Abstract
The effect of pH on the pore-forming ability of two Bacillus thuringiensis toxins, Cry1Ac and Cry1C, was examined with midgut brush border membrane vesicles isolated from the tobacco hornworm, Manduca sexta, and a light-scattering assay. In the presence of Cry1Ac, membrane permeability remained high over the entire pH range tested (6.5 to 10.5) for KCl and tetramethylammonium chloride, but was much lower at pH 6.5 than at higher pHs for potassium gluconate, sucrose, and raffinose. On the other hand, the Cry1C-induced permeability to all substrates tested was much higher at pH 6.5, 7.5, and 8.5 than at pH 9.5 and 10.5. These results indicate that the pores formed by Cry1Ac are significantly smaller at pH 6.5 than under alkaline conditions, whereas the pore-forming ability of Cry1C decreases sharply above pH 8.5. The reduced activity of Cry1C at high pH correlates well with the fact that its toxicity for M. sexta is considerably weaker than that of Cry1Aa, Cry1Ab, and Cry1Ac. However, Cry1E, despite having a toxicity comparable to that of Cry1C, formed channels as efficiently as the Cry1A toxins at pH 10.5. These results strongly suggest that although pH can influence toxin activity, additional factors also modulate toxin potency in the insect midgut.
The insecticidal crystal toxins produced by Bacillus thuringiensis act by forming pores in the midgut luminal membrane of susceptible insects after proteolytic conversion of the crystal proteins to their toxic form and binding to specific receptors (19, 30, 31, 41). The pH of the lepidopteran midgut lumen ranges from about 8 to above 12, depending on species and site along the midgut (8, 9). The highly alkaline and reducing conditions found in the midgut of lepidopteran insects clearly constitute crucial factors in the solubilization of the crystals and proteolytic activation of the toxins (1, 17). However, although high pH is also thought to play a critical role in the activity of the activated toxins (30, 31, 41), most studies on different aspects of the mode of action of B. thuringiensis toxins, including receptor binding and pore formation, have been carried out at near neutral pH.
Insecticidal activity decreases rapidly following exposure of protoxins to pHs below 2 or above 11 (28). In solution, different toxins undergo conformational transitions in response to changes in pH (5–7, 10, 40). The extent of the observed transitions varies considerably, however, apparently depending on the experimental conditions used (10). Furthermore, it remains to be established whether these conformational changes translate into modified toxin activity.
Few studies have examined systematically the effects of pH on the functional properties of the activated toxins. The susceptibility of Cf1 cultured cells to Cry1A toxins is greatly increased as pH is raised from 8 to 10 (14). In contrast, the rate at which Cry1C dissipates an electrical potential generated across the membrane of unilamellar liposomes, by valinomycin-induced efflux of K+ ions, is very similar at pH 7.4 and 10.0, but increases greatly at pH 4.0 (2). In receptor-free planar lipid bilayers, Cry1C forms cation-selective channels at pH 9.5, whereas both cation-selective and anion-selective channels are detected at pH 6.0 (33). In planar lipid bilayers fused to intestinal brush border membrane vesicles isolated from Manduca sexta, the smallest conductance increment observed in the presence of Cry1Ac was 2 nS at pH 8.8 and 13 nS at pH 9.6, suggesting the formation of substantially larger pores at the higher pH (24). However, the internal diameter of the pores formed by Cry1Ac, estimated from the permeability of M. sexta brush border membrane vesicles to neutral solutes of increasing size, only changed from 2.4 nm at pH 8.7 to 2.6 nm at pH 9.8 (4).
In the present study, the membrane-permeabilizing effects of Cry1Ac and Cry1C were examined in M. sexta brush border membrane vesicles over a wide range of pHs (6.5 to 10.5). The pore-forming properties of both toxins varied as a function of pH but followed strikingly different patterns. The role of high pH in toxin function was also investigated by comparing the in vitro pore-forming ability of six B. thuringiensis toxins at pH 10.5 with their toxicities. The results of both assays correlated well for all toxins except Cry1E, which was among the most active toxins in vitro despite its relatively low toxicity for M. sexta larvae.
MATERIALS AND METHODS
Preparation of brush border membrane vesicles.
M. sexta larvae were obtained from the Carolina Biological Supply Company (Burlington, N.C.) and reared on the artificial diet supplied with the insects. Whole midguts were isolated from fifth-instar larvae, freed of attached Malpighian tubules, cut longitudinally to remove the peritrophic membrane and gut contents, and rinsed thoroughly with ice-cold 300 mM sucrose–17 mM Tris-HCl (pH 7.5)–5 mM EGTA. Brush border membrane vesicles were prepared with the Mg2+ precipitation and differential centrifugation method described by Wolfersberger et al. (42).
Toxins.
Cry1Aa, Cry1Ab, Cry1Ac, Cry1B, Cry1C, and Cry1E toxins were produced as insoluble inclusions in Escherichia coli, solubilized, and trypsin activated as described elsewhere (26). Activated toxins were purified by fast protein liquid chromatography using a Mono Q ion-exchange column (Pharmacia Biotech, Montreal, Canada) and eluting bound toxin with a 50 to 500 mM NaCl gradient in 40 mM carbonate buffer (pH 10.5) (26).
