Abstract
Although commercial MRS broth has been designed to allow excellent growth of lactobacilli, most of these bacteria are still subjected to a self-inhibiting process. The most likely explanation is the accumulation of lactic acid or other toxic end products and the depletion of nutrients. In this study, the self-inhibition of Lactobacillus sakei CTC 494 was analyzed in a kinetic way, and a nutrient depletion model was set up to describe the growth inhibition process. This simple model has considerable advantages compared to commonly used descriptive models such as the logistic growth equation. It offers a better fit and a more realistic description of the growth data by taking into account both growth inhibition due to lactic acid production and changes in growth rates due to nutrient depletion. Depending on the fermentation conditions, in MRS broth there appears to be a strong decrease of the specific growth rate over time. Some undefined compounds present in the complex nitrogen source of MRS broth appear to be of crucial importance because of their limited availability. Moreover, nutrient availability affects bacteriocin production through its effect on cell growth as well as on the bacteriocin production per cell. A plateau value for the bacteriocin production by L. sakei CTC 494 was observed.
Bacterial cell growth is strongly dependent on environmental conditions. Factors such as temperature, pH, water activity, redox potential, and the presence of inhibitory compounds determine bacterial growth. Furthermore, the limited availability of certain essential molecules needed for cell metabolism, including energetic compounds and building blocks for cell synthesis, like amino acids, vitamins, minerals, and nucleotides, may slow down the growth rate. Commercial media have been developed to support good growth of lactic acid bacteria, such as de Man-Rogosa-Sharpe (MRS) medium for lactobacilli (7) and M17 medium for lactococci and streptococci (32). These media are often used to study and simulate natural fermentation processes like meat fermentation (11, 20, 21) and sourdough fermentation (12). However, growth of lactic acid bacteria is often inhibited in those media, not only because of inhibition due to the production of organic acids (mainly lactic acid and, in some cases, acetic acid) but also because of nutrient limitation. Organic acids were shown to have a toxic, antimicrobial effect, even for the producer cells. Their antimicrobial mode of action is ascribed to the pH-lowering effect and to the undissociated form of the acid (10). However, the dissociated molecules may play a minor role as well (29, 31). Due to the complexity of both nutrient requirements and availability, it remains rather unclear which compounds are precisely responsible for limitation of the cell growth, but sugars and amino acids seem to be the most crucial factors (2, 13, 16, 23, 33). The need for essential minerals, such as manganese and magnesium, has also been documented (23). Precise modeling of growth data may help to overcome media limitations during data interpretation.
Sigmoidal equations, such as the logistic and the Gompertz equations, have been widely applied to describe the growth of lactic acid bacteria (11, 19, 20, 21, 24, 35). These equations approximate cell growth over time and take into account growth inhibition as cells move into the late growth phase. However, because of their empirical nature, they have little biological meaning, and it may hence be preferred to use models with a more mechanistic approach (3, 27, 28). Such models may help to elucidate the effect of single environmental factors, such as the pH or the lactic acid concentration, on relevant biological parameters, such as the growth rate or the lag phase.
In this study, the growth inhibition process of Lactobacillus sakei CTC 494 cells growing in MRS broth is studied kinetically and is related to the production of lactic acid and the exhaustion of nutrients. Furthermore, the growth-associated bacteriocin production by L. sakei CTC 494, which has previously been described as a function of the environmental factors temperature and pH (20), has been remodeled according to the nutrient depletion growth model that is proposed in this study.
MATERIALS AND METHODS
Microorganisms and media.
L. sakei CTC 494, a producer of the bacteriocin sakacin K (14, 22), was used for growth and bacteriocin production studies. Listeria innocua LMG 13568 was used as an indicator for the estimation of bacteriocin activity. Both strains were maintained as previously described (20). (Modified) MRS broth for the fermentation experiments was prepared from single ingredients (7). Modifications of MRS broth included addition of sodium chloride (Merck, Darmstadt, Germany), changes in the total amount of complex nitrogen source (CNS) (i.e., bacteriological peptone [Oxoid, Basingstoke, United Kingdom], Lab-Lemco powder [Oxoid], and yeast extract [Merck]), or substitution of bacteriological peptone and Lab-Lemco powder by an equal amount of casein tryptone (Oxoid).
Fermentation experiments and sampling.
