Abstract
Background:
Esophagogastroduodenoscopy (EGD) is a valuable tool for diagnosing and treating upper gastrointestinal disease. Prioritizing the use of EGD in resource-limited settings must be customized to local populations to maximize population benefit from the exam.
Methods:
Cross-sectional, retrospective review of EGD reports was conducted at Kamuzu Central Hospital (KCH), Lilongwe, Malawi. Esophageal tumors were defined as obstructive or non-obstructive and esophageal varices were graded on a scale of I to IV. Descriptive statistics were calculated and logistic regression performed for each disease state compared to all other reports.
Results:
One thousand and thirty-four cases were reviewed, with 56% male, and mean age (SD) 44 (17) years. The most common indications were dysphagia (37%), hematemesis (21%), and epigastric pain (16%). The most common diagnoses were normal (36%), esophageal cancer (27%), and esophageal varices (17%). Eighty-six percent of esophageal tumors were obstructive and 45% of esophageal varices were grade III or IV. Normal exams were more likely to be female, younger, and present with dyspepsia. Esophageal cancers were more likely to be male, older, present with dysphagia, and present from districts outside Lilongwe. Esophageal varices were more likely to present with hematemesis.
Conclusions:
EGD is a limited resource at KCH; patient selection should be guided by patient age and indication. The high burden of esophageal cancer and varices in Malawi suggests therapeutic endoscopy would be beneficial.
Introduction
Flexible esophagogastroduodenoscopy (EGD) is widely used for the diagnosis and management of upper gastrointestinal complaints throughout the world, including sub-Saharan Africa. In many cases, accurate diagnosis of pathology can lead to timely, focused treatment. Diagnostic EGD is available in the Department of Surgery at Kamuzu Central Hospital (KCH) in Lilongwe, Malawi, though the capacity to perform endoscopic treatment for identified pathology (such as control of variceal bleeding or esophageal stenting) is not currently available. The prevalence of all upper gastrointestinal pathology and, specifically, those conditions that would benefit from endoscopic treatment has not been assessed at KCH. A system for allocating limited EGD resources based on patient characteristics would lead to more efficient screening of patients to maximize the number of patients who benefit from an EGD.
KCH is a 1000-bed public referral hospital that serves a catchment area of 4 million people. Esophageal cancer is seen frequently at KCH and, until EGD became available in 2008, it was diagnosed based only on a barium swallow. There is a higher prevalence of esophageal squamous cell cancer in specific areas of southeastern Africa, Iran and China, compared to other parts of the world [1–7]. Esophageal varices are also common in sub-Saharan Africa and are frequently associated with schistosomal infection [8, 9].
Previous studies have shown a correlation between presenting symptoms and endoscopic diagnosis that may be used to triage patients in a resource-limited setting. Dysphagia is often characteristic of malignancy [1, 10], while epigastric or abdominal pain are often associated with normal findings [11, 12]. However, indications and endoscopic diagnoses vary widely within sub-Saharan Africa.
The current study describes the prevalence of different disease states in patients undergoing EGD at KCH and associates these disease states with risk factors in order to identify which patients will benefit from and should be referred for EGD. The proportion of pathology that may be amenable to non-invasive endoscopic treatment is determined, as is the geographic distribution of patients with upper gastrointestinal disease that present to KCH.
Materials and Methods
Study Setting
KCH is one of four central referral hospitals in the country, and one of only three to offer EGD. All flexible EGD procedures at KCH are performed through the Department of Surgery in a dedicated Endoscopy Suite.
Study Design and Population
The study design was cross-sectional, performed retrospectively from EGD reports. All reports from September 16, 2008 (when endoscopy equipment first became available) through November 25, 2010 were reviewed. All reports were included except those that were grossly incomplete, inconsistent, or illegible. All EGD studies during this period were performed by one of two general surgeons. The study was approved by the Malawi National Health Sciences Research Council (NHSRC #811) and the University of North Carolina Institutional Review Board (IRB #11-0118).
Data Collection
Data fields included age, sex, home district, primary and secondary indications for endoscopy, findings on endoscopy, and endoscopic diagnosis. In the event an indication was not listed, we categorized the following symptoms: “? esophageal cancer,” “? gastric cancer,” and “? achalasia” as “dysphagia,” and “? peptic ulcer disease” and “? atrophic gastritis” as “dyspepsia.” Esophageal varices were graded by the endoscopist on a scale of I to IV, from least to most severe. When provided, a description of any tumor was recorded. Esophageal tumors were defined as obstructive if a tumor was identified in the esophagus and the report did not include results from the stomach or duodenum (scope was unable to pass the tumor site).
