Abstract
To determine the contribution of sigma B (ςB) to survival of stationary-phase Listeria monocytogenes cells following exposure to environmental stresses, we compared the viability of strain 10403S with that of an isogenic nonpolar sigB null mutant strain after exposure to heat (50°C), ethanol (16.5%), or acid (pH 2.5). Strain viabilities were also determined under the same conditions in cultures that had been previously exposed to sublethal levels of the same stresses (45°C, 5% ethanol, or pH 4.5). The ΔsigB and wild-type strains had similar viabilities following exposure to ethanol and heat, but the ΔsigB strain was almost 10,000-fold more susceptible to lethal acid stress than its parent strain. However, a 1-h preexposure to pH 4.5 yielded a 1,000-fold improvement in viability for the ΔsigB strain. These results suggest the existence in L. monocytogenes of both a ςB-dependent mechanism and a pH-dependent mechanism for acid resistance in the stationary phase. ςB contributed to resistance to both oxidative stress and carbon starvation in L. monocytogenes. The ΔsigB strain was 100-fold more sensitive to 13.8 mM cumene hydroperoxide than the wild-type strain. Following glucose depletion, the ΔsigB strain lost viability more rapidly than the parent strain. ςB contributions to viability during carbon starvation and to acid resistance and oxidative stress resistance support the hypothesis that ςB plays a role in protecting L. monocytogenes against environmental adversities.
Listeria monocytogenes is a gram-positive bacterial pathogen that can cause severe food-borne disease. This organism is responsible for approximately 2,500 illnesses and 500 deaths annually in the United States (18). L. monocytogenes is a common food contaminant, at least in part due to its ubiquitous existence in the environment. This organism's ability to persist and thrive under very different conditions suggests that it is capable of efficiently responding to environmental stress challenges.
In bacteria, the association of appropriate alternative sigma factors with core RNA polymerase provides a mechanism for cellular responses that are mediated through redirection of transcription initiation. In both gram-negative and gram-positive organisms, the alternative sigma factors, RpoS and sigma B (ςB), respectively, regulate expression of numerous genes under environmental stress conditions and upon entry into the stationary phase. The role of RpoS in regulation of genes involved in stress protection has been characterized for various gram-negative bacteria, including Escherichia coli, Salmonella, and Yersinia spp. RpoS has been shown to influence cellular resistance to heat, acid, and high osmolarity in the pathogenic organism E. coli O157:H7 (10) and in Yersinia enterocolitica (4). RpoS also has been shown to contribute to Salmonella virulence. For example, the Salmonella enterica serovar Typhi Ty21 strain used as a live oral vaccine has been shown to have an rpoS mutation that has been demonstrated to contribute to the environmental stress susceptibility of this virulence-attenuated organism (20). Furthermore, in a mouse model system, an S. enterica serovar Typhimurium RpoS mutant has been shown to have potential for use as a live oral vaccine (11).
ςB regulates expression of a large general stress operon in Bacillus subtilis, contributing to transcription of more than 100 genes involved in heat, acid, ethanol, salt, and oxidative stress resistance (2, 3, 14, 22). ςB also has been shown to contribute to heat, acid, and oxidative stress resistance in Staphylococcus aureus (9) and in osmotolerance (5) and acid stress resistance (23) in L. monocytogenes. Becker et al. (5) demonstrated that there is increased L. monocytogenes sigB transcription, which implies that there is increased ςB activity, following exposure of exponentially growing cells to acid, ethanol, osmotic, heat, and oxidative stresses, as well as upon entry into the stationary phase. Furthermore, ςB has been demonstrated to contribute to the ability of stationary-phase L. monocytogenes cells to adapt to and resume growth at reduced temperatures (6). Although sigB and rpoS do not exhibit significant sequence identity beyond that required for conserved functions among sigma factors and while the activities of these transcription factors are regulated in very different ways, the proteins appear to contribute to physiologically similar cellular needs. Furthermore, the B. subtilis ςB and E. coli RpoS regulons share a subset of genes, including genes for nonspecific DNA binding proteins (dps) and catalases (katE) (15). Taken together, these results suggest the possibility that ςB and RpoS have parallel roles in the general stress responses of gram-positive and gram-negative organisms, respectively.