Fermentations were carried out in a computer-controlled 15-liter laboratory fermentor (BiostatC; B. Braun Biotech International, Melsungen, Germany) containing 10 liters of (modified) MRS broth. Preparation of the fermentor, building of the inoculum, and on-line control of the fermentation process (pH, temperature, agitation) were performed as previously described (20). Determination of biomass concentration [X], total lactic acid concentration [L], residual glucose concentration [S], and bacteriocin activity [B], were carried out as described elsewhere (8, 9, 19, 20). Summarizing, biomass (as “cell dry mass” [CDM]) was determined by membrane filtration, lactic acid and glucose were determined by high-pressure liquid chromatography, and bacteriocin activity was determined by a twofold critical dilution method. The optical density at 600 nm (Uvikon 923, Kontron Instruments, Milan, Italy), and CFU were measured and calibrated against CDM measurements to obtain additional CDM data. A change of one unit of optical density during the active growth phase was shown to be equivalent to an increase of 0.39 g of CDM liter−1 (r2 = 0.979). An increase of 108 CFU ml−1 of L. sakei CTC 494 corresponded with 0.13 g of CDM liter−1 (r2 = 0.876).
Model development.
Equations for cell growth are generally based on the specific growth rate μ (per hour), which dictates the evolution of the biomass concentration [X] (in grams of CDM per liter) over time (t) (in hours):
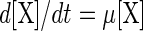 |
1 |
In the early stages of growth, at the end of the lag phase, the specific growth rate μ is at its maximal value (μmax), but it decreases as the biomass concentration increases. Hence, μ equals the maximum specific growth rate μmax multiplied by a dimensionless inhibition function γi:
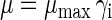 |
2 |
In the case of lactic acid bacteria, the inhibition function γi accounts for the self-inhibition of the cells due to the depletion of sugar and other nutrients and the production of lactic acid. In this study, an attempt was made to represent this growth inhibition as the combined result of the individual γ inhibition functions for lactic acid inhibition γ[HL], sugar limitation γ[S], and nutrient depletion γ[CNS]. In analogy with the γ-concept (34), it was presumed that no interaction effects occur between the individual inhibitory actions, so that
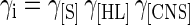 |
3 |
The inhibition function γ[S] is given by the equation of Monod, involving the residual sugar concentration [S] (in grams per liter), and the Monod constant KS (in grams of glucose per liter):
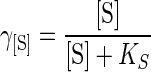 |
4 |
The inhibitory action of lactic acid production (γ[HL]) is due to the toxic effect of the lactic acid molecules produced, the decrease of the pH caused by acid accumulation, and the effect of this decreased pH on the degree of dissociation of the organic acids present in the medium (including acetate and citrate from the MRS broth buffering compounds). If the pH is kept constant, γ[HL] may be simplified as a simple function of the amount of undissociated lactic acid molecules [HL] (in grams per liter), calculated by the equation of Henderson-Hasselbalch. The function further involves a maximum concentration of lactic acid for growth [HL]max (in grams per liter) and a fitting coefficient n (27, 28):
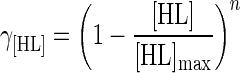 |
5 |
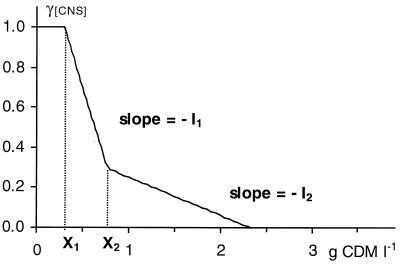
The remaining self-inhibition coefficient, γ[CNS], which is equal to μ [(μmax γ[S] γ[HL])−1], was ascribed to limited availability of nutrients. The actual availability of nutrients equals the initial complex nutrients availability [CNS] minus the consumed amount of nutrients for growth. Due to the complexity of both the nutrient availability and requirements, it is very difficult to obtain a clear kinetic formulation of the growth inhibition as a function of specific, defined compounds. However, since the amount of consumed nutrients is related to the amount of biomass synthesized (2), a relation between γ[CNS] and the produced biomass [X] may be expected. Therefore, the following three-steps inhibition function for nutrient depletion is proposed (Fig. 1):
 |
6 |
 |
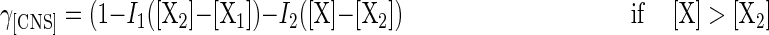 |
The inhibition pattern is composed of a noninhibition phase, and two subsequent phases of decreasing growth rates. The critical biomass concentrations [X1] and [X2] at the start of the two phases of decreasing growth rate, and the inhibition slope coefficients I1 and I2, are dependent on the initial availability of nutrients ([CNS]), as well as the temperature (see Results). In a first step, cells grow exponentially without suffering from nutrient limitations, until a biomass concentration [X1] has been achieved. From then on, at least one unknown nutrient becomes limiting, resulting in a sharp drop of the specific growth rate. At a biomass concentration of [X2] the inhibition slope becomes less steep. At present, the reason for this change in inhibition pattern is not known, but possibly a switch in the metabolism of the cells takes place. Another explanation might be that once the oligopeptides that contain the limiting amino acid are depleted, the cells start taking up the amino acid under its free form, which is thought to be less efficient (2, 16, 33).
