Abstract
A predominant protein of human eosinophils is galectin-10 (Gal-10), also known as Charcot-Leyden crystal protein (CLC-P) because of its remarkable ability to form Charcot-Leyden crystals (CLCs), which are frequently found in tissues from patients with eosinophilic disorders. CLC-P/Gal-10 is highly expressed in human eosinophils and considered a biomarker of eosinophil involvement in inflammation. However, the intracellular sites where large pools of CLC-P/Gal-10 constitutively reside are still unclear, and whether this protein is derived or not from eosinophil granules remains to be established. Here, we applied pre-embedding immunonanogold transmission electron microscopy combined with strategies for optimal antigen and cell preservation and quantitative imaging analysis to investigate, for the first time, the intracellular localization of CLC-P/Gal-10 at high resolution in resting and activated human eosinophils. We demonstrated that CLC-P/Gal-10 is mostly stored in the peripheral cytoplasm of human eosinophils, being accumulated within an area of ~250 nm wide underneath the plasma membrane and not within specific (secretory) granules, a pattern also observed by immunofluorescence. High-resolution analysis of single cells revealed that CLC-P/Gal-10 interacts with the plasma membrane with immunore-active microdomains of high CLC-P/Gal-10 density being found in ~60% of the membrane area. Eosinophil stimulation with CCL11 or TNF-α, which are known inducers of eosinophil secretion, did not change the peripheral localization of CLC-P/Gal-10 as observed by both immunofluorescence and immuno-EM (electron microscopy). Thus, in contrast to other preformed eosinophil proteins, CLC-P/Gal-10 neither is stored within secretory granules nor exported through classical degranulation mechanisms (piecemeal degranulation and compound exocytosis).
Keywords: degranulation, eosinophilic diseases, galectins, inflammation, transmission electron microscopy
1 |. INTRODUCTION
Eosinophil infiltration is a hallmark of many inflammatory diseases and conditions such as asthma and allergic rhinitis.1–3 A common observation at sites of eosinophilic inflammation in tissues, body fluids, and secretions, is the presence of Charcot-Leyden crystals (CLCs), morphologically diverse crystals, which have been associated with eosinophils for more than 100 yr (reviewed in Su4 and Ueki et al.5).
The intriguing relationship between CLCs and eosinophils has been the object of great clinical and scientific interest. Since the first studies establishing that CLCs were crystallized protein, many questions have been raised about the biochemical nature of this protein, originally named CLC-protein (CLC-P), and the ability of human eosinophils to store and secrete it.4 Our group has formerly identified CLC-P as a protein with lysophospholipase activity.6 Structural and genomic similarities between CLC-P and the carbohydrate-binding galectin superfamily have led to its reclassification as a galectin termed galectin-10 (CLC-P/Gal-10).7 CLC-P/Gal-10 can bind to mannose,8 but other ligands for this protein including complex glycoconjugates remain to be determined. Of note, CLC-P/Gal-10 has been exclusively described in human eosinophils, basophils, and regulatory T cells with no evidence for the expression of this protein in the murine system.9
Comprehensive proteomic analyses found that CLC-P/Gal-10 is one of the most abundant proteins within eosinophils from healthy donors.10,11 More recently, we demonstrated that substantial amounts of CLC-P/Gal-10 are released under active eosinophil cytolysis and that increased extracellular concentration of CLC-P/Gal-10 is capable to form CLCs.12
Whereas the extracellular levels of CLC-P/Gal-10 have currently received high attention as a biomarker for eosinophilic diseases2,13 or even potential targets for drugs,14 the intracellular localization of this protein in human eosinophils remains enigmatic. In earlier ultrastructural studies, we found CLC-P to be localized in a minor population of cytoplasmic large vacuoles from mature eosinophils, which were considered core-free “primary granules,” but not in specific (cored) granules.15,16 However, these data are conflicting with the high concentration of CLC-P/Gal-10 existent in human eosinophils.10,11 These cells in normal conditions contain only a single population of specific granules and true core-free (immature) granules, which are rarely found in mature eosinophils, cannot account for such amount of CLC-P/Gal-10.17 Therefore, the intracellular sites where large pools of CLC-P/Gal-10 constitutively reside are still unclear, and whether this protein is contained or not within eosinophil granules remains to be established.
Here, we aimed to investigate the immunolocalization of CLC-P/Gal-10 at high resolution in resting human eosinophils as well as in eosinophils stimulated with agonists to induce degranulation events through piecemeal degranulation (PMD) or compound exocytosis.
