:310. doi: 10.3390/md19060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finamore A., Palmery M., Bensehaila S., Peluso I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017;2017:3247528. doi: 10.1155/2017/3247528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeinalian R., Farhangi M.A., Shariat A., Saghafi-Asl M. The Effects of Spirulina Platensis on Anthropometric Indices, Appetite, Lipid Profile and Serum Vascular Endothelial Growth Factor (VEGF) in Obese Individuals: A Randomized Double Blinded Placebo Controlled Trial. BMC Complement Altern. Med. 2017;17:225. doi: 10.1186/s12906-017-1670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobescu E., Bălan A., Moga M.A., Teodorescu A., Mitrică M., Dima L. Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women? Mar. Drugs. 2020;18:651. doi: 10.3390/md18120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolkers H., Barbosa M.J., Kleinegris D.M.M., Bosma R., Wijffels R.H., Harmsen P.F.H. Microalgae: The Green Gold of the Future?: Large-Scale Sustainable Cultivation of Microalgae for the Production of Bulk Commodities. Wageningen UR—Food & Biobased Research; Wageningen, The Netherlands: 2011. [Google Scholar]
- 22.Coué M., Tesse A., Falewée J., Aguesse A., Croyal M., Fizanne L., Chaigneau J., Boursier J., Ouguerram K. Spirulina Liquid Extract Protects against Fibrosis Related to Non-Alcoholic Steatohepatitis and Increases Ursodeoxycholic Acid. Nutrients. 2019;11:194. doi: 10.3390/nu11010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasbi-Chadli F., Coué M., Aguesse A., Grit I., Souque T., Ferchaud-Roucher V., Ouguerram K. Spirulina Liquid Extract Prevents Metabolic Disturbances and Improves Liver Sphingolipids Profile in Hamster Fed a High-Fat Diet. Eur. J. Nutr. 2021;60:4483–4494. doi: 10.1007/s00394-021-02589-x. [DOI] [PubMed] [Google Scholar]
- 24.Coué M., Croyal M., Habib M., Castellano B., Aguesse A., Grit I., Gourdel M., Billard H., Lépine O., Michel C., et al. Perinatal Administration of C-Phycocyanin Protects Against Atherosclerosis in ApoE-Deficient Mice by Modulating Cholesterol and Trimethylamine-N-Oxide Metabolisms. Arterioscler. Thromb. Vasc. Biol. 2021;41:e512–e523. doi: 10.1161/ATVBAHA.121.316848. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti S., Rinalducci S., Benvenuti F., Francogli S., Pagliarani S., Giorgi L., Micheloni M., D’Amici G.M., Zolla L., Canestrari F. Purification and Characterization of Phycocyanin from the Blue-Green Alga Aphanizomenon Flos-Aquae. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2006;833:12–18. doi: 10.1016/j.jchromb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Qu Y., Fu X., Zhao M., Wang S., Sun L. Isolation, Purification and Properties of an R-Phycocyanin from the Phycobilisomes of a Marine Red Macroalga Polysiphonia Urceolata. PLoS ONE. 2014;9:e87833. doi: 10.1371/journal.pone.0087833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minic S.L., Stanic-Vucinic D., Mihailovic J., Krstic M., Nikolic M.R., Cirkovic Velickovic T. Digestion by Pepsin Releases Biologically Active Chromopeptides from C-Phycocyanin, a Blue-Colored Biliprotein of Microalga Spirulina. J. Proteomics. 2016;147:132–139. doi: 10.1016/j.jprot.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J., Inoguchi T., Sasaki S., Maeda Y., McCarty M.F., Fujii M., Ikeda N., Kobayashi K., Sonoda N., Takayanagi R. Phycocyanin and Phycocyanobilin from Spirulina Platensis Protect against Diabetic Nephropathy by Inhibiting Oxidative Stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R110–R120. doi: 10.1152/ajpregu.00648.2011. [DOI] [PubMed] [Google Scholar]
