Abstract
Although great strides have been achieved, schistosomiasis japonica remains a major public health concern in China. Immunodiagnostics have been widely accepted as the first choice in large-scale screening of Schistosoma japonicum human infections, and indirect hemagglutination test (IHA), enzyme-linked immunosorbent assay (ELISA), and dipstick dye immunoassay (DDIA) are currently the three most common immunological tests for the diagnosis of S. japonicum human infections in China. This meta-analysis aimed to comprehensively assess the performance of IHA, ELISA, and DDIA for the field diagnosis of S. japonicum human infections. A total of 37 eligible publications were enrolled in the final analysis, including 29 Chinese publications and 8 English publications. No significant heterogeneities were detected among the studies reporting ELISA (I2 = 88%, p < 0.05), IHA (I2 = 95%, p < 0.05), or DDIA (I2 = 84%, p < 0.05). DDIA showed the highest pooled sensitivity (90.8%, 95% CI: 84.6% to 94.7%) and IHA presented the highest pooled specificity for detection of S. japonicum human infections (71.6%, 95% CI: 65.9% to 76.7%). Summary receiver operating characteristic (SROC) curve analysis showed that IHA exhibited the highest area under the SROC curve (AUC) (0.88, 95% CI: 0.85 to 0.9), and ELISA presented the lowest AUC (0.85, 95% CI: 0.82 to 0.88). Deeks’ funnel plots indicated no publication bias. IHA presented the highest sensitivity in medium-endemicity regions and the highest specificity for diagnosis of S. japonicum human infections in low-endemicity regions, and ELISA showed the highest diagnostic sensitivity in high-endemicity regions and the highest specificity in medium-endemicity regions, while DDIA exhibited the highest diagnostic sensitivity in high-endemicity regions and the highest specificity in low-endemicity regions. IHA and DDIA presented a higher efficiency for the diagnosis of S. japonicum human infections in marshland and lake regions than in hilly and mountainous regions, while ELISA showed a comparable diagnostic sensitivity between in marshland and lake regions and hilly and mountainous regions (88.3% vs. 88.6%), and a higher specificity in marshland and lake regions than in hilly and mountainous regions (60% vs. 48%). Our meta-analysis demonstrates a comparable diagnostic accuracy of IHA, ELISA, and DDIA for S. japonicum human infections, and the diagnostic sensitivity and specificity of IHA, ELISA, and DDIA vary in types and infection prevalence of endemic regions. DDIA combined with IHA is recommended as a tool for screening chemotherapy targets and seroepidemiological surveys during the stage moving towards schistosomiasis elimination in China. Further studies to examine the effectiveness of combinations of two or three immunological tests for diagnosis of S. japonicum human infections are warranted.
Keywords: Schistosoma japonicum, immunodiagnosis, diagnostic efficiency, indirect hemagglutination test, enzyme-linked immunosorbent assay, dipstick dye immunoassay, meta-analysis
1. Introduction
Schistosomiasis is a neglected global tropical parasitic disease which affects more than 140 million individuals and causes approximately 200,000 annual deaths worldwide [1]. The recent great strides urged the ambitious goal set for elimination of schistosomiasis as a public health problem in all disease-endemic countries in the world by 2030; however, great challenges have been identified to achieve this ambitious goal [2,3]. Unfortunately, the global pandemic of COVID-19 poses negative effects on global schistosomiasis elimination programs, adding challenges to achieve the goal of a schistosomiasis-free world [4,5,6].
Schistosomiasis in China, Indonesia, and the Philippines is caused by infections with Schistosoma japonicum as the predominant species [7]. China was once highly endemic for S. japonicum and suffered from the highest burden of schistosomiasis in the world [8]. After the national schistosomiasis control program was initiated in the 1950s, great successes have been achieved, and schistosomiasis had been eliminated as a public health problem in China according to the World Health Organization-defined criteria as of 2015 [9]. Nevertheless, multiple challenges are identified to completely wipe out the “God of Plague” in the country [10].
Diagnosis is central to the schistosomiasis control program, which is necessary for identification of chemotherapy targets, assessment of chemotherapy efficacy, as well as planning, implementation, and evaluation of the effectiveness of the schistosomiasis control program [11]. Currently, detection of parasite eggs or juvenile parasites with parasitological techniques remains the gold standard for the definitive diagnosis of schistosomiasis japonica; however, these tools suffer from problems of time-consuming procedures, low participation rate, and high false negative rate in low-endemicity regions [12]. A large number of emerging molecular assays have shown potential in precise early diagnosis of schistosomiasis japonica; however, these DNA- or RNA-based assays suffer from problems of laboratory-intensive procedures, high costs, and requirements of professional healthcare education, and there have been no commercial molecular kits available for the clinical diagnosis of S. japonicum human infections until now [13,14,15]. Immunodiagnostics, which are rapid and easy to perform, are currently the most efficacious and practical means for diagnosis of human schistosomiases based on the detection of infection-specific antibodies and have been widely accepted as the first choice in large-scale screening of S. japonicum human infections, seroepidemiological surveys, and assessment of the effectiveness of the schistosomiasis control program in China [16], although the performance of immunodiagnostic assays for early detection of S. japonicum human infections remains to be improved [17,18].
Currently, indirect hemagglutination test (IHA), enzyme-linked immunosorbent assay (ELISA), and dipstick dye immunoassay (DDIA) are the three most common immunological tests for the diagnosis of S. japonicum human infections in China [17,18]. During the stage moving towards elimination of schistosomiasis, the option of immunodiagnostics is a critical part of the national schistosomiasis elimination program in China [19]. However, there have been no pooled estimates of the sensitivity and specificity of commercial immunological tests for the diagnosis of S. japonicum human infections in endemic foci with different epidemic types and levels [20,21,22]. Based on data from public databases, this study aimed to comprehensively assess the performance of the three most common commercial immunodiagnostic assays, including IHA, ELISA, and DDIA, for the diagnosis of S. japonicum human infections in endemic foci of China, so as to provide insights into the option of immunodiagnostics for the national schistosomiasis elimination program in China.
2. Methods
2.1. Literature Search
A joint search was performed in international and national electronic databases, including Web of Science, PubMed, Scopus, Google Scholar, and Chinese electronic databases CNKI (https://www.cnki.net/; accessed on 7 April 2022), Wanfang Data (https://www.wanfangdata.com.cn; accessed on 7 April 2022) and VIP (http://www.cqvip.com/; accessed on 7 April 2022) using the mesh terms ((schistosom *) OR (bilharzia *)) AND ((serologic * test) OR (immunological test) OR (ELISA) OR (IHA) OR (DDIA)) AND ((stool examination) OR (Kato-Katz) OR (miracidium hatching)), to retrieve English and Chinese publications pertaining to the diagnosis of S. japonicum human infections with IHA, ELISA, or DDIA. The time of search was defined as from 1980 to 2021. In addition, the post-text references of retrieved publications were read, and grey literatures, such as institutional annual reports, proceedings, and collections, were artificially searched to track all possibly related studies.
2.2. Inclusion and Exclusion Criteria
We defined the following inclusion criteria: (1) there were parasitological tests as the gold standard for diagnosis of S. japonicum human infections in the studies, such as Kato-Katz technique or miracidium hatching test; (2) there were one or multiple uses of thee three immunological tests (IHA, ELISA, DDIA) in the study; (3) the immunological assay had been commercial and standardized used in the schistosomiasis-endemic foci; (4) there were detailed numbers to calculate true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) in the study; (5) immunological assays were used for detection of S. japonicum human infections in the study; (6) immunological tests were performed independently and blindly; and (7) full-text files were available. All studies that met the following criteria were excluded from the analysis: (1) no parasitological tests; (2) immunological assays were used for detection of S. japonicum infections in animals; (3) case-control studies; (4) no detailed numbers to calculate TP, FP, TN, or FN; (5) review articles or meeting reports; (6) the study sample size was less than 50; or (7) full-text publications were unavailable.
2.3. Data Extraction
Following joint search in electronic databases, repeated publications were excluded, and the title and abstract of screened literatures were carefully read. Then, eligible studies were identified based on defined inclusion and exclusion criteria. Each grey literature was reviewed carefully based on the defined inclusion and exclusion criteria. All data were managed using the software Microsoft Excel 2010 (Microsoft Inc., Redmond, WA, USA). The title, authors, year of publication, serological tests, participants’ age, epidemic types, endemicity, the gold standard used for detection of S. japonicum human infections, TP, FP, TN, and FN were extracted by two independent investigators. If there was a disagreement, an additional investigator was introduced, and the final decision was made by the third investigator.
2.4. Asymmetry Test
The potential presence of publication bias was evaluated using the Deeks’ funnel plot created with the software Stata version 14.0 (Stata Corporation Lakeway, TX, USA) [23]. An asymmetrical funnel plot indicated the presence of publication bias.