Light-scattering assay.
The membrane-permeabilizing effects of B. thuringiensis toxins were analyzed with an osmotic swelling assay as described by Carroll and Ellar (3). Brush border membranes were first resuspended to about 90% of the desired final volume in 10 mM of either MES (morpholineethanesulfonic acid)-KOH (pH 6.5), HEPES-KOH (pH 7.5), Tris-HCl (pH 8.5), 2-[N-cyclohexylamino]ethanesulfonic acid–KOH (pH 9.5), or 3-[cyclohexylamino]-1-propanesulfonic acid–KOH (pH 10.5) and incubated overnight at 4°C. At least 1 h before the start of the osmotic swelling experiments, the suspension was further diluted to a final concentration of 0.4 mg of membrane protein/ml with the appropriate buffer supplemented with enough bovine serum albumin to achieve a final concentration of 1 mg/ml.
In most experiments, the vesicles were preincubated at 23°C for 60 min with the specified toxin concentration, ranging from 5 to 150 pmol of toxin/mg of membrane protein (2 to 60 nM). The assay was initiated by rapidly mixing the vesicles with an equal volume of 10 mM of the appropriate buffer, 1 mg of bovine serum albumin per ml, and either 150 mM KCl, tetramethylammonium chloride, or potassium gluconate or 300 mM sucrose or raffinose with a Hi-Tech Scientific (Salisbury, England) stopped-flow rapid kinetics apparatus. Alternatively, in experiments designed to monitor toxin-induced increases in membrane permeability to KCl, toxin was added to the KCl solution before mixing with the vesicles, without preincubation. Scattered light intensity was monitored at a wavelength of 450 nm, with a photomultiplier tube at a 90° angle from the incident light beam, at 23°C in a PTI spectrofluorometer (Photon Technology International, South Brunswick, N.J.). Data were recorded every 0.1 s.
Data analysis.
Percent volume recovery was defined as 100 (1 − It), where It is the relative scattered light intensity measured at time t. For pore formation kinetic experiments, percent volume recovery was calculated for every experimental point, and values obtained with control vesicles, assayed without added toxin, were subtracted from the experimental values measured in the presence of toxin. Experiments, each carried out in quintuplicate, were performed three times with different vesicle preparations. Because variations were usually much greater between experiments carried out with different batches of vesicles than among replicate assays using the same vesicle preparation, each set of replicate values was averaged, and data are reported as means ± standard error of the mean (SEM) of these average values, each taken as n = 1.
RESULTS
Toxin-induced permeability to KCl.
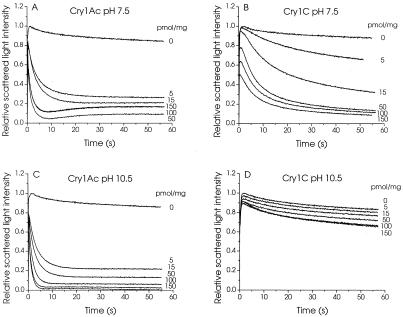
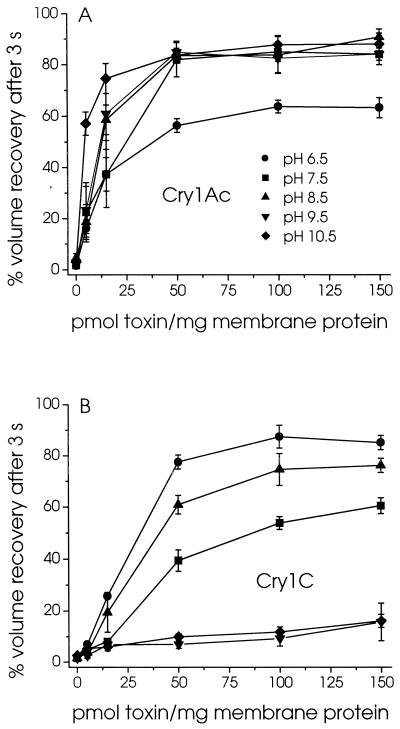
Brush border membrane vesicles isolated from M. sexta midguts were preincubated with either Cry1Ac or Cry1C for 1 h and subjected to a hypertonic shock by rapidly mixing them with an equal volume of 150 mM KCl (Fig. 1). Their volume decreased rapidly, as evidenced by a sharp rise in scattered light intensity. Depending on their permeability to KCl, the vesicles subsequently recovered some of their original volume, as shown by a gradual decrease in scattered light intensity. In the presence of Cry1Ac, the extent of volume recovery after a given time increased rapidly with increasing toxin concentration at both pH 7.5 and 10.5 (Fig. 1A and C). In contrast, in the presence of Cry1C, volume recovery increased much more rapidly as a function of toxin concentration at pH 7.5 (Fig. 1B) than at pH 10.5 (Fig. 1D). Although at pH 7.5 the vesicles swelled to a similar extent in the presence of high concentrations of both toxins, the swelling rates were much higher for Cry1Ac (Fig. 1A) than for Cry1C (Fig. 1B). The results of similar experiments, carried out over a wide range of pHs, are summarized in Fig. 2. pH had very little effect on the activity of Cry1Ac between pH 7.5 and 10.5, but the rates at which the vesicles swelled were significantly lower at pH 6.5 (Fig. 2A). In contrast, whereas the permeability induced by Cry1C was somewhat comparable to that induced by Cry1Ac at pH 6.5, 7.5, and 8.5, it decreased sharply as pH was raised to 9.5 and 10.5 (Fig. 2B).