FIG. 1.
Modeled growth inhibition of L. sakei CTC 494 due to nutrient depletion (γ[CNS]) as a function of the amount of biomass produced (in grams of CDM per liter) in MRS broth at a controlled temperature of 30°C and pH 6.5. I1 and I2 represent the inhibition slopes.
Depletion of fermentable sugar ([S]) (in grams per liter) and its conversion into lactic acid ([L]) (in grams per liter) may be modeled as follows:
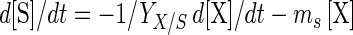 |
7 |
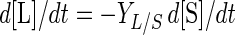 |
8 |
where YX/S is the cell yield coefficient (in grams of CDM per gram of glucose), mS is the maintenance coefficient (in grams of glucose per gram of CDM per hour), and YL/S is the yield coefficient for the production of lactic acid (in grams of lactic acid per gram of glucose). For L. sakei CTC 494, YX/S and mS were shown to be dependent on both the temperature and pH (20). The value of YL/S is, however, not significantly influenced by the environment and equals 1.0 g g−1.
Bacteriocin production by L. sakei CTC 494 is a growth-related process, encompassing simultaneous bacteriocin synthesis and apparent inactivation through adsorption to the producer cells (20, 21). The bacteriocin activity in the cell-free supernatant [B], expressed in mega-arbitrary units (MAU) per liter, is given by the following equation:
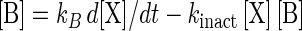 |
9 |
where kB is the specific bacteriocin production (in MAU per gram of CDM), and kinact is the apparent bacteriocin inactivation (in liters per gram of CDM per hour). For an improved fitting of the bacteriocin data, it was useful to introduce a minimum biomass concentration for bacteriocin production ([XB]) (in grams of CDM per liter), below which the value of kB was equal to zero. This makes sense from a physiological point of view, since bacteriocin production by L. sakei CTC 494 is a regulated process which depends on the accumulation of an inducing peptide. At a certain critical cell density enough inducing peptide is present in the environment to switch on bacteriocin production (25).
Modeling procedure.
In a first step, the γ[S] inhibition function (equation 4) was defined by setting KS equal to 0.01 g of glucose liter−1, which is an acceptable value for lactobacilli (15, 28). Next, the γ[HL] inhibition function (equation 5) was calculated by fitting of the μmax data obtained from 100-ml-scale experiments, using small bottles of MRS broth with different concentrations of lactic acid. This γ-function expresses the ratio of μmax to the optimal value of μmax which is obtained when all remaining factors are kept constant. Finally, for each 10-liter fermentation experiment, equations 1, 7, 8, and 9 of the model were integrated with the Euler integration technique in Microsoft Excel (version 7.0) on an IBM-compatible Pentium II computer. The parameters needed for the modeling were estimated by manual adjustment until the best fit was obtained. Previously, the estimation of the various parameters of the model was shown to be a statistically reliable method (20). The lag phase was modeled as a Heaviside function which forces the specific growth rate to zero for the duration of this phase (3, 5). The inhibition function γ[CNS] (equation 6) was equal to the residual growth inhibition that could not be explained by the other inhibition functions.
RESULTS
Inhibition of the growth of L. sakei CTC 494 due to lactic acid production and sugar limitation.
In MRS broth, the homofermentative lactic acid bacterium L. sakei CTC 494 converts 1 mol of glucose to 2 mol of lactic acid.
Upon accumulation, lactic acid becomes more inhibitory to the cells. The theoretical maximum concentration of undissociated lactic acid that still permits cell growth of L. sakei CTC 494 was estimated at ±2.7 g of undissociated lactic acid liter−1, with an adjusting coefficient n equal to 3.1 (equation 5). A total lactic acid concentration of 20 g liter−1 corresponded with an undissociated lactic acid concentration of 0.45 g liter−1 at pH 5.5 and resulted in a growth inhibition of 25% (γ[HL] = 0.75). At pH 6.5, 0.05 g of undissociated lactic acid liter−1 was present, resulting in a growth inhibition of only 6% (γ[HL] = 0.94).
Fresh MRS broth contains 20 g of glucose per liter, available for bacterial growth. If the residual glucose concentration was as low as 1.0 g liter−1, growth rate inhibition due to sugar limitation was not more than 1% (γ[S] = 0.99). Even at 0.1 g liter−1, inhibition did not exceed 9% (γ[S] = 0.91).
Inhibition of the growth of L. sakei CTC 494 due to the exhaustion of nutrients.