2.5. Meta-Analysis
Since the “threshold effect” has been recognized as a notable source of heterogeneity for diagnostic tests [24], the presence of heterogeneity caused by the “threshold effect” was tested using the software Stata version 14.0. Then, the heterogeneity among studies was examined using the software OpenMeta [analyst] version 3.3 prior to pooled estimates. If I2 statistic was <50% and p > 0.05 in the Q test, no significant heterogeneity was identified among studies, and a fixed-effect model was employed for pooled estimates; otherwise, a random-effect model was used. A summary receiver operating characteristic (SROC) curve was plotted using the software Stata version 14.0 in order to compare the diagnostic accuracy of three immunological assays, and the area under the SROC curve (AUC) was calculated. A higher AUC indicated a greater diagnostic accuracy.
Next, subgroup analyses were performed according to the test of heterogeneity, to examine the effects of the endemicity and epidemic types of schistosomiasis on the performance of IHA, ELISA, and DDIA for detection of S. japonicum human infections.
In this study, the epidemic types were classified into marshland and lake regions, plain regions with waterway networks and hilly and mountainous regions [25], and the epidemic level was classified based on the prevalence of S. japonicum human infections: high endemicity, infection prevalence of 10% and higher; medium endemicity, infection prevalence of <10% and no less than 5%; and low endemicity, infection prevalence of <5% [26].
3. Results
3.1. Study Characteristics
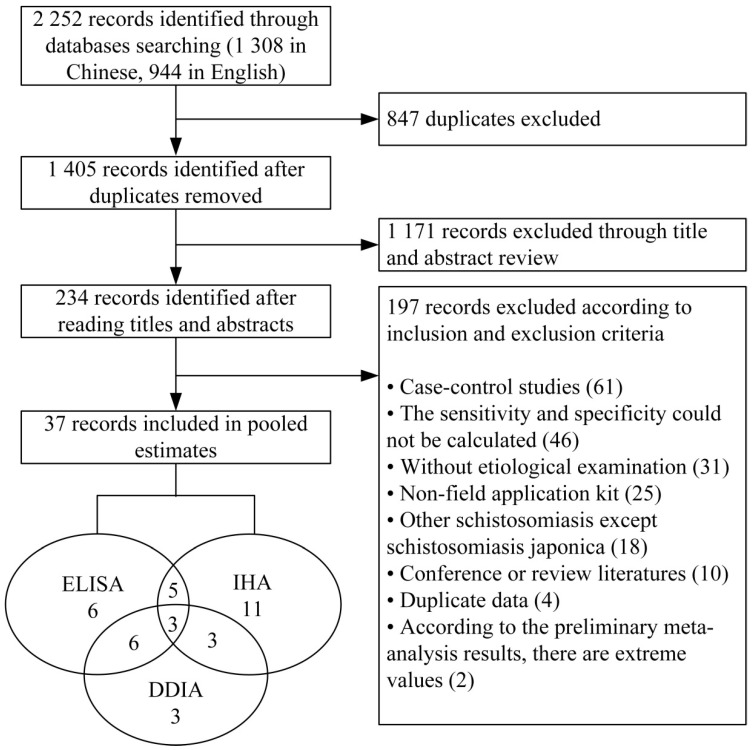
A total of 2252 publications were screened, including 1308 Chinese publications and 944 English publications, and 37 eligible publications that met the inclusion and exclusion criteria were enrolled in the final analysis, including 29 Chinese publications and 8 English publications (Figure 1). Table 1 demonstrates the characteristics of all included studies.
Figure 1.
Flow chart of publication selection.
Table 1.
Subject characteristics of the included studies.
| Publication Year | Subjects’ Age (Years) | Degree of Endemicity | Epidemic Types | Immunological Assay | Parasitological Technique | True Positives | False Negatives | True Negatives | False Positives | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1982 | >15 | High | Hilly and mountainous regions | IHA | Miracidium hatching test (three slides from three stool samples) | 295 | 28 | 1923 | 978 | [27] |
| 1999 | 3 to 70 | High | Plain regions with waterway networks | IHA | Kato-Katz (two slides from one stool sample) | 200 | 80 | 157 | 197 | [28] |
| 2000 | 6 to 60 | Medium | Marshland and lake regions | IHA | Kato-Katz | 413 | 28 | 3723 | 458 | [29] |
| 2002 | 5 to 56 | Low | Marshland and lake regions | IHA | Kato-Katz (three slides from one stool sample) | 12 | 3 | 564 | 37 | [30] |
| DDIA | 14 | 1 | 436 | 165 | ||||||
| 2002 | N/A | Medium | Marshland and lake regions | IHA | Kato-Katz (three slides from one stool sample) | 64 | 14 | 430 | 175 | [31] |
| DDIA | 74 | 4 | 247 | 358 | ||||||
| 2002 | 5 to 60 | N/A | Marshland and lake regions | ELISA | Miracidium hatching test | 10 | 1 | 43 | 20 | [32] |
| 2003 | 6 to 64 | Low | Marshland and lake regions | DDIA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 17 | 1 | 389 | 56 | [33] |
| ELISA | 15 | 3 | 375 | 70 | ||||||
| 2004 | N/A | Low | Marshland and lake regions | DDIA | Kato-Katz | 16 | 1 | 247 | 49 | [34] |
| 2004 | 6 to 60 | Medium | Hilly and mountainous regions | IHA | Miracidium hatching test | 12 | 9 | 30 | 29 | [35] |
| DDIA | 16 | 5 | 19 | 40 | ||||||
| 2004 | 60 to 65 | Low | Marshland and lake regions | DDIA | Miracidium hatching test (three slides from three stool samples) | 13 | 0 | 400 | 52 | [36] |
| 2005 | 15 to 70 | High | Marshland and lake regions | DDIA | Miracidium hatching test (three slides from one stool sample) | 51 | 3 | 237 | 113 | [37] |
| ELISA | 47 | 7 | 228 | 122 | ||||||
| 2005 | 10 to 70 | High | Hilly and mountainous regions | DDIA | Kato-Katz (three slides from one stool sample) | 184 | 3 | 189 | 283 | [38] |
| ELISA | 181 | 6 | 98 | 374 | ||||||
| 2005 | 6 to 65 | High | Hilly and mountainous regions | ELISA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 182 | 5 | 77 | 237 | [39] |
| 2005 | N/A | Low | Hilly and mountainous regions | IHA | Kato-Katz (three slides from one stool sample) | 2 | 0 | 458 | 41 | [40] |
| High | 65 | 1 | 384 | 175 | ||||||
| 2006 | 5 to 65 | N/A | Hilly and mountainous regions, and marshland and lake regions | ELISA | Kato-Katz (six slides from two stool samples) | 73 | 3 | 90 | 4 | [41] |
| IHA | 68 | 8 | 94 | 4 | ||||||
| 2006 | N/A | High | Hilly and mountainous regions | ELISA | Miracidium hatching test | 139 | 25 | 101 | 241 | [42] |
| 2006 | >5 | High, medium, and low | Hilly and mountainous regions | IHA | Miracidium hatching test (three slides from three stool samples) | 31 | 36 | 1589 | 272 | [43] |
| Kato-Katz (two slides from one stool sample) | 57 | 83 | 1542 | 246 | ||||||
