Abstract
Metabolic syndrome is a multifactorial disorder characterized by hyperglycemia, hyperlipidemia, obesity, and hypertension risk factors. Moreover, metabolic syndrome is the most ordinary risk factor for cardiovascular disease (CVD). Numerous chemical drugs are being synthesized to heal metabolic risk factors. Still, due to their abundant side effects, herbal medicines have a vital role in the treatment of these abnormalities. Ginger (Zingiber officinale Roscoe, Zingiberaceae) plant has been traditionally used in medicine to treat disorders, including CVD. The unique ginger properties are attributed to the presence of [6]-gingerol, [8]-gingerol, [10]-gingerol, and [6]-shogaol, which through different mechanisms can be beneficial in metabolic syndrome. Ginger has a beneficial role in metabolic syndrome treatment due to its hypotensive, anti‐obesity, hypoglycemic, and hypolipidemic effects. It can significantly reduce atherosclerotic lesion areas, VLDL and LDL cholesterol levels, and elevate adenosine deaminase activity in platelet and lymphocytes. Also, it promotes ATP/ADP hydrolysis. In the current article review, the critical properties of ginger and its constituents’ effects on the metabolic syndrome with a special focus on different molecular and cellular mechanisms have been discussed. This article also suggests that ginger may be introduced as a therapeutic or preventive agent against metabolic syndrome after randomized clinical trials.
Key Words: Diabetes, Dyslipidemia, Ginger, Hypertension, Metabolic syndrome, Obesity, Zingiber
Introduction
Metabolic syndrome is a complicated disorder coming from an unhealthy diet and low physical activity (1), which is a major public-health challenge worldwide and is a notable cardiovascular risk factor (2). Besides, obesity (3), dyslipidemia (4), hyperglycemia (5), hypertension (6), and insulin resistance (7) are the most accepted unifying theories explaining the metabolic syndrome pathophysiology.
According to the National Cholesterol Education Program (NCEP) criteria, metabolic syndrome diagnosis demands at least three of the following factors to be present: central obesity or abdominal obesity: waist outline > 102 cm and > 89 cm in males and females, respectively (or body mass index (BMI) > 30 kg/m2), elevated plasma triglyceride (TG) ≥150 mg/dL, reduced high-density lipoprotein cholesterol (HDL) < 40 mg/dL or < 50 mg/dL in males and females, respectively; high blood pressure ≥130/85 mmHg, and fast blood sugar (FBS) of 110–125 mg/dl (8).
Cardiovascular disease (CVD) is the most prevalent cause of human morbidity and mortality worldwide and metabolic risk factors for CVD include diabetes, high low-density lipoprotein (LDL)-cholesterol, hypertension, and obesity (9). Therefore, different efforts have been made to inhibit and cure metabolic syndrome regarding the prevention of CVD.
Herbal medicines have been used by patients with CVD around the world, for their significant preventive and therapeutic effects (10). In this regard, different studies have reported the effects of numerous plants and their active constituents in metabolic syndrome, including saffron (11), cinnamon (12), garlic (13), grapes (14), avocado (15), rosemary (16), Chinese hawthorn (17), etc.
Ginger (Zingiber officinale Roscoe, Zingiberaceae), a medicinal plant belonging to the Zingiberaceae family, was classified by an English botanist, William Roscoe (18). Ginger with perpetual tuber or rhizome roots is cultivated naturally in Southern Asia and grows in subtropical and tropical areas (19, 20). It has been used in diet-induced metabolic disorders (19, 21, 22) and also safely in cooking and folk medicine (23). Besides, ginger is used in the treatment of arthritis (24), nonalcoholic fatty liver disease (25), primary dysmenorrhea (26), and nausea caused by pregnancy (27) and chemotherapy (28) in traditional Chinese medicine and the Indian ayurvedic system of medicine.
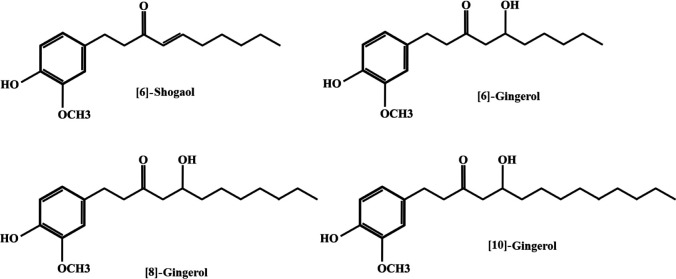
Rhizomes of dried ginger consist of about 6% cellulose, 8–9% fat, 9% protein, 70% carbohydrate, and 4.5% ash (20). Its main non-volatile components include [6]-gingerol, [8]-gingerol, [10]-gingerol, [6]-shogaol, which are responsible for its pharmacological activities (29, 30). The chemical structures of ginger constituents have been shown in Figure 1. Ginger and its active components are strong anti-oxidant agents (31) and have remarkable effects in the treatment of metabolic syndrome abnormalities (32, 33).
Figure 1.
The chemical structures of ginger constituents
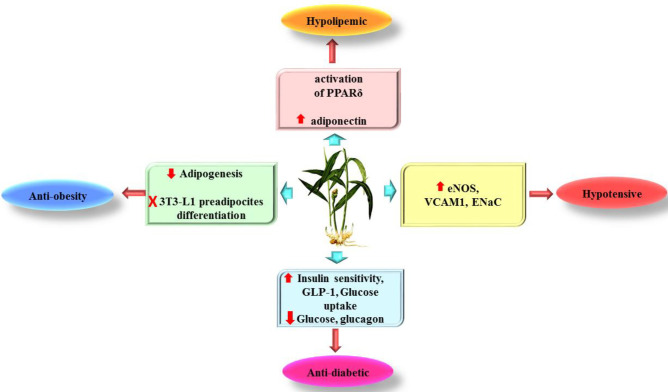
In the current article, the effects of ginger and its active ingredients in metabolic syndrome risk factors, including hypertension, obesity, hyperglycemia, and hyperlipidemia, were reviewed. The role of ginger and its components in metabolic syndrome have been presented in Figure 2.
Figure 2.
Schematic effects of ginger and its active constituents in metabolic syndrome
Methology
The search for the studies published was conducted in 4 databases or search engines (PubMed, Web of Science, Google Scholar, and Scopus). The literature published using the following keywords: Metabolic syndrome, Ginger; Zingiber; Diabetes; Dyslipidemia; Obesity, and Hypertension were selected and reviewed.
Effect on diabetes
Numerous clinical trials have reported that metabolic syndrome is a strong predictor of diabetes incidence (34, 35). Insulin resistance is the main feature of metabolic syndrome leading to type 2 diabetes development (36). The complications attributed to diabetes, including atherosclerosis (37), retinopathy (38), nephropathy (39), and neuropathy persist as significant causes of morbidities and mortalities worldwide (40).
The hypoglycemic properties of ginger and its constituents have been mentioned in various studies. In a study, it has been shown that treatment with 200 mg/kg ethanolic ginger extract ([6]-gingerol) for ten weeks can develop insulin sensitivity in a high-fat high-carbohydrate diet-fed rat model. Thus, insulin resistance can be prevented by [6]-gingerol (41).
In another study, the aqueous extracts of ginger were administered for six weeks (200 and 400 mg/kg- oral administration) in Sprague-Dawley rats. In this study, ginger diminished the blood glucose level and raised insulin plasma levels in obese diabetic rats (42). Similarly, the oral administration of [6]-gingerol (200 mg/kg for 28 days), induced hypoglycemia in type 2 diabetic mice, restored the interrupted endocrine factors and modulated insulin secretion in rodents (43).
