Abstract
The anaerobic microbial oxidation of toluene to CO2 coupled to humus respiration was demonstrated by use of enriched anaerobic sediments from the Amsterdam petroleum harbor (APH) and the Rhine River. Both highly purified soil humic acids (HPSHA) and the humic quinone moiety model compound anthraquinone-2,6-disulfonate (AQDS) were utilized as terminal electron acceptors. After 2 weeks of incubation, 50 and 85% of added uniformly labeled [13C]toluene were recovered as 13CO2 in HPSHA- and AQDS-supplemented APH sediment enrichment cultures, respectively; negligible recovery occurred in unsupplemented cultures. The conversion of [13C]toluene agreed with the high level of recovery of electrons as reduced humus or as anthrahydroquinone-2,6-disulfonate. APH sediment was also able to use nitrate and amorphous manganese dioxide as terminal electron acceptors to support the anaerobic biodegradation of toluene. The addition of substoichiometric amounts of humic acids to bioassay reaction mixtures containing amorphous ferric oxyhydroxide as a terminal electron acceptor led to more than 65% conversion of toluene (1 mM) after 11 weeks of incubation, a result which paralleled the partial recovery of electron equivalents as acid-extractable Fe(II). Negligible conversion of toluene and reduction of Fe(III) occurred in these bioassay reaction mixtures when humic acids were omitted. The present study provides clear quantitative evidence for the mineralization of an aromatic hydrocarbon by humus-respiring microorganisms. The results indicate that humic substances may significantly contribute to the intrinsic bioremediation of anaerobic sites contaminated with priority pollutants by serving as terminal electron acceptors.
Toluene is an important constituent of gasoline, accounting for 5 to 7% (wt/wt) of its composition (39). Due to leaks in underground fuel storage tanks, improper disposal techniques, and spills of all types of petroleum products, widespread contamination with toluene has occurred in soil, sediment, and groundwater. The relatively high aqueous solubility of toluene, 515 mg/liter at 20°C (39), accounts for its mobility in the environment. Due to its toxicity, toluene is considered a priority pollutant by the U.S. Environmental Protection Agency (39). Toluene is a depressant of the central nervous system (39) and an enhancing agent in skin carcinogenesis (12).
Microbial degradation of toluene readily occurs under aerobic conditions (32, 33) by a wide variety of aerobic bacteria utilizing several monooxygenases and a dioxygenase to initiate the attack. However, many polluted sites are often depleted of oxygen. Consequently, alternative degradation pathways under anaerobic conditions are important in determining the fate of toluene. Various investigators have shown that in the absence of oxygen, toluene degradation is linked to methanogenesis and to sulfate, nitrate, and iron reduction (16). Recently, toluene degradation was also shown to be linked to the reduction of manganese oxides (21, 22) and to a fermentative oxidation process with fumarate as a terminal electron acceptor (29). These alternative electron acceptors either occur naturally in groundwater and sediments (e.g., iron) or are possible additives for stimulating in situ biodegradation processes.
In the present study, humus is evaluated as a potential electron acceptor for toluene biodegradation. Humus is the stable organic matter accumulating in sediments and soils (35). Although humus is generally considered to be inert for microbial catabolism, it has recently been reported to play an active role in the anaerobic oxidation of a wide variety of ecologically relevant organic substrates (e.g., acetate and lactate) as well as hydrogen by serving as a terminal electron acceptor (4, 7, 9, 28). These studies have demonstrated that the reduction of humic substances may be an important mechanism for organic substrate oxidation in many anaerobic environments. Quinone moieties of humus have been implicated as the redox active groups (31) accepting the electrons. Anthraquinone-2,6-disulfonate (AQDS) has been used as a defined model for such moieties (7, 9, 17, 28). Most humus-respiring microorganisms are also capable of transferring electrons to AQDS, reducing it to anthrahydroquinone-2,6-disulfonate (AH2QDS); therefore, quinone model compounds imitate the function of humus as a terminal electron acceptor. Since reduced humus and hydroquinones are readily oxidized by Fe(III) and Mn(IV) (28, 36), humus only needs to be present at substoichiometric concentrations to be an effective electron acceptor as long as these metal oxides are abundant in the sediment. Thus, humus can link the degradation of substrates to dissimilatory metal reduction.
Aside from the simple substrates initially tested, evidence is accumulating that more complex substrates are degraded by quinone respiration. The anaerobic microbial oxidation of phenol and p-cresol in granular sludge was recently found to be coupled to the reduction of AQDS (8). The addition of humic acids or AQDS was also shown to stimulate the mineralization of the priority pollutants vinyl chloride and dichloroethene by a humus-respiring consortium under anaerobic conditions (5).
The fact that there are a wide variety of organic compounds which can be utilized by humus-respiring consortia leads to the question of whether humus can also support the anaerobic oxidation of toluene by serving as a terminal electron acceptor. In this study, the capacity of two different sediments for oxidizing toluene with humic acids or AQDS as a terminal electron acceptor was explored. The results constitute a clear quantitative demonstration of the mineralization of an aromatic hydrocarbon priority pollutant by humus-respiring microorganisms.
MATERIALS AND METHODS
Sediments.
Two different sediments were used for the present study. Petroleum harbor sediment was dredged from the Amsterdam petroleum harbor (APH), which was constructed for storage and transshipment of petroleum and coal. Around the APH, industrial activities developed and oil tanks were built. At the beginning of World War II, oil storage tanks were destroyed and large quantities of oil leaked into the harbor, causing major oil contamination of the sediment. Diverse other sources, such as industrial discharges, shipping, and tanker cleaning, have also contributed to contamination of the sediment. As a consequence, APH sediment is contaminated with oil and polycyclic aromatic hydrocarbons (11). Anaerobic Rhine River sediment was collected alongside the banks of the river near Lexkesveer in Wageningen, The Netherlands. This sediment was chosen because toluene, benzene, and naphthalene have been detected as contaminants in Rhine River water (20). This sediment has been previously shown to degrade aromatic compounds, such as toluene and sulfanilic acid, under different redox conditions (21, 37). Both sources of inocula were able to oxidize hydrogen and acetate with AQDS as a terminal electron acceptor (7).
