Abstract
Objective:
Acute respiratory distress syndrome (ARDS) after out-of-hospital cardiac arrest is common and associated with worse outcomes. In the hospital setting, there are many potential risk factors for post-arrest ARDS, such as aspiration, sepsis, and shock. ARDS after in-hospital cardiac arrest (IHCA) has not been characterized.
Methods:
We performed a single-center retrospective study of adult patients admitted to the hospital between 2014–2018 who suffered an IHCA, achieved return of spontaneous circulation (ROSC), and were either already intubated at the time of arrest or within 2 hours of ROSC. Post-IHCA ARDS was defined as meeting the Berlin criteria in the first 3 days following ROSC. Outcomes included alive-and-ventilator free days across 28 days, hospital length-of-stay, hospital mortality, and hospital disposition.
Results:
Of 203 patients included, 146 (71.9%) developed ARDS. In unadjusted analysis, patients with ARDS had fewer alive-and-ventilator-free days over 28 days with a median of 1 (IQR: 0, 21) day, compared to 18 (IQR: 0, 25) days in patients without ARDS (p = 0.03). However, this association was not significant after multivariate adjustment. There was also a non-significant longer hospital length-of-stay (15 [IQR: 7, 26] vs 10 [IQR: 7, 22] days, p = 0.25; median adjusted increase in ARDS patients: 3 [95% CI: −2 to 8] days, p = 0.27) and higher hospital mortality (53% vs 44%, p = 0.26; aOR 1.6 [95% CI: 0.8–2.9], p = 0.17) in the ARDS group.
Conclusion:
Among IHCA patients, almost three-quarters developed ARDS within 3 days of ROSC. As in out of hospital cardiac arrest, post-IHCA ARDS is common.
Keywords: Cardiac arrest, ARDS, Resuscitation
Introduction
Acute respiratory distress syndrome (ARDS) after out-of-hospital cardiac arrest (OHCA) is common and has been associated with worse outcomes.1-3 In one large cohort of patients successfully resuscitated from OHCA, 50% developed ARDS and the development of ARDS was associated with worse outcomes.1 While several studies have examined ARDS after OHCA, ARDS after in-hospital cardiac arrest (IHCA) has not been characterized. IHCA occurs in over 290,000 adults in the United States each year, and there are important differences between the OHCA and IHCA populations in epidemiology, causes, and outcomes.5
Many factors surrounding cardiac arrest place post-arrest patients at high risk of developing lung injury. In addition to common risk factors of ARDS such as aspiration, pneumonia, sepsis, and shock,5 cardiac arrest introduces other potential precipitants including trauma from chest compressions, hemodynamic collapse, ventilator-induced injuries, and reperfusion injury.6,7 Given prior associations of ARDS with worse outcomes in the OHCA setting, strategies to reduce the incidence of ARDS post-resuscitation in IHCA may be important areas of future investigation.
In this study, we characterize the occurrence and associated outcomes of ARDS in the IHCA population.
Methods
Study design and cohort selection
This was a single-center, retrospective analysis utilizing a prospectively collected database of patients experiencing an IHCA at an urban tertiary care center. The inclusion criteria included adult patients (aged 18 or older) admitted to the hospital between 2014 and 2018 who suffered IHCA, achieved return of spontaneous circulation (ROSC), and were either intubated at time of resuscitation or within 2 hours of arrest. Patients who died or were extubated on the day of resuscitation and those with pre-arrest ARDS were excluded. Only the index arrest was included in patients with more than one arrest (Fig. 1). This study was approved by the institutional review board of Beth Israel Deaconess Medical Center.