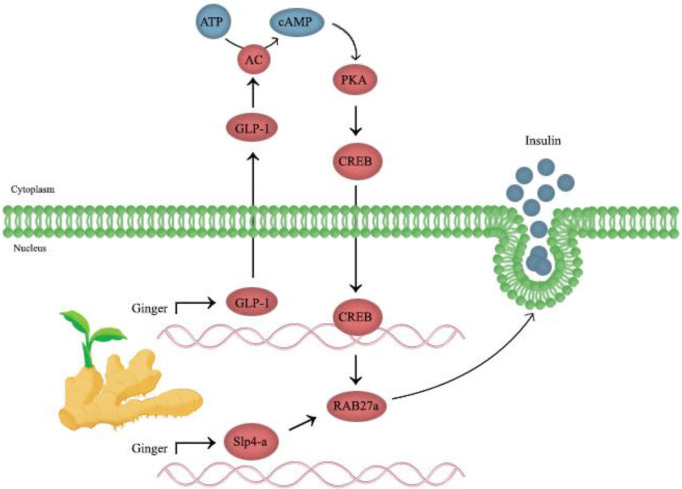
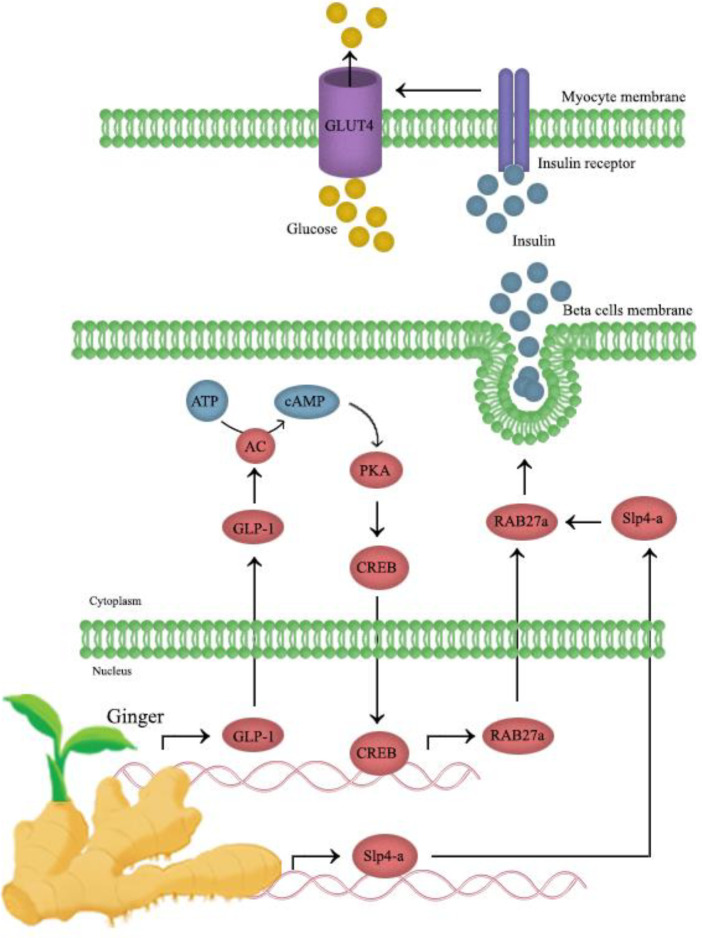
Glucagon-like peptide 1 (GLP-1) is a gut hormone released by the enteroendocrine cell that has a crucial role in stimulating insulin and suppressing glucagon release, preventing gastric depletion, and lowering appetite (44, 45). It is investigated that GLP-1 levels can be regulated through the [6]-gingerol effect on insulin release. Mechanistically, [6]-gingerol upregulates and activates cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), and cAMP response element-binding protein (CREB) in the pancreatic islets, which are major ingredients of the GLP-1-mediated insulin secretion pathway. [6]-Gingerol can upregulate both Rab27a GTPase and Slp4-a expression in pancreatic islets; Also, it improves the exocytosis of insulin-containing secretory granules. [6]-Gingerol can develop glycogen storage in muscles through arising glycogen synthase one (GYS1) activity. Moreover, there are plenty of GLUT4 transporters in the skeletal myocytes membrane, which can be explained by elevating Rab8 and Rab10 GTPases expression that is responsible for GLUT4 exocytosis to the membrane (Figure 3). Therefore, GLP-1 is regulated by the insulinotropic activity of [6]-gingerol and [6]-gingerol treatment. It promotes glucose distribution in skeletal muscles by improving GYS1 activity and boosting the presentation of GLUT4s in the cell membrane (43). The effect of ginger on glucose consumption by myocytes has been shown in Figure 4.
Figure 3.
The role of ginger on insulin release
Figure 4.
Effects of ginger on glucose consumption by skeletal myocytes
The effective role of [6]-gingerol in mouse skeletal muscle C2C12 cells was investigated in other research. [6]-Gingerol has a critical impact on glucose metabolism through the potentiation of insulin-mediated glucose regulation (46).
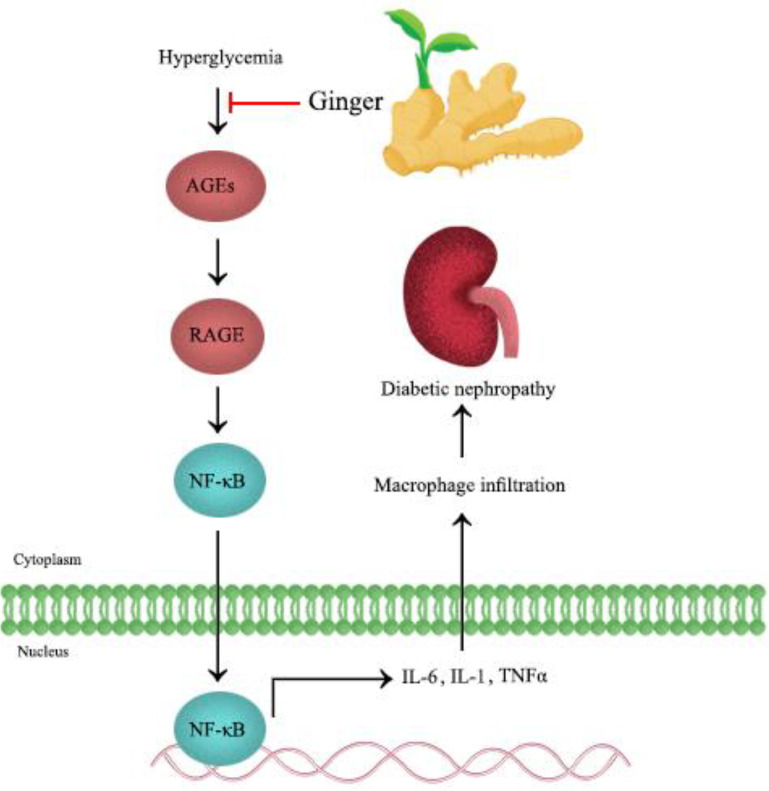
Studies have shown that prolonged hyperglycemia can activate advanced glycation end-products (AGEs) formation and main di-carbonyl compounds levels, including methylglyoxal or glyoxal (the major originators of AGEs and N-carboxymethyl-lysine (CML)) which are significantly higher in diabetic patients (47, 48). Besides, Sampath et al. have investigated the administration of phloretin derived from apple and [6]-gingerol (intraperitoneal (IP) administration) for 17 weeks at two different doses (25 mg/kg and 75 mg/kg) to C57BL/6 mice on a high-fat diet reduced blood sugar, alanine aminotransferase (ALT), aspartate aminotransferase (ASP), AGEs and insulin concentrations. Also, it can reduce AGEs and CML levels via the Nrf2 (nuclear factor erythroid-2-related-factor-2) pathway, elevating the GSH/GSSG ratio, heme oxygenase-1, and glyoxalase 1 in the liver. So, phloretin and [6]-gingerol can attenuate diabetes-induced complications (49).
The comparison of glucose-burning effects of gingerol, shogaol, paradol, and zingerone represented that 6-shogaol and 6-paradol have substantial effects on agitating glucose consumption by 3T3-L1 adipocytes and C2C12 myotubes. Furthermore, 6-paradol, a metabolite of 6-shogaol, lowered blood glucose in the high-fat diet mouse models through oral administration of 2 different low doses (6.75 mg/kg/d) and high doses (33/75 mg/kg/d) for 30 consecutive days. Therefore, 6-paradol can be considered as an active hypoglycemic constituent of ginger (50). The effects of ginger on prolonged hyperglycemia have been presented in Figure 5.
