Abstract
The levels of voltage-gated and synaptic currents in the same neuron type can vary substantially across individuals. Yet, the phase relationships between neurons in oscillatory circuits are often maintained, even in the face of varying oscillation frequencies. We examined whether synaptic and intrinsic currents are matched to maintain constant activity phases across preparations, using the lateral pyloric (LP) neuron of the stomatogastric ganglion (STG) of the crab, Cancer borealis. LP produces stable oscillatory bursts on release from inhibition, with an onset phase that is independent of oscillation frequency. We quantified the parameters that define the shape of the synaptic current inputs across preparations and found no linear correlations with voltage-gated currents. However, several synaptic parameters were correlated with oscillation period and burst onset phase, suggesting they may play a role in phase maintenance. We used dynamic clamp to apply artificial synaptic inputs and found that those synaptic parameters correlated with phase and period were ineffective in influencing burst onset. Instead, parameters that showed the least variability across preparations had the greatest influence. Thus, parameters that influence circuit phasing are constrained across individuals, while those that have little effect simply co-vary with phase and frequency.
Keywords: correlations, dynamic clamp, oscillation, phase maintenance, stomatogastric, synaptic dynamics
Significance Statement
Across individuals, some attributes of circuit output are similar, yet others vary considerably. This was first demonstrated in circuits where bursting neurons show relatively stable activity phases that are independent of variations in cycle frequency, despite substantial variability in ionic currents. We show here that some attributes of synaptic current trajectory covary with cycle period or phase but do not compensate for variability in other currents. Importantly, activity phase is insensitive to experimental variation of the attributes it is most correlated with, but very sensitive to variations of those that are tightly constrained. Therefore, phase similarity is not because of compensatory regulation of intrinsic or synaptic currents but achieved through tight regulation of synaptic attributes that influence phase.
Introduction
Sensory representations and motor outputs are characterized by the relative timing between different circuit neurons, particularly during oscillatory activity (Ainsworth et al., 2012). Distinct phases of activity within each cycle are found both during oscillations associated with cognition and various behavioral states (Hasselmo et al., 2002; Hájos et al., 2004; Somogyi and Klausberger, 2005; Buzsáki and Wang, 2012; Wilson et al., 2015; Buzsáki and Tingley, 2018; Dragoi, 2020), and during rhythmic motor activity, where they underlie the sequential activation of different groups of muscles (Vidal-Gadea et al., 2011; Bucher et al., 2015; Grillner and El Manira, 2015, 2020; Katz, 2016; Kiehn, 2016; Bidaye et al., 2018). The relative timing (phase) of a neuron’s activity within each oscillation cycle is dependent on an interplay of intrinsic membrane currents and total cycle-to-cycle synaptic input (Harris-Warrick, 2002; Oren et al., 2006; Marder, 2011; McDonnell and Graham, 2017; Martinez et al., 2019b). There are two confounding aspects of this interplay. First, in many oscillatory systems, phase is maintained over a range of frequencies, i.e., intrinsic and synaptic properties have to ensure that absolute timing of responses changes proportionally to the speed of rhythmic circuit activity (Grillner, 2006; Mullins et al., 2011; Zhang et al., 2014; Le Gal et al., 2017; Martinez et al., 2019b). Second, phase can be very similar across individual animals despite substantial variability in the individual ionic and synaptic currents (Bucher et al., 2005; Marder and Goaillard, 2006; Calabrese et al., 2011, 2016; Marder, 2011; Roffman et al., 2012; Golowasch, 2014; Hamood and Marder, 2014; Marder et al., 2014a).
The phenomenon that circuit activity is maintained despite substantial variability in underlying conductances has been explored most thoroughly in invertebrate central pattern generators, including those of the crustacean stomatogastric ganglion (STG). In these circuits, the timing of neural activity is critically dependent on voltage-gated ion channels (Harris-Warrick et al., 1995a,b; Kloppenburg et al., 1999). However, such voltage-gated conductances and the associated ion channel expression show substantial interindividual variability (Liu et al., 1998; Golowasch et al., 2002; Marder and Goaillard, 2006; Schulz et al., 2006; Marder, 2011; Hamood and Marder, 2014; Marder et al., 2014a), raising the question how activity can be so similar across preparations. A possible explanation is suggested by the finding that voltage-gated conductances do not vary independently, but in a cell type-specific correlated manner (Khorkova and Golowasch, 2007; Schulz et al., 2007; Ransdell et al., 2012; Temporal et al., 2012; Tran et al., 2019). Theoretical work suggests that homeostatic, compensatory tuning explains correlation of expression levels of different ion channels (Prinz et al., 2004b; O’Leary et al., 2013, 2014; Franci et al., 2020), and there is some experimental evidence that co-regulation of voltage-gated conductances can have compensatory function to preserve circuit activity (MacLean et al., 2003, 2005; Ransdell et al., 2012, 2013; Zhao and Golowasch, 2012; Santin and Schulz, 2019).
Synaptic currents also vary substantially across individuals and their magnitude is correlated with relative timing of the burst onset of the postsynaptic neuron (Goaillard et al., 2009). In theoretical work, the magnitude of synaptic currents has been varied and tuned alongside voltage-gated conductances to show which combinations and possible mechanisms give rise to similar activity (Prinz et al., 2004b; O’Leary et al., 2014), and it has been suggested that the relative synaptic strengths must be different in individual animals to produce observed activity phases (Günay et al., 2019). However, it is unknown whether synaptic currents co-vary with individual voltage-gated currents in a correlated manner to compensate for variability in intrinsic neuronal excitability. Furthermore, the effect of synaptic input on rhythmic patterns is not just dependent on synaptic strength, but also on timing, duration, and details of the temporal trajectory of the synaptic current (Prinz et al., 2003; Martinez et al., 2019b).
We examine how synaptic inputs contribute to phase constancy under normal biological conditions in the face of variability across individuals. For this, we use the identified lateral pyloric (LP) neuron in the STG, a follower neuron of the triphasic oscillatory pyloric circuit, which has a single copy in each animal. We examine the variability of synaptic input to the LP neuron across animals and compare that with its activity phase. We examine correlations among synaptic parameters and between these parameters and intrinsic voltage-gated currents of the LP neuron. We then use the dynamic clamp technique to explore how synaptic parameters influence the activity phase of the LP neuron.
Materials and Methods
Experimental preparation
Adult male crabs (Cancer borealis) were acquired from local distributors and maintained in aquaria filled with chilled (12–13°C) artificial sea water until use. Crabs were anesthetized before dissection by placing them in ice for at least 20 min. The stomatogastric nervous system including the STG, esophageal ganglion, the pair of commissural ganglia, and the motor nerves were dissected from the stomach and pinned to a saline filled, Sylgard-coated (Dow Corning) Petri dish (schematic in Fig. 1A). The STG was desheathed, exposing the somata of the neurons for intracellular impalement. Preparations were superfused with chilled (10–13°C) physiological saline containing: 11 mm KCl, 440 mm NaCl, 13 mm CaCl2 · 2H2O, 26 mm MgCl2 · 6H2O, 11.2 mm Tris base, and 5.1 mm maleic acid with a pH of 7.4.
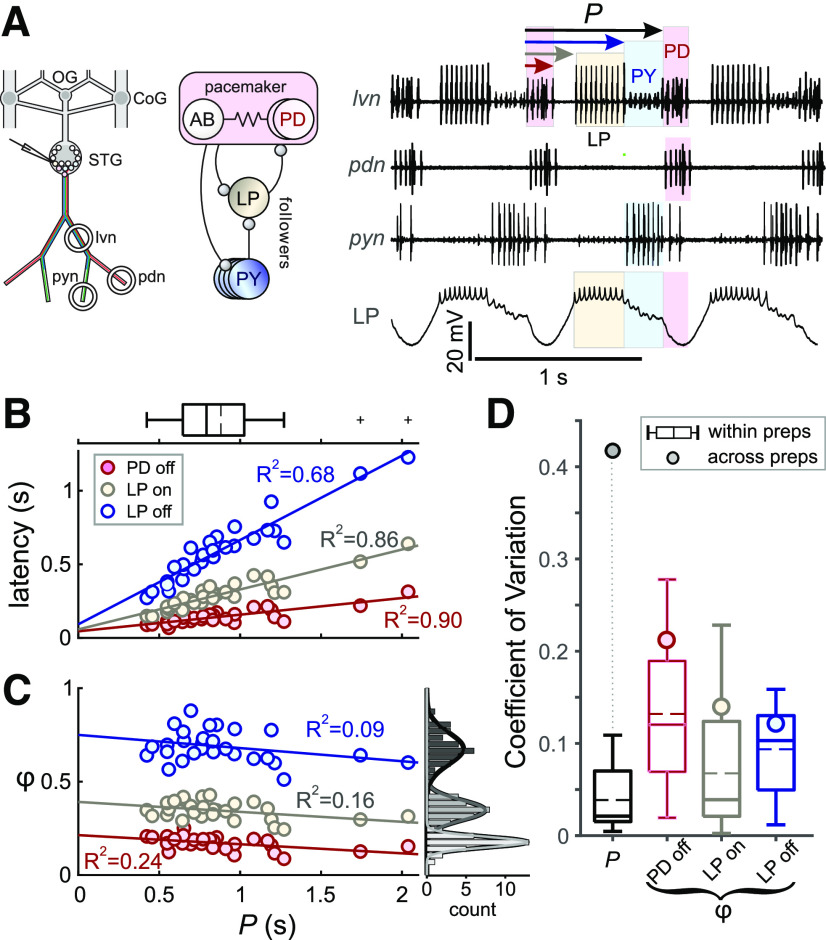
Figure 1.
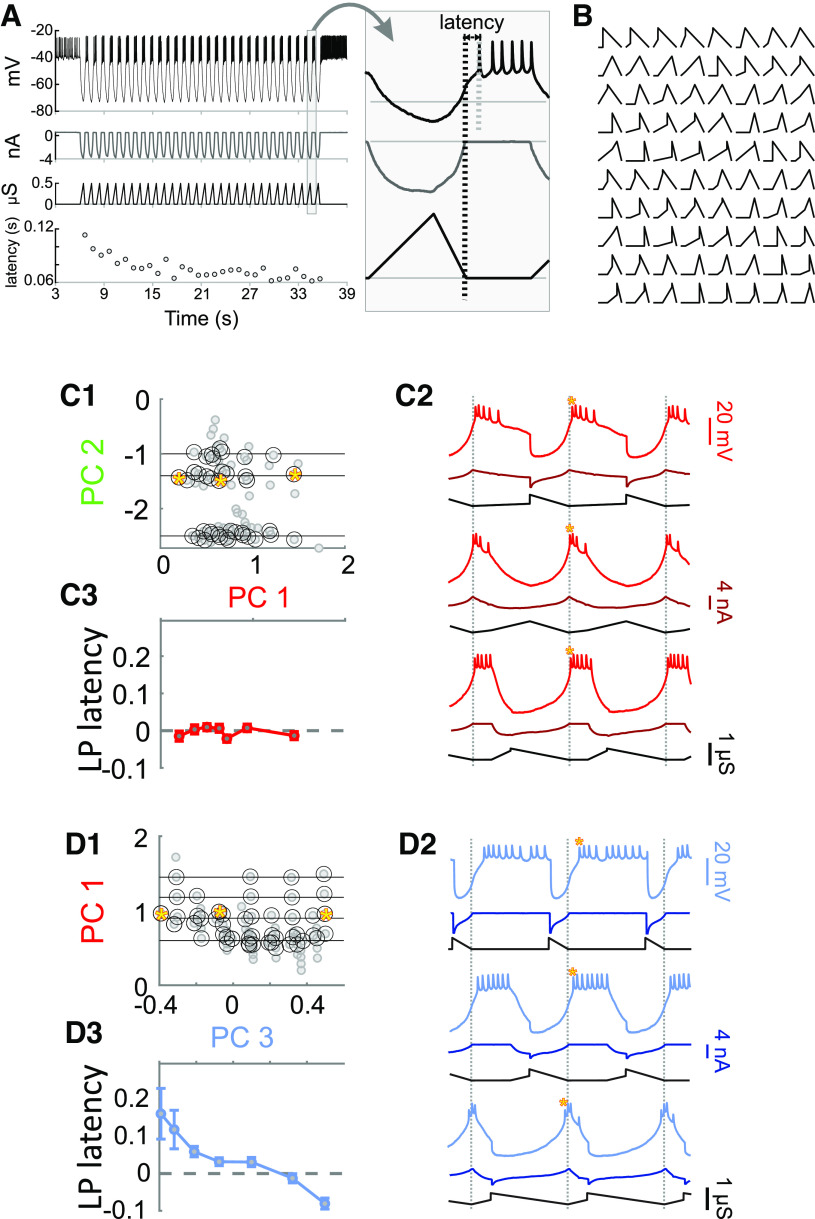
Pyloric activity phases are variable but not correlated with the cycle period. A, Left, Schematic diagram shows the layout of the STNS in vitro and the locations of intracellular (electrode) and extracellular (circles) recordings. OG: esophageal ganglion, CoG: commissural ganglion, STG: stomatogastric ganglion, lvn: the lateral ventricular nerve, pdn: PD nerve, pyn: pyloric nerve. Middle, Schematic diagram shows a simplified circuit diagram of the neurons recorded. Ball-and-stick symbols are inhibitory chemical synapses. Resistor denotes electrical coupling. Right, Simultaneous extracellular and intracellular recordings show the regular triphasic oscillations of the pyloric circuit. Shown are bursting activity of pacemaker neuron PD and follower neurons LP and PY. Cycle period (P) and latencies of the onset and end of each burst (arrows) are calculated from the onset of the PD burst. Extracellular recordings are from the lvn (showing the LP, PD and PY spikes), the pdn (showing the PD spikes), and pyn [showing the PY and the lateral posterior gastric (LPG) neuron spikes]. Intracellular recording from the LP neuron shows bursting activity (yellow) and slow wave oscillations, as well as timing of IPSPs from the PY (light blue) and PD (pink) neurons. B, Burst latencies of the PD and LP neuron in reference to PD burst onset, as marked in panel A, shown versus P. Quartile plot shows the distribution of P, with the dashed line indicating the mean value. Lines indicate best linear fit, showing that latencies grow proportionally with P. C, Phase values (φ = latency/P) shown versus P. Histograms show distribution of φ values. Linear fits indicate a lack of correlation between all φ values and P. D, CV of P and φ values shown to compare variability of the values within preparations (quartile plots) to their variability across preparations (circles).
Extracellular recordings of rhythmic patterns
Extracellular recordings from identified motor nerves were performed using pairs of stainless steel electrodes, placed inside and outside of a petroleum jelly well created to electrically isolate a small section of the nerve, and amplified using a differential AC amplifier (AM Systems, model 1700). All traces were digitized using a Digidata 1332 data acquisition board and recorded in pClamp 10 software (both Molecular Devices).
The activity of three neuron types was used to identify the triphasic pyloric pattern (Marder and Bucher, 2007). The two pyloric dilator (PD) neurons belong to the pyloric pacemaker group of neurons, and we therefore used their burst onset as the reference time that defined each cycle of activity. The pyloric constrictor neurons include the single LP neuron and multiple pyloric (PY) neurons. The constrictor neurons are follower neurons that receive strong inhibition from the pacemaker group and rebound from this inhibition to produce bursting activity at different phases. Spontaneous rhythmic pyloric activity was recorded from the lateral ventricular nerve (lvn), the PD nerve (pdn), and occasionally also from the pyloric nerve (pyn; Fig. 1A, nomenclature after Maynard and Dando, 1974). The lvn contains the axons of all three neurons types, with LP action potentials easily identifiable by their large amplitude. The pdn contains only the axons of the PD neurons, and the pyn only those of the PY neurons.
Intracellular recordings and voltage clamp
For Intracellular impalement of the LP neuron soma, glass microelectrodes were prepared using the Flaming-Brown micropipette puller (P97; Sutter Instruments) and filled with 0.6 m K2SO4 and 20 mm KCl, yielding electrode resistances of 10–30 MΩ. Individual pyloric neurons were sequentially impaled, and the LP neuron was identified by its activity pattern and correspondence of action potentials between the soma recording and the extracellular recording of the lvn (Fig. 1A). Recordings were amplified using Axoclamp 2B and 900A amplifiers (Molecular Devices) and recorded alongside the extracellular signals in pClamp. For current measurements, the LP soma was simultaneously impaled with two electrodes, and membrane potential was controlled in two electrode voltage clamp mode.
