Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in December 2019, causing millions of deaths all over the world, and the lack of specific treatment for severe forms of coronavirus disease 2019 (COVID-19) have led to the development of vaccines in record time, increasing the risk of vaccine safety issues. Recently, several cases of thrombotic thrombocytopenic purpura (TTP) have been reported following COVID-19 vaccination. TTP is a rare disease characterized by thrombocytopenia, microangiopathic hemolytic anemia and ischemic end-organ lesions. It can be either congenital or acquired. Various events such as viral infections, medication, pregnancy, malignancies, and vaccinations may cause TTP. Here, we report two cases of acquired TTP following Sinopharm COVID-19 vaccine (BBIBP-CorV) and Sinovac COVID-19 vaccine (CoronaVac). Diagnosis was based on clinical presentation and confirmed with a severe reduction in the activity of von Willebrand factor-cleaving protease ADAMTS-13 and the presence of inhibitory autoantibodies. The two patients were successfully treated with corticosteroids, plasma exchange therapy and rituximab in the acute phase. In the literature, the reported cases of TTP induced by COVID-19 vaccination occurred after Adenoviral Vector DNA- and SARS-CoV-2 mRNA-Based COVID-19 vaccines. To the best of our knowledge, this is the first report of acquired TTP after inactivated virus COVID-19 vaccination.
Keywords: vaccines, COVID-19, safety, purpura, thrombotic thrombocytopenic
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel virus first detected in Wuhan in December 2019. A few months later, the World Health Organization (WHO) declared a worldwide pandemic. This virus can cause severe viral pneumonia with acute respiratory distress syndrome causing millions of deaths [1,2]. Currently, there is no effective treatment for coronavirus disease 2019 (COVID-19) [3]. However, several vaccines have been developed worldwide to reduce COVID-19 mortality and morbidity [4]. These vaccines have obtained emergency use approval by the WHO in several countries, increasing the risk of vaccine safety issues, and some adverse events have been reported [5,6,7]. Most frequent were injection site reactions or systemic effects (e.g., fatigue, headache, body pain, fever) with rare serious adverse events (e.g., anaphylaxis, Guillain-Barré, thrombosis with thrombocytopenia Syndrome) [8,9,10]. Several cases of thrombotic thrombocytopenic purpura (TTP) induced by COVID-19 vaccination have been reported in the literature [11,12,13,14]. TTP is a rare hematologic disorder classically characterized by the pentad of fever, hemolytic anemia, thrombocytopenia, renal failure, and neurologic dysfunction. However, most patients do not have the entire pentad [15]. This disease is caused by a severe decrease in the activity of the von Willebrand factor-cleaving protease ADAMTS-13 which can be either congenital or acquired due to anti-ADAMTS-13 autoantibodies [11]. Various events may initiate the production of those antibodies such as viral infections, medication, pregnancy, malignancies and, occasionally, vaccinations [16]. Here, we report two cases of acquired TTP after two inactivated COVID-19 vaccines: BBIBP-CorV vaccine, known as the Sinopharm COVID-19 vaccine, and CoronaVac, known as the Sinovac vaccine. To the best of our knowledge, the present cases are the first reported cases of acquired TTP after inactivated virus COVID-19 vaccination.
2. Case Presentations
The Research and Ethics Committee of Farhat Hached University Hospital approved the publication of the retrospectively obtained and anonymized data of the two cases (ID number of the approval: CER 10-2022).
2.1. Case 1
A 38-year-old Caucasian, North African Maghrebian woman with no medical history presenting with dizziness and ecchymosis in her upper limbs was referred to the hematology department. The patient reported that she had received a first dose of an inactivated virus COVID-19 vaccine Sinopharm (BBIBP-CorV) twenty days before symptom onset. Laboratory findings revealed hemoglobin 6 g/dL, platelet count 6 × 109/L, lactate dehydrogenase (LDH) 1074 UI/L, D-dimer 1200 µg/L, haptoglobin 0.54 g/L, creatinine 66 µmol/L, urea 20.7 mg/dL, total bilirubin 3.75 mg/dL and indirect bilirubin 2.88 mg/dL. Peripheral blood smear showed schistocytes (1 to 2%). During her hospital stay, the patient presented left hemi-body heaviness and dysarthria. A brain MRI revealed an ischemic stroke in the territory of the inferoposterior cerebellar artery. A curative anticoagulation was started. A few hours after ICU admission, the patient presented a sudden generalized tonico-clonic seizure with status epilepticus requiring her intubation. Glycemia and electrolytes were within the normal ranges. The patient was promptly given clonazepam and intravenous sodium valproate. Analgo-sedation was prolonged with remifentanyl and midazolam to achieve a Richmond Agitation Sedation Scale (RASS) [17] at −5 to control the status epilepticus and obtain patient–ventilator synchronization. The presence of thrombocytopenia, hemolytic anemia and neurological symptoms was indicative of a presumptive diagnosis of TTP. The PLASMIC score, used to identify patients with ADAMTS-13 deficiency in suspected TTP patients, was at 6 (range, 0–7) indicating a high risk of severe ADAMTS-13 deficiency < 10%. The patient was promptly treated by methylprednisolone 1000 mg daily for three consecutive days, then 1 mg/kg/day in combination with daily plasma exchange therapy (PEX).
Infectious screening tests (e.g., human immunodeficiency virus (HIV), hepatitis, SARS-CoV-2, Epstein-Barr virus, and cytomegalovirus) were negative. Autoimmunity investigations revealed severe ADAMTS-13 deficiency (6%) with positive anti ADAMTS-13 autoantibodies more than 15 U/mL (normal < 12 U/mL) confirming the diagnosis of an acquired TTP.