Fig. 1 –
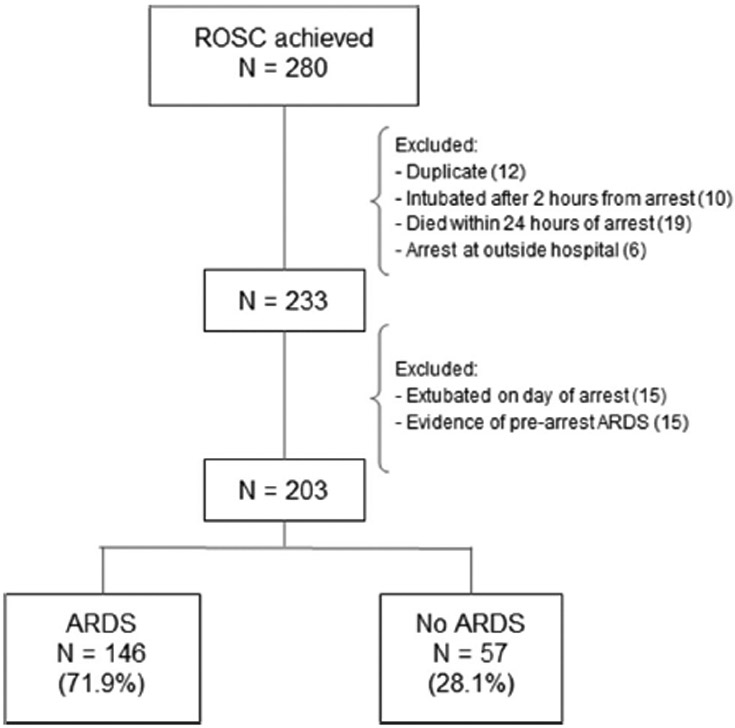
Consort Diagram.
Data collection
Demographic data, comorbidities, risk factors, cardiac arrest characteristics, laboratory data, post-arrest interventions, and outcomes were extracted from an existing database and missing data were gathered via chart review of the electronic medical record. Risk factors for ARDS were categorized as non-cardiogenic shock, aspiration or pneumonia, overdose, sepsis not from pulmonary source, and major trauma. The lowest PaO2 and its corresponding FiO2 were collected across each 24-hour period for 3 days after the day of ROSC. If the PaO2 was not recorded during a 24-hour period, the oxygen saturation in the blood (SpO2) was used as a proxy for PaO2 using a conversion equation.8 The presence of bilateral opacities was determined based on chest imaging radiology reports obtained during the 3-day window. The first GCS recorded after arrest and lactate values within 2 hours of arrest were recorded. Evaluation of clinical practices was performed via chart review and included the use of a continuous midazolam infusion for at least 12 hours, high tidal volume ventilation of > 8 cc/kg for at least 6 consecutive hours, elevated driving pressure > 15 cm H2O for at least 6 consecutive hours, hyperoxia, defined as PaO2 > 300 mmHg, at any time, and fluid balance over 3 days following ROSC.
Primary exposure
ARDS was defined using the Berlin criteria.6 The occurrence of ARDS was evaluated during a 3-day period following ROSC to allow enough time for ARDS to develop but not so long that ARDS was likely to be attributable to other causes. A sensitivity analysis was conducted after removing patients with echocardiograms within 3 days of arrest showing an ejection fraction < 40%, as a possible indicator of cardiogenic pulmonary edema rather than ARDS.
Primary and secondary outcomes
The primary outcome was alive-and-ventilator free days across 28 days. Secondary outcomes included hospital length-of-stay, hospital mortality, and hospital disposition. For the outcome of alive-and-ventilator free days, we used the following definitions and assumptions: 1) a patient was not considered to be on a ventilator if they had an invasive airway (eg, tracheostomy) but were not receiving mechanical ventilation, 2) patients who died within 28 days could have alive-and-ventilator free days across 28 days if they were extubated prior to death, 3) a patient who was discharged to a facility while on mechanical ventilation was assumed to continue on the ventilator for the remainder of the 28 days, and 4) a patient who was discharged to home or a facility was assumed to survive for 28 days. There was one patient discharged to hospice with unknown time of death; time of death was assumed to be the time of discharge.
Statistical analysis
Descriptive statistics were reported as means with standard deviations or medians with interquartile ranges based on the distribution of data. Categorical data were presented as counts with frequencies. Between-group comparisons were made with Fisher’s exact tests or Chi-square tests for categorical data and Student’s t-tests or Wilcoxon ranked sum tests for continuous data. Logistic regression was used post-hoc to evaluate the relationship between the development of post-arrest ARDS (primary exposure) and the outcomes of logged alive-and-ventilator free days (with a value of 0 changed to 0.0001 prior to transforming the variable) and mortality controlling for rhythm, downtime, age, and location of arrest. Downtime was defined as time from cardiac arrest to ROSC. Quantile regression was used post-hoc to determine the median difference in alive-and-ventilator free days and hospital length of stay in all patients, adjusting for rhythm, age, location of arrest, and downtime. These analyses were not repeated in survivors only due to risk of model overfit. Statistical analyses were performed using STATA SE/16 and R-studio. Two tailed p-value < 0.05 were used to determine statistical significance.
