Abstract
The observed sensitivity of Listeria monocytogenes to the toxic proline analogue l-azetidine-2-carboxylic acid (AZ) suggested that proline synthesis in Listeria may be regulated by feedback inhibition of γ-glutamyl kinase (GK), the first enzyme of the proline biosynthesis pathway, encoded by the proB gene. Taking advantage of the Epicurian coli mutator strain XL1-Red, we performed random mutagenesis of the recently described proBA operon and generated three independent mutations in the listerial proB homologue, leading to proline overproduction and salt tolerance when expressed in an E. coli (ΔproBA) background. While each of the mutations (located within a conserved 26-amino-acid region of GK) was shown to confer AZ resistance (AZr) on an L. monocytogenes proBA mutant, listerial transformants failed to exhibit the salt-tolerant phenotype observed in E. coli. Since proline accumulation has previously been linked to the virulence potential of a number of pathogenic bacteria, we analyzed the effect of proline overproduction on Listeria pathogenesis. However, our results suggest that as previously described for proline auxotrophy, proline hyperproduction has no apparent impact on the virulence potential of Listeria.
Genetic and physiological analysis of proline accumulation in both prokaryotic and eukaryotic systems (11, 20) has provided evidence that is consistent with diverse functions of proline, not only as a source of energy, carbon and nitrogen but also as an effective osmolyte (1, 10, 11, 23) and more recently as a potential virulence factor for a number of pathogenic bacteria (2, 12, 33).
While proline can be synthesized from ornithine in both plants and animals (18), glutamate is the primary precursor for proline biosynthesis in bacteria (23) and in osmotically stressed plant cells (14). Bacterial proline synthesis from glutamate occurs via three enzymatic reactions, catalyzed by γ-glutamyl kinase (GK) (proB product, EC 2.7.2.11), γ-glutamyl phosphate reductase (GPR) (proA product, EC 1.2.1.41), and Δ1-pyrroline-5-carboxylate reductase (P5C) (proC product, EC 1.5.1.2). For the majority of bacteria the proB and proA genes constitute an operon, which is distant from proC on the chromosome. In plants, e.g., Vigna aconitifolia and Arabidopsis, the first two steps of proline biosynthesis from glutamate are catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS), a bifunctional enzyme with both GK and GPR activities at the N- and C-terminal domains, respectively (18).
For both prokaryotic and eukaryotic systems, proline synthesis from glutamate is regulated by feedback inhibition of the first enzyme in the pathway. Studies on purified enzymes suggest that in addition to proline-mediated inhibition, the γ-glutamyl kinase activities of GK and P5CS are also modulated to a lesser extent by glutamate and ADP, thereby tuning proline synthesis to cellular substrate and energy availability (37, 39). Proline hyperproducing strains of bacteria, exhibiting reduced proline-mediated feedback inhibition of GK activity (a result of single-base-pair substitutions in either the bacterial proB gene [13, 22, 28, 29, 32] or the 5′ domain of the plant P5CS coding region [39]), have been isolated based on their resistance to toxic proline analogues (l-azetidine-2-carboxylic acid [AZ] [15] and 3,4-dehydro-dl-proline, compounds which inhibit GK activity while not interfering with protein synthesis [23]).
In addition to the obvious advantages for commercial amino acid synthesis (29), the osmoprotective properties of proline overproduction (19) have led to the development of transgenic drought-resistant plants (17). However, since proline may function as a potential virulence factor (2, 12, 33) and is known to facilitate the growth of certain pathogenic bacteria at elevated osmolarities (9), the use of transmissible genetic elements encoding proline hyperproduction may lead to undesirable consequences, if introduced prematurely into the natural environment.
