Abstract
One hundred nineteen isolates from a commercial zucchini purée stored at 4, 10, and 20 to 25°C were fingerprinted using repetitive sequence-based PCR (REP-PCR) and classified into 35 REP types. One representative isolate of each REP type was subsequently identified by API50CHB/20E profile and partial rrs gene sequence analysis. Nine REP types were misidentified by the API system. Strains were misidentified as being in the Bacillus circulans (group 2) API taxon or in taxa with a low number of positive API characters such as Brevibacillus brevis. A phylogenetic analysis pointed to one new species of Bacillus and three new species of Paenibacillus among the misidentified REP types. Bacterial components in zucchini purée were compared phenotypically with those obtained in previous work on broccoli, carrot, leek, potato, and split pea purées, based on simple matching coefficient and unweighted pair group method with averages cluster analysis. Out of 254 strains, 69 strains previously identified as B. circulans (group 2) or B. circulans/B. macerans/B. polymyxa were assigned to a new Paenibacillus taxon phylogenetically related to P. azotofixans. Storage conditions at 4°C favored the development of “B. macroides/B. maroccanus” and Paenibacillus spp. in zucchini purées and Paenibacillus spp. in other purées. Storage conditions at 20 to 25°C favored the development of B. subtilis group (B. licheniformis and B. subtilis) and B. cereus group strains. At 10°C, Paenibacillus spp. were always present at high frequencies, whereas the occurrence of B. macroides/B. maroccanus (in zucchini purées), B. cereus, and B. pumilus varied with the experiment.
Bacillus and its relatives are widely distributed in the natural environment. Their ubiquitous nature favors contamination of many foods, such as fruits, vegetable products, nuts, cereals, rice, dried foods, spices, milk, and dairy products (16, 24). The resistance of their endospores, which may be associated with psychrotrophic or acidophilic properties, causes specific problems for the food industry; they are common food spoilage organisms in milk, pasteurized milk products, and acidic products (7, 12, 20, 33). Several Bacillus species, such as B. cereus, B. licheniformis, and B. subtilis have also been incriminated in food-borne illnesses (30). Because the emergence of pasteurized chilled food containing vegetables is recent, the presence of Bacillus and relatives in these foods is poorly documented. Pasteurized chilled foods usually receive a mild heat treatment and contain no additives or preservatives, in order to support their image of freshness. These foods are stored at refrigeration temperatures, and their shelf life ranges from a few days to 3 months. Spore-forming contaminants survive the mild processing and may develop during refrigerated storage, thus impairing the quality and safety of the product.
Morphological and physiological criteria are widely used for the identification of Bacillus species. However, the API50CHB phenotypic identification system used by Carlin et al. (4) failed to identify 16% of isolates from purées stored at 25°C and 44% of isolates from purées stored at 4 and 10°C. Those difficulties did not allow analysis of the distribution of species according to the storage conditions. Some authors have experienced the same difficulties in identifying Bacillus spp. with phenotypic methods (23, 28). Fortunately, rapid molecular methods that afford a more reliable bacterial taxonomy have been developed. Goto et al. (10) have emphasized the usefulness of 16S ribosomal DNA (rrs) gene sequencing for rapid identification of species in the genus Bacillus. Techniques such as PCR have prompted the development of useful typing methods and favored polyphasic approaches to microbial community analysis. Repetitive sequence-based PCR (REP-PCR) has notably been recognized as a rapid fingerprinting method at the strain level (18, 32). This technique has been applied to many gram-negative bacteria but seldom to Bacillus.
In a preliminary study on pasteurized vegetable purées, including leek, zucchini, broccoli, split pea, carrot, and potato purées, Carlin et al. (4) showed that Bacillus spp. formed the dominant bacterial components in purées kept at different storage temperatures. Among the purées, zucchini purées showed the most rapid bacterial growth and spoilage. B. cereus was significantly present in this product stored at 10°C (3.6 to 5.2 log CFU per g). A bacterial component not found in other purées was dominant in this product at refrigeration temperature and remained unidentified. The zucchini purée microflora thus called for more thorough investigation.
The aim of this work was to study the effect of refrigerated storage on the microfloral composition of zucchini purées using a polyphasic approach enabling reliable identification of isolates. More isolates from zucchini purée were obtained in this work than in the study of Carlin et al. (4). In a first step, REP-PCR typing was applied to all isolates to reduce the number of identifications. API50CHB/20E profiles and 16S ribosomal DNA (rrs) gene sequence analysis were then used to identify each REP type. We also compared the bacterial components of zucchini purées with those previously isolated from two independent experiments on leek, broccoli, split pea, zucchini, carrot, and potato purées (4).
MATERIALS AND METHODS
Reference strains.
The reference strains of Bacillus and Paenibacillus used for cluster analysis of phenotypic features were as follows: B. licheniformis CIP 5271T (Collection of the Institut Pasteur, Paris, France), B. subtilis CIP 5265T, B. cereus NC 7401 (Nogoya City Public Health Research Institute, Nogoya, Japan), B. cereus CIP 6624T, B. circulans NCIB 9374T (National Collection of Industrial Bacteria, Aberdeen, Scotland), Paenibacillus polymyxa ATCC 842T (American Type Culture Collection, Manassas, Va.), P. macerans ATCC 8244T, and P. azotofixans ATCC 35681T. B. alvei CIP 6618T and B. globisporus CIP 103266T were also used as control strains in growth tests at 5 and 10°C (6). The rrs sequence references used for phylogenetic study came from the international databases; the corresponding accession numbers are mentioned in Fig. 2.
FIG. 2.
Phylogenetic relationships between unidentified bacterial components of zucchini purées and Paenibacillus and Bacillus based on rrs sequences. The branching pattern was generated by the NJ method (25). Numbers above branches are bootstrap values (9) of above 90% for NJ analysis. Numbers below branches are bootstrap values of above 90% for maximum-parsimony analysis (15). Bar, 0.01 nucleotide substitution per site (s/s). Unidentified bacterial components are in boldface. The rrs sequences of type strains of 20 Paenibacillus species, 20 Bacillus species, and 1 Brevibacillus species were obtained from the DDBJ-EMBL-GenBank database, and accession numbers are given following the species names.
Isolation of the dominant bacterial component in zucchini purées.