| ELISA | Miracidium hatching test (three slides from three stool samples) | 23 | 18 | 743 | 163 | |||||
| Kato-Katz (two slides from one stool sample) | 28 | 21 | 740 | 158 | ||||||
| 2006 | 6 to 65 | Low | Marshland and lake regions, plain regions with waterway networks, and hilly and mountainous regions | ELISA | Miracidium hatching test (one slide from one stool sample) | 25 | 2 | 249 | 860 | [44] |
| DDIA | 27 | 0 | 521 | 588 | ||||||
| 2007 | >5 | High, medium, and low | Hilly and mountainous regions | ELISA | Miracidium hatching test (one slide from one stool sample) and Kato-Katz (four slides from one stool sample) | 19 | 10 | 185 | 137 | [45] |
| DDIA | 13 | 16 | 219 | 103 | ||||||
| 2007 | 10 to 70 | High | Hilly and mountainous regions | DDIA | Kato-Katz (three slides from one stool sample) | 111 | 9 | 260 | 128 | [46] |
| ELISA | 114 | 6 | 309 | 79 | ||||||
| DDIA | Miracidium hatching test (one slide from one stool sample) | 155 | 8 | 129 | 216 | |||||
| ELISA | 160 | 3 | 82 | 263 | ||||||
| 2007 | 6 to 65 | Medium | Marshland and lake regions | DDIA | Miracidium hatching test (three slides from one stool sample) and Kato-Katz (three slides from one stool sample) | 110 | 36 | 779 | 634 | [47] |
| ELISA | 96 | 50 | 731 | 682 | ||||||
| IHA-A | 125 | 21 | 504 | 909 | ||||||
| IHA-B | 111 | 35 | 899 | 514 | ||||||
| 2007 | 6 to 65 | Medium | Hilly and mountainous regions | ELISA | Miracidium hatching test (three slides from three stool samples) | 6 | 2 | 39 | 61 | [48] |
| 2007 | 5 to 75 | Village A: high | Marshland and lake regions | IHA | Kato-Katz (three slides from one stool sample) | 159 | 31 | 465 | 369 | [49] |
| Village B: medium | IHA | 48 | 4 | 495 | 240 | |||||
| 2007 | N/A | High | Marshland and lake regions | IHA | Kato-Katz (seven slides from one stool sample) and miracidium hatching test | 159 | 39 | 44 | 47 | [50] |
| 2007 | 6 to 65 | Low | Hilly and mountainous regions | ELISA | Kato-Katz (four slides from one stool sample) | 35 | 3 | 726 | 675 | [51] |
| 2008 | N/A | N/A | Marshland and lake regions | IHA | Kato-Katz (twelve slides from two stool samples) | 39 | 17 | 510 | 67 | [52] |
| 2008 | 6 to 65 | Medium | Hilly and mountainous regions | IHA | Kato-Katz (three slides from one stool sample) | 34 | 0 | 436 | 130 | [53] |
| IHA | 41 | 0 | 425 | 134 | ||||||
| IHA | 52 | 0 | 411 | 137 | ||||||
| 2008 | >5 | High | Marshland and lake regions | IHA | Kato-Katz (six slides from two stool samples) | 156 | 27 | 460 | 368 | [54] |
| IHA | 68 | 19 | 323 | 242 | ||||||
| 2008 | >5 | High | Marshland and lake regions | ELISA | Kato-Katz (six slides from two stool samples) | 162 | 24 | 322 | 506 | [55] |
| ELISA | 69 | 18 | 302 | 263 | ||||||
| 2009 | 11 to 46 | Medium | Marshland and lake regions | IHA | Kato-Katz (three slides from one stool sample) | 10 | 2 | 145 | 17 | [56] |
| ELISA | 11 | 1 | 134 | 28 | ||||||
| 2010 | 6 to 65 | Low | Marshland and lake regions | IHA | Miracidium hatching test (three slides from one stool sample) | 4 | 1 | 264 | 18 | [57] |
| DDIA | 4 | 1 | 22 | 260 | ||||||
| ELISA | 4 | 1 | 52 | 230 | ||||||
| 2010 | 5 to 80 | Low | Hilly and mountainous regions | IHA | Kato-Katz (nine slides from three stool samples) and miracidium hatching test | 19 | 0 | 149 | 226 | [58] |
| 2011 | 6 to 65 | Medium and low | Marshland and lake regions, and hilly and mountainous regions | DDIA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 241 | 23 | 3196 | 2825 | [59] |
| 2011 | 6 to 65 | Medium and low | Marshland and lake regions, and hilly and mountainous regions | IHA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 231 | 33 | 3370 | 2254 | [60] |
| ELISA | 252 | 12 | 3137 | 2847 | ||||||
| 2013 | 6 to 65 | Low | Marshland and lake regions, and hilly and mountainous regions | IHA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 68 | 6 | 1772 | 1158 | [61] |
| DDIA | 67 | 7 | 1201 | 1729 | ||||||
| ELISA | 71 | 3 | 1221 | 1709 | ||||||
| 2016 | >5 | N/A | Marshland and lake regions | IHA | Kato-Katz (twenty seven slides from three stool samples) | 3 | 0 | 282 | 232 | [62] |
| 2017 | N/A | N/A | Marshland and lake regions | IHA | Kato-Katz (three slides from one stool sample) and miracidium hatching test | 49 | 12 | 116 | 23 | [63] |
| ELISA | 52 | 9 | 130 | 9 |
N/A, subjects’ age or the degree of the endemicity was not reported in the study.
3.2. Meta-Analysis
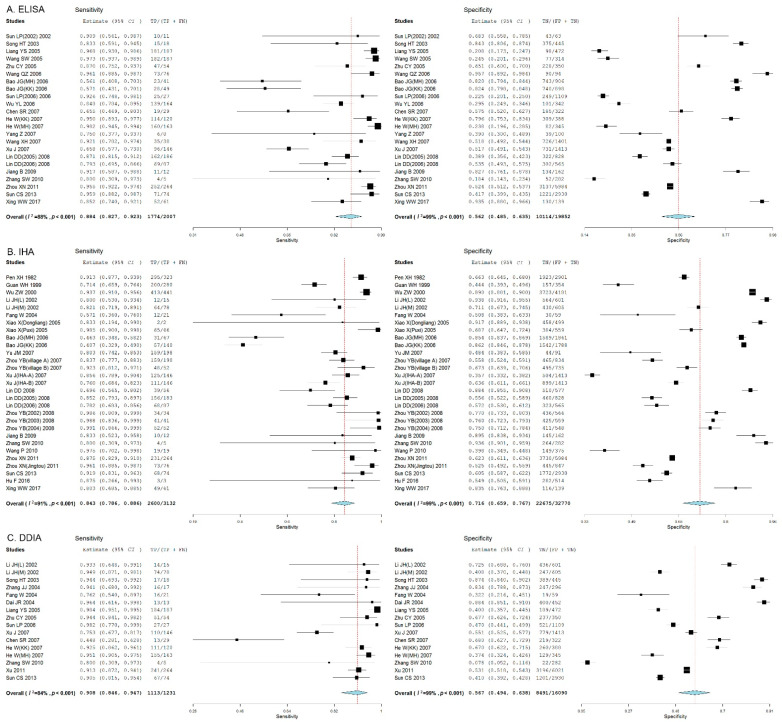
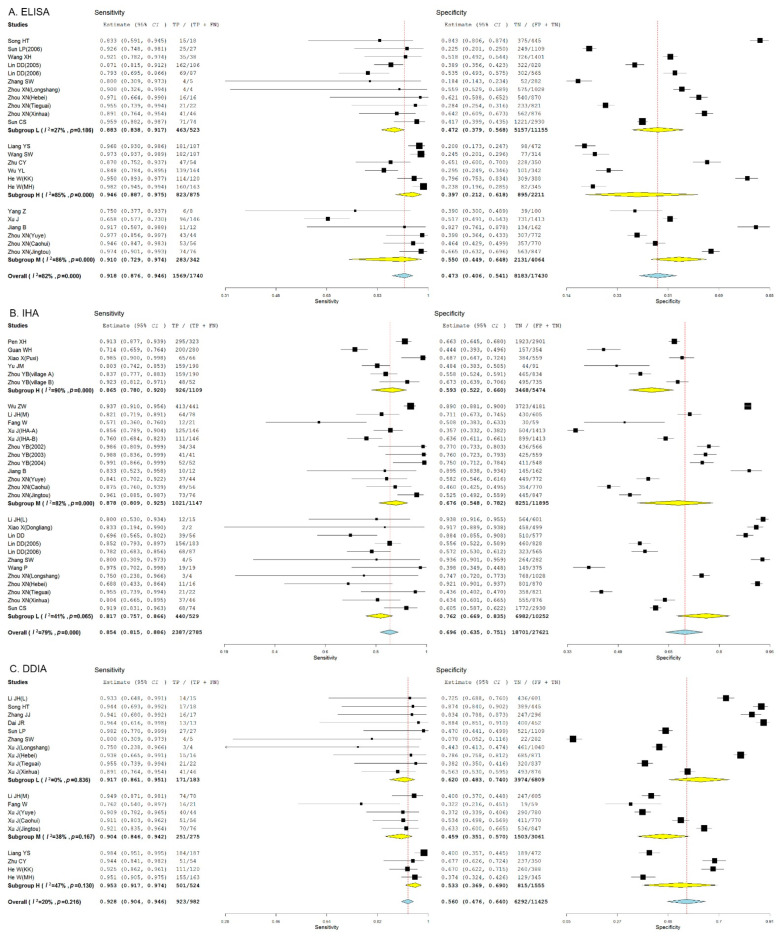
We found that the threshold effect contributed 12%, 1%, and 3% to the heterogeneity for ELISA, IHA, and DDIA, respectively. The test of heterogeneity revealed significant heterogeneities among the studies reporting ELISA (I2 = 88%, p < 0.05), IHA (I2 = 95%, p < 0.05), and DDIA (I2 = 84%, p < 0.05), and a random-effect model was therefore employed for pooled estimates. DDIA showed the highest pooled sensitivity (90.8%, 95% CI: 84.6% to 94.7%) and IHA presented the highest pooled specificity for detection of S. japonicum human infections (71.6%, 95% CI: 65.9% to 76.7%) (Figure 2).
