Abstract
Nitrate (NO3−) supplementation has been reported to enhance intermittent exercise performance; however, its impact on oxygen (O2) cost during intermittent running exercise is unclear. The aim of this study was to assess if acute NO3− supplementation would elicit performance benefits in recreationally active individuals during the Yo–Yo intermittent recovery level 1 (Yo-Yo IR1) test, with its potential benefit on O2 consumption (VO2), in a double-blind, randomized, crossover study, 12 recreational males consumed NO3−-rich (NIT; ~12.8 mmol), and NO3−-depleted (PLA; 0.04 mmol) concentrated beetroot juice 3 h before completing the Yo-Yo IR1 test. VO2 was measured at 160, 280 and 440 m (sub-maximal) and when the test was terminated (peak). Performance in the Yo–Yo IR1 was greater with NIT (990 ± 442.25 m) compared to PLA (870 ± 357.4 m, p = 0.007). The VO2 was not significantly different at 160 m (1.92 ± 0.99 vs. 2.1 ± 0.88 L·min−1), 280 m (2.62 ± 0.94 vs. 2.83 ± 0.94 L·min−1), 440 m (3.26 ± 1.04 vs. 3.46 ± 0.98 L·min−1) and peak (4.71 ± 1.01 vs. 4.92 ± 1.17 L·min−1) between NIT and PLA trials (all p > 0.05). The present study has indicated that acute supplementation of NO3− enhanced intermittent running performance but had no effect on VO2 during the Yo–Yo IR1 test in recreational young adults.
Keywords: nitric oxide, ergogenic aid, sports nutrition, beetroot juice, exercise performance
1. Introduction
The ergogenic effect of dietary nitrate (NO3−) supplementation is attributed to its reduction of NO3− to nitrite (NO2−) and, subsequently, nitric oxide (NO) [1]. This ingestion of NO3−-rich sources is known to increase plasma NO2− and be beneficial for reducing the oxygen (O2) cost for a given workload [2,3,4,5], improving muscle contractile properties [6,7], and supporting fatigue resistance [8,9,10]. Interestingly, it has been shown that a vegetable source is more effective than NO3− salts [11], taken as a supplement (e.g., concentrated beetroot juice). Furthermore, existing evidence also supports the notion that the reduction of NO2− to NO is enhanced in intra-muscular hypoxic conditions such as that observed within the skeletal muscle during high-intensity activity [12,13].The potential benefits of NO3− supplementation were also shown in muscle contractile properties (e.g., evoked contractile force), and these effects seem in preferentially type II compared to type I muscle fibers [14]. This enhancement in muscle contractility after NO3− supplementation has been attributed to improved calcium handling and release [6,7] and improved skeletal muscle blood flow and vascular conductance during submaximal efforts [15]. As such, enhanced intermittent running performance in moderately- [16,17] and well-trained individuals [18] following NO3− supplementation might be associated with the potential type II fibers’ specific impact of NO3− [6,15] as type II fibers are predominantly recruited to satisfy the high muscle contraction demands during high-intensity intermittent exercise [19].
The Yo–Yo intermittent recovery level 1 test (Yo–Yo IR1) is well-established, ecologically valid and commonly used [20]. There are three previous studies that investigated the influence of NO3− on the Yo–Yo IR1 test. Wylie et al. [16] reported an improvement in the Yo–Yo IR1 by 4.2% amongst moderately-trained team sport players following very large dose of NO3− (29 mmol) over 36 h before the test. Subsequent studies observed similar enhancement (3.9% and 3.4%) in the Yo–Yo IR1 test after the ingestion of 6.4 mmol for 5 days in moderately-trained team sport players [17] and a 12.8 mmol/day for 6 days in highly trained soccer players, respectively [18]. Besides the heavy recruitment of type II fibers during a transition from low to high metabolic rate [21,22], intermittent running also leads to a high O2 demand relative to O2 delivery. Therefore, combined with the possible effects on type II fibers, the enhanced Yo–Yo IR1 performance in the previous studies might also link to the potential benefits of NO3− supplementation on exercise efficiency by reducing the O2 cost [8]. Oxygen consumption (VO2) at a given velocity is an endurance performance referred as running economy [23], and it is assessed because it reflects the energy cost of running [24]. Whilst most studies have reported an enhanced economy using acute and/or multiple days of NO3− supplementation during steady-state endurance exercises [25], it is presently unclear whether the enhanced economy effect of NO3− supplementation that was observed during steady-state endurance exercises might also occur during an intermittent exercise as the Yo–Yo IR1 test. As such, further research is required to assess the effect of NO3− supplementation on the response of VO2 during intermittent running in humans.
The previous studies above which reported enhanced intermittent running performance applied short-term supplementation periods [16,17,18], but previous meta-analyses reported that there is no difference between acute (e.g., 2–3 h pre-exercise) and chronic dosing regimens (e.g., 1–15 days) of NO3− on endurance exercise [26,27]. In addition, more recent meta-analyses have revealed that the benefits of NO3− supplementation in power output generally are apparent following acute supplementation [14,28]. Despite consistent effects of NO3− supplementation on the Yo–Yo IR1 test after multiple-day supplementation, to date, its effect after acute supplementation is yet to be determined. This is important to evaluate, as it would be a more practical and applicable nutritional intervention approach for performance in team sports.