Commercial pasteurized chilled zucchini purées were obtained from a processing plant in Normandy, France. Zucchini purée packs (400-g units) were randomly sampled at the end of the processing line and sent to the INRA laboratory in Avignon (France) by refrigerated road transport (2 to 3°C). On arrival at the laboratory, sample packs were stored at 4.0 ± 0.0°C, 10 ± 0.5°C and room temperature (20 to 25°C), for, respectively, 21, 21, and 5 days. Individual packs were inspected for swelling at each sampling time.
The diversity of API50CHB/20E taxa encountered in stored vegetable purées was not higher in 16-purée packs than in 5-purée packs (4). For each storage condition, 10 packs were analyzed as follows. Fifty grams of purée per pack was collected, and samples from two packs were pooled in sterile stomacher bags. The five resulting samples (100 g each) were homogenized for 2 min in 200 ml of sterile distilled water using a stomacher. Homogenates were serially diluted and plated on J-agar (6) for aerobic mesophilic bacteria (AMB) counts and on MYP agar (31) for B. cereus counts. J-agar plates and MYP plates were incubated at 28°C for, respectively, 48 and 24 h. For each sample, all of the colonies from a sector covering 100% to 12.5% (one-eighth) of the plate surface were selected from countable J-agar plates. A total of 119 colonies were purified on J-agar and stored in glycerol (30%, vol/vol) at −20 and −80°C.
DNA isolation technique.
A short protocol was used for processing large numbers of samples. Bacterial cultures were grown overnight at 30°C on J-agar. Cells were suspended in 1 ml of Milli-Q water (optical density at 620 nm = 0.5), pelleted at 13,000 rpm for 15 min, and resuspended in 100 μl of Tris-HCl (10 mM, pH 8.2). The cells were lysed by incubation at 55°C for 2 h with 13 μl of proteinase K (1 mg ml−1) (Sigma, St-Quentin-Fallavier, France). Proteinase K was inactivated by incubating lysates at 100°C for 10 min, and cell debris was removed by centrifugation at 13,000 rpm. Supernatants were then transferred to 0.5-ml sterile tubes and stored at −20°C. Lysates were used directly for PCR.
REP-PCR analysis.
REP-PCR with REP 1R-I (5′ III IGC ICG ICG ICA TCI GGC 3′) and REP 2-I (5′ ICG ICT TAT CIG GCC TAC 3′) (Eurogentec S.A., Seraing, Belgium) was carried out as previously described (32). When REP-PCR failed with primers REP 1R-I and REP 2-I, primers REP 1R-Dt (5′ III NCG NCG NAC TCN GGC 3′) and REP 2-Dt (5′ NGC NCT TAT CNG GCC TAC 3′) were used. Amplification reactions were performed in a final volume of 25 μl containing cell lysate (5 μl), deoxynucleoside triphosphate mix (Eurogentec) (1.25 mM), MgCl2 (4 mM), primers (2.4 μM each), dimethyl sulfoxide (10%, vol/vol), 1.5 U of Goldstar DNA polymerase (Eurogentec), and Goldstar buffer (Eurogentec). A PCR 2400 thermal cycler (Perkin-Elmer, Courtaboeuf, France) was used with the following temperature profile: 25 cycles of 94°C for 1 min, 40°C for 1 min, and 65°C for 8 min and a final extension at 65°C for 16 min. A blank containing Milli-Q water instead of cell lysate and a positive control strain lysate producing a known pattern were included in each PCR experiment. REP-PCR products (5 μl) were separated on a 1.5% (wt/vol) agarose gel. Agarose gels were stained with ethidium bromide, and images were captured under UV illumination by a video system (Bioblock, Illkirch, France). DNA fingerprints of isolates were compared for similarity by visual inspection of band patterns after contiguous realignments of amplificons on agarose gels. Fingerprints were considered similar when all visible bands present in each isolate had the same apparent migration. Variations in intensity and shape did not constitute differences. Isolates were then classified into REP types according to their electrophoretic profiles.
rrs gene sequence analysis.
For the first approach in identification, a representative of each REP type was randomly selected for rrs sequence analysis. The rrs gene DNA PCR products (amplificons) of 38 representative strains, corresponding to positions 9 to 1548 of the B. subtilis rrs gene, were amplified by PCR using the primers 5′ AGA GTT TGA TC(A,C) TGG CTC AG 3′ (forward primer) and 5′ GG(A,C) TAC CTT GTT ACG A(T,C)T TC 3′ (reverse primer). The reactions were performed with a reaction mixture (final volume, 100 μl) containing cell lysate (1 μl), deoxynucleoside triphosphate mix (Eurogentec) (0.2 mM), MgCl2 (2.5 mM), primers (0.5 μM each), 1.5 U of AmpliTaq polymerase (Perkin-Elmer), and AmpliTaq buffer (Perkin-Elmer). Amplifications were performed in a PCR 2400 thermal cycler (Perkin-Elmer), using the following temperature profile: 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 2 min, followed by a final extension at 72°C for 5 min.
The amplification products were purified with a QIAquick Spin PCR purification kit (QIAGEN, Courtaboeuf, France) and were sequenced with an ABI PRISM Dye Terminator Ready Reaction kit (Perkin-Elmer) using a DNA thermal cycler (Omnigene; Hybaid, Middlesex, United Kingdom) for 25 cycles with the following program: 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Five primers were used in the sequencing reaction to obtain a complete rrs gene sequence. These primers corresponded to the following positions in the Escherichia coli rrs sequence: primer S6, position 518 to 534; primer S10, position 906 to 925; primer S12, position 1099 to 1114; primer S15, position 1384 to 1400; and primer S17, position 1493 to 1509. Sequences were determined with an automatic DNA sequencer (ABIPrism 377 DNA sequencer; Perkin-Elmer). The protocols used were as recommended by the manufacturer.
Partial sequences of the rrs gene (about 450 bp) were obtained for all 38 representative strains using primer S6. These sequences were compared with rrs gene sequences from the GenBank using the BLASTN (version 2.0.11) program (2). For 8 of the 38 representative strains, this partial sequence was not closely related (<97% sequence homology) to known species sequences in the databases, and therefore the rrs gene was entirely sequenced (about 1,400 bp) using all of the primers listed above.
Phenotypic identifications.