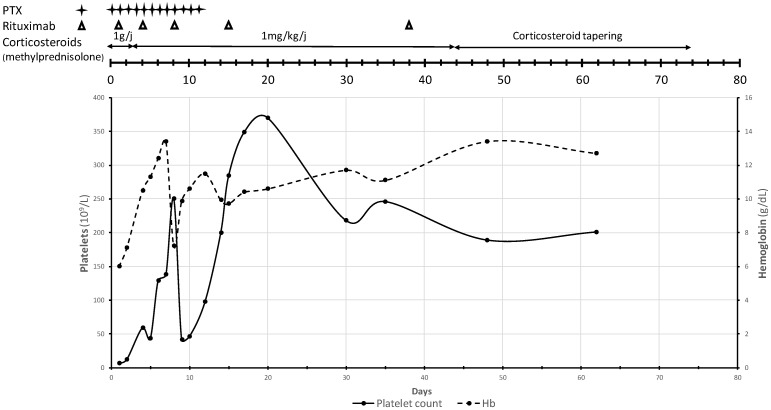
The patient showed clinical improvement after the third PEX with symptom resolution. She remained seizure free and was extubated on day 5 of her ICU stay. The normalization of LDH was achieved 7 days after initiation of PEX, whereas a decrease in total bilirubin to 1 mg/dL was seen on day 10 of treatment. On day 15 in the ICU, a normalization of the platelet count was observed. The patient had fully recovered after a 17-day course of glucocorticoids, 12 sessions of PEX and rituximab. Laboratory parameter improvement trends (platelet and hemoglobin level) are displayed in Figure 1. The patient was discharged with a follow up at the hematology department. Prednisone was tapered off over 5 weeks. The patient made a complete recovery and is currently living a normal life. The latest ADAMTS-13 activity at the 6-month follow-up visit showed 94%.
Figure 1.
Platelet count (×109/L) and hemoglobin level (g/dL) trends throughout the course of corticosteroids, plasma exchange and rituximab during the ICU stay and the follow-up period of case 1.
2.2. Case 2
A previously healthy 30-year-old Caucasian, North-African Maghrebian male presented to the emergency department with headache, fever, dysarthria and right hemiparesis. He had received a second dose of an inactivated COVID-19 vaccine CoronaVac, one month prior to consultation. Laboratory findings showed hemoglobin 7.2 g/dL, platelet count, 9 × 109/L, LDH 1268 UI/L, D-dimer 1890 µg/L, haptoglobin 0.26 g/L and creatinine 105 µmol/L. A peripheral blood smear showed schistocytes (2%). Their PLASMIC score was at 5 (range, 0–7). A presumptive diagnosis of TTP was made. The patient was admitted to the ICU. On the initial examination, the patient had a fluctuating consciousness, dysarthria and right hemiparesis without any petechiae or purpura. The brain CT scan revealed no abnormalities. No triggering factors such as viral infections or medication, alcohol or illicit drug use were identified. Infectious screening tests including SARS-CoV-2 were negative. Investigations revealed severe ADAMTS-13 deficiency (<0.2%) with positive anti ADAMTS-13 autoantibodies (12 U/mL). All other autoimmune tests returned negative.
The patient received methylprednisolone 1000 mg daily for three consecutive days followed by prednisone 1 mg/kg/day in combination with daily PEX. Weekly infusion of rituximab for 4 weeks was started two weeks after admission due to issues concerning the patient’s health insurance.
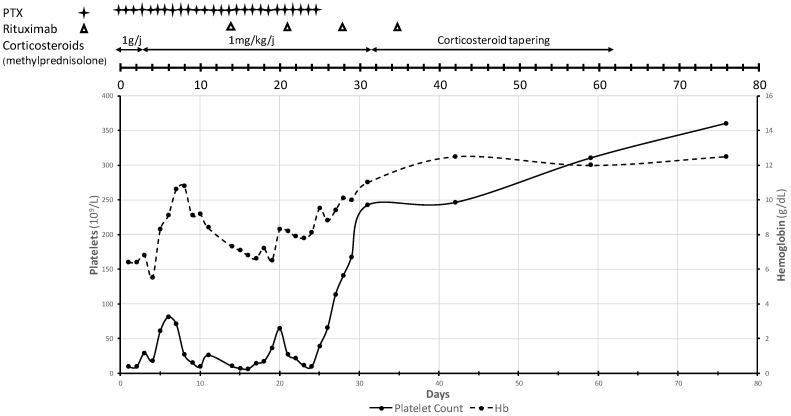
Neurological symptoms resolved gradually after the sixth PEX. However, there was no improvement in platelet count and LDH values, leading to prolongation of PEX therapy in association with rituximab. The laboratory findings showed a complete and sustained response at day 28 of ICU stay. The patient had fully recovered after a 31-day course, which included 26 sessions of PEX (Figure 2). The patient was discharged with hemoglobin at 10 g/dL and platelets at 180 × 109/L with a follow-up at the hematology department. The steroid dose was tapered off over 4 weeks. One month later, the control of activity of ADAMTS-13 was 74%.
Figure 2.
Platelet count (×109/L) and hemoglobin level (g/dL) trends throughout the course of corticosteroids, plasma exchange and rituximab during the ICU stay and the follow-up period of case 2.
3. Discussion
Thrombotic thrombocytopenic purpura (TTP) is a rare blood disorder with an incidence of 3 to 10 cases per million adults per year [16]. It was first described by Eli Moschcowitz in 1924 [18,19]. The pathogenesis of this disorder includes the formation of small-vessel platelet rich thrombi leading to ischemic end organ injury [20]. The historical pentad (fever, hemolytic anemia, thrombocytopenia, neurological or renal dysfunction) is only seen in <10% of the patients [21,22]. Microangiopathic hemolytic anemia (reduced Hb and haptoglobin, increased LDH and presence of schistocytes) and thrombocytopenia are sufficient for presumptive diagnosis of TTP. The PLASMIC score derived by Bendapudi et al. [23] stratifies patients according to their risk of having severe ADAMTS-13 deficiency. When dichotomized at high (score 6–7) vs. low–intermediate risk (score 0–5), the PLASMIC score predicted severe ADAMTS-13 deficiency with positive predictive value at 72%, negative predictive value at 98%, sensitivity 90%, and specificity 92% [24]. A severe reduction in the activity of von Willebrand factor (VWF) cleaving metalloprotease (ADAMTS-13) (less than 10%) and the presence of inhibitory antibodies confirm the diagnosis [20].
TTP can be classified into two types: congenital or acquired (autoimmune TTP). Autoimmune TTP can be triggered by infections, malignancy, pregnancy, medications and vaccines [11,19]. Rarely, some vaccines (e.g., influenza, pneumococcus, rabies and H1N1) have been reported to induce acquired TTP [11,14,21,25,26,27]. Vaccines have been hypothesized to activate the immune system leading to autoantibody formation and hence the development of autoimmune disorders such as TTP [21,28].