FIG. 1.
Effect of pH on the osmotic swelling of M. sexta midgut brush border membrane vesicles induced by Cry1Ac and Cry1C. Vesicles (0.4 mg of membrane protein/ml), isolated from fifth-instar larvae and equilibrated overnight in 10 mM HEPES-KOH (pH 7.5) (A and B) or CAPS-KOH (pH 10.5) (C and D), were incubated for 60 min with the indicated concentrations of Cry1Ac (A and C) or Cry1C (B and D). Vesicles were rapidly mixed with an equal volume of 150 mM KCl, 1 mg of bovine serum albumin per ml, and 10 mM HEPES-KOH (pH 7.5) (A and B) or CAPS-KOH (pH 10.5) (C and D) directly in a cuvette using a stopped-flow apparatus. Scattered light intensity was measured at an angle of 90° in a PTI spectrofluorometer. Each tracing represents the average of five experiments.
FIG. 2.
Effect of pH on the KCl permeability of the pores formed by Cry1Ac and Cry1C in midgut brush border membrane vesicles. Vesicles were incubated for 60 min with the indicated concentrations of Cry1Ac (A) or Cry1C (B) in 1 mg of bovine serum albumin per ml and 10 mM MES-KOH (pH 6.5) (●), HEPES-KOH (pH 7.5) (■), Tris-HCl (pH 8.5) (▴), CHES-KOH (pH 9.5) (▾), or CAPS-KOH (pH 10.5) (⧫). Their permeability to KCl was then assayed by monitoring scattered light intensity after rapid mixing with an equal volume of the same buffer supplemented with 150 mM KCl. Percent volume recovery was calculated as described in Materials and Methods.
A similar pattern was observed when the vesicles were exposed to the toxin at the time that they were mixed with KCl, without preincubation (Fig. 3). In the presence of Cry1Ac, the vesicles swelled rapidly at every pH tested, although a slightly longer delay before the onset of swelling and a slightly reduced rate of swelling were observed at pH 10.5 (Fig. 3A). In contrast, in the presence of Cry1C, both the delay preceding the onset of swelling and the swelling rate decreased gradually with increasing pH (Fig. 3B). This reduction in the permeabilizing effect of the toxin was especially strong at pH 9.5 and 10.5.
FIG. 3.
Effect of pH on the kinetics of pore formation by Cry1Ac and Cry1C. Permeability of the vesicles to KCl was assayed as described in the legend to Fig. 2 except the vesicles were not preincubated with the toxin. Instead, 150 pmol/mg of membrane protein of either Cry1Ac (A) or Cry1C (B) was added to the KCl solution before mixing with the vesicles. Percent volume recovery was calculated for each experimental point, and the values measured for control vesicles, assayed in the absence of toxin, were subtracted from those obtained in the presence of toxin. For clarity, error bars are shown for every 50th experimental point.
Effect of pH on ionic selectivity of Cry1C-induced channels.
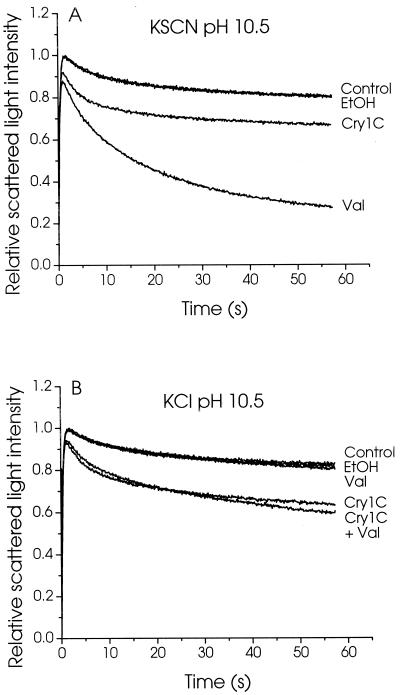
Because, in the presence of a salt gradient, the rate at which the vesicles swell depends on the rate of influx of the least-permeant ionic species (3), the reduced activity observed for Cry1C at the higher pHs could be due to a pH-induced change in the ionic selectivity of its pores. To test the possiblity that the reduced rate of vesicle swelling was due to a lower permeability of toxin channels to the anion, experiments were conducted in which the anionic permeability of the vesicles was artificially increased by replacing chloride with the more highly permeant thiocyanate ion (SCN−) (Fig. 4A). This substitution had little effect on the permeability observed in the presence of Cry1C. Rapid vesicle swelling was nevertheless observed when the permeability to potassium ions was also increased, in the absence of toxin, by addition of valinomycin, confirming that SCN− ions are more permeant than Cl− ions. Increasing the permeability to potassium ions with valinomycin in the presence of KCl also had little effect on the rate of vesicle swelling induced by Cry1C, indicating that the reduced activity of this toxin at high pH was also not due to a reduced permeability of its pores to cations (Fig. 4B). This low membrane permeability to both cations and anions is therefore probably attributable to a reduced number of functional pores formed at high pH.
