ABSTRACT
Lophomoniasis is an emerging protozoan disease that mostly involves the lungs. Because the disease has similar symptoms to other pulmonary infections such as tuberculosis, most cases are underdiagnosed. Here, we present a patient with co-infection of Lophomonas/Mycobacterium.
INTRODUCTION
Tuberculosis (TB) is an ancient disease that has affected humanity for over 4000 years [1]. A total of 1.5 million people died of TB in 2020 (including 214 000 HIV-positive people). TB is the 13th leading cause of global death and the second leading reason for infection-related deaths, following the coronavirus disease of 2019 (COVID-19; above HIV/AIDS) [2]. The most frequently affected organ systems include the respiratory system, the gastrointestinal (GI) system, the lymphoreticular system, the central nervous system, the skin, the musculoskeletal system, the reproductive system and the liver [3]. Pulmonary TB has typical clinical characteristics, including chronic cough, sputum production, loss of appetite, weight loss, fever, night sweats and hemoptysis [4].
Lophomonas is a protozoan that frequently lives as a commensal agent in the guts of some insects, such as cockroaches and termites [5]. However, this emerging pathogenic protozoan can infect both immunocompromised and immunocompetent human hosts, primarily through the upper and lower respiratory tracts [5].
There are several case reports, which present the co-morbidity of Lophomonas and Mycobacterium [5–7], but all are diagnosed by direct microscopic examination. Here, we describe a patient whose lophomoniasis was diagnosed by molecular approach despite previous reports [6–9] that used only direct microscopic examination for the diagnosis of the disease.
CASE REPORT
In June 2018, a 67-year-old man was referred to Imam Khomeini Hospital in Sari, northern Iran, with a 1-month complaint of dyspnea, cough, sputum production and night sweating. His vital signs in the emergency department (ED) were as follows: blood pressure was 110/75 mmHg, temperature was 37.3°C, respiratory rate was 18, radial pulse was 90 BPM and peripheral oxygen saturation (SO2) was 96%. He had a history of diabetes for 20 years, which was poorly controlled (hemoglobin A1C = 9.9%, fasting blood sugar = 200 mg/dl and 2-h postprandial plasma glucose = 240 mg/dl) only with metformin tablets. He also had a history of 30 pack/year smoking. On a physical examination, the positive findings were his pale conjunctiva on both sides, distal symmetric polyneuropathy (stocking-glove pattern) and the crackle heard by pulmonary auscultation. He had previously been treated for a diagnosis of community-acquired pneumonia (CAP) with levofloxacin tablets, which had not improved his symptoms. Next, the patient underwent a chest-computed tomography (CT) scan that showed cavitary lesions with decreased lung parenchymal volume in the right upper lobe and centrilobular nodules with a linear branching pattern (tree in bud) in the right upper lobe and base of both lungs. Bronchiectasis with peribronchial thickening in the right middle and lower lobes was also detected (see Fig. 1). The lab tests found nothing unusual (Table 1). A sputum acid-fast bacillus (AFB) smear test was done based on the patient’s symptoms and the TB prevalence in our region, which was positive. Therefore, the patient was discharged with fixed-dose combination (4FDC) tablets (isoniazid (75 mg), rifampin (150 mg), pyrazinamide (400 mg) and ethambutol (275 mg)) for 2 months, as well as fixed-dose combination (2FDC) tablets (isoniazid (75 mg), rifampin (150 mg)) for 4 months.
Figure 1.
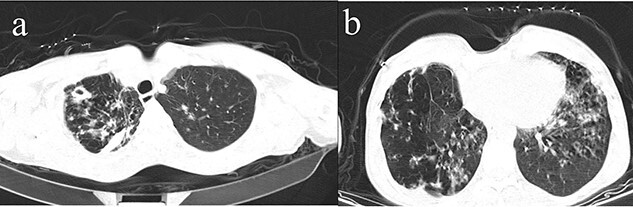
An axial lung CT-scan showing cavitary lesions with decreased lung parenchymal volume in right upper lobe (a). centrilobular nodules with linear branching pattern (tree in bud) in right upper lobe and base of both lungs and bronchiectasis with peribronchial thickening in the right middle and lower lobes was also detected (b).