Worldwide, in response to the COVID-19 pandemic, several vaccines have been developed using various techniques: messenger RNA (mRNA) (Pfizer-BioNTech [BNT162b2], Moderna and CureVac), human or primate adenovirus vectors (Janssen-Johnson & Johnson [Ad26.COV2-S], Astra-Zeneca [chAdOx1 nCoV-19], Sputnik-V, and CanSino) and an inactivated whole-virus SARS-CoV-2 (Bharat Biotech, Sinopharm and Sinovac) [22]. The emergency use authorization of these vaccines in several countries increased the risk of safety issues [5,7]. In the literature, there have been some reported cases of TTP following Adenoviral Vector DNA- and SARS-CoV-2 mRNA-based COVID-19 vaccines [11,29]. Indeed, vaccines against viral pathogens have been reported to be associated with onset and/or relapse of TTP [30]. This rare autoimmune disease may occur after the first or the second dose of COVID-19 vaccines, typically one to two weeks after vaccination [13].
For TTP, vaccine-induced immune thrombotic thrombocytopenia (VITT) is a differential diagnosis. VITT is another adverse event that has been recently reported after COVID-19 vaccination. It is a novel clinical syndrome demonstrating striking similarities to TTP. VITT is diagnosed clinically by the presence of mild to severe thrombocytopenia, documented evidence or suspicion of thrombosis and positive antibodies against platelet factor 4 (PF4) [31,32,33]. In the present two cases, severely reduced ADAMTS-13 activity and the presence of schistocytes or microangiopathic hemolytic anemia on the blood smear support the diagnosis of TTP. Temporal association and absence of other triggering factors for secondary TTP led to the diagnosis that this disorder was induced by COVID-19 vaccination. The mechanism linking TTP with COVID-19 vaccines is poorly understood [12]. However, it has been well established that, in patients with acquired TTP, deficiency of ADAMTS-13 results from autoimmune inhibitors of the ADAMTS-13 protease. The levels of the ADAMTS-13 inhibitors tend to be low (<10 U/mL), often receding to even lower or undetectable levels within weeks or months. Such characteristics of the ADAMTS-13 inhibitors suggest that the immune response is induced by exposure to exogenous antigens with molecular mimicry to ADAMTS-13 [34]. The two cases were recorded within a two-year-long COVID-19 pandemic; including just one year of active vaccination in Tunisia, in which more than five hundred COVID-19 patients were admitted to a 12-bed medical ICU, along with another 600 non-COVID-19 patients in the same two-year period. This highlights the scarcity of such complications in our hospital.
On 5 April 2022, a personal literature review based on a 2020–2022 PubMed search (key items: “Thrombotic thrombocytopenic purpura” AND “COVID-19 vaccines” AND “case report”) found 19 papers including 32 cases published in English language. Among these studies, TTP was reported as an adverse event of, respectively, Pfizer-BioNTech (n = 24), Moderna (n = 3), Astra-Zeneca (n = 4) and Janssen-Johnson & Johnson (n = 1) (Table 1; Results of the 32 Cases, Published During the 2020–2022 Period, Including Thrombotic Thrombocytopenic Purpura following COVID-19 Vaccination) [11,12,13,14,20,21,22,25,26,28,29,30,35,36,37,38,39,40,41].
Table 1.
Results of the 32 Cases, Published During the 2020–2022 Period, Including Thrombotic Thrombocytopenic Purpura (TTP) following COVID-19 Vaccination.
| Authors and Ref | Country (Year) |
Old Gender | Underlying Disease | First Episode | Symptoms | Vaccine | Biology | ADAMTS 13 Activity | Treatments | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Relapse | Dose | Autoantibody * | ||||||||
| Time after Vaccination | ||||||||||
| Chamarti et al. [20] | USA (2021) |
80 | Hypertension | First | Generalized weakness | Pfizer- BioNTech |
Hemoglobin, 4.8 g/dL | <2% | Plasma Exchange Steroids | Improved |
| Male | Diabetes | Malaise | Second dose | Platelets, 48 × 109/L | 182 U/mL | Rituximab | ||||
| Hyperlipidemia | 14 days | Schistocytes, +++ | ||||||||
| Gout | Creatinine, 212.16 µmol/L | |||||||||
| Iron deficiency | LDH, 1118 UI/L | |||||||||
| Anemia | Haptoglobin, <10 mg/dL | |||||||||
| Giuffrida et al. [14] | Italy (2021) |
83 | Undifferentiated connective tissue disease | First | Severe anemia | Pfizer- BioNTech |
Hemoglobin, 6.1 g/dL | <10% | Plasma Exchange Steroids | Death (probably due to a |
| Female | Diabetes | Macro-hematuria | First dose | Retic, 28% | 40 U/mL | Caplacizumab | sudden cardiovascular event) | |||
| Diffuse petechiae | 7 days | Platelets, 46 × 109/L | ||||||||
| Schistocytes, 10% | ||||||||||
| Creatinine, 77.79 µmol/L | ||||||||||
| LDH, 1905 UI/L | ||||||||||
| Haptoglobin, <7 mg/dL | ||||||||||
| 30 | Beta-thalassemia | First | Diffuse petechiae | Pfizer- BioNTech |
Hemoglobin, 8.9 g/dL | <10% | Plasma Exchange Steroids | Improved | ||
| Female | Intense headache | First dose | Retic, 29% | 77.6 U/mL | Caplacizumab | |||||
| Fatigue | 18 days | Platelets, 11 × 109/L | ||||||||
| Schistocytes, 5–10% | ||||||||||