The effect of sugar limitation and inhibition by lactic acid production alone could not fully explain the growth inhibition of L. sakei CTC 494 in MRS medium, especially at pH 6.5, where 99.8% of the lactic acid is dissociated. It seemed that the toxic action and/or the limited availability of some other compound(s) was responsible for major growth inhibition. For this reason, experiments were carried out using altered initial concentrations of the complex nutrients source [CNS] of MRS broth. Increase of [CNS] boosted bacterial growth, while a decrease resulted in a reduction of cell growth and biomass yield (Fig. 2, 3, and 4).
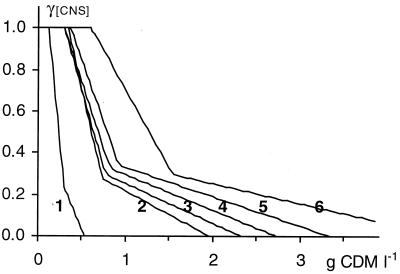
FIG. 2.
Modeled growth inhibition of L. sakei CTC 494 due to nutrient depletion (γ[CNS]) as a function of the amount of biomass produced (in grams of CDM per liter) in tryptone broth at a controlled pH 6.5 and 30°C (1) and in MRS broth at a controlled pH 6.5 and controlled temperature of 35°C (2), 30°C (3), 25°C (4), and 20°C (5), as well as in modified MRS broth with a double complex nutrient concentration at a controlled pH 6.5 and 30°C (6).
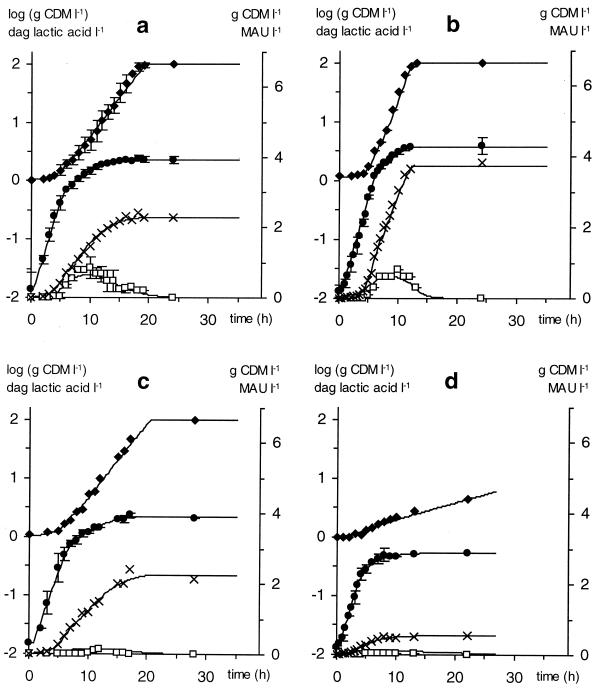
FIG. 3.
Cell growth (×, in grams of CDM per liter; ●, in log [grams of CDM per liter]), lactic acid production (♦, in decagrams per liter), and bacteriocin activity (□, in MAU per liter) for L. sakei CTC 494 in standard MRS broth (a), MRS broth with a double complex nutrient concentration (b), MRS broth with 20 g of NaCl liter−1 (c), and tryptone broth (d). All fermentations were carried out at a controlled temperature of 30°C and pH 6.5. Data points in panel a represent the mean of four repeats. Full lines are according to the model.
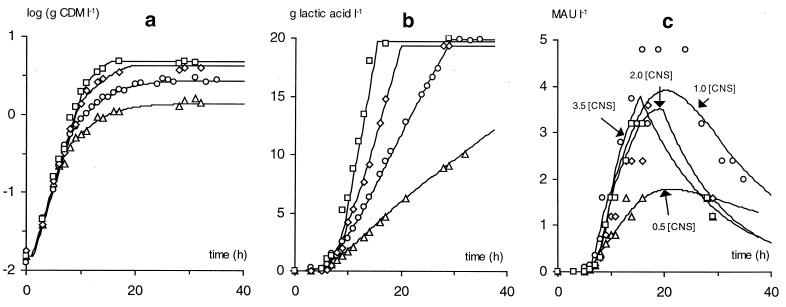
FIG. 4.
Influence of the initial complex nutrient concentration [CNS] (symbols: ▵, 0.5 [CNS]; ○, 1.0 [CNS]; ⋄, 2.0 [CNS]; □, 3.5 [CNS]) on cell growth (a), lactic acid production (b), and bacteriocin activity (c) for L. sakei CTC 494 in (modified) MRS broth at a controlled temperature of 25°C and pH 5.5. Data points for 1.0 [CNS] represent the mean of three repeats. Full lines are according to the model.