One of the investigators (LLW) reviewed paper endoscopy reports and entered data directly into a Microsoft Access database. A random concordance sample (10% of the total reports) was reviewed independently by a second investigator (RI). Of 918 fields in the 102 reports, 875 matched, representing 95% concordance between the entries from the two investigators. Discordant entries were compared to the original reports and corrected when necessary. Hospital pathology files were reviewed over the study period for all patients with specimens taken from gastrointestinal sites and from those with no site reported, and results were entered directly into the database for patients for whom there was also an endoscopy report. During the time frame of this study, there were no pathology services at KCH, and timely pathologic review of specimens was difficult to obtain.
Data Analysis
Frequencies and means were calculated for all variables. Bi-variable relationships (effect of age, sex, and indication on endoscopic diagnosis) were examined. Independent, two-sided t-tests were used to compare means between groups. A multivariate model including all clinically significant variables was created to examine prevalence odds ratios. Statistical significance was demonstrated using p-values and 95% confidence intervals. All analyses were performed using the statistical software Stata (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
One thousand thirty-four diagnostic endoscopy reports were available during the period reviewed. Of these, endoscopy could not be completed in 30 patients (3%), most often due to lack of patient cooperation. Three hundred and twenty-six patients did not have an indication listed; 159 patients with “? esophageal cancer,” 13 patients with “? gastric cancer” and 1 patient with “? achalasia” were assigned an indication of “dysphagia” and 152 patients with “? peptic ulcer disease” and 1 patient with “? atrophic gastritis” were assigned an indication of “dyspepsia.” Fifty-six percent of patients were male with a mean age of 44 years (SD 17, range: 13-96).
The most common indications for endoscopy were dysphagia (37%), hematemesis (21%), epigastric pain (16%), and dyspepsia (16%) (Table 1). The most common endoscopic diagnoses were normal endoscopy (36%), esophageal cancer (27%), and esophageal varices (17%) (Table 2). The mean (SD) age for the most common diagnoses was 37 (15) years for normal endoscopies, 55 (15) for esophageal cancer, and 40 (14) for esophageal varices (Figure 1). These age differences were statistically significant when those with esophageal cancer and esophageal varices were each compared to those with normal endoscopy (p = 0.00, p = 0.04, respectively). Patients with a normal endoscopy result were statistically more likely to be female, younger, and present with dyspepsia. Patients with an endoscopic diagnosis of esophageal cancer were statistically more likely to be male, older, present with dysphagia, and have a home district outside of Lilongwe. The only significant risk factor for patients with an endoscopic diagnosis of esophageal varices was presentation with hematemesis (Table 3).
Table 1:
Study population.
| Variable | n | % |
|---|---|---|
| Age (y) | ||
| <10 | 0 | 0 |
| 11-20 | 66 | 6 |
| 21-30 | 209 | 20 |
| 31-40 | 219 | 21 |
| 41-50 | 180 | 17 |
| 51-60 | 148 | 14 |
| 61-70 | 109 | 11 |
| 71-80 | 65 | 6 |
| 81-90 | 18 | 2 |
| 91-100 | 3 | 0.3 |
| Not recorded | 17 | 2 |
| Sex | ||
| Male | 582 | 56 |
| Female | 443 | 43 |
| Not recorded | 9 | 1 |
| Primary indication for endoscopy | ||
| Dysphagia | 385 | 37 |
| Hematemesis | 217 | 21 |
| Epigastric pain | 170 | 16 |
| Dyspepsia | 168 | 16 |
| Other | 66 | 6 |
| Not recorded | 28 | 3 |
| Total | 1,034 | 100 |
Table 2:
Endoscopic diagnosis.
