Abstract
Background: The present systematic review and meta-analysis investigated the cross-sectional and longitudinal associations between protein intake and sarcopenia in older adults. Methods: Observational studies that investigated the association between protein intake and sarcopenia as the primary or secondary outcome in people aged 60 years and older were included. Studies published in languages other than English, Italian, Portuguese, and Spanish were excluded. Studies were retrieved from MEDLINE, SCOPUS, EMBASE, CINAHL, AgeLine, and Food Science Source databases through January 31, 2022. A pooled effect size was calculated based on standard mean differences. Results: Five cross-sectional studies, one longitudinal study, and one case-control study that investigated 3353 community-dwelling older adults with a mean age of approximately 73 years were included. The meta-analysis of four studies indicated that older adults with sarcopenia consumed significantly less protein than their peers with no sarcopenia. Conclusions: Results of the present study suggest that an inadequate protein intake might be associated with sarcopenia in older adults.
Keywords: nutrition, anorexia, physical function, walking speed, muscle strength, dynapenia, frailty, elderly
1. Introduction
Sarcopenia is a neuromuscular disease characterized by muscle atrophy, dynapenia, and loss of physical function [1,2,3,4,5]. The overall prevalence of sarcopenia might reach up to 86.5% in adults depending on the definition used and the setting of evaluation, and is especially high in the older population [6]. This scenario deserves concern, given that the progression of sarcopenia is associated with the incidence of numerous negative events, including malnutrition, anorexia, physical inactivity, metabolic and osteoarticular disorders, cognitive impairment, falls, depressive symptoms, and death [7,8]. As such, sarcopenia is recognized as a public health problem and the identification of potential strategies to prevent its development and progression is a priority [1,2].
Nutrition is a modifiable lifestyle factor that may be harnessed to foster active and healthy aging [9,10]. In particular, a protein consumption higher than the current recommended dietary allowance (RDA, 0.8 g/kg/day) is proposed as a strategy to preserve muscle mass and physical function in advanced age [11,12,13,14]. This recommendation is based on the fact that the aged muscle requires a greater amount of amino acids (AAs) to maximally stimulate muscle protein synthesis (MPS) in response to hyperaminoacidemia, a phenomenon known as anabolic resistance [15,16,17,18,19,20]. The failure to properly stimulate MPS predisposes to gradual loss of muscle mass, mainly of type II muscle fibers, which impacts muscle strength generation and physical function [21,22]. Although it is commonly believed that an adequate protein intake could prevent the development of sarcopenia or at least attenuate its progression [23,24,25], findings on the matter are inconclusive.
To provide an up-to-date and comprehensive appraisal of the topic, we conducted a systematic review and meta-analysis of cross-sectional and longitudinal studies that explored the relationship between protein intake and sarcopenia in older adults.
2. Materials and Methods
This is a systematic review and meta-analysis of observational studies that investigated cross-sectional and longitudinal associations between protein intake and sarcopenia. The study was fully performed by investigators and no librarian was part of the team. The study complies with the criteria of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [26] and the Cochrane Handbook for Systematic Reviews and Interventions [27].
2.1. Eligibility Criteria
Inclusion criteria were: (1) observational studies (e.g., case-control, cross-sectional, cohort longitudinal studies) that investigated the association between protein intake and sarcopenia; (2) participants aged 60 years or older; (3) sarcopenia identified according to the presence of muscle atrophy plus dynapenia, low muscle power, physical dysfunction, reduced mobility, and/or low scores on batteries of physical performance tests (e.g., Short Physical Performance Battery (SPPB)); (4) published studies in English, Italian, Portuguese, or Spanish language.
To be included in the meta-analysis of cross-sectional studies, investigations should provide mean and standard deviation (SD) of case (i.e., high protein intake (HPI)) and control groups (i.e., low protein intake, (LPI)), or at least two groups divided according to protein consumption and the sample size of each group, or Pearson’s correlation coefficient (r)/Betas (β)/odds ratio (OR) values for the association between protein intake and sarcopenia. For the meta-analysis of longitudinal studies, investigations should provide the number of participants, β, OR, hazard ratio, and/or risk ratio for the development of sarcopenia according to protein consumption levels. We excluded randomized controlled trials, quasi-experimental, cross-over, and preclinical studies, as well as investigations that examined the effects of nutritional interventions alone or combined with other interventions (e.g., physical exercise) on sarcopenia. Studies that enrolled participants with gastrointestinal and/or renal diseases, anorexia, cancer, or any condition that may directly impair protein metabolism (e.g., maple syrup urine disease, tyrosinemia) were also excluded.