A single set of [X1], [X2], I1, and I2 values was able to fit L. sakei CTC 494 fermentations at different salt concentrations (0 and 2% NaCl at 30°C and pH 6.5 [Fig. 3c]; 0, 2, 4, 6, and 8% NaCl at 25°C and pH 5.5 [results not shown]), indicating that salt does not significantly affect the inhibition due to nutrient depletion. Consequently, the amount of nutrients present in the initial growth medium has the potential to construct a certain final biomass regardless of the salt concentration. Moreover, a change in pH from 6.5 to 5.5 did not affect nutrient depletion kinetics at 30°C, nor at 25°C (results not shown). On the other hand, a change in the initial complex nutrients availability ([CNS]) profoundly influenced the values of [X1] and [X2] and of I1 and I2, according to a proportional and an inversely proportional relation, respectively (Fig. 2). This resulted in a strong effect on biomass yields (Fig. 3b and 4). Moreover, high temperatures significantly reduced cell yields. The intersection of the inhibition curve with the abscissa, representing the theoretical final cell concentration, decreased with increasing temperature (Fig. 2). Maximum values of [X1] and [X2] were obtained at 20°C (0.42 and 0.97 g of CDM liter−1, respectively). Hence, the same amount of nutrients was able to produce more biomass at 20°C than at higher temperatures, suggesting that the cell construction mechanism is more efficient at low temperatures.
Substituting meat-based peptone with casein tryptone was clearly not in favor for the growth of the sausage isolate L. sakei CTC 494 (Fig. 3d). If the bacteriological peptone and Lab-Lemco powder of MRS were substituted with equal amounts of casein tryptone, the same maximum specific growth rate was observed (0.85 h−1), but nutrient limitations were much more pronounced. In the latter case, [X1] and [X2] were equal to 0.12 and 0.30 g of CDM liter−1, respectively, whereas I1 and I2 equaled 4.25 and 1.00, respectively.
Comparison of the nutrient depletion model with the logistic growth model for L. sakei CTC 494.
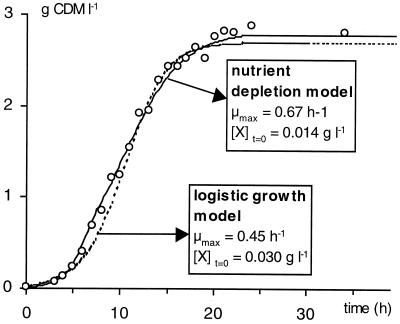
In previous studies, cell growth of L. sakei CTC 494 was described with the logistic equation and was expressed as a function of temperature and pH (20) and as a function of the concentration of salt and nitrite (21). Substituting the logistic equation with the mechanistic nutrient depletion model presented in this study led to significantly higher and more accurate values of μmax. According to the nutrient depletion model, the values of the maximum specific growth rate at 20, 25, 30, and 35°C (constant pH 6.5) were increased from 0.27, 0.45, 0.56, and 0.71 h−1 (20) to 0.41, 0.67, 0.88, and 0.91 h−1, respectively. This resulted in a considerably better fit of the experimental data points from the early exponential growth curve, since, for sufficient fitting of the data set as a whole, the logistic model led to an underestimation of μmax (Fig. 5). Moreover, an overestimated initial biomass concentration had to be assumed (0.030 g of CDM liter−1). For the nutrient depletion model, this value was reduced to 0.014 g of CDM liter−1, which is in perfect agreement with the actual inoculum size (1% [vol/vol], obtained from a culture of L. sakei CTC 494 of 1.3 to 1.5 g of CDM liter−1).
FIG. 5.
Comparison between the logistic growth model and the nutrient depletion model for a fermentation with L. sakei CTC 494 in MRS broth at 25°C and pH 6.5.
Modeling of the bacteriocin production by L. sakei CTC 494.
Initially, at the onset of the fermentation, cells did not produce bacteriocin, but bacteriocin production was switched on at a biomass concentration of [XB]. This concentration equaled 0.10 to 0.20 g of CDM liter−1 for all fermentations (Fig. 3 and 4), except at 35°C, where no bacteriocin activity could be detected during the entire fermentation process. The introduction of the threshold value [XB] into the bacteriocin model led to an improved fitting of the soluble bacteriocin activity data, compared to earlier modeling attempts (20, 21). Bacteriocin activity in the supernatant was dependent on the amount of producing cells but also on the specific bacteriocin production kB, i.e., the production per cell, and the apparent bacteriocin inactivation kinact. As it has been shown previously, the values of kB and kinact are dependent on the environmental conditions temperature, pH, and salt concentration (20, 21). This observation can also be made from the results presented in this study (Fig. 3 and 4). Furthermore, from the nutrient studies at 25°C and pH 5.5 presented in this work, it became evident that the bacteriocin production is also dependent on the complex nutrients concentration (Fig. 4c). An increase in biomass due to higher nutrient availability did not cause a proportional increase of the bacteriocin activity in the supernatant. This was to be explained by a reduction of the specific bacteriocin production at increasing nutrient concentrations. The latter observation was also made at 30°C and pH 6.5 (Fig 3b). Apparently, in a rich environment the production of high amounts of defensive bacteriocin molecules is reduced. On the other hand, a certain minimum nutrient concentration seems to be necessary for optimal bacteriocin activity (Fig. 4c).