Sediment incubations.
Bicarbonate buffered basal medium (pH 7.2) was prepared as previously described (7). For this study, the concentrations of NH4Cl and K2HPO4 were modified to 0.1 and 0.05 g per liter, respectively. The basal medium was supplied with one of the following electron acceptors: AQDS (25 mM), nitrate (10 mM), or sulfate (6.25 mM). AQDS was previously dissolved in boiled water, and then all the components of the basal medium were added. The medium was cooled in a stream of N2-CO2 (80:20). All the media were dispensed into 117-ml glass serum bottles after being with N2-CO2 (80:20) at a final volume of 50 ml (67 ml as headspace), and then inoculation took place by adding 10 g (dry weight) of previously homogenized sediment per liter. The bottles were sealed with Viton stoppers (Maag Technic AG, Dübendorf, Switzerland) and aluminum crimps and were flushed with N2-CO2 (80:20). Sulfate and nitrate were added from anaerobic and sterilized stock solutions in distilled water. Toluene (final concentration, 1 mM) was added from a stock solution in hexadecane. Hexadecane did not exceed 0.2% (vol/vol) of the liquid volume in the bioassays. Biodegradation of toluene was also confirmed in the absence of hexadecane, but the results presented in this study came from experiments in which toluene was added in hexadecane to facilitate minimal processing error during its addition. All the bioassay reaction mixtures were statically incubated in a 30°C room and were manually shaken before sampling to ensure the homogeneous distribution of toluene. Sterile controls were prepared under the same conditions and autoclaved for 20 min at 120°C two times prior to the addition of toluene. Controls without toluene added but with the same amount of hexadecane added were also included to correct for the endogenous reduction of the different electron acceptors provided and to verify the absence of hexadecane metabolism. All the experiments were carried out with triplicate incubations for all the conditions studied. Toluene degradation and reduction of the corresponding electron acceptor were monitored over time as described below.
Metal oxides as terminal electron acceptors for anaerobic toluene degradation.
The capacity of APH sediment for degrading toluene with insoluble metal oxides as terminal electron acceptors was also explored. Vernadite (amorphous MnO2) and goethite (amorphous FeOOH) were prepared as previously described (2, 19). The metal oxide suspensions were washed three times by centrifugation and resuspended in distilled water. Finally, the metal oxides were suspended in basal medium to obtain final concentrations of Mn(IV) and Fe(III) of 25 and 50 mM, respectively. The bicarbonate concentration was set at 2.5 g per liter in these experiments, and HEPES (50 mM, pH 7.2) was included as a buffer. The metal suspensions were flushed with N2-CO2 (80:20) and homogeneously distributed into 117-ml glass serum bottles at a final volume of 50 ml (67 ml as headspace). The bottles were inoculated with 10 g (dry weight) of APH sediment per liter and sealed with Viton stoppers and aluminum crimps. All the bioassays were conducted in an N2-CO2 (80:20) atmosphere. When the impact of humic substances on the biodegradation of toluene with metal oxides was studied, humic acids (Janssen Chimica Belgium; 2 g per liter) were added to the medium and distributed in the same form as described above. Toluene was added to the cultures from a stock solution in hexadecane. Sterile and endogenous controls were prepared in the same manner as described above for the bottles with alternative electron acceptors, and all bioassay reaction mixtures were incubated under the same conditions as described above. Toluene degradation was monitored over time as described below, and reduction of the metal oxides was also measured at the end of the experiment as described below.
Mineralization of [13C]toluene with AQDS and humic substances as terminal electron acceptors.
Bioassay mixtures in which the anaerobic degradation of toluene was observed coupled to the reduction of AQDS were decanted, and the bottles were refilled with anaerobic fresh medium (containing 25 mM AQDS) in an N2-H2 (95:5) atmosphere. The bottles were sealed again with Viton stoppers and aluminum crimps and were flushed with N2-CO2 (80:20) before more toluene (1 mM) was added. The bioassay bottles were refilled three times (when all toluene had been depleted) in the same way before the sediment was transferred to bottles for studies with uniformly labeled [13C]toluene. The basal medium was prepared without bicarbonate for studies with [13C]toluene but was amended with AQDS (5 mM) or with highly purified soil humic acids (HPSHA; 12 g per liter). The media were neutralized by adding sodium hydroxide or hydrochloric acid and were buffered with sodium phosphate (10 mM, pH 7.2). The media were homogeneously dispensed into 57-ml glass serum bottles (a final volume of 25 ml with a headspace of 32 ml), and the enriched sediment was added at 10 g (dry weight) per liter under anaerobic conditions. The bottles containing the enriched sediment were flushed with pure nitrogen gas, and then uniformly labeled [13C]toluene was added from a stock solution in anaerobic and sterile distilled water. All the experiments were carried out with triplicate incubations for all the conditions studied. All the bioassay mixtures were statically incubated in a 30°C room and were manually shaken before sampling to ensure the homogeneous distribution of toluene. The production of 13CO2 from [13C]toluene and the depletion of [13C]toluene were monitored over time as described below. The electrons transferred to AQDS and to HPSHA during [13C]toluene degradation were also monitored as described below. Sterile controls were prepared under the same conditions and autoclaved for 20 min at 120°C two times prior to the addition of [13C]toluene. Controls without [13C]toluene added were also included to correct for the background level of 13CO2 and reduction of AQDS and humus by endogenous substrates in the enrichment culture.
Analytical techniques.