Figure 5.
Effects of ginger on hyperglycemia
Also, ginger may have better hypoglycemic effects in combination with cinnamon (42), garlic (Allium sativum) (51), and clove (52).
It has been proven that ginger can treat type 2 diabetes complications, including nephropathy and neuropathy (53, 54). One notable mechanism involved in these complications is oxidative stress (40). So, ginger may have beneficial effects on type 2 diabetes complications due to its anti-oxidant activity. For example, in a study, rats with type 2 diabetes were treated with 400 or 800 mg/kg/d of ginger extract for six weeks (oral administration). Ginger improved hyperglycemia, hyperlipidemia, and kidney functions in diabetic animal models. Also, it reduced the histological variations in the diabetic rat’s kidney. Chronic hyperglycemia led to a considerable elevation in malondialdehyde (MDA), protein carbonyl, proinflammatory cytokines, cytochrome C, and caspase-3 levels in the rat’s kidney. Ginger extract attenuated oxidative stress, inflammation, and apoptosis; also, it boosted anti-oxidant defenses in the diabetic kidney. Thus, ginger extract is considered for a protective role against diabetic renal injuries through inhibition of oxidative stress, inflammation, and apoptosis (53). Moreover, Mata-Bermudez et al. study suggested that [6]-gingerol in neuropathic rats enhances antiallodynic effects which are mediated by the serotoninergic system including the 5-hydroxytryptamine receptors (5-HT1A/1B/1D/5A receptors) activation. These receptors mediate both excitatory and inhibitory neurotransmissions (54). Another study demonstrated that consuming ginger (5% of daily food for eight weeks) resulted in a reduction of lipid peroxidation, renal nephropathy, and elevation of plasma anti-oxidant capacity in Wistar rats (55).
On the other hand, diabetic retinopathy is a common complication of diabetes (56). Zerumbone (ZER), a compound derived from the rhizomes of zingiber zerumbet, was introduced as a retinal protective agent (57). Tzeng et al. treated STZ-diabetic rats with ZER (40 mg/kg/d- oral administration) for eight weeks; then, they claimed the administration of ZER reduces blood glucose and HbA1C. Also, ZER downregulated AGEs levels and their receptors in retinal cells of diabetic rats. Moreover, ZER attenuated the upregulation of tumor necrosis factor (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) induced by diabetes. Furthermore, it ameliorated the overexpression of vascular endothelial growth factor (VEGF) and intercellular adhesion molecule-1 (ICAM-1); and reduced nuclear factor (NF)-κB activity and apoptosis in rats’ retinal cells. Thus, ZER may have a protective role in retinas due to its anti-inflammatory activity (58).
Furthermore, the hypoglycemic and blood insulin levels increase under the influence of ginger and its active constituents have been demonstrated in a variety of clinical trial studies. In a randomized clinical trial, three-month oral administration of ginger supplementation (3 g/d) in patients with type 2 diabetes, decreased blood glucose concentration, total anti-oxidant capacity, and paraoxonase-1 (PON-1) activity (59).
Makhdoomi Arzati et al. in a clinical trial study indicated that ginger supplementations (2000 mg/d for ten weeks - oral administration) could diminish FBS and hemoglobin A1C (HbA1C) concentrations in type 2 diabetic patients; but, there were no changes in the levels of TG, total cholesterol, LDL, and HDL (60).
In this regard, another clinical trial research identified that daily receiving three 1g microcrystalline-containing capsules of ginger supplementation for eight weeks results in attenuating FBS and HbA1c levels and improving insulin resistance (61). Another clinical trial study on type 2 diabetic patients has shown that oral consumption of ginger (1600 mg/d) for 12 weeks ameliorated insulin sensitivity and reduced C-reactive protein (CRP) and prostaglandin E₂ (PGE₂). Their results demonstrated that ginger significantly decreased FBS, HbA1C, insulin, TG, total cholesterol, CRP, and PGE2 rather than in the placebo group, but there were no noticeable differences in HDL, LDL and TNFα levels. Hence, ginger might have an active role in the treatment of the complications of diabetes (62).
According to these reports, ginger has a preventive or therapeutic effect on type 2 diabetes by multiple mechanisms such as enhancing insulin levels, reducing glucose levels, increasing beta cells, inducing glucose uptakes and phosphorylation of adenosine monophosphate-activated protein kinase (AMPK), reducing insulin resistance, improving the levels of adiponectin and anti-oxidant effects. Since there is not enough evidence to improve diabetic nephropathy in clinical trials, more human studies should be conducted to validate the effects of ginger on diabetic nephropathy (40, 53, 54).
Effect on dyslipidemia
Patients with metabolic syndrome may have dyslipidemia, including hypertriglyceridemia, high blood levels of apolipoprotein B (apo B) and LDL, and low HDL levels (63). It seems that the use of some plants as complementary therapeutics or extraction of their active ingredients along with currently available drugs will improve the management of hypertriglyceridemia in patients (64). Several studies have represented that ginger and its active components modified total cholesterol, total triglyceride, LDL, and HDL in serum; and have indicated that ginger is exactly a hypolipidemic agent (65-67). A study has shown that the marked elevation in total cholesterol, triglycerides (TG), lipoproteins, and phospholipids levels in serum are considerably decreased following administration of the ethanol extract of ginger (200 mg/kg for ten weeks – oral administration) in cholesterol-fed rabbits (68).
Adiponectin directly regulates lipid metabolism. 6-Gingerol (0.2 mg/kg- 7 weeks- oral administration) improves lipid aggregation, insulin resistance, and mitochondrial dysfunction in aging rats’ skeletal muscle. 6-Gingerol decreases the high plasma TG via increasing adiponectin concentrations of plasma and adipose tissue, and it also elevates the expression of muscular adiponectin receptor 1 (AdipoR1) which activates the adenosine monophosphate-activated protein kinase/peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (AMPK/PGC-1α) signaling pathway (69). Li et al. research has revealed that 6-gingerol by affecting lipid metabolism through increasing β-oxidation and reducing lipogenesis, normalizes the hepatic triglyceride level and plasma insulin level in the rat. Thus, the hepatic anti-steatotic effect of 6-gingerol is correlated with the upregulation of fatty acid oxidation and inhibition of the de novo lipogenesis pathway (69).
In a study by Hee-Jeong Kim et al., ginger supplementation (200 mg/kg for 12 weeks- oral administration) reduced plasma TC and TG. It inhibited liver steatosis by regulating hepatic gene expression implicated in lipogenesis and lipolysis (70). Besides these findings, it was found that ginger (oral administration of 200 mg/Kg for seven weeks) protected alcohol-induced myocardial damage by suppressing hyperlipidemia and cardiac biomarkers in Wistar male albino rats. Ginger attenuated the alcohol-induced lipid profile changes except for HDL, so noticeably reduced the alcohol-induced myocardial damage (71).
Moreover, the role of angiotensin-1-converting enzyme (ACE) inhibitors in cardiovascular disease improvement have been established in various research studies. In a study, the inhibitory action of ACE by oral administration of two varieties of ginger (4% and 2%) for three days was investigated on high cholesterol diet-fed rats. In contrast with other studies, white ginger showed the best inhibitory effect and increased plasma lipid profile with an elevation of MDA content in rat liver and heart tissues. However, red and white ginger supplementation caused a considerable reduction in the plasma TC, TG, very low-density-lipoprotein-cholesterol (VLDL-C), LDL, and MDA levels in the tissues. Conversely, ginger supplementation increased the plasma levels of HDL. The inhibition of ACE activity may be involved in this effect of ginger (72).
Also, ginger extract (250 µg/d for ten weeks- oral administration) resulted in ameliorating plasma TG, TC, VLDL, and LDL in mice. Also, in peritoneal macrophages derived from the mice fed with ginger extract, the cholesterol biosynthesis rate was significantly reduced. Also, the ginger extract lowered the concentration of LDL-associated lipid peroxides and prevented LDL accumulation (73).