| Endoscopic diagnosis | n | % |
|---|---|---|
| Normal endoscopy | 377 | 36 |
| Esophageal cancer | 274 | 27 |
| Esophageal varices | 174 | 17 |
| Gastric cancer | 49 | 5 |
| Other | 31 | 3 |
| Procedure unable to be completed | 30 | 3 |
| Duodenal ulcer | 18 | 2 |
| Gastric ulcer | 18 | 2 |
| Duodenitis | 16 | 2 |
| Gastritis | 15 | 1 |
| Gastroesophageal reflux, without Barrett’s | 9 | 1 |
| Esophagitis | 8 | 1 |
| Esophageal candidiasis | 4 | 0.4 |
| Hiatal hernia | 3 | 0.3 |
| Pharyngeal cancer | 3 | 0.3 |
| Achalasia | 2 | 0.2 |
| Esophageal herpes simplex virus | 1 | 0.1 |
| Gastroesophageal reflux, with Barrett’s | 1 | 0.1 |
| Laryngeal cancer | 1 | 0.1 |
| Total | 1,034 | 100 |
Figure 1:
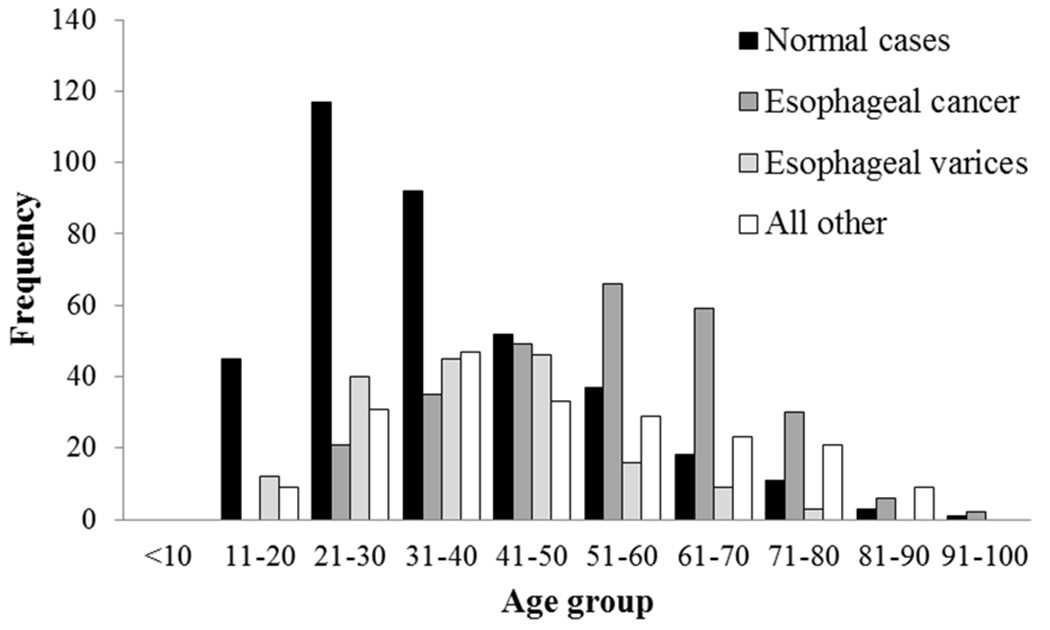
Patient age distribution for normal endoscopy cases, esophageal cancer cases, esophageal varices cases, and all other cases.
Table 3:
Adjusted* prevalence odds ratios for the most common endoscopic diagnoses. NR, not recorded; OR, odds ratio; CI, confidence interval.
| Total | Normal endoscopy | Esophageal cancer | Esophageal varices | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| n | n | OR | CI | p | n | OR | CI | p | n | OR | CI | p | |
| Sex | |||||||||||||
| Female | 443 | 193 | 1.00 | -- | -- | 88 | 1.00 | -- | -- | 74 | 1.00 | -- | -- |
| Male | 582 | 181 | 0.65 | 0.48-0.89 | 0.01 | 183 | 1.52 | 1.02-2.27 | 0.04 | 99 | 0.94 | 0.60-1.48 | 0.79 |
| NR | 9 | 3 | 0.32 | 0.04-2.34 | 0.26 | 3 | 0.86 | 0.09-8.65 | 0.90 | 1 | 5.57 | 0.61-50.75 | 0.13 |
| Age | |||||||||||||
| For each 10-year increase | -- | -- | 0.76 | 0.68-0.84 | 0.00 | -- | 1.23 | 1.09-1.39 | 0.00 | -- | 0.91 | 0.78-1.06 | 0.23 |
| Primary indication | |||||||||||||
| Dyspepsia | 168 | 136 | 1.00 | -- | -- | 2 | 1.00 | -- | -- | 10 | 1.00 | -- | -- |
| Dysphagia | 385 | 56 | 0.06 | 0.04-0.11 | 0.00 | 251 | 110.18 | 26.51-457.82 | 0.00 | 9 | 0.46 | 0.18-1.22 | 0.12 |
| Epigastric pain | 170 | 104 | 0.41 | 0.25-0.69 | 0.00 | 4 | 2.08 | 0.37-11.58 | 0.40 | 5 | 0.49 | 0.16-1.49 | 0.21 |
| Hematemesis | 217 | 42 | 0.06 | 0.03-0.10 | 0.00 | 2 | 0.78 | 0.11-5.64 | 0.81 | 140 | 33.04 | 15.95-68.41 | 0.00 |
| Other | 66 | 30 | 0.28 | 0.15-0.55 | 0.00 | 8 | 9.23 | 1.86-45.74 | 0.01 | 3 | 0.91 | 0.24-3.47 | 0.89 |
| NR | 28 | 9 | 0.15 | 0.06-0.37 | 0.00 | 7 | 23.86 | 4.56-124.76 | 0.00 | 7 | 5.20 | 1.68-16.12 | 0.00 |