FIG. 4.
Effect of thiocyanate ions and valinomycin on the Cry1C-induced permeability of M. sexta brush border membrane vesicles. Membrane vesicles were incubated for 60 min in 1 mg of bovine serum albumin per ml and 10 mM CAPS-KOH (pH 10.5), with or without (Control) 150 pmol of Cry1C per mg of membrane protein, 0.15% (vol/vol) ethanol (EtOH), or 7.5 μM valinomycin (Val), as indicated for each tracing. The vesicles were rapidly mixed with an equal volume of 1 mg of bovine serum albumin per ml, 10 mM CAPS-KOH (pH 10.5), and 150 mM KSCN (A) or KCl (B). Scattered light intensity was monitored as described in the legend to Fig. 1.
Permeability of toxin channels to other solutes.
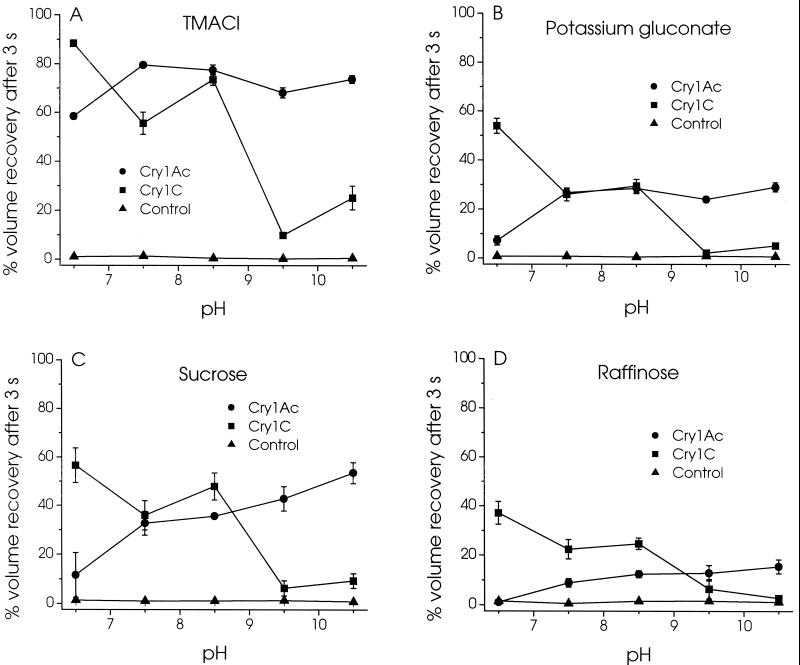
The permeability of the channels formed by Cry1Ac and Cry1C for different charged and neutral solutes was also examined as a function of pH (Fig. 5). In the absence of toxin, the vesicles were very poorly permeable to all solutes tested. As was observed for KCl (Fig. 2A), the permeability to tetramethylammonium chloride (Fig. 5A), potassium gluconate (Fig. 5B), sucrose (Fig. 5C), and raffinose (Fig. 5D) was lower at pH 6.5 than at higher pHs in the presence of Cry1Ac. A gradual increase in the extent of volume recovery after 3 s was also observed as pH was increased from 7.5 to 10.5 in the presence of sucrose (Fig. 5C) and raffinose (Fig. 5D), the largest solutes tested. In contrast, the permeability to all four solutes was significantly higher at pH 6.5, 7.5, and 8.5 than at pH 9.5 and 10.5 in the presence of Cry1C.
FIG. 5.
Effect of pH on the permeability of the pores formed by Cry1Ac and Cry1C to various solutes. Membrane vesicles were incubated for 60 min with 150 pmol/mg of membrane protein of Cry1Ac (●) or Cry1C (■) or without toxin (▴) in 10 mM MES-KOH (pH 6.5), HEPES-KOH (pH 7.5), Tris-HCl (pH 8.5), CHES-KOH (pH 9.5), or CAPS-KOH (pH 10.5). Their permeability was then assayed as described in the legend to Fig. 2 except that 150 mM KCl was replaced with either 150 mM tetramethylammonium chloride (TMACl) (A) or potassium gluconate (B) or 300 mM sucrose (C) or raffinose (D).
Comparison of toxin pore-forming ability at pH 10.5 and toxicity.