Measurements of voltage-gated currents
In LP and other pyloric neurons, three intrinsic voltage-gated currents are relatively straightforward to measure in the intact circuit, without pharmacological manipulation (Zhao and Golowasch, 2012): the high-threshold K+ current (IHTK), the fast transient K+ current (IA), and the hyperpolarization-activated inward current (IH).
IHTK, consisting of the delayed rectifier and calcium-dependent K+ currents (Khorkova and Golowasch, 2007), was measured from the responses to voltage steps following a 270-ms prestep to −40 mV to inactivate IA. Voltage steps (750 ms) were delivered from −60 to +30 mV, in increments of 10 mV. In addition to subtracting the baseline current at −40 mV, the current recorded from the smallest voltage step was used to estimate the leak current, scaled proportionally for all voltage steps, and subtracted offline. The persistent component (IHTKp) was measured by taking an average of current recorded during the last 70 ms of a voltage step (90–99% of step duration). The transient component (IHTKt) was measured by taking the current peak, recorded during the first 150 ms of the voltage step.
IA was obtained by recording the total K+ current (IKtot) and digitally subtracting the previously measured IHTK. The neuron was held at −80 mV to remove inactivation. IKtot was then activated using voltage steps from −60 to +40 mV in 10-mV increments. After subtracting IHTK from IKtot, the difference current was baseline subtracted. Because these currents were recorded without blocking sodium currents, effects of spikes generated in the electrotonically relatively distant axon were seen in the IA traces (Fig. 2A; see also Zhao and Golowasch, 2012). Before measuring the peak amplitude of the currents, we used a robust smoothing function to remove the action potential-mediated transients. The amplitude of IA was measured as the maximum during the first 150 ms of the voltage step.
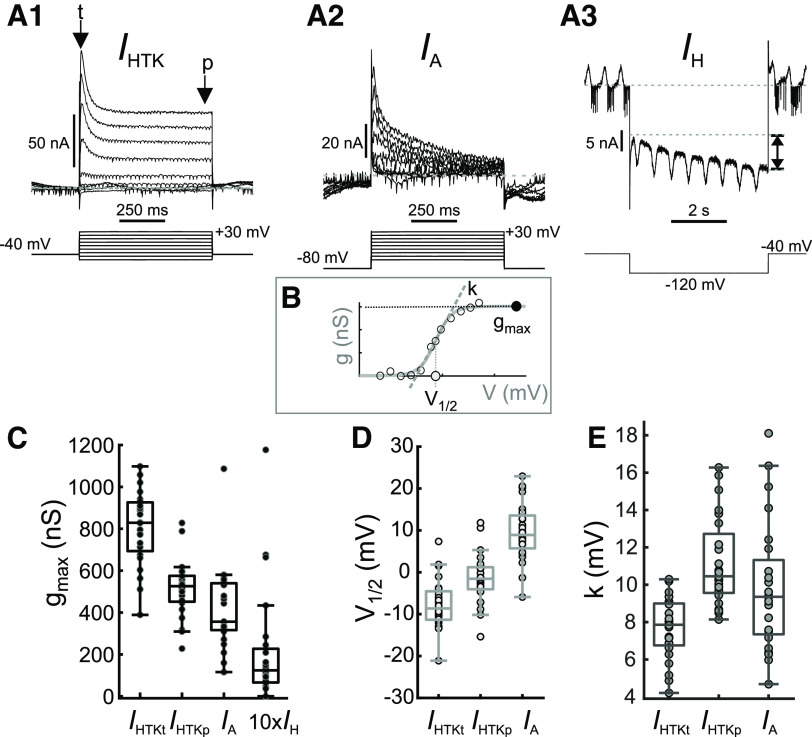
Figure 2.
Parameters defining voltage-gated currents IHTK, IA, and IH show considerable variability. A, Example voltage clamp recordings of high-threshold potassium currents [IHTK, A1; arrows indicate the transient (t) and persistent (p) components], the transient potassium A current (IA, A2) and the H current (IH, A3). Double-arrow in A3 indicates the measured amplitude of IH. B, Schematic diagram of fits in each experiment to the IHTK and IA conductances (g, measured by dividing current by the driving force, assuming EK = −80 mV). Fits were used to calculate maximum conductance (gmax), half-activation voltage (V1/2), and activation slope factor (k, measured from the slope of the dashed line). C, Maximal conductances of the transient and persistent components of IHTK, IA, and IH across preparations. D, Half-activation voltage of the transient and persistent components of IHTK and of IA across preparations. E, Activation slope factor of the transient and persistent components of IHTK and of IA across preparations. Among these parameters, there were some pairwise correlations among the gmax values as well as the parameters V1/2 and k (see Extended Data Fig. 2-1 for plots and Extended Data Fig. 2-2 for all p and R values).
Correlations among parameters of the voltage-gated ionic currents. No fit line in a panel indicates lack of correlation. See Extended Data Figure 2-2 for all p and R values. Download Figure 2-1, EPS file (1.2MB, eps) .
All p and R values for extended data Figure 2-1. Download Figure 2-2, xls file (28.5KB, xls) .
IHTKp, IHTKt, and IA were converted into conductances using the voltage-current relationships and an estimated K+ reversal potential (EK) of −85 mV. We then fit a standard sigmoid equation to a plot of conductance over membrane potential:
(X = HTKp, HTKt, or A). The sigmoid fits yielded values for maximal conductance (gmax), voltage of half- activation (V1/2) and slope factor (k).
IH was measured by holding LP at −40 mV for >1.5 s and then stepping to more negative potentials between −60 and −120 mV for 5 s, in increments of 10 mV. Because of the small and variable size of IH in the LP neuron, it is difficult to measure an accurate activation curve or reversal potential at physiological temperatures, particularly because rhythmic synaptic currents occur at similar amplitudes. Therefore, we only used the response to the step to −120 mV to estimate IH. The current was calculated by taking the difference between the current at the beginning and just before the end of the voltage step. The measured current was converted into conductance using a reversal potential of −30 mV (Buchholtz et al., 1992). In two preparations, the LP neuron did not have any measurable IH.
Measurements of synaptic currents
Pyloric neurons receive mainly graded inhibitory synaptic input. Because LP is a follower neuron, pyloric oscillations continue while the LP neuron is voltage clamped, thus allowing for measurement of the IPSCs (Martinez et al., 2019b). LP was voltage clamped at a holding potential of −50 mV for at least 30 s. Relatively higher level of holding potential was used to allow the network to maintain its natural oscillation frequency with minimal interference and to parameterize contributions of cell specific synaptic inputs more precisely. The current was averaged from the last 5 cycles measured, and a resulting unitary waveform was extracted. This unitary waveform was tagged at five distinct points, t0 to t4 (with the cycle period P = t4 – t0), which were connected using a piecewise linear graph (Fig. 3). The IPSC can be defined as the duration of this waveform from t1 to t4. The baseline of the IPSC (I = 0) was defined as the IPSC onset value at time t1. The IPSC waveform was normalized by P. Thus, the IPSC waveform can be characterized fully using the following parameters:
- Phase parameters:
- DCLP: duty cycle of the LP burst preceding the phases of synaptic input (= (t1 – t0)/P),
- DCPY: duty cycle of the PY component of the IPSC (= (t2 – t1)/P),
- DCPD: duty cycle of the pacemaker component of the IPSC (= (t4 – t2)/P),
- θLP: peak phase of the synapse within the cycle, relative to the onset of the LP burst (= (t3 – t0)/P),
- θPD: peak phase of the synapse within the cycle, relative to the onset of the PD burst (= (t3 – t2)/P),
- Δpk: peak phase of the synapse within the IPSC (= (t3 – t1)/(t4 – t1)).
- Amplitude parameters:
- Itot: the maximum IPSC amplitude,
- IPD: amplitude of the pacemaker component of the IPSC,
- IPY: amplitude of the PY component of the IPSC (= Itot – IPD).
- Slope parameters:
- mPY: rise slope of the PY component (= IPY/(t2 – t1)),
- mPD: rise slope of the pacemaker component (= IPD/(t3 – t2)),
- mfall: decay rate of the IPSC (= Itot/(t4 – t3)).
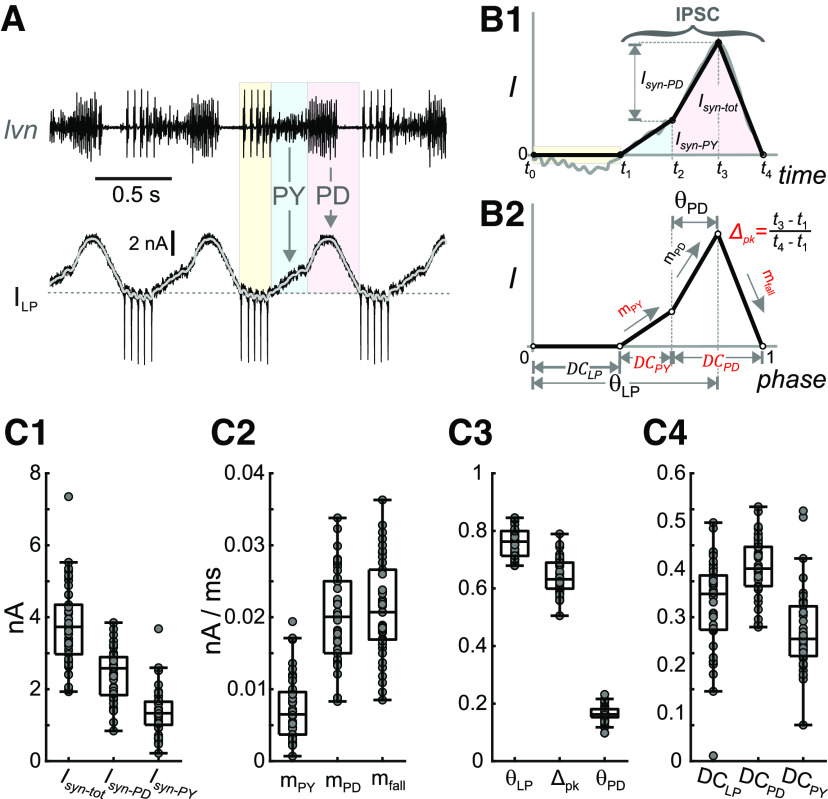
Figure 3.
Parameters that define the synaptic input show considerable variability. A, Total current measured in the LP neuron voltage clamped at a holding potential of −50 mV during the ongoing pyloric rhythm. The pyloric rhythm is recorded extracellularly (lvn), indicating the timing of the LP, PY, and PD neuron bursts. LP action potentials escape the voltage clamp and can be seen in the current and extracellular recordings (pale yellow). The portion of the current outside this range is because of synaptic input (downward arrows) from the PY (light blue) and pacemaker (PD, pink) neurons. The gray curve is the current low-pass filtered (<20 Hz). B1, The synaptic waveform shape (gray curve) during a single cycle of oscillation is approximated by a piecewise-linear curve (black curve), marked by five time points (t0–t4) denoting the borders of the colored regions in panel A. The time range of the IPSC and the amplitudes of the synaptic currents because of the pacemaker neurons (Isyn-PD), because of the PY neurons (Isyn-PY), and the sum of the two (Isyn-tot) are marked. B2, The piecewise-linear curve of B1 shown in phase (time/period). This normalized curve is used to define the parameters of synaptic input to the LP neuron. For definitions, please refer to the main text. Five primary parameters (in red) are chosen for further analysis. C, The interindividual variability of different synaptic parameters, including current amplitudes (C1), slopes (C2), peak phases (C3), and duty cycles (C4).
Clearly, these parameters are not independent and include redundant ones. We defined all parameters to maintain the clarity of the biophysical interpretation of the IPSC and the contributing network components. However, for correlations between synaptic parameters and between synaptic and intrinsic current parameters, we defined the nonredundant subset, which consists of the following five parameters:
The other seven parameters can be calculated from these values using simple geometry:
Note that the synaptic conductance waveform was taken to be identical to the synaptic current waveform measured in voltage clamp, as synaptic current at a constant holding potential simply scales with synaptic conductance.
Dynamic clamp application of artificial synaptic input current
Dynamic clamp was implemented using the NetClamp software (Gotham Scientific) on a 64-bit Windows 7 PC using an NI PCI-6070-E board (National Instruments). We used dynamic clamp to inject artificial synaptic currents (Isyn) into the synaptically isolated LP neuron (Prinz et al., 2004a; Zhao et al., 2010; Chen et al., 2016; Golowasch et al., 2017; Martinez et al., 2019b). In these experiments, the preparations were superfused with saline containing 10−5 m picrotoxin (Sigma-Aldrich) to block the bulk of synaptic input to the LP neuron (Martinez et al., 2019a).
The dynamic clamp injected current Isyn was defined as:
where gsyn is the synaptic conductance and Esyn is the synaptic reversal potential (set to −80 mV). gsyn was defined as a unitary stereotypical piecewise-linear waveform, mimicking the experimentally measured synaptic conductance. The unitary synaptic conductance waveforms were constructed using the following algorithm:
Itot = 1.
DCLP + DCPY + DCPD = 1.
DCLP, DCPY, θPD and IPY were chosen from the values between 0 and 1, in increments of 0.2.
mPD > mPY
DCLP + DCPY + θPD < 1.
These rules yielded a total of 80 waveforms, which included a few duplicates. Each waveform was applied periodically with a cycle period of 1 s and a peak amplitude of 0.5 μS. In each trial, the artificial synaptic input was applied for at least 30 s.
In these experiments, the bursting activity of the LP neuron was quantified by measuring the latency of the burst onset compared with the end of the conductance waveform (t4 in Fig. 3). Note that this is different from the burst latency measured for calculating the LP phase during an ongoing pyloric rhythm (Fig. 1A, right panel), which is measured with respect to the onset of the pacemaker PD neuron bursts. However, our primary goal in these experiments was to understand how changing the shape of the synaptic input influenced the activity of the LP neuron. The corresponding reference point in the dynamic clamp experiments would have been the onset of the pacemaker component of the synaptic input (t2 in Fig. 3). However, had we measured latency with respect to t2, our calculation of latency would have given the appearance that it changes with the waveforms, even if there was absolutely no change in the LP neuron activity. This is because t2 is quite different across the 80 waveforms. The end of the conductance waveform is the only reference point that accurately reports changes in the LP activity.
Data analysis
All analysis was performed using custom scripts written in MATLAB (MathWorks). All linear correlations were measured using MATLAB built-in function ‘corr,’ which computes Pearson’s linear correlation coefficient. Principal component analysis (PCA) was performed using the MATLAB ‘pca’ function. Figures were plotted in MATLAB and panels were assembled in CorelDRAW (version 2020, Corel).
The activity phase (φLP on) of the LP neuron burst onset is defined as the time interval between the onset of the pacemaker PD neuron’s burst to the onset of the LP neuron burst, normalized by the period (P) of that cycle, defined as the time interval between the two consequent PD neuron bursts (Fig. 1A). To examine the effect of changing the synaptic waveform along each principal component (using dynamic clamp) on φLP on, for each principal component PCj (j = 1,…,5), we projected all 80 synaptic waveforms onto the plane defined by PCj and each PCk (k ≠ j). We then found all waveform pairs (say wn and wm) that fell within ±0.1 of each PCk value and were different by at least 0.1 in PCj, and measured φLP on for each waveform. (In this analysis, to exclude any effect of the duration of inhibition, we computed φLP on by calculating the latency as the time-to-first-spike of the burst relative to the end of dynamic clamp inhibition, and then divided this latency by P.) We then calculated the sensitivity of the LP burst onset latency (lat) for this pair of waveforms wn and wm as:
[here, PCj(wn) is assumed to be > PCj(wm)]. We reported the sensitivity of φLP on to PCj, while keeping PCk constant, as the statistical distribution defined by snm values in all preparations (200–500 data points, depending on j and k). The overall sensitivity burst latency to PCj in each preparation was calculated as the mean value of all snm values when changing PCj, while keeping PCk constant, for all k ≠ j, in that preparation.