The indicators of hyperlipidemia can be cutaneous fatty acid-binding protein (C-FABP), retinoid-binding protein (RBP), and heart fatty acid-binding protein (H-FABP), which are the genes involved in lipid metabolisms. The oral administration of 500 mg ginger per day for 12 weeks tends to decrease the expression of RBP mRNA in the liver and visceral fat in hyperlipidemic rats, so, it may develop lipid metabolism in male rats (74).
It is investigated that 6-gingerol (50 to 200 μM/d) regulated hepatic cholesterol metabolism in HepG2 cells, and both cellular total cholesterol and free cholesterol (FC) were reduced. Also, 6-gingerol (100 to 200 μM) raised the LDL receptor (LDLR) protein. Moreover, it has been proven that 6-gingerol by the activation of sterol regulatory element-binding protein 2 (SREBP2), up-regulation of cholesterol efflux-related genes liver X receptor alpha (LXRα) and ATP-binding cassette transporter (ABCA1) can regulate cholesterol metabolism through LDLR up-regulation (75).
In line with other studies which have suggested 6-gingerol has a preventive role in adipogenesis and lipid content accumulation, another study has shown that 6-gingerol prevented the adipogenesis and attenuated mRNA transcription factors expression and the major lipogenic enzymes in the 3T3-L1 cell line. So, the role of 6-gingerol in adipogenic differentiation is associated with motivating the Wnt/β-catenin signaling activation, inducing glycogen synthase kinase-3β (GSK-3β) phosphorylation and aggregating β-catenin in nuclei (76).
Moreover, in hypothyroidism and diabetic rats that received 500 mg/kg ginger extract (oral administration) for 21 days, the level of TC and LDL significantly reduced. Also, glucose levels fundamentally decreased in ginger-treated diabetic rats (77).
Based on the studies, the use of herbal supplements may inhibit most CVDs. A 10-week intensive exercise simultaneously with ginger supplement consumption (3 g of ginger pills per day) in overweight women could ameliorate MCP-1 (type 1 monocytes chemotactic protein) without any considerable impact on ICAM-1 and interleukin 10 (IL-10) (78). Altogether, ginger and its active ingredients reduce hyperlipidemia via multiple mechanisms (72, 73, 77), including anti-oxidant effects and increasing the level of adiponectin (69).
Effect on obesity
Obesity and overweight are accompanied by various disorders, including type 2 diabetes, dyslipidemia, hypertension, and heart disease (79, 80). Obesity is a prevalent disorder worldwide for decades (81, 82). The efficacy and safety of the approved anti-obesity agents are not satisfying, so there is an essential need for new and efficient treatments (83). Based on several research studies on cell lines, animals, and humans, ginger is an anti-obesity agent (84-88).
Research by Suk et al. suggests that gingerenone attenuates diet-induced obesity by decreasing fat mass in mice. It suppresses the development of adipose and inflammation by AMPK activation. So, gingerenone inhibits adipogenic differentiation and lipid aggregation in the 3T3-L1 cell line (89).
The peroxisome proliferator-activated receptor δ (PPARδ) stimulators exhibited anti-obesity effects (90). A combination of 1.3% 6-shogaol and 4.8% 6-gingerol (18 weeks of oral administration) has regulatory effects on PPARδ signaling in C57BL/6J mice and is as PPARδ ligand and motivated PPARδ expression in skeletal muscle myotubes cell line. The findings represented that following the activation of the PPARδ pathway with ginger, obesity was reduced, and exercise tolerance capacity developed by elevating skeletal muscle fat catabolism (91).
Moreover, the oral administration of 5% ginger powder (4 weeks of treatment) significantly decreased body weight which was accompanied by a positive impact on the level of peroxisomal catalase without inhibition of pancreatic lipase level or any effect on bilirubin level in male albino rats (92).
In this regard, a study on male Wistar rats has shown that ginger (oral administration of 50 mg/d for 6 weeks) reduced structural heart abnormalities. They have indicated that the effects of ginger can be associated with the reduction of the leptin and cathepsin G levels via its anti-oxidant effect (93).
A clinical trial study on overweight women exhibited an insignificant effect of ginger (two 1g tablets per day for 12 weeks) on blood glucose and a notable impact on TG, rather than the placebo. Nevertheless, ginger did not show any significant impact on plasma MDA levels (94).
On the other hand, Park et al., in a clinical trial study, acclaimed that 6-shogaol (5.89–8.83 mg/g/d for 12 weeks- oral administration) has an anti-obesity effect without any meaningful side effects. During the supplementation period, the body weight, BMI, and body fat levels in the treatment group were significantly lower than in the placebo group (95).
Due to the different reports, the anti-obesity effect of ginger and its components is moderate. Besides, the preventive and therapeutic effects of ginger in obesity are mediated via multiple mechanisms, such as increasing leptin and HDL-cholesterol levels, elevating skeletal muscle fat catabolism, and activation of AMPK and PPARδ pathways (96-98).
Effect on hypertension
Hypertension is one of the main features of metabolic syndrome (6), a significant cause of CVDs, such as vascular disorders, heart disorders, and coronary artery disease (99, 100). Antihypertensive drugs have several side effects, so herbal medicines have been considered in different studies (101). Numerous studies have proven ginger, and its ingredients may have a hypotensive effect and protective impacts on the cardiovascular system (102-104).
6-Gingerol improves hypertension biomarkers expression and reduces lipid accumulation by increasing phosphorylated endothelial nitric oxide synthase (eNOS) protein, vascular cell adhesion protein 1 (VCAM1), TNFα, epithelial sodium channel (ENaC) protein through PPARδ in mouse preadipocytes (3T3-L1 cells), human embryonal kidney cells (HEK293 cells), and human umbilical vein endothelial cells (HUVECs) (105).
Hypertension is associated with alterations of the platelet that contributes to cardiovascular complications development. Studies demonstrated that the oral administration of 4% of ginger supplementation for two weeks elevated adenosine deaminase (ADA) activity in platelet and lymphocytes. Also, it elevated ATP/ADP hydrolysis and hydrolysis of Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) in hypertensive rats. As well, ginger increased proinflammatory cytokines (IL-1, IL- 6, interferon-γ, and TNF-α) levels with a reduction of anti-inflammatory cytokines (interleukin-10) level (106).
A research study has shown that ginger supplementations (0-2 g/d, 2-4 g/d, and 4-6 g/d- oral administration) have a preventive role on some chronic diseases, including hypertension, hyperlipidemia, type 2 diabetes, fatty liver, and CHD in men and women; also it can decrease the possibility of disorders (107). Altogether, ginger and its constituents improve blood pressure problems and related disorders. Different studies that show the role of ginger and its components on metabolic syndrome risk factors have been summarized in Table 1.
Table 1.