Previously we described the isolation and characterization of the listerial proBA operon (35). In this study we generated proB mutants which overproduce proline, and we assess the contribution of such overproduction to the growth and survival of Listeria monocytogenes, both in hypersaline environments and during infection of an animal (murine) model.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown at 37°C either in Luria Bertani (LB) medium (26) or M9 minimal medium (GIBCO/BRL, Eggenstein, Federal Republic of Germany) containing appropriate additional requirements. L. monocytogenes strains were grown either in brain heart infusion broth (Oxoid, Unipath Ltd., Basingstoke, United Kingdom) or in chemically defined minimal medium (DM) (31). Blood agar plates consisted of blood agar base (Lab M) to which 5% sheep blood was added following autoclaving. Where necessary, proline and its analogues (AZ and 3,4-dehydro-dl-proline) (Sigma Chemical Co., St. Louis, Mo.) were added to the growth medium at the appropriate concentration, as filter-sterilized solutions. Antibiotics when needed were made up as described by Maniatis et al. (26) as concentrated stocks and added to media at the required levels. Where indicated, media osmolarity was adjusted by the addition of NaCl.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| L. monocytogenes | ||
| LO28 | Serotype 1/2c | P. Cossart, Institut Pasteur |
| PSOE | L. monocytogenes LO28 ΔproBA, Pro− | 35 |
| E. coli | ||
| CSH26 | ara Δ(lac proBA) thi Pro− | L. Csonka, Purdue |
| XL1-Red | endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac mutD5 mutS mutT Tn10 (Tetr) | Stratagene |
| Plasmids | ||
| pC1372 | Cmr, 5.7-kb E. coli/L. lactisb shuttle vector | 16 |
| pCPL9 | pC1372::5.5-kb EcoRI insert harboring the LO28 proBA operon | 35 |
| pCPL9mut | Randomly mutated pCPL9 from E. coli XL1-Red | This study |
| pCPL12 | pCPL9 ProB V121I; AZr | This study |
| pCPL13 | pCPL9 ProB A144V; AZr | This study |
| pCPL14 | pCPL9 ProB E146K; AZr | This study |
| pCPL15 | pCPL9 ProB E146K; ProA I328V; AZr | This study |
| pCPL16 | pCPL9 ProB V121I; AZr | This study |
AZr, AZ resistance; Cmr, chloramphenicol resistance; Tetr, tetracycline resistance.
L. lactis, Lactococcus lactis.
DNA manipulations and sequence analysis.
Routine DNA manipulations were performed as described by Maniatis et al. (26). Plasmid DNA was isolated using the Qiagen QIAprep Spin Miniprep Kit (Qiagen, Hilden, Federal Republic of Germany). E. coli was transformed by standard methods (26), and electrotransformation of L. monocytogenes was achieved by the protocol outlined by Park and Stewart (30). PCR reagents (Taq polymerase and deoxynucleoside triphosphates) were purchased from Boehringer GmbH (Mannheim, Germany) and used according to the manufacturer's instructions with a Hybaid (Middlesex, United Kingdom) PCR express system. Oligonucleotide primers for PCR and sequence purposes were synthesized on an oligo 1000M DNA synthesizer (Beckman Instruments Inc., Fullerton, California). Nucleotide sequence determination was performed on an ABI 373 automated sequencer using the BigDye Terminator sequence kit (Lark Technologies, Inc. Essex, United Kingdom). Nucleotide and protein sequence analysis were done using Lasergene (DNASTAR, Ltd., London, United Kingdom). The nucleotide sequence of the proBA operon in L. monocytogenes can be accessed from the GenBank database (accession no. AF282880).
Generation of proline analogue-resistant mutants.
The plasmid pCPL9, harboring the listerial proBA operon, was transformed into the mutator strain Epicurian coli XL1-Red (Stratagene), and transformants were selected on LB plates containing chloramphenicol (30 μg/ml). Transformants were then pooled and grown overnight at 37°C in LB broth. Randomly mutated plasmid DNA extracted from this culture was then used to transform the proline synthesis mutant E. coli CSH26. Mutations leading to proline overproduction were selected by plating transformants on M9 minimal medium containing 5 mM AZ. These transformants were then pooled and grown in M9 containing 4% added NaCl, to select for mutations encoding proline hyperproduction leading to osmotolerance. Plasmids isolated from the resultant osmotolerant AZr CSH26 clones were then used to transform L. monocytogenes PSOE (ΔproBA) before screening for proline analogue resistance (AZr at 10 mM concentrations) and salt tolerance (growth in DM plus 4% added NaCl).
Analysis of proline production.
Proline hyperproduction was assayed using a modification of the proline bioassay described by Kosuge and Hoshino (22). The cell-free supernatant from overnight cultures of proline-producing strains, in proline-deficient minimal media, was spotted (in 5-μl volumes) onto M9 plates containing no added proline and seeded with the E. coli proline auxotroph CSH26 indicator. Proline overproduction and excretion was confirmed by subsequent growth of the indicator cells. Quantitative analysis of the proline in the cell extract of putative proline overproducers was carried out using a 6300 amino acid analyzer (Beckman Instruments Ltd., High Wycombe, United Kingdom).