| District | |||||||||||||
| Lilongwe | 252 | 88 | 1.00 | -- | -- | 44 | 1.00 | -- | -- | 112 | 1.00 | -- | -- |
| Other | 782 | 289 | 0.88 | 0.61-1.28 | 0.51 | 230 | 1.83 | 1.12-2.99 | 0.02 | 62 | 1.12 | 0.68-1.83 | 0.65 |
Odds ratios are adjusted for all other variables displayed in the table.
Of 385 patients presenting with dysphagia, 251 (65%) had an endoscopic diagnosis of esophageal cancer, with 220 (88%) presenting with obstructive tumors. Of the total of 274 patients with an endoscopic diagnosis of esophageal cancer, 236 (86%) presented with an obstructive tumor. The majority of reports did not comment on whether a patient with an obstructive tumor would be a candidate for stenting. Of the 174 patients with esophageal varices, 79 (45%) were assessed as grade III or grade IV.
A biopsy was reported on the EGD report in 43 patients. Of these, the primary diagnosis recorded on the EGD form was esophageal cancer in 19, gastric cancer in 9, gastritis in 4, gastric ulcer in 3, normal endoscopy in 3 (healed ulcer, “suspicious looking mucosa,” “suspicious metaplasia”), esophageal varices in 2 (both with “mild inflammation near the pylorus”), and duodenal ulcer, duodenitis, and fibrotic stenosis due to esophagitis in 1 each. Histology results were actually available in the hospital pathology files for only 11 patients. In those with histology results, the endoscopic and histologic diagnoses were concordant in 5 of 11 patients. Histology results were available for 4 patients with an endoscopic diagnosis of esophageal cancer. One sample was inadequate for evaluation. The histologic diagnoses for the remaining samples were esophageal adenocarcinoma, high grade squamous dysplasia, and esophageal ulcer.
Sixty-three percent of all patients presented from districts in the central region of Malawi, including 59% of those with esophageal cancer and 68% of those with esophageal varices (Figure 2). However, patients presented to this central region referral hospital for EGD from every district in Malawi. Compared to those with normal results, cases of esophageal cancer were nearly twice as likely to be found outside Lilongwe district. There was no difference between the geographic distribution of normal results and cases of esophageal varices (Table 4).