2.2. Search Strategy and Selection Criteria
Studies published on or before 31 January 2022 were retrieved from the following six electronic databases by one investigator: (1) MEDLINE (PubMed interface); (2) SCOPUS (Elsevier interface); (3) EMBASE (OVID interface), (4) CINAHL (EBSCO interface); (5) AgeLine (EBSCO interface); and (6) Food Science Source (EBSCO interface). Further eligible articles were identified by checking the reference lists of retrieved articles. In addition, citation searches on key articles were performed in Google Scholar and ResearchGate. Initially, a search strategy was designed using keywords, MeSH terms, and free text words, such as "protein intake", "sarcopenia", and "older adults". Afterwards, keywords and subject headings were exhaustively combined using Boolean operators. The complete search strategy is shown in Supplementary Material S1.
2.3. Data Extraction, Quality Assessment, and Risk of Bias
Titles and abstracts of retrieved articles were screened for eligibility by two researchers (HJCJ, RC). The full text was consulted if the abstract did not provide enough information for final evaluation. Two reviewers (HJCJ, RC) extracted the coded variables (i.e., methodological quality, risk of bias, and characteristics of the studies) using a standardized coding form. A third researcher was consulted to solve disagreements (EM), if necessary. The quality of reporting for each study was performed by two researchers (HJCJ, RC) using the Quality Assessment Tool for Observational Cohort and Cross-Sectional of the National Institutes of Health [28]. This tool contains 14 questions that assess several aspects associated with the risk of bias, type I and type II errors, transparency, and confounding factors. The studies were positive for item 8 if they investigated protein sources and/or distribution. Items 6, 7, and 13 do not refer to cross-sectional studies and were removed from the quality analysis. The maximum scores for cross-sectional and prospective studies were 11 and 14, respectively. The agreement rate for quality assessment between reviewers was 98%.
2.4. Statistical Analysis
The meta-analysis was conducted using Revman 5.4.1 (Cochrane Collaboration, Copenhagen, Denmark). Effect sizes (ESs) were measured using means and SDs. Central and dispersion values were obtained from included studies or were calculated according to the Cochrane guidelines [27]. Specifically, medians were assumed as means when studies presented symmetrical data. SDs were calculated from confidence intervals (CIs) and standard errors (SEs), according to the following formulas:
| SD1 = √N × (Upper limit − Lower limit)/3.92 | (1) |
| SD2 = SE × √N | (2) |
From the interquartile range, SDs were obtained according to the formulas proposed by Luo [29] and Shi [30]. A single pairwise comparison was created when multiple studies referred to the same database, using the formulas proposed by the Cochrane guidelines [27]. The pooled ES was calculated based on standard mean differences (SMDs), because studies used different tests and/or protocols to operationalize sarcopenia. Due to the variability of sample characteristics, a random-effect model was used to calculate the pooled ES. Additionally, the I2 index was classified as "might not be important" (0–40%), "may represent moderate heterogeneity" (30–60%), "may represent substantial heterogeneity" (50–90%), or "may represent considerable heterogeneity" (75–100%) [27]. Forest plots were used to illustrate the summary statistics and the variation (heterogeneity) across studies.
3. Results
3.1. Literature Search
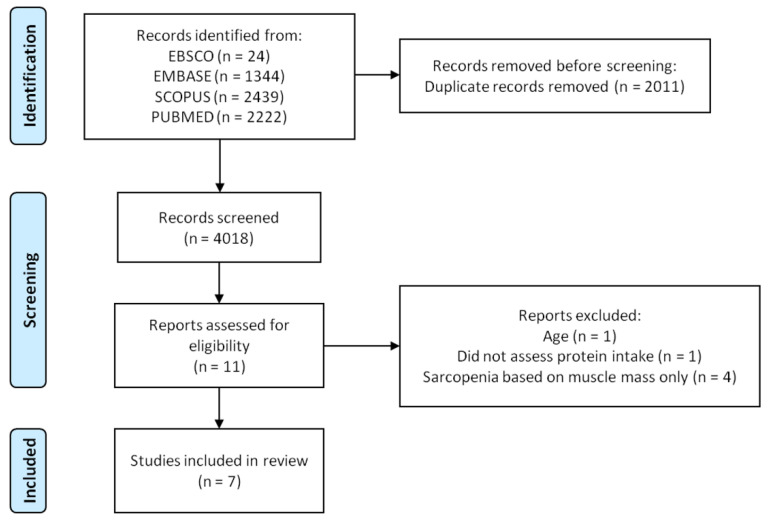
Six-thousand and twenty-nine records were identified through database and hand searches. Of these, 2011 were excluded based on duplicated data, and 4018 titles and abstracts were evaluated. Eleven articles were fully assessed for eligibility and four studies were excluded based on selection criteria (Supplementary Material S2). Seven articles were included in the investigation. The flowchart of the study is shown in Figure 1.