Virulence assays.
Bacterial virulence was determined by intraperitoneal and peroral inoculation of 8-to 12-week-old BALB/c mice. Intraperitoneal inoculations were carried out as described previously (34), using overnight cultures of mutant and wild-type Listeria (4 × 105 cells), suspended in 0.2 ml of phosphate-buffered saline. For peroral inoculations, mutant and wild-type strains suspended in buffered saline with gelatin were mixed at a ratio of 1:1. Mice were infected with approximately 2 × 109 cells (total) using a micropipette tip placed immediately behind the incisors. At 3 days postinfection mice were euthanized, and listerial numbers were determined by spread plating homogenized samples onto brain heart infusion broth (for liver and spleen) and blood agar (for Peyer's patches and small intestine wall and contents) with and without added chloramphenicol (10 μg/ml). Maintained resistance to both chloramphenicol and AZ following passage through the mouse model confirmed plasmid stability phenotypically.
RESULTS AND DISCUSSION
Random mutagenesis of the listerial proBA operon.
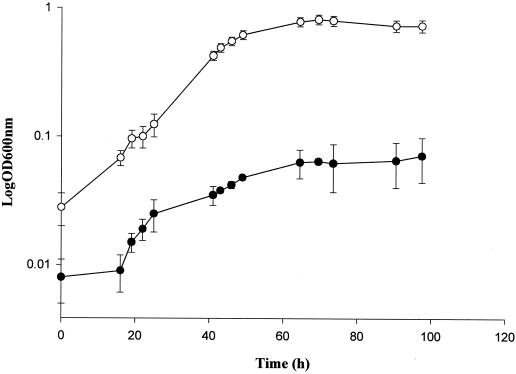
The observed AZ-mediated inhibition of L. monocytogenes (Fig. 1) indicated that as with the majority of systems (both prokaryotic and eukaryotic), listerial proline biosynthesis from glutamate may be regulated by proline-dependent feedback inhibition of the GK activity. Mutations leading to proline analogue resistance (and consequential proline hyperproduction) have been described for a number of organisms and have in each case been linked to mutations in GK, leading to a decreased sensitivity of the enzyme for its allosteric effector proline and its analogues (13, 22, 28, 29, 32).
FIG. 1.
Growth of L. monocytogenes LO28 (●) and PSOE (pCPL12) (○) bacteria in DM containing 10 mM AZ as determined using a spectronic 20D+ spectrophotometer. Growth curves of Listeria containing plasmids with the other ProB mutations (pCPL13–16) described in the text were identical to that of PSOE (pCPL12), but for clarity they are excluded from this graph. Log OD 600nm, log optical density at 600 nm.
In an effort to generate proline-hyperproducing strains of L. monocytogenes, we used a random mutagenesis strategy to introduce point mutations into the cloned listerial proBA operon. Plasmid pCPL9 (harboring the listerial proBA locus) was transformed into the E. coli mutator strain XL1-Red. Mutations in three of the primary DNA repair pathways of this strain result in a mutation rate which is ∼5,000-fold higher than that of the wild type; hence pCPL9 replication within XL1-Red led in the introduction of point mutations throughout the operon. The randomly mutated pCPL9 “bank,” designated pCPL9mut, was subsequently transformed into the E. coli proline auxotroph CSH26, and transformants were selected on minimal medium containing 5 mM AZ. While no colonies were obtained following a control transformation with unmutated pCPL9, transformation efficiencies of 75 CFU/μg of DNA were achieved from pCPL9mut, colonies appearing after 36 h at 37°C. Following overnight growth at elevated osmolarities, five AZr transformants were chosen at random for further analysis. Proline production levels of the five analogue resistant strains were tested using the proline bioassay in combination with amino acid analysis (Fig. 2A). Complementation of the proline auxotrophic indicator strain showed that each clone exhibited proline overproduction and excretion compared to the parent containing pCPL9. Proof that the observed phenotype was the result of mutations in the cloned listerial proBA operon was obtained by recomplementation studies, in which plasmid isolated from each of the complementing clones once again conferred AZr, not only on the recipient E. coli CSH26 strain but also on the listerial proline auxotroph PSOE (Fig. 1).