Effects of ginger and its active components on metabolic syndrome risk factors
| Effect | Compound | Study design | Dose | Result | Ref. |
|---|---|---|---|---|---|
| Anti-diabetic | 6-gingerol | High cholesterol-high carbohydrate fed rats | 200 mg/kg P.O. | ↓Insulin resistance | (41) |
| Aqueous extracts of ginger | Obese diabetic Sprague-Dawley rats | 200 and 400 mg/kg P.O. | ↓ BG, ↑ Insulin | (42) | |
| Ginger | Diabetic patients | 3g/d P.O. | ↓ BG | (59) | |
| Ginger supplementation | Diabetic patients | 2000 mg/d P.O. | ↓FBS, ↓ HbA1C | (60) | |
| Ginger powder | Diabetic patients | 3 one-gr capsules P.O. | Improve insulin resistance | (61) | |
| Ginger supplementation | Diabetic patients | 1600 mg/d P.O. | ↑ Insulin sensitivity, ↑CRP | (62) | |
| Ginger | Diabetic Wistar rats | 5% P.O. | ↓ Diabetic nephropathy | (55) | |
| Rhizome of Ginger | High-fat diet-fed rats | 100, 200 and 400 mg/kg P.O. | ↑ Insulin | (108) | |
| Ginger methanolic extracts | Diabetic dyslipidemic rats | 300mg P.O. | ↓FBS | (109) | |
| Ginger | High-fat diet-induced type 2 diabetic rabbits | 12.5% P.O. | ↑ Insulin, ↓leptin | (110) | |
| Ginger + unripe plantain | STZ- induced Diabetic Rats | 710:100 g/kg P.O. | Not effective | (111) | |
| Juice of Ginger methanolic extract |
STZ- induced Diabetic Rats | 4 mL/kg PO. | ↓ BG | (112) | |
| Ginger extract | Diabetic Rats | 500mg/kg P.O. | ↓ BG | (113) | |
| Ethanolic ginger extract | Mice and rats | 50-800 mg/kg I.P. | ↓ BG | (114) | |
| [6]-Gingerol | As Intoxicated mice | 50 mg/kg P.O. | ↓ BG | (115) | |
| Aqueous extracts of raw ginger | Alloxan-induced diabetic and insulin-resistant diabetic rats | 500mg/mLP.O. | ↓ BG | (116) | |
| Ginger capsule | Diabetic patients | 1 g/d P.O. | ↓MDA | (117) | |
| Ginger | L6 myotubes | 400 µg/mL | ↓ BG | (118) | |
| Antilipidemic | Ethanol ginger extract | Cholesterol-fed rabbits | 200 mg/kg P.O. | ↓Hyperlipidemia | (68) |
| 6-gingerol | Ageing rats | 0.2 mg/ kg P.O. | ↓ TG, ↑Adiponectin | (69) | |
| Ginger supplementation | C57BL/6J mice | 200 mg/kg PO. | ↓ TG, ↓TC | (70) | |
| Ginger | Male wistar rats | 200 mg/Kg P.O. | ↓Hyperlipidemia | (71) | |
| Red and White Ginger | High cholesterol diet-fed rats | 4% or 2% P.O. | ↓TC, ↓TG,↓MDA | (72) | |
| Ginger extract | Mice | 25 or 250 µg/d P.O. | ↓Oxidize LDL, | (73) | |
| Ginger | Male rat | 500 mg P.O. | ↑Lipid metabolism | (74) | |
| Ginger extract | Hypothyroidism rats | 500 mg/kg P.O. | ↓TC, ↓LDL | (77) | |
| 6-gingerol | Poloxamer induced hyperlipidemic rats | 3 mg/ kg I.P. | ↓Hyperlipidemia | (119) | |
| aqueous ginger extract | Alloxan monohydrate-induced diabetic rats | 1000 mg/kg P.O. | ↓Hyperlipidemia, ↓TC, ↓LDL | (120) | |
| Gingerenone A | 3T3-L1 cell line | 40 μmol | ↓Adipogenesis lipid accumulation | (89) | |
| Anti-obesity | 6-Shogaol, 6-gingerol | C57BL/6J mice | 0.3% P.O. | ↓Obesity | (91) |
| Ginger powder | Male albino rats | 5% P.O. | ↓Bodyweight | (92) | |
| Ginger | Male wistar rats | 50 mg/d P.O. | ↓Leptin, ↓Cathepsin | (93) | |
| High-hydrostatic pressure ginger extract | High-fat diet-fed rats | 8 g/kg P.O. | ↓Obesity | (121) | |
| Extract of Ginger | Wistar rats | Unknown | ↓Bodyweight | (122) | |
| Ginger supplements | Obese women | 2 one-g tablets/d P.O. | ↓Obesity | (123) | |
| Extract of Ginger | High-fat diet-fed rats | 0.1 mL/80 g body weight P.O. | ↑Muscle mitochondrial biogenesis, ↑ HDL-C, ↓Obesity | (124) | |
| Ethanol extract of black ginger | Diabetic NSY Mice | 100 mg/kg PO. | Prevent obesity | (125) | |
| Ginger aqueous extract | Obese diabetic rats | 200 and 400 mg/kg P.O. | ↓Obesity | (42) | |
| Ginger aqueous extract | Obese diabetic rats | 100 and 200 mg/kg P.O. | ↓Obesity | (126) | |
| Hypotensive | 6-gingerol | 3T3-L1 cells/ HEK293 cells | 50 μmol/d | ↓ VCAM1, ↓ TNFα, Hypertension improvement | (105) |
| Ginger | Hypertensive rats | 4% P.O. | ↑ ADA, ↓ l-NAME | (106) | |
| Ginger | Hypertensive rats | 4% P.O. | ↑Proinflammatory cytokines, ↑ ADA, ↓ Anti-inflammatory cytokines | (106) | |
| Ginger | Anesthetized rats | 0.3-3 mg/kg I.V. | ↓ Arterial blood pressure | (127) | |
| Ginger aqueous extract | Anesthetized rats | 3.0-10.0 mg/kg I.V. | ↓ Arterial blood pressure | (128) | |
| Red and White Ginger | High cholesterol diet-fed rats | 2-4% P.O. | Hypertension improvement | (72) | |
| Ginger | Patient with hypertension | 0-6 g/d P.O. | ↓Probability hypertension | (107) |
Kg: kilogram; ml: milliliter; mg: milligram; PO: Per os (oral administration); IV: Intravenous; BG: blood glucose; FBS: fast blood sugar, HbA1C: hemoglobin A1c; CRP: c-reactive protein; TG: triglyceride; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein
Conclusion
Generally, ginger and its constituents are effective agents in the treatment of metabolic syndrome by reducing lipid accumulation by increasing the level of eNOS protein, VCAM1, TNFα, and ENaC. Also, ginger can play preventive or therapeutic roles in metabolic syndrome by diminishing FBS, and HbA1C by reducing insulin resistance, and anti-oxidant effects. It decreases some lipid profiles, blood pressure, and adiponectin and also increases leptin, and HDL-cholesterol levels, and elevates skeletal muscle fat catabolism as well, by activating the AMPK signaling pathway.
Authors’ Contributions
SS Searched the literature, wrote the original draft, and revised the manuscript. FY Designed the Figures. SIH Supervised. HH and SM Designed the study and revised the manuscript.
Conflicts of Interest
The authors declare not to have any conflicts of interest.
