Abstract
Ischaemic heart disease is the most common cause of death in males and the second in the female gender. Yet we often only focus on identification and treatment of this foremost cause of death in adulthood. The review asks the question what form of coronary disease do we encounter in childhood, what predisposing factors give rise to atherosclerosis and what strategies in childhood could we employ to detect and reduce atherosclerosis development in later life.
Keywords: atherosclerosis, childhood, coronary artery abnormalities, ischaemia, myocardial in farction, prevention
1. Introduction
Ischaemic heart disease is the most common cause of death in men and the second in women [1]. In a simplistic way, myocardial ischemia occurs when blood flow to the heart is reduced, preventing the cardiac muscle from getting enough oxygen. The reduced blood flow is usually the consequence of a partial or complete blockage of coronary arteries.
Although often considered as a condition affecting just adult people, life threatening myocardial ischaemia can present dramatically in paediatric age as well. This is the case of anomalous left coronary artery from the pulmonary artery (ALCAPA) and arterial calcification syndrome.
Not only that, but atherosclerosis—the most frequent aetiology of myocardial ischaemia—is a long process, which starts early since childhood in the form of fatty streaks. These first-stage lesions, which are characterised by an anomalous and reversible collection of oxidized lipoprotein particles in the inner layer of arteries, may lead to coronary arteries narrowing due to the progressive build-up of atheromatous plaque as time goes by. Fatty streaks are the first step of the atherosclerotic process [2]. Fatty streaks can be found in the aorta of a majority of children over the age of 3 years, augmenting quickly during adolescence, with coronary artery involvement which begins approx. a decade later [3]. Some characteristics of cardiovascular dysfunction during adulthood may even be influenced prenatally by means of a combination of genetic and unfavourable intrauterine environment [4].
Furthermore, type 2 diabetes is on an upward trajectory among children and adolescents. As per the report by the US Centers for Disease Control and Prevention (CDC), more than 5000 children and adolescents/year between the ages of 10 and 19, develop type 2 diabetes [5]. It is caused by insulin resistance as well as nonautoimmune β-cell failure usually starting amid puberty. However, youth-onset type 2 diabetes shares unique aspects, such as quicker β-cell decline and faster development of atherosclerosis and diabetes complications compared to the form developing in adulthood [6]. Microvascular complications such as endothelial dysfunction and risk factors for macrovascular complications are already evident at the time of diagnosis of type 2 diabetes in youth [7].
In addition, since the obesity epidemic is becoming a matter of serious concern worldwide, emerging countries included, this should be taken into account, since it represents an important risk factor for developing atherosclerosis. In fact, obesity onset in early life triggers atherosclerosis development in vessels such as the aorta and the coronary arteries [8]. There are also a couple of conditions in childhood which predispose to dramatically accelerated atherosclerosis, such as Kawasaki disease (KD) and familial hypercholesterolemia (FHC).
This review asks the question what forms of coronary disease do we encounter in childhood, what predisposing factors give rise to atherosclerosis and what strategies in childhood could we employ to detect and reduce atherosclerosis development in later life.
2. What Forms of Coronary Disease Do We Encounter in Childhood?
The forms of coronary disease seen in children can be subdivided into congenital and acquired. Those which are congenital can be symptomatic and life-threatening in paediatric age. Among them, the ALCAPA is worth mentioning.
ALCAPA is a well-recognised but rare congenital coronary artery anomaly affecting approximately 1 in 300,000 people [9]. Much less frequently encountered is the anomalous origin of either the right coronary artery or one of the conal branches off the pulmonary artery. ALCAPA typically presents in infancy resulting in myocardial ischemia and cardiac failure. The first reported case of a 3-month-old boy was by Bland, White and Garland in 1933 [10]. Other authors have reported several fatal ALCAPA, mostly fatal during the first year of life [11]. ALCAPA is usually an isolated entity, although it has been described in association with specific cardiac lesions (e.g., ventricular septal defect, Tetralogy of Fallot and Hypoplastic left heart syndrome) [12].
The pathophysiology of ALCAPA is intrinsically related to the decrease in pulmonary vascular resistance and pulmonary arterial pressure after birth. Therefore, the child often remains asymptomatic until a few weeks of age when the neonatal transitional circulation develops. With the drop in pulmonary arterial pressure, left coronary artery perfusion pressure decreases resulting in decreased antegrade coronary artery flow. The right coronary artery acts as the coronary blood supply as the pulmonary artery diastolic pressure drops further with reduced pulmonary vascular resistance, resulting in a coronary artery steal of the left coronary artery into the pulmonary artery. This induces myocardial ischemia conditioned by the extent of coronary arterial collateralisation, which impacts the timing of presentation [13].
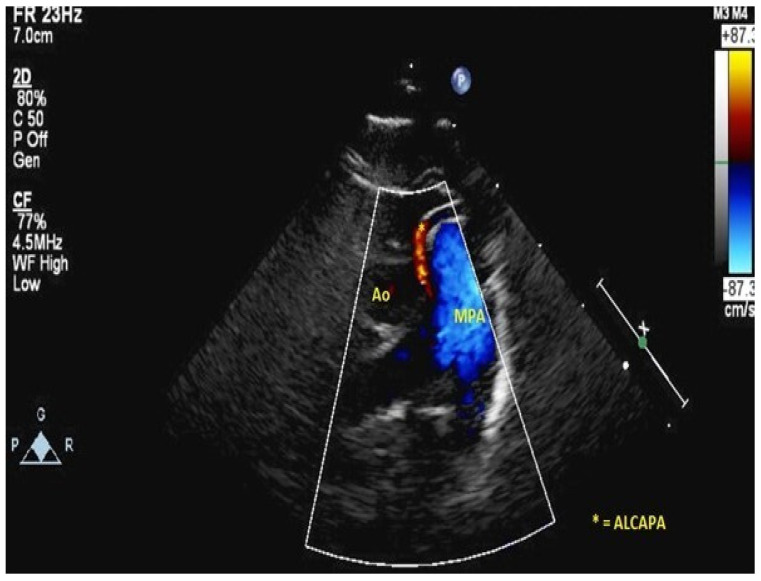
There are two types of presentation. Infants and small children typically have symptoms of ischemia during the first months of life (irritability, crying), poor feeding and congestive heart failure (left ventricular [LV] systolic dysfunction, LV dilatation, mitral regurgitation, enlarged right coronary artery with retrograde flow up the left coronary artery identified as a jet into the main pulmonary artery. See Figure 1).
Figure 1.
ALCAPA coming off the main pulmonary artery.
Acute presentations such as sudden cardiac death are rare [14]. Acute clinical presentation may also be challenging to differentiate from myocarditis or dilated cardiomyopathy. The second type of presentation is in older patients with well collateralised coronary circulation who may present late in life and even be detected incidentally on postmortem examination.
Diagnosis depends on a combination of clinical findings, ECG abnormalities and imaging studies. The classic ECG presentation is Q waves in 1, aVL and leads V4–V6. Transthoracic echocardiography identifies an enlarged right coronary artery, retrograde flow in the left coronary artery into the pulmonary artery in combination with an ischemic mitral valve with mitral regurgitation and significantly dilated LV with reduced LV function [15]. Rarely, catheterisation or CT imaging is required to confirm the anatomical findings [16].
Treatment is surgical once the child is stabilised. Direct reimplantation of the coronary artery into the aorta is the preferable surgical procedure to ligation of the coronary artery [17,18]; although, in certain cases the creation of an intrapulmonary tunnel to baffle the coronary artery through the main pulmonary trunk to the aorta (Takeuchi procedure) is used [19]. Given its unique strategy, the Takeuchi procedures are associated with supravalvar pulmonary stenosis and baffle leaks [20]. A lot of patients demonstrate a dramatic clinical and echocardiographic improvement, with remodelling seen through reduction in LV size, improved LV function and reduction in mitral regurgitation [21]. Subsequent additional surgery such as mitral valve repair may be required in some patients and transplantation is rarely required after revascularisation [22,23,24].