FIG. 2.
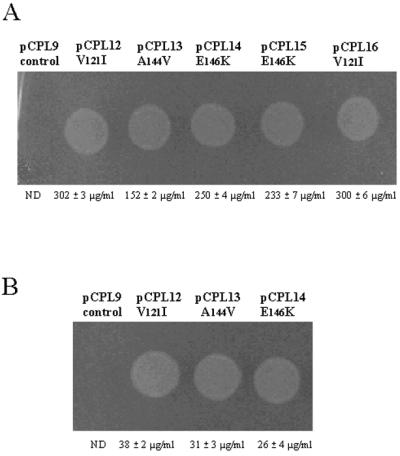
Bioassay for proline overproduction using E. coli CSH26 (ΔproBA) as the indicator strain (as described in Materials and Methods) and concentrations of proline in the cell extract (as determined by high-pressure liquid chromatography analysis of the cell-free supernatant) following transformation of E. coli CSH26 (A) and Listeria PSOE (B) with mutated proB genes. In each case unmutated pCPL9 expressed against a ΔproBA background (CSH26 for panel A and PSOE for panel B) served as the control. Results of high-pressure liquid chromatography analysis represent the mean value of three independent experiments. ND, not detected. Similar results were obtained when the mutants were cultured at elevated osmolarity and in complex broth in the presence of exogenous osmolytes.
Sequence analysis of the mutated proBA genes.
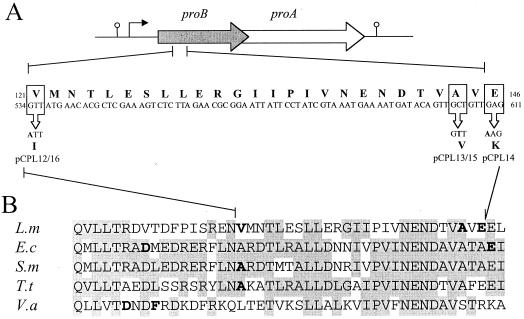
Plasmid DNA isolated from the five proline-overproducing CSH26 clones (pCPL12–16; Table 1) was in each case subjected to sequence analysis of the cloned listerial proBA operon. Nucleotide sequence comparisons with the wild-type proBA genes revealed a small number of base substitutions in the mutated operons (Fig. 3A). Interestingly the base changes, each of which results in an amino acid substitution within a defined (26-amino-acid) region of the GK enzyme, map closely to previously isolated mutations leading to proline overproduction in other genera (13, 22, 28, 29, 32, 39) (Fig. 3B). This highly conserved region almost certainly represents an important regulatory domain, most probably the enzyme allosteric binding site. Alternatively, substitutions in this domain may lead to conformational changes resulting in a loss of the enzyme's allosteric properties.
FIG. 3.
(A) Point mutations in the listerial proBA operon leading to proline overproduction and AZ resistance. (B) Feedback-resistant mutations in the GKs of L. monocytogenes (L.m), E. coli (E.c), S. marcescens (S.m), T. thermophilus (T.t), and V. aconitifolia (V.a). Residues affected by mutations conferring AZ resistance are in boldface. Conserved residues are shaded.
In all, three independent mutations leading to an altered GK were obtained: V121I (pCPL12 and pCPL16), A144V (pCPL13), and E146K (pCPL14 and pCPL15). In addition, pCPL15 also contains an A-to-G silent mutation at nucleotide 390 of the proB gene, as well as an I328V substitution in the GPR protein. Interestingly, mutations leading to proline overproduction have been observed in very similar positions in other genera, although the actual residues vary. For example, the amino acid corresponding to the listerial V121I mutation is also altered in both Serratia marcescens and Thermus thermophilus, but in both those cases from A to V (Fig. 3B). Thus, a change from valine in the listerial GK is matched by a change to valine at the equivalent position in these other genera. The other mutations at positions 144 and 146 are also close to a mutation at a similar position in E. coli, illustrating that this also functions as an important region in the GK allosteric site.
Effects of proB mutations on salt tolerance.