DISCUSSION
Sigmoidal growth equations are generally able to describe the growth of lactic acid bacteria. The logistic equation, used in our previous work, enabled a satisfactory description of cell growth by L. sakei CTC 494 (20, 21). However, this approach had some major drawbacks since it leads to an underestimation of the initial growth rate, an overestimation of the initial biomass concentration, and a loss of fit in the early and late stages of the growth curve. Moreover, the logistic model is descriptive and generates little mechanistic insight. Finally, the assumption that growth inhibition is a continuous process does not seem to hold in the case of L. sakei CTC 494, since a sudden shortage of essential nutrients will result in a discontinuous inhibition pattern. In contrast with the logistic growth model, the relation between the decline of the growth rate and the availability of nutrients is not necessarily linear (30).
In this study, a nutrient depletion model was set up as an alternative to the logistic equation, offering a considerably better fit of the experimental growth data. The model consists of a three-step equation, which relates growth inhibition to biomass concentration, as a measure for the exhaustion of nutrients. Previously, the growth of L. sakei CCUG 42687 in a tryptone medium could be divided into four growth phases based on the profile of the acid production rate (1). Substituting the logistic equation with the nutrient depletion model results in an overall model that is no longer purely descriptive, but one that creates the link between the microbial environment and the cell growth of L. sakei CTC 494. The nutrient depletion model distinguishes between the inhibition due to lactic acid production and the growth reduction due to the exhaustion of nutrients. Obviously, the self-inhibition γi of growing L. sakei CTC 494 cells at a certain defined moment of time is related to the biomass concentration at that moment. In the case of the logistic equation, γi is indeed approximated by (1 − X/Xmax), with Xmax being the maximum attainable biomass concentration (in grams of CDM liter−1) for a given set of environmental conditions. This may be understood by the fact that the biomass concentration reflects the amount of lactic acid produced as well as the amount of nutrients consumed. Lactic acid inhibition alone cannot explain the strong reduction in growth rate by L. sakei CTC 494 over time, especially at pH values of 6.5, where very few undissociated lactic acid molecules are present. Likewise, the large amounts of lactic acid produced by a Lactococcus lactis subsp. lactis strain were theoretically responsible for only 6% of the overall growth inhibition in a synthetic MS10 medium (23). In this work, the depletion of complex nutrients was used as the basis to model the growth reduction of L. sakei CTC 494 in MRS broth. An increase of the initial complex nutrients source of MRS broth does not affect the maximum specific growth rate but clearly boosts final biomass yields as well as the production of lactic acid due to a prolonged availability of nutrients. To a lesser extent, the same observation was made for the exopolysaccharide-producing Streptococcus thermophilus LY03 strain (6). Manganese and magnesium, which are important minerals for stimulating the growth of lactic acid bacteria, were shown to be sufficiently available in MRS broth, allowing growth of L. sakei CTC 494 without limitations (F. Leroy and L. De Vuyst, unpublished results). Therefore, the limiting component in MRS broth is likely to be a vitamin, an amino acid, or a peptide.
Despite the limitations in cell growth, the amount of nutrients present in MRS broth favors bacteriocin production by L. sakei CTC 494. Surprisingly, an increase of the cell growth through addition of higher amounts of nutrients does not lead to an increase of maximum bacteriocin levels in the culture supernatant. The increase in the amount of bacteriocin-producing cells was counterbalanced by a substantial decrease of the bacteriocin production per cell. It has been observed previously that the cultivation of a L. lactis subsp. lactis strain with a low nutrient concentration supported a higher relative specific nisin production rate compared with one cultivated with higher nutrient concentrations (17). The total level of nisin could not be raised despite the availability of excess nutrients. Likewise, the specific bacteriocin production of Lactobacillus amylovorus DCE 471 was reduced if the CNS of MRS broth was increased (4). Apparently, cells present in a rich environment reduce their defensive bacteriocin production mechanism, underlying the potential role of bacteriocin production in natural, competitive, and nutrient-depleted ecosystems. A possible reason for the microbial behavior in an artificial cultivation environment is self-protection. High levels of bacteriocin in the cellular environment might inhibit further bacteriocin production because of a limited immunity of the producer cells to their own bacteriocin. In other words, the cells reduce their bacteriocin production in a way such that a certain critical plateau activity is not exceeded, to avoid levels of bacteriocin in the cellular environment that would be toxic to the producer cells. On the other hand, it was noticed that a too-poor environment is harmful for bacteriocin production by L. sakei CTC 494. In this matter, an increase of the yeast extract content of a tryptone medium increased the specific bacteriocin production by L. sakei CCUG 42687 (1), whereas alteration of the nitrogen source of MRS broth did not seem to affect the plantaricin production rate by Lactobacillus plantarum TMW1.25 (18). In view of practical applications, it seems to be of crucial importance that the bacteriocin-producing strain is perfectly adapted to the nutrient environment. L. sakei CTC 494, isolated from naturally fermented sausage (14), produces high amounts of bacteriocin in a meat-like environment (MRS broth), but bacteriocin production completely fails in a casein tryptone medium. The latter medium does not offer a nutrient spectrum that permits good cell growth, nor does it support bacteriocin production. In conclusion, extremely rich environments will not necessarily lead to a gain of bacteriocin activity. An increase of the nutrient availability will increase bacteriocin production due to improved cell growth, but only until a certain plateau value is reached. From then on, bacteriocin activity levels cannot further be increased due to limitations of the bacteriocin production mechanism, for instance immunity. A plateau value for the bacteriocin production by L. sakei CTC 494 was noticed earlier for combinations of pH and temperature (20). There are, however, indications that this characteristic is widespread in bacteriocin-producing lactic acid bacteria (17, 19, 26).