Therefore, the aim of this study was to assess if acute supplementation of NO3− would elicit performance benefits in recreationally active individuals during the Yo–Yo IR1 test, with its potential benefit on VO2 consumption. It was hypothesized that NO3− supplementation would enhance performance and reduce VO2 cost during the Yo–Yo IR1 test.
2. Materials and Methods
2.1. Participants
The sample size of this study was based on a priori calculation using G*Power software (version 3.1.9.4, Universität, Düsseldorf, Germany). In determining the minimal estimated sample, we have considered two key outcomes, namely total distance achieved and the difference in overall change of VO2. Considering total distance, a standardized mean difference of 1.03 was used based on the work of Nyakayiru et al. [18]. Considering the difference in change of VO2, a standardized mean difference of 0.93 was used based on the work of Bailey et al. [8]. In both instances, a t-test family was used with matched pairs, a power of 0.80, a two-tailed approach, and α was set at 0.05. The results from these estimations indicated that sample of 10–12 participants would be sufficient to detect a difference between NO3− (NIT) and placebo (PLA) supplementation. Twelve recreationally active males (mean ± SD: age 27 ± 10 years, body mass 78.1 ± 11.8 kg, stature 180.5 ± 5.3 cm) were recruited for this study. All participants were involved in regular moderate-intensity exercise ~3 days per week and muscle-strengthening activities ~2 days per week. The participants were non-smokers, healthy, and did not use dietary supplements at the time of data collection. All participants were university students in the Sport Science department and were familiar with the Yo–Yo IR1 test. Ethics approval for this study was given by the Manchester Metropolitan University Research Ethics Committee (Reference no: 33132). All participants were informed of the nature and possible risks of the experimental procedures before providing written informed consent.
2.2. Experimental Design
The participants visited the testing facility on two separate occasions. Participants were assigned in a randomized, double-blind, placebo-controlled, crossover design to consume either a NO3−-rich (NIT) or a NO3−-depleted concentrated beetroot juice (PLA). The experimental trials were all carried out at the similar time of the day (±2 h). Mean and standard deviation of ambient temperature, humidity and pressure during the two trials were 17 ± 2.1 °C, 56.0 ± 4.3% and 1018 ± 2 mbar, respectively. A four- to six-day washout period separated the supplementation periods, as suggested by Wylie et al. [9]. Each participant was asked to record their dietary intake in the 24 h before the first experimental trial and replicate this in the 24 h before the subsequent trial. Participants were instructed to avoid strenuous exercise and the consumption of alcohol and caffeine for at least 24 h before each experimental trial. Participants avoided using antibacterial mouthwash throughout the duration of the study due to its prevention of the reduction of NO3− to NO2− in the oral cavity [29].
2.3. Supplementation Protocol
The participants received 140 mL of concentrated beetroot juice (~12.8 mmol of NO3−; Beet it, James White Drinks Ltd., Ipswich, UK) or NO3−-depleted beetroot juice (~0.04 mmol of NO3−) as PLA. This chosen dose was based on current recommendation for the optimal ergogenic effect of NO3− supplementation (5–13 mmol of NO3−) [1,9,27]. Participants consumed 2 × 70 mL the supplement 3 h before the test to coincide with peak plasma [NO2−] [9].
2.4. Procedures
Upon arrival at the testing facility (an indoor fourth-generation artificial grass pitch), participants completed a standardized warm-up (10 min), ending with the first two shuttles of the Yo–Yo IR1 test in order to familiarize themselves with the audio and initial speeds. After 10 min of passive recovery, a resting capillary blood lactate (BLa) sample was taken from the pad of the index finger of the left hand using the Lactate Pro-2 (Lactate Pro analyser, Arkay, Kyoto, Japan). Immediately after the Yo-Yo IR1, a second capillary BLa sample was taken.
Participants had an online gas analyzer fitter using a custom-made harness with the device positioned on their back. Pulmonary gas exchange was measured continuously using a Cosmed K5 (Cosmed, K5, Cosmed, Rome, Italy) with the system set for breath-by-breath analysis. The calibration of the K5 gas analyzer was performed before each test, according to the manufacturer’s instructions including a gas, volume, carbon dioxide (CO2) and breathing frequency. Breath-by-breath VO2, CO2 production (VCO2) and minute ventilation (VE) data from each test were linearly interpolated to provide second-by-second values. Subsequently, mean VO2, VCO2 and VE were assessed during each run and recovery period and averaged to provide the overall mean VO2, VCO2 and VE during the run and recovery periods for each stage of the Yo–Yo IR1 test. The mean sub-maximal values of 160, 280 and 440 m were based on those previously used [30]. Peak values for each variable were considered as the highest value achieved during the test. Previous literature has reported that the COSMED K5 had excellent reliability for VO2 (CV: 4.4%, CI: 3.2–6.7%, concordance correlation coefficient [CCC]: 0.95), VCO2 (CV: 6.2%, CI: 4.5–9.7%, CCC: 0.92) and VE (CV: 6.9%, CI: 4.9–10.7%, CCC: 0.89) during more than 2 h of continuous field tests [31].