The role of proline as an osmoprotectant was first described by Christian (7, 8), who in 1955 reported that addition of the amino acid to media of elevated osmolarity could relieve bacterial growth inhibition. Based on these observations, Csonka (9) isolated a proline-overproducing mutant of Salmonella enterica serovar Typhimurium, exhibiting increased salt tolerance. The mutation (E. coli ProB D107N [13]) was located on the E. coli episome, F′128, and could thus be easily transferred to other enteric bacteria (9, 24).
The role of proline as an effective osmolyte has since been described for a variety of bacteria, including Listeria (3, 4). While each of the three mutations described in the previous section conferred a similar level of resistance to the proline analogue AZ in E. coli, the ProB V121I mutation conferred the highest level of osmotolerance at 4% NaCl relative to the control strain. The remaining mutations, while not as osmotolerant as ProB V121I, still showed significant increases in growth rate relative to the control at elevated osmolarities (data not shown).
Recently we described the isolation and disruption of the listerial proBA operon, revealing a significant role for proline synthesis in contributing to the growth and survival of L. monocytogenes in environments of elevated osmolarity (35). In order to further assess the importance of proline synthesis, we analyzed the effect of overproducing proline on the same characteristics: osmotolerance and virulence. We introduced all three independent proB mutations leading to proline overproduction and analogue resistance into the Listeria PSOE (ΔproBA) background. While each of the mutated genes conferred AZr on PSOE, the observed levels of proline overproduction were found to be approximately 10-fold lower than those of E. coli CSH26 (Fig. 2B).
While this evidence (AZr and proline overproduction, albeit at a reduced level) indicated a physiological consequence of the introduced mutations, none of the mutants exhibited an osmotolerant phenotype (data not shown). There are a number of possible explanations for this phenomenon, the most plausible of which concerns the extreme turgor requirement of gram-positive bacteria, which can be as much as seven times that of their gram-negative counterparts (21). Maintenance of elevated turgor requires the accumulation of high cytoplasmic concentrations of compatible solutes: e.g., while 0.5 mM proline is sufficient to promote maximal growth stimulation in E. coli at elevated osmolarities (9), upwards of 10 mM proline is required to facilitate growth of Listeria at a similar salt concentration (4). While this observed difference in proline concentration may well reflect the difference in turgor requirements of Listeria and E. coli, less efficient proline transport, coupled possibly with a more rapid breakdown of the accumulated proline against the Listeria background, cannot be ruled out. In any case the levels of proline overproduction observed, while sufficient to permit growth of E. coli at otherwise inhibitory salt concentrations, seem inadequate to restore sufficient turgor to PSOE bacteria.
Thus, increasing the capacity to produce proline alone may not be enough to confer osmotolerance. In S. marcessens, maximal proline production (and consequential osmotolerance) resulted not only from mutations in the proB gene leading to proline hyperproduction (29) but also from an unknown mutation leading to an increased production of glutamate (the substrate for GK), in combination with mutations in the putA gene, which result in a decreased rate of proline catabolism (38). The lack of an observed salt tolerance phenotype, when the proB mutations are transformed into the Listeria background, thus may reflect either a limiting concentration of glutamate (and/or ATP) or degradation of excess proline by the listerial PutA equivalent. Strain-specific effects may also contribute to the observed drop in proline production and excretion in Listeria, given that the proB mutations were originally isolated against an E. coli background and as such are presumably optimized for this environment.
Effects of proline overproduction on the virulence potential of L. monocytogenes.
In addition to its role as an osmolyte, which in itself could potentially provide a distinct growth advantage to Listeria when exposed to the elevated osmolarity (equivalent to 0.3 M NaCl [6]) of the gastrointestinal tract, proline has also been suggested to function as a potential virulence factor in certain pathogenic bacteria (2, 12, 33). Recent evidence suggests that at least in plant cells, proline may also act as a free radical scavenger, protecting the cells from the damaging effects of oxidative stress (17). Since an oxygen-dependent respiratory burst is one of the major mechanisms by which neutrophils and macrophages kill bacteria (25), proline hyperproduction may shield Listeria from the oxidative stress encountered within the macrophage phagosome.