2 |. MATERIAL AND METHODS
2.1 |. Eosinophil isolation, viability, and stimulation
Eosinophils were isolated from the peripheral blood of healthy donors by negative selection.18 Eosinophil viability and purity were >99%. Purified eosinophils (106 cells/mL) were stimulated with TNF-α (200 ng/mL; R&D Systems: Minneapolis, MN, USA) or recombinant human CCL11 (100 ng/mL; R&D Systems) in RPMI-1640 medium plus 0.1% ovalbumin (Sigma: St. Louis, MO, USA), or medium alone at 37°C, for 1 h.19 In parallel studies, cells were stimulated with PMA (10 ng/mL; Sigma) at 37°C for 15 min.
2.2 |. Ethics statement
This study was carried out in accordance with the ethical principles taken from the Declaration of Helsinki and written informed consent was obtained from all donors under Institutional Review Board approved protocols.
2.3 |. Immunostaining of biopsy samples and isolated eosinophils
Surgically obtained nasal polyps from 8 patients with severe eosinophilic chronic rhinosinusitis (ECRS, Supporting Information material 1), diagnosed as described,20 were stained for CLC/Gal-1012 (Supporting Information material 1). Whole slides were evaluated by a skilled technician (Noriko Tan) and CLCs defined as CLC-P/Gal-10-positive typical bipyramidal shape in the interstitial tissues. Immunostaining procedures of isolated eosinophils and colocalization studies (CLC-P/Gal-10 and major basic protein 1 [MBP-1])12 as well as image acquisition (conventional and super-resolution microscopy) are described in Supporting Information material 1. The intracellular expression of CLC-P/Gal-10 was assessed with flow cytometry (Supporting Information material 1).
2.4 |. Conventional and pre-embedding immunonanogold electron microscopy (EM)
ECRS tissue samples were fixed in 1% paraformaldehyde and 1.25% glutaraldehyde in 1 M sodium cacodylate buffer (4 h, room temperature [RT]) and prepared for conventional transmission electron microscopy (TEM) as before.21 Immunonanogold EM was carried out on entire permeabilized cells or cryosections before standard EM processing. Cell preparation for immunonanogold EM and all labeling steps22 are detailed in Supporting Information material 2. Samples were viewed with a transmission electron microscope (Tecnai G2 Spirit, FEI/Thermo Fisher: Waltham, MA, USA) at 80 KV.
2.5 |. Quantitative imaging analysis
For all quantification studies, randomly taken electron micrographs of eosinophil sections showing the entire cell profile and nucleus (n = 101) were evaluated. Images (tiff files uncompressed 8-bit gray-scale with 366 DPI) were processed and analyzed using Fiji software (National Institutes of Health, Bethesda, MD, USA) as detailed in Supporting Information material 2.
2.6 |. Statistical analyses
The Student’s t test or 2-way ANOVA followed by Tukey’s multiple comparisons test were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA).
3 |. RESULTS AND DISCUSSION
CLCs are frequently formed in diseases such as ECRS associated with tissue eosinophilia (Fig. 1A).12,14 To evaluate the presence of CLC-P/Gal-10-immunorreactive CLCs in vivo, biopsy samples from patients with ECRS (Supporting Information material 1) were analyzed by immunofluorescence. Free CLC-P/Gal-10-stained CLCs with typical morphology were identified at tissue sites of eosinophil infiltration (Fig. 1B–D) in 6 out 8 patients (75%). These data confirm that CLCs formed in vivo are composed of CLC-P/Gal-10, as previously observed.12,14
FIGURE 1. Tissue Charcot-Leyden crystals (CLCs) observed in biopsies of allergic patients.

(A) A representative electron micrograph shows bypiramidal and hexagonal CLCs in an area with cytolytic eosinophils. Chromatolytic nuclei (N) and free eosinophil extracellular granules (arrowheads) are observed. Samples were prepared for conventional transmission electron microscopy. (B) Typical CLCs immunolabeled for CLC-P/Gal-10 (green) are indicated (arrows). (C) CLC-P/Gal-10-positive CLCs (arrows) are seen in higher magnification. (D) Control isotype antibody-treated tissue. Nuclei are labeled blue with Hoechst 33342 in (B-D). Data are representative of 6 patients. Surgically obtained nasal polyps with severe eosinophilic chronic rhinosinusitis (ECRS) were diagnosed as described.20 Subject information is provided in Supporting Information material 1
Having detected extracellular CLC-P/Gal-10-immunoreactive CLCs in clinically relevant biopsies, the same antibody was then applied to in vitro studies to investigate the intracellular content of CLC-P/Gal-10 in human eosinophils isolated from the peripheral blood. First, resting permeabilized eosinophils were evaluated by flow cytometry and immunofluorescence. These cells showed a large intracellular pool of Gal-10 (Fig. 2A) and this protein was mostly observed in the eosinophil peripheral cytoplasm (Fig. 2B). When a double staining for both CLC-P/Gal-10 and MBP-1, a classical marker for eosinophil specific (secretory) granules,17 was performed, no marked colocalization was observed (Fig. 2C).