Results
A total of 203 patients were included. The majority of patients had initial nonshockable rhythms (n = 148, 76%). Patients with ARDS were more likely to have had an initial rhythm of asystole compared to those without ARDS (13% vs 2%, p = 0.02). The suspected causes of arrest were cardiac in 81 (40%) patients and respiratory in 64 (32%) patients. The median arrest downtime was 9 minutes (IQR: 5, 15) with 20% having a downtime of 20 minutes or greater. The remainder of the baseline characteristics can be found in Table 1.
Table 1 –
Baseline characteristics.
| Overall n = 203 |
ARDS n = 146 |
No ARDS n = 57 |
P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 69 (58–77) | 69 (56–77) | 67 (61–76) | 0.85 |
| Female, n (%) | 72 (35) | 54 (37) | 18 (32) | 0.47 |
| White race, n (%) | 111 (55) | 82 (56) | 29 (51) | 0.60 |
| White race (excluding unknown), n (%) | 111/172 (65) | 82/124 (66) | 29/48 (60) | 0.48 |
| Comorbidities, n (%) | ||||
| Congestive heart failure | 43 (21.2) | 30 (20.5) | 13 (22.8) | 0.72 |
| Coronary artery disease | 64 (31.5) | 42 (28.8) | 22 (38.6) | 0.18 |
| Chronic pulmonary disease | 31 (15.3) | 27 (18.5) | 4 (7.0) | 0.05 |
| Diabetes mellitus | 80 (39.4) | 54 (37.0) | 26 (45.6) | 0.26 |
| Chronic kidney disease | 54 (26.6) | 34 (23.3) | 20 (35.1) | 0.09 |
| Substance use disorder | 53 (26.1) | 37 (25.3) | 16 (28.1) | 0.69 |
| Arrhythmia | 32 (15.8) | 23 (15.8) | 9 (15.8) | >0.99 |
| Malignancy | 41 (20.2) | 29 (19.9) | 12 (21.1) | 0.85 |
| Cirrhosis | 18 (8.9) | 14 (9.6) | 4 (7.0) | 0.78 |
| Stroke | 17 (8.4) | 10 (6.8) | 7 (12.3) | 0.26 |
| Dementia | 9 (4.4) | 8 (5.5) | 1 (1.8) | 0.45 |
| ARDS risk factors, n (%) | ||||
| Any | 145 (71.4) | 108 (74.0) | 37 (64.9) | 0.20 |
| Shock, non-cardiogenic | 94 (46.3) | 70 (47.9) | 24 (42.1) | 0.45 |
| Aspiration or pneumonia | 68 (33.5) | 54 (37.0) | 14 (24.6) | 0.09 |
| Overdose | 6 (3.0) | 6 (4.1) | 0 (0) | 0.19 |
| Sepsis, not from pulmonary source | 38 (18.7) | 28 (19.2) | 10 (17.5) | 0.79 |
| Major trauma | 8 (3.9) | 5 (3.4) | 3 (5.3) | 0.69 |
Overall, 146/203 (72%) patients developed ARDS during the 3 days following ROSC. Among those with ARDS, the mean PaO2 to FiO2 ratio was 188 mmHg (SD 110) in the first 24-hour period following the day of ROSC, 206 mmHg (SD 83) in the second 24-hour period, and 211 mmHg (SD 81) in the third period. Of those with ARDS, most had mild (PaO2:FiO2 201–300 mmHg, 30%) or moderate (PaO2:FiO2 101–200 mmHg, 47%) disease (Table 3). There were no significant differences in the demographics, comorbidities, ARDS risk factors, or arrest interventions between patients with post-arrest ARDS and those without (Table 2). 69% of those who had post-arrest ARDS had an initial GCS score of 3 after ROSC compared to 46% without ARDS (p < 0.01).