All isolates were examined for colony morphology; tested for catalase reaction, anaerobic growth on anaerobic agar (6), and typical reaction on MYP agar (31); and examined for spore shape and position under phase-contrast microscopy (magnification, ×1,000). Further characterizations were performed for the 38 representative strains. The production of acid metabolites from 49 carbohydrates was tested with API50CHB strips (bioMérieux, Marcy-L'Etoile, France), and tests for proteolysis of gelatin, activities of different enzymes (nitrate reductase, galactosidase, urease, and tryptophanase), H2S formation, production of acetoin, and citrate utilization were carried out with API20E strips (bioMérieux) (17). Data for the phenotypic features from both the API50CH and API20E tests (API50CHB/20E) were submitted for identification by APILAB Plus software version 3.3.3 (bioMérieux).
Data analysis.
To determine the phylogenies of the representative strains for which the entire rrs gene was sequenced, phylogenetic trees were constructed by (i) the neighbor-joining (NJ) method (25) using the two-parameter substitution rate method of Kimura (14) and (ii) the maximum-parsimony method (15) with, in all instances, the no-gap option. Bootstrap estimates (9) were obtained from 100 replicates for both the NJ and maximum-parsimony methods. The different analyses were implemented using the Phylo-win software (22). Graphic representation of the resulting tree was obtained using NJPlot software (22).
The cluster analysis of phenotypic features included 76 characters from the following tests: API50CHB/20E system; anaerobic growth; spore shape, position, and swelling; and growth at 5 and 10°C (6). A total of 254 phenotypic profiles previously obtained during two independent experiments (4) from strains isolated from stored leek, zucchini, broccoli, split pea, carrot, and potato purées were included in the numerical analysis. The phenotypic profiles of the identified strains representative of each REP type in zucchini purées (35 strains) and of eight reference strains were also included for comparison and delineation of taxa. A distance matrix was calculated using the simple matching coefficient (SSM) of Sokal and Michener (27), and the clusters were analyzed by the unweighted pair group method with averages (UPGMA), with SPSS version 10.0 software.
Fisher's exact test (34) was used to determine if storage conditions or the type of vegetable purée had a significant effect on the frequency of each of the different bacterial groups isolated. The test was conducted using Statistica software (Kernel version 5.5 A; StatSoft, Inc., Paris, France) for all pairwise combinations of storage conditions or type of vegetable within each experimental block.
Nucleotide sequence accession numbers.
The rrs gene sequences of unidentified bacterial strains have been deposited in the EMBL database under the following accession numbers: AJ297712 (strain P14-7), AJ297713 (strain P22-9), AJ297714 (strain P15-9), AJ297715 (strain P51-3), AJ297716 (strain P53-6), AJ297717 (strain P51-5), AJ297718 (strain P53-2), and AJ297719 (strain P54-2).
RESULTS
REP-PCR typing.
A complex pattern with 8 to 15 DNA fragments was obtained after gel electrophoresis of the REP-PCR product for each of the 119 strains isolated from zucchini purées. This technique has seldom been used for Bacillus strains, and an adaptation of the protocol was necessary for some isolates. For isolates with highly mucous colonies (26 isolates) classified as having colony morphology types IV and V (Table 1), additional centrifugation (11,000 × g) in Tris-HCl before lysis with proteinase K improved REP-PCR amplification. Primers REP1R-I and REP2-I did not give discriminating PCR products for isolates classified as having colony morphology types I, IV3, and III (number of DNA fragments, <4) (Table 1). Primers REP1R-Dt and REP2-Dt gave acceptable PCR products for all of these isolates. Among the 119 isolates, 53 isolates were fingerprinted using primers REP1R-Dt and REP2-Dt. The patterns observed for the control strains (one for each primer pair) were always the same for separate DNA preparations and for the separate PCR experiments done during this study. Occasionally, a variation in the intensity of minor bands was observed, probably due to DNA concentration (not shown).
TABLE 1.
Distribution of 119 zucchini purée isolates according to REP type and identities of representative strainsa
| REP typeb with primer group:
|
No. of isolates | Colonies on:
|
Representative straine | API 50CHB/20E profile
|
16S ribosomal DNA sequence analysis
|
||||
|---|---|---|---|---|---|---|---|---|---|
| REP | REP-Dt | MYP agar c | J-agard | Taxon | Identity (%) | Species closely related | Identity (%) | ||
| P1 | 1 | − | II5 | P14-7 | B. circulans group 2 | 91.2 | P. azotofixans | 96 | |
| P2 | 1 | − | II5 | P22-9 | B. circulans group 2 | 98.8 | P. azotofixans | 96 | |
| C | 9 | − | II3 | P52-3 | B. circulans group 2/P. macerans | 83/16 | P. amylolyticus | 99 | |
| A | 4 | L+ | III | P12-9 | P. polymyxa | 99.5 | P. polymyxa | 99 | |
| B | 21 | L+ | II2 | P51-3 | P. polymyxa | 99.9 | P. polymyxa | 96 | |
| L | 1 | − | II4 | P15-9 | B. lentus | 99.9 | P. ilinoisensis | 90 | |
| Id | E1 | 5 | − | IV3 | P53-2 | Brevibacillus brevis/B. sphaericus | 65/35 | B. macroides/B. maroccanus | 98 |
| E2 | 2 | − | IV1 | P51-5 | Brevibacillus brevis | 97.3 | B. macroides/B. maroccanus | 99 | |
| F | 1 | − | IV2 | P54-2 | Brevibacillus brevis/B. sphaericus | 34/65 | B. macroides/B. maroccanus | 99 | |
| I | 1 | − | II6 | P22-5 | B. circulans group 1/B. macerans | 53/23 | B. circulans | 99 | |
| R | 2 | − | II6 | P22-10 | B. circulans group 1 | 99.7 | B. circulans | 99 | |
| D | 1 | − | VII | P53-6 | Brevibacillus brevis | 99.9 | B. sphaericus | 94 | |
| G | 13 | − | V1, V2, V3 | P22-8 | B. licheniformis/B. subtilis | 54/46 | B. licheniformis | 99 | |
| K1 | 1 | − | V2 | P23-12 | B. licheniformis | 99.9 | B. licheniformis | 99 | |
| K2 | 2 | − | V4 | P22-11 | B. licheniformis | 98.2 | B. licheniformis | 99 | |
| K3 | 2 | − | V1 | P23-17 | B. licheniformis | 99.9 | B. licheniformis | 98 | |
| H1 | 1 | − | V12 | P22-17 | B. licheniformis/B. subtilis | 80/20 | B. subtilis | 99 | |
| H2 | 3 | − | V11 | P23-5 | B. amyloliquefaciens/B. subtilis | 95/5 | B. subtilis | 99 | |
| Id | J | 1 | − | I3 | P23-18 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M1 | 6 | − | I1, I2 | P15-7 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M2 | 2 | − | I2 | P15-4′ | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M3 | 1 | − | I1 | P13-7 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M4 | 2 | − | I1 | P13-5 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M5 | 1 | − | I1 | P14-2′ | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | M6 | 6 | − | I1, I2 | P12-10 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | N | 9 | − | I1, I2 | P12-4 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | 01 | 1 | − | I1 | P14-4 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | O2 | 3 | − | I1, I2 | P14-5 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | Q1 | 1 | − | I3 | P23-2 | B. pumilus | 99.9 | B. pumilus | 98 |
| Id | Q2 | 1 | − | I2 | P11-7 | B. pumilus | 99.9 | B. pumilus | 98 |
| Id | S | 2 | − | I3 | P23-3 | B. pumilus | 99.9 | B. pumilus | 99 |
| Id | T | 4 | + | III | P22-3 | B. mycoides | 98.8 | B. cereus group | 99 |
| Id | U | 1 | + | III | P22-4 | B. mycoides/B. cereus | 98/1 | B. cereus group | 99 |
| Id | V | 1 | + | III | P15-3 | B. cereus group 1/B. mycoides | 52/48 | B. cereus group | 99 |
| Id | W | 6 | + | III | P13-4 | B. cereus group 1 | 99.5 | B. cereus group | 99 |
Results for which the AP150CHB/20E system was in disagreement with rrs gene sequence identity are in boldface.