Acknowledgment
This study was supported by Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome; a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 3.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Halpern A, Mancini MC, Magalhães MEC, Fisberg M, Radominski R, Bertolami MC, et al. Metabolic syndrome, dyslipidemia, hypertension and type 2 diabetes in youth: From diagnosis to treatment. Diabetol Metab Syndr. 2010;2:55. doi: 10.1186/1758-5996-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumeron F, Lamri A, Khalil CA, Jaziri R, Porchay-Baldérelli I, Lantieri O, et al. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome: Results from a French prospective study, Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2011;34:813–817. doi: 10.2337/dc10-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004;43:1817–1822. doi: 10.1016/j.jacc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Mikhail N. The metabolic syndrome: insulin resistance. Curr Hypertens Rep. 2009;11:156. doi: 10.1007/s11906-009-0027-4. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 9.Lakka H-M, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The Metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 10.Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavi BM, Hosseinzadeh H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97:1679–1685. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- 12.Mollazadeh H, Hosseinzadeh H. Cinnamon effects on metabolic syndrome: A review based on its mechanisms. Iran J Basic Med Sci. 2016;19:1258–1270. doi: 10.22038/ijbms.2016.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest . 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 14.Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 15.Tabeshpour J, Razavi BM, Hosseinzadeh H. Effects of avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytother Res. 2017;31:819–837. doi: 10.1002/ptr.5805. [DOI] [PubMed] [Google Scholar]
- 16.Hassani FV, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn-Schmiedeb Arch Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 17.Dehghani S, Mehri S, Hosseinzadeh H. The effects of Crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iran J Basic Med Sci. 2019;22:460–468. doi: 10.22038/IJBMS.2019.31964.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macit MS, Sözlü S, Kocaadam B, Acar-Tek N. Evaluation of ginger (Zingiber Officinale Roscoe) on energy metabolism and obesity: systematic review and meta-analysis. Food Rev Int. 2019;35:685–706. [Google Scholar]
- 19.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan H. Growing and plant characteristics of ginger (Zingiber officinale Roscoe) DERIM. 2014;22:1–9. [Google Scholar]
- 21.Yang M, Liu C, Jiang J, Zuo G, Lin X, Yamahara J, et al. Ginger extract diminishes chronic fructose consumption-induced kidney injury through suppression of renal overexpression of proinflammatory cytokines in rats. BMC Complement Altern Med. 2014;14:174. doi: 10.1186/1472-6882-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suthisut D, Fields PG, Chandrapatya A. Contact toxicity, feeding reduction, and repellency of essential oils from three plants from the ginger family (Zingiberaceae) and their major components against Sitophilus zeamais and Tribolium castaneum. J Econ Entomol. 2011;104:1445–1454. doi: 10.1603/EC11050. [DOI] [PubMed] [Google Scholar]
- 23.Torkzadeh-Mahani S, Nasri S, Esmaeili-Mahani S. Ginger (Zingiber officinale Roscoe) prevents morphine-induced addictive behaviors in conditioned place preference test in rats. Addiction & health. 2014;6:65–72. [PMC free article] [PubMed] [Google Scholar]
- 24.Funk JL, Frye JB, Oyarzo JN, Chen J, Zhang H, Timmermann BN. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition. 2016;4:123–131. doi: 10.1016/j.phanu.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahimlou M, Yari Z, Hekmatdoost A, Alavian SM, Keshavarz SA. Ginger supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Hepat Mon. 2016:16. doi: 10.5812/hepatmon.34897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirooye P, Hashem-Dabaghian F, Hamzeloo-Moghadam M, Afrakhteh M, Bioos S, Mokaberinejad R. A clinical comparative study of oral and topical ginger on severity and duration of primary dysmenorrhea. Res J Pharmacogn. 2017;4:23–32. [Google Scholar]
- 27.Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai. 2007;90:1703–1708. [PubMed] [Google Scholar]
- 28.Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479–1489. doi: 10.1007/s00520-011-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative anti-oxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Xie Z, Koo HJ, McLaughlin SP, Timmermann BN, Gang DR. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc) Phytochemistry. 2006;67:1673–1685. doi: 10.1016/j.phytochem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Morakinyo A, Oludare G, Aderinto O, Tasdup A. Anti-oxidant and free radical scavenging activities of aqueous and ethanol extracts of Zingiber officinale. Biol Med. 2011;3:25–30. [Google Scholar]
- 32.Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: A review. Ann N Y Acad Sci. 2017;1398:83–98. doi: 10.1111/nyas.13375. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Chen H, Song Z, Wang X, Sun Z. Effects of ginger (Zingiber officinale Roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:5692962. doi: 10.1155/2018/5692962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadaegh F, Ghasemi A, Padyab M, Tohidi M, Azizi F. The metabolic syndrome and incident diabetes: Assessment of alternative definitions of the metabolic syndrome in an Iranian urban population. Diabetes Res Clin Pract. 2008;80:328–334. doi: 10.1016/j.diabres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 36.Taskinen M-R. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 37.Sardu C, De Lucia C, Wallner M, Santulli G. Diabetes mellitus and its cardiovascular complications: new insights into an old disease. J Diabetes Res. 2019;2019:1905194. doi: 10.1155/2019/1905194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibru T, Aga F, Boka A. Prevalence of diabetic retinopathy and associated factors among type 2 diabetes patients at tikur anbessa hospital ethiopia. J Diabetes Metab . 2019;10:1–7. [Google Scholar]
- 39.Khoury CC, Chen S, Ziyadeh FN. Pathophysiology of diabetic nephropathy. chronic renal disease. Elsevier; 2020. pp. 279–296. [Google Scholar]
- 40.Lipscombe LL, Hux JE. Trends in Diabetes Prevalence, Incidence, and Mortality in Ontario, Canada 1995-2005: A Population-Based Study. Lancet. 2007;369:750–756. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Tran VH, Kota BP, Nammi S, Duke CC, Roufogalis BD. Preventative effect of Zingiber officinale on insulin resistance in a high-fat high-carbohydrate diet-fed rat model and its mechanism of action. Basic Clin Pharmacol Toxicol. 2014;115:209–215. doi: 10.1111/bcpt.12196. [DOI] [PubMed] [Google Scholar]
- 42.Shalaby MA, Saifan HY. Some pharmacological effects of cinnamon and ginger herbs in obese diabetic rats. J Intercult Eethnopharmacol. 2014;3:144–153. doi: 10.5455/jice.20140818050741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samad MB, Mohsin M, Razu BA, Hossain MT, Mahzabeen S, Unnoor N, et al. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic beta-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complement Altern Med. 2017;17:395. doi: 10.1186/s12906-017-1903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. The Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 45.Rameshrad M, Razavi BM, Lalau J-D, De Broe ME, Hosseinzadeh H. An overview of glucagon-like peptide-1 receptor agonists for the treatment of metabolic syndrome: A drug repositioning. Iran J Basic Med Sci. 2020;23:556–568. doi: 10.22038/ijbms.2020.41638.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JO, Kim N, Lee HJ, Moon JW, Lee SK, Kim SJ, et al. [6]-Gingerol affects glucose metabolism by dual regulation via the AMPKalpha2-Mediated AS160-Rab5 pathway and AMPK-mediated insulin sensitizing effects. J Cell Biochem. 2015;116:1401–1410. doi: 10.1002/jcb.25100. [DOI] [PubMed] [Google Scholar]
- 47.Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int J Mol Sci. 2017;18:984. doi: 10.3390/ijms18050984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J-H, Lin X, Bu C, Zhang X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutrition and Metabolism. 2018;15:72–72. doi: 10.1186/s12986-018-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampath C, Rashid MR, Sang S, Ahmedna M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017;226:79–88. doi: 10.1016/j.foodchem.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 50.Wei C-K, Tsai Y-H, Korinek M, Hung P-H, El-Shazly M, Cheng Y-B, et al. 6-Paradol and 6-shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int J Mol Sci. 2017;18:168. doi: 10.3390/ijms18010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Islam MS, Choi H. Comparative effects of dietary ginger (Zingiber officinale) and garlic (Allium sativum) investigated in a type 2 diabetes model of rats. J Med Food. 2008;11:152–159. doi: 10.1089/jmf.2007.634. [DOI] [PubMed] [Google Scholar]