Table 3 –
Incidence of ARDS.
| Overall n = 203 |
ARDS n = 146 |
No ARDS n = 57 |
|
|---|---|---|---|
| Incidence, n (%) | 146 (71.9%) | N/A | N/A |
| PaO2:FiO2 ratio, mean (SD) | |||
| Day 1a | 218 (143) | 188 (110) | 295 (186) |
| Day 2 | 234 (115) | 206 (83) | 309 (153) |
| Day 3 | 235 (111) | 211 (81) | 307 (152) |
| Severity of ARDS, n (%) | |||
| Mild (PaO2 to FiO2 ratio 201–300 mmHg) | N/A | 44 (30.1%) | N/A |
| Moderate (PaO2 to FiO2 ratio 101–200 mmHg) | N/A | 68 (46.6%) | N/A |
| Severe (PaO2 to FiO2 ratio 0–100 mmHg) | N/A | 34 (23.3%) | N/A |
Abbreviations: PaO2 (partial pressure of oxygen in arterial blood), FiO2 (fraction of inspired oxygen).
Days 1–3 correspond with each 24-hour period for 3 days after the day of ROSC.
Table 2 –
Cardiac arrest characteristics.
| Overall n = 203 |
ARDS n = 146 |
No ARDS n = 57 |
P-value | |
|---|---|---|---|---|
| Characteristics | ||||
| Initial rhythm, n (%)a | 0.10 | |||
| Asystole | 19 (9.7) | 18 (12.9) | 1 (1.8) | 0.02 |
| Pulseless electrical activity | 129 (66.2) | 90 (64.3) | 39 (70.9) | 0.38 |
| Ventricular fibrillation | 19 (9.7) | 13 (9.2) | 6 (10.9) | 0.79 |
| Ventricular tachycardia | 28 (14.4) | 19 (13.6) | 9 (16.4) | 0.65 |
| Location, n (%) | 0.16 | |||
| Emergency department | 36 (17.7) | 28 (19.2) | 8 (14.0) | |
| Intensive care unit | 61 (30.0) | 47 (32.2) | 14 (24.6) | |
| Floor | 60 (29.6) | 44 (30.1) | 16 (28.1) | |
| Other | 46 (22.7) | 27 (18.5) | 19 (33.3) | |
| Suspected cause, n (%) | 0.93 | |||
| Cardiac | 81 (39.9) | 57 (39.0) | 24 (42.1) | |
| Respiratory | 64 (31.5) | 48 (32.9) | 16 (28.1) | |
| Other | 25 (12.3) | 18 (12.3) | 7 (12.3) | |
| Unknown | 33 (16.3) | 23 (15.8) | 10 (17.5) | |
| Arrest downtime in minutes, median (IQR) | 9 (5–15) | 9 (5–15) | 7 (4–15) | 0.27 |
| <10, n (%) | 124 (61.1) | 86 (58.9) | 38 (66.7) | |
| 10–19, n (%) | 40 (19.7) | 30 (20.5) | 10 (17.5) | |
| ≥20, n (%) | 39 (19.2) | 30 (20.5) | 9 (15.8) | |
| Data | ||||
| Post-ROSC GCS 3, n (%) | 127 (62.6) | 101 (69.2) | 26 (45.6) | 0.002 |
| Lactate within 2 hours, median (IQR)b | 5.9 (3.0–9.0) n = 198 |
6.0 (3.2–9.2) n = 142 |
5.5 (2.5–8.2) n = 56 |
0.13 |
| Ejection fraction %, mean (SD)c | 39 (20) n = 158 |
38 (19) | 43 (21) | 0.11 |
| ≤ 40, n (%) | 90 (57.0) | 68 (59.6) | 22 (50.0) | |
| > 40, n (%) | 68 (43.0) | 46 (40.4) | 22 (50.0) | |
| Interventions, n (%) | ||||
| Targeted temperature management | 77 (37.9) | 57 (39.0) | 20 (35.1) | 0.60 |
| EEG (within 1 day) | 81 (39.9) | 58 (39.7) | 23 (40.4) | 0.94 |
| Vasopressor (within 1 day) | 176 (86.7) | 129 (88.4) | 47 (82.5) | 0.27 |
| Coronary angiogram (within 3 days) | 49 (24.1) | 33 (22.6) | 16 (28.1) | 0.41 |
Abbreviations: GCS (glascow coma scale), EEG (electroencephalography).