Letters represent major different REP types; numbers following letters represent related subgroups differentiated by one or two minor bands on electrophoretic patterns; Id, low discriminative electrophoretic pattern (fewer than four bands).
+, lecithinase positive and manitol negative; L+, lecithinase positive only; −, lecithinase negative and manitol negative.
I to VII, major distinct morphotypes; numbers following morphotypes represent subgroups according to mucosal aspect (types I, IV, V, and VI) or according to the colony size and thickness (type II).
Strain randomly selected from each REP type for API50CHB/20E identification and rrs gene sequencing.
Clustering of isolates and identification of representative strains.
REP-PCR patterns were compared, and the 119 isolates were classified into 35 REP types. An example of electrophoretic profile comparison is shown in Fig. 1. The distribution of isolates according to REP types and purée storage temperatures is shown in Tables 1 and 2. All isolates in a same REP type showed similar characteristics for catalase reaction, anaerobic growth, colony morphology, and spore morphology. Strain identification was achieved for one representative isolate of each REP type with API50CHB/20E profile analysis and partial rrs gene sequence analysis.
FIG. 1.
Examples of REP-PCR patterns of isolates from zucchini purée (gels a and b). Lanes 1 to 12, REP type G. Lanes 13 to 21, REP type N. Lane 22, REP type M4. Lane 23, REP type M5. Lane 24, REP type M3. Lanes M, DNA molecular size marker X (Roche Diagnostic, Meylan, France).
TABLE 2.
Composition of zucchini purée microflora according to storage temperature
| Species determined by rrs gene sequence identificationa | REP type(s) | No. (%) of isolates under the following storage conditions for purée packs:
|
Total no. of isolates | ||
|---|---|---|---|---|---|
| 21 days at 4°C | 21 days at 10°C | 5 days at 20–25°C | |||
| B. macroides/B. maroccanus | E1, E2, F | 8 (31) | 8 | ||
| P. amyloliticus | C | 9 (34) | 9 | ||
| Bacillus species 1 (B. sphaericus) | D | 1 (4) | 1 | ||
| P. polymyxa | A | 1 (4) | 3 (6) | 4 | |
| Paenibacillus species 1 (P. polymyxa) | B | 7 (27) | 14 (28) | 21 | |
| Paenibacillus species 2 | L | 1 (2) | 1 | ||
| Paenibacillus species 3 (P. azotofixans) | P1, P2 | 1 (2) | 1 (2) | 2 | |
| B. cereus group | T, U, V, W | 6 (12) | 6 (13) | 12 | |
| B. pumilus | J, M1, M2, M3, M4, M5, M6, N, O1, O2, Q1, Q2, S | 25 (50) | 11 (27) | 36 | |
| B. licheniformis | G, K1, K2, K3 | 18 (42) | 18 | ||
| B. subtilis | H1, H2 | 4 (9) | 4 | ||
| B. circulans | I, R | 3 (7) | 3 | ||
| Total | 35 | 26 (100) | 50 (100) | 43 (100) | 119 |
The most related species is in parentheses.
For 25 REP types, representing 50% of the 119 isolates from J-agar, identification was unambiguous: taxa obtained with the API system were confirmed by partial rrs sequence analysis (Table 1). These isolates were identified as B. circulans group 1 (REP type R; 2 isolates), P. polymyxa (REP type A; 4 isolates), B. licheniformis (REP types K1, K2, and K3; five isolates), B. pumilus (all 13 REP types; 36 isolates), and B. cereus group (all four REP types; 12 isolates) (Table 1). In the B. cereus group, B. cereus, B. thuringiensis, B. anthracis, B. weihenstephanensis, and B. mycoides could not be discriminated with the API50CHB system and partial rrs gene sequencing. For four REP types (G, H1, H2, and I), representing 15% of the 119 isolates from J-agar, API50CHB profiles were weakly discriminative and gave two putative species names. The rrs partial sequence analysis was necessary to determine to which species these isolates belonged (Table 1). They belonged to B. circulans (REP type I; 1 isolate), B. licheniformis (REP type G; 13 isolates), and B. subtilis (REP types H1 and H2; 4 isolates). These four REP types also represented 40% of the 53 isolates picked on J-agar from zucchini purées stored at 20 to 25°C.
For the nine remaining REP types (B, C, D, E1, E2, F, P1, P2, and L), the identification using the API50CHB system did not agree with the rrs partial sequence analysis. These nine REP types represented 35% of the total isolates picked on J-agar. They also represented 32 to 96% of isolates from zucchini purées stored at low temperature. REP type C (nine isolates), designated “B. circulans group 2/B. macerans” according to the API50CHB system, was clearly identified as Paenibacillus amylolyticus by rrs partial sequence analysis (99% sequence homology) (Table 1). The other eight REP types (B, D, E, E1, F, P, P1, and L; 31 isolates) remained unidentified, and so phylogenetic identification was performed to clarify their taxonomic positions.