Figure 1.
Flowchart of the study.
3.2. Characteristics of the Included Studies
The main characteristics of the included studies are shown in Table 1. Five cross-sectional studies [31,32,33,34,35], one longitudinal study [36], and one case-control study [37] that investigated 3353 community-dwelling older adults with a mean age of approximately 73 years from Australia, Belgium, Finland, India, and the Netherlands were included. One study [32] included participants from Italy, Poland, the Netherlands, and the United Kingdom. Nutritional habits were assessed using 24-h dietary recall, 3- and 7-day food records, diet history, and food frequency questionaries. Sarcopenia was operationalized according to the European Working Group on Sarcopenia in Older People (EWGSOP) [1], EWGSOP2 [2], and the Foundation for the National Institutes of Health (FNIH) sarcopenia project [5]. One study compared all three sarcopenia frameworks [34], and one study [32] diagnosed sarcopenia according to the presence of low skeletal muscle index (SMI) and SPPB score.
Table 1.
Characteristics of the included studies.
| Year | Author | Study Type | Follow-Up (Years) | Country | Sample Characteristics | Sample Size | Mean Age (Years) | Mean Daily Protein Intake | Dietary Intake Assessment Method | Sarcopenia Assessment Method |
|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Veerlan et al. [37] | Case-Control | — | Netherland | Community-dwelling older adults | 132 | ~71 | ~73.9 g | 3-d food record | (a) SMI and (b) SPPB |
| 2019 | Beaudart et al. [35] | Cross-sectional | — | Belgium | Community-dwelling older adults | 331 | 74.8 | ~82.7 g | Food frequency questionnaire | EWGSOP |
| 2020 | Das et al. [34] | Cross-sectional | — | Australia | Community-dwelling older men | 794 | 81.1 | — | Diet history questionnaire | FNIH, EWGSOP, and EWGSOP2 |
| 2020 | Granic et al. [36] | Longitudinal | 3 | United Kingdom | Community-dwelling older adults | 757 | 85+ | — | 24-h dietary recall | EWGSOP |
| 2020 | Jyväkorpi et al. [33] | Cross-sectional | — | Finland | Community-dwelling older adults | 126 | ~87.4 | ~0.93 g/kg BW | 3-d food record | EWGSOP2 |
| 2020 | Montiel-Rojas et al. [32] | Cross-sectional | — | Europe | Community-dwelling women | 986 | ~71 | — | 7-d food record | EWGSOP2 |
| 2021 | Rahman et al. [31] | Cross-sectional | — | Indian | Community-dwelling women | 227 | 65.1 | ~52.2 g | Diet history | EWGSOP |
BW= body weight; EWGSOP = European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; SMI = Skeletal muscle index; SPPB = Short Physical Performance Battery.
3.3. Quality Assessment
Quality assessment scores are shown in Supplementary Material S3. The overall score of cross-sectional studies [31,32,33,34,35] ranged from six to seven. All of the studies clearly stated the research question (item 1), specified the study population (item 2), recruited participants from the same or a similar population (item 4), clearly defined and used valid and reliable exposure (item 9) and outcome (item 11) measures. Four investigations reported a participation rate of eligible persons of at least 50% (item 3), two studies investigated different levels of exposure (item 8), and three investigations adjusted their results according to confounding parameters (item 14). No studies justified the sample size (item 5) or reported whether investigators were blinded to the exposure of participants (item 12).
The longitudinal study [36] had an overall score of 10. The study established the research question (item 1), specified the study population (item 2), investigated a study population with a participation rate of eligible persons of at least 50% (item 3), recruited participants from the same or a similar population (item 4), justified the sample size (item 5), measured the exposure of interest before the outcome being measured (item 6), used a timeframe sufficient to expect to see an association between exposure and outcome (item 7), clearly defined and used valid and reliable exposure (item 9) and outcome measures (item 11), and adjusted their results according to confounding parameters (item 14). The study did not investigate different levels of exposure (item 8), did not assess the exposure more than once (item 10), and did not report whether investigators were blinded to the exposure of participants (item 12).