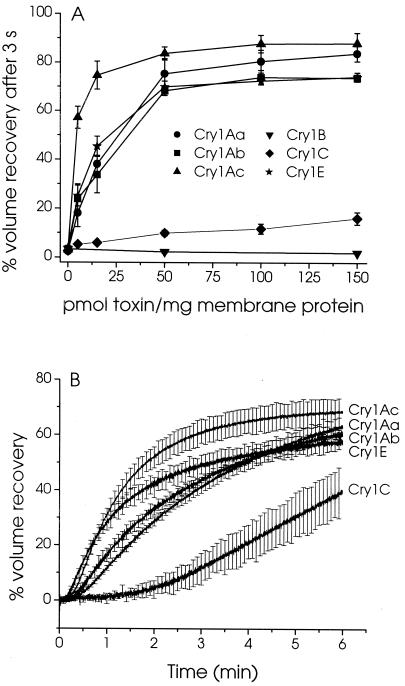
The reduced activity of Cry1C at high pH correlates well with its weaker toxicity toward M. sexta larvae (16, 39). The pore forming ability of six B. thuringiensis toxins was therefore assayed at pH 10.5 (Fig. 6) and compared with their toxicities (Table 1). Whereas Cry1Aa, Cry1Ab, and Cry1Ac were strongly active in light-scattering experiments and highly toxic to the larvae, Cry1B was unable to form pores in membrane vesicles and nontoxic. In contrast, the in vitro pore-forming activity of Cry1E was comparable to that of the Cry1A toxins (Fig. 6A) in spite of a much weaker toxicity, comparable to that of Cry1C (Table 1). A similar pattern was observed when vesicles were exposed simultaneously to a KCl gradient and toxin without preincubation. Under these conditions, the vesicles began to swell after a delay which was considerably longer for Cry1C than for the other toxins. The shortest delays preceding vesicle swelling and the fastest initial rates of swelling were observed for Cry1Ac and Cry1E (Fig. 6B).
FIG. 6.
Pore-forming ability and kinetics of pore formation of various toxins in M. sexta midgut brush border membrane vesicles at pH 10.5. To assay pore-forming ability (A), vesicles were incubated for 60 min with the indicated concentrations of either Cry1Aa (●), Cry1Ab (■), Cry1Ac (▴), Cry1B (▾), Cry1C (⧫), or Cry1E (★) in 1 mg of bovine serum albumin per ml and 10 mM CAPS-KOH (pH 10.5). Their permeability to KCl was assayed as described in the legend to Fig. 2. Kinetics of pore formation (B) were assayed at pH 10.5 as described in the legend to Fig. 3, with 150 pmol of the indicated toxins per mg of membrane protein.
TABLE 1.
Comparison of the activity of B. thuringiensis toxins determined by in vivo bioassays on M. sexta larvae and in vitro light-scattering assays at pH 10.5
| Toxin | Bioassay (LC50 [ng/cm2])a
|
Light-scattering assay (ED50 [pmol/mg])b | |
|---|---|---|---|
| Protoxin | Activated toxin | ||
| Cry1Aa | 5.2 | 20 | 26 |
| Cry1Ab | 8.6 | 20 | 31 |
| Cry1Ac | 5.3 | 9 | 4 |
| Cry1B | >625 | —c | NA |
| Cry1C | 74 | 111 | >150 |
| Cry1E | 72 | 73 | 22 |
The 50% lethal concentration (LC50) values are those reported by Höfte et al. (16) and Van Rie et al. (38, 39).
The 50% effective dose (ED50) values are the toxin concentrations required to cause 50% volume recovery after 3 s and were obtained from the curves shown in Fig. 6A. NA, not active.
—, data not available.
DISCUSSION
This report presents the first evidence of differential effects of pH on the functional properties of closely related, lepidopteran-specific B. thuringiensis toxins. The use of brush border membrane vesicles, in addition to ensuring the presence of a full complement of membrane receptors for the toxins, is particularly well suited for the study of toxin activity over a wide range of pHs. In the absence of toxin, vesicle permeability to all five solutes studied was low over the entire range of pHs tested. Problems associated with tissue damage that occur, for example, when cultured insect cells are exposed to high pH (14, 15; V. Vachon, unpublished observations) were thus avoided.
Considerable differences were observed in the effects of pH on pore-forming properties between Cry1Ac and Cry1C. In the presence of Cry1Ac, membrane permeability was considerably higher under alkaline conditions than at pH 6.5. Because this lower permeability at pH 6.5 was more pronouced for potassium gluconate, sucrose, and raffinose, the larger solutes tested, than for KCl and tetramethylammonium chloride, these results strongly suggest a reduction in both the number and the size of the pores formed by Cry1Ac at acid pH. Membrane permeability to the larger solutes also increased, although much more gradually, as pH was increased from 7.5 to 10.5. These results are consistent with a slight increase in pore diameter, in agreement with earlier estimates based on osmotic swelling measurements (4). They are difficult, however, to reconcile with estimates based on the conductance of the channels formed by Cry1Ac in planar lipid bilayers into which brush border membrane vesicles were incorporated (24, 41). The pore diameter estimated at pH 8.8 using this latter approach, 0.9 nm (41), is comparable to the effective hydrodynamic diameter of sucrose (0.93 nm) (22) and smaller than that of raffinose (1.2 nm) (32), two molecules that diffused readily across the pores formed by Cry1Ac at alkaline pH. The comparison of pore size estimates based on single-channel conductance and those based on membrane permeability to uncharged solutes may not be entirely justified, however, since the former are based on the assumption that the pores are filled with a solution having the same conductance as the external aqueous phase and that electrostatic interactions and friction between the ions and the wall of the pore are negligible. Pore size estimates based on these two approaches can indeed differ considerably, as was found for a bacterial porin (37) and for spider α-latrotoxin (21).