We also examined the sensitivity of latency to the onset of the LP burst to changing the synaptic parameters, in our dynamic clamp experiments, along a predetermined fixed direction (DC = DCPD + DCPY) as opposed to any other direction in the parameter space. Because our parameter space is 5D (DCPY, DCPD, Δpk, mPY, mfall), this space can be equivalently described by an orthonormal basis with one unit vector in the direction of DC and four unit vectors orthogonal to DC and one another. Thus, the first basis vector was the unit vector in the direction of DC. To choose the other four basis vectors, we projected all synaptic waveforms into the four-dimensional hyperspace (DC⊥) perpendicular to DC and performing a PCA to obtain the four PCs that define the basis of the DC⊥ hyperspace. Note that these four PCs are distinct from those described above.
Results
Variability of phase
The goal of this study was to identify mechanisms that allow a follower pyloric neuron to maintain constant activity phase across preparations, despite considerable variability in cycle period, synaptic input, and voltage-gated conductances. We chose the LP neuron to explore these mechanisms, because it exists as a single copy and is readily identifiable. The LP neuron does not have intrinsic oscillatory activity but receives periodic inhibitory synaptic input from the pacemaker neurons AB and PD, and the follower PY neurons. In each cycle, it rebounds from inhibition to produce a burst of action potentials (Fig. 1A).
The triphasic pyloric activity pattern was continuously present in all preparations, with the temporal sequence of each PD burst being followed after some delay by the LP burst, and then the PY burst (Fig. 1A). To quantify the variability in phase and its consistency across different cycle periods (P), we measured the latencies of the LP neuron burst onset (LPon) and termination (LPoff) across 28 preparations, from at least 30 s of pyloric activity in each. All latencies were measured with respect to the burst onset of the pacemaker group PD neurons. We also kept track of the burst end phase (PDoff) of the PD neurons, to quantify the degree to which the pacemakers maintain a constant duty cycle (Abbott et al., 1991). We did not quantify the PY neuron burst onset and end phases, because in C. borealis, they are virtually identical to LPoff of the same cycle, and PDon of the subsequent cycle (Goaillard et al., 2009). First, we determined the mean values for latencies, P, and phases (φ = latency/P) in each preparation. Across preparations, P ranged from 423 to 2038 ms, with a mean of 880 ms (±368 SD). As reported previously (Bucher et al., 2005; Goaillard et al., 2009), the latency values of PDoff, LPon, and LPoff increased roughly proportionally with P (Fig. 1B). Consequently, phases did not change significantly with P (Fig. 1C).
It is noteworthy that a lack of correlation with P does not mean that phases were completely invariant, as the histograms in Figure 1C indicate. We compared the variability of mean phases and P across preparations with the cycle-to-cycle variability observed across individual preparations. Figure 1D shows box plots of coefficients of variation (CVs) within individual preparations, alongside the single CV values calculated from the means across preparations. Variations in phase were in the same range within and across preparations. In contrast, there was a much larger variability of mean P across preparations than within each preparation. These results confirm that phases are under much tighter control across preparations than cycle period.
Interindividual variability of voltage-gated currents and synaptic inputs
The maximal conductances (gmax) of voltage-gated ionic (henceforth called intrinsic) currents in identified pyloric neurons, including LP, show large variability across animals (Marder and Goaillard, 2006; Schulz et al., 2006; Goaillard et al., 2009; Marder, 2011; Golowasch, 2014; Marder et al., 2014a). Variability of gmax is well correlated with variability in transcript levels of the underlying ion channel genes, and therefore serves as a good proxy for variability of ion channel numbers (Schulz et al., 2006).
We measured intrinsic currents in LP for two reasons. First, variability has previously only been determined for gmax, and we wanted to also examine the variability of voltage dependence. Second, we measured synaptic currents in the same preparations to establish whether there was co-variation that could indicate compensatory regulation of intrinsic and synaptic currents. We performed these measurements of synaptic and intrinsic currents during ongoing rhythmic pyloric activity, restricting ourselves to the subset of intrinsic currents that under these conditions can be measured without pharmacological manipulation (Zhao and Golowasch, 2012). They included the high-threshold voltage-gated K+ current (IHTK), the transient K+ current (IA), and the hyperpolarization-activated inward current (IH; Fig. 2A). Currents were converted to conductance values, and for K+ currents, the activation curves in each individual preparation were fit with a sigmoid to determine gmax, voltage of half activation (V1/2), and the slope factor (k; Fig. 2B). For IHTK, we obtained these parameters for both the transient (IHTKt) and the persistent (IHTKp) components.
Like previous reports (Schulz et al., 2006; Khorkova and Golowasch, 2007), gmax values of IHTK, IA, and IH showed large variability (Fig. 2C). In addition, we found that for both IHTK and IA, the parameters V1/2 and k were also subject to large variability (Fig. 2D,E). We interpret this as an indication that not only the number of channels, but also their gating properties can vary substantially across individuals. Additionally, among these parameters, there were some pairwise correlations among the gmax values as well as the parameters V1/2 and k (Extended Data Fig. 2-1 for plot and Extended Data Fig. 2-2 for p and R values).
To examine variability of synaptic inputs across preparations, we recorded the LP neuron’s graded IPSCs in response to PD and PY neuron input during ongoing pyloric activity (Fig. 3A). The shape of the recorded IPSCs varied considerably across preparations. We used 12 parameters to quantify the IPSC characteristics (Fig. 3B; see Materials and Methods). The distributions of these parameters showed that the IPSC in the LP neuron varies greatly across preparations (Fig. 3C).
The latency of the LP burst onset relative to the pacemakers is shaped by the interaction between its intrinsic voltage-gated ionic currents and the synaptic input that it receives. Notably, hyperpolarization during inhibition de-inactivates IA and activates IH (Harris-Warrick et al., 1995a,b). This plays an important role in controlling the timing of the burst onset, because IH increases the strength of the rebound burst and advances its onset, while IA delays it (MacLean et al., 2005). The activation levels of IH and IA in each cycle depend on the strength, duration, and history of the inhibition.
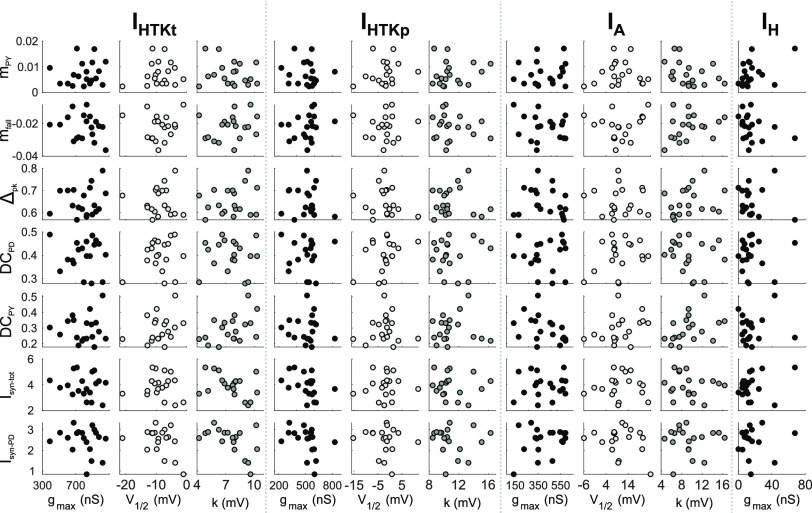
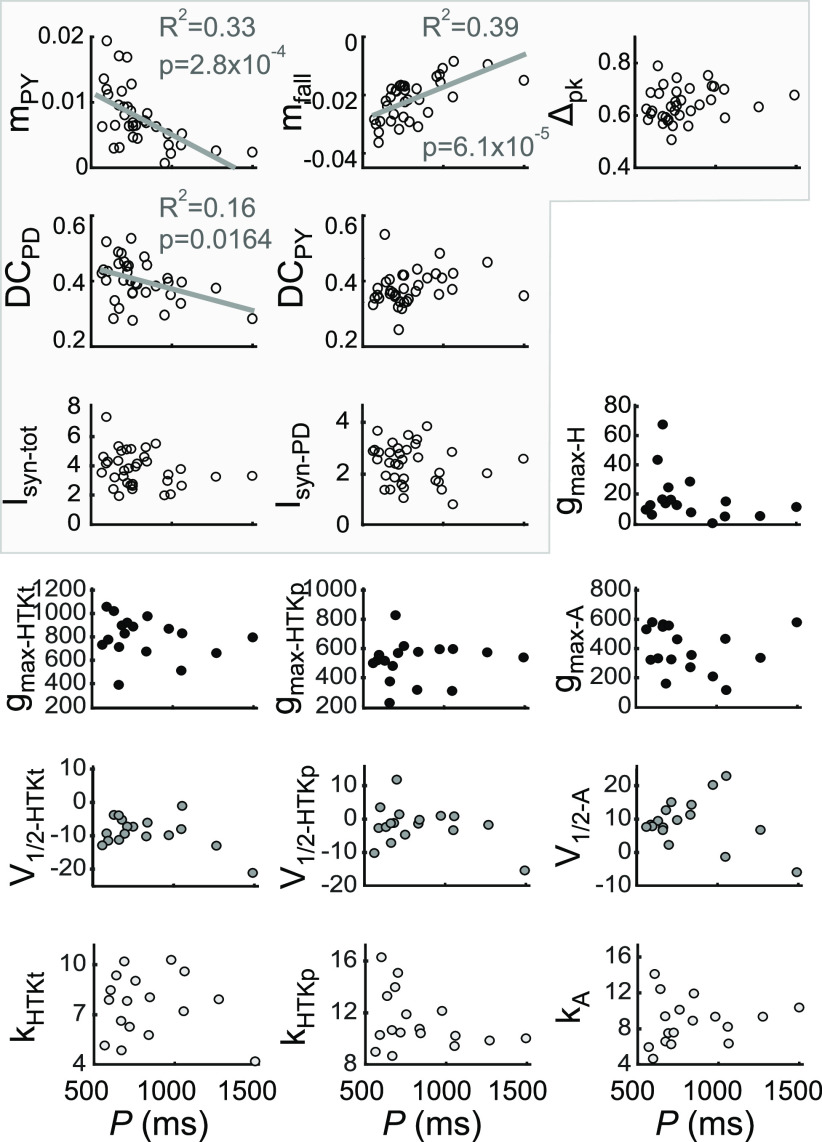
In addition, φLP on is sensitive to changes in both magnitude and temporal trajectory of synaptic inputs (Goaillard et al., 2009; Martinez et al., 2019b). We hypothesized that the synaptic inputs to the LP neuron may covary in a compensatory fashion with its intrinsic properties, thus resulting in a relatively constrained activity phase across animals. We therefore examined the extent to which the synaptic input parameters may be coregulated with gmax of these ionic currents, as well as IHTK. We also tested for any correlations of synaptic parameters with V1/2 and k values of the K+ currents. We did not find any significant pairwise linear correlations between any of the synaptic and intrinsic current parameters (Fig. 4; all p values in linear regression analysis > 0.05; N = 19).
Figure 4.
There are no pairwise linear correlations between any of the synaptic parameters and parameters of the voltage-gated ionic currents. Data shown are from experiments in which every parameter was measured in a single preparation (N = 19). The parameters are defined in Figures 2 and 3. Each dot represents an LP neuron from an individual animal.
The LP burst onset is influenced by synaptic parameters
Our results suggest that the consistency of phase across individuals and its independence of cycle period do not simply arise from pairwise correlations between synaptic and intrinsic parameters. We therefore asked whether individual synaptic or intrinsic current parameters are good candidates for playing a substantial role in controlling phase. To this end, we made use of the variability of mean P and the limited variability of mean φLP on across individuals and performed correlational analyses. For synaptic currents, we included the maximum IPSC amplitude and the amplitude of the pacemaker IPSC, as these are commonly used synaptic parameters. Otherwise, we restricted the analysis to the nonredundant set of parameters. As described in Materials and Methods, the five nonredundant parameters are the subset of measures that are sufficient to describe synaptic current trajectory and can theoretically vary independently of each other.
First, we tested whether variability of current parameters was correlated with P. We found that a subset of the parameters describing the trajectory of synaptic currents, but none of the intrinsic parameters, showed correlations with P (Fig. 5). The IPSC slope parameters mPY and mfall were strongly correlated with P. This means that the slopes of the DCPD also showed a weak (negative) correlation with P. Both these slopes are therefore shallower for slower oscillations. The latter is somewhat surprising, as we found a negative trend but no correlation between φPD off and P in the pyloric pattern analysis shown in Figure 1C. This difference could be because of the fact that DCPD, as measured from the IPSC, includes an additional interval in which the synaptic current persists beyond the PD burst offset.
Figure 5.
A subset of the LP neuron synaptic, but not intrinsic, parameters are correlated with the pyloric cycle period. The five primary synaptic parameters, the amplitudes of the total and pacemaker-component of the synaptic current, and the intrinsic current parameters are compared with the pyloric cycle period (P) across preparations. Three synaptic parameters, but no intrinsic parameter, covary with P. The synaptic parameters are highlighted.
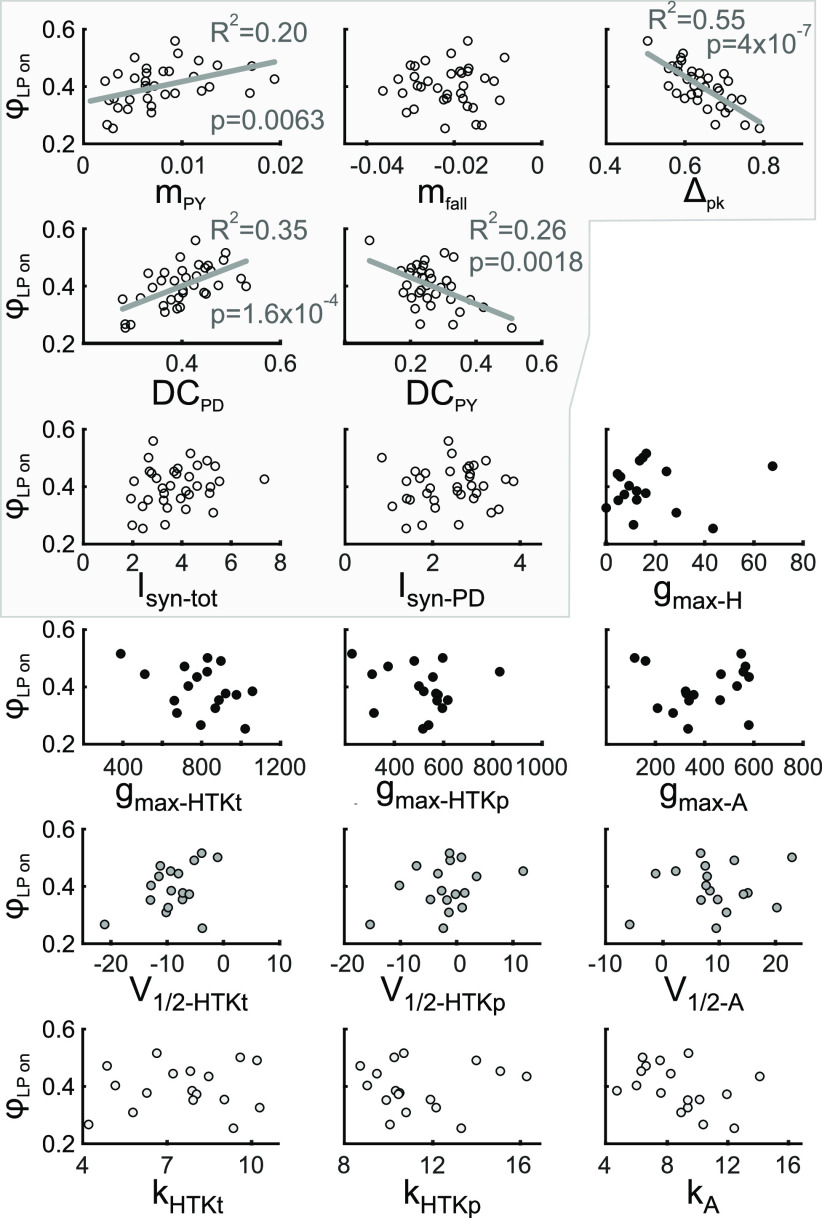
Next, we explored whether φLP on was correlated with any of the current parameters (Fig. 6). Once again, we found correlations with a subset of the parameters describing the trajectory of synaptic currents, but none with intrinsic parameters. φLP on was weakly positively correlated with mPY, and strongly negatively correlated with Δpk. Interestingly, φLP on was correlated strongly with both DCPD and DCPY, with opposite signs. This suggests that synaptic inputs from both the pacemakers and the PY neurons may influence φLP on, although the input from the PY neurons is primarily responsible for the termination of the LP neuron burst, not its onset (Marder and Bucher, 2007). In comparison with Figure 5, mPY and DCPD were correlated with both φLP on and P, whereas mfall was only correlated with P, and Δpk and DCPY only with φLP on.