FIGURE 2. Intracellular localization of Charcot-Leyden crystal protein (CLC-P)/Gal-10 in human resting eosinophils.
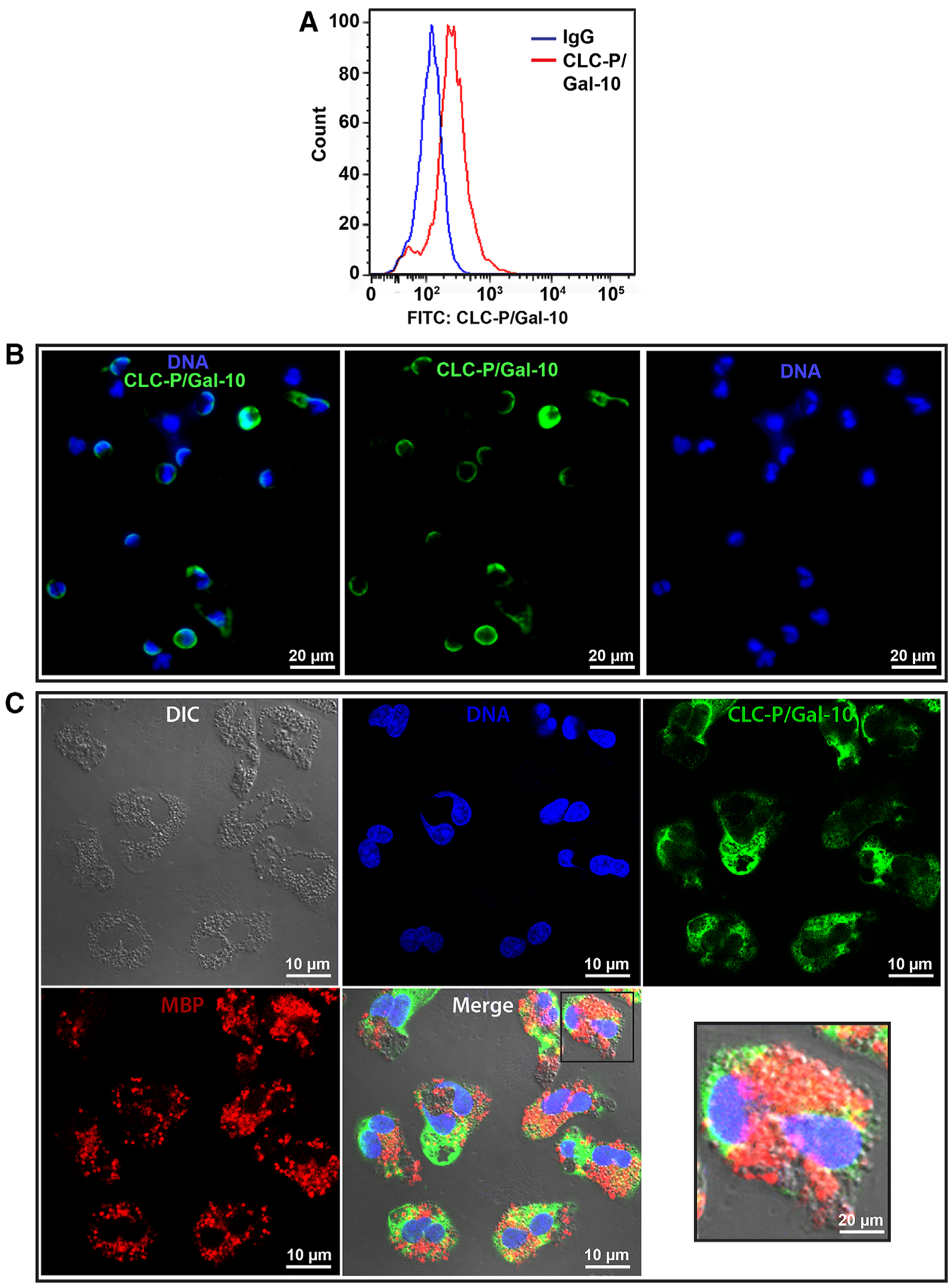
(A) Representative histogram from flow cytometric analyses. (B) Immunofluorescence staining shows peripheral labeling of CLC-P/Gal-10 (green) within eosinophils. Nuclei are labeled blue. (C) Identical fields of eosinophils imaged with differential interference contrast (DIC) microscopy to accentuate the population of specific granules and fluorescence microscopy for DNA (blue), CLC-P/Gal-10 (green), and MBP-1 (red). Note that CLC-P/Gal-10 and MBP-1 are not colocalized. Data are representative of 6 subjects. Freshly isolated eosinophils were fixed and permeabilized before immunolabeling
Next, we applied pre-embedding immunonanogold EM for precise high-resolution imaging of the intracellular locations of CLC-P/Gal-10. This method combines several strategies for ultrastructure and antigen preservation in conjunction with robust blocking of nonspecific binding sites and improved antibody penetration22 and has been used to understand the intracellular localization of cytokines and other immune mediators in human eosinophils (reviewed in Melo and Weller23). Immunolabeling is performed before all EM procedures because antigen preservation may be hampered by conventional processing for EM such as dehydration and resin embedding, resulting in weak or negative labeling.22 Visualization is accomplished with very small gold particles with just 1.4 nm diameter covalently conjugated with Fab’ fragments, which are only one third the size of a whole IgG molecule, thus improving antibody penetration.18,22