The Yo–Yo IR1 test has been described elsewhere [20]. Briefly, the test consists of repeated 2 × 20 m runs, interspersed by a 10 s active recovery period, at progressively increasing speeds controlled by audio bleeps from a portable audio system. First four shuttles were at the speed of 10–13 km·h−1 (0–160 m), then three shuttles at 13.5 km·h−1 (200–280 m) and four shuttles at 14.0 km·h−1 (320–440 m); thereafter, the speed increased 0.5 km·h−1 every eight shuttles (i.e., 760, 1080, 1400 m, etc.). The final distance successfully covered was recorded after the second failed attempt to meet the start/finish line in the allocated time.
2.5. Statistical Analysis
All data were presented as means ± SD. Differences between NIT and PLA in distance covered during the Yo–Yo IR1 test, VO2peak, VCO2peak and VEpeak, RERpeak and BLa pre- and post-exercise test were analyzed using a paired samples t-test. Effect sizes (d) were calculated through Cohen’s d as: large d > 0.8, moderate d = 0.8 to 0.5, small d = 0.5 to 0.2, and trivial d < 0.2 [32]. Differences in VO2, VCO2, VE and RER at 160, 280 and 440 Im were determined using two-way repeated-measure ANOVA (supplement × distance). In addition, effect size was calculated as partial eta-squared (ŋp2) varying small (<0.25), medium (0.26–0.63) and large (>0.63) [33]. All data were analyzed using SPSS 27.0 (IBM Corp., Armonk, NY, USA). Significance was determined at p < 0.05.
3. Results
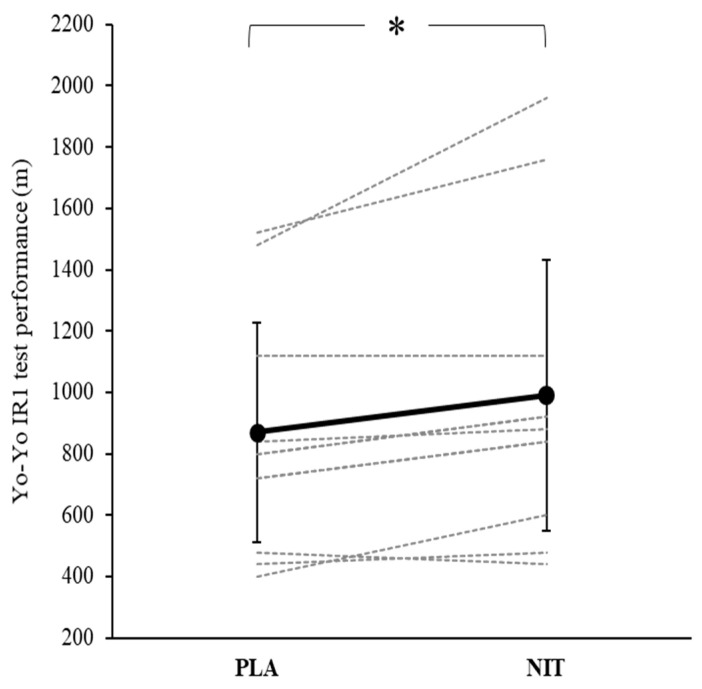
The distance covered in the Yo–Yo IR1 test was significantly greater in NIT (990 ± 442.25 m) compared to PLA (870 ± 357.4 m, p = 0.007, d = 0.30, Figure 1).
Figure 1.
The distance covered in the Yo–Yo IR1 test was 14% greater with NIT compared to PLA. The dashed lines indicate the responses of individual participants. The solid line indicates the group mean (±SD). * p < 0.05.
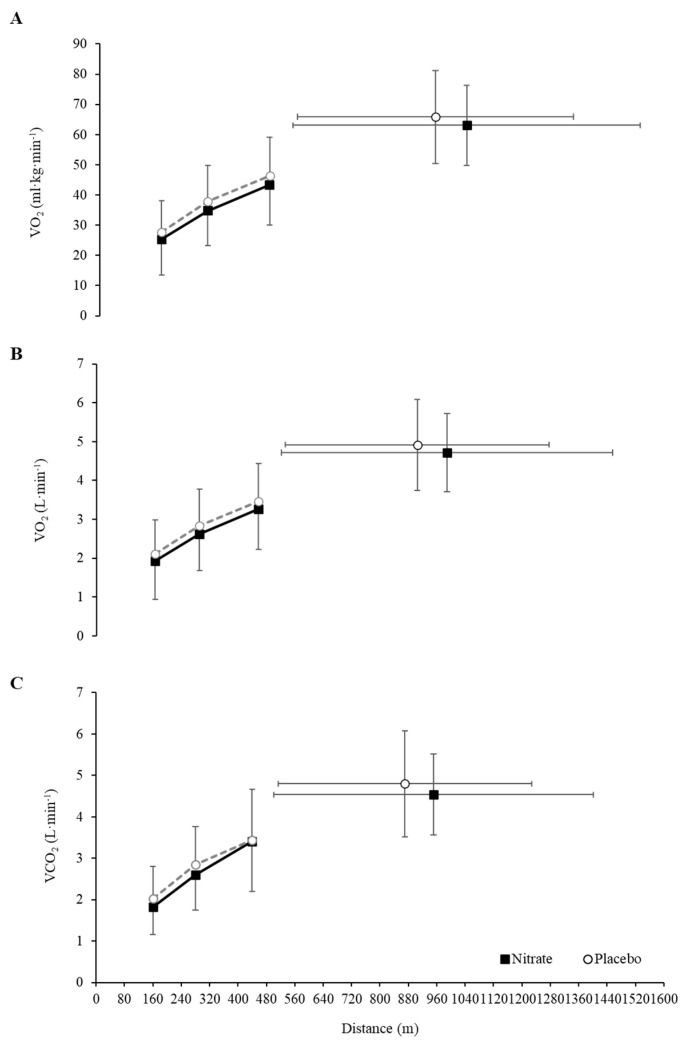
The group mean relative and absolute VO2 and absolute VCO2 responses at 160 m, 280 m, 440 m and peak during the Yo–Yo IR1 following both NIT and PLA supplementation were shown in Figure 2, and values (including VE and RER values) were reported in Table 1. The mean relative VO2 responses at submaximal distances were similar for NIT and PLA (ANOVA: supplementation, F = 2.27; p = 0.160; ŋp2 = 0.171; supplementation × distance, F = 2.32, p = 0.131, ŋp2 = 0.169), while these increased with distance (ANOVA: distance, F = 63.56; p < 0.001; ŋp2 = 0.85). Similarly, the mean absolute VO2 responses at submaximal distances did not differ between NIT and PLA (ANOVA: supplementation, F = 1.14; p = 0.308; ŋp2 = 0.094; supplementation × distance, F = 0.35, p = 