Phylogenetic identification of unidentified strains.
For phylogenetic identification, complete rrs sequences of unidentified strains were analyzed. Three phylogenetic methods were then used, and a tree showing relationships with known Bacillus and Paenibacillus species was built (Fig. 2). The Bacillus and Paenibacillus genera each formed a monophyletic group. REP types E1, E2, F, and D grouped with the Bacillus genus, and REP types B, P1, P2, and L grouped with the Paenibacillus genus. The three REP types E1, E2, and F, related to the “Brevibacillus brevis/B. sphaericus ” API taxa, formed a robust monophyletic unit with the species complex “B. macroides/B. maroccanus ” (Fig. 2). In this group, the divergence between the most distant sequences never exceeded 1.2%, and so the three REP types probably belong to this complex. REP type D, identified as Brevibacillus brevis according to the API system, was related to B. sphaericus and B. fusiformis, with a 5.4% divergence. This REP type probably belongs to a new species.
REP type B was related to P. polymyxa and P. peoriae, with 4.1 and 4.8% sequence divergence, respectively, and its phenotypic characteristics were closely similar to those of P. polymyxa (99.9% identity according to the APILAB system). DNA-DNA hybridizations will be necessary to find out whether REP type B may be considered a new species close to P. polymyxa or whether it belongs to P. polymyxa. REP types P1 and P2, designated B. circulans group 2 according to the API50CHB system, formed a robust monophyletic unit and were close to P. azotofixans (2.9 and 3% sequence divergence). These REP types belong to the same new species, the description of which is in progress (O. Berge et al., unpublished data). REP type L, designated B. lentus according to the API50CHB system, held a separate position in Paenibacillus branch, unrelated to any known Paenibacillus species. The divergence of its rrs sequence was more than 5.8% from the nearest sequences, and so this REP type is probably a new Paenibacillus species. It therefore seems that REP type D belongs to one new species in the genus Bacillus and that REP types B, P1, P2, and L belong to three new species in the genus Paenibacillus. Among these new species, REP types B and D represented 31% of the isolates from zucchini purées stored at 4°C, and REP types B, P, and L represented 32% of the isolates from zucchini purées stored at 10°C.
Microbial diversity in zucchini purées and spoilage.
P. amylolyticus, Paenibacillus species 1 (REP type B), and B. macroides/B. maroccanus (Rep types E1, E2, and F) were the most frequently isolated species at 4°C (Table 2). The level of AMB was low (3.9 ± 1.0 log CFU/g) at this storage temperature, and no spoilage appeared after 21 days. At 10°C, B. pumilus and Paenibacillus species 1 dominated, and slight spoilage started after 14 days of storage. Purée pack swelling appeared after 21 days, with an AMB level of 7.5 ± 0.3 log CFU/g. At room temperature (20 to 25°C), the purée microflora was dominated by B. licheniformis and B. pumilus. At this storage temperature, microbial counts were high (7.8 ± 0.1 log CFU/g) and spoilage appeared after 5 days. B. cereus was present at variable concentrations in purées stored at 10°C (4.6 ± 1.9 log CFU/g) and at a high level in purées stored at 20 to 25°C (6.4 ± 0.5 log CFU/g); the level was <1.7 log CFU/g in purées stored at 4°C.
Phenotypic relationship between bacterial components of various purées.
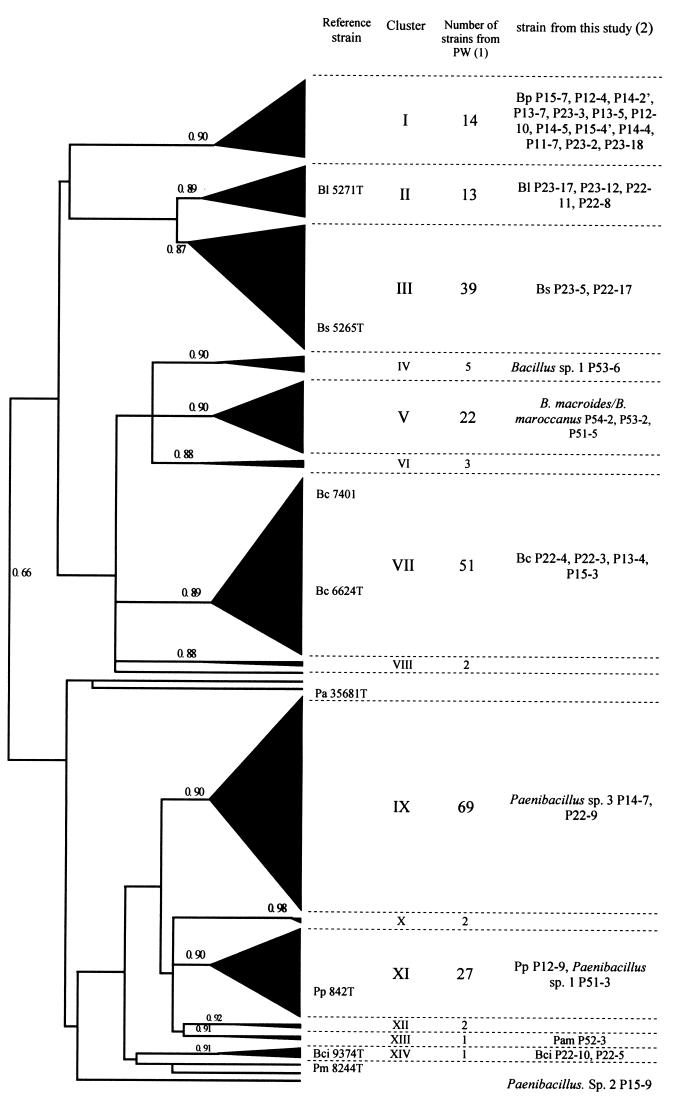
After elucidating the taxonomy and phylogeny of isolates obtained from zucchini purées, we compared them phenotypically to those previously obtained from broccoli, carrot, leek, potato, zucchini, and split pea purées by Carlin et al. (4). The results of the cluster analysis performed on the phenotypic characters are summarized in a simplified dendrogram in Fig. 3. Two main groups were formed at a 66% similarity level, one containing all Bacillus species except B. circulans group 1 and the other containing all Paenibacillus species and B. circulans group 1, including reference strain B. circulans NCIB 9374T, emphasizing the phenotypic similarity between B. circulans sensu stricto and some Paenibacillus spp. Bacterial components of broccoli, carrot, leek, potato, zucchini, and split pea purées formed 14 clusters at an 87% similarity level, with four unclustered strains. Six major phenotypic taxa were found in many purées: B. pumilus (cluster I), B. licheniformis (cluster II), B. subtilis (cluster III), B. cereus (cluster VII), Paenibacillus species 3 (cluster IX), and P. polymyxa (cluster XI). One major cluster (cluster V) representing B. macroides/B. maroccanus was isolated only from zucchini purées stored at 4 and 10°C. Clusters IV (related to B. sphaericus), VI (unidentified strains), VIII (unidentified strains), X and XII (related to B. circulans group 2/B. macerans), XIII (related to P. amylolyticus), and XIV (B. circulans sensu stricto) contained very few strains.