To analyze the effects of proline hyperproduction on the virulence potential of L. monocytogenes, the plasmid carrying the ProB V121I mutation, which gave rise to the most pronounced osmotolerant phenotype in E. coli, was used to transform L. monocytogenes PSOE. The resulting strain (ProB++) was used to infect BALB/c mice, via the intraperitoneal and peroral routes. Similar to results obtained previously for proline auxotrophy (27, 35), proline hyperproduction did not affect colonization of the upper small intestine, nor did it disrupt invasion and spread to the internal organs (Table 2). Thus we conclude that neither proline hyperproduction nor inactivation of proline synthesis has any measurable effect on Listeria pathogenesis. Given that carnitine is most likely the predominant osmolyte in animal tissues (5), the effects if any of mutating proBA might well be masked by carnitine uptake, a hypothesis further evidenced by the fact that mutations in OpuC (a carnitine transport system) result in a significant reduction in the ability of Listeria to colonize the upper small intestine and cause subsequent systemic infection following peroral inoculation (36).
TABLE 2.
Recovery of L. monocytogenes LO28 and the ProB V121I mutant from the tissues of infected mice 3 days after intraperitoneal and peroral infection
| Type of inoculation and organ or tissue | Log10 no. of bacteria per organ or tissue (± SD)a
|
|
|---|---|---|
| ProB | ProB V121I | |
| Intraperitoneal | ||
| Liver | 5.2 (0.4) | 5.6 (0.4) |
| Spleen | 5.5 (0.4) | 5.8 (0.2) |
| Peroral | ||
| Liver | 4.5 (0.6) | 4.6 (0.3) |
| Spleen | 3.8 (0.2) | 3.7 (0.3) |
| Small intestine wall and contents | 4.1 (0.4) | 4.0 (0.5) |
| Peyer's patches | 2.6 (0.2) | 2.6 (0.4) |
Values are averages for four inoculated animals.
ACKNOWLEDGMENTS
We thank László Csonka (Purdue University) for providing E. coli CSH26.
This work has been supported by funding from BioResearch Ireland and the Irish Government under the National Development Plan 2000–2006. C.G.M.G. is the recipient of a Health Research Board (Ireland) Post-Doctoral Research Fellowship.
REFERENCES
- 1.Baumberg S, Klingel U. Biosynthesis of arginine, proline, and related compounds. In: Sonenshine A L, Hock J A, Losick R, editors. Bacillus subtilis and other Gram-positive bacteria: biochemistry, physiology, and molecular Genetics. Washington, D. C.: American Society for Microbiology; 1993. pp. 299–306. [Google Scholar]
- 2.Bayer A S, Coulter S N, Stover C K, Schwan W R. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect Immun. 1999;67:740–744. doi: 10.1128/iai.67.2.740-744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayles D O, Wilkinson B J. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett Appl Microbiol. 2000;30:23–27. doi: 10.1046/j.1472-765x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 4.Beumer R R, Te Giffel M C, Cox L J, Rombouts F M, Abee T. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl Environ Microbiol. 1994;60:1359–1363. doi: 10.1128/aem.60.4.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieber L L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury R, Sahu G K, Das J. Stress response in pathogenic bacteria. J Biosci. 1996;21:149–160. [Google Scholar]
- 7.Christian J H B. The influence of nutrition on the water relations of Salmonella oranienburg. Austr J Biol Sci. 1955;8:75–82. [Google Scholar]
- 8.Christian J H B. The water relations of growth and respiration of Salmonella oranienburg at 30°C. Austr J Biol Sci. 1955;8:490–497. [Google Scholar]
- 9.Csonka L N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182:82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- 10.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 12.Culham D E, Dalgado C, Gyles C L, Mamelak D, MacLellan S, Wood J M. Osmoregulatory transporter ProP influences colonization of the urinary tract by Escherichia coli. Microbiology. 1998;144:91–102. doi: 10.1099/00221287-144-1-91. [DOI] [PubMed] [Google Scholar]
- 13.Dandekar A M, Uratsu S L. A single base pair change in proline biosynthesis genes causes osmotic stress tolerance. J Bacteriol. 1988;170:5943–5945. doi: 10.1128/jb.170.12.5943-5945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delauney A J, Hu C-A A, Kavi Kishor P B, Verma D P S. Cloning of ornitine δ-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem. 1993;268:18673–18678. [PubMed] [Google Scholar]
- 15.Grant M M, Brown A S, Corwin L M, Troxler R F, Franzblau C. Effect of l-azetidine 2-carboxylic acid on growth and proline metabolism in Escherichia coli. Biochim Biophys Acta. 1975;404:180–187. doi: 10.1016/0304-4165(75)90324-4. [DOI] [PubMed] [Google Scholar]