The case-control study [37] had an overall score of eight. The study clearly stated the research question (item 1), specified the study population (item 2), recruited control and case participants from the same or a similar population (item 4), clearly defined the inclusion and exclusion criteria (item 5), clearly defined and differentiated cases from controls (item 6), selected the participants randomly from eligible candidates (item 7), used concurrent control (item 8), and clearly defined and used valid and reliable exposure (item 10). The study did not justify the sample size (item 3), did not confirm whether the exposure occurred before the development of sarcopenia (item 9), did not report whether assessors were blinded to case or control participants (item 11), and did not adjust results according to potential covariates (item 12).
3.4. Cross-Sectional Association between Protein Intake and Sarcopenia
The cross-sectional association between protein intake and sarcopenia is shown in Figure 2. Four studies were included in the pooled analysis [31,33,35,37]. Older adults with sarcopenia consumed significantly less protein than their non-sarcopenic counterparts (SMD = 0.37, 95% CI = 0.19–0.55, p < 0.0001). Heterogeneity was classified as "might not be important" (I2 = 18%, p = 0.30).
Figure 2.
Standard mean differences of protein intake between older adults with and without sarcopenia [31,33,35,37].
3.5. Cross-Sectional Association between Protein Sources and Sarcopenia
One study investigated the association between protein sources and sarcopenia [32]. Montiel-Rojas et al. [32] enrolled 986 older European adults and explored the association between protein sources and sarcopenia, diagnosed according to the presence of low SMI plus reduced handgrip strength. The authors found that the risk of sarcopenia was lower in those with greater protein consumption. In addition, the replacement of animal-derived proteins with an equal amount of plant-derived proteins was associated with a reduced risk of sarcopenia.
3.6. Longitudinal Associations between Protein Intake and Sarcopenia
One study investigated the longitudinal association between protein intake and incident sarcopenia [36]. Compared with older adults on a low-butter diet, those eating a traditional British diet (i.e., rich in butter, red meat, gravy, and potato) had an increased risk of sarcopenia over a 3-year follow-up even if protein intake was ≥1 g/kg of body weight (BW)/d. Results were similar when the HPI threshold was set at ≥0.8 g/kg of BW/d. However, the risk of incident sarcopenia at three years was no longer significant in the fully adjusted model.
4. Discussion
The present systematic review and meta-analysis investigated the association between protein intake and sarcopenia in older adults. The pooled analysis of cross-sectional studies indicated that older adults with sarcopenia have a lower intake of proteins compared with non-sarcopenic peers. Two additional potentially important results were observed. First, the consumption of plant-based protein was cross-sectionally associated with a low prevalence of sarcopenia. Second, older adults on a high-fat/high-energy diet may be at high risk of sarcopenia even if their protein intake is greater than the RDA.
HPI has long been considered to be a modifiable lifestyle factor that might potentially counteract sarcopenia [23,24,25]. This assumption is based on the effects of AAs on muscle protein metabolism. Muscle mass is regulated by a dynamic equilibrium between MPS and muscle protein breakdown (MPB) [38,39,40,41,42]. Adequate protein ingestion is expected to increase AA availability and stimulate sarcoplasmic and myofibrillar protein synthesis by activating the mammalian target of rapamycin (mTOR) and its downstream targets [38,39,40,41,42]. However, the aged muscle frequently shows anabolic resistance, a state of submaximal MPS in response to hyperaminoacidemia, suggesting that greater amounts of protein are required to properly stimulate muscle anabolism in older adults [15,16,17,18,19,20].
If the anabolic resistance is not overcome through the diet, an imbalance in muscle metabolism in favor of MPB might occur, promoting muscle loss [43]. Muscle atrophy occurs preferably in type II muscle fibers [21,22,44], those that contract faster and have a greater capacity to generate tension [21,22]. Hence, it may be expected that older adults with HPI might experience less muscle atrophy and neuromuscular dysfunction.
However, such a view is not supported by the only longitudinal investigation included in the present study. Granic et al. [36] observed that older adults on a traditional British diet and with a protein intake ≥1 g/kg of BW/d had an increased risk of developing sarcopenia compared with those on a low-butter diet during three years of follow-up. These findings have some possible explanations.