The toluene concentrations in 100-μl headspace samples were determined by gas chromatography (Hewlett-Packard 5890 series II) with a flame ionization detector. The chromatograph was equipped with a CP-sil 8CB column, and helium (4.3 ml per min) was used as a carrier gas. The temperatures of the injection port, oven, and detector were 225, 120, and 225°C, respectively. Standards were prepared with basal medium containing the same amount of sediment (10 g [dry weight] per liter) as that used for the experiments and therefore reflect the equilibrium in toluene concentrations between the headspace and the sediment. Toluene was added to the standard bottles from a stock solution in hexadecane. The standard bottles had been autoclaved for 20 min at 120°C two times and incubated at 30°C overnight before toluene was added (4 h before analysis).
Concentrations of AH2QDS were determined spectrophotometrically by monitoring the absorbance at 450 nm in an anaerobic chamber as previously described (7). Mn(II) production was estimated by measuring the accumulation of soluble manganese in 0.5 N hydrochloric acid at the end of the experiment as previously described (25). Samples were collected in an anaerobic chamber with an N2-H2 (96:4) atmosphere. After 30 min, acidified culture medium (1 ml) was filtered through a 0.2-μm-pore-diameter filter and properly diluted before the concentration of Mn(II) was determined by atomic absorption spectroscopy (SpectrAA-300; Varian Nederland B. V.). An air-acetylene flame was used, the wavelength was 403.1 nm, and the lamp current was 5 mA. Fe(II) production was determined by measuring the accumulation of HCl-soluble Fe(II) at the end of the experiment. As previously described (24), the amount of Fe(II) that was soluble after a 30-min extraction in 0.5 N hydrochloric acid was determined with ferrozine. Samples for Fe(II) determinations were also collected in an anaerobic chamber with an N2-H2 (96:4) atmosphere. Methane production was determined as previously described (7).
Electrons transferred to humic substances were quantified as previously described (28). Samples were collected in an anaerobic chamber with an N2-H2 (96:4) atmosphere and filtered through a 0.2-μm-pore-diameter filter. Anaerobic Fe(III) citrate solution (final concentration, 10 mM) was added to filtrates, and after 30 min of reaction, subsamples were taken for Fe(II) determinations. When no Fe(III) citrate was added to liquid samples and Fe(II) determinations were carried out, negligible recovery of electrons was achieved beyond that seen with the endogenous control, indicating the lack of iron bound in the sources of humus applied.
Sulfate concentrations were determined by injecting 30-μl samples with an autosampler (Marathon) into a high-performance liquid chromatograph equipped with a VYDAC ion chromatography column (302 IC; 250 by 4.6 mm). The temperatures of the column and detector (Waters 431 conductivity detector) were 20 and 35°C, respectively. As an eluent, 0.018 M potassium biphthalate was used at a rate of 1.2 ml per min. Samples for sulfate analysis were fixed by two- to fourfold dilution with a 0.1 M zinc acetate solution, centrifuged (10,000 × g, 3 min), and diluted with demineralized water. Nitrate and nitrite concentrations were also determined with a high-performance liquid chromatograph equipped with the same column as that used for sulfate analysis and at the same temperatures. Thirty-microliter samples were also injected with an autosampler. Potassium dihydrogen phosphate (10 g per liter, pH 3) adjusted with phosphoric acid was used as an eluent at a flow rate of 1.5 ml per min. Nitrate and nitrite were detected with a UV detector (783 UV Detector-Kratos Analytical USA) at a wavelength of 205 nm. All samples were centrifuged (10,000 × g, 3 min) before analysis.
The production of 13CO2 from [13C]toluene was quantified based on the ratio of 13CO2 to 12CO2 in 100-μl headspace samples. Carbon has two stable isotopes, with 12C comprising 98.89% and 13C comprising 1.11% of the total abundance (14). Samples were injected into a gas chromatograph (Hewlett-Packard 5890 series II ) equipped with a fused-silica capillary column (PoraplotQ; Chrompack, Bergen op Zoom, The Netherlands), which was connected to a mass spectrometer-selective detector (Hewlett-Packard 5971 series). Helium was used as a carrier gas at a flow rate of 1.5 ml per min. The temperatures of the injector port and detector were 100 and 280°C, respectively. The oven temperature was maintained at 40°C during the first 3 min and then gradually (20°C per min) was increased to 240°C for achieving [13C]toluene quantification in the same samples. The extent of mineralization of [13C]toluene was calculated according to the concentrations of 13CO2 measured in the headspace, which were corrected for the theoretical amount of 13CO2 dissolved in the liquid phase based on Henry's law. These data were corroborated by carrying out representative bioassays at the end of the experiments from which total recovery of 13CO2 was achieved by acidification with concentrated hydrochloric acid. The data obtained from these representative cultures were very closely related (more than 90% similar) to those theoretically calculated.
Chemicals.
AQDS was purchased from Aldrich Chemical (Milwaukee, Wis.). Toluene (99.5%) and humic acid sodium salt were purchased from Janssen Chimica (Geel, Belgium). Hexadecane (99%) was purchased from Acros Organics (Geel, Belgium). Uniformly labeled [13C]toluene (99% 13C) was purchased from Campro Scientific (Veenendaal, The Netherlands). HPSHA were purchased from the International Humic Substances Society. The elemental composition of the soil humic acids was as follows (in percent dry weight): carbon, 58.1; hydrogen, 3.7; oxygen, 34.1; nitrogen, 4.1; and sulfur, 0.4. The phenolic OH content was 1.73 mol per kg of dry humus. Further information can be obtained at the website of the International Humic Substances Society (http://www.ihss.gatech.edu). All other chemicals were obtained from E. Merck AG (Darmstadt, Germany).
RESULTS
Biodegradation of toluene with alternative electron acceptors.