Another symptomatic and usually deadly form of congenital ischaemic heart disease in infants is arterial calcification syndrome.
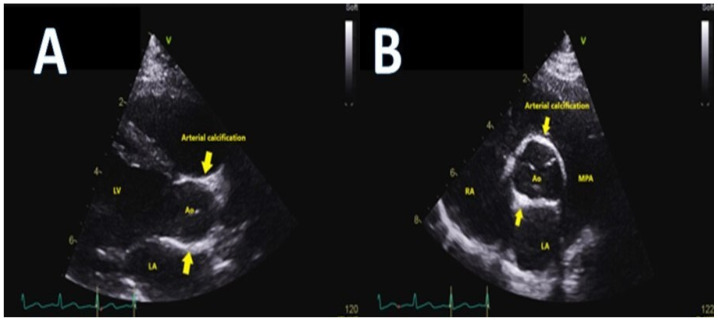
Idiopathic calcification of the coronary arteries is an extremely uncommon inheritable disease that typically presents in early infancy [17]. Diffuse calcification of many of the arteries but particularly the coronary arteries results in cardiac ischemia, congestive cardiac failure and systemic hypertension [18,19]. The left and right coronary arteries are typically thick-walled, with significant luminal narrowing. The aorta, great vessels and renal vessels demonstrate thickening of the wall as well (Figure 2).
Figure 2.
Thickening of the aorta patient seen in echocardiographic parasternal long axis (panel (A)) and short axis (panel (B)) views in arterial calcification syndrome.
Histological examination demonstrates extensive accumulation of calcium in the inner elastic layer of all the vessels. Mutations in ENPP1 gene are linked with this rare syndrome in several patients [20].
Children typically present as very unwell, with congestive heart failure and arterial hypertension. Their combination should alert one to this diagnosis. Some children will present with angina-type symptoms and may even be inconsolable [21]. The pulses are often impalpable due to calcification, which can be visualised on plain X-rays of the arms and legs. Echocardiography is diagnostic demonstrating extensive aortic wall calcification and calcification of the coronary arteries [22]. Computed tomography may provide further delineation of the extent of coronary, aortic, great vessels and renal artery involvement [23]. Treatment consists of bisphosphonate therapy with some patients responding well to treatment [24,25]. Despite this, many patients die from hypertension, LV hypertrophy and myocardial infarction (MI) in the first three months of life, prompting some centres to offer cardiac transplantation [26,27,28,29,30,31,32,33,34,35].
Coronary artery disease in infants can also arise in subjects undergoing arterial switch operation—which implies coronary reimplantation—for transposition of the great vessels, a rare congenital heart disease. During surgery, the coronary arteries are translocated and coronary artery stenosis and blockage have been shown by angiography and computed tomography in up to 5–7% of subjects [36,37,38].
3. What Predisposing Factors Give Rise to Atherosclerosis and What Strategies in Childhood Could We Employ to Detect and Reduce Atherosclerosis Development in Later Life?
To summarise, there are two main acquired conditions predisposing to atherosclerosis development in children: Kawasaki disease (KD) and familial hypercholesterolemia (FHC).
KD is a systemic vasculitis with an unknown origin, maybe viral. It is the most common acquired cardiovascular disease in developed countries as well [39,40]. Although intravenous immunoglobulin infusion is an effective treatment for the disease, some patients still develop coronary aneurysms, which is an abnormal dilatation of the same [41,42]. Even if coronary artery aneurysm reversion is demonstrated in approx. 75% of patients, long-term outcomes of the inflamed arterial wall are uncertain, mostly in individuals with KD and giant aneurysms without regression [43]. These coronary aneurysms undergo remodelling with time, causing intimal (the inner coronary artery layer) thickening and calcification [44,45,46]. This triggers the onset of stenosis close to the aneurysms or blockage of the coronary arteries, causing ischemic heart disease [39,47]. Generally speaking, KD patients with previous small to medium coronary artery aneurysms show regression of the dilatation. Conversely, those with large coronary aneurysms (e.g., those with a maximal internal coronary artery diameter ≥ 8.0 mm in terms of absolute dimension or those with a z-score ≥ 10 after correction for body size area, as per the American Heart Association classification) are classified as having giant coronary aneurysms which do not revert, but continue or develop stenosis, causing acute MI easily [47,48]. In one of the largest studies on patients with KD and giant aneurysms and who were lost to follow-up for about 25 years, 46% of the sample exhibited a progression to coronary stenosis or complete obstruction. Of note 30.6% had MI and 15.4% died.
The other patients had persistent coronary aneurysms without any noteworthy stenosis in the coronary artery over a follow-up between 10 and 25 years.
Again, 21.7% of the patients showed obstructive coronary lesions that may trigger myocardial ischemia as time goes by. Similar findings are reported in smaller studies [43]. Ongoing remodelling of coronary arteries with giant aneurysms might continue long after acute KD, thus progressing to coronary stenosis, even 25 years following the occurrence of KD. The ongoing remodelling may be triggered by underlying ongoing inflammation of the coronary arterial wall, as shown by high blood levels of inflammatory markers in these patients [49]. In a 33 subject case series, abnormalities, such as arterial wall fibrosis, cellular infiltrates, hyperplasia of the intima or neovascularization, were found even in a few coronary artery portions without previous dilatation by echocardiography. Similar injuries were proved in significantly higher proportions in coronary tracts with long lasting aneurysms, followed by segments with angiographically regressed aneurysms and segments with coronary artery dilatation without any previous aneurysms [50].
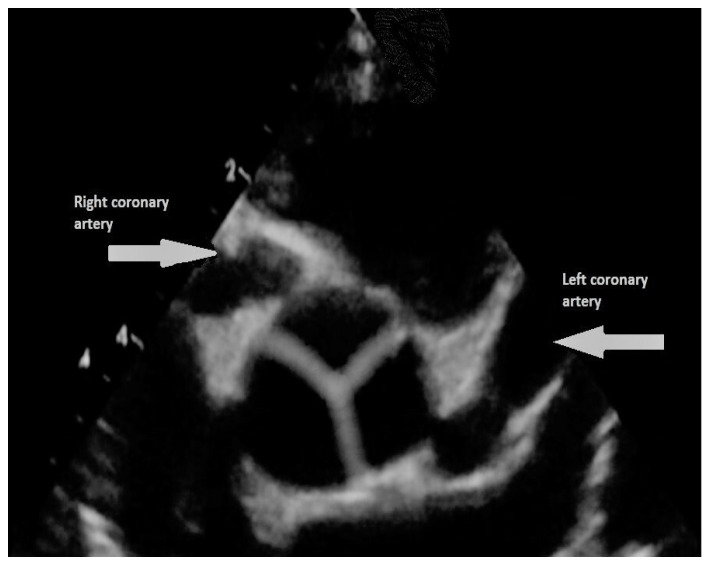
The overall long-term survival of KD patients and giant aneurysms is quite good for up to three decades (survival rate is 90% at 20 years and 87% at 30 years) with various kinds of invasive interventions used in over 50% of the patients [43]. Adverse events are predicted by the extent of damage to the coronary arteries; patients with large coronary artery aneurysms are at high risk of complications. However, even those with remodelled aneurysms are not totally risk-free [44,49]. In accordance with the 2017 American Heart Association KD guidelines, long-term follow-up is mandatory for those with coronary artery involvement [51]. Patients with persisting large aneurysms require follow-up during their whole life with yearly or biyearly checks [51]. Blood thinning therapy in terms of primary prevention is suggested [51]. So far, no randomized clinical trials have been set up to evaluate the safety and efficacy of antithrombotic therapy to prevent coronary clots in KD. The current scientific evidence is based on small retrospective studies. Antiplatelet agents, such as low-dose aspirin, are considered standard of care to prevent clotting in individuals with coronary artery aneurysms. Patients with large or giant aneurysms are at very high risk of intracoronary thrombosis and MI (see Figure 3).