| Creatinine, 79.56 µmol/L | ||||||||||
| LDH, 900 UI/L | ||||||||||
| Haptoglobin, <7 mg/dL | ||||||||||
| Karabulut et al. [11] | USA (2021) |
48 | No | First | Acute-onset, transient right-sided weakness | Moderna Biotech | Hemoglobin, 8.8 g/dL | <3% | Plasma Exchange Steroids | Improved |
| Male | Slurred speech lasting | First dose | Platelets, 10 × 109/L | 6.6 BEU | Rituximab | |||||
| 5 days | Schistocytes, 2–3% | |||||||||
| Creatinine, 83.98 µmol/L | ||||||||||
| LDH, 884 UI/L | ||||||||||
| Haptoglobin, <10 mg/dL | ||||||||||
| Lee et al. [28] | UK (2021) | 50 | Hypertension | First | Dysphasia | AstraZeneca | Hemoglobin, 9.9 g/dL | 0% | Plasma Exchange Steroids | Improved |
| Female | Acute upper limb numbness | First dose | Retic, 6.9% | 94.93 U/mL | Rituximab | |||||
| 12 days | Platelets, 33 × 109/L | |||||||||
| Schistocytes, + | ||||||||||
| LDH, 359 UI/L | ||||||||||
| Maayan et al. [29] | Israel (2021) |
40 | No | First | Somnolence | Pfizer- BioNTech |
Hemoglobin, 9.9 g/dL | 0% | Plasma Exchange | Improved |
| Female | Fever | Second dose | Platelets, 12 × 109/L | 51 U/mL | Steroids | |||||
| Macroscopic hematuria | 8 days | Schistocytes, 6% | Caplacizumab | |||||||
| Creatinine, 81.35 µmol/L | ||||||||||
| LDH, 7129 UI/L | ||||||||||
| 28 | Morbid obesity | First | Dysarthria | Pfizer- BioNTech |
Hemoglobin, 9.1 g/dL | 0% | Plasma Exchange Steroids | Improved | ||
| Male | Second dose | Platelets, 38 × 109/L | 113 U/mL | Caplacizumab Rituximab | ||||||
| 28 days | Schistocytes, 6% | |||||||||
| Creatinine, 132.63 µmol/L | ||||||||||
| LDH, 3063 UI/L | ||||||||||
| 31 | TTP | Relapse | Vaginal bleeding | Pfizer- BioNTech |
Hemoglobin, 7.7 g/dL | 0% | Plasma Exchange Steroids | Continu caplacizumab | ||
| Female | Purpura | First dose | Platelets, 17 × 109/L | 64 U/mL | Caplacizumab | |||||
| 13 days | Schistocytes, 10% | Rituximab | ||||||||
| Creatinine, 106 µmol/L | ||||||||||
| LDH, 4000 UI/L | ||||||||||
| 30 | TTP | Relapse | Purpura | Pfizer- BioNTech |
Hemoglobin, 8.3 g/dL | 0% | Plasma Exchange Steroids | Improved | ||
| Male | Second dose | Retic, 8% | 21 U/mL | Caplacizumab | ||||||
| 8 days | Platelets, 14 × 109/L | Rituximab | ||||||||
| Schistocytes, 14% | ||||||||||
| Renal function, normal | ||||||||||
| LDH, 1138 UI/L | ||||||||||
| Osmanodja et al. [35] | Germany (2021) |
25 | No | First | Persisting malaise | Moderna Biotech | Hemoglobin, 7.4 g/dL | <5% | Plasma Exchange Steroids | Continu caplacizumab |
| Male | Fever | First dose | Retic, 233.1 109/L | 72.2 U/ml | Caplacizumab | |||||
| Headache | 13 days | Platelets, 29 × 109/L | Rituximab | |||||||
| Word-finding difficulties | Schistocytes, 2.1% | |||||||||
| Nausea, vomiting | Creatinine, 132.6 µmol/L | |||||||||
| Petechial bleeding | LDH, 999 UI/L | |||||||||
| Hematuria | Haptoglobin, <8 mg/dL | |||||||||
| Pavenski et al. [30] | Canada (2021) |
84 | TTP | Relapse | Lethargy | Pfizer- BioNTech |
Hemoglobin, 7.2 g/dL | <1% | Plasma Exchange | Improved |
| Male | Prostate cancer Hypertension Diabetes | Myalgias | First dose | Retic, elevated | >15 U/mL | Steroids | ||||
| Gout | Anorexia | 7 days | Platelets, 58 × 109/L | Rituximab | ||||||
| Hypercholesterolemia | Schistocytes, + | |||||||||
| Creatinine, 77 µmol/L | ||||||||||
| LDH, 594 UI/L | ||||||||||
| Sissa et al. [36] | Italy (2021) | 48 | TTP | Relapse | Ecchymosis | Pfizer- BioNTech |
Hemoglobin, 11.5 g/dL | <3% | Plasma Exchange | Improved |
| Female | Second dose | Platelets, 94 × 109/L | 88 U/mL | Steroids | ||||||
| 6 days | Schistocytes, 10% | |||||||||
| Renal function, normal | ||||||||||
| LDH, 637 UI/L | ||||||||||
| Waqar et al. [22] | USA (2021) |
69 | Hypertension Chronic kidney disease | First | Severe fatigue | Pfizer- BioNTech |
Hemoglobin, 9.3 g/dL | 2% | Plasma Exchange Steroids | Improved |
| Male | HIV | Shortness of breath | Second dose | Retic, 2.8% | >90 U/mL | Rituximab | ||||
| Chronic hepatitis B | 7 days | Platelets, 22 × 109/L | ||||||||
| Deep | Schistocytes, ++ | |||||||||
| vein thrombosis | Creatinine, 177.68 µmol/L | |||||||||
| LDH, 1229 UI/L | ||||||||||
| Yucum et al. [37] | USA (2021) |
62 | Hypertension | first | Acute onset of altered mental status | Johnson and Johnson | Hemoglobin, 8.2 g/dL | <12% | Plasma Exchange Steroids | Improved |
| Female | Hyperlipidemia | First dose | Retic, 8% | NA | Hemodialysis | |||||
| Hypothyroidism | 37 days | Platelets, 11 × 109/L | ||||||||
| Creatinine, 530 µmol/L | ||||||||||
| LDH, >2500 UI/L | ||||||||||
| ASAT/ALAT, 982/231 U/L | ||||||||||
| Al Ahmad et al. [21] | Kuwait (2021) |
37 | Secondary polycythemia | first | Dizziness, fatigue | AstraZeneca-Oxford | Hemoglobin, 8.3 g/dL | 2.60% | Plasma Exchange Steroids | Improved |
| Male | Headache | First dose | Retic, 8% | Positive | Rituximab | |||||
| Shortness of breath | 10 days | Platelets, 14 × 109/L | ||||||||
| Palpitation | Schistocytes, 14% | |||||||||
| Dark urine and petechiae | Renal function, normal | |||||||||
| LDH, 1138 UI/L | ||||||||||