In the presence of Cry1C, pH-induced changes in membrane permeability followed a completely different pattern from that observed for Cry1Ac. For all five solutes tested, membrane permeability was substantially higher between pH 6.5 and 8.5 than at pH 9.5 and 10.5. These results strongly suggest that the ability of Cry1C to form channels in the midgut apical membrane drops sharply above pH 8.5. This abrupt decrease in toxin activity could clearly not be attributed to a pH-induced change in the ion selectivity of the channels since, in the presence of Cry1C, membrane permeability at high pH could not be modified by artificially increasing the permeability of the membrane to the cation (with valinomycin) or to the anion (by replacing Cl− by SCN−).
These findings do not exclude, however, that the channels have a certain ionic selectivity that could be modulated by changes in pH, as was suggested earlier (33). A detailed comparison with previous results is nevertheless made difficult by the fact that individual channels were analyzed previously in planar lipid bilayers (33), whereas osmotic swelling experiments, such as those presented here, examine the overall effects of the toxins on membrane permeability. Planar lipid bilayer studies have revealed that B. thuringiensis toxins can form a variety of channels differing in conductance, ion selectivity, and kinetic properties, at least in the absence of membrane receptors (33, 36). The effects of pH on the properties and relative proportion of these different channels could vary considerably. Under the experimental conditions used in the present study, such effects of pH on the ionic selectivity of the pores may be masked by the strong effects on pore size, observed for Cry1Ac, and on the number of pores formed, observed for Cry1C. In addition, it cannot at present be excluded that the pores formed by B. thuringiensis toxins in the presence of membrane receptors may differ from those formed in their absence, as in planar lipid bilayer studies (20, 35, 41).
Our results confirm that the high pH of the midgut lumen plays an important role in toxin potency against lepidopteran insects. Previous studies have documented examples of toxins with a strong in vitro pore-forming ability despite a modest in vivo toxicity (23, 25, 29). The results obtained in the present study for Cry1C suggest that this discrepancy may be due, at least in part, to the fact that the in vitro experiments were carried out at pHs below those found in the insect midgut. Experiments performed under mildly alkaline conditions do not necessarily overestimate toxin activity, however, as evidenced by the relatively small changes that were observed in the properties of Cry1Ac between pH 7.5 and 10.5. Furthermore, the use of a high pH does not necessarily ensure full agreement between a toxin's pore-forming ability and its toxicity. Although pore formation at pH 10.5 correlated well with toxicity for most toxins studied, Cry1E had a pore-forming ability comparable to that of Cry1Ac despite a toxicity similar to that of Cry1C. Additional factors such as ionic strength, presence of proteases, and membrane potential could therefore possibly influence toxin activity in the midgut environment.
The present study also raises a number of questions concerning the mechanism by which pH affects toxin pore-forming properties. Pore formation is the result of a toxin's binding to a specific receptor, followed by insertion into the membrane and assembly of a functional pore. Toxin activity could thus be modified by titration of charged residues not only on the toxin molecule, but also on its receptor. To our knowledge, few studies have examined the effect of pH on the binding properties of B. thuringiensis toxin receptors (18, 38), and most binding experiments were carried out at pH 7.4. Only minor differences in the binding of Cry1Ab to M. sexta brush border membrane vesicles were observed when pH was increased to 10.0 (38). Similarly, Cry1Ac bound to its receptor, purified from Lymantria dispar, with the same affinity at pH 7.4 and 9.7 (18). Although these findings are consistent with the results of the present study, it remains to be ascertained whether the binding of other toxins such as Cry1C is also largely insensitive to changes in pH.
The insertion of Cry1C into the membrane could also be rendered much less efficient at high pH by an increase in the relative number of negative charges at the surface of the membrane due to the titration of positively charged groups on membrane phospholipids and proteins. Finally, assembly of a functional pore probably involves the oligomerization of toxin molecules (11–13, 27, 34). This step could also be affected by changes in pH. Clearly, further work will be required to distinguish among these different possibilities. A detailed analysis of pH effects on each of these steps should contribute to a better understanding of the mechanisms by which pores are formed by B. thuringiensis toxins.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Sciences and Engineering Council of Canada and the Fonds pour la formation de chercheurs et l'aide à la recherche of Quebec.