- 52.Al-Attar AM, Zari TA. Modulatory effects of ginger and clove oils on physiological responses in streptozotocin-induced diabetic rats. Int J Pharmacol. 2007;3:34–40. [Google Scholar]
- 53.Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother. 2018;106:381–389. doi: 10.1016/j.biopha.2018.06.148. [DOI] [PubMed] [Google Scholar]
- 54.Mata-Bermudez A, Izquierdo T, de Los Monteros-Zuniga E, Coen A, Godinez-Chaparro B. Antiallodynic effect induced by [6]-gingerol in neuropathic rats is mediated by activation of the serotoninergic system and the nitric oxide-cyclic guanosine monophosphate-adenosine triphosphate-sensitive K(+) channel pathway. Phytother Res. 2018;32:2520–2530. doi: 10.1002/ptr.6191. [DOI] [PubMed] [Google Scholar]
- 55.Afshari AT, Shirpoor A, Farshid A, Saadatian R, Rasmi Y, Saboory E, et al. The effect of ginger on diabetic nephropathy, plasma anti-oxidant capacity and lipid peroxidation in rats. Food Chem. 2007;101:148–153. [Google Scholar]
- 56.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes care. 2004;27:84–87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 57.Kalantari K, Moniri M, Boroumand Moghaddam A, Abdul Rahim R, Bin Ariff A, Izadiyan Z, et al. A review of the biomedical applications of zerumbone and the techniques for its extraction from ginger rhizomes. Molecules. 2017;22:1645. doi: 10.3390/molecules22101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzeng TF, Liou SS, Tzeng YC, Liu IM. Zerumbone, a phytochemical of subtropical ginger, protects against hyperglycemia-induced retinal damage in experimental diabetic rats. Nutrients. 2016;8:449. doi: 10.3390/nu8080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complement Integr Med. 2015;12:165–170. doi: 10.1515/jcim-2014-0021. [DOI] [PubMed] [Google Scholar]
- 60.Makhdoomi Arzati M, Mohammadzadeh Honarvar N, Saedisomeolia A, Anvari S, Effatpanah M, Makhdoomi Arzati R, et al. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int J Endocrinol Metab. 2017;15:e57927. doi: 10.5812/ijem.57927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mozaffari-Khosravi H, Talaei B, Jalali B-A, Najarzadeh A, Mozayan MR. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014;22:9–16. doi: 10.1016/j.ctim.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65:515–520. doi: 10.3109/09637486.2014.880671. [DOI] [PubMed] [Google Scholar]
- 63.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med. 2003;115:24–28. doi: 10.1016/j.amjmed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Mollazadeh H, Mahdian D, Hosseinzadeh H. Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. Phytomedicine. 2019;53:43–52. doi: 10.1016/j.phymed.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 65.Arzati MM, Honarvar NM, Saedisomeolia A, Anvari S, Effatpanah M, Arzati RM, et al. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int J Endocrinol Metab. 2017;15:e57927. doi: 10.5812/ijem.57927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Las Heras N, Valero-Muñoz M, Martín-Fernández B, Ballesteros S, López-Farré A, Ruiz-Roso B, et al. Molecular factors involved in the hypolipidemic-and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Applied Physiology, Nutrition, and Metabolism. 2017;42:209–215. doi: 10.1139/apnm-2016-0374. [DOI] [PubMed] [Google Scholar]
- 67.Fatima A, Niaz K, Suhail B, Murad S. Ginger pasted-powder prevents dyslipidemia and body weight. Pakistan J. Medical Health Sci. 2018;12:974–976. [Google Scholar]
- 68.Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol. 1998;61:167–171. doi: 10.1016/s0378-8741(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Wang S, Yao L, Ma P, Chen Z, Han TL, et al. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2019;362:125–135. doi: 10.1016/j.taap.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Kim H-J, Kim B, Mun E-G, Jeong S-Y, Cha Y-S. The anti-oxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice. Nut Res Prac. 2018;12:503–511. doi: 10.4162/nrp.2018.12.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Subbaiah GV, Mallikarjuna K, Shanmugam B, Ravi S, Taj PU, Reddy KS. Ginger treatment ameliorates alcohol-induced myocardial damage by suppression of hyperlipidemia and cardiac biomarkers in rats. Pharmacogn Mag. 2017;13:69–75. doi: 10.4103/0973-1296.203891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akinyemi AJ, Ademiluyi AO, Oboh G. Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger (Zingiber officinale) in rats fed a high cholesterol diet. J Med Food. 2014;17:317–323. doi: 10.1089/jmf.2012.0264. [DOI] [PubMed] [Google Scholar]
- 73.Fuhrman B, Rosenblat M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130:1124–1131. doi: 10.1093/jn/130.5.1124. [DOI] [PubMed] [Google Scholar]
- 74.Matsuda A, Wang Z, Takahashi S, Tokuda T, Miura N, Hasegawa J. Upregulation of mRNA of retinoid binding protein and fatty acid binding protein by cholesterol enriched-diet and effect of ginger on lipid metabolism. Life Sci. 2009;84:903–907. doi: 10.1016/j.lfs.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Guo J, Liang N, Jiang X, Song Y, Ou S, et al. 6-Gingerol regulates hepatic cholesterol metabolism by up-regulation of LDLR and cholesterol efflux-related genes in HepG2 cells. Front Pharmacol. 2018;9:159 . doi: 10.3389/fphar.2018.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C, Zhou L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/beta-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol In vitro. 2015;30:394–401. doi: 10.1016/j.tiv.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 77.Al-Noory AS, Amreen AN, Hymoor S. Antihyperlipidemic effects of ginger extracts in alloxan-induced diabetes and propylthiouracil-induced hypothyroidism in (rats) Pharmacognosy Res. 2013;5:157–161. doi: 10.4103/0974-8490.112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayebifar S, Afzalpour ME, Kazemi T, Eivary SH, Mogharnasi M. The effect of a 10-week high-intensity interval training and ginger consumption on inflammatory indices contributing to atherosclerosis in overweight women. J Res Med Sci. 2016;21:116. doi: 10.4103/1735-1995.193507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenfield DM, Snowden JA. Cardiovascular diseases and metabolic syndrome. The EBMT Handbook. Springer; 2019. pp. 415–420. [PubMed] [Google Scholar]
- 80.Maksimovic M, Vlajinac H, Radak D, Marinkovic J, Maksimovic J, Jorga J. Association of overweight and obesity with cardiovascular risk factors in patients with atherosclerotic diseases. J Med Biochem. 2019;39:215–223. doi: 10.2478/jomb-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96:3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- 82.Blüher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 83.Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: New drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balogun FO, AdeyeOluwa ET, Ashafa AOT. Pharmacological potentials of ginger. Studies on Ginger: IntechOpen. 2019 [Google Scholar]
- 85.Panda VS, Shah T. A herbal premix containing Macrotyloma uniflorum, ginger and whey curtails obesity in high fat diet fed rats by a novel mechanism. Appl Physiol Nutr Metab. 2020;45:25–35. doi: 10.1139/apnm-2019-0139. [DOI] [PubMed] [Google Scholar]
- 86.Park S-H, Jung S-J, Choi E-K, Ha K-C, Baek H-I, Park Y-K, et al. The effects of steamed ginger ethanolic extract on weight and body fat loss: a randomized, double-blind, placebo-controlled clinical trial. Food Sci Biotechnol. 2020;29:265–273. doi: 10.1007/s10068-019-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srinivasan K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition. 2017;5:18–28. [Google Scholar]
- 88.Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci. 2017;1398:83–98. doi: 10.1111/nyas.13375. [DOI] [PubMed] [Google Scholar]
- 89.Suk S, Kwon GT, Lee E, Jang WJ, Yang H, Kim JH, et al. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. Mol Nutr Food Res. 2017;61:10. doi: 10.1002/mnfr.201700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perreault M, Erbe DV, Tobin JF. PPARδ Agonism for the treatment of obesity and associated disorders: challenges and opportunities. PPAR Res. 2008;2008:125387. doi: 10.1155/2008/125387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Misawa K, Hashizume K, Yamamoto M, Minegishi Y, Hase T, Shimotoyodome A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor delta pathway. J Nutr Biochem. 2015;26:1058–1067. doi: 10.1016/j.jnutbio.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Mahmoud RH, Elnour WA. Comparative evaluation of the efficacy of ginger and orlistat on obesity management, pancreatic lipase and liver peroxisomal catalase enzyme in male albino rats. Eur Rev Med Pharmacol Sci. 2013;17:75–83. [PubMed] [Google Scholar]