Figure 3.
Persisting giant right and left coronary aneurysm (arrows) in a boy with previous Kawasaki syndrome (echocardiographic short-axis view).
In aneurysmatic coronary arteries portions, clotting is induced by significantly abnormal slow blood flow and stasis. Because of that, patients are provided with a combination of antiplatelet and anticoagulant agents, usually low-dose aspirin and warfarin [51]. An important issue is when and how to detect myocardial ischaemia in patients long after the occurrence of the inflammatory disease. Even though it is suggested by the Guidelines as a useful tool to detect ischaemia during follow-up, a treadmill exercise stress test may not detect any ischemic alterations on ECG in patients with chest discomfort, while at cardiac catheterization a significant coronary artery stenosis is detected [51,52]. In agreement with this finding, a study suggested that the treadmill exercise stress test is the worst technique to detect myocardial ischemia in patients with KD [52]. Other possible modalities include stress echocardiography, stress magnetic resonance imaging and myocardial scintigraphy. They proved to be useful in terms of risk stratification, though some of them imply harmful radiations for such young patients [51]. Cardiac catheterization of the coronary arteries may be used for diagnosis and prognosis during the first year following KD and for recurrent follow-up every 1 to 5 years thereafter [51]. The reality is that it is still unknown how stopping the early progress of atherosclerosis in KD is complicated by coronary aneurysms.
Another recently manifested form of ischaemic heart disease in paediatric age is that triggered by COVID-19. The latter can infect children as well, thus causing the so-called Multisystem Inflammatory Syndrome in Children (MIS-C) also termed Paediatric Inflammatory Multisystem Syndrome (PIMS). MIS-C mimics some features of KD, toxic shock syndrome and macrophage activation syndrome. That is the reason why, at its first outbreak, some authors called it a Kawasaki-like disease. The relationship between MIS-C and COVID-19 infection suggests that the pathogenesis involves a post-infectious marked immune system dysregulation. MIS-C has an incidence of 0.2–0.6% of all paediatric COVID-19 infections [53,54,55]. Similarly to KD, MIS-C can also cause cardiac involvement with shock, myocardial dysfunction, ECG changes and coronary dilations/aneurysms development. The latter present in 15% of the MIS-C cases with cardiac involvement. The features of coronary aneurysms in MIS-C appear to be different compared with classic KD aneurysms. The most important differences are a lower likelihood of aneurysm formation than in classic KD, a reduced number of giant forms, a tendency towards aneurysm regression and less thrombotic events [56]. The long-term prognosis of this new form of coronary artery disease in children is still unknown, due to its too recent presentation.
Atherosclerosis is a lengthy chronic inflammatory and degenerative process, which begins in paediatric age and maybe even since foetal life, as shown in a number of studies [57,58]. It can be detected early as endothelial dysfunction, a preclinical abnormal reaction in which arteries constrict rather than dilating as a response to an appropriate trigger. Endothelial dysfunction is caused by reduced production and/or availability of nitric oxide and/or a mismatch in the relative contribution of endothelium-derived relaxing and contracting agents. It occurs before the development of atherosclerotic plaques. Endothelial dysfunction is involved in lesion development by promoting early and late pathways of atherosclerosis such as upregulation of adhesion molecules, increased chemokine production and leukocyte adhesion, raised cell permeability, enhanced low-density lipoprotein oxidation, platelet activation/adhesion/aggregation, cytokine release and arterial smooth muscle cell multiplication and migration [59].
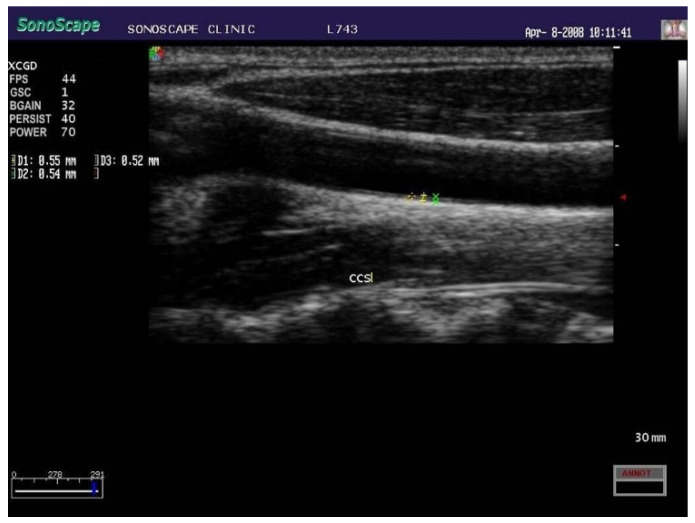
FHC exacerbates the above stated process. Raised inflammatory markers and early endothelial dysfunction have been demonstrated in children with FHC in association with increased intima-media thickness (Figure 4) and low-density lipoproteins (LDL) [60,61,62,63].
Figure 4.
Carotid increased intima-media thickness in a child with FHC (ultrasound scan).
Coronary artery atherosclerotic plaques have been detected in 25% of children and adolescents with FHC and aged 11–23. Aortic atherosclerotic lesions can be found in a vast majority of adolescents with homozygous familial hypercholesterolemia (HoFHC) [64].
FHC is a genetic disorder with autosomal dominant transmission. FHC was the term coined by Carl Muller in 1938 to term the association of hypercholesterolemia, tendon xanthomas, xanthelasmas and occurrence of early ischaemic heart disease. Calcified aortic valve disease is not rare either [65,66]. LDL receptor (LDL-R) discovery by Goldstein and Brown in 1985 was the cornerstone to understand pathophysiology of LDL accumulation and atherosclerosis development [67,68,69]. Classic FHC (85–95% of cases) is caused by chromosome 19p13.2 mutations. It encodes for LDL-R. About 2000 possible mutations have been reported so far. LDL-R is a transmembrane glycoprotein that is responsible for degrading approx. two-thirds of circulating LDL. However, apolipoprotein B (~5–10%) as well as proprotein convertase subtilisin/kexin type 9 (PCSK9) (1–2%>) mutations can lead to a similar phenotype. More rarely, mutations can involve low density lipoprotein receptor adaptor protein 1 (LDLRAP1) (<1%, recessive FHC) or apolipoprotein E (APOE) (<<1%). Recessive FHC is diffused in some isolated geographic areas such as the island of Sardinia in Italy with a prevalence of 1:38,000 [70,71].
Regarding the prevalence of the disease, initially heterozygous familial hypercholesterolemia (HeFHC) was assumed to affect 1 in 500 individuals, while HoFHC prevalence was thought to be 1:1,000,000 [72]. The most recent epidemiologic investigations, however, have pointed out that the prevalence of HeFHC is 1:217–1:300, whereas that of HoFHC is 1:300,000. It means that HoFHC affects 30,000,000 individuals in the world [70,73,74].
The main feature of FHC is high LDL levels since infancy. Identifying children with FHC as early as possible is pivotal to start a treatment and improve their prognosis [75,76]. Unfortunately, missing FHC diagnosis is quite common in Europe with harmful aftermaths owing to the dramatically increased risk of MI after the age of 20 [75,77,78].
In adult patients, FHC diagnosis is made on the basis of increased LDL levels associated with normal triglycerides values, xanthomas, corneal arcus and family history of early MI [75,76].
Conversely, making diagnosis of FHC in childhood is by far more difficult. The only certain clinical feature is increased LDL. Xanthomas are rare. This is the reason why some different screening strategies to identify HeFHC have been suggested [79,80,81].
Over the last decade, many Guidelines and documents on FHC diagnosis and treatment have been published [75,82]. The criteria for suspecting FHC in paediatric age are summarised in Table 1 [83,84,85].
