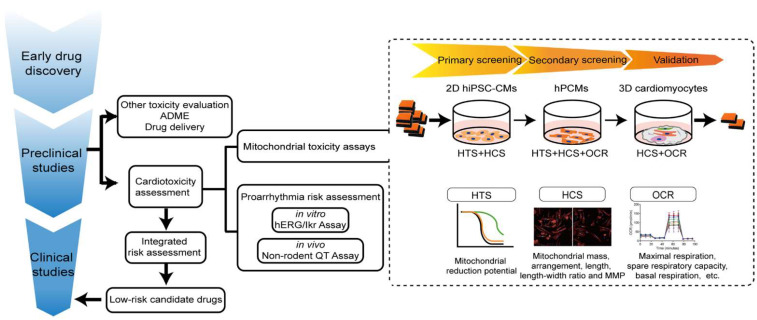
Figure 3.
Proposed workflow of mitochondrial toxicity evaluation during preclinical cardiotoxicity profiling. Mitochondrial toxicity assays can be conducted in parallel with the existing proarrhythmic risk assessments to aid the selection of safer drugs for subsequent clinical studies. In our proposed workflow, 2D hiPSC-CMs, hPCMs, and 3D hiPSC-derived cardiomyocytes models can be cultured in 96- or 384-well assay plates, and treated with candidate drugs for primary screening, secondary screening, and subsequent validation, respectively. Primary assays can be performed firstly by PrestoBlue staining for measurement of reduction potential. Then, the fluorescent images can be captured in a high-content manner after MitoTracker and TMRM staining. Secondary screening combines microplate reading, HCS, and OCR measurement. Validation can be conducted with HCS and OCR measurement. These readouts (reduction potential, mitochondrial mass, distribution, morphology, MMP, and OCR) can be subsequently quantified to identify mitochondrially safe drugs. Abbreviations: ADME: absorption, distribution, metabolism, and excretion; hERG: human ether-a-go-go-related current; 2D hiPSC-CMs: two-dimensional human induced pluripotent-stem-cell-derived cardiomyocytes; HCS: high-content screening; HTS: high-throughput screening; OCR: oxygen consumption rate.