Figure 2:
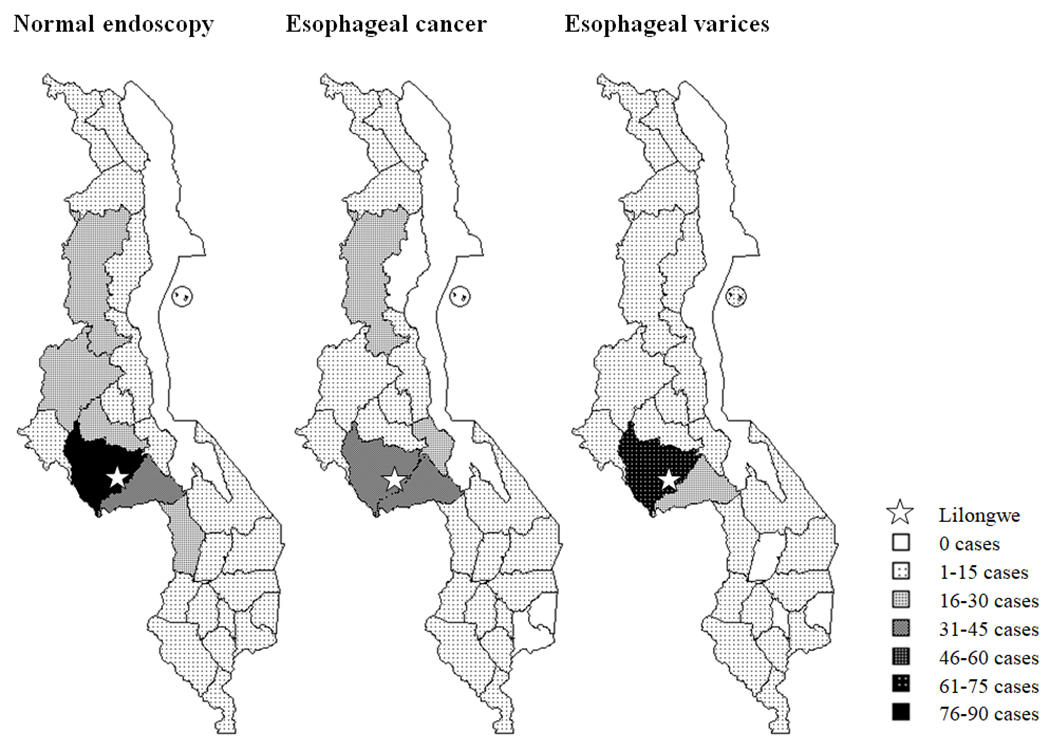
Geographic distribution of normal endoscopy cases, esophageal cancer cases, and esophageal varices cases by district.
Table 4:
Adjusted* prevalence odds ratios comparing home district for esophageal cancer and esophageal varices cases to normal endoscopy cases. OR, odds ratio; CI, confidence interval.
| Cases by district (n) | Lilongwe district | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total | Lilongwe | Other | OR | CI | p | |
| Endoscopic diagnosis | ||||||
| Normal endoscopy | 377 | 88 | 289 | 1.00 | -- | -- |
| Esophageal cancer | 274 | 44 | 230 | 0.57 | 0.33-0.97 | 0.04 |
| Other | 383 | 120 | 263 | 1.00 | 0.68-1.45 | 0.99 |
| Normal endoscopy | 377 | 88 | 289 | 1.00 | -- | -- |
| Esophageal varices | 174 | 62 | 112 | 0.84 | 0.50-1.42 | 0.52 |
| Other | 483 | 102 | 381 | 0.89 | 0.60-1.33 | 0.56 |
Odds ratios are adjusted for age, sex, and primary indication.
Discussion
Demand for endoscopy in Malawi is high, with indications of dysphagia and hematemesis presenting most commonly. These indications had a high percentage of endoscopic findings of esophageal cancer and varices, respectively, and warrant EGD investigation. Patients typically presented with severe disease (obstructive tumors, grade III/IV esophageal varices). At the same time, many patients have normal EGD exams. The proportion of normal exams varies in endoscopy series from African settings, from 6% in Nigeria to 42% in Sudan [13, 14], and was 36% in the current study (Table 5). At KCH, nearly 60% of patients under 30 years have normal results, highlighting an opportunity to reallocate endoscopy resources in this setting.
Table 5:
Review of African EGD data in studies that include “all-comers” for endoscopy.