REFERENCES
- 1.Bietlot H P L, Vishnubhatla I, Carey P R, Pozsgay M, Kaplan H. Characterization of the cysteine residues and disulphide linkages in the protein crystal of Bacillus thuringiensis. Biochem J. 1990;267:309–315. doi: 10.1042/bj2670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butko P, Cournoyer M, Pusztai-Carey M, Surewicz W K. Membrane interactions and surface hydrophobicity of Bacillus thuringiensis δ-endotoxin Cry1C. FEBS Lett. 1994;340:89–92. doi: 10.1016/0014-5793(94)80178-9. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J, Ellar D J. An analysis of Bacillus thuringiensis δ-endotoxin action on insect-midgut-membrane permeability using a light-scattering assay. Eur J Biochem. 1993;214:771–778. doi: 10.1111/j.1432-1033.1993.tb17979.x. [DOI] [PubMed] [Google Scholar]
- 4.Carroll J, Ellar D J. Analysis of the large aqueous pores produced by a Bacillus thuringiensis protein insecticide in Manduca sexta midgut-brush-border-membrane vesicles. Eur J Biochem. 1997;245:797–804. doi: 10.1111/j.1432-1033.1997.00797.x. [DOI] [PubMed] [Google Scholar]
- 5.Choma C T, Kaplan H. Folding and unfolding of the protoxin from Bacillus thuringiensis: evidence that the toxic moiety is present in an active conformation. Biochemistry. 1990;29:10971–10977. doi: 10.1021/bi00501a015. [DOI] [PubMed] [Google Scholar]
- 6.Convents D, Cherlet M, Van Damme J, Lasters I, Lauwereys M. Two structural domains as a general fold of the toxic fragment of the Bacillus thuringiensis δ-endotoxins. Eur J Biochem. 1991;195:631–635. doi: 10.1111/j.1432-1033.1991.tb15747.x. [DOI] [PubMed] [Google Scholar]
- 7.Convents D, Houssier C, Lasters I, Lauwereys M. The Bacillus thuringiensis δ-endotoxin: evidence for a two domain structure of the minimal toxic fragment. J Biol Chem. 1990;265:1369–1375. [PubMed] [Google Scholar]
- 8.Dow J A T. Extremely high pH in biological systems: a model for carbonate transport. Am J Physiol. 1984;246:R633–R635. doi: 10.1152/ajpregu.1984.246.4.R633. [DOI] [PubMed] [Google Scholar]
- 9.Dow J A T. pH gradients in lepidopteran midgut. J Exp Biol. 1992;172:355–375. doi: 10.1242/jeb.172.1.355. [DOI] [PubMed] [Google Scholar]
- 10.Feng Q, Becktel W J. pH-induced transitions of CryIA(a), CryIA(c), and CryIIIA δ-endotoxins in Bacillus thuringiensis. Biochemistry. 1994;33:8521–8526. doi: 10.1021/bi00194a017. [DOI] [PubMed] [Google Scholar]
- 11.Gazit E, Bach D, Kerr I D, Sansom M S P, Chejanovsky N, Shai Y. The α-5 segment of Bacillus thuringiensis δ-endotoxin: in vitro activity, ion channel formation and molecular modelling. Biochem J. 1994;304:895–902. doi: 10.1042/bj3040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazit E, La Rocca P, Sansom M S P, Shai Y. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis δ-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc Natl Acad Sci USA. 1998;95:12289–12294. doi: 10.1073/pnas.95.21.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazit E, Shai Y. The assembly and organization of the α5 and α7 helices from the pore-forming domain of Bacillus thuringiensis δ-endotoxin; relevance to a functional model. J Biol Chem. 1995;270:2571–2578. doi: 10.1074/jbc.270.6.2571. [DOI] [PubMed] [Google Scholar]
- 14.Gringorten J L, Milne R E, Fast P G, Sohi S S, van Frankenhuyzen K. Suppression of Bacillus thuringiensis δ-endotoxin activity by low alkaline pH. J Invertebr Pathol. 1992;60:47–52. [Google Scholar]
- 15.Guihard G, Vachon V, Laprade R, Schwartz J-L. Kinetic properties of the channels formed by the Bacillus thuringiensis insecticidal crystal protein Cry1C in the plasma membrane of Sf9 cells. J Membr Biol. 2000;175:115–122. doi: 10.1007/s002320001060. [DOI] [PubMed] [Google Scholar]
- 16.Höfte H, Van Rie J, Jansens S, Van Houtven A, Vanderbruggen H, Vaeck M. Monoclonal antibody analysis and insecticidal spectrum of three types of lepidopteran-specific insecticidal crystal proteins of Bacillus thuringiensis. Appl Environ Microbiol. 1988;54:2010–2017. doi: 10.1128/aem.54.8.2010-2017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaquet F, Hütter R, Lüthy P. Specificity of Bacillus thuringiensis delta-endotoxin. Appl Environ Microbiol. 1987;53:500–504. doi: 10.1128/aem.53.3.500-504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins J L, Lee M K, Valaitis A P, Curtiss A, Dean D H. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J Biol Chem. 2000;275:14423–14431. doi: 10.1074/jbc.275.19.14423. [DOI] [PubMed] [Google Scholar]
- 19.Knowles B H. Mechanism of action of Bacillus thuringiensis insecticidal δ-endotoxins. Adv Insect Physiol. 1994;24:275–308. [Google Scholar]
- 20.Knowles B H, Dow J A T. The crystal δ-endotoxins of Bacillus thuringiensis: models for their mechanism of action on the insect gut. Bioessays. 1993;15:469–476. [Google Scholar]