| De Bruijn et al. [25] | Belgium (2021) |
38 | No | First | Spontaneous | Pfizer- BioNTech |
Hemoglobin, 10.5 g/dL | 0% | Plasma Exchange | Improved |
| Female | bruising and petechiae | First dose | Retic, 263 109/L | 106.8 BEU | Steroids | |||||
| 14 days | Platelets, 46 × 109/L | Caplacizumab | ||||||||
| Schistocytes, 3% | Rituximab | |||||||||
| Creatinine, 83.98 µmol/L | ||||||||||
| LDH, 631 UI/L | ||||||||||
| Alislambouli et al. [12] | USA (2022) |
61 | No | First | Confusion | Pfizer- BioNTech |
Hemoglobin, 6.5 g/dL | <3% | Plasma Exchange | Improved |
| Male | Fever | First dose | Retic, 8% | NA | Steroids | |||||
| Headache | 5 days | Platelets, 6 × 109/L | Rituximab | |||||||
| Emesis | Schistocytes, 8% | |||||||||
| Dark urine | LDH, 1757 UI/L | |||||||||
| Leg ecchymosis | Haptoglobin, <8 mg/dL | |||||||||
| Deucher et al. [38] | USA (2022) |
28 | TTP | Relapse | Bruising on arms | Pfizer- BioNTech |
Hemoglobin, 10.5 g/dL | <2.5% | Caplacizumab | Improved |
| Female | First dose | Platelets, 84 × 109/L | Positive | Steroids | ||||||
| 5 days | Schistocytes, ++ | Rituximab | ||||||||
| LDH, 205 UI/L | ||||||||||
| Haptoglobin, undetectable | ||||||||||
| Innao et al. [26] | Italy (2022) |
33 | Hodgkin Lymphoma | First | Asthenia | Pfizer- BioNTech |
Hemoglobin, 6.8 g/dL | 8% | Plasma Exchange | Improved |
| Female | Gray Zone Lymphoma | Drowsiness | First dose | Retic, 896 × 109/L | 5 U/mL (not valuable due to defects in the sample) | Steroids | ||||
| Headache | 9 days | Platelets, 12 × 109/L | Caplacizumab | |||||||
| Nausea | Schistocytes, 3% | |||||||||
| Abdominal pain | Creatinine, 122 µmol/L | |||||||||
| Lower extremity purpura | LDH, 1280 UI/L | |||||||||
| Haptoglobin, <6 mg/dL | ||||||||||
| Kirpalani et al. [39] | Japan (2022) |
14 | Anxiety | First | Fatigue | Pfizer- BioNTech |
Hemoglobin, 6.3 g/dL | <1% | Plasma Exchange Steroids | Improved |
| Female | Iron | Headache | First dose | Platelets, 10 × 109/L | 72 U/mL | Caplacizumab | ||||
| Deficiency | Confusion | 14 days | Schistocytes, + | Rituximab | ||||||
| Bruising | LDH, 626 UI/L | |||||||||
| Haptoglobin, <10 mg/dL | ||||||||||
| Ruhe et al. [40] | Germany (2022) | 84 | No | First | Partial hemiplegia | Pfizer- BioNTech |
Hemoglobin, 7.9 g/dL | 1.60% | Plasma Exchange Steroids | Improved |
| Female | Scattered petechiae | First dose | Platelets, 45 × 109/L | 82.2 U/mL | Rituximab | |||||
| 16 days | Schistocytes, 4.2% | |||||||||
| Creatinine, 172.38 µmol/L | ||||||||||
| Haptoglobin, <10 mg/dL | ||||||||||
| Yoshida et al. [13] | Japan (2022) |
57 | Acute hepatitis of unknown cause | First | Fatigue | Pfizer- BioNTech |
Hemoglobin, 5.5 g/dL | <0.5% | Plasma Exchange Steroids | Improved |
| Male | Loss of appetite | First dose | Retic, 496 × 109/L | 1.9 BU/mL | Rituximab | |||||
| Jaundice | 7 days | Platelets, 9 × 109/L | ||||||||
| Schistocytes, 17.6% | ||||||||||
| Creatinine, 138.87 µmol/L | ||||||||||
| LDH, 2275 UI/L | ||||||||||
| Haptoglobin, 3 mg/dL | ||||||||||
| Picod et al. [41] | France (2022) |
36 | Systemic lupus erythematosus | First | Bruising | Pfizer- BioNTech |
Hemoglobin, 10 g/dL | <5% | Plasma Exchange Steroids | Improved |
| Female | Headache | First dose | Platelets, 10 × 109/L | 0.5 BU/mL | Rituximab | |||||
| 6 days | Schistocytes, 3% | |||||||||
| Creatinine, 86.24 µmol/L | ||||||||||
| 54 | TTP | Relapse | Bruising | Moderna Biotech |
Hemoglobin, 11.5 g/dL | <5% | Plasma Exchange Steroids | Improved | ||
| Male | Diffuse | First dose | Platelets, 17 × 109/L | 1.1 BU/mL | Rituximab Caplacizumab | |||||
| mucocutaneous | First dose | Schistocytes, 2% | ||||||||
| bleeding | 23 days | Creatinine, 149.6 µmol/L | ||||||||
| Headache | ||||||||||
| Amnesia | ||||||||||
| 60 | TTP | Relapse | Cerebellar | Pfizer- BioNTech |
Hemoglobin, 10.8 g/dL | <10% | Plasma Exchange Steroids | Improved | ||
| Female | Syndrome | First dose | Platelets, 27 × 109/L | Positive | Rituximab | |||||
| 10 days | Schistocytes, 2% | |||||||||
| Creatinine, 66.88 µmol/L | ||||||||||
| 60 | No | First | Cerebellar | Pfizer- BioNTech |
Hemoglobin, 6.5 g/dL | 5% | Plasma Exchange Steroids | Improved | ||
| Female | Syndrome | First dose | Platelets, 20 × 109/L | 52 U/mL | Caplacizumab | |||||
| Aphasia | 12 days | Schistocytes, 6% | ||||||||
| Confusion | Creatinine, 80.96 µmol/L | |||||||||
| Chest pain | ||||||||||
| 38 | No | First | Fever | Pfizer- BioNTech |
Hemoglobin, 6.6 g/dL | <1% | Plasma Exchange Steroids | Improved | ||
| Male | Headache | Second dose | Platelets, 9 × 109/L | Positive | Rituximab Caplacizumab | |||||
| Hemiparesis | 30 days | Schistocytes, 5% | ||||||||
| Bruising | Creatinine, 88.88 µmol/L | |||||||||
| 68 | Mixed connective tissue disease | Relapse | Dizziness | Pfizer- BioNTech |
Hemoglobin, 10.9 g/dL | 2% | Plasma Exchange Steroids | Improved | ||
| Male | TTP | First dose | Platelets, 39 × 109/L | - | Rituximab Caplacizumab | |||||
| 17 days | Schistocytes, 1% | |||||||||
| Creatinine, 69.52 µmol/L | ||||||||||