There were 8 unknown rhythms that were not included in the analysis.
There were 5 missing lactate values that were not collected within 2 hours of ROSC.
There were 45 missing ejection fraction values that were not collected within 2 hours of ROSC.
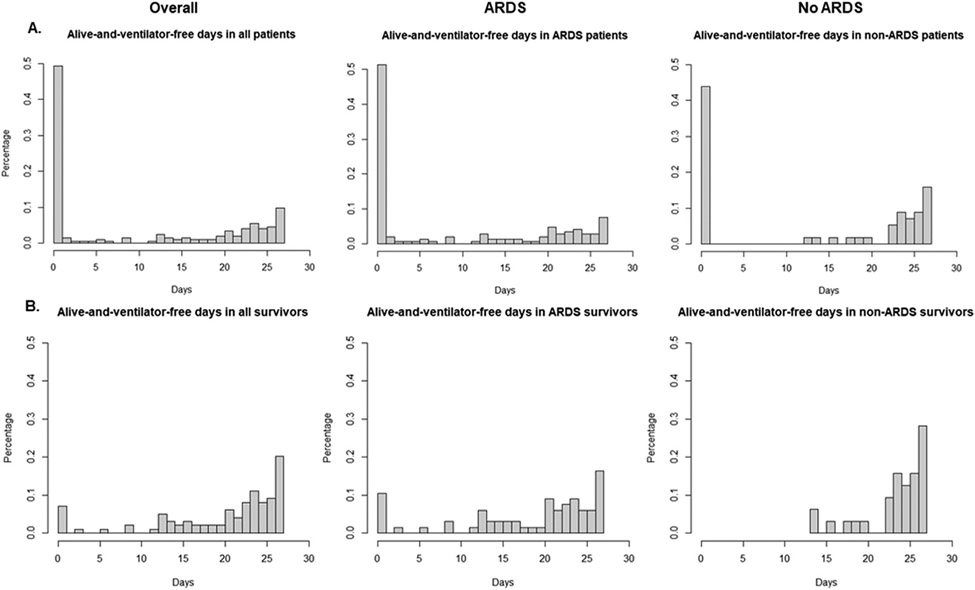
Patients with post-arrest ARDS had fewer alive-and-ventilator-free days over 28 days with a median of 1 (IQR: 0, 21) day, compared to 18 (IQR: 0, 25) days in patients without post-arrest ARDS (p = 0.03) (Fig. 2a). Among survivors to 28 days, the median ventilator-free days in those with ARDS was significantly less than those without ARDS (22 [IQR: 15, 25] vs 25 [IQR: 23, 27] days, p < 0.01) (Fig. 2b). In adjusted analysis, patients with post-arrest ARDS had a median of 8 (95% CI: −1 to 16, p = 0.07) fewer alive-and-ventilator-free days over 28 days compared to those without post-arrest ARDS, which no longer met the a priori alpha threshold for statistical significance. This is similar to what was found when using the logged value of alive-and-ventilator-free days over 28 days (adjusted odds ratio: 0.42 [95% CI: 0.06–2.75], p = 0.4). There was longer hospital length-of-stay (15 [IQR: 7, 26] vs 10 [IQR: 7, 22] days, p = 0.25; median adjusted increase in ARDS patients: 3 [95% CI: −2 to 8] days, p = 0.274) and higher hospital mortality (53% vs 44%, p = 0.26; aOR 1.6 [95% CI: 0.8–2.9], p = 0.173) in the patients with post-arrest ARDS, although these did not reach statistical significance. There was no difference in use of midazolam, low tidal volume ventilation, driving pressures, hyperoxia, or fluid balance between patients who developed ARDS and those who did not (Table 4). Controlling for rhythm, age, and arrest location, an increase in arrest downtime was associated with increased likelihood of developing ARDS, although this also did not reach statistical significance (adjusted odds ratio 1.4 per 10 minute increase in arrest duration, 95% CI [1.0–1.9]; p = 0.08).
Fig. 2 –
Alive-and-ventilator free days in all patients and survivors of 28 days. Histograms of alive-and-ventilator free days over 28 days in all patients (A, n = 203) and survivors to 28 days (B, n = 99).