0.966, ŋp2 = 0.003), while these increased with distance (ANOVA: distance, F = 60.51; p < 0.001; ŋp2 = 0.85). The mean VCO2 response were also very similar at submaximal distances between NIT and PLA (ANOVA: supplementation, F = 1.85; p = 0.201; ŋp2 = 0.144; supplementation × distance, F = 1.84; p = 0.183; ŋp2 = 0.143), while these increased with distance (ANOVA: distance, F = 40.26; p < 0.001; ŋp2 = 0.785). There were also no significant differences between trials for the mean VE response (ANOVA: supplementation, F = 2.94; p = 0.114; ŋp2 = 0.211: supplementation × distance, F = 2.12; p = 0.144; ŋp2 = 0.161), while VE responses increased with distance (ANOVA: distance, F = 43.05; p < 0.001; ŋp2 = 0.796). There were also no significant differences between conditions for RER (ANOVA: supplement, F = 0.46; p = 0.513; ŋp2 = 0.040; distance, F = 0.34; p = 0.713; ŋp2 = 0.030; supplementation × distance, F = 0.02; p = 0.946; ŋp2 = 0.002).
Figure 2.
Relative (A) and absolute (B) pulmonary oxygen consumption (VO2), and absolute carbon dioxide production (VCO2) (C) responses during the Yo–Yo intermittent recovery level 1 test following dietary nitrate and placebo supplementation. Data are mean ± SD.
Table 1.
Submaximal and peak pulmonary gas responses during the Yo–Yo IR1 test, and pre- and post-exercise BLa.
| NIT | PLA | |
|---|---|---|
| VO2 (mL·kg·min−1) | ||
| 160 m | 24.43 ± 11.92 | 27.75 ± 10.43 |
| 280 m | 34.78 ± 11.43 | 37.80 ± 12.00 |
| 440 m | 43.37 ± 13.26 | 46.34 ± 12.81 |
| Peak | 63.07 ± 13.21 | 65.87 ± 15.40 |
| VO2 (L·min−1) | ||
| 160 m | 1.92 ± 0.99 | 2.10 ± 0.88 |
| 280 m | 2.62 ± 0.94 | 2.83 ± 0.94 |
| 440 m | 3.26 ± 1.04 | 3.46 ± 0.98 |
| Peak | 4.71 ± 1.01 | 4.92 ± 1.17 |
| VCO2 (L·min−1) | ||
| 160 m | 1.82 ± 0.66 | 2.03 ± 0.78 |
| 280 m | 2.61 ± 0.85 | 2.85 ± 0.92 |
| 440 m | 3.42 ± 1.21 | 3.44 ± 1.23 |
| Peak | 4.54 ± 0.98 | 4.80 ± 1.28 |
| VE (L·min−1) | ||
| 160 m | 46.65 ± 22.17 | 55.21 ± 26.04 |
| 280 m | 71.11 ± 25.91 | 79.93 ± 28.42 |
| 440 m | 87.69 ± 32.48 | 89.39 ± 34.68 |
| Peak | 138.01 ± 25.59 | 143.27 ± 31.10 |
| RER (VCO2/VO2) | ||
| 160 m | 1.03 ± 0.19 | 1.01 ± 0.15 |
| 280 m | 1.04 ± 0.13 | 1.02 ± 0.07 |
| 440 m | 1.01 ± 0.15 | 0.98 ± 0.12 |
| Peak | 1.06 ± 0.23 | 0.98 ± 0.10 |
| Pre-BLa (mmol·L−1) | 2.1 ± 0.6 | 1.9 ± 0.5 |
| Post-BLa (mmol·L−1) | 12.8 ± 1.6 | 13.7 ± 2.1 |
There were no significant differences in the VO2peak p = 0.114, d = 0.19), VCO2peak (p = 0.085, d = 0.22), VEpeak (p = 0.295, d = 0.18), and RERpeak (p = 0.079, d = 0.80) between conditions. There were no significant differences in pre-BLa (p = 0.45, d = 0.36) and post-BLa (p = 0.24, d = 0.48) between NIT and PLA.