Figure 2.
Forest plots show the pooled sensitivity and specificity of ELISA, IHA, and DDIA for the diagnosis of Schistosoma japonicum human infections. (A) Forest plot of the pooled sensitivity and specificity of ELISA for the diagnosis of S. japonicum human infections; (B) Forest plot of the pooled sensitivity and specificity of IHA for the diagnosis of S. japonicum human infections; (C) Forest plot of the pooled sensitivity and specificity of DDIA for the diagnosis of S. japonicum human infections.
3.3. Comparison of the Diagnostic Accuracy of Three Immunodiagnostic Assays
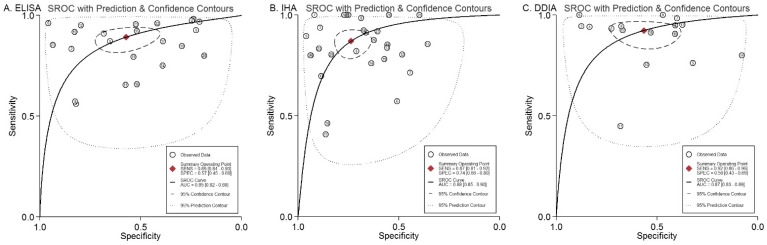
SROC curve analysis showed that IHA exhibited the highest AUC (0.88, 95% CI: 0.85 to 0.9), and ELISA presented the lowest AUC (0.85, 95% CI: 0.82 to 0.88) (Figure 3), indicating that IHA has the highest accuracy for the diagnosis of S. japonicum human infections.
Figure 3.
SROC curves for the diagnostic accuracy of ELISA, IHA, and DDIA for the detection of Schistosoma japonicum human infections. (A) SROC curve for the diagnostic accuracy of ELISA for the detection of S. japonicum human infections; (B) SROC curve for the diagnostic accuracy of IHA for the detection of S. japonicum human infections; (C) SROC curve for the diagnostic accuracy of DDIA for the detection of S. japonicum human infections.
3.4. Publication Bias
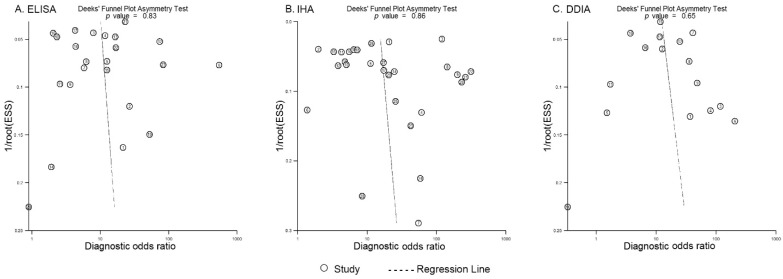
Deeks’ funnel plots were created to evaluate the publication bias of included studies. All three funnel plots were found to be almost symmetrical (Figure 4), and asymmetry test revealed no statistical significances (p > 0.05), indicating no publication bias.
Figure 4.
Deeks’ funnel plots of studies reporting ELISA, IHA, and DDIA. (A) Deeks’ funnel plot of studies reporting ELISA; (B) Deeks’ funnel plot of studies reporting IHA; (C) Deeks’ funnel plot of studies reporting DDIA. An asymmetrical funnel plot indicates the presence of publication bias.
3.5. Subgroup Analysis
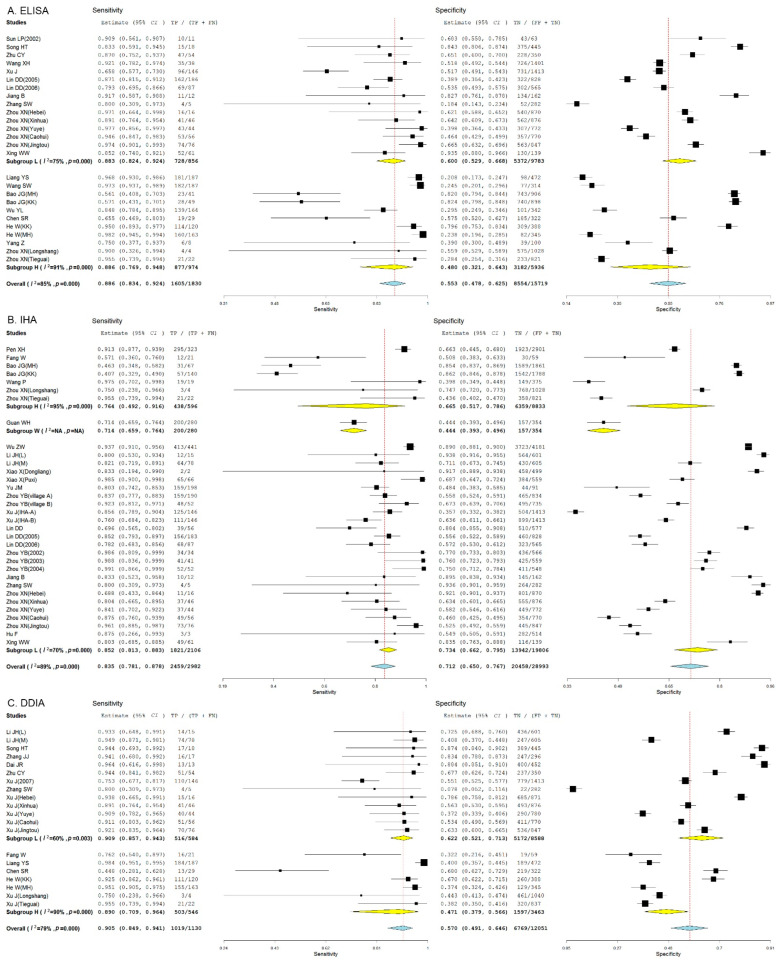
ELISA presented the highest sensitivity in high-endemicity regions (94.6%, 95% CI: 88.7% to 97.5%), and the highest specificity for diagnosis of S. japonicum human infections in medium-endemicity regions (55%, 95% CI: 44.9% to 64.8%) (Figure 5A), and IHA showed the highest diagnostic sensitivity in medium-endemicity regions (87.8%, 95% CI: 80.9% to 92.5%) and the highest diagnostic specificity in low-endemicity regions (76.2%, 95% CI: 66.9% to 83.5%) (Figure 5B), while DDIA exhibited the highest diagnostic sensitivity in high-endemicity regions (95.3%, 95% CI: 91.7% to 97.4%), and the highest diagnostic specificity in low-endemicity regions (62%, 95% CI: 48.3% to 74%) (Figure 5C). Overall, DDIA presented the highest pooled sensitivity (92.8%, 95% CI: 90.4% to 94.6%), and IHA showed the highest pooled specificity for diagnosis of S. japonicum human infections (69.6%, 95% CI: 63.5% to 75.1%).
Figure 5.
Forest plots show the pooled sensitivity and specificity of ELISA, IHA, and DDIA for the diagnosis of Schistosoma japonicum human infections in regions with different endemic levels. (A) Forest plot of the pooled sensitivity and specificity of ELISA for the diagnosis of Schistosoma japonicum human infections in regions with different endemic levels; (B) Forest plot of the pooled sensitivity and specificity of IHA for the diagnosis of Schistosoma japonicum human infections in regions with different endemic levels; (C) Forest plot of the pooled sensitivity and specificity of DDIA for the diagnosis of Schistosoma japonicum human infections in regions with different endemic levels.
ELISA presented a comparable sensitivity between in hilly and mountainous regions (88.6%, 95% CI: 76.9% to 94.8%) and marshland and lake regions (88.3%, 95% CI: 82.4% to 92.4%), and a higher specificity for diagnosis of S. japonicum human infections in marshland and lake regions (60%, 95% CI: 52.9% to 66.8%) than in hilly and mountainous regions (48%, 95% CI: 32.1% to 64.3%) (Figure 6A), and IHA showed a higher pooled diagnostic sensitivity (85.2%, 95% CI: 81.3% to 88.3%) and specificity (73.4%, 95% CI: 66.2% to 79.5%) in marshland and lake regions than in hilly and mountainous regions (76.4%, 95% CI: 49.2% to 91.6%; 66.5%, 95% CI: 51.7% to 78.6%) and plain regions with waterway networks (71.4%, 95% CI: 65.9% to 76.4%; 44.4%, 95% CI: 39.3% to 49.6%) (Figure 6B), while DDIA exhibited a higher pooled diagnostic sensitivity (90.9%, 95% CI: 85.7% to 94.3%) and specificity (62.2%, 95% CI: 52.1% to 71.3%) in marshland and lake regions than in hilly and mountainous regions (89%, 95% CI: 70.9% to 96.4%; 47.1%, 95% CI: 39.7% to 56.6%) (Figure 6C). Similarly, DDIA presented the highest pooled sensitivity (90.5%, 95% CI: 84.9% to 94.1%) and IHA showed the highest pooled specificity for diagnosis of S. japonicum human infections (71.2%, 95% CI: 65% to 76.7%).