Table 1.
| Criterion | Title 2 |
|---|---|
| 1 | Occasional finding of LDL ≥ 4 mmol/L (160 mg/dL) without any bodily sign of note and with a parent suffering from hypercholesterolemia or ischaemic heart disease (<55 years in males and <60 years in females) |
| 2 | LDL-C ≥ 5.0 mmol/L (190 mg/dL) in two different checks after 3 months of low cholesterol diet |
| 3 | LDL-C ≥ 3.5 mmol/L (130 mg/dL) and one parent with a FHC genetic diagnosis |
| 4 | LDL-C ≥ 13 mmol/L (500 mg/dL) with cutaneous xanthomas (dominant or recessive HoFHC) |
When FHC is suspected in a child aged 5 years or more, LDL should be checked. Secondary hypercholesterolemia should be ruled out as well [85]. Definite FHC diagnosis is based on genetic test. Making a diagnosis of FHC implies a cascade family screening as well [85,86].
The first therapeutic approach to FHC is based on lifestyle change (6–12 months), with family support. Calories and fat are important during children growth, but total fat intake should not go beyond 30% of overall calories. Saturated fat should not go beyond 7% of daily calories requirement [87].
Nutraceutical products have demonstrated the ability to reduce LDL in studies enrolling a small number of children. However, stanols and vegetable sterols are not suggested in children aged less than 6 years [88,89,90,91]. In children and adolescents with FHC, high viscosity glucomannan is capable of reducing LDL, blood pressure and weight [92,93]. Red yeast rice extract and policosanols decrease total cholesterol, LDL and apolipoprotein B in children affected by FHC [94].
Daily physical exercise (no less than 60 min/day) and no longer than 2 h/day spent on the TV, computer, smartphone, are important as well [95,96].
Lifestyle and diet change themselves are often inadequate in significantly reducing LDL levels in FHC children. As such, it means that taking medications is necessary.
Statins are the first line treatment in paediatric patients with FHC. The current Guidelines suggest that low dose statins should be provided in these subjects from the age of 8–10 years. The dose should be up titrated, when needed, to reach the goal of a 50% LDL reduction in comparison with baseline in children between 8 and 10 years or ≤3.5 mmol/L (130 mg/dL) in children aged ≥10 years, especially when additional risk factors, increased lipoprotein(a) included, are present [83]. In children with HoFHC, medical treatment should be started immediately after diagnosis [83].
In this setting, early statins administration is able to slow down atherosclerosis progression, as testified by measuring carotid intima-media thickness. The latter is capable of predicting major cardiovascular adverse events [97,98]. Statins have proved to be well tolerated in paediatric age so far, with rare side effects [99].
Ezetimibe, usually co-administered with statins, lowers LDL levels in FHC paediatric patients [100,101].
Monoclonal antibody therapy against PCSK9 (e.g., evolocumab) is indicated in HeFHC children and adolescents when lifestyle change and the highest tolerated dose of statins are not enough to normalise LDL. This treatment is usually efficient and well tolerated even in paediatric age [102].
More recently, other drugs (lomitapide, mipomersen, bempedoic acid and anacetrapib) acting through different mechanisms have been proposed in the most severe forms of FHC that are resistant to statins, but they have not been properly tested in children yet. In HoFHC children, lipoprotein apheresis to remove LDL and lipoprotein(a) from the blood is likely the best therapeutic option, though it is invasive and expensive [103].
Another poorly studied form of ischaemic heart disease is that manifested during neonatal asphyxia. Electrocardiogram changes with onset of T wave inversion and Q waves and cardiac enzymes fluctuations, troponin included, are similar to that observed in adults with heart attack. Echocardiography shows left ventricular dilatation and wall motion abnormalities. So far, the long-term clinical consequences of this neonatal myocardial infarction are still unknown [104].
4. Discussion
Ischaemic heart disease and atherosclerosis are often considered two conditions affecting just middle-aged subjects. On the contrary, they can occur and/or begin during childhood too. Ischaemic heart disease in paediatric age is congenital in its origin and it is usually a life-threatening condition in the affected infants. ALCAPA has a mortality rate up to 90% within the first year of life if left untreated. However, there are several cases reported in adolescents and adults. This is attributed to the development of collaterals between the right and left coronary arteries [105]. Without appropriate treatment, idiopathic infantile arterial calcification also has high morbidity and mortality, although studies to clarify them are still lacking, due to the rarity of the disease. On postmortem evaluation, calcifications of the large arteries are the main feature, but the most common gross findings are myocardial hypertrophy and stiff coronary arteries tortuosity [106]. These two conditions represent a real ischemic heart disease occurring in paediatric age.
On the other hand, fat accumulation and atherosclerosis are matters of concern from childhood. This is true not only in the general population, with paediatric obesity epidemic contributing to and exacerbating atherosclerosis through many pathways such as a rise in blood pressure and glucose level, anomalous lipid profiles and systemic inflammation, but mostly in KD and FHC patients. There is a strong link between LDL blood levels in children and the LDL levels in the same subjects during adulthood. A number of studies (Bogalusa Heart Study, Muscatine Study, Cardiovascular Risk in Young Finns Study, Coronary Artery Risk Development in Young Adults [CARDIA]) have shown that, since cholesterol tends to remain within the same percentiles throughout life, high cholesterol can be tracked from childhood to adulthood. Thus, children with higher cholesterol levels are more likely to become hypercholesterolemic adults. Family history of hypercholesterolemia is quite common as well and represents by far a significant risk factor for encountering major adverse cardiovascular events at a young age [107,108,109,110,111].
Childhood obesity is worrisome in terms of public health. A 2016 study estimated that 124 million children aged between five and nineteen were obese and nearly double that number overweight [112]. Landmark studies including the Bogalusa study showed a direct relationship between increasing body mass index and atherosclerotic lesions in prematurely deceased young people [113] Combined analysis from the International Childhood Cardiovascular Cohort identified obesity and overweight as positively correlating with high-carotid intima-media thickness, a noninvasive biomarker of structural atherosclerotic disease in young adults [114]. The pathophysiological basis of this relationship is multifactorial involving endothelial dysfunction, dyslipidaemia, chronic low-level inflammation and increasing arterial stiffness [115].
The aim of preventing or intervening on atherosclerosis development in childhood means methods for its earlier detection need to be developed. Serum biomarkers provide an exciting opportunity for enhanced, individualised risk prediction but progress in their use in the paediatric population for atherosclerosis has been slow. Targets that have been investigated include markers of oxidative stress, inflammatory markers, lipoproteins and endothelial adhesion molecules [116]. Noninvasive imaging techniques using high resolution ultrasound such as carotid and aortic intimal-medial thickness are validated to assess early atherosclerotic changes. Children with hypercholesterolaemia have increased carotid intimal thickness compared to those with normal cholesterol levels [117]. It has the benefit of offering direct information about the individual’s vascular health, but further longitudinal studies are required to assess its usefulness in the prediction of adult cardiovascular disease in paediatric patients [118]. Metabolomics, the most promising of omics sciences, which is able to take a picture of the metabolic state of an individual in physiological as well pathological settings, may represent an important tool to unveil a number of still obscure points [119].
5. Conclusions
While some strategies have been developed to identify those people at increased risk of atherosclerosis-related cardiac ischaemia and MI, and to treat them in terms of primary prevention, the lack of robust data on the effectiveness of the latter is still a weakness and much more research in the field will be needed in the foreseeable future.