4. Discussion
This study set out to determine whether the acute supplementation of NO3− would enhance the metabolic responses to intermittent running performance as measured via the Yo–Yo IR1 test among recreationally active adults. The original finding of the present study was that the acute supplementation of NO3− via beetroot juice significantly enhanced intermittent running performance in the Yo–Yo IR1 test (by 14%, p = 0.007). However, the metabolic responses (e.g., VO2, VCO2 and VE) did not alter at submaximal distances (at 160, 280 and 440 m) or peak during the Yo–Yo IR1 test after acute NO3− supplementation compared to placebo. These findings indicate that acute NO3− supplementation can enhance performance without altering exercise efficiency during intermittent running.
The present study shows that distance covered in the Yo–Yo IR1 test increased by 14% following the acute supplementation of NO3−. This finding is line with three previous studies that reported an enhanced performance in the Yo–Yo IR1 test by 4.2%, 3.9% and 3.4% after a large NO3− dose (29 mmol) over 36 h before testing [16], a moderate NO3− dose (6.4 mmol) for 5 days [17] and a high NO3− dose (12.8 mmol) for 6 days [18], respectively. Our finding extends the findings of those previous studies and suggests that a similar performance benefit can be gained by an acute high dose of NO3− supplementation (~12.8 mmol of NO3− 3 h pre-exercise). It is interesting to see some of the best participants in the present study showed some of the greatest improvements. Whilst these individual greatest improvements might be an explanation of the much greater improvement in the Yo–Yo IR1 test in the present study compared to previous observations, this also supports the existence of potential responders and non-responders to NO3− supplementation [1,34]. Marked differences exist between individuals in the erogenicity of NO3− supplementation. Assuming the potential type II preference of NO3− is on those factors, it might be speculated that because some of the participants in the present study had a high proportion of type II muscle fibers, this might have theoretically increased the ergogenic potential of NO3− [34]. The greater improvement in the present study compared to the previous observations would be also due to a different population being used (recreational individuals vs. moderately/highly trained team-sport players) given that existing evidence has shown that potential ergogenic effect of NO3− supplementation is more evident in individuals who have low levels of aerobic fitness [1,10]. Highly endurance-trained athletes could be less responsive to NO3− supplementation mediated by a lower fraction of type II muscle fibers or other factors such as greater NO synthase activity, mitochondrial efficiency or better muscle oxygenation compared to moderately trained subjects [35]. Therefore, further research is required to determine whether acute (2–3 h pre-exercise) high-dose supplementation of NO3− (>6 mmol) can benefit in highly trained team sports athletes. Given the considerable differences in technical requirements when executing specific skills, the metabolic cost of the activity and the participants’ characteristics, extrapolating these findings to every team sport activity and/or players is fraught. Future studies are therefore warranted to explore whether the findings of the present study can be reproduced in different team sports activities and/or athletes.
To best of our knowledge, this is the first study that assessed pulmonary VO2, VCO2 and VE responses following NO3− supplementation during the Yo–Yo IR1 test. This study shows that NO3− supplementation had no effect onVO2 response during the Yo–Yo IR1 test. These findings are consistent with observations that have reported an enhancement in intermittent exercise performance, consisting of repeated sprints, but no alteration in VO2 [16,36]. However, more recent studies reported inconsistent findings regarding the impact of NO3− supplementation to improve performance or/and metabolic responses during different high-intensity intermittent exercise [37,38]. Whilst Kent at al. [37] found that supplementation of NO3− did not enhance repeated-sprints performance in hypoxia, but may reduce VO2, Sousa et al. [38] showed no impact of NO3− supplementation either on VO2 or repeated-sprint training. Besides inter-study differences (e.g., participant training status, supplementation regimen and environmental conditions), previous studies applied considerably different exercise modalities and exercise protocols regarding work-to-rest ratio (e.g., intensities, durations, and numbers of work and/or rest) [17,36,37,38,39]. In earlier studies, reduced VO2 following NO3− supplementation during submaximal exercise [2,8,9,25] was attributed to a reduced adenosine-tri-phosphate (ATP) cost of muscle force production. The lack of effect in VO2 in this study and in previous studies conducted intermittent-exercise protocols might be, at least partly, due to the regular fluctuations in exercise intensity (e.g., non-steady-state conditions). Therefore, the findings of the present study suggest that the observed ergogenic effect of NO3− in intermittent exercise may work through divergent mechanisms of action independent of alterations in the efficiency of oxidative metabolism.