- 29.Libby P. Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romay C., Ledón N., González R. Further Studies on Anti-Inflammatory Activity of Phycocyanin in Some Animal Models of Inflammation. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al. 1998;47:334–338. doi: 10.1007/s000110050338. [DOI] [PubMed] [Google Scholar]
- 31.Thangam R., Suresh V., Asenath Princy W., Rajkumar M., Senthilkumar N., Gunasekaran P., Rengasamy R., Anbazhagan C., Kaveri K., Kannan S. C-Phycocyanin from Oscillatoria Tenuis Exhibited an Antioxidant and in Vitro Antiproliferative Activity through Induction of Apoptosis and G0/G1 Cell Cycle Arrest. Food Chem. 2013;140:262–272. doi: 10.1016/j.foodchem.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 32.Romay C., Armesto J., Remirez D., González R., Ledon N., García I. Antioxidant and Anti-Inflammatory Properties of C-Phycocyanin from Blue-Green Algae. Inflamm. Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 33.Romay C., González R., Ledón N., Remirez D., Rimbau V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 34.Ku C.S., Yang Y., Park Y., Lee J. Health Benefits of Blue-Green Algae: Prevention of Cardiovascular Disease and Nonalcoholic Fatty Liver Disease. J. Med. Food. 2013;16:103–111. doi: 10.1089/jmf.2012.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Sámano J., Torres-Montes de Oca A., Luqueño-Bocardo O.I., Torres-Durán P.V., Juárez-Oropeza M.A. Spirulina Maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs. 2018;16:496. doi: 10.3390/md16120496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romay C., Delgado R., Remirez D., González R., Rojas A. Effects of Phycocyanin Extract on Tumor Necrosis Factor-Alpha and Nitrite Levels in Serum of Mice Treated with Endotoxin. Arzneimittelforschung. 2001;51:733–736. doi: 10.1055/s-0031-1300107. [DOI] [PubMed] [Google Scholar]
- 37.Rajasekar P., Palanisamy S., Anjali R., Vinosha M., Elakkiya M., Marudhupandi T., Tabarsa M., You S., Prabhu N.M. Isolation and Structural Characterization of Sulfated Polysaccharide from Spirulina Platensis and Its Bioactive Potential: In Vitro Antioxidant, Antibacterial Activity and Zebrafish Growth and Reproductive Performance. Int. J. Biol. Macromol. 2019;141:809–821. doi: 10.1016/j.ijbiomac.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Ngo D.-H., Kim S.-K. Sulfated Polysaccharides as Bioactive Agents from Marine Algae. Int. J. Biol. Macromol. 2013;62:70–75. doi: 10.1016/j.ijbiomac.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 39.Pak W., Takayama F., Mine M., Nakamoto K., Kodo Y., Mankura M., Egashira T., Kawasaki H., Mori A. Anti-Oxidative and Anti-Inflammatory Effects of Spirulina on Rat Model of Non-Alcoholic Steatohepatitis. J. Clin. Biochem. Nutr. 2012;51:227–234. doi: 10.3164/jcbn.12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spahis S., Borys J.-M., Levy E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017;26:445–461. doi: 10.1089/ars.2016.6756. [DOI] [PubMed] [Google Scholar]
- 41.Medina-Vera I., Gómez-de-Regil L., Gutiérrez-Solis A.L., Lugo R., Guevara-Cruz M., Pedraza-Chaverri J., Avila-Nava A. Dietary Strategies by Foods with Antioxidant Effect on Nutritional Management of Dyslipidemias: A Systematic Review. Antioxidants. 2021;10:225. doi: 10.3390/antiox10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Q., Liu L., Miron A., Klímová B., Wan D., Kuča K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 43.Serban M.-C., Sahebkar A., Dragan S., Stoichescu-Hogea G., Ursoniu S., Andrica F., Banach M. A Systematic Review and Meta-Analysis of the Impact of Spirulina Supplementation on Plasma Lipid Concentrations. Clin. Nutr. 2016;35:842–851. doi: 10.1016/j.clnu.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Bohórquez-Medina S.L., Bohórquez-Medina A.L., Benites Zapata V.A., Ignacio-Cconchoy F.L., Toro-Huamanchumo C.J., Bendezu-Quispe G., Pacheco-Mendoza J., Hernandez A.V. Impact of Spirulina Supplementation on Obesity-Related Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. NFS J. 2021;25:21–30. doi: 10.1016/j.nfs.2021.09.003. [DOI] [Google Scholar]