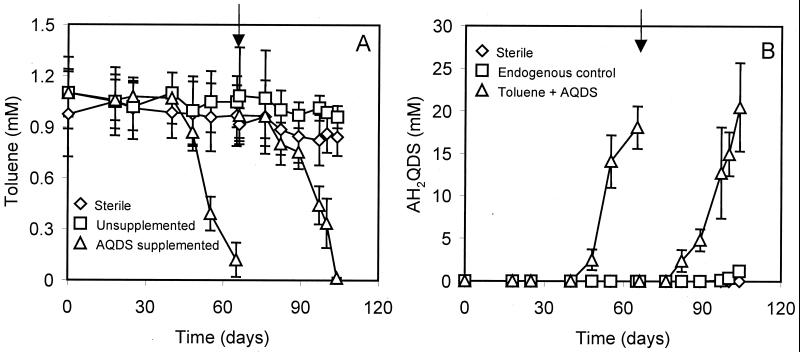
APH sediment degraded toluene in the absence of oxygen when AQDS was included in the medium. During the initial exposure, toluene (1 mM) was completely eliminated after 2 months of incubation (with a lag phase of 40 days), and there was a concomitant reduction of AQDS to AH2QDS. When the contents of the bioassay bottles were decanted and the bottles were refilled with fresh medium containing AQDS (25 mM) and toluene (1 mM), the lag phase was significantly decreased and the same rate of toluene degradation was observed (Fig. 1A). There was no significant loss of toluene when bicarbonate was provided as a sole electron acceptor, nor was methane production detectable. When toluene was incubated with AQDS in autoclaved sediment, no significant loss of toluene was observed. In the biologically active sediment, the consumption of toluene agreed with the reduction of AQDS (Fig. 1B). The ratio of AQDS reduction (corrected for the endogenous control) to toluene degradation was 20.2 ± 5.2 (mean and standard error; n = 3); this value is very close to the stoichiometric value (Table 1), suggesting that toluene was probably completely converted to carbon dioxide under these conditions. Only negligible endogenous AQDS reduction occurred when toluene was omitted from the cultures but the same amount of hexadecane (0.2% [vol/vol]) was included. No reduction of AQDS was detected in the sterilized control.
FIG. 1.
Simultaneous toluene conversion (A) and AQDS reduction (B) by APH sediment in anaerobic culture bottles containing bicarbonate-buffered basal medium supplemented with 25 mM AQDS. The unsupplemented control was prepared in the same manner but without AQDS. The endogenous control (without toluene addition) contained the same amount of hexadecane (0.2% [vol/vol]) as that used for toluene addition. AQDS reduction was quantified spectrophotometrically as the increase in absorbance at 450 nm. Data are means and standard deviations for triplicate incubations in each treatment. Arrows indicate the addition of fresh medium containing AQDS and toluene in depleted bioassay mixtures.
TABLE 1.
Thermodynamic comparisons of the biodegradation of toluene with alternative electron acceptorsa
| Reaction | ΔG°′ (kJ/mol) |
|---|---|
| C7H8 + 36 Fe3+ + 21 H2O → 36 Fe2+ + 43 H+ + 7 HCO3− | −3,629.6 |
| C7H8 + 7.2 NO3− + 0.2 H+ → 3.6 N2 + 0.6 H2O + 7 HCO3− | −3,554.8 |
| C7H8 + 18 MnO2 + 18 H2CO3 → 7 CO2 + 18 MnCO3 + 22 H2O | −3,358.8b |
| C7H8 + 36 FeO(OH) + 36 H+ → 7 CO2 + 36 Fe(OH)+ + 22 H2O | −1,443.6b |
| C7H8 + 18 AQDS + 21 H2O → 18 AH2QDS + 7 H+ + 7 HCO3− | −319.7 |
| C7H8 + 4.5 SO42− + 3 H2O → 4.5 HS− + 2.5 H+ + 7 HCO3− | −205.2 |
| C7H8 + 7.5 H2O → 4.5 CH4 + 2.5 H+ + 2.5 HCO3− | −130.7 |
The possibility that toluene degradation in APH sediment was linked to the reduction of other anoxic electron acceptors was explored. Of all the alternative electron acceptors tested, only nitrate, Mn(IV), and AQDS supported toluene degradation. No toluene degradation was detected under sulfate-reducing or methanogenic conditions after 4 months of incubation. Also, no degradation of toluene was observed when Fe(III) in the form of goethite was used as a direct electron acceptor during the same incubation period. These results coincided with the absence of methane production and Fe(II) production as well as the lack of sulfate elimination during the experiments.
The conversion of toluene agreed with the reduction of nitrate by APH sediment, and the ratio of nitrate reduction (corrected for the endogenous control) to toluene degradation was 5.9 ± 0.7 (mean and standard error; n = 3); this value is very close to the stoichiometric value (Table 1). Toluene conversion by APH sediment was also evident with the addition of amorphous MnO2 in the medium. In parallel with toluene conversion in the MnO2-supplemented cultures was the partial recovery of acid-extractable Mn(II), accounting for 40% of the electron equivalents in toluene consumed.
Humic acid stimulation of toluene biodegradation linked to metal oxide reduction.