Ultrastructural immunolabeling for CLC-P/Gal-10 was performed first in intact permeabilized eosinophils immediately after purification. TEM showed the characteristic ultrastructure of eosinophils, with a major population of intact specific granules exhibiting their unique morphology—an internal often electron-dense crystalline core and outer electron-lucent matrix surrounded by a delimiting membrane (Fig. 3). Our immunonanogold approach in accord with immunofluorescent data (Fig. 2) revealed, for the first time at nanoscale level using a monoclonal antibody against Gal-10, that CLC-P/Gal-10 accumulates in the eosinophil peripheral cytoplasm, under the plasma membrane and not within secretory granules (Fig. 3). This pattern was very consistent being observed in 100% of all randomly analyzed cell sections showing the entire cell profile and nucleus (n = 37). Because permeabilization might hypothetically affect the antibody ability to access deeper cytoplasmic areas including the highly compacted, full of contents, membrane-bound specific granules, we next used a different strategy to label CLC-P/Gal-10 in unstimulated eosinophils. We performed immunolabeling on cryosections of eosinophils so that the antibody could be applied directly on sectioned granules.22 Our results showed the same pattern of immunolabeling, confirming that cytoplasmic pools of preformed CLC-P/Gal-10 mostly resides in the peripheral cytosol and that granules are not storage sites for this protein (Fig. S1). Cells in which the primary antibody was replaced by an irrelevant antibody were negative (Fig. S1).
FIGURE 3. Charcot-Leyden crystal protein (CLC-P)/Gal-10 is predominantly stored in the eosinophil peripheral cytoplasm and not within granules.