FIG. 3.
Simplified dendrogram showing the phenotypic relationship between the bacterial strains from vegetable purées based on the SSM coefficient (27) and UPGMA clustering techniques. Bl, B. licheniformis; Bs, B. subtilis; Bp, B. pumilus; Bc, B. cereus; Pa, P. azotofixans; Pp, P. polymyxa; Bci, B. circulans; Pm, P. macerans; Pam, P. amylolyticus. PW, previous work. (1) Number of strains isolated during two previous independent experiments from broccoli, carrot purée, potato, leek, zucchini, and split pea purées. (2) Strains from zucchini purées identified in this work. The numbers associated with clusters represent the absolute distance coefficient SSM.
Among the 254 strains characterized by Carlin et al. (4), 111 strains were unidentified strains or strains with uncertain identity (B. circulans group 2/B. macerans/B. polymyxa). Based on phenotypic characters, 103 of these strains could be related to known species in zucchini purées studied in the present work and some reference strains. Twenty-two strains were clustered with B. macroides/B. maroccanus (cluster V), five strains were clustered with Bacillus species 1 (cluster IV, related to B. sphaericus), and one strain was clustered with B. circulans sensu stricto (NCIB 9374T). Among strains previously identified as B. circulans group 2 or B. circulans group 2/B. macerans/B. polymyxa according to the API50CHB/20E system, 69 strains were clustered with Paenibacillus species 3 (cluster IX), one strain was clustered with P. amylolyticus (cluster XIII), one strain was clustered with P. polymyxa, and four strains formed two unidentified clusters in the Paenibacillus branch (clusters X and XII). Furthermore, strains designated B. subtilis/B. amyloliquefaciens in the APILAB database were grouped with B. subtilis CIP 5265T and B. subtilis strains in zucchini purées (cluster III).
This dendrogram grouped many strains in the homogeneous phenon P. polymyxa (cluster XI), which may contain different genospecies closely related to P. polymyxa, as Paenibacillus species 1.
Effect of storage conditions and type of vegetable purée on frequency of bacterial groups.
Storage conditions had a significant effect on the frequency with which several of the clusters or groups of bacteria were isolated. The frequency of isolation of Paenibacillus spp. and the B. macroides/B. maroccanus group (cluster V) tended to increase as the temperature of storage decreased (Fig. 4). Paenibacillus spp. were isolated at significantly (P < 0.05) lower frequencies after 5 days of storage at 20°C than for either of the other storage conditions from zucchini (in the isolations made for this present study) and from all vegetable purées considered together for experiment B (Fig. 4B) and experiment C (Fig. 4C). Likewise, the frequency of isolation of the B. macroides/B. maroccanus group was significantly lower for storage at 20°C and 5 days than for storage at 4°C and 21 days.
FIG. 4.
Percentages of strains isolated from zucchini purées in this study (A) and from six types of vegetable purées in two previous independent experiments (B and C) after storage at 4°C for 21 days, at 10°C for 21 days, and at 20°C for 5 to 14 days that were identified as Paenibacillus spp., B. licheniformis/B. subtilis (clusters II and III), B. macroides/B. maroccanus (cluster V), B. cereus (cluster VII), or related to B. sphaericus (cluster IV) or were in the B. pumilus cluster or the remaining minor cluster (other). The numbers associated with bars represent the absolute number of strains isolated for each bacterial group.
Storage conditions of 20°C for 5 days also led to an increase in the frequency of isolation of B. cereus (cluster VII) and of the B. licheniformis/B. subtilis group (clusters II and III) compared to the other storage conditions. The frequency of isolation of B. cereus was significantly greater after 5 days of storage of purées at 21°C than under the other storage conditions for experiment B (Fig. 4B) and experiment C (Fig. 4C), although this effect was not statistically significant in the present study (Fig. 4A). Strains of the B. licheniformis/B. subtilis group were detected only after 5 days of storage at 20°C, and they constituted 30 to 67% of the total strains isolated from each of the different experiments.
Although the occurrence of strains from other clusters, which were essentially represented by B. pumilus (Fig. 4, other) and cluster IV, varied with the source of isolation, no other statistically significant or consistent effects of storage conditions were observed.
The type of vegetable purée had little effect on the frequency of isolation of the different bacterial groups. Although frequencies of the different bacterial groups varied among the types of vegetable purées from which isolations were made, most of these differences were not statistically significant. However, the B. macroides/B. maroccanus group was detected only in zucchini purées throughout the three experiments.
DISCUSSION
REP-PCR allows rapid fingerprinting at an intraspecific level (32), strongly reducing the number of identifications (38 identifications for 140 isolates) without reducing the discriminatory power. This tool has also been previously used with success for B. sporothermodurans (13). In our work, variations occurred in minor-band intensity between DNA preparations or between experiments. Herman et al. (13) showed that variations in band position between batches of primers could also occur. Thus, we strongly recommend the use of the same batch of PCR reagents and the comparison of strains from the same PCR experiment or, failing that, the inclusion of control strains in each PCR experiment. We also found that REP-PCR needs to be adapted to the Bacillus species studied. Notably, primers REP1R-Dt and REP2-Dt seem to be better adapted than REP1R-I and REP2-I to B. pumilus, B. macroides/B. maroccanus, and B. cereus.