L. monocytogenes (17) and B. subtilis (22) have been shown to display enhanced resistance to lethal stresses following preexposure to sublethal levels of the same stresses. In B. subtilis, this stress adaptation response appears to be ςB dependent (22). Pretreatment with mild heat (48°C) or mild salt (4%) stress was shown to enhance survival of the B. subtilis wild-type strain but not a ΔsigB strain when cultures were exposed to a more severe level of the same stress (54°C or 10% ethanol). Furthermore, ςB activation in the absence of specific stresses was also shown to confer enhanced resistance to subsequent exposure to lethal stress (22). Specifically, sigB was placed under control of the isopropyl-β-d-thiogalactopyranoside-inducible promoter Pspac in a B. subtilis strain carrying a null mutation in rsbW, the primary negative regulator of ςB activity. Addition of isopropyl-β-d-thiogalactopyranoside prior to heat or salt treatment enhanced cell survival following exposure to stress, leading to the conclusion that activation of ςB expression alone may protect cells from subsequent stresses.
In this study, we characterized the survival phenotypes of L. monocytogenes sigB null mutant stationary-phase cells under multiple stress conditions, including heat, ethanol, acid, and oxidative stresses. We also examined the role of ςB in the L. monocytogenes stationary-phase stress adaptation response.
MATERIALS AND METHODS
Bacterial strains.
L. monocytogenes 10403S (8) and FSL A1-254 (23) were used throughout this study. L. monocytogenes 10403S, a serotype 1/2a strain, was obtained from D. Portnoy (University of California, Berkeley). L. monocytogenes FSL A1-254 was generated by creating a 600-bp sigB fragment with an in-frame 297-bp deletion between nucleotides 1490 and 1788 of the sigB operon in 10403S (GenBank accession no. AF032446 [20]). Stock cultures were stored at −80°C in brain heart infusion (BHI) broth (Difco, Sparks, Md.) with 15% glycerol and streaked onto BHI agar plates prior to inoculation of the working cultures.
Resistance to ethanol, heat, and acid following preadaptation.
Strains were tested as described by Lou and Yousef (17), with the following modifications. Briefly, 5 ml of BHI broth was inoculated from an isolated colony of L. monocytogenes 10403S or FSL A1-254, and the culture was grown to the stationary phase (12 h) at 37°C with rotary shaking (250 rpm) in a series 25 incubator-shaker (New Brunswick Scientific, Edison, N. J.). Such cultures were used to inoculate fresh tubes containing 5 ml of BHI broth (1:100), which were then incubated with shaking at 37°C for another 12 h. The resulting stationary-phase cultures were centrifuged at 4,000 × g for 5 min. The pellets were resuspended in 5 ml of either BHI broth containing 5% ethanol or BHI broth acidified to pH 4.5 and then incubated for 1 h at 37°C. For heat preadaptation, cells harvested in the stationary phase were resuspended in 5 ml of BHI broth as described above and incubated at 45°C for 1 h. Following 1-h adaptation periods in 5% ethanol and at pH 4.5, cells were harvested, and the pellets exposed to sublethal conditions were resuspended in BHI broth with 16.5% ethanol and in BHI broth (pH 2.5), respectively, and then incubated at 37°C for 3 h. In both cases, the broth media had been prewarmed to 37°C. The pellets from cultures preadapted at 45°C were resuspended in BHI broth (prewarmed to 50°C) and incubated at 50°C for up to 8 h. Nonadapted stationary-phase cell pellets were resuspended directly under the following lethal conditions: BHI broth with 16% ethanol, BHI broth (pH 2.5), and BHI broth at 50°C. These control cultures were incubated in parallel with the preadapted samples. Aliquots were removed for viable cell determination at suitable intervals. Serial dilutions in phosphate-buffered saline were plated in duplicate onto BHI agar plates and then incubated at 37°C for 48 h. Colonies were enumerated, and the results are presented below as percentages of cell survival. Experiments were performed in duplicate and repeated independently at least two times.
Resistance to carbon starvation.
Cultures of L. monocytogenes 10403S and FSL A1-254 were grown from isolated colonies in a defined medium (1) supplemented with a limiting level of glucose (0.04%, wt/vol). Culture density was monitored by determining optical density at 600 nm (OD600), and cell viability was measured by standard plate counting using samples taken at various times for up to 12 h after cultures had stopped growing due to carbon limitation (OD600, ∼0.2).
Resistance to oxidative stress.
Oxidative resistance responses of the mutant and wild-type cultures were assessed by using the oxidative agent cumene hydroperoxide (CHP) (Sigma, St. Louis, Mo.). CHP survival assays were conducted as described by Antelmann et al. (2), with the following modifications. Briefly, cell cultures that had been grown to the stationary phase at 37°C with shaking (250 rpm) for 12 h were inoculated into 5 ml of BHI broth (1:100). Following 12 h of incubation at 37°C, 900-μl portions of the cultures were transferred to tubes containing 100 μl of 138 mM CHP diluted in dimethyl sulfoxide (Fisher Scientific, Fair Lawn, N.J.), which yielded a final CHP concentration of 13.8 mM. Control cultures were transferred to tubes containing 100 μl of dimethyl sulfoxide. All tubes, including the controls, were then incubated for 15 min at 37°C with shaking. Cell viability was assessed by standard plate counting on BHI agar plates that had been incubated at 37°C. The results described below reflect the data from two independent experiments, each performed in triplicate.