(A, B) Note a substantial pool of CLC-P/Gal-10 underneath the plasma membrane detected by immunonanogold electron microscopy. Secretory granules (Gr) showing typical morphology with and electron-dense core and electron-lucent matrix are completely negative for CLC-P/Gal-10. Ultrastructural immunolabeling for CLC-P/Gal-10 was applied to intact eosinophils immediately after isolation from the blood. Cells were fixed and permeabilized before immunolabeling. N, nucleus
To extract quantitative information, imaging analysis was applied to resting eosinophils isolated from 3 patients and ultrastructurally immunolabeled for CLC-P/Gal-10. Our findings demonstrated that the majority of intracellular CLC-P/Gal-10 concentrates in an area of the eosinophil peripheral cytoplasm, measuring ~250 nm wide from the plasma membrane (Fig. 4A and B). By comparing CLC-P/Gal-10 ultrastructural immunolabeling performed in intact (permeabilized) and sectioned cells, we found the same pattern of peripheral localization in all cells evaluated after these two procedures with 80% of all cytosolic immunolabeling for CLC-P/Gal-10 accumulated within this narrow band at the cell periphery (Fig. 4C). We also quantitated the density of CLC-P/Gal-10 labeling per square micrometer of cytoplasm. Our results clearly demonstrated higher density at cell periphery (Fig. 4D). Interestingly, our high-resolution analysis of single cells identified that CLC-P/Gal-10 not only concentrates under the plasma membrane but also interacts with it (Fig. 5A, B, and Bi). CLC-P/Gal-10-immunoreactive microdomains with high density were distributed at the plasma membrane being found 1.04 ± 0.16 (mean ± SEM) microdomains per linear micrometer of plasma membrane (Fig. 5). This means that CLC-P/Gal-10 in human eosinophils is functionally linked to the plasma membrane. This is in accord with the intracellular distribution of other galectin members, which also accumulate under the plasma membrane and are able to interact with membrane lipids in other cells (reviewed in Popa et al.24). Our data also support earlier findings upon subcellular fractionation of eosinophils, in which CLC-P colocalized with the eosinophil membrane fraction and not with the granule fraction.6 However, the functional significance of such association is still unknown. In eosinophils, this interaction maybe particularly important to understand how preformed pools of CLC/Gal-10 are kept in the cytoplasm and promptly released/crystalized in activated cells undergoing plasma membrane disruption (cytolysis).12 The high concentration of CLC-P/Gal-10 in the peripheral eosinophil cytoplasm, prompt to be released, might explain the high levels of Gal-10 found in the sputum of patients with asthma and allergic bronchopulmonary aspergillosis.2 Moreover, the subplasmalemmal localization of CLC-P/Gal-10 may facilitate its roles on regulating cell surface secretion mediated by regulatory palmitoylation, as we consider in a companion manuscript.25
FIGURE 4. Quantitative imaging analysis of Charcot-Leyden crystal protein (CLC-P)/Gal-10 accumulation within human resting eosinophils.

(A, B) Representative sections of eosinophils highlighting the peripheral cytoplasmic area (250 nm wide from the plasma membrane) in which most CLC-P/Gal-10 is concentrated. The entire cell profiles are shown in (Ai and Bi) with gold particles highlighted in blue. Note absence of granule immunoreactivity for CLC-P/Gal-10. (C, D) Quantitative analyses comparing immunolabeling for CLC-P/Gal-10 performed on intact cells and sectioned cells revealed the same pattern of protein localization at cell periphery. Data are representative of 3 donors. A total of 37 cells was analyzed using Fiji software. ****P < 0.0001 vs. inner cell
FIGURE 5. Charcot-Leyden crystal protein (CLC-P)/Gal-10 microdomains with high density are distributed at the eosinophil plasma membrane.

(A) Arrowheads indicate plasma membrane regions (microdomains) of resting eosinophils in which CLC-P/Gal-10 is concentrated. (B and Bi) The membrane area occupied by these microdomains (highlighted in green in Bi) was measured. (C) Quantitative imaging analyses revealed that 60% of the plasma membrane contains high-density-CLC-P/Gal-10 microdomains with a mean of one microdomain localized in each linear micrometer of this membrane. Note in (A) that some scattered labeling on the nucleus (N). Data are representative of 3 patients. A total of 37 cells showing the entire cell profile and nucleus was analyzed using Fiji software
In earlier ultrastructural studies, we found that CLC-P was completely absent from specific granules of human eosinophils obtained from different origins (blood, tissue, and cultures) whereas cytoplasmic, granule-poor areas, including areas beneath the plasma membrane were extensively labeled.15,26–28 This pattern did not change regardless of the methodology used for cell processing. For example, eosinophils evaluated after purification from the blood or directly in blood buffy coats,15 cultures,27 or biopsies26 had the same pattern of immunolabeling.