The nutrient depletion model presented in this study, together with the kinetic description of the production of lactic acid and bacteriocin, permits the description of the in vitro behavior of the bacteriocin-producing L. sakei CTC 494 strain. Together with data from earlier presented work (20, 21), these data improve our understanding of the excellent competitive features of L. sakei CTC 494 under sausage fermentation conditions (14). This work will make it possible to develop a semimechanistic, dynamic model to monitor and control sausage fermentation processes. In meat, however, bacteria grow as microcolonies on the surface of meat particles, suggesting the need for spatial models.
ACKNOWLEDGMENTS
We acknowledge financial support from the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research-Flanders (FWO), the Institute for the Encouragement of Scientific and Technological Research in the Industry (IWT) (in particular the STWW-project Functionality of novel starter cultures in traditional fermentation processes), and the European Commission (grant FAIR-CT97-3227). F.L. was supported by a grant from the IWT.
L. sakei CTC 494 was kindly provided by M. Hugas (Institut de Recerca i Tecnología Agroalimentáries, Centre de Tecnología de la Carn, Monells, Spain).
REFERENCES
- 1.Aasen I M, Møretrø T, Katla T, Axelsson L, Storrø I. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol. 2000;53:159–166. doi: 10.1007/s002530050003. [DOI] [PubMed] [Google Scholar]
- 2.Benthin S, Villadsen J. Amino acid utilization by Lactococcus lactis subsp. cremoris FD1 during growth on yeast extract or casein peptone. J Appl Bacteriol. 1996;80:65–72. [Google Scholar]
- 3.Breidt F, Fleming H P. Modeling of the competitive growth of Listeria monocytogenes and Lactococcus lactis in vegetable broth. Appl Environ Microbiol. 1998;64:3159–3165. doi: 10.1128/aem.64.9.3159-3165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callewaert R, De Vuyst L. Bacteriocin production with Lactobacillus amylovorus DCE 471 is improved and stabilized by fed-batch fermentation. Appl Environ Microbiol. 2000;66:606–613. doi: 10.1128/aem.66.2.606-613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornu M, Delignette-Muller M L, Flandrois J-P. Characterization of unexpected growth of Escherichia coli O157:H7 by modeling. Appl Environ Microbiol. 1999;65:5322–5327. doi: 10.1128/aem.65.12.5322-5327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degeest B, De Vuyst L. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl Environ Microbiol. 1999;65:2863–2870. doi: 10.1128/aem.65.7.2863-2870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 8.De Vuyst L, Callewaert R, Pot B. Characterization and antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst Appl Microbiol. 1996;19:9–20. [Google Scholar]
- 9.De Vuyst L, Callewaert R, Crabbé K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 10.de Wit J C, Rombouts F M. Antimicrobial activity of sodium lactate. Food Microbiol. 1989;7:113–120. [Google Scholar]
- 11.Dossmann M U, Vogel R F, Hammes W P. Mathematical description of the growth of Lactobacillus sake and Lactobacillus pentosus under conditions prevailing in fermented sausages. Appl Microbiol Biotechnol. 1996;46:334–339. doi: 10.1007/BF00166226. [DOI] [PubMed] [Google Scholar]
- 12.Gänzle M G, Ehrmann M, Hammes W P. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl Environ Microbiol. 1998;64:2616–2623. doi: 10.1128/aem.64.7.2616-2623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helinck S, Richard J, Juillard V. The effects of adding lactococcal proteinase on the growth rate of Lactococcus lactis in milk depend on the type of enzyme. Appl Environ Microbiol. 1997;63:2124–2130. doi: 10.1128/aem.63.6.2124-2130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugas M, Garriga M, Aymerich M T, Monfort J M. Inhibition of Listeria in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC 494. J Appl Bacteriol. 1995;79:322–330. [Google Scholar]
- 15.Iwasaki K, Nakajima M, Sasahara H. Rapid continuous lactic acid fermentation by immobilised lactic acid bacteria for soy sauce production. Proc Biochem. 1993;28:39–45. [Google Scholar]