RESULTS AND DISCUSSION
Role of ςB in resistance to heat, ethanol, and acid stress.
The survival of the L. monocytogenes 10403S wild-type strain was compared with that of an isogenic nonpolar sigB null mutant strain following exposure to lethal levels of heat (50°C), ethanol (16.5%), and acid (pH 2.5). To investigate the role of ςB in L. monocytogenes stress adaptation, we also compared strain survival under these lethal environmental stress conditions following a 1-h preexposure to sublethal levels of the same stresses (45°C, 5% ethanol, and pH 4.5). ςB-dependent survival phenotypes were assessed in stationary-phase cells as (i) ςB activity increases upon entry into the stationary phase (5) and (ii) ςB previously has been shown to contribute to L. monocytogenes acid stress survival under these conditions (23).
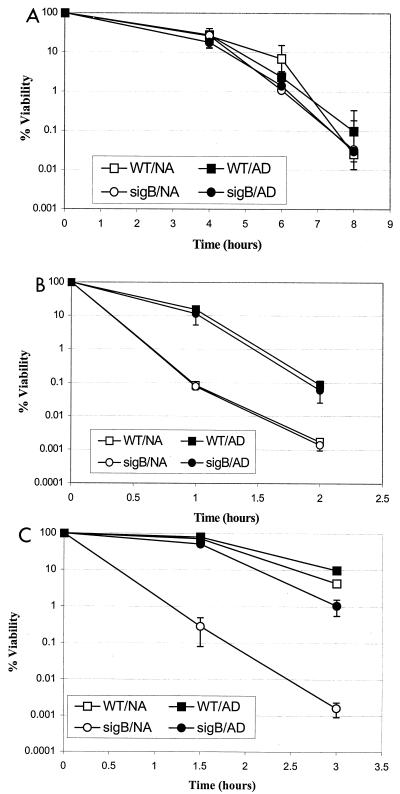
Heat stress resistance and ethanol stress resistance were found to be at least partially ςB independent in L. monocytogenes under the conditions used in this study. Similar culture viabilities were observed for both the L. monocytogenes ΔsigB and wild-type strains when they were exposed to either 50°C (Fig. 1A) or 16.5% ethanol (Fig. 1B). Preadaptation to reduced heat stress (45°C) did not enhance survival of either strain following exposure to a more lethal level of heat stress (50°C) (Fig. 1A). However, preadaptation to a sublethal level of ethanol (5%) enhanced cell survival by 10- to 100-fold in the presence of a more lethal level of ethanol (16.5%) for both the ΔsigB and wild-type strains. The ethanol adaptation response mechanism in L. monocytogenes appears to be ςB independent as the ΔsigB and wild-type strains exhibited similar recoveries from exposure to 16% ethanol following preadaptation in 5% ethanol (Fig. 1B). Although heat and ethanol have been shown to induce sigB transcription in exponentially growing L. monocytogenes cells (5), our results suggest that stationary-phase resistance to heat and ethanol stresses is at least partially ςB independent in this organism. These results parallel those obtained with S. aureus, in which cellular exposure to ethanol or NaCl enhanced production of a sigB transcript but did not differentially affect survival of the ςB mutant and its wild-type counterpart (9). Similarly, in preliminary experiments, the levels of survival of the L. monocytogenes 10403S wild-type strain and the ΔsigB mutant were identical after 24 h of exposure to up to 25% NaCl in BHI broth (incubated at 37°C with shaking) (data not shown) despite the previous observation that there was increased sigB transcription in the presence of 4% NaCl (5). We speculate that the environmental stress conditions used in our study, as well as those used by Chan et al. (9), may have been sufficiently lethal to have overwhelmed possible ςB contributions to cellular survival. Alternatively, it is possible that although ςB activity is induced during exposure to ethanol or salt (5), the resulting general stress response does not provide appropriate cellular defenses against these specific stresses. This hypothesis is supported, at least in part, by the observation that preexposure to 5% ethanol enhances L. monocytogenes survival in the presence of 16.5% ethanol in a ςB-independent manner. The specific mechanism(s) responsible for this adaptive response is currently unknown.
FIG. 1.