Some other mechanistic underpinnings, such as improved muscle contractility, for the effect of NO3− supplementation have been also identified [1]. Animal-based studies reported that the effect of NO3− supplementation is more apparent in type II compared with type I fibers regarding physiological response and performance, such as improving force output selectively in type II fibers via increasing sarcoplasmic reticulum calcium handling and/or release [6,7]. Despite remaining to be elucidated in human, this mechanism may better explain the ergogenic basis for the enhanced performance in the Yo–Yo IR1 test where a greater recruitment of type II fibers is expected [19]. Improved performance in the Yo–Yo IR1 might be related to delaying fatigue as a result of preserving the reduction in muscle excitability [40,41]. Wylie et al. [9] have previously attributed enhanced performance in the Yo–Yo IR1 test to attenuated muscle excitability due to a net loss of potassium. Further, it has been reported that NO3− supplementation attenuated the increase in motor unit action potential (MUP) duration [42]. Shorter MUP duration may result in a faster muscle fiber conduction velocity and greater sarcoplasmic reticulum calcium release and thus maintained force production in the face of fatigue development [43,44]. Taken together, these potential impacts of NO3− supplementation in neuromuscular function and motor unit activity in combination of the potential type II preference of NO3− might account, at least partly, for the enhanced intermittent running performance in the present study. However, it has yet to be fully understood what specific mechanism may underline ergogenic potential of NO3− supplementation, and therefore, it is important to understand that these proposed mechanisms would work independently or in combination to contribute to enhance intermittent exercise performance following dietary NO3− supplementation.
The ergogenic effect of NO3− supplementation has been attributed to its capacity to elevate plasma NO2− level with the subsequent reduction of circulating NO2− to NO [1]. Although plasma NO2− was not assessed after ~12.8 mmol of NO3− supplementation in the present study, doses of 6.4–16.8 mmol of NO3− have been consistently shown to enhance plasma NO2− by a magnitude that would be expected to enhance exercise performance [1,28]. Indeed, acute supplementation with similar and/or lower doses of NO3− has previously shown to enhance performance [9,45] and/or reduce VO2 [9,10,46,47]. The present study aimed consciously not to interfere in dietary intake behavior to accomplish lifestyle adequate exercise conditions, particularly as the dose of supplemented NO3− was much higher than its intake in regular diet. Although we did not standardize the participants’ diets, they were instructed to recorded and reproduce their dietary intake first and subsequent trials in order to exclude a possible effect of dietary intake on performance on trial days. Considering BLa as an indicator of the anaerobic metabolism [48], the results of this study suggest the absence of effect of NO3− supplementation in the anaerobic glycolytic. However, this study has reported a trend to statistical differences in RERpeak with a large effect size (p = 0.079, d = 0.80) with a higher contribution of carbohydrate to metabolism at the end of the Yo–Yo IR1 test. Based on the analysis of the effect sizes of an increased RERpeak within changes in BLa suggest a possible increase in the oxidative (and not anaerobic) metabolism of glucose at high intensity. This could be explained by hemodynamic and metabolic functions of NO as increased blow flow to muscles or an enhanced glucose uptake in the active muscles [49]. In fact, a higher utilization of oxidation of glucose during high intensity efforts could partially explain the enhancement in the time-to-exhaustion during high-intensity exercises [39,50]. Future studies should analyze the effect of the metabolic contributions of NO3− supplementation during high-intensity efforts.
5. Conclusions
The present study has shown, for the first time, that acute high-dose supplementation of NO3− enhanced the Yo–Yo IR1 test performance in recreational young adults. However, NO3− supplementation had no effect on VO2 responses during the intermittent running, suggesting that the observed performance enhancement is likely related to other potential physiological influence of NO3− (e.g., its type II fiber-specific effect and potential impact on neuromuscular functions), but not to its potential effect on O2 cost. These findings suggest that acute supplementation with NO3−-rich beetroot juice may be a nutritional ergogenic aid during intermittent running in recreational adults, and that further mechanistic research is required.