Abbreviations
| ALCAPA | anomalous left coronary artery from the pulmonary artery |
| KD | Kawasaki disease |
| FHC | familial hypercholesterolemia |
| LV | left ventricular |
| ECG | electrocardiogram |
| Echo | echocardiogram |
| CT | computed tomography |
| MI | myocardial infarction |
| LDL | low density lipoprotein |
| HoFHC | homozygous family hypercholesterolemia |
| HeFHC | heterozygous hypercholesterolemia |
| LDL-R | low density lipoprotein receptor |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan M.A., Hashim M.J., Mustafa H., Baniyas M.Y., Al Suwaidi S.K.B.M., AlKatheeri R., Alblooshi F.M.K., Almatrooshi M.E.A.H., Alzaabi M.E.H., Al Darmaki R.S., et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong J.P. Landmark perspective: Coronary atherosclerosis in soldiers. A clue to the natural history of atherosclerosis in the young. JAMA. 1986;256:2863–2866. doi: 10.1001/jama.1986.03380200101029. [DOI] [PubMed] [Google Scholar]
- 4.Hertiš P.T., Petek T., Močnik M., Marčun Varda N. Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants. 2022;11:894. doi: 10.3390/antiox11050894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazeli Farsani S., van der Aa M.P., van der Vorst M.M.J., Knibbe C.A., de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: A systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471–1488. doi: 10.1007/s00125-013-2915-z. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau K.J., Anderson B.J., Berg E.G., Chiang J.L., Chou H., Copeland K.C., Hannon T.S., Huang T.T., Lynch J.L., Powell J., et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 2016;39:1635–1642. doi: 10.2337/dc16-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland K.C., Zeitler P., Geffner M., Guandalini C., Higgins J., Hirst K., Kaufman F.R., Linder B., Marcovina S., McGuigan P., et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J. Clin. Endocrinol. Metab. 2011;96:159–167. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj M. Obesity and cardiovascular risk in children and adolescents. Indian J. Endocrinol. Metab. 2012;16:13–19. doi: 10.4103/2230-8210.91176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith J.D. Anomalous origin of the coronary artery from the pulmonary artery. Br. Heart J. 1959;21:149–161. doi: 10.1136/hrt.21.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland E.F., White P.D., Garland J. Congenital anomalies of the coronary arteries? Report of an unusual case associated with cardiac hypertrophy. Am. Heart J. 1933;8:787–801. doi: 10.1016/S0002-8703(33)90140-4. [DOI] [Google Scholar]
- 11.Fontana R.S., Edwards J.E. Congenital Cardiac Disease: A Review of 357 Cases Studied Pathologically. WB Saunders; Philadelphia, PA, USA: 1962. [Google Scholar]
- 12.Wesselhoeft H., Fawcett J.S., Johnson A.L. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968;38:403425. doi: 10.1161/01.CIR.38.2.403. [DOI] [PubMed] [Google Scholar]
- 13.Frommelt M.A., Miller W., Williamson J., Bergstrom S. Detection of septal coronary collaterals by color flow Doppler imaging is a marker for anomalous origin of a coronary artery from the pulmonary artery. J. Am. Soc. Echocardiogr. 2002;15:259–263. doi: 10.1067/mje.2002.115658. [DOI] [PubMed] [Google Scholar]
- 14.Alsara O., Kalavakunta J.K., Hajjar V., Alsarah A., Cho N., Dhar G. Surviving sudden cardiac death secondary to anomalous left coronary artery from the pulmonary artery: A case report and literature review. Heart Lung. 2014;43:476–480. doi: 10.1016/j.hrtlng.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Patel S.G., Frommelt M.A., Frommelt P.C., Kutty S., Cramer J.W. Echocardiographic diagnosis, surgical treatment, and outcomes of anomalous left coronary artery from the pulmonary artery. J. Am. Soc. Echocardiogr. 2017;30:896–903. doi: 10.1016/j.echo.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Ojha V., Sh C., Vadher A., Singh Malhi A., Kothari S., Jagia P. Anomalous left coronary artery from right pulmonary artery (ALCARPA) with dual left anterior descending arteries- a hitherto unreported combination of coronary anomalies diagnosed on dual source CT. J. Cardiovasc. Comput. Tomogr. 2020;14:e69–e70. doi: 10.1016/j.jcct.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Alexi-Meskishvili V., Hetzer R., Weng Y., Lange P.E., Jin Z., Berger F., Loebe M. Anomalous origin of the left coronary artery from the pulmonary artery. Early results with direct aortic reimplantation. J. Thorac. Cardiovasc. Surg. 1994;108:354–362. doi: 10.1016/S0022-5223(94)70018-4. [DOI] [PubMed] [Google Scholar]
- 18.Fehrenbacher T.A., Mitchell M.E., Ghanayem N.S., Tweddell J.S. Surgery and critical care for anomalous coronary artery from the pulmonary artery. Cardiol. Young. 2010;20((Suppl. S3)):35–43. doi: 10.1017/S1047951110001071. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi S., Imamura H., Katsumoto K., Hayashi I., Katohgi T., Yozu R., Ohkura M., Inoue T. New surgical method for repair of anomalous left coronary artery from pulmonary artery. J. Thorac. Cardiovasc. Surg. 1979;78:7–11. doi: 10.1016/S0022-5223(19)38154-1. [DOI] [PubMed] [Google Scholar]
- 20.Chin A.J., Larsen R.L., Seliem M.A., Andrews B., Jones A., Vetter J., Lieb D. Noninvasive imaging of intraarterial baffles in infants and children. J. Am. Soc. Echocardiogr. 1993;6:45–50. doi: 10.1016/S0894-7317(14)80255-1. [DOI] [PubMed] [Google Scholar]
- 21.Naqvi N., Babu-Narayan S.V., Krupickova S., Muthialu N., Maiya S., Chandershekar P., Cheang M.H., Kostolny M., Tsang V., Marek J. Myocardial Function Following Repair of Anomalous Origin of Left Coronary Artery from the Pulmonary Artery in Children. J. Am. Soc. Echocardiogr. 2020;33:622–630. doi: 10.1016/j.echo.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Radman M., Mastropietro C.W., Costello J.M., Amula V., Flores S., Caudill E., Karki K., Migally K., Narasimhulu S., Piggott K., et al. Collaborative Research from the Pediatric Cardiac Intensive Care Society (CoRe-PCICS) Investigators. Intermediate Outcomes After Repair of Anomalous Left Coronary Artery From the Pulmonary Artery. Ann. Thorac. Surg. 2021;112:1307–1315. doi: 10.1016/j.athoracsur.2020.06.130. [DOI] [PubMed] [Google Scholar]
- 23.Sasikumar D., Dharan B.S., Arunakumar P., Gopalakrishnan A., Sivasankaran S., Krishnamoorthy K.M. The outcome of mitral regurgitation after the repair of anomalous left coronary artery from the pulmonary artery in infants and older children. Interact. Cardiovasc. Thorac. Surg. 2018;27:238–242. doi: 10.1093/icvts/ivy022. [DOI] [PubMed] [Google Scholar]
- 24.Kececioglu D., Deng M.C., Schmid C., Kehl H.G., Baba H.A., Yelbuz M., Scheld H.H., Vogt J. Anomalous origin of the left coronary artery from the pulmonary artery with large anterior myocardial infarction and ischemia: Successful tunnel repair and concomitant heterotopic heart transplantation as biological bridge to recovery. Transpl. Int. 1997;10:161–163. doi: 10.1111/j.1432-2277.1997.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 25.Hault K., Sebire N.J., Ho S.Y., Sheppard M.N. The difficulty in diagnosing idiopathic arterial calcification of infancy, its variation in presentation, and the importance of autopsy. Cardiol. Young. 2008;18:624–627. doi: 10.1017/S1047951108003168. [DOI] [PubMed] [Google Scholar]
- 26.Sebire N.J., Ramsay A., Sheppard M. Idiopathic arterial calcification presenting with cardiac failure and sudden death in an 11-year-old girl. Pediatr. Dev. Pathol. 2002;5:412–414. doi: 10.1007/s10024-001-0256-2. [DOI] [PubMed] [Google Scholar]