Figure 6.
Forest plots show the pooled sensitivity and specificity of ELISA, IHA, and DDIA for the diagnosis of Schistosoma japonicum human infections in endemic foci with different epidemic types. (A) Forest plot of the pooled sensitivity and specificity of ELISA for the diagnosis of Schistosoma japonicum human infections in endemic foci with different epidemic types; (B) Forest plot of the pooled sensitivity and specificity of IHA for the diagnosis of Schistosoma japonicum human infections in endemic foci with different epidemic types; (C) Forest plot of the pooled sensitivity and specificity of DDIA for the diagnosis of Schistosoma japonicum human infections in endemic foci with different epidemic types.
4. Discussion
Precise diagnosis, which is a prerequisite to chemotherapy, is extremely helpful in implementing strategies for the control and elimination of schistosomiasis, which plays a pivotal role in schistosomiasis control programs [64]. Following the concerted efforts for more than seven decades, the epidemiological features of schistosomiasis are characterized by low prevalence and low-infection intensity in China [65,66,67]. Conventional parasitological tools, which remain the gold standard for the diagnosis of schistosomiasis, fail to meet the needs of precise identification of S. japonicum human infections in endemic foci of China, because of its high rate of missing diagnosis in low-endemicity regions [15]. To achieve early, precise identification of S. japonicum infections, multiple PCR assays have been developed and shown potential for the field detection of S. japonicum human infections; however, these assays require specific equipment and are high in costs, making them unlikely to be used for large-scale screening and epidemiological surveys in schistosomiasis-endemic foci [68,69,70]. In addition, loop-mediated isothermal amplification (LAMP) assays were developed for accurate, visualized, and early detection of S. japonicum human infections; however, these assays are extremely likely to be contaminated, resulting in false positives [71,72,73]. Recently, amplification recombinase-aided isothermal amplification (RAA) and recombinase polymerase amplification (RPA) assays have been developed for early detection of S. japonicum human infections; however, the performance of RAA and RPA assays remains to be investigated for detection of S. japonicum infections in large-scale clinical studies [74,75,76,77,78].
Antibody-based immunodiagnostics have been accepted as the first choice for large-scale screening and seroepidemiological surveys of S. japonicum human infections [64]. Currently, there are four commercial serological kits used for diagnosis of S. japonicum human infections in China, including IHA, ELISA, DDIA, and dot immunogold filtration assay (DIGFA), and IHA, ELISA, and DDIA are the three most common approaches used for schistosomiasis immunodiagnosis because of simple, rapid procedures and low costs [17]. Since the diagnostic effectiveness of IHA, ELISA, and DDIA for schistosomiasis has been reported to vary in endemic foci, a precise and comprehensive assessment of the performance of these serological tests for detection of S. japonicum human infections is therefore of great significance to optimize the option of immunodiagnostic assays in various endemic foci of China.
In this study, a total of 37 eligible studies that met the inclusion and exclusion criteria were enrolled in meta-analysis, and no publication bias was detected among studies by the asymmetry test. The highest pooled sensitivity for detection of S. japonicum human infections was seen for DDIA, with the lowest for IHA, and the highest pooled specificity was seen IHA, with the lowest for ELISA, which was in agreement with a previous meta-analysis reporting that IHA, ELISA, and DDIA presented the pooled sensitivities of 0.83, 0.87, and 0.90 and specificities of 0.69, 0.60, and 0.62 for diagnosis of schistosomiasis japonica [20]. In addition, SROC curve analysis showed that IHA exhibited the highest accuracy and ELISA presented the lowest accuracy for the diagnosis of S. japonicum human infections, which was inconsistent with previous results showing 0.89, 0.96, and 0.92 AUCs for IHA, ELISA, and DDIA [20]. This may be attributed to the difference of included studies. However, our findings are in agreement with two previous meta-analyses reporting a higher accuracy of IHA than ELISA for diagnosis of schistosomiasis japonica [21,22].
Since there are three types of endemic foci in China [25], we performed a subgroup analysis to estimate the pooled sensitivity and specificity for detection of S. japonicum human infections in endemic foci with different epidemic types. Our findings showed that IHA and DDIA presented a higher efficiency for the diagnosis of S. japonicum human infections in marshland and lake regions than in hilly and mountainous regions; however, ELISA showed a comparable diagnostic sensitivity between in marshland and lake regions and hilly and mountainous regions (88.3% vs. 88.6%), and a higher specificity in marshland and lake regions than in hilly and mountainous regions (60% vs. 48%). All these three immunological tests are antibody-based assays, and the differences in diagnostic sensitivity and specificity are considered to be explained by that the antigens used for preparation of these three immunodiagnostics are derived from S. japonicum isolates from the marshland and lake regions.
To compare the performance of three immunological tests for detection of S. japonicum human infections in regions with different endemic levels, a subgroup analysis was performed. Our findings showed the highest diagnostic sensitivity of IHA in medium-endemicity regions and the highest specificity in low-endemicity regions, the highest diagnostic sensitivity of ELISA in high-endemicity regions and highest specificity in medium-endemicity regions, and the highest diagnostic sensitivity of DDIA in high-endemicity regions and the highest specificity in low-endemicity regions. Results from a previous meta-analysis showed that the sensitivities of IHA, ELISA, and DDIA were 0.84, 0.76, and 0.94; 0.88, 0.80, and 0.93; and 0.93, 0.81, and 0.93 in high-, medium-, and low-endemicity regions, and the specificities were 0.73, 0.64, and 0.73; 0.59, 0.59, and 0.62; and 0.66, 0.69, and 0.59 in high-, medium-, and low-endemicity regions, respectively [20], which was inconsistent with our data. This may be attributed to the variation of included studies.
To accelerate the achievements of the target for the elimination of schistosomiasis as a public health problem and the interruption of transmission in humans in selected countries by 2030 set out in the WHO road map “Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030” [79], a new guideline for the control and elimination of human schistosomiasis was released by WHO in February 2022 [80]. In this new guideline, six evidence-based recommendations were proposed for the control and elimination of human schistosomiasis in disease-affected countries, including diagnostic strategies for assessment of schistosomiasis infection in humans [80]. In this study, we found a diverse diagnostic sensitivity and specificity of IHA, ELISA, and DDIA for detection of S. japonicum human infections in different types and infection prevalence of endemic regions of China, and DDIA presented the highest pooled sensitivity, while IHA showed the highest pooled specificity for diagnosis of S. japonicum human infections. Large-scale diagnostic tests to compare the performance of IHA, ELISA, and DDIA for detection of S. japonicum human infections in same settings are encouraged. Based on successful experiences from the national schistosomiasis control program, China had been supporting schistosomiasis elimination programs in disease-endemic countries along the Belt and Road Initiative [81]. China-made praziquantel and chemical molluscicides have shown effective for schistosomiasis control in African countries [82,83]. Although IHA, ELISA, and DDIA are produced based on the antigens from S. japonicum isolates, previous studies have shown the effectiveness of DDIA and IHA for the detection of S. mekongi, S. mansoni, and S. haematobium human infections [84,85,86,87]. Further large-scale diagnostic tests to investigate the performance of China-made commercial immunodiagnostic assays for the diagnosis of African schistosomiasis seem justified, with may provide tools to support the elimination of schistosomiasis in African continents. In addition, improvements of China-made immunodiagnostics with antigens from S. mansoni and S. haematobium isolates may improve the sensitivity and specificity for diagnosis of S. mansoni and S. haematobium human infections.
This study has some limitations. First, the immunodiagnostics were provided by different manufacturers; however, we did not compare the diagnostic performance of immunodiagnostics by different manufacturers for schistosomiasis immunodiagnosis, since the suppliers of some immunodiagnostics were not available in publications. Second, we did not perform a subgroup analysis to compare the effectiveness of immunodiagnostic assays among participants with different ages.
In summary, the results of our meta-analysis demonstrate a comparable diagnostic accuracy of IHA, ELISA, and DDIA for S. japonicum human infections, and the diagnostic sensitivity and specificity of IHA, ELISA, and DDIA vary in endemic regions with different epidemic types and endemic levels. In addition, DDIA presents the highest pooled sensitivity and IHA shows the highest pooled specificity for diagnosis of S. japonicum human infections. Since schistosomiasis is currently low in prevalence and infection intensity in China, DDIA in combination with IHA is recommended as a tool for screening chemotherapy targets and seroepidemiological surveys. Further studies to examine the effectiveness of combinations of two or three immunological tests for diagnosis of S. japonicum human infections are warranted.