Author Contributions
Conceptualization, O.E. and R.K.; methodology, O.E. and R.K.; formal analysis, O.E., R.K. and R.D.; investigation, O.E.; data curation, O.E. and R.K.; writing—original draft preparation, O.E. and R.K.; writing—review and editing, O.E. and R.D.; visualization, O.E., R.K.; supervision, R.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Manchester Metropolitan University Research Ethics Committee (protocol code 33132 and date of approval 1 October 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions privacy.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones A.M., Thompson C., Wylie L.J., Vanhatalo A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018;38:303–328. doi: 10.1146/annurev-nutr-082117-051622. [DOI] [PubMed] [Google Scholar]
- 2.Bailey S.J., Fulford J., Vanhatalo A., Winyard P.G., Blackwell J.R., DiMenna F.J., Wilkerson D.P., Benjamin N., Jones A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 3.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Vanhatalo A., Bailey S.J., Blackwell J.R., DiMenna F.J., Pavey T.G., Wilkerson D.P., Benjamin N., Winyard P.G., Jones A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 5.Esen O., Nicholas C., Morris M., Bailey S.J. No effect of beetroot juice supplementation on 100-m and 200-m swimming performance in moderately trained swimmers. Int. J. Sports Physiol. Perform. 2019;14:706–710. doi: 10.1123/ijspp.2018-0654. [DOI] [PubMed] [Google Scholar]
- 6.Hernández A., Schiffer T.A., Ivarsson N., Cheng A.J., Bruton J.D., Lundberg J.O., Weitzberg E., Westerblad H. Dietary nitrate increases tetanic [Ca2+] i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey S.J., Gandra P.G., Jones A.M., Hogan M.C., Nogueira L. Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological. J. Physiol. 2019;597:5429–5443. doi: 10.1113/JP278494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., DiMenna F.J., Wilkerson D.P., Tarr J., Benjamin N., Jones A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 9.Wylie L.J., Kelly J., Bailey S.J., Blackwell J.R., Skiba P.F., Winyard P.G., Jeukendrup A.E., Vanhatalo A., Jones A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 10.Porcelli S., Ramaglia M., Bellistri G., Pavei G., Pugliese L., Montorsi M., Rasica L., Marzorati M. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med. Sci. Sports Exerc. 2015;47:1643–1651. doi: 10.1249/MSS.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 11.Jonvik K.L., Nyakayiru J., Pinckaers P.J., Senden J.M., van Loon L.J., Verdijk L.B. Nitrate-Rich Vegetables Increase Plasma Nitrate and Nitrite Concentrations and Lower Blood Pressure in Healthy Adults. J. Nutr. 2016;146:986–993. doi: 10.3945/jn.116.229807. [DOI] [PubMed] [Google Scholar]
- 12.Modin A., Björne H., Herulf M., Alving K., Weitzberg E., Lundberg J.O.N. Nitrite-derived nitric oxide: A possible mediator of ‘acidic–metabolic’vasodilation. Acta. Physiol. Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 13.Richardson R.S., Noyszewski E.A., Kendrick K.F., Leigh J.S., Wagner P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Investig. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coggan A.R., Baranauskas M.N., Hinrichs R.J., Liu Z., Carter S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021;18:66. doi: 10.1186/s12970-021-00463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson S.K., Hirai D.M., Copp S.W., Holdsworth C.T., Allen J.D., Jones A.M., Musch T.I., Poole D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wylie L.J., Mohr M., Krustrup P., Jackman S.R., Ermιdis G., Kelly J., Black M.I., Bailey S.J., Vanhatalo A., Jones A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013;113:1673–1684. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 17.Thompson C., Vanhatalo A., Jell H., Fulford J., Carter J., Nyman L., Bailey S.J., Jones A.M. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide. 2016;61:55–61. doi: 10.1016/j.niox.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Nyakayiru J., Jonvik K.L., Trommelen J., Pinckaers P.J., Senden J.M., Van Loon L.J., Verdijk L.B. Beetroot juice supplementation improves high-intensity intermittent type exercise performance in trained soccer players. Nutrients. 2017;9:314. doi: 10.3390/nu9030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krustrup P., Mohr M., Steensberg A., Bencke J., Kjær M., Bangsbo J. Muscle and blood metabolites during a soccer game: Implications for sprint performance. Med. Sci. Sports Exerc. 2006;38:1165–1174. doi: 10.1249/01.mss.0000222845.89262.cd. [DOI] [PubMed] [Google Scholar]
- 20.Krustrup P., Mohr M., Amstrup T., Rysgaard T., Johansen J., Steensberg A., Pedersen P.K., Bangsbo J. The yo-yo intermittent recovery test: Physiological response, reliability, and validity. Med. Sci. Sports Exerc. 2003;35:697–705. doi: 10.1249/01.MSS.0000058441.94520.32. [DOI] [PubMed] [Google Scholar]
- 21.Krustrup P., Söderlund K., Mohr M., Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflügers Arch. 2004;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- 22.Krustrup P., Söderlund K., Relu M.U., Ferguson R.A., Bangsbo J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: Effect of thigh occlusion. Scand. J. Med. Sci. Sports. 2009;19:576–584. doi: 10.1111/j.1600-0838.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 23.Saunders P.U., Pyne D.B., Telford R.D., Hawley J.A. Factors affecting running economy in trained distance runners. Sports Med. 2004;34:465–485. doi: 10.2165/00007256-200434070-00005. [DOI] [PubMed] [Google Scholar]
- 24.Barnes K.R., Kilding A.E. Strategies to improve running economy. Sports Med. 2015;45:37–56. doi: 10.1007/s40279-014-0246-y. [DOI] [PubMed] [Google Scholar]