- 27.Guimarães S., Lopes J.M., Oliveira J.B., Santos A. Idiopathic infantile arterial calcification: A rare cause of sudden unexpected death in childhood. Patholog. Res. Int. 2010;2010:185314. doi: 10.4061/2010/185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutsch F., Ruf N., Vaingankar S., Toliat M.R., Suk A., Höhne W., Schauer G., Lehmann M., Roscioli T., Schnabel D., et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat. Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 29.Chong C.R., Hutchins G.M. Idiopathic infantile arterial calcification: The spectrum of clinical presentations. Pediatr. Dev. Pathol. 2008;11:405–415. doi: 10.2350/07-06-0297.1. [DOI] [PubMed] [Google Scholar]
- 30.Kutty S., Cava J.R., Frommelt M.A. Idiopathic infantile arterial calcification: A case report of prenatal and postnatal echocardiographic diagnosis. Echocardiography. 2009;26:862–864. doi: 10.1111/j.1540-8175.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 31.Van der Sluis I.M., Boot A.M., Vernooij M., Meradji M., Kroon A.A. Idiopathic infantile arterial calcification: Clinical presentation, therapy and long-term follow-up. Eur. J. Pediatr. 2006;165:590–593. doi: 10.1007/s00431-006-0146-8. [DOI] [PubMed] [Google Scholar]
- 32.Patel M., Andronikou S., Solomon R., Sinclair P., McCulloch M. Idiopathic arterial calcification in childhood. Pediatr. Radiol. 2004;34:652–655. doi: 10.1007/s00247-004-1166-z. [DOI] [PubMed] [Google Scholar]
- 33.Nitschke Y., Rutsch F. Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Curr. Osteoporos. Rep. 2017;15:255–270. doi: 10.1007/s11914-017-0370-3. [DOI] [PubMed] [Google Scholar]
- 34.Inwald D.P., Yen Ho S., Shepherd M.N., Daubeney P.E. Idiopathic infantile arterial calcification presenting as fatal hypertensive cardiomyopathy. Arch. Dis. Child. 2006;91:928. doi: 10.1136/adc.2006.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glatz A.C., Pawel B.R., Hsu D.T., Weinberg P., Chrisant M.R. Idiopathic infantile arterial calcification: Two case reports, a review of the literature and a role for cardiac transplantation. Pediatr. Transpl. 2006;10:225–233. doi: 10.1111/j.1399-3046.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 36.Bonhoeffer P., Bonnet D., Piéchaud J.F., Stümper O., Aggoun Y., Villain E., Kachaner J., Sidi D. Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J. Am. Coll. Cardiol. 1997;29:202–206. doi: 10.1016/S0735-1097(96)00433-0. [DOI] [PubMed] [Google Scholar]
- 37.Ou P., Celermajer D.S., Marini D., Agnoletti G., Vouhé P., Brunelle F., Le Quan Sang K.H., Thalabard J.C., Sidi D., Bonnet D. Safety and accuracy of 64-slice computed tomography coronary angiography in children after the arterial switch operation for transposition of the great arteries. JACC Cardiovasc. Imaging. 2008;1:331–339. doi: 10.1016/j.jcmg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Ou P., Khraiche D., Celermajer D.S., Agnoletti G., Le Quan Sang K.H., Thalabard J.C., Quintin M., Raisky O., Vouhe P., Sidi D., et al. Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2013;145:1263–1269. doi: 10.1016/j.jtcvs.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 39.White P.H., Cooley W.C., Transitions Clinical Report Authoring Group. American Academy of Pediatrics. American Academy of Family Physicians. American College of Physicians Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2018;142:e20182587. doi: 10.1542/peds.2018-2587. [DOI] [PubMed] [Google Scholar]
- 40.Cooley W.C., Sagerman P.J., American Academy of Pediatrics. American Academy of Family Physicians. American College of Physicians. Transitions Clinical Report Authoring Group Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182–200. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- 41.Sawicki G.S., Lukens-Bull K., Yin X., Demars N., Huang I.C., Livingood W., Reiss J., Wood D. Measuring the transition readiness of youth with special healthcare needs: Validation of the TRAQ–Transition Readiness Assessment Questionnaire. J. Pediatr. Psychol. 2011;36:160–171. doi: 10.1093/jpepsy/jsp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamiyama H., Ayusawa M., Ogawa S., Saji T., Hamaoka K. Health-care transition after Kawasaki disease in patients with coronary artery lesion. Pediatr. Int. 2018;60:232–239. doi: 10.1111/ped.13500. [DOI] [PubMed] [Google Scholar]
- 43.Dahdah N., Kung S.C., Friedman K.G., Marelli A., Gordon J.B., Belay E.D., Baker A.L., Kazi D.S., White P.H., Tremoulet A.H., et al. Falling Through the Cracks: The Current Gap in the Health Care Transition of Patients With Kawasaki Disease: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2021;10:e023310. doi: 10.1161/JAHA.121.023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuda E., Tsujii N., Hayama Y. Stenotic lesions and the maximum diameter of coronary artery aneurysms in Kawasaki disease. J. Pediatr. 2018;194:165–170. doi: 10.1016/j.jpeds.2017.09.077. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs A.H., McCrindle B.W. So hard to say goodbye: Transition from paediatric to adult cardiology care. Nat. Rev. Cardiol. 2014;11:51–62. doi: 10.1038/nrcardio.2013.172. [DOI] [PubMed] [Google Scholar]
- 46.Makino N., Nakamura Y., Yashiro M., Kosami K., Matsubara Y., Ae R., Aoyama Y., Yanagawa H. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr. Int. 2019;61:397–403. doi: 10.1111/ped.13809. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura Y., Yashiro M., Yamashita M., Aoyama N., Otaki U., Ozeki Y., Sano T., Kojo T., Ae R., Aoyama Y., et al. Cumulative incidence of Kawasaki disease in Japan. Pediatr. Int. 2018;60:19–22. doi: 10.1111/ped.13450. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y.T., Manlhiot C., Ching J.C., Han R.K., Nield L.E., Dillenburg R., Pepelassis D., Lai L.S., Smythe J.F., Chahal N., et al. Repeated systematic surveillance of Kawasaki disease in Ontario from 1995 to 2006. Pediatr. Int. 2010;52:699–706. doi: 10.1111/j.1442-200X.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 49.Daniels L.B., Gordon J.B., Burns J.C. Kawasaki disease: Late cardiovascular sequelae. Curr. Opin. Cardiol. 2012;27:572–577. doi: 10.1097/HCO.0b013e3283588f06. [DOI] [PubMed] [Google Scholar]
- 50.Dionne A., Ibrahim R., Gebhard C., Benovoy M., Leye M., Dery J., Lapierre C., Girard P., Fournier A., Dahdah N. Difference between persistent aneurysm, regressed aneurysm, and coronary dilation in Kawasaki disease: An optical coherence tomography study. Can. J. Cardiol. 2018;34:1120–1128. doi: 10.1016/j.cjca.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 51.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 52.Mackie A.S., Fournier A., Swan L., Marelli A.J., Kovacs A.H. Transition and transfer from pediatric to adult congenital heart disease care in Canada: Call for strategic implementation. Can. J. Cardiol. 2019;35:1640–1651. doi: 10.1016/j.cjca.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children. 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassareo P.P., Calcaterra G., Fanos V. Coronavirus disease 2019, Kawasaki disease, and multisystem inflammatory syndrome in children. J. Pediatr. 2020;224:184. doi: 10.1016/j.jpeds.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calcaterra G., Mehta J.L., Fanos V., Bassareo P.P. Insights on Kawasaki disease and multisystem inflammatory syndrome: Relationship with COVID-19 infection. Minerva. Pediatr. 2021;73:203–208. doi: 10.23736/S2724-5276.20.06140-X. [DOI] [PubMed] [Google Scholar]