Acknowledgments
We would like to thank Jing Xu and Shizhu Li for their kind help during the preparation of this manuscript.
Author Contributions
Conceptualization, T.J. and W.W.; methodology, T.J.; software, Z.M.; validation, T.J. and W.W.; formal analysis, Z.M.; investigation, S.L. and L.T.; resources, S.L. and L.T.; data curation, W.W.; writing—original draft preparation, W.W.; writing—review and editing, T.J.; visualization, Z.M.; supervision, T.J.; project administration, T.J.; funding acquisition, T.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available upon request by contact with the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interests.
Funding Statement
This study was supported by the grants from the National Natural Science Foundation of China (No.32161143036) and the National Key Research and Development Program of China (No. 2021YFC2300800, 2021YFC2300804).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verjee M.A. Schistosomiasis: Still a Cause of significant morbidity and mortality. Res. Rep. Trop. Med. 2019;10:153–163. doi: 10.2147/RRTM.S204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giboda M., Bergquist R., Utzinger J. Schistosomiasis at the crossroad to elimination: Review of eclipsed research with emphasis on the post-transmission agenda. Trop. Med. Infect. Dis. 2022;7:55. doi: 10.3390/tropicalmed7040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deol A.K., Fleming F.M., Calvo-Urbano B., Walker M., Bucumi V., Gnandou I., Tukahebwa E.M., Jemu S., Mwingira U.J., Alkohlani A., et al. Schistosomiasis—Assessing progress toward the 2020 and 2025 global goals. N. Engl. J. Med. 2019;381:2519–2528. doi: 10.1056/NEJMoa1812165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G., Xu D., Hu Y., Xu M., Zhang L., Du X., Zhang L., Sun C., Xie Y., Tan X. Impact of the coronavirus disease 2019 lockdown on Schistosoma host Oncomelania hupensis density in Wuhan. Acta Trop. 2022;226:106224. doi: 10.1016/j.actatropica.2021.106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toor J., Adams E.R., Aliee M., Amoah B., Anderson R.M., Ayabina D., Bailey R., Basáñez M.G., Blok D.J., Blumberg S., et al. Predicted impact of COVID-19 on neglected tropical disease programs and the opportunity for innovation. Clin. Infect. Dis. 2021;72:1463–1466. doi: 10.1093/cid/ciaa933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantica G., Martini M., Riccardi N. The possible impact of SARS-CoV-2 on neglected tropical diseases in Europe: The out of spotlights emerging of schistosomiasis. J. Prev. Med. Hyg. 2021;62:E3–E4. doi: 10.15167/2421-4248/jpmh2021.62.1.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.N. Schistosomiasis. Nat. Rev. Dis. Primers. 2018;4:13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 8.Hong Z., Li L., Zhang L., Wang Q., Xu J., Li S., Zhou X.N. Elimination of schistosomiasis japonica in China: From the One Health perspective. China CDC Wkly. 2022;4:130–134. doi: 10.46234/ccdcw2022.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., Li S.Z., Zhang L.J., Bergquist R., Dang H., Wang Q., Lv S., Wang T.P., Lin D.D., Liu J.B., et al. Surveillance-based evidence: Elimination of schistosomiasis as a public health problem in the Peoples’ Republic of China. Infect. Dis. Poverty. 2020;9:63. doi: 10.1186/s40249-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Bergquist R., King C.H., Yang K. Elimination of schistosomiasis in China: Current status and future prospects. PLoS Negl. Trop. Dis. 2021;15:e0009578. doi: 10.1371/journal.pntd.0009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utzinger J., Becker S.L., van Lieshout L., van Dam G.J., Knopp S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015;21:529–542. doi: 10.1016/j.cmi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Lindholz C.G., Favero V., Verissimo C.M., Candido R.R.F., de Souza R.P., Dos Santos R.R., Morassutti A.L., Bittencourt H.R., Jones M.K., St Pierre T.G., et al. Study of diagnostic accuracy of Helmintex, Kato-Katz, and POC-CCA methods for diagnosing intestinal schistosomiasis in Candeal, a low intensity transmission area in northeastern Brazil. PLoS Negl. Trop. Dis. 2018;12:e0006274. doi: 10.1371/journal.pntd.0006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray D.J., Ross A.G., Li Y.S., McManus D.P. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LoVerde P.T. Schistosomiasis. Adv. Exp. Med. Biol. 2019;1154:45–70. doi: 10.1007/978-3-030-18616-6_3. [DOI] [PubMed] [Google Scholar]
- 15.Lv C., Deng W., Wang L., Qin Z., Zhou X., Xu J. Molecular techniques as alternatives of diagnostic tools in China as schistosomiasis moving towards elimination. Pathogens. 2022;11:287. doi: 10.3390/pathogens11030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz R., Schwarz N.G., Hahn A., Frickmann H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell Probes. 2017;31:2–21. doi: 10.1016/j.mcp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Chen C., Guo Q., Fu Z., Liu J., Lin J., Xiao K., Sun P., Cong X., Liu R., Hong Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2021;213:105743. doi: 10.1016/j.actatropica.2020.105743. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J.F., Xu J., Bergquist R., Yu L.L., Yan X.L., Zhu H.Q., Wen L.Y. Development and application of diagnostics in the national schistosomiasis control programme in the People’s Republic of China. Adv. Parasitol. 2016;92:409–434. doi: 10.1016/bs.apar.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y., Chen Y., Jiang Q. History of human schistosomiasis (bilharziasis) in China: From discovery to elimination. Acta Parasitol. 2021;66:760–769. doi: 10.1007/s11686-021-00357-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang X.Y., Yang K. Serological diagnosis methods of schistosomiasis japonica at different prevalence: A meta-analysis. Chin. J. Schisto. Control. 2016;28:18–25. [PubMed] [Google Scholar]
- 21.Zhu H., Yu C., Xia X., Dong G., Tang J., Fang L., Du Y. Assessing the diagnostic accuracy of immunodiagnostic techniques in the diagnosis of schistosomiasis japonica: A meta-analysis. Parasitol. Res. 2010;107:1067–1073. doi: 10.1007/s00436-010-1970-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Li Y., Li H., Xing Y., Qu G., Dai J., Liang Y. Immunodiagnostic efficacy of detection of Schistosoma japonicum human infections in China: A meta analysis. Asian Pac. J. Trop. Med. 2012;5:15–23. doi: 10.1016/S1995-7645(11)60238-1. [DOI] [PubMed] [Google Scholar]
- 23.Liu J. The role of the funnel plot in detecting publication and related biases in meta-analysis. Evid. Based Dent. 2011;12:121–122. doi: 10.1038/sj.ebd.6400831. [DOI] [PubMed] [Google Scholar]
- 24.Hartzes A.M., Morgan C.J. Meta-analysis for diagnostic tests. J. Nucl. Cardiol. 2019;26:68–71. doi: 10.1007/s12350-018-01485-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X.N., Guo J.G., Wu X.H., Jiang Q.W., Zheng J., Dang H., Wang X.H., Xu J., Zhu H.Q., Wu G.L., et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg. Infect. Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao C.L., Zhang L.J., Deng W.P., Li Y.L., Lv C., Dai S.M., Feng T., Qin Z.Q., Duan L.P., Zhang H.B., et al. Contributions and achievements on schistosomiasis control and elimination in China by NIPD-CTDR. Adv. Parasitol. 2020;110:1–62. doi: 10.1016/bs.apar.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Peng X.H., Hou C.S., Shi C.C. Evaluating the value of indirect hemagglutination assay for the field diagnosis of schistosomiasis japonica. Sichuan Med. 1982;3:72–73. [Google Scholar]
- 28.Guan W.H., Yuan H.C., Zhao G.M., Yu J.M., Yang Q.J. Survey of schistosomiasis epidemic in dam-circle marsh region. Chin. J. Public Health. 1999;15:686–688. [Google Scholar]