Figure 6.
The LP neuron burst onset phase is correlated with multiple synaptic, but not intrinsic, parameters. The LP burst onset phase (φLP on) is compared with the five primary synaptic parameters, the amplitudes of the total and pacemaker-component of the synaptic current, and the intrinsic current parameters across preparations. φLP on covaries with four synaptic parameters, but not with intrinsic parameters. The synaptic parameters are highlighted.
The influence of synaptic parameter variation on the LP neuron’s burst onset
Given that our results revealed no correlations between single intrinsic current parameters and φLP on, but correlations between φLP on and several synaptic parameters, we further explored which aspects of the overall synaptic current trajectory were important. Because we found no correlations between synaptic current amplitudes and φLP on or P, we restricted the analysis to the nonredundant parameters. As stated above, these five parameters can theoretically be varied independently to change synaptic current trajectory. However, this does not mean that they actually varied independently in the measured experimental data. Indeed, we found that most parameter pairs were correlated, some strongly and others weakly (Fig. 7A). In particular, Δpk and DCPD were strongly correlated, as was expected for parameters that quantify the contribution of the pacemakers. However, mfall, which also depends on the strength and the timing of the pacemaker inputs, was not correlated with Δpk or DCPD. In contrast, mfall was strongly correlated with mPY. This is somewhat surprising, because mPY quantifies the rise of the PY to LP synapse, whereas mfall is because of the decay of the pacemaker to LP synapse.
Figure 7.
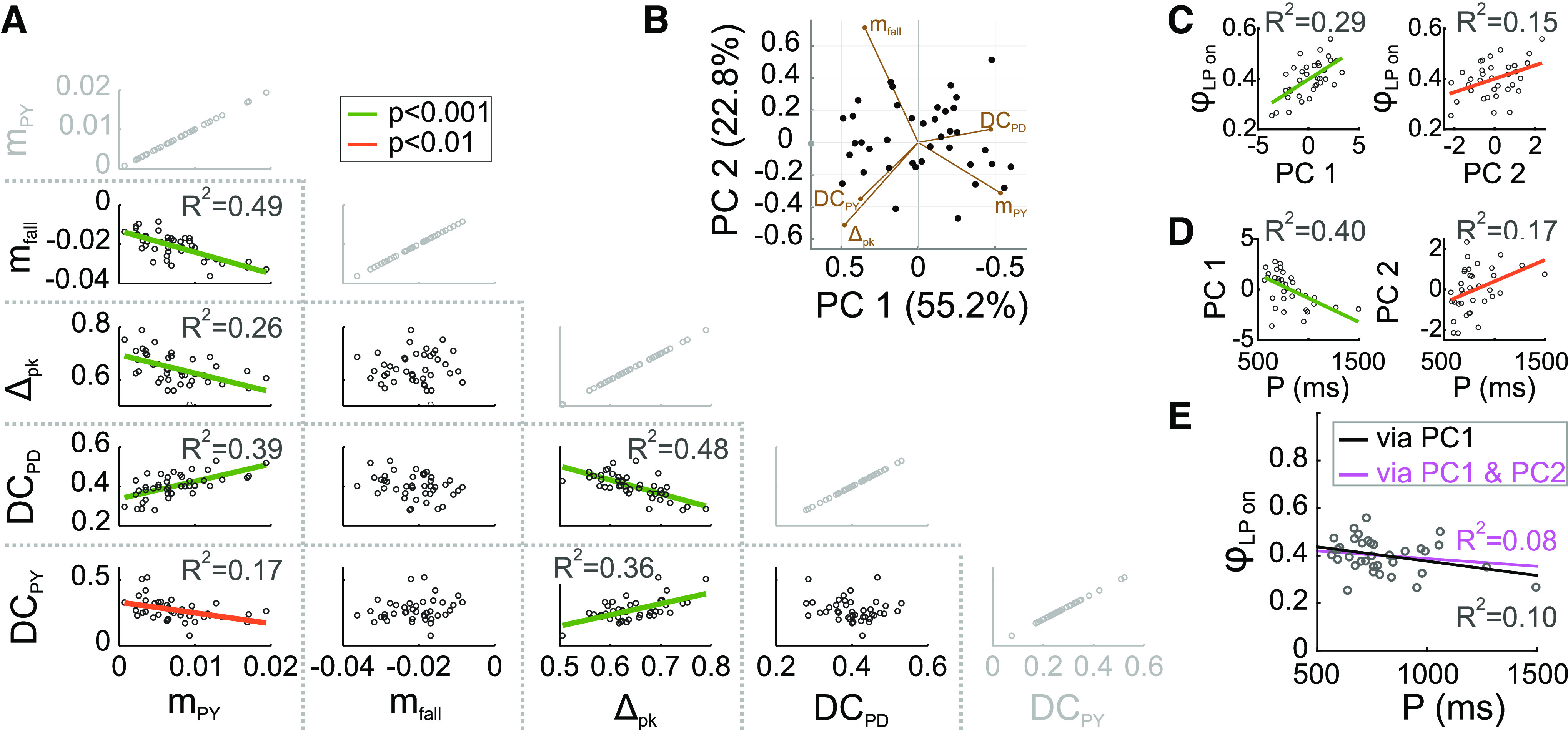
The primary synaptic parameters are correlated. A, The five primary synaptic parameters were compared pairwise across preparations. Of the 10 nontrivial comparisons (shown in black), 6 showed significant correlations. The trivial comparisons (gray) are shown for clarity. B, PCA was used to find directions of largest variability among the five synaptic parameters. The first two principal components described 78% of the variability in synaptic parameters. Filled circles show all recorded synaptic waveforms, projected down to the PC1-PC2 plane. Percentages on axis labels indicate the extent of variability in the direction of the PC. The directions of the five primary synaptic parameters in the PC1-PC2 plane are indicated by brown line segments (biplot). See Extended Data Figure 7-1 for projections onto all PC subplanes. C, Across preparations, the LP burst onset phase (ϕLP) is correlated with both PC1 and PC2 (but not PC3, PC4, or PC5). D, Across preparations, both PC1 and PC2 (but not PC3, PC4, or PC5) are correlated with the pyloric cycle period (P). E, Using the PC1 and PC2 correlations with ϕLP and P (lines in left graphs of panels C, D) to calculate a linear relationship (black line) between ϕLP and P correctly predicts a lack of correlation between these two factors. Including both the PC1 and PC2 correlations (all lines in panels C, D) to do the linear prediction (magenta line) does not greatly improve the prediction.
Biplots of the principal components in all projection planes. Percentages on axis labels indicate the extent of variability in the direction of the PC. The directions of the five primary synaptic parameters are indicated by olive line segments. Download Figure 7-1, EPS file (835.1KB, eps) .
Our correlational analysis indicates that multiple synaptic parameters co-vary across experiments. It is important to note that these correlations do not indicate any inherent dependency among any of these parameters, but that these correlations arise because of (periodic) interactions among the pyloric circuit neurons. Only circuit-level interactions can, for example, relate characteristics of the pacemaker to LP synapses to those of the PY to LP synapse.
The correlation of multiple synaptic parameters with φLP on (as seen in Fig. 6) indicates that these five parameters in fact covary across preparations and therefore the variation in synaptic shape are not because of five independent parameters, but a smaller combination of these five. Thus, it is possible to simplify the correlational analysis by determining which combination of parameters explains the observed variability in φLP on. To reduce the dimensionality of the IPSC parameter space, we performed PCA. We found that 95% of the total variability of IPSC parameters were explained by the first three PCs (PC1: 55.2%; PC2: 22.8%; PC3: 16.5%). Figure 7B shows all synaptic waveforms in the plane of the first two PCs, as only PC1 and PC2 were significantly correlated with φLP on (PC1: p < 0.001; PC2: p = 0.013; Fig. 7C). Interestingly, both PC1 and PC2 (and only these) were also significantly correlated with P (PC1: p < 0.001; PC2: p =0.024; Fig. 7D).
To examine whether the coordinated variation of synaptic parameters in the direction of PC1 was sufficient to explain phase maintenance, we used the linear regression fit equations of PC1 versus φLP on and PC1 versus P (Fig. 7C,D, left panels) to predict a linear relationship between φLP on and P. In Figure 7E, we compare this prediction (black line) with the data for LP on over P shown in Figure 1C, open circles. This comparison produced a coefficient of determination of R2 = 0.10, which was comparable with the linear fit obtained in Figure 1C (R2 = 0.16 for φLP on). This indicates that variation of the synaptic conductance trajectory with P along PC1 is sufficient to remove the correlation between φLP on and P, thus predicting phase maintenance across preparations. Additionally correcting this prediction by adding the linear regression fit equations of PC2 (Fig. 7C,D, right panels) did not greatly change this prediction (Fig. 7E, violet line, R2 = 0.08).
Our analysis of data obtained during spontaneous pyloric rhythmic activity revealed combinations of synaptic parameters whose coordinated variation could potentially result in relatively constant φLP on across preparations, despite variation in P. However, there are two caveats. First, correlation may result from causation in some cases, but not in others. A synaptic parameter (or a principal component, such as PC1) that is correlated with φLP on may in fact causally influence φLP on. If so, the system must adjust this parameter at different cycle periods to produce phase maintenance. For example, this could explain why PC1 is correlated with both φLP on and P. In contrast, a parameter may simply change with φLP on but not influence it, in which case its change with P would not contribute to phase maintenance. Similarly, a parameter such as Δpk that is correlated with φLP on but not P may also causally influence φLP on (Martinez et al., 2019b) and would therefore be kept constant across animals to maintain phase. Second, causation may not necessarily reveal itself as a correlation. In our data, φLP on (and all other pyloric phases) varied in a fairly limited range, independent of the large variability of P. Therefore, simply analyzing correlations in data obtained from spontaneous rhythms constrained the maximum effect a synaptic parameter may have on φLP on to the same limits. Within these limits, a parameter that has no correlation with P or φLP on may in fact have a strong influence on φLP on and, for this reason, be kept constant across animals (and thus show no correlation with P).
For these reasons, establishing a causal influence of synaptic parameters on φLP on requires experimentally controlling and systematically varying them. To this end, we performed a set of experiments in which the LP neuron was synaptically isolated, and synaptic conductance waveforms were artificially applied using the dynamic clamp technique. Conductance trajectories were constructed to resemble the current trajectories and adhering to the same decomposition into parameters shown in Figure 3B. We kept the cycle period constant at 1 s and injected the waveforms periodically until the LP burst activity attained a steady state (∼30 cycles; Fig. 8A). In each experiment, this procedure was repeated with 80 different synaptic trajectories in randomized order (Fig. 8B). Because our focus here is on variability and activity phase, we did not do a complete analysis of these dynamic clamp experiments on LP activity and only considered the effect on the LP neuron’s burst onset at steady state, measured as the latency from the end of the artificial synaptic input (Fig. 8A, inset; also see Materials and Methods).
Figure 8.
Using dynamic clamp to inject a periodic synaptic conductance waveform into the synaptically-isolated LP neuron to measure the latency of LP burst onset. A, A predetermined conductance waveform (one of 80) is injected into the synaptically-isolated LP neuron as an inhibitory synapse for 30 cycles at a cycle period of 1 s. The latency of the LP burst onset, measured form the end of the conductance waveform (long vertical dashed line in inset), reaches a steady state value after several cycles. Inset shows the last cycle. B, A total of 80 synaptic conductance waveforms were used periodic dynamic clamp injection in each LP neuron, as described in A. C, An example analysis of the sensitivity of LP burst onset latency to changes in synaptic waveform shape along PC1, while PC2 remains constant (but other PCs vary freely). C1, The 80 synaptic waveforms (gray filled circles) used in dynamic clamp experiments were sorted by projecting the shape down to the PC1-PC2 plane. Pairs of waveforms (black circles) that fell along horizontal lines of constant PC2 (and were apart by at least 0.05 in PC1 units) were chosen for sensitivity analysis. C2, Example responses of the LP neuron to dynamic clamp injection of synaptic conductance waveforms marked by the yellow stars in C1. C3, The change in LP burst latency (see A) as a function of the change in PC1 value (in bins of 0.1), averaged across constant PC2. The slope of this change (Δlatency/ΔPC) is used as a measure of sensitivity. D, Same as C, but changing the waveform along PC3 while keeping PC1 constant.
The correlations obtained from the PCA imply that varying synaptic waveform along PC1 while retaining the respective correlations with P and φLP on shown in Figure 7C,D should keep φLP on independent of P, which would be sufficient to describe phase constancy across preparations. We used our dynamic clamp data to examine whether changing the synaptic waveform along PC1 in fact influenced the LP burst onset latency. To do so, we first described our 80 synaptic waveforms in terms of PC1-PC5. Because visualization of 5D space is difficult, if not impossible, we show the waveform shapes projected down to the PC1-PC2 and PC1-PC3 planes (Fig. 8C1,D1, respectively). To analyze the effect of changing the synaptic shape in the direction of each PC, we first measured the sensitivity of the LP burst onset latency to changing that PC, while keeping another PC constant (see Materials and Methods). We did this analysis for each pair of PCs. Two examples are shown in Figure 8C,D. Surprisingly, the LP burst onset latency showed little sensitivity when the synaptic waveform was changed along PC1 while keeping PC2 constant (example in Fig. 8C2; average effects in Fig. 8C3; one-way ANOVA: p = 0.26 and F = 1.32). In contrast, changing the synaptic waveform along PC3 while keeping PC1 constant produced a very large decrease the burst onset latency (example in Fig. 8D2; averages in Fig. 8D3; one-way ANOVA: p < 0.001 and F = 8.21 using). This result is surprising because it implies that changing the synaptic waveform along PC1 does not result in any change in the LP burst onset, which contradicts our initial interpretation of the correlations observed in Figure 7C,D. If changing the synaptic waveform along PC1 does not produce any change in the LP burst onset, then it makes no sense to claim that the mechanism for phase constancy across preparations with different cycle periods is by changing the synaptic waveform along PC1. Similarly, the synaptic waveforms showed no correlation between PC3 and either P or φLP on. Yet, experimentally changing the synaptic waveform along PC3 produces a large effect on the LP burst onset.
In Figure 9, we summarize the statistics of the effect of changing the synaptic waveform (with dynamic clamp) along each PC, while keeping one other PC constant. Figure 9A is an illustration of how synaptic waveform changes along each of the five PCs. Figure 9B shows the sensitivity of the LP neuron’s burst onset latency to these changes, either grouped by the PC that was systematically varied while one other was fixed (Fig. 9B1) or grouped by the PC that was fixed while one other was systematically varied (Fig. 9B2). On average, changing the synaptic waveform along each of the PCs, except for PC2, had some effect on the burst onset latency (Fig. 9C). PC3 had the largest effect, followed by PC5. In addition, fixing PC3 made the LP neuron’s burst onset latency insensitive to varying any of the other PCs, while fixing any of the other PCs did not have that effect (Fig. 9D). These results suggest that the synaptic parameters (PC1 and PC2) that show the largest variation across preparations have little influence on the burst onset of the LP neuron, whereas two of the synaptic parameters (PC3 and PC5) which have large effect on the burst onset show little variability and are kept relatively constant across preparations.
Figure 9.