On the other hand, we also reported immunolabeling on a minimal (~5%) population of large vacuoles considered coreless granules (“primary granules”).15,16 Additional former ultrastructural work using mature and immature eosinophils found that both core-containing granules and coreless granules were mainly negative for CLC-P.29 Therefore, regardless of the eosinophil source or maturation stage, CLC-P was always absent from specific granules with a minor population of coreless granules (<5%) being found positive for CLC-P.15,26–28 These previous works also demonstrated that CLC-P was predominantly cytosolic. Calafat and colleagues raised several concerns about the presence of CLC-P within a limited number of eosinophil granules.29 “How to explain that few granules contain such a high density of CLC-P?”29 However, subsequent to these initial works during the 1990s, no attention was given to the subcellular localization of CLC-P within human eosinophils. With the contemporary recognitions that human eosinophils do not contain “primary granules” but just a single population of specific granules,17 and that CLC-P/Gal-10 is clearly not stored within granules, as demonstrated here, it is conceivable that the “primary granules” originally positive for CLC-P represent in fact distinct membrane compartments from intracellular pathways, such as the endocytic pathway. It is documented that galectins can reenter different cells by endocytosis30 and are able to populate endocytic and recycling compartments.31,32 Moreover, cytosolic galectins rapidly accumulate in disrupted endocytic compartments due to their ability of binding to their ligands (glycans), which are normally present in the lumen of these endocytic vacuoles, but under disruption, are exposed to the cytosol (reviewed in Johannes et al.33). This event has been demonstrated for Gal-3 and Gal-8 after endocytosis of pathogens in vacuoles that undergo lysis or in disrupted endosomes (reviewed in Johannes et al.33). To test the hypothesis that CLCP/Gal-10 accumulates in endocytic vacuoles in human eosinophils, we treated these cells for 15 minutes with PMA, which causes rapid formation of plasma membrane-derived vacuoles in the cytoplasm of leukocytes and macrophages.34–36 As expected, PMA activation, although it did not change the predominant CLC-P/Gal-10 localization at the cell periphery, led to accentuated eosinophil vacuolization with endocytic vacuoles consistently labeled for CLC-P/Gal-10 (Fig. S2). Of note, the population of specific granules including enlarged, activated granules was not labeled for CLC-P/Gal-10 after PMA treatment (Fig. S2). Thus, we can assume that CLC-P/Gal-10-positive vacuoles occasionally seen in the eosinophil cytoplasm in the present work (Fig. S3) or in our former studies (“primary granules”) are likely endocytic compartments.
Finally, we next addressed whether CLC-P/Gal-10 locations in the cytoplasm could be affected by eosinophil degranulation. We wondered if stimulation with agonists would change the CLC-P/Gal-10 distribution or direct CLC-P/Gal-10 to the secretory granules. We then performed ultrastructural immunolabeling of eosinophils after stimulation with CCL11 or TNF-α, which are known inducers of eosinophil activation and secretion37–39 leading to eosinophil secretion through different mechanisms—PMD or compound exocytosis, respectively.19 Eosinophil activation with these stimuli did not change the cytoplasmic localization of CLC-P/Gal-10 as observed by both immunofluorescence, including super-resolution microscopy (Fig. 6A) and immunoEM (Fig. 6B). Protrusive structures such as filopodia and uropods observed at the eosinophil surface (shape changes), a feature of leukocyte activation and secretion,40 were remarkably labeled for CLC-P/Gal-10 whereas secretory granules remained negative (Fig. 6C and Fig. S3). Quantitative imaging analyses of cell sections obtained from 3 patients demonstrated that more than 80% of all cytoplasmic CLC-P/Gal-10 immunolabeling was kept at cell periphery after stimulation, within a 250 nm band wide from the plasma membrane (Fig. 6D). Yet, when the density of gold particles was quantitated per square micrometer of cytoplasm, labeling was clearly concentrated at this peripheral region (Fig. 6E), as observed for resting eosinophils (Fig. 4D) and not associated with secretory granules. The distribution of CLC-P/Gal-10 in the entire cytoplasm in nonstimulated and stimulated cells is also shown in Figure S4. The majority of the immunolabeling, as noted, was observed in the cytosol whereas nuclei and vacuoles displayed less positivity (Fig. S4). Our data using CCL11 and TNF-α as stimuli indicate that CLC-P/Gal-10 is not directed to specific granules nor exported through degranulation mechanisms involving PMD or compound exocytosis.
FIGURE 6. Eosinophil activation with degranulating stimuli did not change the intracellular localization of Charcot-Leyden crystal protein (CLC-P)/Gal-10.