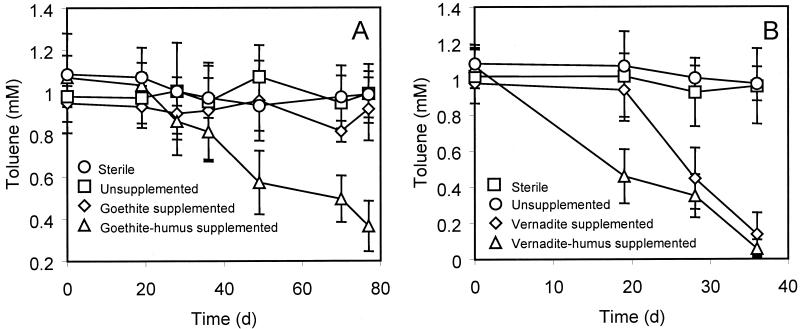
To explore the potential link between the biodegradation of toluene and the dissimilatory reduction of metal oxides by channeling of electrons via humus respiration, APH sediment incubation mixtures were supplemented either with goethite (FeOOH; 50 mM) or with vernadite (MnO2; 25 mM) together with a substoichiometric amount of humic acids (2 g per liter). The electron-accepting capacity of these humic acids was determined as previously described (28) with an acetate-oxidizing humus-respiring enrichment culture; the average electron uptake was 0.306 milliequivalent per g of humic acids. Thus, the addition of these humic acids at this level could account for the biodegradation of only 1.7% of the toluene added to the cultures (1 mM). Nevertheless, when this low level of humic acids was added, more than 65% of the toluene was depleted in the goethite-humus-supplemented cultures by APH sediment after 11 weeks of incubation (Fig. 2A). The consumption of toluene in these cultures paralleled the partial recovery of acid-extractable Fe(II), accounting for 30% of the electron equivalents in toluene consumed. Negligible conversion of toluene and release of acid-extractable Fe(II) occurred in the goethite-supplemented cultures when humic acids were omitted from the medium. Likewise, none of these phenomena appeared in sterilized incubation mixtures with autoclaved sediment supplemented with goethite and humic acids (Fig. 2A).
FIG. 2.
Conversion of toluene by APH sediment in anaerobic culture bottles containing HEPES-buffered basal medium supplemented with amorphous ferric oxyhydroxide (goethite, 50 mM) (A) or amorphous manganese dioxide (vernadite, 25 mM) (B). Goethite-humus- and vernadite-humus-supplemented cultures also contained 2 g of humic acids per liter. Unsupplemented controls were prepared in the same manner but without metal oxide and humus. Sterile controls contained both metal oxide and humus with autoclaved sediment. Data are means and standard deviations for triplicate incubations in each treatment. d, days.
When the same source of humic acids was applied to vernadite-supplemented cultures at the same level, toluene conversion proceeded with a lag phase shorter than that observed in the absence of humic acids (Fig. 2B). Toluene was completely depleted in both instances after 5 weeks of incubation. This result coincided with the partial recovery of acid-extractable Mn(II), accounting for 34% of the electron equivalents in toluene consumed.
[13C]toluene conversion to 13CO2 with AQDS and humic substances as terminal electron acceptors.
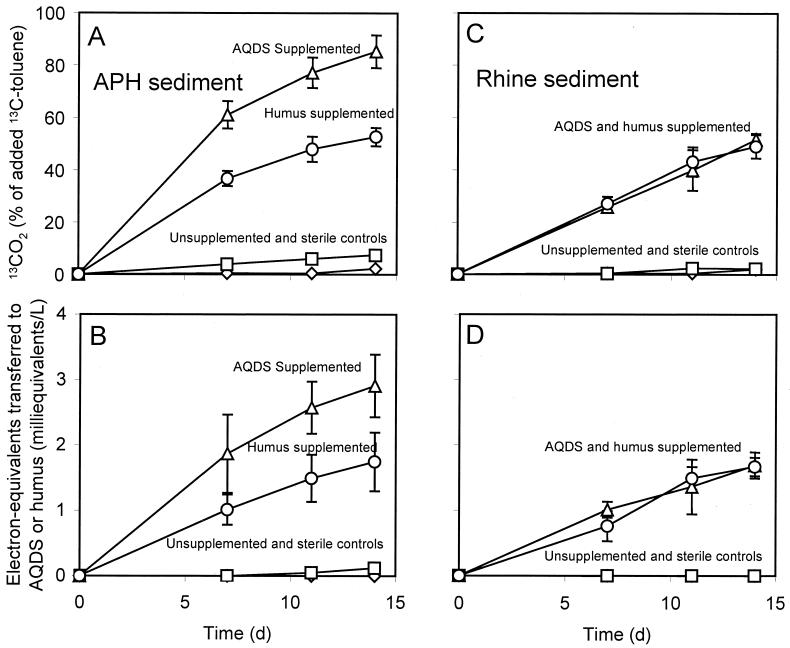
To confirm the mineralization of toluene to CO2 under anoxic quinone- and humus-respiring conditions, enrichment cultures from sediment samples were incubated with uniformly labeled [13C]toluene. Enriched APH sediment was able to convert [13C]toluene to 13CO2 in medium supplemented with AQDS (5 mM) or with HPSHA (12 g per liter) without any lag phase (Fig. 3A). There was negligible recovery of 13CO2 in the endogenous control in the absence of [13C]toluene and in the presence of [13C]toluene and HPSHA incubated with autoclaved sediment. In the absence of AQDS and HPSHA, less than 7% of the added [13C]toluene was recovered as 13CO2, probably due to the presence of small amounts of AQDS remaining in the sediment from previous enrichment. This finding was confirmed by the slight orange color developed in these controls and by the slight reduction of Fe(III) citrate by the culture fluid from these controls (Table 2). The conversion of [13C]toluene to 13CO2 by APH sediment was concomitantly coupled with an increase in electrons recovered as AH2QDS or as reduced humus in the cultures (Fig. 3B). In fact, there were high levels of recovery of both [13C]carbon and electrons in the AQDS- and HPSHA-containing cultures (Table 2). Enriched sediment obtained from the Rhine River was also able to convert [13C]toluene to 13CO2 with AQDS or HPSHA as a terminal electron acceptor, but the rate of toluene mineralization was lower than that observed with enriched APH sediment (Fig. 3C). Controls showed no significant recovery of 13CO2 or [13C]toluene conversion. The extent of [13C]toluene mineralization observed (about 1.8 ± 0.1 milliequivalents per liter in both instances) paralleled the stoichiometric recovery of electrons as AH2QDS or as reduced humus (Fig. 3D). There was negligible recovery of electrons in the sterilized and endogenous (without [13C]toluene addition) controls.