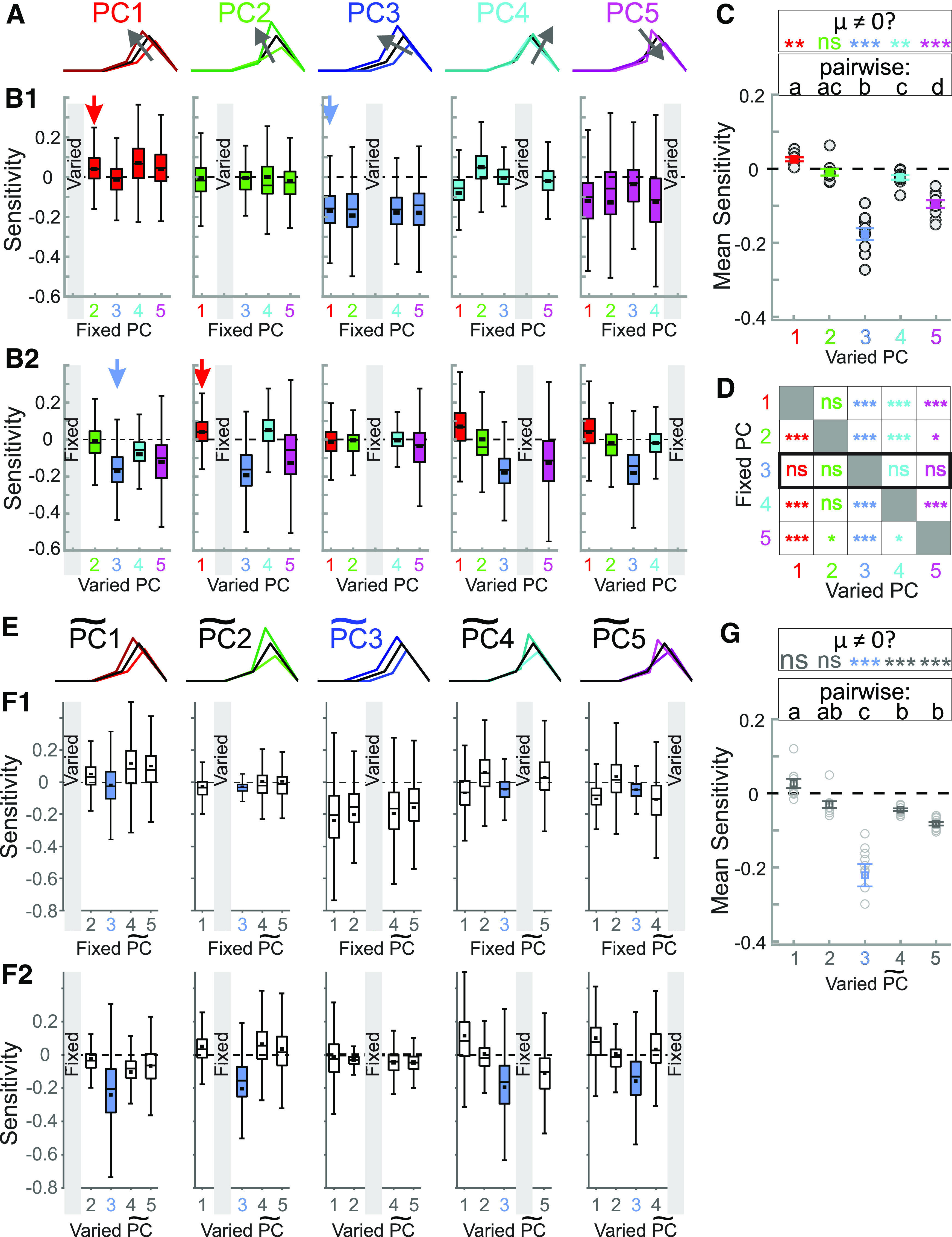
Varying synaptic waveform shape along each principal component while keeping another principal component constant. A, Graphical representation of how the shape of a representative synaptic waveform (black) changes when synaptic parameters are shifted by 25% in the direction of each principal component (in the direction of the arrow). B1, Sensitivity of LP burst onset latency to changing the principal component along a single PC (marked by gray box in each panel) while a single other PC is kept constant (and the other three are not controlled). Examples of the process (marked by arrows) are shown in Figure 8C,D. In these panels, quartile plots are from data including every individual sensitivity value (200–500 data points) in each experiment (N = 10 animals). Black squares show mean values. B2, The same data as in B1, reorganized so that each panel shows data when a single PC (gray box) is kept constant while a single other PC is varied (and the other 3 are not controlled). The red and blue arrows point to the same data as they do in B1. C, Overall sensitivity of LP burst latency (Fig. 8) to changing the synaptic waveform along each PC, measured as an overall average of the values shown in panel B. In this graph, only the mean sensitivity values in each experiment are used as data points (N = 10). These sensitivities were significantly different (one-way RM-ANOVA p < 0.001). Different letters (a–d) indicate p < 0.01 with post hoc Tukey’s test; shared letters indicate p > 0.05. Asterisks indicate post hoc analysis indicating whether the mean value (μ) ≠ 0, **p < 0.001, ***p < 0.0001. D, Statistical summary of panel A data, indicating how varying one PC, while keeping another PC constant (and not controlling others), would produce a change in LP burst latency. Asterisks indicate post hoc analysis indicating whether μ ≠ 0, *p < 0.01, **p < 0.001, ***p < 0.0001. E, The direction of DC is marked as for visual comparison with PC3 in panels A–D. The other s are roughly (but not exactly) in similar directions as the corresponding PCs of A–D. F1, Sensitivity of LP burst onset latency to changing the principal component along a single (marked by gray box in each panel) while a single other is kept constant (and the other three are not controlled). Data are the same as in B. Black squares show mean values. F2, The same data as in F1, reorganized so that each panel shows data when a single PC (gray box) is kept constant while a single other PC is varied (and the other 3 are not controlled). G, Overall sensitivity of LP burst latency to changing the synaptic waveform along each (as in panel F), measured as an overall average of the values shown in F. Notations the same as in C. See Extended Data Figure 9-1 for values of PCs and s.
Description of the derivation of the PCs from the PCs in Figure 9, by assuming that PC3 is fixed in the direction of DC = DCPD + DCPY. Download Figure 9-1, EPS file (1.9MB, eps) .
To provide a more intuitive understanding of the changes in the direction of PC3, we restricted the changes to the main components in the original synaptic parameters, which were to simultaneously increase DCPD and DCPY (see Extended Data Fig. 9-1). A simple concurrent change of these two parameters (i.e., changing DCPD + DCPY) simply implies changing the proportion of the cycle that the synapse is active (see Fig. 3B). Thus, changing DCPD + DCPY provides a way to intuit the major effect of changing the synapse in the direction of PC3, which is a change in the proportion of each cycle during which the LP neuron is inhibited. We therefore fixed the direction of PC3 to be exactly that of DCPD + DCPY (henceforth simply called DC) and asked how the dynamic clamp changes in this direction influence the LP latency. To do this comparison, we had to redefine the directions of our 5D parameter space to be either along DC or perpendicular to it (DC⊥). The derivation of the principal components in the DC⊥ hyperspace is described in Materials and Methods. Thus, our parameter space can be described in terms of five new directions, one in the direction of DC, which is approximately that of PC3. For clarity, we call this direction . The other four directions are the principal components in the DC⊥ hyperspace (derivation described in Materials and Methods) which are approximately aligned with the directions PC1, PC2, PC4, and PC5 (Fig. 9E; Extended Data Fig. 9-1). Again, for clarity, we call these directions, respectively, (Extended Data Fig. 9-1). We then re-analyzed the dynamic clamp data using the new directions to measure the sensitivity of the LP neuron’s burst onset latency to changing the parameters in these directions.
We found that varying the synaptic waveform in the direction of DC ( ) produced the largest effect on the LP neuron burst latency compared with any of the directions in (Fig. 9F). Similarly, when the synaptic waveforms were fixed in the direction of , changes in any other direction produced little effect on the burst latency (Fig. 9F2, middle panel). These effects are summarized in the statistics shown in Figure 9G.
Discussion
Variability of activity phases within and across individuals
During oscillatory circuit activity, differences in sensory, descending, and modulatory inputs often result in different activity phases between different neurons, whereas similar behavioral settings and circuit states produce characteristic phase relationships (Marder and Bucher, 2001; Wang, 2010; Grillner and El Manira, 2015, 2020; Wilson et al., 2015; Frigon, 2017). These activity phases can even be maintained over a wide range of rhythm frequencies within individuals, which has been demonstrated in many motor systems (DiCaprio et al., 1997; Wenning et al., 2004; Marder et al., 2005; Grillner, 2006; Le Gal et al., 2017).
Across individuals, activity patterns can vary, particularly in cycle period, but retain enough consistency in the activity phases to be readily matched across the same individuals. In the pyloric circuit, spontaneous in vitro rhythmic patterns in individual preparations show some cycle-to-cycle variability in the bursting neurons’ activity phases, consistent with cycle-to-cycle variability in cycle period (Bucher et al., 2005; Elices et al., 2019). However, mean phases are well maintained when mean cycle period is experimentally altered (Hooper, 1997; Tang et al., 2012; Soofi et al., 2014). Across individuals, phases also show some limited variability but are insensitive to substantial differences in mean cycle period (Bucher et al., 2005; Goaillard et al., 2009). We confirm here that phases can vary across individuals but do not correlate with mean cycle period (Fig. 1C). We also show that the variability of neuronal activity phases across individuals is within the same ranges as cycle-to-cycle variability within individuals, although cycle period varies substantially more across individuals than it does within individuals (Fig. 1D). This raises the question of whether these activity phases are constrained to a small range of variability. We assert that there is no absolute measure for how much variability constitutes a lot or a little, and such an assessment should depend on a reference value. For example, in the leech heartbeat system, variability in phase has been interpreted as being large because phase values varied as a substantial fraction of the reference cycle (Wenning et al., 2018). In the pyloric circuit, variability of phases under control conditions is limited in the sense that phases are largely constrained to values that differ from those under different neuromodulatory conditions (Marder and Bucher, 2007; Harris-Warrick, 2011). In our dataset, variability in phase was large enough to allow us to search for correlations with intrinsic and synaptic current parameters, but the fact that phases remain independent of cycle period justified asking which parameters may be constrained or may co-vary in a compensatory manner to achieve consistent circuit output phases across individuals.
Variability of intrinsic and synaptic currents
Activity phases are shaped by both intrinsic and synaptic currents, both of which can vary substantially across individuals. In the LP neuron, the known intrinsic currents vary several-fold across animals (Liu et al., 1998; Schulz et al., 2006, 2007; Golowasch, 2014). We found that the voltage-gated currents do not just vary in magnitude, but that the half-activation voltage and slope factors were also quite variable across preparations (Fig. 2). Different studies have reported a range of different values for these parameters (Zhao and Golowasch, 2012). Some variability may be because of recordings in different cell types or species (Harris-Warrick et al., 1995b), but the mean values of the parameters that we recorded are consistent with the original recordings of ionic currents in the crab LP neuron (Golowasch and Marder, 1992). It should be noted that the magnitude of K+ conductances correlate well with corresponding channel gene mRNA copy numbers (Schulz et al., 2006), which serves as independent confirmation that variability is not solely because of noise or experimental error. We cannot provide a similar independent confirmation for variability in voltage dependence, and it is not obvious to which degree half-activation and slope factor measurements may be more affected by experimental error than gmax is. However, variability in voltage dependence may be because of posttranslational modifications of ion channels (Jindal et al., 2008; Voolstra and Huber, 2014; Laedermann et al., 2015) or their phosphorylation state (Ismailov and Benos, 1995; Hofmann et al., 2014).
Not only did we not find any correlations between intrinsic and synaptic currents in the LP neuron, but we also did not find any correlations between intrinsic current parameters with either cycle period (Fig. 5) or φLP on (Fig. 6). This does not mean that intrinsic currents do not play an important role in controlling phase. Intrinsic properties, as determined by voltage-gated ionic currents, pump currents and even leak currents, are primary determinants of its activity. In the LP neuron, intrinsic properties have a great influence on φLP on, as can be seen for example from the slow response of this neuron to repetitive dynamic clamp application of the same artificial synaptic input (Fig. 7A). This slow response is indicative of a form of short-term memory over a timescale of many cycles that is attributed to intrinsic properties (Goaillard et al., 2010; Schneider et al., 2021). Some aspects of phase regulation are in fact dominated by intrinsic properties. For example, the phase difference between the LP and PY neurons is largely determined by differences in intrinsic currents, as experimentally applying identical synaptic input into both neuron types preserves their relative timing (Rabbah and Nadim, 2005).
Varying synaptic current amplitudes across individuals can still give rise to similar CPG output, for example in the leech heartbeat system (Norris et al., 2007, 2011). In the pyloric circuit, similar values for φLP on are achieved across individuals despite large variability of pacemaker synaptic input during ongoing rhythmic activity (Goaillard et al., 2009). We confirm the substantial variability in pacemaker to LP synaptic current amplitudes and in addition describe similar variability for PY to LP input (Fig. 3C1). However, phase also depends on the relative timing, duration, and precise temporal trajectory of synaptic inputs (Prinz et al., 2003; Martinez et al., 2019b). In particular, φLP on is exquisitely sensitive to the shape and amplitude of synaptic input within preparations (Martinez et al., 2019b), and we show here that attributes describing the trajectory of the total synaptic current input to LP vary substantially across individuals (Fig. 3C2–C4). Therefore, similar values of φLP on are found across individuals despite varying intrinsic and synaptic currents.
In general, phase is dependent on an interplay of intrinsic and synaptic currents. Because φLP on adjusts over several cycles, any such interplay must occur at a much slower timescale than that of an individual cycle. Synaptic inhibition activates IH and de-inactivates IA, which plays a critical role in determining rebound delay in follower neurons at different cycle periods (Harris-Warrick et al., 1995a,b; MacLean et al., 2005). IH and IA promote phase maintenance in individuals, particularly in conjunction with short-term synaptic depression, which results in an increase of inhibition with increasing cycle periods (Nadim and Manor, 2000; Manor et al., 2003; Bose et al., 2004; Greenberg and Manor, 2005; Mouser et al., 2008). Goaillard et al. (2009) recorded pyloric circuit activity and subsequently measured mRNA expression levels of the channel genes coding for IH and IA in LP, and also found no correlations with φLP on. However, they did find φLP on to be correlated with the maximum value of a neuropeptide-activated current, which was also correlated with synaptic currents. Therefore, a lack of correlations between cycle period or φLP on and single intrinsic current parameters across individuals may simply mean that variability is well compensated across different currents.
The total synaptic current to the LP neuron is a combination of inputs from the pacemaker neurons AB and PD, and the 3–5 PY neurons, and therefore has a complex waveform shape (Fig. 3). Of the five parameters that defined the synaptic waveform, three showed significant correlation with P across different animals (Fig. 5), and four parameters had a strong correlation with φLP on (Fig. 6). Surprisingly, these parameters did not include the strength of the synaptic input from the pacemaker or PY neurons. Goaillard et al. (2009) did not consider PY synaptic inputs to LP but separated AB and PD inputs by their different reversal potentials and found φLP on correlated with peak values of both, albeit with different sign. It is unclear whether this different finding simply results from the different way we defined synaptic strengths. However, the duty cycle and peak phase of the synapse, which strongly influence the LP phase in individuals (Martinez et al., 2019b) were among the correlated parameters. A linear dimensionality reduction using PCA showed only two parameters (the first two principal components PC1 and PC2) sufficiently explained the correlation between cycle period and φLP on. Consistent with these correlations, using the first two principal components to connect cycle period across preparations with the variability of φLP on was sufficient to explain phase maintenance across animals.
Variability and co-regulation
Variability of intrinsic currents in STG neurons may be compensated by cell-type-specific co-regulation of different voltage-gated channels (Khorkova and Golowasch, 2007; Schulz et al., 2007; Temporal et al., 2012; Tran et al., 2019), but it is not known to which degree synaptic currents may be co-regulated. Variability of synaptic currents could be compensated for by variability in intrinsic currents, as has been suggested for the leech heartbeat system (Günay et al., 2019), and as is implicit in theoretical work that shows similar circuit activity with different combinations of intrinsic and synaptic current levels (Prinz et al., 2004b; Onasch and Gjorgjieva, 2020). Alternatively, compensatory co-regulation of intrinsic currents could lead to consistent neuronal excitability on its own, and variability of synaptic trajectory then must be constrained to allow for consistent phases.