Table 4 –
Outcomes and clinical practices.
| Overall n = 203 |
ARDS n = 146 |
No ARDS n = 57 |
P-value | |
|---|---|---|---|---|
| Alive and vent free days at 28 days, median (IQR) | 2 (0–23) | 1 (0–21) | 18 (0–25) | 0.03 |
| Alive and vent free days at 28 days, 28-day survivorsa, median (IQR) | 23 (17–26) | 22 (15–25) | 25 (23–27) | <0.01 |
| Hospital length-of-stay, median (IQR) | 13 (7–25) | 15 (7–26) | 10 (7–22) | 0.25 |
| Hospital mortality, n (%) | 102 (50.3) | 77 (52.7) | 25 (43.9) | 0.26 |
| Hospital disposition, n (%) | 0.13 | |||
| Home | 23 (11.3) | 12 (8.2) | 11 (19.3) | |
| Rehabilitation center | 73 (36.0) | 53 (36.3) | 20 (35.1) | |
| Death or hospice | 106 (52.2) | 80 (54.8) | 26 (45.6) | |
| Other | 1 (0.5) | 1 (0.7) | 0 (0) | |
| Use of midazolam, n (%) | 53 (26.1) | 43 (29.5) | 10 (17.5) | 0.08 |
| Not LTTV, Vt > 8 cc/kg, n (%) | 32 (15.8) | 22 (15.1) | 10 (17.5) | 0.66 |
| Driving pressure > 15 cmH2O, n (%)b | 50 (25.3) | 34 (23.9) | 16 (28.6) | 0.50 |
| Hyperoxia, PaO2 > 300 mmHg, n (%)c | 57 (28.5) | 37 (25.7) | 20 (35.7) | 0.16 |
| Fluid balance over 3 days in cc, median (IQR) | 3622 (170–7500) | 3450 (112–7600) | 3800 (600–6700) | 0.67 |
Abbreviations: LTTV (low tidal volume ventilation), Vt (tidal volume).
This included 99 patients who survived to 28 days.
Excludes 5 unknown driving pressures.
Excludes 3 unknown hyperoxia levels.
A sensitivity analysis excluding 90 patients with ejection fraction < 40% showed an incidence of post-arrest ARDS of 67.7%. There were fewer median alive-and-ventilator-free days in patients with ARDS (0 [IQR: 0, 20] vs 20 [IQR: 0, 26] days, p = 0.07; adjusted median difference: −7 [95% CI: −19–5] days, p = 0.28) compared to patients without ARDS, although this difference was not statistically significant.
Discussion
In this study, we found a high incidence (72%) of ARDS after ROSC in patients with IHCA. The occurrence of ARDS was associated with fewer alive-and-ventilator-free days, although this did not reach the level of statistical significance after multivariate adjustment–potentially owing to lack of statistical power and resultant Type II error. These findings suggest that ARDS after IHCA is common and could be a potential target for future intervention.
In comparison to prior studies in the OHCA population, this study shows a high incidence of ARDS after IHCA. Almost three quarters of patients developed post-arrest ARDS, which is likely an underrecognized diagnosis in this population. A retrospective study by Johnson et al. found an incidence of 48% with mean P:F ratio of 155 mmHg in mechanically ventilated OHCA patients.1 The higher incidence of ARDS in our study can be explained by different characteristics in the IHCA compared to OHCA populations. For example, IHCA may be more likely to occur due to other acute illnesses (eg, sepsis), which are known risk factors for ARDS. Our study also used a longer window of 3 days after resuscitation to evaluate for ARDS rather than the 48 hours used by Johnson et al.
In combination with the existing literature, our results suggest that patients with post-arrest ARDS are at higher risk of longer mechanical ventilation and death as compared to those without ARDS. Alive-and-ventilator-free days is a commonly used tool in ARDS studies9 that is not as commonly applied to the post-arrest population. Alive-and-ventilator free days may be a useful composite outcome for post-cardiac arrest studies since mechanical ventilation is used not just for respiratory failure but also as a supportive measure for a range of organ failures (eg, neurologic failure). Our study shows a median of 17 fewer alive-and-ventilator-free days in patients with post-arrest ARDS than without ARDS. While this difference was not statistically significant (p-value 0.07) after adjustment for rhythm, age, downtime, and location of arrest, this lack of significance could be due to insufficient statistical power to detect a true difference. Similarly, there was a 9% absolute increase in mortality in the post-arrest patients with ARDS, although this also was not statistically significant. This study’s findings can serve as a basis for future studies on ARDS in the post-arrest population.