FIG. 3.
Mineralization of [13C]toluene to 13CO2 (A and C) coupled to the reduction of AQDS or humus (B and D) by enriched APH (A and B) or Rhine River (C and D) sediments in anaerobic culture bottles containing phosphate-buffered basal medium supplemented with AQDS (5 mM) or with highly purified soil humic acids (12 g per liter). Uniformly labeled [13C]toluene was added at an initial concentration of 100 μM relative to the liquid volume. Unsupplemented controls were prepared in the same manner but without AQDS and humus. All data were corrected relative to the endogenous control (without [13C]toluene addition). Data are means and standard deviations for triplicate incubations in each treatment. d, days.
TABLE 2.
Balances of electrons and [13C]carbon for the anaerobic conversion of uniformly labeled [13C]toluene with AQDS and HPSHA as terminal electron acceptors by enriched APH sediment after 2 weeks of incubationa
| Balance | Culture | [13C]toluene added | Product and toluene remaining | Total recovery (%)b |
|---|---|---|---|---|
| Electron equivalent (meq/liter)c | Unsupplemented | 3.7 | [13C]toluene remaining (3.4) | |
| Fe(III) citrate reduced (0.1) | ||||
| Total (3.5) | 95 | |||
| AQDS supplemented | 3.4 | [13C]toluene remaining (NDd) | ||
| AH2QDS (2.9) | ||||
| Total (2.9) | 85 | |||
| HPSHA supplemented | 3.5 | [13C]toluene remaining (0.9) | ||
| Fe(III) citrate reduced (1.7) | ||||
| Total (2.6) | 74 | |||
| [13C]carbon (mmol of 13C/liter)e | Unsupplemented | 0.75 | [13C]toluene remaining (0.69) | |
| 13CO2 (0.05) | ||||
| Total (0.74) | 99 | |||
| AQDS supplemented | 0.69 | [13C]toluene remaining (ND) | ||
| 13CO2 (0.63) | ||||
| Total (0.63) | 91 | |||
| HPSHA supplemented | 0.71 | [13C]toluene remaining (0.18) | ||
| 13CO2 (0.39) | ||||
| Total (0.57) | 80 |
Data represent means obtained from triplicate incubations for the different conditions applied, and standard deviations were, in general, within 10% of the means. Negligible conversion of [13C]toluene and reduction of the corresponding electron acceptor occurred in sterilized incubations with autoclaved sediment.
Total recovery of electrons = (electrons recovered in electron acceptor + [13C]toluene not consumed)/[13C]toluene added. Total recovery of [13C]carbon = (carbon recovered as 13CO2 + [13C]toluene not consumed)/[13C]toluene added.
Corrected for endogenous controls. Less than 2% of endogenous reduction occurred in all instances.
ND, not detected.
Corrected for the background level of 13CO2 found in the absence of [13C]toluene.
DISCUSSION
Humic substances as terminal electron acceptors for the anoxic microbial oxidation of toluene.
In the present study, humic acids and the humic model compound AQDS were explored as potential electron acceptors for achieving anoxic microbial oxidation of toluene by different inocula. Toluene biodegradation was coupled to the reduction of humic acids and AQDS by APH and Rhine River sediments. The results from this study provide multiple lines of evidence that humic compounds are implicated in the anoxic biodegradation of toluene. First, toluene biodegradation became feasible when the anaerobic sediments were supplied with HPSHA and AQDS. Second, the electron equivalents from the consumed toluene were recovered at high levels as AH2QDS (85%) and reduced humic acids (65%) when AQDS and HPSHA, respectively, served as the terminal electron acceptors. Third, uniformly labeled [13C]toluene was mineralized to 13CO2, and the recovery of 13C-labeled carbon as 13CO2 accounted for 74 to 91% of the [13C]toluene consumed. The results constitute a clear quantitative demonstration of anoxic aromatic hydrocarbon biodegradation linked to the reduction of quinones and humic acids.
Previously, Lovley et al. (28) hypothesized that humus served as a direct electron acceptor during benzene biodegradation when humic acids were added as chelators to increase Fe(III) oxide bioavailability for a benzene-degrading Fe(III)-reducing consortium in contaminated sediment. This hypothesis was based on the observation that humic acids stimulated benzene biodegradation better than synthetic chelators (e.g., EDTA and nitrilotriacetic acid) even though humus had inferior chelating properties (27). The mechanism proposed implies that benzene was degraded with humic substances acting as the direct electron acceptors and that the obtained reduced humus was recycled back to the oxidized form by chemical reaction with Fe(III) oxides. The impact of AQDS on anaerobic benzene oxidation was also studied at three different sites of Fe(III)-reducing sediments (1). Stimulation of benzene oxidation was observed at one site when 600 μM AQDS was applied; this result may have been due to the use of AQDS as an electron acceptor, but the reduction of AQDS was not demonstrated. The same sediment sample did not oxidize benzene when 300 μM AQDS was applied, yet the amount of benzene added (12 μM) would have required only 180 μM AQDS for complete oxidation. Strains of Geobacter have been isolated that can oxidize toluene with Fe(III) as an electron acceptor (10). The same strains can also reduce AQDS with acetate; thus, it is conceivable that they could couple toluene oxidation to AQDS reduction.
The Gibbs free energy of toluene degradation linked to AQDS reduction is more favorable than that of degradation linked to sulfate reduction and methanogenesis (Table 1). Thermodynamic differences might partly explain why toluene degradation in this study readily occurred with AQDS but not with sulfate or bicarbonate as electron acceptors. Biodegradation of toluene coupled to sulfate reduction (3, 30) and methanogenesis (13, 18, 40) has previously been reported to occur, but these processes usually require long lag periods before rates become appreciable.