- 16.Hayes F. Physical and genetic characterisation of plasmid DNA from Lactococcus lactis subsp. lactis UC317. Ph.D. thesis. Cork, Ireland: University College Cork; 1990. [Google Scholar]
- 17.Hong Z, Lakkineni K, Zhang Z, Verma D P S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C-A A, Delauney A J, Verma D P S. A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakowec M W, Smith L T, Dandekar A M. Recombinant plasmid conferring proline overproduction and osmotic tolerance. Appl Environ Microbiol. 1985;50:441–446. doi: 10.1128/aem.50.2.441-446.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavi Kishor P B, Hong Z, Miao G, Hu C, Verma D P S. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline overproduction and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolarity environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 22.Kosuge T, Hoshino T. Construction of a proline-producing mutant of the extremely thermophilic eubacterium Thermus thermophilus HB27. Appl Environ Microbiol. 1998;64:4328–4332. doi: 10.1128/aem.64.11.4328-4332.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisinger T. Biosynthesis of proline. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 434–441. [Google Scholar]
- 24.Le Rudulier D, Yang S S, Conka L N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982;719:273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- 25.Mahan M J, Slauch J M, Mekalanos J J. Environmental regulation of virulence gene expression in Escherichia, Salmonella, and Shigella spp. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd Edition. Washington, D.C.: American Society for Microbiology; 1996. pp. 1075–1090. [Google Scholar]
- 26.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 27.Marquis H, Archie Bouwer H G, Hinrichs D J, Portnoy D A. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61:3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massarelli I, Forlani G, Ricca E, De Felice M. Enhanced and feedback-resistant γ-glutamyl kinase activity of an Escherichia coli transformant carrying a mutated proB gene of Streptococcus thermophilus. FEMS Microbiol Lett. 2000;182:143–147. doi: 10.1111/j.1574-6968.2000.tb08888.x. [DOI] [PubMed] [Google Scholar]
- 29.Omori K, Suzuki S-I, Imai Y, Komatsubara S. Analysis of the mutant proBA operon from a proline-producing strain of Serratia marcescens. J Gen Microbiol. 1992;138:693–699. doi: 10.1099/00221287-138-4-693. [DOI] [PubMed] [Google Scholar]
- 30.Park S F, Stewart G S A B. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 31.Premaratne R J, Lin W-J, Johnson E A. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rushlow K E, Deutch A H, Smith C J. Identification of a mutation that relieves gamma-glutamyl kinase from allosteric feedback inhibition by proline. Gene. 1984;39:109–112. doi: 10.1016/0378-1119(85)90115-5. [DOI] [PubMed] [Google Scholar]
- 33.Schwan W R, Coulter S N, Ng E Y W, Langhorne M H, Ritchie H D, Brody L L, Westbrock-Wadman S, Bayer A S, Folger K R, Stover C K. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect Immun. 1998;66:567–572. doi: 10.1128/iai.66.2.567-572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleator R D, Gahan C G M, O'Driscoll B, Hill C. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int J Food Microbiol. 2000;60:261–268. doi: 10.1016/s0168-1605(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 35.Sleator R D, Gahan C G M, Hill C. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl Environ Microbiol. 2001;67:2571–2577. doi: 10.1128/AEM.67.6.2571-2577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sleator R D, Wouters J, Gahan C G M, Abee T, Hill C. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl Environ Microbiol. 2001;67:2692–2698. doi: 10.1128/AEM.67.6.2692-2698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith C J, Deutch A H, Rushlow K E. Purification and characteristics of a γ-glutamyl kinase involved in Escherichia coli proline biosynthesis. Appl Environ Microbiol. 1984;157:545–551. doi: 10.1128/jb.157.2.545-551.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura M, Kisumi M. Proline-hyperproducing strains of Serratia marcescens: enhancement of proline analogue-mediated growth inhibition by increasing osmotic stress. Appl Environ Microbiol. 1985;49:782–786. doi: 10.1128/aem.49.4.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C-S, Lu Q, Verma D P S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem. 1995;270:20491–20496. doi: 10.1074/jbc.270.35.20491. [DOI] [PubMed] [Google Scholar]