Protein quality refers to the anabolic response elicited by protein sources [45]. Numerous studies found that animal-based proteins produced greater muscular anabolism than plant-based proteins [40,46,47]. These divergent anabolic responses are attributed to differences in digestion and absorption rates, and branched-chain AA (BCAA) content [45,48]. Indeed, animal proteins are characterized by digestibility rates higher than 90%, whereas digestibility rates barely reache 50% with plant proteins [45,48]. Furthermore, animal proteins have a greater content of BCAAs in comparison to plant-based proteins [14,45]. Such data are important because BCAAs, and mainly leucine, are considered to be major stimulators of MPS [42,49,50].
Hence, it is possible that older adults with HPI who developed sarcopenia had a protein consumption mostly based on plant sources, providing an insufficient supply of AAs to properly stimulate MPS. Although this hypothesis offers a reasonable explanation for the report by Granic et al. [36], other investigations found that a high intake of plant-based proteins was associated with faster walking speed [51] and lower prevalence of frailty [52]. Experts in the field interpreted these findings as the indication that an adequate intake of vegetable proteins may also properly stimulate muscle anabolism [14]. Another possible explanation to the findings by Granic et al. [36] is that the traditional British diet is characterized by a high intake of fat and energy. Such dietary regimes are associated with an increased risk of obesity which, in turn, promotes the development of insulin resistance, oxidative stress, low-grade systemic inflammation, and hormonal changes [53]. All of these factors play a role in the pathophysiology of sarcopenia. Finally, results by Granic et al. [36] were not controlled for many covariables that might impact the association between protein intake and sarcopenia, including the practice of physical exercise [54,55], the presence of frailty [56], and oral health [57].
Only one study investigated the association between protein sources and sarcopenia [32]. Montiel-Rojas et al. [32] observed that the consumption of plant protein was negatively associated with the presence of sarcopenia. Additional studies investigating the potential role of protein sources on the development of sarcopenia are warranted.
Our study has limitations that deserve discussion. First, all of the investigations included examined community-dwelling older adults, and extrapolations to hospitalized patients and people living in long-term institutions should be made with caution. Second, our pooled analysis was conducted to identify differences in means and SDs, given the limited number of studies that performed regression analyses. This indicates that results were not adjusted for numerous covariables. Third, substantial heterogeneity was observed in the way protein consumption data were presented (e.g., absolute, adjusted according to BW, percentage of calories). Fourth, the limited number of included studies did not allow meta-regression, dose-response, risk of bias, or “trim and fill” analysis to be conducted. Fifth, the findings on the longitudinal association between protein intake and those on protein sources with sarcopenia were based on one study each. Sixth, different studies were included in the cross-sectional and longitudinal analyses, which might produce divergent results. Seventh, although most studies used EWGSOP criteria to identify people with sarcopenia, different instruments, cutoff points, and other operational definitions of sarcopenia were also utilized. This aspect deserves concern because protein intake might be associated with each one of those variables, therefore influencing our results. In fact, vegetal protein has been associated with walking speed, but not with muscle strength [51,58]. Eighth, most of the investigations were conducted in Europe. Finally, no studies took into account the severity of sarcopenia.
Notwithstanding, our study provides directions for future investigations. The finding that sarcopenic older adults consumed significantly less protein than their non-sarcopenic counterparts partially supports the assumption that protein intake is associated with sarcopenia and encourages the conduct of large multicentric, cross-sectional and longitudinal studies to better explore the subject. Future investigations should take into consideration several nutritional and sarcopenia-related aspects that are still lacking in the literature, including differences between protein sources, diagnostic criteria for sarcopenia, and sociocultural factors. The impact of relevant covariables should also be explored. The lack of this information still limits extrapolations of the current findings to clinical practice.
5. Conclusions
Our pooled analysis indicate that older adults with sarcopenia consumed significantly less protein than their non-sarcopenic counterparts. These results were based on differences in means and SDs, given the lack of investigations that conducted regression analyses. One cross-sectional study noted that plant-based protein might be negatively associated with the prevalence of sarcopenia. On the other hand, a longitudinal study observed that older adults following a traditional British dietary pattern had an increased risk of sarcopenia even if protein intake was high. These findings suggest that more cross-sectional and longitudinal studies, with deeper statistical approaches and more comprehensive analyses of protein-related parameters are required to confirm and expand the current results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19148718/s1, Supplementary Material S1: Search strategy, Supplementary Material S2: Reasons for study exclusion, Supplementary Material S3: Quality analysis.