Table 1.
Lab parameter results of the patient at ED
| Lab parameter | Results | Normal range |
|---|---|---|
| Ph | 7.39 | 7.35–7.45 mmHg |
| Pco2 | 40 mmol/l | 35–45 mmHg |
| Hco3 | 25 mmol/l | 22–28 mEq/l |
| FBS | 70 mg/dl | 90–110 mg/dl |
| AST | 36 U/l | 10–44 IU/l |
| ALT | 15 U/l | ˂45 IU/l |
| ALP | 100 U/l | 80–306 IU/l |
| WBC | 8200 U/l | 4000–10 000/mm3 |
| RBC | 4.5*106 | 4.2–5.4*106 cells/mcL |
| HB | 13.5 g/dl | 14–18 g/dl |
| PLT | 250 000 | 145 000–450 000/mm3 |
| HCT | 35 | 35.5–44.9% |
| MCV | 85 fl | 80–100 fl |
| MCH | 27 pg | 27–31 picograms/cell |
| MCHC | 32 | 32–36 grams/deciliter (g/dl) |
| Neutrophils | 55.6% | 55–70% |
| Lymphocyte | 32.4% | 20–35% |
| Monocyte | 6.8% | 3–8% |
| Eosinophil | 5.2% | 0–6% |
| CRP | 3 mg/l | <6 mg/l |
| ESR | 2 mm/h | <20 mm/h |
| Urea | 29 mg/dl | 15–45 mg/dl |
| Cr | 1 mg/dl | 0.5–1.3 mg/dl |
| Na | 135 mEq/l | 135–145 mEq/l |
| K | 4.5 mEq/l | 3.5–5.5 mEq/l |
| Mg | 2.3 mg/dl | 1.8–2.5 mg/dl |
The sputum AFB smear of the patient was checked weekly till it became negative after 8 weeks of treatment. At the end of his 6-month regimen of treatment, the patient’s cough persisted, and he was again referred to our clinic. Negative AFB smears until the end of the sixth month of treatment reassure us of resolved TB. But the persistence of respiratory symptoms led us to investigate more serious diseases like cancers and etc. Thus, the patient is a candidate for bronchoscopy. Bronchoscopic findings revealed a slightly narrow trachea, a widening carina, and mild mucosal edema and hyperemia in the right bronchus. A bronchoalveolar lavage fluid (BALF) sample was also obtained.
Moreover, the BALF sample was used to detect Lophomonas infection by microscopic examination (using both wet smear observation and Papanicolaou staining methods) and conventional polymerase chain reaction (PCR) technique [10]. Afterward, under light microscopy, an ovoid multifllagelated protozoan which has granular cytoplasm with no defined nucleus was observed, which indicated Lophomonas spp., trophozoite (see Fig. 2), and then Lophomonas infection was confirmed using the conventional PCR method (see Fig. 3). Finally, after the diagnosis of Lophomonas infection, the patient was discharged with metronidazole tablets three times a day for 2 weeks. During the follow-up, the symptoms were completely resolved, and the patients had no complaints.
Figure 2.
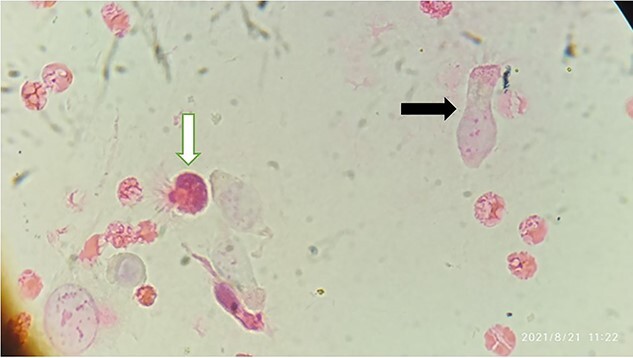
Papanicolaou stained smear of BALF showing Lophomonas trophozoite (white arrow) and bronchial epithelial cell (black arrow) (magnification × 400)
Figure 3.