We found no evidence of co-regulation between intrinsic and synaptic currents (Fig. 4), which suggests that phase constancy across preparations is not because of any obvious linear correlations that matched synaptic inputs to intrinsic properties. However, there are caveats to this analysis. We only performed pairwise linear correlations, and it is possible that we missed higher dimensional or nonlinear interactions. In addition, the nature of the intrinsic and synaptic current attributes we considered are somewhat mismatched. We described intrinsic voltage-gated currents with standard biophysical parameters, obtaining values for gmax and voltage dependence. These parameters can be direct targets of cellular regulation, but it is not trivial to determine how their variability translates to variability in current magnitude and trajectory during ongoing circuit activity. In contrast, we assessed the magnitude and temporal trajectory of synaptic currents during ongoing pyloric activity, which are determined by presynaptic and postsynaptic properties as well as the voltage trajectories of the presynaptic neurons (Goaillard et al., 2009). Our synaptic current attributes therefore describe well the dynamics of synaptic interactions during circuit activity but can only serve as an indirect assessment of biophysical parameters that would be the targets of cellular regulation. In STG neurons, maximal synaptic currents or conductances and the dependence on presynaptic voltage have been assessed for their sensitivity to different neuromodulators (Zhao et al., 2011; Garcia et al., 2015; Li et al., 2018), but cannot be measured during ongoing circuit activity and their interindividual variability has not been directly addressed.
Correlation versus causation
Correlational analyses from spontaneous rhythmic activity restricted us to the limited variability of circuit output and did not afford us control of the variability of synaptic attributes. We therefore used the dynamic clamp, a technique that allows precise manipulation of synaptic inputs to individual neurons, which can be used to explore the role of a synapse in circuit activity (Bartos et al., 1999; Wright and Calabrese, 2011a,b; Martinez et al., 2019b). These experiments clearly showed that the LP burst onset is quite sensitive to the shape of the synaptic input waveform in a manner that was consistent across preparations. To our surprise, changing the waveform along PC1 or PC2, the two major directions of variability in the parameter space obtained from spontaneous activity, did not produce the largest influence on the LP burst onset. Instead, changing the waveform along PC5 and PC3, directions that did not show significant change with either cycle period or the LP burst onset across preparations, had the largest effect on the LP burst onset. In fact, when the waveform shape was kept constant along PC3, changing it along any other PC did not influence the LP burst onset at all (Fig. 9D). Conversely, changing the waveform along PC3, while keeping any other PC constant, produced the strongest effects on the LP burst onset.
As with all methods of dimensionality reduction, PCA does not yield readily intuitive results. Consequently, describing the effect of parameter changes along principal components does not necessarily produce conclusions that are easily described in terms of the original synaptic parameters. In the case of PC3, however, we found that the major effect of this principal component was to simultaneously change the duty cycles of the pacemaker (PD) and PY synaptic inputs to the LP neuron. When we made the further approximation that this was a proportional change, this meant that PC3 was mainly influencing the proportion (DC) of each cycle where the LP neuron receives synaptic input (see DCPY + DCPD in Fig. 3B2). Furthermore, changing the synaptic waveform in the direction of DC, as opposed to other parameter directions, had almost the same effect as changing it in the direction of PC3, as opposed to the other PCs. The fact that the duration of synaptic input has a significant influence on the LP burst phase onset is at first view not too surprising. But the pyloric rhythm is driven by the pacemakers, including the PD neuron, resulting in an LP neuron rebound burst followed by a burst of the PY neurons. The PY to LP synapse is therefore thought to control the end of the LP burst, not its onset (Fig. 1A). The fact that DC is kept under strict control in each preparation implies that the PY to LP synapse also influences the subsequent LP burst, although one would assume that the much stronger pacemaker to LP synapse overwhelms this influence. These findings indicated that, across preparations, the synaptic waveform is tuned by the circuit to remain unchanged along this direction of maximum sensitivity. Thus, in this subcircuit, phase constancy across preparations is achieved partly by a precise control of the synaptic parameters that have the largest influence on phase.
It is tempting to interpret the correlation of underlying properties with attributes of circuit output as an indication that these attributes are controlled by these properties. However, properties may simply change with circuit output and not determine it. Similarly, one may interpret the lack of correlation as a sign of absence of influence. However, functional influence may be masked by the necessity to simply constrain parameters with large influence on output to a range that keeps output stable. The correlational relationships between the synaptic parameters and cycle period or φLP on that we described from spontaneous circuit output could statistically explain phase maintenance across animals. However, this explanation does not hold the test of causation. The same parameters that statistically predict φLP on, or vary systematically with cycle period, have little influence on the burst onset when varied experimentally. Conversely, we only found the synaptic waveform attributes that are important for the control of phase by systematically varying them experimentally. Thus, our correlational explanation (Fig. 7E) is in fact a consistency argument: if some synaptic parameters change with cycle period, then the same parameter must also change with φLP on in a manner that predicts phase constancy.
Many neural processes are found to co-vary across animals and correlations are often argued to be essential for the function of neural circuits (Golowasch, 2019; Santin and Schulz, 2019). It is important to remember that, despite the levels of degeneracy observed in the parameter space defining circuit output (Goldman et al., 2001; Bucher et al., 2005; Swensen and Bean, 2005), correlations may simply be coincidental to the fact that the varying parameters do not have a meaningful influence on the function of interest (Hudson and Prinz, 2010; O’Leary et al., 2013).
Considering the numerous parameters that can influence the output of a neural circuit, interindividual variability is neither surprising nor avoidable. Yet a consistent output pattern requires some essential combination of circuit parameters to be tightly constrained. Those that are not show variability across individuals and, because of the constraints of the output pattern, are forced to co-vary with output quantities that may also be relatively unconstrained, such as cycle frequency. Thus, parameters correlated with circuit output may contribute little to the output pattern, but rather become correlated because of constraints on this pattern.
Differential control of activity phases
We addressed here how activity phases can stay consistent under control conditions, i.e., in the same circuit state. However, synaptic function and activity phases can be different between different circuit states, for example through the influence of neuromodulators (Harris-Warrick, 2011; Marder, 2012; Bucher and Marder, 2013; Marder et al., 2014b; Nadim and Bucher, 2014; Daur et al., 2016; Brzosko et al., 2019). In motor systems, the functional impact of such adjustments can be particularly transparent, as circuit reconfiguration through neuromodulation is for example a core mechanism for adjusting locomotion gait and speed (Harris-Warrick, 2011; Miles and Sillar, 2011; Bucher et al., 2015; Kiehn, 2016; Grillner and El Manira, 2020). Neuromodulators can affect neurotransmitter release, receptor properties, and postsynaptic intrinsic response properties (Nadim and Bucher, 2014). In addition, synaptic function can change because the activity profile of the presynaptic neuron is modified, as has been shown for STG neurons (Johnson et al., 2005, 2011; Zhao et al., 2011). All these actions of neuromodulators can alter the temporal trajectory of synaptic responses. Therefore, our results provide a useful framework for understanding which aspects of the temporal dynamics of synaptic inputs can be altered by neuromodulators to change phase, and which changes produce robustness for phase relationships.
Synthesis
Reviewing Editor: Arianna Maffei, Stony Brook University
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: NONE.
The reviewers agree that this is an important manuscript reporting interesting and novel results. Experiments are overall well-designed and executed, and the data analysis is appropriate.
The primary weaknesses identified by both reviewers regard the clarity of the data presentation. There are concerns that the description may be too technical to be accessible to readers interested in the topic who lack a specific background in the experimental design. There are also a few concerns regarding the wide use of correlations and more specific concerns regarding measurements of a small number of parameters.
The detailed comments of the reviewers follow below.
Reviewer #1:
In this article, the authors investigate the contribution of cell-to-cell variability in intrinsic and synaptic properties to the output pattern of the LP neuron of the stomatogastric ganglion. The authors first use voltage-clamp measurements of voltage-gated and synaptic conductances during the ongoing rhythm and correlative analysis to decipher which parameters may co-vary and determine preparation-to-preparation phase constancy of the LP neuron. The authors conclude from these measurements that synaptic parameters are not correlated with intrinsic properties, and that only synaptic parameters predict LP output (mainly phase and period). The authors then use principal component analysis to define composed synaptic parameters that best predict variations in LP output. Finally, the authors use dynamic-clamp to determine whether the correlations observed indicate causal relationships between synaptic parameters (or their PC counterparts) and LP output. The surprising (or not) conclusion of this analysis is that the synaptic parameters that are most correlated with LP output do not seem to influence it significantly, while the synaptic parameters that show little preparation-to-preparation variability strongly determine LP output variations. This suggests that the synaptic parameters efficiently controlling LP output are in fact kept quite constant from preparation to preparation, such that correlative analysis cannot reveal their importance. The article is well written, and the experiments and analysis are well performed. Nonetheless, the conclusions of the last set of experiments (dynamic-clamp experiments analyzed using PCA) are quite abstract and the biological relevance of these findings is difficult to grasp. Additional analysis and/or rewriting would be necessary to improve that last part of the manuscript and its overall conclusions.
MAJOR POINTS
1) The results section ends with a description of which principal components describing the synaptic parameters’ collective variation have a significant effect on LP burst onset. This analysis is sound and leads to the conclusion that the less variable PCs have the strongest influence on LP burst onset while the most variable PCs have little effect on LP burst onset. Although going back to the individual synaptic parameters is not straightforward (by the nature of PCA and dimensionality reduction), this effort should be made by the authors. In other words, it would be nice to read conclusions about the specific synaptic parameters underlying the most critical PCs. Without it, the main results remain quite abstract and lack biological relevance. Since the synaptic parameters contributing the most to PC3 and PC5 seem to have the strongest influence on LP burst onset, it would be really helpful to show the relationships between individual synaptic parameters and these PCs (such as what is done in Figure 7B for PC1 and PC2) in order to then provide an interpretation of the dynamic-clamp experiments not only in terms of PCs but in terms of biologically relevant synaptic parameters. Some supplementary analysis or at least some rewriting would be necessary to provide the readers with a “biological” interpretation of which synaptic parameters appear to critically control LP firing.
2) A major part of the study is based on measurements of the synaptic currents received by the LP neuron. I was somewhat surprised to see that the authors measured these synaptic currents by clamping the LP neuron at -50mV. This voltage is rather depolarized, such that unclamped action currents are visible on the recordings (Figure 3). Even though the authors are clamping the neuron for 30s to reach a steady state of activation/inactivation for voltage-gated conductances, the unclamped action currents suggest that the state of intrinsic conductances may still be variable in remote unclamped compartments. The authors need to justify this choice, as a more hyperpolarized potential is usually a safer choice to avoid any risk of contamination by voltage-gated conductances (IH can easily be dealt with).
3) The authors report a half-activation voltage for IA much more depolarized than usually reported. In fact, IA activation voltage is more depolarized than for the IHTK current, which is a bit surprising. The work from Harris-Warrick et al. in particular demonstrated that IH and IA interact to define rebound properties in pyloric neurons, however this is only compatible with IA behaving as a “low-threshold” potassium current. The authors need to explain why IA properties are so different in their experimental conditions.
4) Much of the analysis in the first part of the manuscript relies on correlations and linear regressions. Since the authors perform many correlations for a given set of variables (such as in Figure 4, 5 and 6 in particular), it is critical to correct for false discovery rate. The authors may have performed it and omitted to mention it in the methods section. Otherwise, some of the weak correlations reported in Figure 5 and 6 may not resist the correction for multiple correlations. Either way, this needs to be corrected.
5) When analyzing the properties of voltage-gated currents, the authors only report conductance value for IH. I understand that measuring precisely IH properties during the ongoing rhythm is particularly difficult, but this is a real shame, because IH activation voltage in particular is one of the parameters that we might expect to act in synergy with synaptic inputs.
Reviewer #2:
This is a detailed and sophisticated study on how the LP neuron of the Cancer borealis STG maintains a constant activity phase across preparations (different crabs) despite big variations in cycle period and synaptic and intrinsic currents. For this, the authors first carried out voltage clamp recordings from the LP neuron during normal spontaneous oscillating activity. It is established that while the latency for the LP neuron to fire increases with increasing cycle periods, the activity phase is relatively stable over varying cycle periods. Notably, the amount of variability in cycle period across preparations is found to be markedly greater than the degree of variation in the LP activity phase across the same preparations. To determine how the stable activity phase is achieved, synaptic inputs (IPSCs) to the LP and several different voltage-dependent intrinsic currents expressed by the LP neuron were quantified. Correlation analysis indicated several synaptic parameters to be correlated with LP burst onset, not just one. Thus the authors carry out a principle component analysis followed by ingenious use of dynamic clamp to test the different waveforms generated by the different PCs. The two synaptic parameters that were determined to be most effective in altering LP onset were also determined to be relatively stable across crabs, while the synaptic parameters that had little effect on burst onset were more variable. Overall, this study identifies specific synaptic parameters that are stable and appear to work to maintain stability of phase. The results of this work add significant and novel insight into how the activity phases of individual neurons that comprise an oscillatory circuit can be relatively stable across vastly different cycle periods and oscillation frequencies. The experiments are well-designed and logical. The figures are effective, especially the schematics in figure 1. Focusing on just one neuron type of the STG in detail is unique and effective approach. Injecting all the different synaptic waveforms into the LP using dynamic clamp is a great use of dynamic clamp.
The main weakness of this study is in the way that it is written. There are many sentences that are full of acronyms and no re-phrasing to help the reader understand. It is challenging. Some of the ideas being conveyed seem to be contradictory, but that is probably just because of poor phrasing. Below are suggestions on how to improve clarity.
Significance statement
1. The Significance Statement should include both the significance of the question being addressed and the significance of the findings. This significance statement appears to describe only the significance of the question being addressed but does not describe the findings of the study and their significance. It should probably be re-written to include this.
2. Line 17 and Line 20: There are two seemingly contradictory ideas conveyed in this Significance Statement. The first sentence (shown below) conveys the idea that neural circuits produce similar outputs across individuals and the third sentence conveys the idea that circuit output shows variability across individuals. Perhaps this could be re-phrased to convey a more cohesive idea.
Line 17 “Neural circuits produce a similar output across individuals despite a large variability in parameters...”
Line 20: “.....circuit output shows considerable variability across individuals, and, additionally there are correlations among circuit parameters.”
Introduction
3. line 61 “Synaptic currents also vary substantially across individuals and their magnitude is correlated with relative timing of the postsynaptic neuron.”
What is meant by “the relative timing of the postsynaptic neuron”? Timing of what, exactly? How can the synaptic currents (recorded from postsynaptic neuron) be correlated with the relative timing of the postsynaptic neuron? This sentence should be rephrased to clarify the idea being conveyed.
Results
Line 329: (Results shown in figure 5) “The IPSC slope parameters mpy and mfall were strongly correlated with P, but in opposite directions. DCPD also showed a weak (negative) correlation with P.”
Adding an addition sentence which paraphrases this one, and adding a brief interpretation of these results would improve the clarity of this section.
Line 339: (Results shown in Figure 6) “In comparison with Figure 5, Mpy and DCPD were correlated with both φ LP on and P, whereas mfall was only correlated with P, and deltapk and DCpy only with φ LP on.”
This requires an incredible amount of effort to comprehend. Adding an addition sentence which paraphrases this one, and adding a brief interpretation and summary of these results would improve the ease in understanding this section.
Line 350- 354: This section would benefit with more detailed explanation, sans the acronyms.
353: Why exactly is this surprising?
Line 358: What is a lower dimensional parameter space? Why is it an issue?
Figures
Figure 1
Legend
A.
What are LPG spikes?
"Intracellular recording from the LP neuron shows bursting activity (blue) and slow wave oscillations, as well as timing of IPSCs from the PY (green) and PD (pink) neurons.”