- 93.Ilkhanizadeh B, Shirpoor A, Nemati S, Rasmi Y. Protective effects of ginger (Zingiber officinale) extract against diabetes-induced heart abnormality in rats. Diabetes Metab J. 2016;40:46–53. doi: 10.4093/dmj.2016.40.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Attari VE, Mahluji S, Jafarabadi MA, Ostadrahimi A. Effects of supplementation with ginger (Zingiber officinale Roscoe) on serum glucose, lipid profile and oxidative stress in obese women: a randomized, placebo-controlled clinical trial. J Pharm Sci. 2015;21:184–191. [Google Scholar]
- 95.Park S-H, Jung S-J, Choi E-K, Ha K-C, Baek H-I, Park Y-K, et al. The effects of steamed ginger ethanolic extract on weight and body fat loss: a randomized, double-blind, placebo-controlled clinical trial. Food Sci Biotechnol. 2020;29:265–273. doi: 10.1007/s10068-019-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ebrahimzadeh Attari V, Malek Mahdavi A, Javadivala Z, Mahluji S, Zununi Vahed S, Ostadrahimi A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother Res. 2018;32:577–585. doi: 10.1002/ptr.5986. [DOI] [PubMed] [Google Scholar]
- 97.Maharlouei N, Tabrizi R, Lankarani KB, Rezaianzadeh A, Akbari M, Kolahdooz F, et al. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59:1753–1766. doi: 10.1080/10408398.2018.1427044. [DOI] [PubMed] [Google Scholar]
- 98.Taghizadeh M, Farzin N, Taheri S, Mahlouji M, Akbari H, Karamali F, et al. The effect of dietary supplements containing green tea, capsaicin and ginger extracts on weight loss and metabolic profiles in overweight women: A randomized double-blind placebo-controlled clinical trial. Ann Nutr Metab. 2017;70:277–285. doi: 10.1159/000471889. [DOI] [PubMed] [Google Scholar]
- 99.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension. 2016;68:67–94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayosi BM, Cupido B, Lawrenson J. Cardiovascular diseases. In: Magill, A.J, Ryan, E.T, editors. hunter’s tropical medicine and emerging infectious diseases. Elsevier; 2020. pp. 8–15. [Google Scholar]
- 101.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 102.Elkhishin IA, Awwad IA. A study of the cardiovascular toxic effects of Zingiber officinale (ginger) in adult male albino rats and its possible mechanisms of action. MJFCT. 2009;17:109–127. [Google Scholar]
- 103.Ghayur MN, Gilani AH. Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. J Cardiovas Pharmacol. 2005;45:74–80. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 104.Wen J, Zhang L, Wang J, Wang J, Wang L, Wang R, et al. Therapeutic effects of higenamine combined with [6]‐gingerol on chronic heart failure induced by doxorubicin via ameliorating mitochondrial function. J Cell Mol Med. 2020;24:4036–4050. doi: 10.1111/jcmm.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee Y-J, Jang Y-N, Han Y-M, Kim H-M, Seo HS. 6-Gingerol Normalizes the Expression of Biomarkers Related to Hypertension via PPARδ in HUVECs, HEK293, and Differentiated 3T3-L1 Cells. PPAR Res. 2018;2018:6485064. doi: 10.1155/2018/6485064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akinyemi AJ, Thomé GR, Morsch VM, Bottari NB, Baldissarelli J, de Oliveira LS, et al. Effect of ginger and turmeric rhizomes on inflammatory cytokines levels and enzyme activities of cholinergic and purinergic systems in hypertensive rats. Planta Med. 2016;82:612–620. doi: 10.1055/s-0042-102062. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Yu H, Zhang X, Feng Q, Guo X, Li S, et al. Evaluation of daily ginger consumption for the prevention of chronic diseases in adults: A cross-sectional study. Nutrition. 2017;36:79–84. doi: 10.1016/j.nut.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 108.Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2009;104:366–373. doi: 10.1111/j.1742-7843.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 109.Hussain N, Hashmi AS, Wasim M, Akhtar T, Saeed S, Ahmad T. Synergistic potential of Zingiber officinale and Curcuma longa to ameliorate diabetic-dyslipidemia. Pak J Pharm Sci. 2018;31:491–498. [PubMed] [Google Scholar]
- 110.Abdulrazak A, Tanko Y, Mohammed A, Mohammed KA, Sada NM, Dikko AA. Effects of clove and fermented ginger on blood glucose, leptin, insulin and insulin receptor levels in high fat dietinduced type 2 diabetic rabbits. Niger J Physiol Sci. 2018;33:89–93. [PubMed] [Google Scholar]
- 111.Iroaganachi M, Eleazu CO, Okafor PN, Nwaohu N. Effect of unripe plantain (musa paradisiaca) and ginger (Zingiber officinale) on blood glucose, body weight and feed intake of streptozotocin-induced diabetic rats. Open Biochem J. 2015;9:1–6. doi: 10.2174/1874091X01509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akhani S, Vishwakarma S, Goyal R. Antidiabetic activity of zingiber officinal roscoe in streptozotocin-induced non-insulin dependent diabetic rats. Indian J Pharm sci. 2005;67:553. [Google Scholar]
- 113.Jafri SA, Abass S, Qasim M. Hypoglycemic effect of ginger (Zingiber officinale) in alloxan induced diabetic rats (Rattus norvagicus) Pak Vet J. 2011;31:160–162. [Google Scholar]
- 114.Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 115.Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol Lett. 2012;210:34–43. doi: 10.1016/j.toxlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Iranloye BO, Arikawe AP, Rotimi G, Sogbade AO. Anti-diabetic and anti-oxidant effects of Zingiber officinale on alloxan-induced and insulin-resistant diabetic male rats. Niger J Physiol Sci. 2011;26:89–96. [PubMed] [Google Scholar]
- 117.Mohammadi H, Avandi SM. Effect of eight weeks resistance training with ginger supplementation on malondialdehyde and body compostion index in type 2 diabetes patients. koomesh. 2019;21:73–82. [Google Scholar]
- 118.Noipha K, Ninla-Aesong P. Antidiabetic activity of Zingiber officinale Roscoe rhizome extract: An In vitro study. Hayati J Biosci. 2018;25:160–168. [Google Scholar]
- 119.Shao Y, Yu Y, Li C, Yu J, Zong R, Pei C. Synergistic effect of quercetin and 6-gingerol treatment in streptozotocin induced type 2 diabetic rats and poloxamer P-407 induced hyperlipidemia. RSC Advances. 2016;6:12235–12242. [Google Scholar]
- 120.Al-Qudah MM, Haddad MA, EL-Qudah JM. The effects of aqueous ginger extract on pancreas histology and on blood glucose in normal and alloxan monohydrate-induced diabetic rats. Biomed Res. 2016;7:350–356. [Google Scholar]
- 121.Kim S, Lee M-S, Jung S, Son H-Y, Park S, Kang B, et al. Ginger extract ameliorates obesity and inflammation via regulating MicroRNA-21/132 expression and AMPK activation in white adipose tissue. Nutrients. 2018;10:1567. doi: 10.3390/nu10111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bin-Meferij MM, Shati AA, Eid RA, El-Kott AF. Anti-obesity and anti-hepatosteatosis effects of dietary Zingiber officinale extract in male obese rats. Int J Pharmacol. 2017;13:620–627. [Google Scholar]
- 123.Ebrahimzadeh Attari V, Asghari Jafarabadi M, Zemestani M, Ostadrahimi A. Effect of zingiber officinale supplementation on obesity management with respect to the uncoupling protein 1 -3826A>G and β3-adrenergic receptor Trp64Arg polymorphism. Phytother Res. 2015;29:1032–1039. doi: 10.1002/ptr.5343. [DOI] [PubMed] [Google Scholar]
- 124.Oh S, Lee M-S, Jung S, Kim S, Park H, Park S, et al. Ginger extract increases muscle mitochondrial biogenesis and serum HDL-cholesterol level in high-fat diet-fed rats. J Funct Foods. 2017;29:193–200. [Google Scholar]
- 125.Ochiai M, Takeuchi T, Nozaki T, Ishihara Ko, Matsuo T. Kaempferia parviflora ethanol extract, a peroxisome proliferator‐activated receptor γ ligand‐binding agonist, improves glucose tolerance and suppresses fat accumulation in diabetic NSY mice. J Food Sci. 2019;84:339–348. doi: 10.1111/1750-3841.14437. [DOI] [PubMed] [Google Scholar]
- 126.Ismail NS. Protective effects of aqueous extracts of cinnamon and ginger herbs against obesity and diabetes in obese diabetic rat. World J Dairy Food Sci. 2014;9:145–153. [Google Scholar]
- 127.Ghayur MN, Gilani AH. Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. J Cardiovasc Pharmacol. 2005;45:74–80. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 128.Ghayur MN, Gilani AH, Afridi MB, Houghton PJ. Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vascul Pharmacol. 2005;43:234–241. doi: 10.1016/j.vph.2005.07.003. [DOI] [PubMed] [Google Scholar]