Percent viabilities of L. monocytogenes ΔsigB (circles) and wild-type (WT) (squares) stationary-phase cultures during exposure to lethal stresses, including heat (50°C) (A), ethanol (16.5%) (B), and acid (pH 2.5) (C), with (AD) (solid symbols) and without (NA) (open symbols) preadaptation for 1 h at 45°C, in the presence of 5% ethanol, and at pH 4.5, respectively. The results are averages based on at least two repetitions, each performed in duplicate. The error bars indicate standard deviations.
As shown in Fig. 1C, acid resistance in L. monocytogenes stationary-phase cells exposed to pH 2.5 appears to be at least partially ςB dependent. The percent survival of the L. monocytogenes ΔsigB strain was almost 10,000-fold lower than that of its parent strain after 3 h of exposure to pH 2.5. However, a 1-h preexposure to a sublethal level of acid stress (pH 4.5) improved ΔsigB strain viability by more than 1,000-fold following exposure to pH 2.5, suggesting that L. monocytogenes stationary-phase acid tolerance can be induced by exposure to reduced pH in a ςB-independent manner.
Previous work has shown that L. monocytogenes possesses a pH-dependent log-phase acid tolerance response (ATR), as well as a stationary-phase-dependent ATR (12). Our data suggest that stationary-phase acid tolerance depends on at least two mechanisms, a ςB-dependent mechanism and a pH-dependent mechanism that is at least partially ςB independent, as cell viability at pH 2.5 following preadaptation was not fully recovered in the ΔsigB strain. These results are similar to the partially ςB-dependent acid adaptive response observed in S. aureus cells that had been preexposed to pH 4 prior to pH 2 treatment (9). By comparison, three mechanisms contributing to acid tolerance have been reported in S. enterica serovar Typhimurium (16). This pathogenic gram-negative bacterium displays two distinct pH-dependent ATRs, a log-phase ATR and a stationary-phase ATR, as well as pH-independent stationary-phase acid tolerance, which is RpoS dependent.
Role of ςB in survival during carbon starvation.
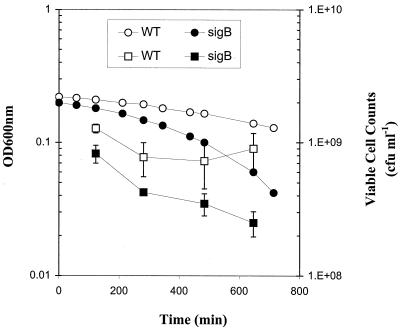
The relative viabilities of the L. monocytogenes ΔsigB and 10403S wild-type strains grown in a defined medium with limiting glucose (0.04%, wt/vol) were compared to assess the role of ςB in survival during carbon starvation. Plate counts were used to determine culture viabilities for up to 12 h following growth arrest at an OD600 of ∼0.2 due to glucose depletion. The ΔsigB strain lost viability more rapidly than the parent strain under carbon starvation conditions (Fig. 2). After 12 h in a glucose-depleted medium, ΔsigB strain viability was reduced by 85%, compared with a 25% reduction for the wild-type culture. The viability loss may have been due to enhanced cell lysis, as suggested by the ∼10-fold reduction in OD600 for the ΔsigB strain. These data suggest an important role for ςB in L. monocytogenes survival during carbon starvation. Expression of more than eight proteins has been shown to be induced by ςB in glucose-starved B. subtilis cells (7). Furthermore, glucose-starved B. subtilis (2) and S. aureus (9) ΔsigB strains have been shown to be less resistant to oxidative stress than their wild-type parents.
FIG. 2.
Viabilities of L. monocytogenes ΔsigB (solid symbols) and wild-type (WT) (open symbols) cultures that had been grown in a defined medium with limiting glucose (0.04%, wt/vol). The x axis indicates the time following growth cessation in the defined medium. OD600 (circles) and viable numbers (squares) were recorded for up to 12 h after the cultures had stopped growing due to carbon limitation. The error bars indicate standard deviations.
Role of ςB in resistance to oxidative stress.
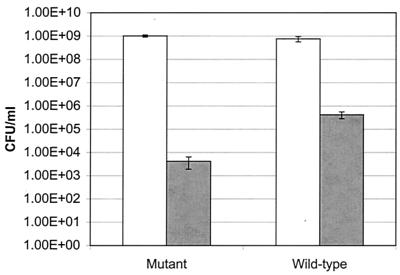
The role of ςB in protecting L. monocytogenes against oxidative stress was assessed by comparing the viability of the ΔsigB strain with that of the 10403S parent following exposure to 13.8 mM CHP for 15 min. The viability of the ΔsigB strain was 100-fold lower than that of its wild-type parent (Fig. 3), suggesting that oxidative stress resistance is at least partially ςB dependent in stationary-phase L. monocytogenes cells. In combination with the observed increase in L. monocytogenes sigB transcription following exposure to 0.15% H2O2 (5), these results provide further evidence that ςB contributes to cellular survival under oxidizing conditions.