The bursting activity of the LP neuron looks to be happening in the yellow-shaded part (not blue), and the PD IPSCs look to be shaded in blue , not green (in fact, there is no green region in this figure, as far as I can see.)
Figure 4.
Is figure 4 missing its legend?
What does each dot represent?
Figure 8:
C1: The legend states that the pairs of waveforms that fall along horizontal lines and that were chosen for sensitivity analysis are indicated by red circles. There are no red circles in panel C1....is this referring to C3?
Also the legend for C1 refers to an inset above C1 that shows waveform shapes ...what inset exactly? Is this inset part of panel A or B? It is difficult to know what inset is being referred to here.
C2. “...Synaptic conductance waveforms marked by the stars in A1...”
There are no stars marked in A1. There are stars in C1....
Discussion
While the discussion is comprehensive it would be interesting to hear more on the authors’ interpretation / ideas about the PC3 synaptic waveform - i.e. how changing the waveform along this particular PC is able to alter the burst onset latency of the LP neuron. ..what makes the LP neuron most sensitive to this particular waveform? How exactly could this /these synaptic parameters work to maintain stable activity phase despite varying cycle periods and frequencies?
Also - what may be the effect of decreasing onset latency of the LP neuron (by injecting PC3 waveform via dynamic clamp) on the overall oscillation of the circuit? Does altering the onset latency of the LP neurons disrupt the overall cycle period or overall oscillation pattern?
Author Response
Dear Editors,
We would like to thank you and the reviewers for considering our manuscript suitable for publishing in eNeuro. We would also like to thank you all for raising useful criticism which will help improve our paper’s impact. We have addressed all the questions raised by the reviewers. Please find below a point by point response to the reviewer’s comments.
The detailed comments of the reviewers follow below.
Reviewer #1:
MAJOR POINTS
1) The results section ends with a description of which principal components describing the synaptic parameters’ collective variation have a significant effect on LP burst onset. This analysis is sound and leads to the conclusion that the less variable PCs have the strongest influence on LP burst onset while the most variable PCs have little effect on LP burst onset. Although going back to the individual synaptic parameters is not straightforward (by the nature of PCA and dimensionality reduction), this effort should be made by the authors. In other words, it would be nice to read conclusions about the specific synaptic parameters underlying the most critical PCs. Without it, the main results remain quite abstract and lack biological relevance. Since the synaptic parameters contributing the most to PC3 and PC5 seem to have the strongest influence on LP burst onset, it would be really helpful to show the relationships between individual synaptic parameters and these PCs (such as what is done in Figure 7B for PC1 and PC2) in order to then provide an interpretation of the dynamic-clamp experiments not only in terms of PCs but in terms of biologically relevant synaptic parameters. Some supplementary analysis or at least some rewriting would be necessary to provide the readers with a “biological” interpretation of which synaptic parameters appear to critically control LP firing.
We took the advice of the reviewer to heart and extended Figure 9 to explain the role of PC3 in more intuitive terms. We also added a paragraph to the discussion to address these points.
2) A major part of the study is based on measurements of the synaptic currents received by the LP neuron. I was somewhat surprised to see that the authors measured these synaptic currents by clamping the LP neuron at -50mV. This voltage is rather depolarized, such that unclamped action currents are visible on the recordings (Figure 3). Even though the authors are clamping the neuron for 30s to reach a steady state of activation/inactivation for voltage-gated conductances, the unclamped action currents suggest that the state of intrinsic conductances may still be variable in remote unclamped compartments. The authors need to justify this choice, as a more hyperpolarized potential is usually a safer choice to avoid any risk of contamination by voltage-gated conductances (IH can easily be dealt with).
Thank you for raising this question. Indeed it was a difficult choice for us because the main goal in this study was to measure the synaptic and intrinsic current during ongoing rhythm while minimally interfering with the rhythm properties. Though the concern of the reviewer is valid, we had to make the choice of holding potential to allow pyloric rhythm to continue with natural frequency. Additionally, a previous study (Martinez et al, 2019, J Neurophys) shows that the shape of the synaptic input is independent of the holding potential. We have included the following sentence in the manuscript to address the reason for our choice. Please see L154 in the methods section as “Relatively higher level of holding potential was used to allow the network maintain its natural oscillation frequency with minimal interference and to parameterize contributions of cell specific synaptic inputs more precisely”.
3) The authors report a half-activation voltage for IA much more depolarized than usually reported. In fact, IA activation voltage is more depolarized than for the IHTK current, which is a bit surprising. The work from Harris-Warrick et al. in particular demonstrated that IH and IA interact to define rebound properties in pyloric neurons, however this is only compatible with IA behaving as a “low-threshold” potassium current. The authors need to explain why IA properties are so different in their experimental conditions.
The reviewer is correct about this. However, there have been a variety of reports on ionic current activation in the LP neuron and these differ among different species. Our numbers are consistent with the original values in this neuron, reported by Golowasch and Marder, 1992 Figure 9. We now include this point in the manuscript.
4) Much of the analysis in the first part of the manuscript relies on correlations and linear regressions. Since the authors perform many correlations for a given set of variables (such as in Figure 4, 5 and 6 in particular), it is critical to correct for false discovery rate. The authors may have performed it and omitted to mention it in the methods section. Otherwise, some of the weak correlations reported in Figure 5 and 6 may not resist the correction for multiple correlations. Either way, this needs to be corrected.
The reviewer is absolutely correct. We have corrected this error. Please note, however, that this does not affect any of the conclusions of the study.
5) When analyzing the properties of voltage-gated currents, the authors only report conductance value for IH. I understand that measuring precisely IH properties during the ongoing rhythm is particularly difficult, but this is a real shame, because IH activation voltage in particular is one of the parameters that we might expect to act in synergy with synaptic inputs.
We agree that measuring properties of low-voltage threshold currents like IH precisely would have helped address the question in a much better way but unfortunately to measure the currents under natural oscillating conditions we could not measure IH by blocking high voltage activated currents.
Reviewer #2:
This is a detailed and sophisticated study on how the LP neuron of the Cancer borealis STG maintains a constant activity phase across preparations (different crabs) despite big variations in cycle period and synaptic and intrinsic currents. For this, the authors first carried out voltage clamp recordings from the LP neuron during normal spontaneous oscillating activity. It is established that while the latency for the LP neuron to fire increases with increasing cycle periods, the activity phase is relatively stable over varying cycle periods. Notably, the amount of variability in cycle period across preparations is found to be markedly greater than the degree of variation in the LP activity phase across the same preparations. To determine how the stable activity phase is achieved, synaptic inputs (IPSCs) to the LP and several different voltage-dependent intrinsic currents expressed by the LP neuron were quantified. Correlation analysis indicated several synaptic parameters to be correlated with LP burst onset, not just one. Thus the authors carry out a principle component analysis followed by ingenious use of dynamic clamp to test the different waveforms generated by the different PCs. The two synaptic parameters that were determined to be most effective in altering LP onset were also determined to be relatively stable across crabs, while the synaptic parameters that had little effect on burst onset were more variable. Overall, this study identifies specific synaptic parameters that are stable and appear to work to maintain stability of phase. The results of this work add significant and novel insight into how the activity phases of individual neurons that comprise an oscillatory circuit can be relatively stable across vastly different cycle periods and oscillation frequencies. The experiments are well-designed and logical. The figures are effective, especially the schematics in figure 1. Focusing on just one neuron type of the STG in detail is unique and effective approach. Injecting all the different synaptic waveforms into the LP using dynamic clamp is a great use of dynamic clamp.
The main weakness of this study is in the way that it is written. There are many sentences that are full of acronyms and no re-phrasing to help the reader understand. It is challenging. Some of the ideas being conveyed seem to be contradictory, but that is probably just because of poor phrasing. Below are suggestions on how to improve clarity.
Significance statement
1. The Significance Statement should include both the significance of the question being addressed and the significance of the findings. This significance statement appears to describe only the significance of the question being addressed but does not describe the findings of the study and their significance. It should probably be re-written to include this.
We have rewritten the significance statement.
2. Line 17 and Line 20: There are two seemingly contradictory ideas conveyed in this Significance Statement. The first sentence (shown below) conveys the idea that neural circuits produce similar outputs across individuals and the third sentence conveys the idea that circuit output shows variability across individuals. Perhaps this could be re-phrased to convey a more cohesive idea.
Line 17 “Neural circuits produce a similar output across individuals despite a large variability in parameters...”
Line 20: “.....circuit output shows considerable variability across individuals, and, additionally there are correlations among circuit parameters.”
The reviewer is absolutely correct. We have reworded this to say that certain output attributes remain similar whereas others show variability.
Introduction
3. line 61 “Synaptic currents also vary substantially across individuals and their magnitude is correlated with relative timing of the postsynaptic neuron.”
What is meant by “the relative timing of the postsynaptic neuron”? Timing of what, exactly? How can the synaptic currents (recorded from postsynaptic neuron) be correlated with the relative timing of the postsynaptic neuron? This sentence should be rephrased to clarify the idea being conveyed.
Thank you for pointing out the missing description. It is “the relative timing of the burst onset of the postsynaptic neuron” and we have fixed it in the manuscript.
Results
Line 329: (Results shown in figure 5) “The IPSC slope parameters mpy and mfall were strongly correlated with P, but in opposite directions. DCPD also showed a weak (negative) correlation with P.”
Adding an addition sentence which paraphrases this one, and adding a brief interpretation of these results would improve the clarity of this section.
Done.
Line 339: (Results shown in Figure 6) “In comparison with Figure 5, Mpy and DCPD were correlated with both φ LP on and P, whereas mfall was only correlated with P, and deltapk and DCpy only with φ LP on.”
This requires an incredible amount of effort to comprehend. Adding an addition sentence which paraphrases this one, and adding a brief interpretation and summary of these results would improve the ease in understanding this section.
We have added a sentence to clarify this.
Line 350- 354: This section would benefit with more detailed explanation, sans the acronyms.
353: Why exactly is this surprising?
We attempted to clarify what exactly is surprising in these correlations.
Line 358: What is a lower dimensional parameter space? Why is it an issue?
We have rewritten this for clarity.
Figures
Figure 1
Legend
A.
What are LPG spikes?
Now defined in the legend.
"Intracellular recording from the LP neuron shows bursting activity (blue) and slow wave oscillations, as well as timing of IPSCs from the PY (green) and PD (pink) neurons.”
The bursting activity of the LP neuron looks to be happening in the yellow-shaded part (not blue), and the PD IPSCs look to be shaded in blue, not green (in fact, there is no green region in this figure, as far as I can see.)
Thank you for pointing out mislabeled legend. We have made corrections now.
Figure 4.
Is figure 4 missing its legend?
What does each dot represent?
Fixed.
Figure 8:
C1: The legend states that the pairs of waveforms that fall along horizontal lines and that were chosen for sensitivity analysis are indicated by red circles. There are no red circles in panel C1....is this referring to C3?
Also the legend for C1 refers to an inset above C1 that shows waveform shapes ...what inset exactly? Is this inset part of panel A or B? It is difficult to know what inset is being referred to here.
C2. “...Synaptic conductance waveforms marked by the stars in A1...”
There are no stars marked in A1. There are stars in C1....
Thank you for catching inaccuracies in the description of the Figure 8 legend. All the concerns have been addressed.
Discussion
While the discussion is comprehensive it would be interesting to hear more on the authors’ interpretation / ideas about the PC3 synaptic waveform - i.e. how changing the waveform along this particular PC is able to alter the burst onset latency of the LP neuron. ..what makes the LP neuron most sensitive to this particular waveform? How exactly could this /these synaptic parameters work to maintain stable activity phase despite varying cycle periods and frequencies?
Please see response to Comment 1 of Reviewer 1.
Also - what may be the effect of decreasing onset latency of the LP neuron (by injecting PC3 waveform via dynamic clamp) on the overall oscillation of the circuit? Does altering the onset latency of the LP neurons disrupt the overall cycle period or overall oscillation pattern?
The cycle period of network oscillation is primarily driven by the pacemaker PD neurons and the synaptic feedback these neurons receive. It is therefore unlikely that the cycle would be disrupted by changing the shape of the input to a follower neuron. What the reviewer asks would be extremely difficult to address experimentally if cycle frequency is not controlled. If it is, however, the pattern of activity can be greatly influenced by changing PC3, as seen even in the one example of the LP neuron activity that we show in Figure 8D2. Although we have the dynamic clamp data, we have not done a comprehensive analysis of how the circuit parameters are affected by changing each PC. Considering how dense the data in the current study are, we think it would be unwise to add this analysis too.