- 29.Wu Z.W., Liu Z.C., Yang G.F. Reliability of application of IHA method for determine chemotherapy targets of schistosomiasis in moderately endemic areas of lake region. Chin. J. Schist. Control. 2000;12:21–23. [Google Scholar]
- 30.Li J.H., Wang T.P., Xiao X., Wu W.D., Lv D.B., Fang G.R., Cai W., Zheng J., Xu J., Wang R.R. Cost-effectiveness analysis on different schistosomiasis case screen methods in hypo-endemic area. J. Pract. Parasit. Dis. 2002;10:145–148. [Google Scholar]
- 31.Li J.H., Wang T.P., Xiao X., Wu W.D., Lv D.B., Fang G.R., Cai W., Zheng J., Xu J., Wang R.R. Cost-effectiveness analysis on different schistosomiasis case screen methods in hypo-endemic area. Chin. J Schist. Control. 2002;14:418–421. [Google Scholar]
- 32.Sun L.P., Hong Q.B., Zhou X.N., Huang Y.X., Wu F., Zhang Y.P., Yang G.J. Field evaluation of fraction antigen of SEA applied in screening of schistosomiasis. Chin. Parasit. Dis Control. 2002;15:42–44. [Google Scholar]
- 33.Song H.T., Liang Y.S., Dai J.R., Li H.J., Ji C.S., Shen X.H., Li L.G., Yin F. Cost-effectiveness of three immunoassays for diagnosis of schistosomiasis in lower endemic area. Chin. J. Schist. Control. 2003;15:300–301. [Google Scholar]
- 34.Zhang J.J., Xu L., Song H.T. Application of DDIA for screening schistosomiasis in high-risk populations. J. Trop. Dis. Parasitol. 2004;2:50–51. [Google Scholar]
- 35.Fang W., Gan Z.M., Dong P.H., Yang T.L., Chen F., Luo B.R., Qiu Z.L. Field application of dipstick dye immunoassay in schistosomiasis-endemic areas in Yunnan Province. Chin. J. Schist. Control. 2004;16:325–326. [Google Scholar]
- 36.Dai J.R., Zhu Y.C., Liang Y.S., Zhao S., Li H.J., Xu Y.L., Hua W.Q., Cao G.Q., Xu M. Study on scheme for screening schistosomiasis in low endemic areas. Chin. J. Schist. Control. 2004;16:13–15. [Google Scholar]
- 37.Zhu Y.C., He W., Dai J.R., Xu M., Liang Y.S., Tang J.X., Hua W.Q., Cao G.Q., Chen H.G., Lou P.A. Application of dipstick dye immunoassay (DDIA) kit on detection of schistosomiasis japonica on large scale in endemic areas. Chin. J. Schist. Control. 2004;16:13–15. [Google Scholar]
- 38.Liang Y.S., Zhu Y.C., Dai J.R., He W., Xu M., Li Y.L., Wang S.W., Tang J.X., Hua W.Q., Li H.J., et al. Field application of dipstick dye immunoassay (DDIA) kit for detecting schistosomiasis in mountainous endemic regions in Yunnan Province. Chin. J. Schist. Control. 2005;17:405–408. [Google Scholar]
- 39.Wang S.W., Yang Z., Yin G.L., Li Y.L., Yang J., Zhao J.B., Luo B.R., Zuo X.F., Zou H.M., Zhang J.P. Application of antibody-based ELISA for detection of schistosomiasis in highly endemic regions. Parasit. Infect. Dis. 2005;3:79–80. [Google Scholar]
- 40.Xiao X., Wang T., Ye H., Qiang G., Wei H., Tian Z. Field evaluation of a rapid, visually-read colloidal dye immunofiltration assay for Schistosoma japonicum for screening in areas of low transmission. Bull. World Health Organ. 2005;83:526–533. [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q.Z., Wang F.F., Yin X.M., Zhu L., Zhang G.H., Fang G.R., Wang T.P., Xiao X., Jiang Q.W. Evaluation of screening effects of ELISA and IHA techniques in different epidemic areas of schistosomiasis. J. Trop. Dis. Parasitol. 2006;4:135–139. [Google Scholar]
- 42.Wu Y.L., Cheng M., Meng W., Li H.X. Comparison between effect of two schistosomiassi diagnostic kit application to the multimountain area in Yunnan Province. BMC J. 2006;29:168–169. [Google Scholar]
- 43.Bao J.G., Qiang G.X., Deng Y.J., Zhang R., Chen Y. Comparison of four diagnostic assays for the field detection of schistosomiasis. J. Trop. Dis. Parasitol. 2006;4:33–34. [Google Scholar]
- 44.Sun L.P., Hong Q.B., Huang Y.X., Liang Y.S., Xu M., Zhang L.H., Gao Y., Zhou M., Yang K., Zhu Y.C. Comparison of two immunoassays for schistosomiaisis diagnosis in the field. Chin. J. Schist. Control. 2004;16:192–196. [Google Scholar]
- 45.Chen S.R., Chen F., Zhou X.N., Li H.J., Stenmann P.J., Yang Z., Li Y.L. Comparison of aetiological and serological diagnosis methods in schistosomiasis mountainous endemic area. Parasitol. Infect. Dis. 2007;5:1–4. [Google Scholar]
- 46.He W., Zhu Y.C., Liang Y.S., Dai J.R., Xu M., Tang J.X., Cao G.Q., Hua W.Q., Li Y.L., Yang Z. Comparison of stool examination and immunodiagnosis for schistosomiasis. Chin. J. Schist. Control. 2007;19:107–109. [Google Scholar]
- 47.Xu J., Chen N.G., Feng T., Wang E.M., Wu X.H., Chen H.G., Wang T.P., Zhou X.N., Zheng J. Effectiveness of routinely used assays for the diagnosis of schistosomiasis japonica in the field. Chin. Parasitol. Parasit. Dis. 2007;25:175–179. [PubMed] [Google Scholar]
- 48.Yang Z., Yin G.L., Fan C.Z., Luo B.R., Liu Y.H., Duan Y.C., Cui Y.H., Yang Y.N., Sun H.Y., Wang S.W. Effect of gold labeling immunoassay for diagnosis of schistosomiasis. Parasitol. Infect. Dis. 2007;5:97–98. [Google Scholar]
- 49.Zhou Y.B., Yang M.X., Wang Q.Z., Zhao G.M., Wei J.G., Peng W.X., Jiang Q.W. Field comparison of immunodiagnostic and parasitological techniques for the detection of Schistosomiasis japonica in the People’s Republic of China. Am. J. Trop. Med. Hyg. 2007;76:1138–1143. doi: 10.4269/ajtmh.2007.76.1138. [DOI] [PubMed] [Google Scholar]
- 50.Yu J.M., de Vlas S.J., Jiang Q.W., Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Wang X.H., Zhou X.N., Li Y.L., Lv S., Li L.H., Jia T.W., Chen S.R., Yang Z., Fang W., Chen F. Evaluation of two tests for detecting Schistosoma japonicum infection using a Bayesian approach. Chin. J. Health Stat. 2007;24:361–364. [Google Scholar]
- 52.Lin D.D., Liu Y.M., Hu F., Tao B., Wang X.M., Zuo X.X., Li J.Y., Wu G.L. Evaluation on application of common diagnosis methods for schistosomiasis japonica in endemic areas of China I Evaluation on estimation of prevalence of Schistosoma japonicum infection by IHA screening method. Chin. J. Schist. Control. 2008;20:179–183. [PubMed] [Google Scholar]
- 53.Zhou Y.B., Yang M.X., Tao P., Jiang Q.L., Zhao G.M., Wei J.G., Jiang Q.W. A longitudinal study of comparison of the Kato-Katz technique and indirect hemagglutination assay (IHA) for the detection of schistosomiasis japonica in China, 2001–2006. Acta Trop. 2008;107:251–254. doi: 10.1016/j.actatropica.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Lin D.D. Evaluation of the Performance of Commonly Used Diagnostic Assays for the Field Detection of Schistosomiasis Japonica in China. Nanjing Medical University; Nanjing, China: 2008. [Google Scholar]
- 55.Lin D.D., Xu J.M., Zhang Y.Y., Liu Y.M., Hu F., Xu X.L., Li J.Y., Gao Z.L., Wu H.W., Kurtis J., et al. Evaluation of IgG-ELISA for the diagnosis of Schistosoma japonicum in a high prevalence, low intensity endemic area of China. Acta Trop. 2008;107:128–133. doi: 10.1016/j.actatropica.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Jiang B., Zhou Y.D., Meng Q.Y., Luo Q.L., Shen J.L. Study on IgY immunoglobulin-based double antibody sandwich ELISA for schistosomiaisis diagnosis in the field. J. Trop. Med. Parasitol. 2009;7:138–140. [Google Scholar]
- 57.Zhang S.W., Cheng B., Qu H.J., Chen Z.M., Zou Q., Chu L.P., Zhang L., He H.R., Tang S.H., Huang X.P., et al. Validity evaluation of dipstick dye immuno-assay (DDIA) for screening in low endemic areas of schistosomiasis. Chin. J. Schist. Control. 2010;22:171–173. [Google Scholar]
- 58.Wang P., Ren C.P., Wang T.P., Shen J.J. Evaluation of recombinant 29 000 extra membranous protein for the ummunodiagnosis of schistosomiasis japonica. Chin. J. Parasitol. Parasit. Dis. 2010;28:284–286. [PubMed] [Google Scholar]