- 16.Juillard V, Le Bars D, Kunji E R S, Konings W N, Gripon J-C, Richard J. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol. 1995;61:3024–3030. doi: 10.1128/aem.61.8.3024-3030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim W S, Hall R J, Dunn N W. The effect of nisin concentration and nutrient depletion on nisin production of Lactococcus lactis. Appl Microbiol Biotechnol. 1997;48:449–453. doi: 10.1007/s002530051078. [DOI] [PubMed] [Google Scholar]
- 18.Klostermaier P, Heiko Scheyhing C, Ehrmann M, Vogel R F. Mathematical evaluation of plantaricin formation supports an auto-induced production mechanism. Appl Microbiol Biotechnol. 1999;51:462–469. [Google Scholar]
- 19.Lejeune R, Callewaert R, Crabbé K, De Vuyst L. Modelling the growth and bacteriocin production by Lactobacillus amylovorus DCE 471 in batch cultivation. J Appl Microbiol. 1998;84:159–168. [Google Scholar]
- 20.Leroy F, De Vuyst L. Temperature and pH conditions that prevail during the fermentation of sausages are optimal for the production of the antilisterial bacteriocin sakacin K. Appl Environ Microbiol. 1999;65:974–981. doi: 10.1128/aem.65.3.974-981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leroy F, De Vuyst L. The presence of salt and a curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl Environ Microbiol. 1999;65:5350–5356. doi: 10.1128/aem.65.12.5350-5356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy F, De Vuyst L. Sakacins. In: Naidu N, editor. Natural antimicrobial systems. Boca Raton, Fla: CRC Press LLC; 2000. pp. 589–610. [Google Scholar]
- 23.Loubière P, Cocaign-Bousquet M, Matos J, Goma G, Lindley N D. Influence of end-products inhibition and nutrient limitations on the growth of Lactococcus lactis subsp. lactis. J Appl Microbiol. 1997;82:95–100. [Google Scholar]
- 24.Mercier P, Yerushalmi L, Rouleau D, Dochain D. Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J Chem Tech Biotechnol. 1992;55:111–121. [Google Scholar]
- 25.Nes I F, Diep D B, Håvarstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie van Leeuwenhoek Int. J Gen Mol Microbiol. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 26.Nilsen T, Nes F, Holo H. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC 492. J Bacteriol. 1998;180:1848–1854. doi: 10.1128/jb.180.7.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passos F, Fleming H P, Ollis D F, Hassan H M, Felder R M. Modeling the specific growth rate of Lactobacillus plantarum in cucumber extract. Appl Microbiol Biotechnol. 1993;40:143–150. [Google Scholar]
- 28.Passos F, Fleming H P, Ollis D F, Felder R M, McFeeters R F. Kinetics and modeling of lactic acid production by Lactobacillus plantarum. Appl Environ Microbiol. 1994;60:2627–2636. doi: 10.1128/aem.60.7.2627-2636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Presser K A, Ratkowsky D A, Ross T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl Environ Microbiol. 1997;63:2355–2360. doi: 10.1128/aem.63.6.2355-2360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiwu C, Lawson G J. Study on models of single populations: an expansion of the logistic and exponential equations. J Theor Biol. 1982;98:645–659. [Google Scholar]
- 31.Ray B, Sandine W E. Acetic, propionic and lactic acids of starter culture bacteria as biopreservatives. In: Ray B, Daeschel M, editors. Food biopreservatives of microbial origin. Boca Raton, Fla: CRC Press LLC; 1992. pp. 103–136. [Google Scholar]
- 32.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Niel E W J, Hahn-Hägerdal B. Nutrient requirements of lactococci in defined growth media. Appl Microbiol Biotechnol. 1999;52:617–627. [Google Scholar]
- 34.Wijtzes T, de Wit J C, Huis in 't Veld J H J, van 't Riet K, Zwietering M H. Modelling bacterial growth of Lactobacillus curvatus as a function of acidity and temperature. Appl Environ Microbiol. 1995;61:2533–2539. doi: 10.1128/aem.61.7.2533-2539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwietering M H, Jongenburger I, Rombouts F M, van 't Riet R. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]