FIG. 3.
Viabilities of L. monocytogenes ΔsigB and wild-type stationary-phase cultures that were not exposed to CHP (open bars) or were exposed to 13.8 mM CHP for 15 min (shaded bars). The error bars indicate standard deviations.
Antelmann et al. (2) also observed that a B. subtilis ΔsigB strain was more sensitive to CHP than wild-type cells were; however, the cells had been grown under glucose depletion conditions. Conversely, ςB-dependent oxidative stress resistance induced by starvation has been observed in B. subtilis (13) and in S. aureus (9) strains exposed to hydrogen peroxide. ςB has been shown to control expression of several genes involved in oxidative stress resistance in B. subtilis, including katE, which encodes a catalase protein (2, 14), and dps, which encodes a DNA-protecting protein (3). In the gram-negative organism S. enterica serovar Typhimurium (21) and in E. coli (19), oxidative stress resistance has been shown to depend at least partially on RpoS.
In conclusion, this study showed that ςB contributes to survival of stationary-phase L. monocytogenes cells under acid and oxidative stress conditions. ςB also contributes to survival of growth-arrested L. monocytogenes cells during carbon starvation. Furthermore, our data reveal the existence of at least two mechanisms of acid resistance in L. monocytogenes stationary-phase cells: a pH-independent ςB-dependent mechanism and a pH-dependent mechanism that is at least partially ςB independent. We hypothesize that in L. monocytogenes acid and oxidative stress protection conferred by ςB may contribute to the virulence of this invasive pathogen, as this organism must survive acid and oxidative stresses imposed by the host to cause illness.
ACKNOWLEDGMENT
A. Ferreira was supported by a fellowship from CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil).
REFERENCES
- 1.Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;141:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 2.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badger J L, Miller V L. Role of RpoS in survival of Yersinia enterocolitica to a variety of environmental stresses. J Bacteriol. 1995;177:5370–5373. doi: 10.1128/jb.177.18.5370-5373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker L A, Cetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker L A, Evans S N, Hutkins R W, Benson A K. Role of ςB in adaptation of Listeria monocytogenes to growth at low temperatures. J Bacteriol. 2000;182:7083–7087. doi: 10.1128/jb.182.24.7083-7087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 8.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 9.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheville A M, Arnold K W, Buchrieser C, Cheng C M, Kaspar C W. rpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1996;62:1822–1824. doi: 10.1128/aem.62.5.1822-1824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (ςS) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 12.Davis M J, Coote P J, O'Byrne C P. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology. 1996;142:2975–2982. doi: 10.1099/13500872-142-10-2975. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann S, Hecker M. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann S, Lindner C, Hecker M. Cloning, nucleotide sequence, and regulation of katE encoding a sigma B-dependent catalase in Bacillus subtilis. J Bacteriol. 1995;177:5598–5605. doi: 10.1128/jb.177.19.5598-5605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee I S, Slonczewski J L, Foster J W. A low-pH-inducible, stationary-phase acid tolerance response in Salmonella typhimurium. J Bacteriol. 1994;176:1422–1426. doi: 10.1128/jb.176.5.1422-1426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou Y, Yousef A E. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl Environ Microbiol. 1997;63:1252–1255. doi: 10.1128/aem.63.4.1252-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michan C, Manchado M, Dorado G, Pueyo C. In vivo transcription of the Escherichia coli oxyR regulon as a function of growth phase and in response to oxidative stress. J Bacteriol. 1999;181:2759–2764. doi: 10.1128/jb.181.9.2759-2764.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbe-Saule V, Coynault C, Norel F. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol Lett. 1995;126:171–176. doi: 10.1111/j.1574-6968.1995.tb07412.x. [DOI] [PubMed] [Google Scholar]
- 21.Seymour R L, Mishra P V, Khan M A, Spector M P. Essential roles of core starvation-stress response loci in carbon-starvation-inducible cross-resistance and hydrogen peroxide-inducible adaptive resistance to oxidative challenge in Salmonella typhimurium. Mol Microbiol. 1996;20:497–505. doi: 10.1046/j.1365-2958.1996.5451068.x. [DOI] [PubMed] [Google Scholar]
- 22.Völker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedmann M, Arvik T J, Hurley R J, Boor K J. General stress transcription factor ςB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]