(A) Imunofluorescence of stimulated or not human eosinophils shows the same pattern of CLC-P/Gal-10 immunolocalization (green) at cell periphery. (B, Bi) A representative electron micrograph of a CCL11-stimulated eosinophil showing Gal-10 accumulation underneath the plasma membrane. Note a filopodium (arrow) with many CLC-P/Gal-10 clusters. Immunolabeling was highlighted in blue in (Bi). (C) A surface protrusion (uropod, arrow) from a TNF-α-stimulated eosinophil is densely labeled for CLC-P/Gal-10. Granule fusions (*), typical of compound exocytosis, were induced by cell activation. Note in (B and C) that CLC-P/Gal-10 is concentrated in the peripheral cytoplasm but not within secretory granules. Eosinophils isolated from the blood were stimulated or not with CCL11 or TNF-α for 1 h and processed for immunofluorescence or immunonanogold electron microscopy. A total of 111 electron micrographs showing the entire cell profile and nucleus (N) was analyzed using Fiji software. Data are representative of 3 patients. ****P < 0.0001 vs. inner cell
In previous works from our group, CLC-P ultrastructural localization was also investigated in activated eosinophils. For example, in eosinophils that developed in cultures supplemented with recombinant human IL 5 (rhIL-5), we found that all mature eosinophils were activated and displayed PMD.27 Specific granules and their emptied containers did not contain any label for CLC-P. These actively secreting eosinophils displayed substructural sites of CLC-P identical to those that we have described in activated tissue eosinophils (skin, hypereosinophilic syndrome [HES]).26 Altogether, our current and previous works are consistent in demonstrating that CLC-P/Gal-10 is not a protein localized in secretory granules, either within resting or activated intact eosinophils.
Our findings support a current consensus that galectins members are synthesized as cytosolic proteins, reside in the cytosol or nucleus for much of their lifetime, and are exported from cells without direct movement through the classical secretory pathway.24 Indeed, galectins lack typical amino-terminal signal peptides and therefore are not fed into the endoplasmic reticulum/Golgi complex, staying in the cytosol as a soluble pool.24 However, if small amounts of CLC-P/Gal-10 are released directly across the plasma membrane, for example, through extracellular vesicle formation (microvesicles), in response to agonists, remains to be determined. On the other hand, recent studies have identified massive release of CLC-P/Gal-10 and crystallization into CLCs associated with specific mechanisms of active cytolysis and formation of extracellular traps (ETosis) by human eosinophils.12,14
In summary, the present work unveils the intracellular localization of CLC-P/Gal-10 in human eosinophils as a preformed cytosolic protein residing in the peripheral cytoplasm in association with the plasma membrane and not localized within granules neither exported through degranulation events involving PMD nor compound exocytosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health (NIH), USA, grants R37AI020241 and R01AI022571; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), grants 309734/2018-5 and 434914/2018-5); Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil), grant CBB-APQ-03647-16; Japan Agency for Medical Research and Development (Grant 19ek0410055); the Mochida Memorial Foundation for Medical and Pharmaceutical Research; and the Japanese Society of Laboratory Medicine Fund for Promotion of Scientific Research. The authors are grateful to Noriko Tan for outstanding technical assistance. We apologize to investigators whose relevant work has not been cited because of space constraints.
Abbreviations:
- CLC-P
Charcot-Leyden crystal protein
- ECRS
eosinophilic chronic rhinosinusitis
- EM
electron microscopy
- Gal-10
galectin-10
- HES
hypereosinophilic syndrome
- MBP1
major basic protein 1
- PMD
piecemeal degranulation
- rhIL-5
recombinant human IL 5
- RT
room temperature
- TEM
transmission electron microscopy
Footnotes
SUPPORTING INFORMATION
Additional information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Ghafouri B, Irander K, Lindbom J, Tagesson C, Lindahl M. Comparative proteomics of nasal fluid in seasonal allergic rhinitis. J Proteome Res. 2006;5:330–338. [DOI] [PubMed] [Google Scholar]
- 2.Chua JC, Douglass JA, Gillman A, O’Hehir RE, Meeusen EN. Galectin-10, a potential biomarker of eosinophilic airway inflammation. PLoS One. 2012;7:e42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su J A brief history of Charcot-Leyden crystal protein/galectin-10 research. Molecules. 2018;23:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueki S, Miyabe Y, Yamamoto Y, et al. Charcot-Leyden crystals in eosinophilic inflammation: active cytolysis leads to crystal formation. Curr Allergy Asthma Rep. 2019;19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller PF, Goetzl EJ, Austen KF. Identification of human eosinophil lysophospholipase as the constituent of Charcot-Leyden crystals. Proc Natl Acad Sci U S A. 1980;77:7440–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerman SJ, Corrette SE, Rosenberg HF, et al. Molecular cloning and characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). Similarities to IgE binding proteins and the S-type animal lectin superfamily. J Immunol. 1993;150:456–468. [PubMed] [Google Scholar]