- 56.Cinteză E., Voicu C., Filip C., Ioniță M., Popescu M., Bălgrădean M., Nicolescu A., Mahmoud H. Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis. Diagnostics. 2022;12:884. doi: 10.3390/diagnostics12040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. National Heart, Lung, and Blood Institute Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128((Suppl. S5)):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercuro G., Bassareo P.P., Flore G., Fanos V., Dentamaro I., Scicchitano P., Laforgia N., Ciccone M.M. Prematurity and low weight at birth as new conditions predisposing to an increased cardiovascular risk. Eur. J. Prev. Cardiol. 2013;20:357–367. doi: 10.1177/2047487312437058. [DOI] [PubMed] [Google Scholar]
- 59.Hadi H.A., Carr C.S., Al Suwaidi J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 60.Martino F., Loffredo L., Carnevale R., Sanguigni V., Martino E., Catasca E., Zanoni C., Pignatelli P., Violi F. Oxidative stress is associated with arterial dysfunction and enhanced intima-media thickness in children with hypercholesterolemia: The potential role of nicotinamide-adenine dinucleotide phosphate oxidase. Pediatrics. 2008;122:e648–e655. doi: 10.1542/peds.2008-0735. [DOI] [PubMed] [Google Scholar]
- 61.Loffredo L., Martino F., Carnevale R., Pignatelli P., Catasca E., Perri L., Calabrese C.M., Palumbo M.M., Baratta F., Del Ben M., et al. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J. Pediatr. 2012;161:1004–1009. doi: 10.1016/j.jpeds.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 62.Masoura C., Pitsavos C., Aznaouridis K., Skoumas I., Vlachopoulos C., Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis. 2011;214:129–138. doi: 10.1016/j.atherosclerosis.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Narverud I., Retterstøl K., Iversen P.O., Halvorsen B., Ueland T., Ulven S.M., Ose L., Aukrust P., Veierød M.B., Holven K.B. Markers of atherosclerotic development in children with familial hypercholesterolemia: A literature review. Atherosclerosis. 2014;235:299–309. doi: 10.1016/j.atherosclerosis.2014.05.917. [DOI] [PubMed] [Google Scholar]
- 64.Awan Z., Alrasadi K., Francis G.A., Hegele R.A., McPherson R., Frohlich J., Valenti D., de Varennes B., Marcil M., Gagne C., et al. Vascular calcifications in homozygote familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2008;28:777–785. doi: 10.1161/ATVBAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 65.Rader D.J., Cohen J., Hobbs H.H. Monogenic hypercholesterolemia: New insights in pathogenesis and treatment. J. Clin. Investig. 2003;111:1795–1803. doi: 10.1172/JCI200318925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu H., Cheng J., Lin S., Wang S., Chen X. Calcified Aortic Valve Disease in Patients With Familial Hypercholesterolemia. J. Cardiovasc. Pharmacol. 2020;76:506–513. doi: 10.1097/FJC.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Defesche J.C., Gidding S.S., Harada-Shiba M., Hegele R.A., Santos R.D., Wierzbicki A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers. 2017;3:17093. doi: 10.1038/nrdp.2017.93. [DOI] [PubMed] [Google Scholar]
- 69.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benn M., Watts G.F., Tybjærg-Hansen A., Nordestgaard B.G. Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur. Heart J. 2016;37:1384–1394. doi: 10.1093/eurheartj/ehw028. [DOI] [PubMed] [Google Scholar]
- 71.Fellin R., Arca M., Zuliani G., Calandra S., Bertolini S. The history of Autosomal Recessive Hypercholesterolemia (ARH). From clinical observations to gene identification. Gene. 2015;555:23–32. doi: 10.1016/j.gene.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 72.Goldstein J.L., Schrott H.G., Hazzard W.R., Bierman E.L., Motulsky A.G. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Investig. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 74.EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) Lancet. 2021;398:1713–1725. doi: 10.1016/S0140-6736(21)01122-3. [DOI] [PubMed] [Google Scholar]
- 75.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J., National Lipid Association Expert Panel on Familial Hypercholesterolemia Familial hypercholesterolemias: Prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011;5((Suppl. S3)):S9–S17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 77.Singh S., Bittner V. Familial hypercholesterolemia--epidemiology, diagnosis, and screening. Curr. Atheroscler. Rep. 2015;17:482. doi: 10.1007/s11883-014-0482-5. [DOI] [PubMed] [Google Scholar]
- 78.Singh A., Gupta A., Collins B.L., Qamar A., Monda K.L., Biery D., Lopez J.A.G., de Ferranti S.D., Plutzky J., Cannon C.P., et al. Familial Hypercholesterolemia Among Young Adults with Myocardial Infarction. J. Am. Coll. Cardiol. 2019;73:2439–2450. doi: 10.1016/j.jacc.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 79.Pang J., Martin A.C., Mori T.A., Beilin L.J., Watts G.F. Prevalence of Familial Hypercholesterolemia in Adolescents: Potential Value of Universal Screening? J. Pediatr. 2016;170:315–316. doi: 10.1016/j.jpeds.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Avis H.J., Kusters D.M., Vissers M.N., Huijgen R., Janssen T.H., Wiegman A., Kindt I., Kastelein J.J., Wijburg F.A., Hutten B.A. Follow-up of children diagnosed with familial hypercholesterolemia in a national genetic screening program. J. Pediatr. 2012;161:99–103. doi: 10.1016/j.jpeds.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 81.O’Brien E.C., Roe M.T., Fraulo E.S., Peterson E.D., Ballantyne C.M., Genest J., Gidding S.S., Hammond E., Hemphill L.C., Hudgins L.C., et al. Rationale and design of the familial hypercholesterolemia foundation CAscade SCreening for Awareness and DEtection of Familial Hypercholesterolemia registry. Am. Heart J. 2014;167:342–349.e17. doi: 10.1016/j.ahj.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 82.Marks D., Thorogood M., Neil H.A., Humphries S.E. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/S0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 83.Wiegman A., Gidding S.S., Watts G.F., Chapman M.J., Ginsberg H.N., Cuchel M., Ose L., Averna M., Boileau C., Borén J., et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015;36:2425–2437. doi: 10.1093/eurheartj/ehv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramaswami U., Futema M., Bogsrud M.P., Holven K.B., Roeters van Lennep J., Wiegman A., Descamps O.S., Vrablik M., Freiberger T., Dieplinger H., et al. Comparison of the characteristics at diagnosis and treatment of children with heterozygous familial hypercholesterolaemia (FH) from eight European countries. Atherosclerosis. 2020;292:178–187. doi: 10.1016/j.atherosclerosis.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bertolini S., Calandra S., Arca M., Averna M., Catapano A.L., Tarugi P., Italian Study Group of Homozygous Familial Hypercholesterolemia Homozygous familial hypercholesterolemia in Italy: Clinical and molecular features. Atherosclerosis. 2020;312:72–78. doi: 10.1016/j.atherosclerosis.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 86.Sturm A.C., Knowles J.W., Gidding S.S., Ahmad Z.S., Ahmed C.D., Ballantyne C.M., Baum S.J., Bourbon M., Carrié A., Cuchel M., et al. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J. Am. Coll Cardiol. 2018;72:662–680. doi: 10.1016/j.jacc.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 87.Molven I., Retterstøl K., Andersen L.F., Veierød M.B., Narverud I., Ose L., Svilaas A., Wandel M., Holven K.B. Children and young adults with familial hypercholesterolaemia (FH) have healthier food choices particularly with respect to dietary fat sources compared with non-FH children. J. Nutr. Sci. 2013;2:e32. doi: 10.1017/jns.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cicero A.F.G., Colletti A., Bajraktari G., Descamps O., Djuric D.M., Ezhov M., Fras Z., Katsiki N., Langlois M., Latkovskis G., et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017;75:731–767. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 89.Steinberg D. The rationale for initiating treatment of hypercholesterolemia in young adulthood. Curr. Atheroscler. Rep. 2013;15:296. doi: 10.1007/s11883-012-0296-2. [DOI] [PubMed] [Google Scholar]