Author Contributions
Conceptualization, H.J.C.-J. and E.M.; methodology, H.J.C.-J., D.A., R.C., A.P., M.T., F.L., M.C. and E.M.; formal analysis, H.J.C.-J., D.A., R.C., A.P., M.T., F.L., M.C. and E.M.; investigation, H.J.C.-J., R.C. and D.A.; resources, H.J.C.-J., D.A., M.C. and E.M.; data curation, H.J.C.-J., D.A., R.C., A.P., M.T., F.L., M.C. and E.M.; writing—original draft preparation, H.J.C.-J.; writing—review and editing, D.A., R.C., A.P., M.T., F.L., M.C. and E.M.; supervision, M.C. and E.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partially funded by an Intramural Research Grant from the Università Cattolica del Sacro Cuore (D1.2020; E.M.) and the nonprofit research foundation Centro Studi Achille e Linda Lorenzon (A.P., E.M., H.J.C.-J. and R.C.). The APC was funded by Ministero della Salute—Ricerca Corrente 2022.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M., et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing. 2018;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen L.-K., Liu L.-K., Woo J., Assantachai P., Auyeung T.-W., Bahyah K.S., Chou M.-Y., Chen L.-Y., Hsu P.-S., Krairit O., et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Dam T.-T., Peters K.W., Fragala M., Cawthon P.M., Harris T.B., McLean R., Shardell M., Alley D.E., Kenny A., Ferrucci L., et al. An Evidence-Based Comparison of Operational Criteria for the Presence of Sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petermann-Rocha F., Balntzi V., Gray S.R., Lara J., Ho F.K., Pell J.P., Celis-Morales C. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle. 2022;13:86–99. doi: 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Q., Hu K., Yan C., Zhao B., Mei F., Chen F., Zhao L., Shang Y., Ma Y., Ma B. Associated Factors of Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:4291. doi: 10.3390/nu13124291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koon-Yee Lee G., Chun-Ming Au P., Hoi-Yee Li G., Chan M., Li H.-L., Man-Yung Cheung B., Chi-Kei Wong I., Ho-Fun Lee V., Mok J., Hon-Kei Yip B., et al. Sarcopenia and Mortality in Different Clinical Conditions: A Meta-Analysis. Osteoporos. Sarcopenia. 2021;7:S19–S27. doi: 10.1016/j.afos.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvani R., Miccheli A., Landi F., Bossola M., Cesari M., Leeuwenburgh C., Sieber C.C., Bernabei R., Marzetti E. Current Nutritional Recommendations and Novel Dietary Strategies to Manage Sarcopenia. J. Frailty Aging. 2013;2:38–53. doi: 10.14283/jfa.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Arai H., Assantachai P., Akishita M., Chew S.T.H., Dumlao L.C., Duque G., Woo J. Roles of Nutrition in Muscle Health of Community-dwelling Older Adults: Evidence-based Expert Consensus from Asian Working Group for Sarcopenia. J. Cachexia Sarcopenia Muscle. 2022;13:1653–1672. doi: 10.1002/jcsm.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the Prot-Age Study Group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Coelho-Junior H.J., Marzetti E., Picca A., Cesari M., Uchida M.C., Calvani R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients. 2020;12:2915. doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deer R.R., Volpi E. Protein Intake and Muscle Function in Older Adults. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:248–253. doi: 10.1097/MCO.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landi F., Calvani R., Tosato M., Martone A.M., Ortolani E., Savera G., D’Angelo E., Sisto A., Marzetti E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients. 2016;8:295. doi: 10.3390/nu8050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpi E., Mittendorfer B., Rasmussen B.B., Wolfe R.R. The Response of Muscle Protein Anabolism to Combined Hyperaminoacidemia and Glucose-Induced Hyperinsulinemia Is Impaired in the Elderly. J. Clin. Endocrinol. Metab. 2000;85:4481–4490. doi: 10.1210/jc.85.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. Aging Is Associated with Diminished Accretion of Muscle Proteins after the Ingestion of a Small Bolus of Essential Amino Acids. Am. J. Clin. Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 17.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. A High Proportion of Leucine Is Required for Optimal Stimulation of the Rate of Muscle Protein Synthesis by Essential Amino Acids in the Elderly. Am. J. Physiol. Endocrinol. Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 18.Wall B.T., Gorissen S.H., Pennings B., Koopman R., Groen B.B.L., Verdijk L.B., van Loon L.J.C. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE. 2015;10:e0140903. doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore D.R., Churchward-Venne T.A., Witard O., Breen L., Burd N.A., Tipton K.D., Phillips S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older versus Younger Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 20.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P.M., Rennie M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005;19:1–22. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 21.Nilwik R., Snijders T., Leenders M., Groen B.B.L., van Kranenburg J., Verdijk L.B., Van Loon L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Scott W., Stevens J., Binder-Macleod S. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001;81:1810–1816. doi: 10.1093/ptj/81.11.1810. [DOI] [PubMed] [Google Scholar]