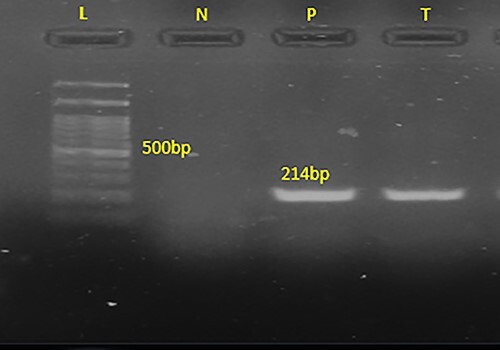
The 214 bp band of the PCR product is shown in 1.5% agarose gel electrophoresis, confirming Lophomonas spp., in the BALF sample of the patient. L (ladder): 100-bp DNA marker; N: negative sample; P: positive sample, T: the patient specimen.
DISCUSSION
Lophomonas infection is poorly understood, and many characteristics of the protozoan remain unknown [5]. Based on registry data, the prevalence of lophomoniasis in the Iranian population was estimated at ~22% [5, 9]. Because the clinical symptoms of this disease overlap with those of other respiratory infections, diagnosing Lophomonas infection is more difficult, especially when it co-exists with other respiratory infections. Clinicians should consider the possibility of other pathogen comorbidities, especially when patient’s symptoms do not resolve [5].
Direct microscopic examination in wet mount and/or stained smears remains the standard diagnostic approach for Lophomonas infection [5, 9]. Wet mounting allows for the rapid examination of a fresh clinical specimen, which may be mistaken for ciliated respiratory epithelial cells. On the other hand, stained smears allow more detailed morphological characteristics of parasites to be identified, which increases the possibility of detecting them from suspected cells [5, 9]. However, recently, Fakhar et al. [10] introduced a first PCR test for detecting Lophomonas infection on patients’ respiratory specimens to avoid misdiagnosis of it from bronchial and or other respiratory ciliated cells such as creola bodies as well as allow low density of the parasite load in the clinical specimens to be detected [5]. Accordingly, to avoid these potential diagnostic pitfalls, a PCR test to confirm the microscopic examination is highly recommended.
In this report, we met a patient whose symptoms did not improve despite receiving first-line TB treatment regimens. On the other hand, his sputum AFB smear became negative, which means that TB has resolved, but symptoms remain worrisome. We followed up the patient based on the directly observed treatment, short-course (DOTs) strategy, and we were completely sure about the patient’s compliance with the treatment.
Because all BALF samples taken in our teaching hospital (Imam Khomeini hospital) were submitted to the Iranian National Registry Center for Lophomoniasis (INRCL) for diagnosing lophomoniasis, the presence of Lophomonas in our patient’s sample was an incidental finding. Although we could not suggest a strategy for Lophomonas diagnosis in practice, in the near future, we aim to be able to provide a good diagnostic approach for Lophomonas, especially after a case of lung cavitation caused by this protozoan was published [11]. This indicates that this newly discovered protozoan may be more hazardous than previously imagined.
Another case of Lophomonas/TB co-infection was reported in India [7], but they used a light microscope to diagnose lophomoniasis, which has lower sensitivity and specificity than the PCR technique. There was also evidence that reported the co-infection of Lophomonas with other respiratory infections like COVID-19 [12] and aspergillosis [13].
In conclusion, although it is rational to go forward with guidelines for more well-known diseases (like TB here), it is also beneficial to keep an eye on this emerging parasite when it is co-infected with other pathogens, especially in the endemic region. It means that more watchfulness should be exercised with regard to co-infection. As a whole, our report sheds light on the possible presence of Lophomonas/TB co-infection, which leads to a decreasing potential risk of underdiagnosis and or its severe complications.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
FUNDING
None declared.
ETHICAL APPROVAL AND C ONSENT TO PARTICIPATE
This research was reviewed and approved by the research ethics committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1397.2969). Written informed consent was taken from the patient to include the clinical details.
CONSENT
Informed consent for publication of this case report was taken from the patient.