- 59.Xu J., Feng T., Lin D.D., Wang Q.Z., Tang L., Wu X.H., Guo J.G., Peeling R.W., Zhou X.N. Performance of a dipstick dye immunoassay for rapid screening of Schistosoma japonicum infection in areas of low endemicity. Parasit. Vectors. 2011;4:87. doi: 10.1186/1756-3305-4-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X.N., Xu J., Chen H.G., Wang T.P., Huang X.B., Lin D.D., Wang Q.Z., Tang L., Guo J.G., Wu X.H., et al. Tools to support policy decisions related to treatment strategies and surveillance of Schistosomiasis japonica towards elimination. PLoS Negl. Trop. Dis. 2011;5:e1408. doi: 10.1371/journal.pntd.0001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun C.S., Wang F.F., Wang Y., Zhou L., Yin X.M., Wang Q.Z., Zhang L.S., Wang E.M., Zhang S.Q. Effectiveness of an indirect hemagglutination assay kit at diagnosing schistosomiasis in the field. J. Parasit. Biol. 2013;8:982–985. [Google Scholar]
- 62.Hu F., Li Z.J., Li Y.F., Yuan M., Xie S.Y., Liu Y.M., Li J.Y., Gao Z.L., Pu Y., Wang J.M., et al. Study on cut-off value of IHA method for schistosomiasis diagnosis in different endemic areas. Chin. J. Schist. Control. 2016;28:644–647, 682. doi: 10.16250/j.32.1374.2016181. [DOI] [PubMed] [Google Scholar]
- 63.Xing W., Yu X., Feng J., Sun K., Fu W., Wang Y., Zou M., Xia W., Luo Z., He H., et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017;17:164. doi: 10.1186/s12879-017-2182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weerakoon K.G., Gobert G.N., Cai P., McManus D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang S.Q., Sun C.S., Wang M., Lin D.D., Zhou X.N., Wang T.P. Epidemiological features and effectiveness of schistosomiasis control programme in lake and marshland region in the People’s Republic of China. Adv. Parasitol. 2016;92:39–71. doi: 10.1016/bs.apar.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Shi L., Li W., Wu F., Zhang J.F., Yang K., Zhou X.N. Epidemiological features and control progress of schistosomiasis in waterway-network region in the People’s Republic of China. Adv. Parasitol. 2016;92:97–116. doi: 10.1016/bs.apar.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Zhou Y.B., Li R.Z., Wan J.J., Yang Y., Qiu D.C., Zhong B. Epidemiological features and effectiveness of schistosomiasis control programme in mountainous and hilly region of the People’s Republic of China. Adv. Parasitol. 2016;92:73–95. doi: 10.1016/bs.apar.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 68.He P., Gordon C.A., Williams G.M., Li Y., Wang Y., Hu J., Gray D.J., Ross A.G., Harn D., McManus D.P. Real-time PCR diagnosis of Schistosoma japonicum in low transmission areas of China. Infect. Dis. Poverty. 2018;7:8. doi: 10.1186/s40249-018-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fung M.S., Xiao N., Wang S., Carlton E.J. Field evaluation of a PCR test for Schistosoma japonicum egg detection in low-prevalence regions of China. Am. J. Trop. Med. Hyg. 2012;87:1053–1058. doi: 10.4269/ajtmh.2012.12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lier T., Johansen M.V., Hjelmevoll S.O., Vennervald B.J., Simonsen G.S. Real-time PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 2008;105:74–80. doi: 10.1016/j.actatropica.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Xu J., Guan Z.X., Zhao B., Wang Y.Y., Cao Y., Zhang H.Q., Zhu X.Q., He Y.K., Xia C.M. DNA detection of Schistosoma japonicum: Diagnostic validity of a LAMP assay for low-intensity infection and effects of chemotherapy in humans. PLoS Negl. Trop. Dis. 2015;9:e0003668. doi: 10.1371/journal.pntd.0003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J., Rong R., Zhang H.Q., Shi C.J., Zhu X.Q., Xia C.M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) Int. J. Parasitol. 2010;40:327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Wang C., Chen L., Yin X., Hua W., Hou M., Ji M., Yu C., Wu G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasit. Vectors. 2011;4:164. doi: 10.1186/1756-3305-4-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song Z., Ting L., Kun Y., Wei L., Jian-Feng Z., Li-Chuan G., Yan-Hong L., Yang D., Qing-Jie Y., Hai-Tao Y. Establishment of a recombinase-aided isothermal amplification technique to detect Schistosoma japonicum specific gene fragments. Chin. J. Schisto. Control. 2018;30:273–277. doi: 10.16250/j.32.1374.2018120. [DOI] [PubMed] [Google Scholar]
- 75.Ye Y.Y., Zhao S., Liu Y.H., Zhang J.F., Xiong C.R., Ying Q.J., Yang K. Establishment of a nucleic acid dipstick test for detection of Schistosoma japonicum specific gene fragments based on the recombinase-aided isothermal amplification assay. Chin. J. Schist. Control. 2021;33:334–338. doi: 10.16250/j.32.1374.2021016. [DOI] [PubMed] [Google Scholar]
- 76.Zhao S., Liu Y.H., Li T., Li W., Zhang J.F., Guo L.C., Ying Q.J., Yang H.T., Yang K. Rapid detection of Schistosoma japonicum specific gene fragment by recombinase aided isothermal amplification combined with fluorescent probe. Chin. J. Parasitol. Parasit. Dis. 2019;37:23–27. [Google Scholar]
- 77.Sun K., Xing W., Yu X., Fu W., Wang Y., Zou M., Luo Z., Xu D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasit. Vectors. 2016;9:476. doi: 10.1186/s13071-016-1745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng W., Wang S., Wang L., Lv C., Li Y., Feng T., Qin Z., Xu J. Laboratory evaluation of a basic recombinase polymerase amplification (RPA) assay for early detection of Schistosoma japonicum. Pathogens. 2022;11:319. doi: 10.3390/pathogens11030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. [(accessed on 6 June 2022)]. Available online: https://www.who.int/publications/i/item/9789240010352.
- 80.WHO WHO Guideline on Control and Elimination of Human Schistosomiasis. [(accessed on 6 June 2022)]. Available online: https://www.who.int/publications/i/item/9789240041608. [PubMed]
- 81.Chen J., Bergquist R., Zhou X.N., Xue J.B., Qian M.B. Combating infectious disease epidemics through China’s Belt and Road Initiative. PLoS Negl. Trop. Dis. 2019;13:e0007107. doi: 10.1371/journal.pntd.0007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X.Y., He J., Juma S., Kabole F., Guo J.G., Dai J.R., Li W., Yang K. Efficacy of China-made praziquantel for treatment of schistosomiasis haematobium in Africa: A randomized controlled trial. PLoS Negl. Trop. Dis. 2019;13:e0007238. doi: 10.1371/journal.pntd.0007238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xing Y.T., Dai J.R., Yang K., Jiang T., Jiang C.G., Mohammed S.J., Kabole F. Bulinus snails control by China-made niclosamide in Zanzibar: Experiences and lessons. In: Yang K., Mehlhorn H., editors. Sino-African Cooperation for Schistosomiasis Control in Zanzibar. Springer Nature Switzerland AG; Cham, Switzerland: 2021. pp. 147–159. [Google Scholar]
- 84.Zhang L.J., Mwanakasale V., Xu J., Sun L.P., Yin X.M., Zhang J.F., Hu M.C., Si W.M., Zhou X.N. Diagnostic performance of two specific Schistosoma japonicum immunological tests for screening Schistosoma haematobium in school children in Zambia. Acta Trop. 2020;202:105285. doi: 10.1016/j.actatropica.2019.105285. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Y.C., Socheat D., Bounlu K., Liang Y.S., Sinuon M., Insisiengmay S., He W., Xu M., Shi W.Z., Bergquist R. Application of dipstick dye immunoassay (DDIA) kit for the diagnosis of schistosomiasis mekongi. Acta Trop. 2005;96:137–141. doi: 10.1016/j.actatropica.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Hua H.Y., Wang W., Cao G.Q., Tang F., Liang Y.S. Improving the management of imported schistosomiasis haematobia in China: Lessons from a case with multiple misdiagnoses. Parasit. Vectors. 2013;6:260. doi: 10.1186/1756-3305-6-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y.C., Hassen S., He W., Cao G.Q. Preliminary study on detection of schistosomiasis mansoni with dipstick dye immunoassay (DDIA) kit. Chin. J. Schist. Control. 2006;18:419–421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this study are available upon request by contact with the corresponding authors.