- 25.Pawlak-Chaouch M., Boissiere J., Gamelin F.X., Cuvelier G., Berthoin S., Aucouturier J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide. 2016;53:65–76. doi: 10.1016/j.niox.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 26.McMahon N.F., Leveritt M.D., Pavey T.G. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: A systematic review and meta-analysis. Sports Med. 2017;47:735–756. doi: 10.1007/s40279-016-0617-7. [DOI] [PubMed] [Google Scholar]
- 27.Senefeld J.W., Wiggins C.C., Regimbal R.J., Dominelli P.B., Baker S.E., Joyner M.J. Ergogenic effect of nitrate supplementation: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2020;52:2250. doi: 10.1249/MSS.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esen O., Dobbin N., Callaghan M. The effect of dietary nitrate on the contractile properties of human skeletal muscle: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2022. in press . [DOI] [PubMed]
- 29.Govoni M., Jansson E.Å., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Dobbin N., Moss S.L., Highton J., Twist C. An examination of a modified Yo-Yo test to measure intermittent running performance in rugby players. Eur. J. Sport. Sci. 2018;18:1068–1076. doi: 10.1080/17461391.2018.1475509. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Suarez I., Martin-Rincon M., Gonzalez-Henriquez J.J., Fezzardi C., Perez-Regalado S., Galvan-Alvarez V., Juan-Habib J.W., Morales-Alamo D., Calbet J.A. Accuracy and precision of the COSMED K5 portable analyser. Front. Physiol. 2018:1764. doi: 10.3389/fphys.2018.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 33.Ferguson C.J. An effect size primer: A guide for clinicians and researchers. In: Kazdin A.E., editor. Methodological Issues and Strategies in Clinical Research. American Psychological Association; Worcester, MA, USA: 2016. pp. 301–310. [DOI] [Google Scholar]
- 34.Jones A.M., Vanhatalo A., Seals D.R., Rossman M.J., Piknova B., Jonvik K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021;53:280–294. doi: 10.1249/MSS.0000000000002470. [DOI] [PubMed] [Google Scholar]
- 35.Wilkerson D.P., Hayward G.M., Bailey S.J., Vanhatalo A., Blackwell J.R., Jones A.M. Influence of acute dietary nitrate supplementation on 50 miles time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012;112:4127–4134. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 36.Wylie L.J., Bailey S.J., Kelly J., Blackwell J.R., Vanhatalo A., Jones A.M. Influence of beetroot juice supplementation on intermittent exercise performance. Eur. J. Appl. Physiol. 2016;116:415–425. doi: 10.1007/s00421-015-3296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kent G.L., Dawson B., McNaughton L.R., Cox G.R., Burke L.M., Peeling P. The effect of beetroot juice supplementation on repeat-sprint performance in hypoxia. J. Sports Sci. 2019;37:339–346. doi: 10.1080/02640414.2018.1504369. [DOI] [PubMed] [Google Scholar]
- 38.Sousa A., Viana J.L., Milheiro J., Reis V.M., Millet G.P. Effect of hypoxia and nitrate supplementation on different high-intensity interval-training sessions. Eur. J. Appl. Physiol. 2021;121:2585–2594. doi: 10.1007/s00421-021-04726-0. [DOI] [PubMed] [Google Scholar]
- 39.Aucouturier J., Boissière J., Pawlak-Chaouch M., Cuvelier G., Gamelin F.X. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide. 2015;49:16–25. doi: 10.1016/j.niox.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 40.McKenna M.J., Bangsbo J., Renaud J.M. Muscle K+, Na+, and Cl− disturbances and Na+-K+ pump inactivation: Implications for fatigue. J. Appl. Physiol. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen O.B., de Paoli F.V. Regulation of Na+–K+ homeostasis and excitability in contracting muscles: Implications for fatigue. Appl. Physiol. Nutr. Metab. 2007;32:974–984. doi: 10.1139/H07-099. [DOI] [PubMed] [Google Scholar]
- 42.Esen O., Faisal A., Zambolin F., Bailey S.J., Callaghan M.J. Effect of nitrate supplementation on skeletal muscle motor unit activity during isometric blood flow restriction exercise. Eur. J. Appl. Physiol. 2022;122:1683–1693. doi: 10.1007/s00421-022-04946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farina D., Arendt-Nielsen L., Graven-Nielsen T. Effect of temperature on spike-triggered average torque and electrophysiological properties of low-threshold motor units. J. Appl. Physiol. 2005;99:197–203. doi: 10.1152/japplphysiol.00059.2005. [DOI] [PubMed] [Google Scholar]
- 44.McManus L., Hu X., Rymer W.Z., Lowery M.M., Suresh N.L. Changes in motor unit behavior following isometric fatigue of the first dorsal interosseous muscle. J. Neurophysiol. 2015;113:3186–3196. doi: 10.1152/jn.00146.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shannon O.M., Duckworth L., Barlow M.J., Deighton K., Matu J., Williams E.L., Woods D., Xie L., Stephan B., Siervo M., et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front. Physiol. 2017;8:401. doi: 10.3389/fphys.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Nyberg M., Christensen P.M., Blackwell J.R., Hostrup M., Jones A.M., Bangsbo J. Nitrate-rich beetroot juice ingestion reduces skeletal muscle O2 uptake and blood flow during exercise in sedentary men. J. Physiol. 2021;599:5203–5214. doi: 10.1113/JP281995. [DOI] [PubMed] [Google Scholar]
- 48.Davis H.A., Gass G.C. Blood lactate concentrations during incremental work before and after maximum exercise. Br. J. Sports Med. 1979;13:165–169. doi: 10.1136/bjsm.13.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domínguez R., Cuenca E., Maté-Muñoz J.L., García-Fernández P., Serra-Paya N., Estevan M.C., Herreros P.V., Garnacho-Castaño M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients. 2017;9:43. doi: 10.3390/nu9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson K., Turnerb L., Prichardb J., Doddb F., Kennedyb D., Haskellb C., Blackwell J.R., Jones A.M. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir. Physiol. Neurobiol. 2014;193:11–20. doi: 10.1016/j.resp.2013.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions privacy.