- 8.Swaminathan GJ, Leonidas DD, Savage MP, Ackerman SJ, Acharya KR. Selective recognition of mannose by the human eosinophil Charcot-Leyden crystal protein (galectin-10): a crystallographic study at 1.8 a resolution. Biochemistry. 1999;38:15406. [DOI] [PubMed] [Google Scholar]
- 9.Kubach J, Lutter P, Bopp T, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110: 1550–1558. [DOI] [PubMed] [Google Scholar]
- 10.Straub C, Pazdrak K, Young TW, et al. Toward the proteome of the human peripheral blood eosinophil. Proteomics Clin Appl. 2009;3: 1151–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkerson EM, Johansson MW, Hebert AS, et al. The Peripheral blood eosinophil proteome. J Proteome Res. 2016;15:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueki S, Tokunaga T, Melo RCN, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018;132:2183–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Re V, Simula MP, Cannizzaro R, et al. Galectin-10, eosinophils, and celiac disease. Ann N Y Acad Sci. 2009;1173:357–364. [DOI] [PubMed] [Google Scholar]
- 14.Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science. 2019;364: aaw4295. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak AM, Letourneau L, Login GR, Weller PF, Ackerman SJ. Ultrastructural localization of the Charcot-Leyden crystal protein (lysophospholipase) to a distinct crystalloid-free granule population in mature human eosinophils. Blood. 1988;72:150–158. [PubMed] [Google Scholar]
- 16.Dvorak AM, Letourneau L, Weller PF, Ackerman SJ. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) to intracytoplasmic crystals in tumor cells of primary solid and papillary epithelial neoplasm of the pancreas. Lab Invest. 1990;62:608–615. [PubMed] [Google Scholar]
- 17.Melo RCN, Weller PF. Contemporary understanding of the secretory granules in human eosinophils. J Leukoc Biol. 2018;104:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmo LAS, Bonjour K, Spencer LA, Weller PF, Melo RCN. Single-cell analyses of human eosinophils at high resolution to understand compartmentalization and vesicular trafficking of interferon-gamma. Front Immunol. 2018;9:1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmo LAS, Bonjour K, Ueki S, et al. CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J Leukoc Biol. 2016;100:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC study. Allergy. 2015;70:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo RCN, Spencer LA, Perez SA, et al. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melo RCN, Morgan E, Monahan-Earley R, Dvorak AM, Weller PF. Pre-embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nature Protocols. 2014;9:2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melo RCN, Weller PF. Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy. Exp Cell Res. 2016;347:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa SJ, Stewart SE, Moreau K. Unconventional secretion of annexins and galectins. Semin Cell Dev Biol. 2018;83:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller PF, Wang H, Melo RCN. The Charcot-Leyden crystal protein revisited—a lysopalmitoylphospholipase and more. J Leuk Biol. 2020;108:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorak AM, Weller PF, Monahan-Earley RA, Letourneau L, Ackerman SJ. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab Invest. 1990;62:590–607. [PubMed] [Google Scholar]
- 27.Dvorak AM, Furitsu T, Letourneau L, Ishizaka T, Ackerman SJ. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- 29.Calafat J, Janssen H, Knol EF, Weller PF, Egesten A. Ultrastructural localization of Charcot-Leyden crystal protein in human eosinophils and basophils. Eur J Haematol. 1997;58:56–66. [DOI] [PubMed] [Google Scholar]
- 30.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289:845–850. [DOI] [PubMed] [Google Scholar]
- 31.Stechly L, Morelle W, Dessein AF, et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 2009;10:438–450. [DOI] [PubMed] [Google Scholar]
- 32.Straube T, von Mach T, Honig E, Greb C, Schneider D, Jacob R. pH-dependent recycling of galectin-3 at the apical membrane of epithelial cells. Traffic. 2013;14:1014–1027. [DOI] [PubMed] [Google Scholar]
- 33.Johannes L, Jacob R, Leffler H. Galectins at a glance. J Cell Sci. 2018;131: jcs208884. [DOI] [PubMed] [Google Scholar]
- 34.Repine JE, White JG, Clawson CC, Holmes BM. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974;54:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phaire-Washington L, Wang E, Silverstein SC. Phorbol myristate acetate stimulates pinocytosis and membrane spreading in mouse peritoneal macrophages. J Cell Biol. 1980;86:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsson A, Nixon JB, McPhail LC. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. J Leukoc Biol. 2000;67:396–404. [DOI] [PubMed] [Google Scholar]
- 37.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179: 4840–4848. [DOI] [PubMed] [Google Scholar]
- 38.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. [DOI] [PubMed] [Google Scholar]
- 39.Spencer LA, Szela CT, Perez SA, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fais S, Malorni W. Leukocyte uropod formation and membrane/cytoskeleton linkage in immune interactions. J Leukoc Biol. 2003;73:556–563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