- 23.Martone A.M., Marzetti E., Calvani R., Picca A., Tosato M., Santoro L., Di Giorgio A., Nesci A., Sisto A., Santoliquido A., et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed Res. Int. 2017;2017:2672435. doi: 10.1155/2017/2672435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer J., Morley J.E., Schols A.M.W.J., Ferrucci L., Cruz-Jentoft A.J., Dent E., Baracos V.E., Crawford J.A., Doehner W., Heymsfield S.B., et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle. 2019;10:956–961. doi: 10.1002/jcsm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landi F., Marzetti E., Martone A.M., Bernabei R., Onder G. Exercise as a Remedy for Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 26.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Green S., Higgins J. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2005. [Google Scholar]
- 28.Study Quality Assessment Tools|NHLBI, NIH. [(accessed on 27 December 2021)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 29.Luo D., Wan X., Liu J., Tong T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 30.Shi J., Luo D., Weng H., Zeng X.-T., Lin L., Chu H., Tong T. Optimally Estimating the Sample Standard Deviation from the Five-Number Summary. Res. Synth. Methods. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 31.Rahman R., Wilson B.P., Paul T.V., Yadav B., Kango Gopal G., Viggeswarpu S. Prevalence and Factors Contributing to Primary Sarcopenia in Relatively Healthy Older Indians Attending the Outpatient Department in a Tertiary Care Hospital: A Cross-Sectional Study. Aging Med. 2021;4:257–265. doi: 10.1002/agm2.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montiel-Rojas D., Nilsson A., Santoro A., Bazzocchi A., de Groot L.C.P.G.M., Feskens E.J.M., Berendsen A.A.M., Madej D., Kaluza J., Pietruszka B., et al. Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins. Nutrients. 2020;12:3601. doi: 10.3390/nu12123601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jyväkorpi S.K., Urtamo A., Kivimäki M., Strandberg T.E. Macronutrient Composition and Sarcopenia in the Oldest-Old Men. Clin. Nutr. 2020;39:3839–3841. doi: 10.1016/j.clnu.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Das A., Cumming R.G., Naganathan V., Blyth F., Le Couteur D.G., Handelsman D.J., Waite L.M., Ribeiro R.V., Simpson S.J., Hirani V. Associations between Nutrient Intakes and Dietary Patterns with Different Sarcopenia Definitions in Older Australian Men: The Concord Health and Ageing in Men Project. Public Health Nutr. 2021;24:4490–4505. doi: 10.1017/S1368980020003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaudart C., Locquet M., Touvier M., Reginster J.Y., Bruyère O. Association between Dietary Nutrient Intake and Sarcopenia in the SarcoPhAge Study. Aging Clin. Exp. Res. 2019;31:815–824. doi: 10.1007/s40520-019-01186-7. [DOI] [PubMed] [Google Scholar]
- 36.Granic A., Mendonça N., Sayer A.A., Hill T.R., Davies K., Siervo M., Mathers J.C., Jagger C. Effects of Dietary Patterns and Low Protein Intake on Sarcopenia Risk in the Very Old: The Newcastle 85+ Study. Clin. Nutr. 2020;39:166–173. doi: 10.1016/j.clnu.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verlaan S., Aspray T.J., Bauer J.M., Cederholm T., Hemsworth J., Hill T.R., McPhee J.S., Piasecki M., Seal C., Sieber C.C., et al. Nutritional Status, Body Composition, and Quality of Life in Community-Dwelling Sarcopenic and Non-Sarcopenic Older Adults: A Case-Control Study. Clin. Nutr. 2017;36:267–274. doi: 10.1016/j.clnu.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Atherton P.J., Etheridge T., Watt P.W., Wilkinson D., Selby A., Rankin D., Smith K., Rennie M.J. Muscle Full Effect after Oral Protein: Time-Dependent Concordance and Discordance between Human Muscle Protein Synthesis and MTORC1 Signaling. Am. J. Clin. Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 39.Bohé J., Low A., Wolfe R.R., Rennie M.J. Human Muscle Protein Synthesis Is Modulated by Extracellular, Not Intramuscular Amino Acid Availability: A Dose-Response Study. J. Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 41.Greenhaff P.L., Karagounis L.G., Peirce N., Simpson E.J., Hazell M., Layfield R., Wackerhage H., Smith K., Atherton P., Selby A., et al. Disassociation between the Effects of Amino Acids and Insulin on Signaling, Ubiquitin Ligases, and Protein Turnover in Human Muscle. Am. J. Physiol. Endocrinol. Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson D.J., Hossain T., Hill D.S., Phillips B.E., Crossland H., Williams J., Loughna P., Churchward-Venne T.A., Breen L., Phillips S.M., et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013;591:2911–2923. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkinson D.J., Piasecki M., Atherton P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lexell J., Taylor C.C., Sjöström M. What Is the Cause of the Ageing Atrophy?. Total Number, Size and Proportion of Different Fiber Types Studied in Whole Vastus Lateralis Muscle from 15- to 83-Year-Old Men. J. Neurol. Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 45.van Vliet S., Burd N.A., van Loon L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015;145:1981–1991. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 46.Anthony T.G., McDaniel B.J., Knoll P., Bunpo P., Paul G.L., McNurlan M.A. Feeding Meals Containing Soy or Whey Protein after Exercise Stimulates Protein Synthesis and Translation Initiation in the Skeletal Muscle of Male Rats. J. Nutr. 2007;137:357–362. doi: 10.1093/jn/137.2.357. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell C.J., Della Gatta P.A., Petersen A.C., Cameron-Smith D., Markworth J.F. Soy Protein Ingestion Results in Less Prolonged P70S6 Kinase Phosphorylation Compared to Whey Protein after Resistance Exercise in Older Men. J. Int. Soc. Sports Nutr. 2015;12:6. doi: 10.1186/s12970-015-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinu M., Abbate R., Gensini G.F., Casini A., Sofi F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017;57:3640–3649. doi: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 49.Bolster D.R., Vary T.C., Kimball S.R., Jefferson L.S. Leucine Regulates Translation Initiation in Rat Skeletal Muscle Via Enhanced EIF4G Phosphorylation. J. Nutr. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 50.Dardevet D., Sornet C., Balage M., Grizard J. Stimulation of in Vitro Rat Muscle Protein Synthesis by Leucine Decreases with Age. J. Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 51.Coelho-Junior H.J., Calvani R., Gonçalves I.O., Rodrigues B., Picca A., Landi F., Bernabei R., Uchida M.C., Marzetti E. High Relative Consumption of Vegetable Protein Is Associated with Faster Walking Speed in Well-Functioning Older Adults. Aging Clin. Exp. Res. 2019;31:837–844. doi: 10.1007/s40520-019-01216-4. [DOI] [PubMed] [Google Scholar]
- 52.Schoufour J.D., Franco O.H., Kiefte-de Jong J.C., Trajanoska K., Stricker B., Brusselle G., Rivadeneira F., Lahousse L., Voortman T. The Association between Dietary Protein Intake, Energy Intake and Physical Frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019;121:393–401. doi: 10.1017/S0007114518003367. [DOI] [PubMed] [Google Scholar]
- 53.Hong S.-H., Choi K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020;21:494. doi: 10.3390/ijms21020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coelho-Júnior H.J., Uchida M.C., Picca A., Bernabei R., Landi F., Calvani R., Cesari M., Marzetti E. Evidence-Based Recommendations for Resistance and Power Training to Prevent Frailty in Community-Dwellers. Aging Clin. Exp. Res. 2021;33:2069–2086. doi: 10.1007/s40520-021-01802-5. [DOI] [PubMed] [Google Scholar]
- 55.Izquierdo M., Merchant R.A., Morley J.E., Anker S.D., Aprahamian I., Arai H., Aubertin-Leheudre M., Bernabei R., Cadore E.L., Cesari M., et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging. 2021;25:824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- 56.Coelho-Júnior H.J., Rodrigues B., Uchida M., Marzetti E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018;10:1334. doi: 10.3390/nu10091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Marchi R.J., Hugo F.N., Hilgert J.B., Padilha D.M.P. Association between Oral Health Status and Nutritional Status in South Brazilian Independent-Living Older People. Nutrition. 2008;24:546–553. doi: 10.1016/j.nut.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 58.Coelho-Junior H.J., Calvani R., Picca A., Gonçalves I.O., Landi F., Bernabei R., Cesari M., Uchida M.C., Marzetti E. Association between Dietary Habits and Physical Function in Brazilian and Italian Older Women. Nutrients. 2020;12:1635. doi: 10.3390/nu12061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the manuscript.