Some species, such as B. pumilus and the B. cereus group, were easily identified with the API50CHB/20E system. For these, phenotypic identification is sufficient. For the P. polymyxa group, certain strains identified as P. polymyxa with 99.9% similarity with the APILAB system may belong to a distinct genospecies closely related to P. polymyxa, as illustrated by Paenibacillus species 1. In the B. subtilis group, the discrimination between the species B. subtilis, B. licheniformis, and B. amyloliquefaciens can be low using the API50CHB/20E system. Furthermore, some strains were completely misidentified with the API system, which assigned them to an incorrect genus. The number of misidentifications was particularly high for isolates from refrigerated products. Strains of B. macroides/B. maroccanus or strains related to B. sphaericus were identified as Brevibacillus brevis by the API system. This was mainly due to the low number of positive characters in the API50CHB strips; these strains are mesophilic, and the temperature used to incubate API strips was not a limiting factor for growth. In this case, the spore morphology easily showed the misidentification. In contrast, misidentifications of Paenibacillus strains as B. circulans group 2 by the API system could not be detected by other phenotypic characters, and rrs gene sequence analysis was necessary to detect the mistake. As described by Ash et al. (3), the combination of morphology and physiology was sufficient to distinguish rRNA group 3 bacilli (i.e., Paenibacillus) from other mesophilic species of Bacillus, with the exception of B. circulans. The heterogeneity of the species B. circulans Jordan 1980 was previously shown by DNA-DNA relatedness (21). Emendation of B. circulans started recently with the description of P. illinoisensis (26). Paenibacillus sp. strain TOD45 and Paenibacillus sp. strain RSA19, which are closely related to P. azotofixans (1), had also previously been identified as B. circulans group 2 by phenotypic characterization (API50CHB). In our work, two strains identified as B. circulans sensu stricto by their rrs sequences were related to the B. circulans group 1 API taxon. These results suggest that strains related to the B. circulans group 1 API taxon may belong to B. circulans sensu stricto, whereas strains related to the B. circulans group 2 API taxon may belong to Paenibacillus spp. In purées, B. circulans sensu stricto was isolated from purées stored at room temperature, whereas Paenibacillus spp. were isolated preferentially from purées stored at a low temperature.
Storage conditions strongly influenced the microfloral composition of purées. Storage at 4°C favored the development of B. macroides/B. maroccanus and Paenibacillus spp. in zucchini purées and of Paenibacillus spp. in other purées. Storage at 20 to 25°C favored the development of the B. subtilis group (B. licheniformis and B. subtilis) and the B. cereus group. In the intermediate storage condition at 10°C, the development of Paenibacillus spp. was favored, whereas the occurrence of others species, such as B. macroides/B. maroccanus (in zucchini purées), B. cereus, or B. pumilus varied with the experiment. The psychrotrophic properties of B. macroides/B. maroccanus and of Paenibacillus spp. (4, 6, 8) and the rapid growth of the B. subtilis and B. cereus groups at room temperature (6, 8) probably explain this distribution. The selective effect of temperature on purée microflora suggests that the relative abundance of certain species could serve as an indicator of storage conditions of purées. The preferential occurrence of B. circulans, B. polymyxa, and B. macerans at low temperature was also observed in milk and pasteurized milk (7, 11, 19, 29); the species B. polymyxa and B. macerans have since been transferred into the Paenibacillus genus as P. polymyxa and P. macerans (3), and it has been shown that the heterogeneous B. circulans complex harbors Paenibacillus species (1, 21, 26).
Spoilage, shown by pack swelling, occurred in zucchini purées after storage at 10°C and at room temperature. Temperatures of 10°C may often occur during the distribution of the product or in the refrigerators of consumers, and spoilage may develop before the use-by date specified by the manufacturers (21 days). In this study, the prevailing species at 10°C were P. pumilus and a species phenotypically and genotypically related to P. polymyxa, Paenibacillus species 1. As opposed to B. pumilus, P. polymyxa (previously named B. polymyxa) is well known for its ability to produce gas from different carbon sources and in spoiled milk (6, 20). Consequently, Paenibacillus species 1 could be the cause of pack swelling at 10°C by gas production.
In conclusion, the composition of the microflora of pasteurized chilled vegetable purées is highly determined by storage conditions. Compliance with the storage temperature recommended by the manufacturer (4°C) should prevent spoilage and growth of potential human pathogens such as B. cereus, B. licheniformis, and B. subtilis. Fluctuations of storage temperature before consumption of the product may induce growth of B. cereus; some strains from pasteurized chilled vegetable purées were able to grow at temperatures below 10°C (5). This work also emphasizes the possibility of misidentifying Bacillus or Paenibacillus isolates in foods when using phenotypic methods alone. In particular, the entry for B. circulans group 2 of the API database needs to be revised, as some isolates assigned to this taxon apparently belong to Paenibacillus spp. This revision should encompass a more thorough phylogenetic investigation involving the complete reassessment of the B. circulans complex. This is especially important, as strains from this complex are often psychrotrophic contaminants of refrigerated pasteurized foods. The discrimination between Bacillus species exhibiting a low number of positive API characters is also problematic. Partial sequencing of the rrs gene is a good alternative for problematic phenotypic identifications and seems truly necessary in the case of Bacillus and relatives. This has become easier since the advent of commercial automatic sequencers. The present sequence databases offer a powerful tool for identification. The principal limit to the application of rrs gene sequencing is probably the high number of identifications needed in ecological studies. This work gives an example of a strategy used to reduce the number of identifications: inclusion of a rapid preliminary REP-PCR typing step to cluster related isolates.
ACKNOWLEDGMENTS
This work was supported in part by research projects no. 95G0086 (Ministère de la Recherche, France) and FAIR CT97-3159 (European Commission).
We thank Alain Huart and Louis Gardan (Station de Pathologie Végétale, INRA, Angers, France) for their help in obtaining phenograms and Mohamed Barakat and Catherine Brutesco (CEA Cadarache, Saint-Paul-lez-Durance, France) for their technical contributions.