GUARANTOR
Dr Mahdi Fakhar.
AUTHORS’ CONTRIBUTIONS
AT involved in the clarification and collecting of data and writing of the manuscript draft. MF and ASh involved in editing of the manuscript. ESB, ASh and MN is involved in critically revising the whole manuscript. MF and AT are responsible for presenting data and submitting the manuscript. All authors reviewed and approved the final version of the manuscript.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
DATA AVAILABILITY STATEMENT
The data are available with the correspondence author and can be reached on request.
Contributor Information
Amirmasoud Taheri, Parasitology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran.
Mahdi Fakhar, Parasitology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran.
Ali Sharifpour, Parasitology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran.
Maryam Nakhaei, Parasitology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran.
Elham Sadat Banimostafavi, Parasitology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran; Radiology Department, Iranian National Registry Center for Lophomoniasis (INRCL), Mazandaran University of Medical Sciences, Sari, Iran.
REFERENCES
- 1. Zaman K. Tuberculosis: a global health problem. J Health Popul Nutr 2010;28:111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World health organization , Tuberculosis 14 October 2021, (1 December 2021, date last accessed): https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- 3. Mbuh TP, Ane-Anyangwe I, Adeline W, Thumamo Pokam BD, Meriki HD, Mbacham WF. Bacteriologically confirmed extra pulmonary tuberculosis and treatment outcome of patients consulted and treated under program conditions in the littoral region of Cameroon. BMC Pulm Med 2019;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loddenkemper R, Lipman M, Zumla A. Clinical aspects of adult tuberculosis. Cold Spring Harb Perspect Med 2015;6:a017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fakhar M, Sharifpour A, Nakhaei M, Banimostafavi ES, Ghasemi M, Abedian Set al. Lophomonas and Lophomoniasis: Biology, Etiology,Epidemiology, Pathogenesis, Diagnosis and Treatment, 1st edn. Gorgan, Iran: Noruzi Publisher, 2021. [Google Scholar]
- 6. Xue J, Li Y-L, Yu X-M, Li D-K, Liu M-F, Qiu J-Fet al. Bronchopulmonary infection of Lophomonas blattarum: a case and literature review. Korean J Parasitol 2014;52:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verma S, Verma G, Singh DV, Mokta J, Negi RS, Jhobta Aet al. Dual infection with pulmonary tuberculosis and Lophomonas blattarum in India. Int J Tuberc Lung Dis 2015;19:368–9. [DOI] [PubMed] [Google Scholar]
- 8. Ding Q, Shen K. Pulmonary infection with Lophomonas blattarum. Indian J Pediatr 2021;88:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fakhar M, Nakhaei M, Sharifpour A, Safanavaei S, Abedi S, Tabaripour Ret al. Morphological and molecular identification of emerged Lophomonas blattarum infection in Mazandaran Province, Northern Iran: first registry-based study. Acta Parasitol 2021;66:1510–6. [DOI] [PubMed] [Google Scholar]
- 10. Fakhar M, Nakhaei M, Sharifpour A, Kalani H, Banimostafavi ES, Abedi Set al. First molecular diagnosis of Lophomoniasis: the end of a controversial story. Acta Parasitol 2019;64:390–3. [DOI] [PubMed] [Google Scholar]
- 11. Taheri A, Fakhar M, Sharifpour A, Banimostafavi E. Cavitary pulmonary lesions following emerging lophomoniasis: a novel perspective. Respirol Case Rep 2022;10:e0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakhaei M, Fakhar M, Sharifpour A, Banimostafavi ES, Zakariaei Z, Mehravaran Het al. First co-morbidity of Lophomonas blattarum and COVID-19 infections: confirmed using molecular approach. Acta Parasitol 2021;22:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharifpour A, Zakariaei Z, Fakhar M, Banimostafavi ES, Nakhaei M, Soleymani M. Post-COVID-19 co-morbidity of emerged Lophomonas infection and invasive pulmonary aspergillosis: first case report. Clin Case Rep 2021;9:e04822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available with the correspondence author and can be reached on request.