| Location | Year | Author | n | Department | Most common indications | Most common diagnoses |
|---|---|---|---|---|---|---|
| Eastern Africa | ||||||
| Malawi | 2011 | Current study | 1,034 | Surgery | Dysphagia (37%) Hematemesis (21%) Epigastric pain (16%) |
Normal (36%) Esophageal cancer (27%) Esophageal varices (17%) |
| Malawi | 2009 | Mothes et al. [11] | 441 | Medicine and Surgery | Dysphagia (47%) Abdominal pain (29%) Epigastric pain (16%) |
Esophageal carcinoma (28%) Normal (24%) Gastritis (10%) |
| Uganda | 2008 | Ocama et al. [1] | 287 | Medicine | Epigastric pain (59%) Dysphagia (25%) Vomiting (23%) |
Normal (26%) Gastritis (21%) Esophageal cancer (20%) |
| Zambia | 2008 | Kelly et al. [12] | 2,132 | Not reported | Epigastric pain (37%) Non-localized abdominal pain (28%) Gastrointestinal bleeding (11%) |
Normal (31%) Gastritis (20%) Esophageal candidiasis (13%) |
| Kenya | 2005 | Lodenyo et al. [19] | 768 | Not reported | Not reported | Gastritis (26%) Esophageal varices (14%) Normal (11%) |
| Ethiopia | 2004 | Taye et al. [20] | 10,000 | Medicine | Dyspepsia (59%) Upper gastrointestinal bleeding (18%) Liver disease (11%) |
Duodenal ulcer (32%) Normal (25%) Esophageal varices (10%) |
| Tanzania | 1991 | Missalek et al. [21] | 4,000 | Medicine | Not reported | Normal (30%) Duodenal ulcer (22%) Gastritis (11%) |
| Sudan | 1983 | Fedail et al. [13] | 2,500 | Medicine | Not reported | Normal (42%) Duodenal ulcer (17%) Esophageal varices (9%) |
| Ethiopia | 1983 | Kefenie [22] | 720 | Medicine | Dyspepsia (74%) Gastrointestinal hemorrhage (18%) Dysphagia (4%) |
Gastritis (46%) Duodenal ulcer (21%) Normal (12%) |
| Ethiopia | 1981 | Tsega [23] | 1,084 | Not reported | Dyspepsia (55%) Upper gastrointestinal bleeding (11%) Dysphagia (3%) |
Chronic gastritis (36%) Normal (32%) Duodenal ulcer (14%) |
| Kenya | 1979 | Wankya et al. [24] | 120 | Medicine | Not reported | Duodenitis and erosions (34%) Duodenal ulcer (30%) Gastritis (28%) |
| Ethiopia | 1977 | Tsega [25] | 300 | Medicine | Dyspepsia (62%) Gastrointestinal hemorrhage (17%) Dysphagia (6%) |
Gastritis (42%) Normal (26%) Duodenal ulcer (16%) |
| Western Africa | ||||||
| Nigeria | 2009 | Olokoba et al. [26] | 269 | Medicine | Dyspepsia (61%) Upper gastrointestinal bleeding (15%) Gastroesophageal reflux (10%) |
Not reported |
| Nigeria | 2008 | Onyekwere et al. [27] | 170 | Medicine | Dyspepsia/epigastric pain (36%) Chronic peptic ulcer disease (20%) Upper gastrointestinal bleeding (18%) |
Gastroesophageal reflux disease (40%) Gastroduodenitis (39%) Peptic ulcer disease (35%) |
| Ghana | 2007 | Aduful et al. [17] | 6,977 | Medicine and Surgery | Epigastric pain (43%) Dyspepsia (33%) Hematemesis/melena (14%) |
Normal (41%) Chronic duodenal ulcer (20%) Acute gastritis (13%) |
| Nigeria | 2006 | Agbakwuru et al. [14] | 882 | Surgery | Not reported | Acute gastritis (35%) Duodenal ulcer (25%) Reflux esophagitis (20%) |
| Nigeria | 1999 | Danbauchi et al. [28] | 790 | Medicine | Dyspepsia (70%) Dyspepsia and vomiting (9%) Hematemesis (9%) |
Normal (41%) Gastritis (37%) Duodenitis (27%) |
| Nigeria | 1995 | Andrew et al. [29] | 326 | Not reported | Not reported | Normal (36%) Duodenitis (22%) Esophagitis (21%) |
| Benin | 1991 | Kodjoh et al. [30] | 930 | Not reported | Epigastric pain (32%) Typical ulcerative syndrome (12%) Gastrointestinal bleeding (11%) |
Gastritis (47%) Duodenitis (29%) Esophagitis (22%) |
| Nigeria | 1990 | Malu et al. [31] | 431 | Medicine | Dyspepsia (78%) Upper gastrointestinal bleeding (12%) Portal hypertension (4%) |
Normal (33%) Duodenal ulcer (27%) Deformed bulb or pylorus (27%) |
| Nigeria | 1978 | Adesola et al. [32] | 501 | Surgery | Peptic ulcer diathesis (66%) Upper gastrointestinal hemorrhage (14%) Suspicion for gastric carcinoma (10%) |
Gastritis/duodenitis (42%) Duodenal ulcer (31%) Esophagitis (9%) |
| Nigeria | 1978 | Lewis et al. [33] | 144 | Medicine | Dyspepsia (62%) Hematemesis/melena (20%) Suspicion for neoplasm (8%) |
Gastritis (27%) Normal (13%) Duodenitis (11%) Duodenal ulcer (11%) |
Patients presenting with dysphagia had an increased odds ratio of endoscopic diagnosis of esophageal cancer compared to patients who present with dyspepsia, and 27% of all patients undergoing EGD had endoscopic findings consistent with esophageal cancer. Although these patients were older on average than patients with normal findings or varices, they were still relatively young, with a median age of 56 (range: 22-96). This younger age distribution was also seen in Zambia, where 28% of esophageal squamous cell carcinomas were seen in patients under 45 years [12]. In contrast, data from the Surveillance Epidemiology and End Results cancer registry in the United States show a much older age distribution, with a median age of 68 years [15].