The anoxic biodegradation of toluene with humic substances as terminal electron acceptors was not evident at all sites and was found only at historically polluted sites, indicating long-term enrichment of hydrocarbon-degrading microorganisms after prolonged exposure to aromatic hydrocarbon pollutants. Sludge, soil, and sediment materials from pristine sites previously reported to degrade readily biodegradable compounds with AQDS as a terminal electron acceptor (7) were not able to degrade toluene under AQDS-reducing conditions (data not shown).
APH sediment showed the capacity to utilize other, more favorable electron acceptors [nitrate and Mn(IV)] to support the biodegradation of toluene, reflecting the notion that this consortium may contain a wide variety of microorganisms with different capacities to degrade aromatic hydrocarbons or that microorganisms involved in toluene biodegradation may achieve this anoxic process with different electron acceptors. Other consortia have previously shown the capacity to degrade toluene with both nitrate and Mn(IV) as terminal electron acceptors (23).
Humic acid stimulation of toluene biodegradation linked to metal oxide reduction.
Goethite was not utilized directly as an electron acceptor by APH sediment to achieve the anoxic biodegradation of toluene. Conversion of toluene was made feasible only by supplementing the goethite-containing cultures with substoichiometric levels of humic acids in terms of electron-accepting equivalents. The electron-accepting capacity of the humic acids tested could account for only 1.7% of the potentially degradable toluene, and yet 65% of the toluene was degraded in these experiments. The stimulation can thus be accounted for only by a chelating effect of humic acids with Fe(III) (27) or a redox-mediating effect (28). Based on previous observations in the literature that demonstrated the involvement of humic substances as redox mediators linking the oxidation of simple substrates (e.g., acetate) to goethite reduction (17, 26, 28), it is plausible that goethite reduction by toluene degraders in APH sediment was a result of reduced humic acids acting as electron shuttles in the goethite-humus bioassays. Non-iron-reducing bacteria, e.g., Propionibacterium freudenreichii, were recently reported to channel electrons from anaerobic oxidation via humic acids toward Fe(III) reduction, suggesting that dissimilatory iron reduction in soil and sediment may not be exclusively related to iron-reducing microorganisms (4). Hydroquinones in humus can reach micropores that remain inaccessible to Fe(III)-reducing microorganisms (41) and may eliminate the need for direct contact between humus-reducing microorganisms and metal oxides as a prerequisite for achieving anoxic organic matter oxidation.
The partial recovery of electron equivalents from converted toluene either as Mn(II) or as Fe(II) in the metal oxide-humus-supplemented cultures may be explained by a series of postreduction biogeochemical reactions. Biogenic Fe(II) and Mn(II) might have undergone sorption to bacteria or to the residual metal oxide surface, as well as precipitation with sulfide (6, 42), which may have accounted for decreased recovery during the acid extraction technique applied.
Ecological implications.
The results presented in this study for toluene and previous results obtained with vinyl chloride and dichloroethene (5) suggest that humus, the most abundant organic matter in nature, may be a more important electron acceptor for the bioremediation of contaminated environments than previously thought. Biodegradation of recalcitrant contaminants may take place in sediments, wetlands, and eutrophic lakes rich in organic matter and in microniches in compost, where humic substances could serve as potential electron acceptors for the anoxic microbial oxidation of a wide variety of organic pollutants. Moreover, quinone- or humus-reducing bacteria and activities have previously been found in many organic matter-rich environments (7, 9). Therefore, intrinsic bioremediation may be much more prevalent in these habitats than previously considered. Humic substances may also greatly stimulate the anoxic biodegradation of organic contaminants in oligotrophic environments as well by linking the biodegradation of these pollutants to the reduction of other electron acceptors. In particular, quinones in humus may channel electrons from anoxic pollutant oxidation to metal oxide reduction by serving as redox mediators, a scenario which was shown to occur for the anoxic oxidation of methyl tert-butyl ether (15).
ACKNOWLEDGMENTS
This research was financially supported by the Council of Science and Technology of Mexico (CONACyT).
We thank Wim Roelofsen for technical assistance during measurements of labeled toluene and Alfons Stams for critical review.
REFERENCES
- 1.Anderson R T, Lovley D R. Naphthalene and benzene degradation under Fe(III)-reducing conditions in petroleum-contaminated aquifers. Bioremediat J. 1999;3:121–135. [Google Scholar]
- 2.Atkinson R J, Posner A M, Quirk J P. Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface. J Phys Chem. 1967;71:550–558. [Google Scholar]
- 3.Beller H R, Spormann A M, Sharma P K, Cole J R, Reinhard M. Isolation and characterization of a novel toluene-degrading sulfate-reducing bacterium. Appl Environ Microbiol. 1996;62:1188–1196. doi: 10.1128/aem.62.4.1188-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz M, Schink B, Brune A. Humic acid reduction by Propionibacterium freudenreichii and other fermentative bacteria. Appl Environ Microbiol. 1998;64:4507–4512. doi: 10.1128/aem.64.11.4507-4512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley P M, Chapelle F H, Lovley D R. Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl Environ Microbiol. 1998;64:3102–3105. doi: 10.1128/aem.64.8.3102-3105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdige D J, Nealson K H. Chemical and microbiological studies of sulfide-mediated manganese reduction. Geomicrobiol J. 1986;4:361–387. [Google Scholar]