| 66 | No | First | Facial paralysis | AstraZeneca-Oxford | Hemoglobin, 7.9 g/dL | <5% | Plasma Exchange | Improved | ||
| Male | First dose | Platelets, 11 × 109/L | - | Steroids | ||||||
| 8 days | Schistocytes, 4% | Rituximab | ||||||||
| Creatinine, 81.84 µmol/L | Caplacizumab | |||||||||
| 70 | Ischemic strokes | First | Coma | AstraZeneca-Oxford | Hemoglobin, 8 g/dL | 11% | Intravenous Immunoglobulins Plasma Infusion Steroids | Death 2 month | ||
| Female | Hypertension | Hemiparesis | First dose | Platelets, 6 × 109/L | 140 U/mL | Rituximab Caplacizumab | after presentation | |||
| 10 days | Schistocytes, 2% | |||||||||
| Creatinine, 79.2 µmol/L | ||||||||||
| 22 | No | First | Coma | Pfizer- BioNTech |
Hemoglobin, 6.8 g/dL | 6% | Plasma Exchange Steroids | Improved | ||
| Male | Seizures | Second dose | Platelets, 10 × 109/L | Positive | Rituximab | |||||
| Purpura | 18 days | Schistocytes, 2% | ||||||||
| Fever | Creatinine, 101.2 µmol/L | |||||||||
| 20 | Systemic lupus erythematosus | First | Systemic lupus erythematosus Flare | Pfizer- BioNTech |
Hemoglobin, 5.3 g/dL | <10% | Plasma Infusion Steroids | Improved | ||
| Female | Polyarthritis | First dose | Platelets, 51 × 109/L | 50 U/mL | ||||||
| Erythema | 25 days | Schistocytes, 3% | ||||||||
| Creatinine, 88 µmol/L |
* Autoantibodies to ADAMTS-13 was assessed either as the titer of total autoantibodies with a simplified enzyme-linked immunosorbent assay (ELISA) and expressed in arbitrary units (U/mL; normal < 12 U/mL) or as the titer of inhibitory antibodies using an alternative methodology (Bethesda assay) expressed in Bethesda Units (BU/mL; normal < 1 BU/mL) or BEU (normal < 0.4). NA: Not available. Retic, reticulocytes; LDH, Lactate dehydrogenase; +++, semi-quantitative appreciation of schistocytes.
The strength of the present study is that this is the first report of acquired TTP after inactivated virus COVID-19 vaccination. Vaccination status, vaccine name and date of doses were verified by checking the patients’ vaccination certificate in the national register of vaccination (Government’s EVAX website). In the current cases, TTP occurred 20 days after the first dose of Sinopharm and 30 days after second dose of CoronaVac. The two cases were reported to the regional pharmacovigilance center.
The present study has some limitations. First, it is a case report of only two patients. Second, addressing the question of possible prior SARS-CoV-2 infection was very difficult to prove definitely, unless checking for seroconversion, which could also result from the vaccine. In the present two cases, the causality relationship between the TTP and the vaccine was made very probable on a bundle of anamnestic, clinical and laboratory arguments and the chronology between vaccination and the onset of symptoms. Third, the short review was not a systematic one and used only one database.
Healthcare workers involved in COVID-19 vaccination programs need to educate the recipients of the COVID-19 vaccines about the possible adverse events. Careful clinical auto-surveillance must be conducted in the post-vaccine period. There are currently no recommended screenings for TTP when a patient has no signs or symptoms. However, clinicians should consider the possibility of TTP when evaluating thrombocytopenia following vaccination. Without the prompt initiation of adequate treatment, TTP is a life-threatening thrombotic microangiopathy. It is a medical emergency requiring rapid diagnosis and treatment, usually in intensive care units. According to the International Society of Thrombosis and Haemostasis, PEX represents the cornerstone of TTP treatment with strong recommendation for adding corticosteroids [28,42]. Rituximab (a monoclonal anti-CD20 antibody) and Caplacizumab (an anti-VWF antibody fragment) can improve TTP outcomes and decrease the duration of PEX. Caplacizumab is not yet available worldwide, and it has a significant cost [43].
4. Conclusions
This report highlights potential safety issues that can be encountered after COVID-19 vaccination. The benefits of vaccination in fighting the ongoing pandemic outweigh the risk of side effects. Additional surveillance is required in the post-vaccine period to detect adverse events in a timely fashion. TTP is a very rare life-threatening complication of COVID-19 vaccination. It is a medical emergency that is almost always fatal if adequate treatment is not initiated early. Further research should be conducted to correctly identify the mechanism linking thrombotic microangiopathic disorders with COVID-19 vaccines.
Author Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: I.B.S., I.M., R.T., E.B., H.B.I. and M.B.; Drafting the article or revising it critically for intellectual content: I.B.S., I.M., R.T., E.B., C.B.S. and M.B.; Final approval of the version to be published: I.B.S., I.M., R.T., E.B., H.B.I., C.B.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research and Ethics Committee of Farhat Hached University Hospital (ID number of the approval: CER 10-2022).