REFERENCES
- 1.Achouak W, Normand P, Heulin T. Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Int J Syst Bacteriol. 1999;49:961–967. doi: 10.1099/00207713-49-3-961. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash C, Priest F G, Collins M D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbank and Collins) using PCR probe test, proposal for the creation of a new genus Paenibacillus. Antonie Leeuwenhoek. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- 4.Carlin F, Guinebretiere M H, Choma C, Pasqualini R, Braconnier A, Nguyen-The C. Spore-forming bacteria in commercial cooked, pasteurized and chilled vegetable purées. Food Microbiol. 2000;17:153–165. [Google Scholar]
- 5.Choma C, Guinebretiere M H, Carlin F, Schmitt P, Velge P, Granum P E, Nguyen-The C. Prevalence, characterization and growth of Bacillus cereus in commercial cooked chilled foods containing vegetable. J Appl Microbiol. 2000;88:617–625. doi: 10.1046/j.1365-2672.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 6.Claus D, Berkeley R C W. Genus Bacillus Cohn 1872, 174AL. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1105–1139. [Google Scholar]
- 7.Dommett T W. Spoilage of aseptically packaged pasteurized liquid dairy products by thermoduric psychrotrophs. Food Aust. 1992;44:459–461. [Google Scholar]
- 8.Emberger O. A contribution to cultivation methods for the detection of aerobic sporeforming bacteria. Zentbl Bakteriol Abt 2 Bd. 1970;125:555–564. [PubMed] [Google Scholar]
- 9.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Goto K, Omura T, Hara Y, Sadaie Y. Application of the partial 16S rDNA sequence as an index for rapid identification of species in the genus Bacillus. J Gen Appl Microbiol. 2000;46:1–8. doi: 10.2323/jgam.46.1. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths M W, Phillips J D. Incidence, source and some properties of psychrotrophic Bacillus spp in raw and pasteurized milk. J Soc Dairy Technol. 1990;43:62–66. [Google Scholar]
- 12.Hanlin J H. Spoilage of acidic products by Bacillus species. Dairy Food Environ Sanit. 1998;18:655–659. [Google Scholar]
- 13.Herman L, Heyndrickx M, Waes G. Typing of Bacillus sporothermodurans and other Bacillus species isolated from milk by repetitive element sequence based PCR. Lett Appl Microbiol. 1998;26:183–188. doi: 10.1046/j.1472-765x.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 15.Kluge A G, Farris J S. Quantitative phyletics and the evolution of anurans. Syst Zool. 1969;18:1–32. [Google Scholar]
- 16.Kramer J M, Gilbert R J. Bacillus cereus and other Bacillus species. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker; 1989. pp. 21–70. [Google Scholar]
- 17.Logan N A, Berkeley R C W. Identification of Bacillus strains using the API system. J Gen Microbiol. 1984;130:1871–1882. doi: 10.1099/00221287-130-7-1871. [DOI] [PubMed] [Google Scholar]
- 18.Louws F J, Fulbright D W, Taylor Stephens C, De Bruijn F J. Specific genomic fingerprinting of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequence and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr R, Eppert I, Scherer E. Incidence and identification of psychrotrophic (7°C-tolerant) Bacillus spp. in German HTST pasteurized milk. Milchwissenschaft. 1999;54:26–60. [Google Scholar]
- 20.Meer R R, Baker J, Bodyfelt F W, Griffiths M W. Psychrotrophic Bacillus spp. in fluid milk products: a review. J Food Prot. 1991;54:969–997. doi: 10.4315/0362-028X-54.12.969. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura L K, Swezey J. Taxonomy of Bacillus circulans Jordan 1890: base composition and reassociation of deoxyribonucleic acid. Int J Syst Bacteriol. 1983;33:46–52. [Google Scholar]
- 22.Perriere G, Gouy M. WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie. 1996;78:364–369. doi: 10.1016/0300-9084(96)84768-7. [DOI] [PubMed] [Google Scholar]
- 23.Pirttijärvi T S M, Ahonen L M, Maunuksela L M, Salkinoja-Salonen M S. Bacillus cereus in a whey process. Int J Food Microbiol. 1998;44:31–41. doi: 10.1016/s0168-1605(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 24.Roberts D, Watson G N, Gilbert R J. Contamination of food plants and plant products with bacteria of public health significance. In: Rhodes-Roberts M E, Skinner F A, editors. Bacteria and plants. The Society for Applied Bacteriology symposium series no. 10. London, United Kingdom: Academic Press; 1982. pp. 169–195. [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Shida O, Takagi H, Kadowaki K, Nakamura L K, Komagata K. Emended description of Paenibacillus amylolyticus and description of Paenibacillus. illinoisensis sp. nov. and Paenibacillus chibensis sp. nov. Int J Syst Bacteriol. 1997;47:299–306. doi: 10.1099/00207713-47-2-299. [DOI] [PubMed] [Google Scholar]
- 27.Sokal R R, Michener C D. A statistical method for evaluating systematic relationships. Kansas Univ Sci Bull. 1958;38:1409–1438. [Google Scholar]
- 28.Tatzel R, Ludwig W, Schleifer K H, Wallnöfer P R. Identification of Bacillus strains isolated from milk and cream with classical and nucleic acid hybridization methods. J Dairy Res. 1994;61:529–535. doi: 10.1017/s0022029900028454. [DOI] [PubMed] [Google Scholar]
- 29.Ternström A, Lindberg A M, Molin G. Classification of the spoilage flora of raw and pasteurized bovine milk, with special reference to Pseudomonas and Bacillus. J Appl Bacteriol. 1993;75:25–34. doi: 10.1111/j.1365-2672.1993.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull P C B, Kramer J M, Melling J. Bacillus. In: Parker M T, Duerden B I, editors. Topley and Wilson's principles of bacteriology, virology and immunity. 8th ed. Vol. 2. Sevenoaks, Kent, United Kingdom: Edward Arnold; 1990. pp. 187–210. [Google Scholar]
- 31.Van Netten P, Kramer J M. Media for the detection and enumeration of Bacillus cereus in foods: a review. Int J Food Microbiol. 1992;17:85–99. doi: 10.1016/0168-1605(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 32.Versalovic J, Schneider M, De Bruijn F J, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 33.Walls I, Chuyate R. Alicyclobacillus—historical perspectives and preliminary characterization study. Dairy Food Environ Sanit. 1998;18:499–503. [Google Scholar]
- 34.Zar J H. Biostatistical analysis. Englewood Cliffs, N.J: Prentice Hall; 1984. [Google Scholar]