- 7.Cervantes F J, van der Velde S, Lettinga G, Field J A. Competition between methanogenesis and quinone respiration for ecologically important substrates in anaerobic consortia. FEMS Microbiol Ecol. 2000;34:161–171. doi: 10.1111/j.1574-6941.2000.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes F J, van der Velde S, Lettinga G, Field J A. Quinones as terminal electron acceptors for anaerobic microbial oxidation of phenolic compounds. Biodegradation. 2000;11:313–321. doi: 10.1023/a:1011118826386. [DOI] [PubMed] [Google Scholar]
- 9.Coates J D, Ellis D J, Roden E, Gaw K, Blunt-Harris E L, Lovley D R. Recovery of humic-reducing bacteria from a diversity of sedimentary environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates J D, Bhupathiraju V K, Achenbach L A, Mclnerney M J, Lovley D R. Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, three new, strict anaerobic, dissimilatory Fe(III)-reducers. Int J Syst Evol Microbiol. 2001;51:581–588. doi: 10.1099/00207713-51-2-581. [DOI] [PubMed] [Google Scholar]
- 11.Cuypers M P, Grotenhuis J T C, Rulkens W H. Characterisation of PAH-contaminated sediments in a remediation perspective. Water Sci Technol. 1998;37:157–164. [Google Scholar]
- 12.Dean B J. Genetic toxicology of benzene, toluene, xylenes and phenols. Mutat Res. 1978;47:75–97. doi: 10.1016/0165-1110(78)90014-3. [DOI] [PubMed] [Google Scholar]
- 13.Edwards E A, Grbiæ-Galiæ D. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microbiol. 1994;60:313–322. doi: 10.1128/aem.60.1.313-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faure G. Principles of isotope geology. New York, N.Y: John Wiley & Sons, Inc.; 1986. [Google Scholar]
- 15.Finneran K T, Lovley D R. Anaerobic degradation of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) Environ Sci Technol. 2001;35:1785–1790. doi: 10.1021/es001596t. [DOI] [PubMed] [Google Scholar]
- 16.Frazer A C, Coschigano P W, Young L Y. Toluene metabolism under anaerobic conditions: a review. Anaerobe. 1995;1:293–303. doi: 10.1006/anae.1995.1030. [DOI] [PubMed] [Google Scholar]
- 17.Fredrickson J K, Kostandarithes H M, Li S W, Plymale A E, Daly M J. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol. 2000;66:2006–2011. doi: 10.1128/aem.66.5.2006-2011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grbić-Galić D, Vogel T M. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987;53:254–260. doi: 10.1128/aem.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostka J, Nealson K H. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria. In: Burlage R S, Atlas R, Stahl D, Geesey G, Sayler G, editors. Techniques in microbial ecology. New York, N.Y: Oxford University Press; 1998. pp. 58–78. [Google Scholar]
- 20.Langenhoff A A M. Ph.D. thesis. Biodegradation of toluene, benzene and naphthalene under anaerobic conditions. Wageningen, The Netherlands: Wageningen University; 1997. [Google Scholar]
- 21.Langenhoff A A M, Zehnder A J B, Schraa G. Behavior of toluene, benzene and naphthalene under anaerobic conditions in sediment columns. Biodegradation. 1996;7:267–274. [Google Scholar]
- 22.Langenhoff A A M, Brouwers-Ceiler D L, Engelberting J H L, Quist J J, Wolkenfelt J G P N, Zehnder A J B, Schraa G. Microbial reduction of manganese coupled to toluene oxidation. FEMS Microbiol Ecol. 1997;22:119–127. [Google Scholar]
- 23.Langenhoff A A M, Briglia M, Nijenhuis I, Tan N C G, Zehnder A J B, Schraa G. Characterization of a manganese-reducing, toluene-degrading enrichment culture. FEMS Microbiol Ecol. 1997;24:113–125. [Google Scholar]
- 24.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron and manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 27.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 29.Meckenstock R U. Fermentative toluene degradation in anaerobic defined syntrophic cocultures. FEMS Microbiol Lett. 1999;177:67–73. doi: 10.1111/j.1574-6968.1999.tb13715.x. [DOI] [PubMed] [Google Scholar]
- 30.Rabus R, Nordhaus R, Ludwig W, Widdel F. Complete oxidation of toluene under strictly anoxic conditions by a new sulfate-reducing bacterium. Appl Environ Microbiol. 1993;59:1444–1451. doi: 10.1128/aem.59.5.1444-1451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott D T, McKnight D M, Blunt-Harris E L, Kolesar S E, Lovley D R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]
- 32.Smith M R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1:191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- 33.Smith M R. The physiology of aromatic hydrocarbon degrading bacteria. In: Ratledge C, editor. Biochemistry of microbial degradation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 347–378. [Google Scholar]
- 34.Sober H A. Handbook of biochemistry: selected data for molecular biology. Cleveland, Ohio: The Chemical Rubber Co.; 1970. [Google Scholar]
- 35.Stevenson F J. Humus chemistry: genesis, composition, reactions. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 36.Stone A T, Morgan J J. Reduction of manganese(III) and manganese(IV) oxides by organics. 1. Reaction with hydroquinone. Environ Sci Technol. 1984;18:450–456. doi: 10.1021/es00124a011. [DOI] [PubMed] [Google Scholar]
- 37.Tan N C G, Prenafeta-Boldú F X, Opsteeg J L, Lettinga G, Field J A. Biodegradation of azo dyes in cocultures of anaerobic granular sludge with aerobic aromatic amine degrading enrichment cultures. Appl Microbiol Biotechnol. 1999;51:865–871. doi: 10.1007/s002530051475. [DOI] [PubMed] [Google Scholar]
- 38.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Public Health Service. Toxicological profile for toluene. Publication ATSDR/TP-89/23. Atlanta, Ga: Agency for Toxic Substances and Disease Registry, U.S. Public Health Service; 1989. [Google Scholar]
- 40.Wilson B H, Smith G B, Rees J F. Biotransformation of selected alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic aquifer material: a microcosm study. Environ Sci Technol. 1986;20:997–1002. doi: 10.1021/es00152a005. [DOI] [PubMed] [Google Scholar]
- 41.Zachara J M, Fredrickson J K, Li S M, Kennedy D W, Smith S C, Gassman P L. Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am Mineralogist. 1998;83:1426–1443. [Google Scholar]
- 42.Zachara J M, Fredrickson J K, Smith S C, Gassman P L. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim Cosmochim Acta. 2001;65:75–93. [Google Scholar]