Informed Consent Statement
Written informed consent has been obtained from the two patients to publish this paper.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon a reasonable request.
Conflicts of Interest
All the authors certify that they have no affiliations with/or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saida I.B., Ennouri E., Nachi R., Meddeb K., Mahmoud J., Thabet N., Jerbi S., Boussarsar M. Very Severe COVID-19 in the Critically Ill in Tunisia. Pan Afr. Med. J. 2020;35 doi: 10.11604/pamj.supp.2020.35.2.24753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaghoubi A., Amel Jamehdar S., Movaqar A., Milani N., Soleimanpour S. An Effective Drug against COVID-19: Reality or Dream? Expert Rev. Respir. Med. 2021;15:505–518. doi: 10.1080/17476348.2021.1854092. [DOI] [PubMed] [Google Scholar]
- 4.Yadav T., Srivastava N., Mishra G., Dhama K., Kumar S., Puri B., Saxena S.K. Recombinant Vaccines for COVID-19. Hum. Vaccines Immunother. 2020;16:2905–2912. doi: 10.1080/21645515.2020.1820808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.-C., Chen I.-T., Chao C.-M., Lee P.-I., Ko W.-C., Hsueh P.-R. COVID-19 Vaccines: Concerns beyond Protective Efficacy and Safety. Expert Rev. Vaccines. 2021;20:1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 6.Al Khames Aga Q.A., Alkhaffaf W.H., Hatem T.H., Nassir K.F., Batineh Y., Dahham A.T., Shaban D., Al Khames Aga L.A., Agha M.Y.R., Traqchi M. Safety of COVID-19 Vaccines. J. Med. Virol. 2021;93:6588–6594. doi: 10.1002/jmv.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros-Ribeiro A.C., Aikawa N.E., Saad C.G.S., Yuki E.F.N., Pedrosa T., Fusco S.R.G., Rojo P.T., Pereira R.M.R., Shinjo S.K., Andrade D.C.O., et al. Immunogenicity and Safety of the CoronaVac Inactivated Vaccine in Patients with Autoimmune Rheumatic Diseases: A Phase 4 Trial. Nat. Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 8.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., Pletcher M.J., Marcus G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA. 2021;4:e2140364. doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcec R., Likic R. Postvaccination Anaphylaxis and MRNA-Based SARS-CoV-2 Vaccines—Much Ado about Nothing? Br. J. Clin. Pharmacol. 2021;87:3632–3633. doi: 10.1111/bcp.14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jęśkowiak I., Wiatrak B., Grosman-Dziewiszek P., Szeląg A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines. 2021;9:502. doi: 10.3390/vaccines9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karabulut K., Andronikashvili A., Kapici A.H. Recurrence of Thrombotic Thrombocytopenic Purpura after MRNA-1273 COVID-19 Vaccine Administered Shortly after COVID-19. Case Rep. Hematol. 2021;2021:4130138. doi: 10.1155/2021/4130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alislambouli M., Veras Victoria A., Matta J., Yin F. Acquired Thrombotic Thrombocytopenic Purpura Following Pfizer COVID-19 Vaccination. Haematologica. 2022;3:207–210. doi: 10.1002/jha2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida K., Sakaki A., Matsuyama Y., Mushino T., Matsumoto M., Sonoki T., Tamura S. Acquired Thrombotic Thrombocytopenic Purpura Following BNT162b2 MRNA Coronavirus Disease Vaccination in a Japanese Patient. Intern. Med. 2022;61:407–412. doi: 10.2169/internalmedicine.8568-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuffrida G., Condorelli A., Di Giorgio M.A., Markovic U., Sciortino R., Nicolosi D., Di Raimondo F. Immune-Mediated Thrombotic Thrombocytopenic Purpura Following Pfizer-BioNTech COVID-19 Vaccine. Haematologica. 2021;107:1008–1010. doi: 10.3324/haematol.2021.279535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully M., Hunt B.J., Benjamin S., Liesner R., Rose P., Peyvandi F., Cheung B., Machin S.J., on behalf of British Committee for Standards in Haematology Guidelines on the Diagnosis and Management of Thrombotic Thrombocytopenic Purpura and Other Thrombotic Microangiopathies. Br. J. Haematol. 2012;158:323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- 16.Reese J.A., Muthurajah D.S., Hovinga J.A.K., Vesely S.K., Terrell D.R., George J.N. Children and Adults with Thrombotic Thrombocytopenic Purpura Associated with Severe, Acquired Adamts13 Deficiency: Comparison of Incidence, Demographic and Clinical Features. Pediatr. Blood Cancer. 2013;60:1676–1682. doi: 10.1002/pbc.24612. [DOI] [PubMed] [Google Scholar]
- 17.Sessler C.N., Gosnell M.S., Grap M.J., Brophy G.M., O’Neal P.V., Keane K.A., Tesoro E.P., Elswick R.K. The Richmond Agitation-Sedation Scale: Validity and Reliability in Adult Intensive Care Unit Patients. Am. J. Respir. Crit. Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 18.MOSCHCOWITZ E. Hyaline Thrombosis of the Terminal Arterioles and Capillaries: A Hitherto Undescribed Disease. Proc. N. Y. Pathol. Soc. 1924;24:21–24. [Google Scholar]
- 19.Knöbl P. Thrombotic Thrombocytopenic Purpura. Memo. 2018;11:220–226. doi: 10.1007/s12254-018-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamarti K., Dar K., Reddy A., Gundlapalli A., Mourning D., Bajaj K. Thrombotic Thrombocytopenic Purpura Presentation in an Elderly Gentleman Following COVID Vaccine Circumstances. Cureus. 2021;13:e16619. doi: 10.7759/cureus.16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Ahmad M., Al-Rasheed M., Shalaby N.A.B. Acquired Thrombotic Thrombocytopenic Purpura with Possible Association with AstraZeneca-Oxford COVID-19 Vaccine. EJHaem. 2021;2:534–536. doi: 10.1002/jha2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waqar S.H.B., Khan A.A., Memon S. Thrombotic Thrombocytopenic Purpura: A New Menace after COVID Bnt162b2 Vaccine. Int. J. Hematol. 2021;114:626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bendapudi P.K., Hurwitz S., Fry A., Marques M.B., Waldo S.W., Li A., Sun L., Upadhyay V., Hamdan A., Brunner A.M., et al. Derivation and External Validation of the PLASMIC Score for Rapid Assessment of Adults with Thrombotic Microangiopathies: A Cohort Study. Lancet Haematol. 2017;4:e157–e164. doi: 10.1016/S2352-3026(17)30026-1. [DOI] [PubMed] [Google Scholar]