- 90.Ras R.T., Geleijnse J.M., Trautwein E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gylling H., Plat J., Turley S., Ginsberg H.N., Ellegård L., Jessup W., Jones P.J., Lütjohann D., Maerz W., Masana L., et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232:346–360. doi: 10.1016/j.atherosclerosis.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 92.Martino F., Martino E., Morrone F., Carnevali E., Forcone R., Niglio T. Effect of dietary supplementation with glucomannan on plasma total cholesterol and low density lipoprotein cholesterol in hypercholesterolemic children. Nutr. Metab. Cardiovasc. Dis. 2005;15:174–180. doi: 10.1016/j.numecd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Martino F., Puddu P.E., Pannarale G., Colantoni C., Martino E., Niglio T., Zanoni C., Barillà F. Low dose chromium-polynicotinate or policosanol is effective in hypercholesterolemic children only in combination with glucomannan. Atherosclerosis. 2013;228:198–202. doi: 10.1016/j.atherosclerosis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Guardamagna O., Abello F., Baracco V., Stasiowska B., Martino F. The treatment of hypercholesterolemic children: Efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 2011;21:424–429. doi: 10.1016/j.numecd.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 95.Manousaki D., Barnett T.A., Mathieu M.E., Maximova K., Simoneau G., Harnois-Leblanc S., Benedetti A., McGrath J.J., Henderson M., QUALITY Cohort Collaborative Group Tune out and turn in: The influence of television viewing and sleep on lipid profiles in children. Int. J. Obes. 2020;44:1173–1184. doi: 10.1038/s41366-020-0527-5. [DOI] [PubMed] [Google Scholar]
- 96.Authors/Task Force Members. ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 97.Luirink I.K., Wiegman A., Kusters D.M., Hof M.H., Groothoff J.W., de Groot E., Kastelein J.J.P., Hutten B.A. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N. Engl. J. Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 98.Grabarczyk M., Pomianowska K., Zaręba-Głód T., Zachurzok A., Małecka-Tendera E., Matusik P. Statin therapy and lipids-lowering supplements-safe and effective treatment of lipids disturbances in children. Pediatr. Endocrinol. Diabetes Metab. 2022;28:46998. doi: 10.5114/pedm.2022.116114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dombalis S., Nash A. The Effect of Statins in Children and Adolescents with Familial Hypercholesterolemia: A Systematic Review. J. Pediatr. Health Care. 2021;35:292–303. doi: 10.1016/j.pedhc.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Van der Graaf A., Cuffie-Jackson C., Vissers M.N., Trip M.D., Gagné C., Shi G., Veltri E., Avis H.J., Kastelein J.J. Efficacy and safety of coadministration of ezetimibe and simvastatin in adolescents with heterozygous familial hypercholesterolemia. J. Am. Coll. Cardiol. 2008;52:1421–1429. doi: 10.1016/j.jacc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 102.Santos R.D., Ruzza A., Hovingh G.K., Wiegman A., Mach F., Kurtz C.E., Hamer A., Bridges I., Bartuli A., Bergeron J., et al. Evolocumab in Pediatric Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020;383:1317–1327. doi: 10.1056/NEJMoa2019910. [DOI] [PubMed] [Google Scholar]
- 103.Luirink I.K., Hutten B.A., Greber-Platzer S., Kolovou G.D., Dann E.J., de Ferranti S.D., Taylan C., Bruckert E., Saheb S., Oh J., et al. Practice of lipoprotein apheresis and short-term efficacy in children with homozygous familial hypercholesterolemia: Data from an international registry. Atherosclerosis. 2020;299:24–31. doi: 10.1016/j.atherosclerosis.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 104.Bassareo P.P., Abella R., Fanos V., Mercuro G. Biomarkers of corticosteroid-induced hypertrophic cardiomyopathy in preterm babies. Front. Biosci. 2010;2:1460–1471. doi: 10.2741/e205. [DOI] [PubMed] [Google Scholar]
- 105.Peña E., Nguyen E.T., Merchant N., Dennie C. ALCAPA syndrome: Not just a pediatric disease. Radiographics. 2009;29:553–565. doi: 10.1148/rg.292085059. [DOI] [PubMed] [Google Scholar]
- 106.Palmas G., Tumbarello R., Abbruzzese P., Fanos V. Idiopathic infantile arterial calcification: Case report. Minerva. Pediatr. 2008;60:457–460. [PubMed] [Google Scholar]
- 107.De Ferranti S.D., Rodday A.M., Mendelson M.M., Wong J.B., Leslie L.K., Sheldrick R.C. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 108.Skinner A.C., Perrin E.M., Skelton J.A. Prevalence of obesity and severe obesity in US children, 1999-2014. Obesity. 2016;24:1116–1123. doi: 10.1002/oby.21497. [DOI] [PubMed] [Google Scholar]
- 109.Garrido-Miguel M., Cavero-Redondo I., Álvarez-Bueno C., Rodríguez-Artalejo F., Moreno L.A., Ruiz J.R., Ahrens W., Martínez-Vizcaíno V. Prevalence and Trends of Overweight and Obesity in European Children From 1999 to 2016: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019;173:e192430. doi: 10.1001/jamapediatrics.2019.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belay B., Belamarich P.F., Tom-Revzon C. The use of statins in pediatrics: Knowledge base, limitations, and future directions. Pediatrics. 2007;119:370–380. doi: 10.1542/peds.2006-0787. [DOI] [PubMed] [Google Scholar]
- 111.Newman W.P., III, Freedman D.S., Voors A.W., Gard P.D., Srinivasan S.R., Cresanta J.L., Williamson G.D., Webber L.S., Berenson G.S. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N. Engl. J. Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 112.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berenson G.S., Srinivasan S.R., Bao W., Newman W.P., III, Tracy R.E., Wattigney W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998;338:1650–6165. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 114.Koskinen J., Juonala M., Dwyer T., Venn A., Thomson R., Bazzano L., Berenson G.S., Sabin M.A., Burns T.L., Viikari J.S.A., et al. Impact of Lipid Measurements in Youth in Addition to Conventional Clinic-Based Risk Factors on Predicting Preclinical Atherosclerosis in Adulthood: International Childhood Cardiovascular Cohort Consortium. Circulation. 2018;137:1246–1255. doi: 10.1161/CIRCULATIONAHA.117.029726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Skilton M.R., Celermajer D.S. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int. J. Obes. 2006;30:1041–1049. doi: 10.1038/sj.ijo.0803397. [DOI] [PubMed] [Google Scholar]
- 116.Canas J.A., Sweeten S., Balagopal P.B. Biomarkers for cardiovascular risk in children. Curr. Opin. Cardiol. 2013;28:103–114. doi: 10.1097/HCO.0b013e32835dd0ce. [DOI] [PubMed] [Google Scholar]
- 117.Tonstad S., Joakimsen O., Stensland-Bugge E., Leren T.P., Ose L., Russell D., Bønaa K.H. Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler. Thromb. Vasc. Biol. 1996;16:984–991. doi: 10.1161/01.ATV.16.8.984. [DOI] [PubMed] [Google Scholar]
- 118.Dalla Pozza R., Ehringer-Schetitska D., Fritsch P., Jokinen E., Petropoulos A., Oberhoffer R., Association for European Paediatric Cardiology Working Group Cardiovascular Prevention Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. 2015;238:380–387. doi: 10.1016/j.atherosclerosis.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 119.Pintus R., Bassareo P.P., Dessì A., Deidda M., Mercuro G., Fanos V. Metabolomics and Cardiology: Toward the Path of Perinatal Programming and Personalized Medicine. Biomed. Res. Int. 2017;2017:6970631. doi: 10.1155/2017/6970631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.