- 45.Hatami E., Ghalishourani S.-S., Najafgholizadeh A., Pourmasoumi M., Hadi A., Clark C.C.T., Assaroudi M., Salehi-sahlabadi A., Joukar F., Mansour-Ghanaei F. The Effect of Spirulina on Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Diabetes Metab. Disord. 2021;20:883–892. doi: 10.1007/s40200-021-00760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H., Liao D., Pu R., Cui Y. Quantifying the Effects of Spirulina Supplementation on Plasma Lipid and Glucose Concentrations, Body Weight, and Blood Pressure. Diabetes Metab. Syndr. Obes. Targets Ther. 2018;11:729–742. doi: 10.2147/DMSO.S185672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazloomi S.M., Samadi M., Davarpanah H., Babajafari S., Clark C.C.T., Ghaemfar Z., Rezaiyan M., Mosallanezhad A., Shafiee M., Rostami H. The Effect of Spirulina Sauce, as a Functional Food, on Cardiometabolic Risk Factors, Oxidative Stress Biomarkers, Glycemic Profile, and Liver Enzymes in Nonalcoholic Fatty Liver Disease Patients: A Randomized Double-blinded Clinical Trial. Food Sci. Nutr. 2021;10:317–328. doi: 10.1002/fsn3.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazokopakis E.E., Papadomanolaki M.G., Fousteris A.A., Kotsiris D.A., Lampadakis I.M., Ganotakis E.S. The Hepatoprotective and Hypolipidemic Effects of Spirulina (Arthrospira Platensis) Supplementation in a Cretan Population with Non-Alcoholic Fatty Liver Disease: A Prospective Pilot Study. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2014;27:387–394. [PMC free article] [PubMed] [Google Scholar]
- 49.Ou Y., Lin L., Pan Q., Yang X., Cheng X. Preventive Effect of Phycocyanin from Spirulina Platensis on Alloxan-Injured Mice. Environ. Toxicol. Pharmacol. 2012;34:721–726. doi: 10.1016/j.etap.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Gupta M., Dwivedi U.N., Khandelwal S. C-Phycocyanin: An Effective Protective Agent against Thymic Atrophy by Tributyltin. Toxicol. Lett. 2011;204:2–11. doi: 10.1016/j.toxlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birkett M.A., Day S.J. Internal Pilot Studies for Estimating Sample Size. Stat. Med. 1994;13:2455–2463. doi: 10.1002/sim.4780132309. [DOI] [PubMed] [Google Scholar]
- 53.Browne R.H. On the Use of a Pilot Sample for Sample Size Determination. Stat. Med. 1995;14:1933–1940. doi: 10.1002/sim.4780141709. [DOI] [PubMed] [Google Scholar]
- 54.Julious S.A. Sample Size of 12 per Group Rule of Thumb for a Pilot Study. Pharm. Stat. 2005;4:287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 55.Hertzog M.A. Considerations in Determining Sample Size for Pilot Studies. Res. Nurs. Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 56.Sim J., Lewis M. The Size of a Pilot Study for a Clinical Trial Should Be Calculated in Relation to Considerations of Precision and Efficiency. J. Clin. Epidemiol. 2012;65:301–308. doi: 10.1016/j.jclinepi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Teare M.D., Dimairo M., Shephard N., Hayman A., Whitehead A., Walters S.J. Sample Size Requirements to Estimate Key Design Parameters from External Pilot Randomised Controlled Trials: A Simulation Study. Trials. 2014;15:264. doi: 10.1186/1745-6215-15-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.