- 24.Li A., Khalighi P.R., Wu Q., Garcia D.A. External Validation of the PLASMIC Score: A Clinical Prediction Tool for Thrombotic Thrombocytopenic Purpura Diagnosis and Treatment. J. Thromb. Haemost. 2018;16:164–169. doi: 10.1111/jth.13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruijn S., Maes M., de Waele L., Vanhoorelbeke K., Gadisseur A. First Report of a de Novo ITTP Episode Associated with an MRNA-based Anti-COVID-19 Vaccination. J. Thromb. Haemost. 2021;19:2014–2018. doi: 10.1111/jth.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Innao V., Urso S., Insalaco M., Borraccino A., Consoli U. Immune Thrombotic Thrombocytopenic Purpura Following Pfizer-BioNTech Anti-COVID-19 Vaccination in a Patient Healed from Lymphoma after Allogeneic Hematopoietic Stem Cell Transplantation. Thromb. Res. 2022;210:91–93. doi: 10.1016/j.thromres.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivieri B., Betterle C., Zanoni G. Vaccinations and Autoimmune Diseases. Vaccines. 2021;9:815. doi: 10.3390/vaccines9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H.P., Selvaratnam V., Rajasuriar J.S. Thrombotic Thrombocytopenic Purpura after ChAdOx1 NCoV-19 Vaccine. BMJ Case Rep. 2021;14:e246049. doi: 10.1136/bcr-2021-246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maayan H., Kirgner I., Gutwein O., Herzog-Tzarfati K., Rahimi-Levene N., Koren-Michowitz M., Blickstein D. Acquired Thrombotic Thrombocytopenic Purpura: A Rare Disease Associated with BNT162b2 Vaccine. J. Thromb. Haemost. 2021;19:2314–2317. doi: 10.1111/jth.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavenski K. Relapse of Immune Thrombotic Thrombocytopenic Purpura Following Vaccination with COVID19 MRNA Vaccine. TH Open. 2021;5:e335–e337. doi: 10.1055/s-0041-1732342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klok F.A., Pai M., Huisman M.V., Makris M. Vaccine-Induced Immune Thrombotic Thrombocytopenia. Lancet Haematol. 2022;9:e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aleem A., Nadeem A.J. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Coronavirus (COVID-19) Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) [PubMed] [Google Scholar]
- 33.Tews H.C., Driendl S.M., Kandulski M., Buechler C., Heiss P., Stöckert P., Heissner K., Paulus M.G., Kunst C., Müller M., et al. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia with Venous Thrombosis, Pulmonary Embolism, and Adrenal Haemorrhage: A Case Report with Literature Review. Vaccines. 2022;10:595. doi: 10.3390/vaccines10040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai H.-M. Pathophysiology of Thrombotic Thrombocytopenic Purpura. Int. J. Hematol. 2010;91:1–19. doi: 10.1007/s12185-009-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmanodja B., Schreiber A., Schrezenmeier E., Seelow E. First Diagnosis of Thrombotic Thrombocytopenic Purpura after SARS-CoV-2 Vaccine—Case Report. BMC Nephrol. 2021;22:411. doi: 10.1186/s12882-021-02616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sissa C., Al-Khaffaf A., Frattini F., Gaiardoni R., Mimiola E., Montorsi P., Melara B., Amato M., Peyvandi F., Franchini M. Relapse of Thrombotic Thrombocytopenic Purpura after COVID-19 Vaccine. Transfus. Apher. Sci. 2021;60:103145. doi: 10.1016/j.transci.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yocum A., Simon E.L. Thrombotic Thrombocytopenic Purpura after Ad26.COV2-S Vaccination. Am. J. Emerg. Med. 2021;49:441.e3–441.e4. doi: 10.1016/j.ajem.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deucher W., Sukumar S., Cataland S.R. Clinical Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura Following COVID-19 Vaccination. Res. Pract. Thromb. Haemost. 2022;6:e12658. doi: 10.1002/rth2.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirpalani A., Garabon J., Amos K., Patel S., Sharma A.P., Ganesan S.L., Barton M., Cacciotti C., Leppington S., Bakovic L., et al. Thrombotic Thrombocytopenic Purpura Temporally Associated with BNT162b2 Vaccination in an Adolescent Successfully Treated with Caplacizumab. Br. J. Haematol. 2022;196:e11–e14. doi: 10.1111/bjh.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruhe J., Schnetzke U., Kentouche K., Prims F., Baier M., Herfurth K., Schlosser M., Busch M., Hochhaus A., Wolf G. Acquired Thrombotic Thrombocytopenic Purpura after First Vaccination Dose of BNT162b2 MRNA COVID-19 Vaccine. Ann. Hematol. 2022;101:717–719. doi: 10.1007/s00277-021-04584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picod A., Rebibou J.-M., Dossier A., Cador B., Ribes D., Vasco-Moynet C., Stephan C., Bellal M., Wynckel A., Poullin P., et al. Immune-Mediated Thrombotic Thrombocytopenic Purpura Following COVID-19 Vaccination. Blood. 2022;139:2565–2569. doi: 10.1182/blood.2021015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X.L., Vesely S.K., Cataland S.R., Coppo P., Geldziler B., Iorio A., Matsumoto M., Mustafa R.A., Pai M., Rock G., et al. ISTH Guidelines for Treatment of Thrombotic Thrombocytopenic Purpura. J. Thromb. Haemost. 2020;18:2496–2502. doi: 10.1111/jth.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanlon A., Metjian A. Caplacizumab in Adult Patients with Acquired Thrombotic Thrombocytopenic Purpura. Ther. Adv. Hematol. 2020;11:2040620720902904. doi: 10.1177/2040620720902904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon a reasonable request.