ARDS is a heterogeneous entity in its clinical presentation, course, and outcomes.10-12 While it is often referred to as a distinct entity, the clinical syndrome of ARDS may be a collection of distinct types of lung injuries with similar appearances on chest radiographs and clinical presentations. Specifically within the peri-arrest context, a multitude of factors could precipitate and contribute to the development of ARDS such as aspiration, ventilator-induced injury, reperfusion injury, and pulmonary contusion. As such, post-arrest lung injury meeting the clinical criteria for ARDS likely encompasses a variety of types of injury that have yet to be further characterized on a clinical and biological level. As ARDS continues to have high mortality without targeted therapies,13 it is important to recognize the heterogeneity of the syndrome and classify its phenotypes by etiology to guide better diagnosis and treatment.12,14-16.
Limitations/challenges
Our study has several limitations. First, there was a relatively small sample size which likely led to inadequate power to detect a significant difference for the outcomes. The period 2014–2018 was selected to represent a relatively contemporary cohort, but also one that was not impacted by epidemiological changes related to the COVID-19 pandemic. Second, the single center study may limit generalizability of results. Third, the extent of which cardiogenic pulmonary edema could fully explain respiratory failure was difficult to assess via chart review and this may have overestimated the incidence of ARDS. Lastly, we chose a time frame of three days after ROSC to assess for the development of ARDS, which may have captured ARDS developing from other causes besides cardiac arrest.
Conclusions
Among IHCA patients at a single center, almost three-quarters developed ARDS within 3 days of ROSC. ARDS was not associated with worse outcomes, such as alive-and-ventilator-free days or mortality, although the study may have been underpowered to detect a difference.
Acknowledgements
AM was funded by K23GM128005. MD was funded by K24HL127101 and R01HL136705.
Footnotes
Conflict of Interest
The manuscript has not been published previously and is not under consideration in another journal. The authors report no external funding source for this study and declare no conflict of interest.
CRediT authorship contribution statement
All authors contributed substantially to the paper and are accountable for all aspects of the work. JS, AM, KB, and MD were involved in conceptualization. JS, HR, MI, and AG were involved in data curation and formal analysis. All authors participated in writing and reviewing of the manuscript.
REFERENCES
- 1.Johnson NJ, Caldwell E, Carlbom DJ, et al. The acute respiratory distress syndrome after out-of-hospital cardiac arrest: Incidence, risk factors, and outcomes. Resuscitation 2019;135:37–44. 10.1016/j.resuscitation.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Kim J-S, Kim Y-J, Kim M, et al. The impact of severity of acute respiratory distress syndrome following cardiac arrest on neurologic outcomes. Ther Hypothermia Temp Manag 2021;11:96–102. 10.1089/ther.2019.0047. [DOI] [PubMed] [Google Scholar]
- 3.Elmer J, Wang B, Melhem S, et al. Exposure to high concentrations of inspired oxygen does not worsen lung injury after cardiac arrest. Crit Care 2015;19:105. 10.1186/s13054-015-0824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-Hospital Cardiac Arrest: A Review. JAMA 2019;321:1200–10. 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:1904–5. 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NJ, Carlbom DJ, Gaieski DF. Ventilator management and respiratory care after cardiac arrest: oxygenation, ventilation, infection, and injury. Chest 2018;153:1466–77. 10.1016/j.chest.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410–7. 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 9.Schoenfeld DA, Bernard GR, Network ARDS. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772–7. [DOI] [PubMed] [Google Scholar]
- 10.Maley JH, Thompson BT. Embracing the heterogeneity of ARDS. Chest 2019;155:453–5. 10.1016/j.chest.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care 2020;24:102. 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan YA, Fan E, Ferguson ND. Precision medicine and heterogeneity of treatment effect in therapies for ARDS. Chest 2021;160:1729–38. 10.1016/j.chest.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med 2017;5:524–34. 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 14.Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II. Rapidly improving ARDS in therapeutic randomized controlled trials. Chest 2019;155:474–82. 10.1016/j.chest.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018;6:691–8. 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611–20. 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]