References
- Abbott LF, Marder E, Hooper SL (1991) Oscillating networks: control of burst duration by electrically coupled neurons. Neural Comput 3:487–497. 10.1162/neco.1991.3.4.487 [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Traub RD, Kopell NJ, Whittington MA (2012) Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron 75:572–583. 10.1016/j.neuron.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP (1999) Coordination of fast and slow rhythmic neuronal circuits. J Neurosci 19:6650–6660. 10.1523/JNEUROSCI.19-15-06650.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaye SS, Bockemühl T, Büschges A (2018) Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol 119:459–475. 10.1152/jn.00658.2017 [DOI] [PubMed] [Google Scholar]
- Bose A, Manor Y, Nadim F (2004) The activity phase of postsynaptic neurons in a simplified rhythmic network. J Comput Neurosci 17:245–261. 10.1023/B:JCNS.0000037685.71759.1a [DOI] [PubMed] [Google Scholar]
- Brzosko Z, Mierau SB, Paulsen O (2019) Neuromodulation of spike-timing-dependent plasticity: past, present, and future. Neuron 103:563–581. 10.1016/j.neuron.2019.05.041 [DOI] [PubMed] [Google Scholar]
- Bucher D, Marder E (2013) SnapShot: neuromodulation. Cell 155:482–482.e1. 10.1016/j.cell.2013.09.047 [DOI] [PubMed] [Google Scholar]
- Bucher D, Prinz AA, Marder E (2005) Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci 25:1611–1619. 10.1523/JNEUROSCI.3679-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Haspel G, Golowasch J, Nadim F (2015) Central pattern generators. In: eLS. Chichester: Wiley, Ltd. [Google Scholar]
- Buchholtz F, Golowasch J, Epstein IR, Marder E (1992) Mathematical model of an identified stomatogastric ganglion neuron. J Neurophysiol 67:332–340. 10.1152/jn.1992.67.2.332 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Tingley D (2018) Space and time: the hippocampus as a sequence generator. Trends Cogn Sci 22:853–869. 10.1016/j.tics.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A, Wright TM (2011) Coping with variability in small neuronal networks. Integr Comp Biol 51:845–855. 10.1093/icb/icr074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A (2016) The neural control of heartbeat in invertebrates. Curr Opin Neurobiol 41:68–77. 10.1016/j.conb.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li X, Rotstein HG, Nadim F (2016) Membrane potential resonance frequency directly influences network frequency through electrical coupling. J Neurophysiol 116:1554–1563. 10.1152/jn.00361.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daur N, Nadim F, Bucher D (2016) The complexity of small circuits: the stomatogastric nervous system. Curr Opin Neurobiol 41:1–7. 10.1016/j.conb.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCaprio R, Jordan G, Hampton T (1997) Maintenance of motor pattern phase relationships in the ventilatory system of the crab. J Exp Biol 200:963–974. 10.1242/jeb.200.6.963 [DOI] [PubMed] [Google Scholar]
- Dragoi G (2020) Cell assemblies, sequences and temporal coding in the hippocampus. Curr Opin Neurobiol 64:111–118. 10.1016/j.conb.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices I, Levi R, Arroyo D, Rodriguez FB, Varona P (2019) Robust dynamical invariants in sequential neural activity. Sci Rep 9:9048. 10.1038/s41598-019-44953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franci A, O’Leary T, Golowasch J (2020) Positive dynamical networks in neuronal regulation: how tunable variability coexists with robustness. IEEE Control Syst Lett 4:946–951. 10.1109/LCSYS.2020.2997214 [DOI] [Google Scholar]
- Frigon A (2017) The neural control of interlimb coordination during mammalian locomotion. J Neurophysiol 117:2224–2241. 10.1152/jn.00978.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia VJ, Daur N, Temporal S, Schulz DJ, Bucher D (2015) Neuropeptide receptor transcript expression levels and magnitude of ionic current responses show cell type-specific differences in a small motor circuit. J Neurosci 35:6786–6800. 10.1523/JNEUROSCI.0171-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, Marder E (2009) Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci 12:1424–1430. 10.1038/nn.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Pulver SR, Marder E (2010) Slow and persistent postinhibitory rebound acts as an intrinsic short-term memory mechanism. J Neurosci 30:4687–4692. 10.1523/JNEUROSCI.2998-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Golowasch J, Marder E, Abbott LF (2001) Global structure, robustness, and modulation of neuronal models. J Neurosci 21:5229–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J (2014) Ionic current variability and functional stability in the nervous system. Bioscience 64:570–580. 10.1093/biosci/biu070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J (2019) Neuromodulation of central pattern generators and its role in the functional recovery of central pattern generator activity. J Neurophysiol 122:300–315. 10.1152/jn.00784.2018 [DOI] [PubMed] [Google Scholar]
- Golowasch J, Marder E (1992) Ionic currents of the lateral pyloric neuron of the stomatogastric ganglion of the crab. J Neurophysiol 67:318–331. 10.1152/jn.1992.67.2.318 [DOI] [PubMed] [Google Scholar]
- Golowasch J, Goldman MS, Abbott LF, Marder E (2002) Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol 87:1129–1131. 10.1152/jn.00412.2001 [DOI] [PubMed] [Google Scholar]
- Golowasch J, Bose A, Guan Y, Salloum D, Roeser A, Nadim F (2017) A balance of outward and linear inward ionic currents is required for generation of slow-wave oscillations. J Neurophysiol 118:1092–1104. 10.1152/jn.00240.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg I, Manor Y (2005) Synaptic depression in conjunction with A-current channels promote phase constancy in a rhythmic network. J Neurophysiol 93:656–677. 10.1152/jn.00640.2004 [DOI] [PubMed] [Google Scholar]
- Grillner S (2006) Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52:751–766. 10.1016/j.neuron.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Grillner S, El Manira A (2015) The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31:244–249. 10.1016/j.conb.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Grillner S, El Manira A (2020) Current principles of motor control, with special reference to vertebrate locomotion. Physiol Rev 100:271–320. 10.1152/physrev.00015.2019 [DOI] [PubMed] [Google Scholar]
- Günay C, Doloc-Mihu A, Lamb DG, Calabrese RL (2019) Synaptic strengths dominate phasing of motor circuit: intrinsic conductances of neuron types need not vary across animals. eNeuro 6:ENEURO.0417-18.2019. 10.1523/ENEURO.0417-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF (2004) Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 24:9127–9137. 10.1523/JNEUROSCI.2113-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood AW, Marder E (2014) Animal-to-animal variability in neuromodulation and circuit function. Cold Spring Harb Symp Quant Biol 79:21–28. 10.1101/sqb.2014.79.024828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM (2002) Voltage-sensitive ion channels in rhythmic motor systems. Curr Opin Neurobiol 12:646–651. 10.1016/s0959-4388(02)00377-x [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM (2011) Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol 21:685–692. 10.1016/j.conb.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J (1995a) Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol 74:1404–1420. 10.1152/jn.1995.74.4.1404 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S (1995b) Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci 15:342–358. 10.1523/JNEUROSCI.15-01-00342.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP (2002) A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14:793–817. 10.1162/089976602317318965 [DOI] [PubMed] [Google Scholar]
- Hofmann F, Flockerzi V, Kahl S, Wegener JW (2014) L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 94:303–326. 10.1152/physrev.00016.2013 [DOI] [PubMed] [Google Scholar]
- Hooper SL (1997) Phase maintenance in the pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion. J Comput Neurosci 4:191–205. 10.1023/a:1008822218061 [DOI] [PubMed] [Google Scholar]
- Hudson AE, Prinz AA (2010) Conductance ratios and cellular identity. PLoS Comput Biol 6:e1000838. 10.1371/journal.pcbi.1000838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailov II, Benos DJ (1995) Effects of phosphorylation on ion channel function. Kidney Int 48:1167–1179. 10.1038/ki.1995.400 [DOI] [PubMed] [Google Scholar]
- Jindal HK, Folco EJ, Liu GX, Koren G (2008) Posttranslational modification of voltage-dependent potassium channel Kv1.5: COOH-terminal palmitoylation modulates its biological properties. Am J Physiol Heart Circ Physiol 294:H2012–2021. 10.1152/ajpheart.01374.2007 [DOI] [PubMed] [Google Scholar]
- Johnson BR, Schneider LR, Nadim F, Harris-Warrick RM (2005) Dopamine modulation of phasing of activity in a rhythmic motor network: contribution of synaptic and intrinsic modulatory actions. J Neurophysiol 94:3101–3111. 10.1152/jn.00440.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Brown JM, Kvarta MD, Lu JY, Schneider LR, Nadim F, Harris-Warrick RM (2011) Differential modulation of synaptic strength and timing regulate synaptic efficacy in a motor network. J Neurophysiol 105:293–304. 10.1152/jn.00809.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS (2016) Evolution of central pattern generators and rhythmic behaviours. Philos Trans R Soc Lond B Biol Sci 371:20150057. 10.1098/rstb.2015.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorkova O, Golowasch J (2007) Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci 27:8709–8718. 10.1523/JNEUROSCI.1274-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O (2016) Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17:224–238. 10.1038/nrn.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM (1999) Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol 81:29–38. 10.1152/jn.1999.81.1.29 [DOI] [PubMed] [Google Scholar]
- Laedermann CJ, Abriel H, Decosterd I (2015) Post-translational modifications of voltage-gated sodium channels in chronic pain syndromes. Front Pharmacol 6:263. 10.3389/fphar.2015.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal JP, Dubuc R, Smarandache-Wellmann C (2017) Coordination of rhythmic movements. In: Neurobiology of motor control: fundamental concepts and new directions. (Hooper SL, Buschges A, eds). Hoboken: Wiley-Blackwell. [Google Scholar]
- Li X, Bucher D, Nadim F (2018) Distinct co-modulation rules of synapses and voltage-gated currents coordinate interactions of multiple neuromodulators. J Neurosci 38:8549–8562. 10.1523/JNEUROSCI.1117-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Golowasch J, Marder E, Abbott LF (1998) A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J Neurosci 18:2309–2320. 10.1523/JNEUROSCI.18-07-02309.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM (2003) Activity-independent homeostasis in rhythmically active neurons. Neuron 37:109–120. 10.1016/s0896-6273(02)01104-2 [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Goeritz ML, Casey R, Oliva R, Guckenheimer J, Harris-Warrick RM (2005) Activity-independent co-regulation of IA and Ih in rhythmically active neurons. J Neurophysiol 94:3601–3617. [DOI] [PubMed] [Google Scholar]
- Manor Y, Bose A, Booth V, Nadim F (2003) Contribution of synaptic depression to phase maintenance in a model rhythmic network. J Neurophysiol 90:3513–3528. 10.1152/jn.00411.2003 [DOI] [PubMed] [Google Scholar]
- Marder E (2011) Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci U S A 108:15542–15548. 10.1073/pnas.1010674108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E (2012) Neuromodulation of neuronal circuits: back to the future. Neuron 76:1–11. 10.1016/j.neuron.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11:R986–R996. 10.1016/s0960-9822(01)00581-4 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D (2007) Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316. 10.1146/annurev.physiol.69.031905.161516 [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM (2006) Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7:563–574. 10.1038/nrn1949 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL (2005) Invertebrate central pattern generation moves along. Curr Biol 15:R685–R699. 10.1016/j.cub.2005.08.022 [DOI] [PubMed] [Google Scholar]
- Marder E, Goeritz ML, Otopalik AG (2014a) Robust circuit rhythms in small circuits arise from variable circuit components and mechanisms. Curr Opin Neurobiol 31:156–163. 10.1016/j.conb.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, O’Leary T, Shruti S (2014b) Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci 37:329–346. 10.1146/annurev-neuro-071013-013958 [DOI] [PubMed] [Google Scholar]
- Martinez D, Santin JM, Schulz D, Nadim F (2019a) The differential contribution of pacemaker neurons to synaptic transmission in the pyloric network of the Jonah crab, Cancer borealis. J Neurophysiol 122:1623–1633. 10.1152/jn.00038.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Anwar H, Bose A, Bucher DM, Nadim F (2019b) Short-term synaptic dynamics control the activity phase of neurons in an oscillatory network. Elife 8:e46911. 10.7554/eLife.46911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus and Panulirus argus (Decapoda Crustacea). Philos Trans R Soc Lond B 268:161–220. [DOI] [PubMed] [Google Scholar]
- McDonnell MD, Graham BP (2017) Phase changes in neuronal postsynaptic spiking due to short term plasticity. PLoS Comput Biol 13:e1005634. 10.1371/journal.pcbi.1005634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Sillar KT (2011) Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 26:393–411. 10.1152/physiol.00013.2011 [DOI] [PubMed] [Google Scholar]
- Mouser C, Nadim F, Bose A (2008) Maintaining phase of the crustacean tri-phasic pyloric rhythm. J Math Biol 57:161–181. 10.1007/s00285-007-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Buchanan JT, Friesen WO (2011) Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems. Prog Neurobiol 93:244–269. 10.1016/j.pneurobio.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Manor Y (2000) The role of short-term synaptic dynamics in motor control. Curr Opin Neurobiol 10:683–690. 10.1016/s0959-4388(00)00159-8 [DOI] [PubMed] [Google Scholar]
- Nadim F, Bucher D (2014) Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29:48–56. 10.1016/j.conb.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris BJ, Weaver AL, Wenning A, García PS, Calabrese RL (2007) A central pattern generator producing alternative outputs: pattern, strength, and dynamics of premotor synaptic input to leech heart motor neurons. J Neurophysiol 98:2992–3005. 10.1152/jn.00877.2007 [DOI] [PubMed] [Google Scholar]
- Norris BJ, Wenning A, Wright TM, Calabrese RL (2011) Constancy and variability in the output of a central pattern generator. J Neurosci 31:4663–4674. 10.1523/JNEUROSCI.5072-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Caplan JS, Marder E (2013) Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci U S A 110:E2645–E2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Franci A, Marder E (2014) Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron 82:809–821. 10.1016/j.neuron.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onasch S, Gjorgjieva J (2020) Circuit stability to perturbations reveals hidden variability in the balance of intrinsic and synaptic conductances. J Neurosci 40:3186–3202. 10.1523/JNEUROSCI.0985-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hájos N (2006) Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci 26:9923–9934. 10.1523/JNEUROSCI.1580-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Thirumalai V, Marder E (2003) The functional consequences of changes in the strength and duration of synaptic inputs to oscillatory neurons. J Neurosci 23:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Abbott LF, Marder E (2004a) The dynamic clamp comes of age. Trends Neurosci 27:218–224. 10.1016/j.tins.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E (2004b) Similar network activity from disparate circuit parameters. Nat Neurosci 7:1345–1352. 10.1038/nn1352 [DOI] [PubMed] [Google Scholar]
- Rabbah P, Nadim F (2005) Synaptic dynamics do not determine proper phase of activity in a central pattern generator. J Neurosci 25:11269–11278. 10.1523/JNEUROSCI.3284-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransdell JL, Nair SS, Schulz DJ (2012) Rapid homeostatic plasticity of intrinsic excitability in a central pattern generator network stabilizes functional neural network output. J Neurosci 32:9649–9658. 10.1523/JNEUROSCI.1945-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransdell JL, Nair SS, Schulz DJ (2013) Neurons within the same network independently achieve conserved output by differentially balancing variable conductance magnitudes. J Neurosci 33:9950–9956. 10.1523/JNEUROSCI.1095-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman RC, Norris BJ, Calabrese RL (2012) Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol 107:1681–1693. 10.1152/jn.00903.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin JM, Schulz DJ (2019) Membrane voltage is a direct feedback signal that influences correlated ion channel expression in neurons. Curr Biol 29:1683–1688.e2. 10.1016/j.cub.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AC, Fox D, Itani O, Golowasch J, Bucher D, Nadim F (2021) Frequency-dependent action of neuromodulation. eNeuro 8:ENEURO.0338-21.2021. 10.1523/ENEURO.0338-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E (2006) Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 9:356–362. 10.1038/nn1639 [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder EE (2007) Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A 104:13187–13191. 10.1073/pnas.0705827104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562:9–26. 10.1113/jphysiol.2004.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi W, Goeritz ML, Kispersky TJ, Prinz AA, Marder E, Stein W (2014) Phase maintenance in a rhythmic motor pattern during temperature changes in vivo. J Neurophysiol 111:2603–2613. 10.1152/jn.00906.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP (2005) Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci 25:3509–3520. 10.1523/JNEUROSCI.3929-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LS, Taylor AL, Rinberg A, Marder E (2012) Robustness of a rhythmic circuit to short- and long-term temperature changes. J Neurosci 32:10075–10085. 10.1523/JNEUROSCI.1443-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temporal S, Desai M, Khorkova O, Varghese G, Dai A, Schulz DJ, Golowasch J (2012) Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J Neurophysiol 107:718–727. 10.1152/jn.00622.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T, Unal CT, Severin D, Zaborszky L, Rotstein HG, Kirkwood A, Golowasch J (2019) Ionic current correlations are ubiquitous across phyla. Sci Rep 9:1687. 10.1038/s41598-018-38405-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A, Topper S, Young L, Crisp A, Kressin L, Elbel E, Maples T, Brauner M, Erbguth K, Axelrod A, Gottschalk A, Siegel D, Pierce-Shimomura JT (2011) Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci U S A 108:17504–17509. 10.1073/pnas.1108673108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Huber A (2014) Post-translational modifications of TRP channels. Cells 3:258–287. 10.3390/cells3020258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ (2010) Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–1268. 10.1152/physrev.00035.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning A, Hill AA, Calabrese RL (2004) Heartbeat control in leeches. II. Fictive motor pattern. J Neurophysiol 91:397–409. 10.1152/jn.00528.2003 [DOI] [PubMed] [Google Scholar]
- Wenning A, Norris BJ, Günay C, Kueh D, Calabrese RL (2018) Output variability across animals and levels in a motor system. Elife 7:e31123. 10.7554/eLife.31123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Varela C, Remondes M (2015) Phase organization of network computations. Curr Opin Neurobiol 31:250–253. 10.1016/j.conb.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM Jr, Calabrese RL (2011a) Patterns of presynaptic activity and synaptic strength interact to produce motor output. J Neurosci 31:17555–17571. 10.1523/JNEUROSCI.4723-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM Jr, Calabrese RL (2011b) Contribution of motoneuron intrinsic properties to fictive motor pattern generation. J Neurophysiol 106:538–553. 10.1152/jn.00101.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Guy RD, Mulloney B, Zhang Q, Lewis TJ (2014) Neural mechanism of optimal limb coordination in crustacean swimming. Proc Natl Acad Sci U S A 111:13840–13845. 10.1073/pnas.1323208111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Golowasch J (2012) Ionic current correlations underlie the global tuning of large numbers of neuronal activity attributes. J Neurosci 32:13380–13388. 10.1523/JNEUROSCI.6500-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Golowasch J, Nadim F (2010) Pacemaker neuron and network oscillations depend on a neuromodulator-regulated linear current. Front Behav Neurosci 4:21. 10.3389/fnbeh.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F (2011) Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci 31:13991–14004. 10.1523/JNEUROSCI.3624-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations among parameters of the voltage-gated ionic currents. No fit line in a panel indicates lack of correlation. See Extended Data Figure 2-2 for all p and R values. Download Figure 2-1, EPS file (1.2MB, eps) .
All p and R values for extended data Figure 2-1. Download Figure 2-2, xls file (28.5KB, xls) .
Biplots of the principal components in all projection planes. Percentages on axis labels indicate the extent of variability in the direction of the PC. The directions of the five primary synaptic parameters are indicated by olive line segments. Download Figure 7-1, EPS file (835.1KB, eps) .
Description of the derivation of the PCs from the PCs in Figure 9, by assuming that PC3 is fixed in the direction of DC = DCPD + DCPY. Download Figure 9-1, EPS file (1.9MB, eps) .