- 21.Krasilnikov O V, Sabirov R Z. Comparative analysis of latrotoxin channels of different conductance in planar lipid bilayers: evidence for cluster organization. Biochim Biophys Acta. 1992;1112:124–128. doi: 10.1016/0005-2736(92)90262-k. [DOI] [PubMed] [Google Scholar]
- 22.Krasilnikov O V, Sabirov R Z, Ternovsky P G, Muratkhodjaev J N. A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol Immunol. 1992;105:93–100. doi: 10.1111/j.1574-6968.1992.tb05891.x. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Banks D, Adang M J. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry1 δ-endotoxins using brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl Environ Microbiol. 1999;65:457–464. doi: 10.1128/aem.65.2.457-464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin F G, Wolfersberger M G. Bacillus thuringiensis δ-endotoxin and larval Manduca sexta midgut brush-border membrane vesicles act synergistically to cause very large increases in the conductance of planar lipid bilayers. J Exp Biol. 1995;198:91–96. doi: 10.1242/jeb.198.1.91. [DOI] [PubMed] [Google Scholar]
- 25.Masson L, Mazza A, Gringorten L, Baines D, Aneliunas V, Brousseau R. Specificity domain localization of Bacillus thuringiensis insecticidal toxins is highly dependent on the bioassay system. Mol Microbiol. 1994;14:851–860. doi: 10.1111/j.1365-2958.1994.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 26.Masson L, Préfontaine G, Péloquin L, Lau P C K, Brousseau R. Comparative analysis of the individual protoxin components in P1 crystals of Bacillus thuringiensis subsp. kurstaki isolates NDR-12 and HD-1. Biochem J. 1989;269:507–512. doi: 10.1042/bj2690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson L, Tabashnik B E, Liu Y-B, Brousseau R, Schwartz J-L. Helix 4 of the Bacillus thuringiensis Cry1Aa toxin lines the lumen of the ion channel. J Biol Chem. 1999;274:31996–32000. doi: 10.1074/jbc.274.45.31996. [DOI] [PubMed] [Google Scholar]
- 28.Nishiitsutsuji-Uwo J, Oshawa A, Nishimura M S. Factors affecting the insecticidal activity of δ-endotoxin of Bacillus thuringiensis. J Invertebr Pathol. 1977;29:162–169. [Google Scholar]
- 29.Peyronnet O, Vachon V, Brousseau R, Baines D, Schwartz J-L, Laprade R. Effect of Bacillus thuringiensis toxins on the membrane potential of lepidopteran insect midgut cells. Appl Environ Microbiol. 1997;63:1679–1684. doi: 10.1128/aem.63.5.1679-1684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajamohan F, Lee M K, Dean D H. Bacillus thuringiensis insecticidal proteins: molecular mode of action. Progr Nucleic Acid Res Mol Biol. 1998;60:1–27. doi: 10.1016/s0079-6603(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 31.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz S G, Solomon A K. Determination of the effective hydrodynamic radii of small molecules by viscometry. J Gen Physiol. 1961;44:1189–1199. doi: 10.1085/jgp.44.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz J-L, Garneau L, Savaria D, Masson L, Brousseau R, Rousseau E. Lepidopteran-specific crystal toxins from Bacillus thuringiensis form cation- and anion-selective channels in planar lipid bilayers. J Membr Biol. 1993;132:53–62. doi: 10.1007/BF00233051. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J-L, Juteau M, Grochulski P, Cygler M, Préfontaine G, Brousseau R, Masson L. Restriction of intramolecular movements within the Cry1Aa toxin molecule of Bacillus thuringiensis through disulfide bond engineering. FEBS Lett. 1997;410:397–402. doi: 10.1016/s0014-5793(97)00626-1. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J-L, Lu Y-J, Söhnlein P, Brousseau R, Laprade R, Masson L, Adang M J. Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEBS Lett. 1997;412:270–276. doi: 10.1016/s0014-5793(97)00801-6. [DOI] [PubMed] [Google Scholar]
- 36.Slatin S L, Abrams C K, English L. Delta-endotoxins form cation-selective channels in planar lipid bilayers. Biochem Biophys Res Commun. 1990;169:765–772. doi: 10.1016/0006-291x(90)90397-6. [DOI] [PubMed] [Google Scholar]
- 37.Vachon V, Laprade R, Coulton J W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986;861:74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- 38.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis δ-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990;56:1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venugopal M G, Wolfersberger M G, Wallace B A. Effects of pH on conformational properties related to the toxicity of Bacillus thuringiensis δ-endotoxin. Biochim Biophys Acta. 1992;1159:185–192. doi: 10.1016/0167-4838(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 41.Wolfersberger M G. Permeability of Bacillus thuringiensis CryI toxin channels. In: Clark J M, editor. Molecular action of insecticides on ion channels. Washington, D.C.: American Chemical Society; 1995. pp. 294–301. [Google Scholar]
- 42.Wolfersberger M, Luethy P, Maurer A, Parenti P, Sacchi F V, Giordana B, Hanozet G M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol. 1987;86A:301–308. [Google Scholar]