While dyspepsia or epigastric pain was the most common indication for endoscopy in all other published studies from sub-Saharan Africa, both the current study and the prior study from Malawi found dysphagia to be the most common complaint (Table 5). Both studies also found a similarly high prevalence of esophageal cancer. This suggests either a different spectrum of disease in Malawi or different patterns in referral for endoscopy. Referral patterns may be associated with the department responsible for providing endoscopy; surgical disease may be referred for endoscopy more frequently when the endoscopies are performed by surgeons. Endoscopy in the current study is based in the Surgery Department, and was based in both the departments of Surgery and Medicine in the prior study in Malawi [11].
The high proportion of esophageal cancer found in this study is consistent with previous reports of a high burden of esophageal squamous cell carcinoma in southeastern Africa [1–4]. In the prior study from Malawi, esophageal cancer was the most common diagnosis, found in 28% of patients overall [11]. In contrast to southeastern Africa, esophageal squamous cell carcinoma is rare in western Africa, where many of the people live with the same environmental exposures as in Malawi (subsistence farming and reliance on a single crop, aflatoxin grain contamination, HIV disease) [16, 17]. Esophageal squamous cell carcinoma is also common in regions of China and Iran [5–7]. Although the disease has been associated with tobacco use, this does not explain the striking geographic distribution. Our study suggests a geographic distribution of esophageal cancer within Malawi, with varied patterns of home district for patients with normal EGD and esophageal cancer. Notably, esophageal cancer cases presented more commonly from rural districts than from the district that includes the city of Lilongwe; however, this may reflect referral practices for different conditions.
The majority of esophageal cancers in the current study were obstructive, suggesting many esophageal cancers in Malawi may be amenable to dilatation and stenting for palliation. Though endoscopic treatment is not currently available at KCH, it has been implemented successfully in Malawi and other African settings. In the prior study in Malawi, esophageal stenting was performed in 11 cases with early malignant stenosis, and gastrostomy was offered to eligible patients with dysphagia [11]. In the Zambia study, 3% of the endoscopies were therapeutic, including 15 esophageal dilatation procedures for fixed strictures or achalasia and 40 variceal ligations [12]. In a study of patients presenting with dysphagia in Sudan, 83% of the endoscopies were therapeutic, with 87 cases of dilatation and 2 stents placed [10]. These studies suggest that implementation of endoscopic treatments is feasible in a setting like KCH.
Patients presenting with hematemesis had an increased odds ratio of having esophageal varices compared to patients presenting with dyspepsia and 17% of all patients had varices. Esophageal varices are a consequence of portal hypertension, which is commonly due to chronic schistosomiasis infection in Malawi. In a study of Malawian adults with acute gastrointestinal bleeding, 69% of those with esophageal varices were found to have S. mansoni infection [8]. Although banding is used in many countries to treat esophageal varices, it is not currently available at KCH. Instead, patients identified with varices on EGD are often managed operatively with a modified Hassab decongestion procedure (splenectomy, devascularization of the distal 7 cm of the esophagus and the proximal stomach) [18]. Diagnostic EGD is therefore important to identify patients with varices who are otherwise well enough to undergo this procedure.
A limitation of the current study is that the indication for endoscopy was often recorded as “rule out” a particular diagnosis instead of a true indication. While this does not affect the validity of our main results, it may have an impact on the associations between indications and diagnoses. A second limitation is the lack of confirmed pathology for patients with cancer, although clinical diagnosis is likely to have a reasonably high concordance with pathologic diagnosis. It was also not possible to accurately assess the concordance between endoscopic and histologic diagnosis in the current study, due to the very small sample size of patients with histologic results. In examining a geographic distribution of disease, we did not have patient location information that was more specific than the district level; this made it difficult to make accurate urban and rural comparisons, as at least three districts contain both urban and rural areas. Finally, the data reviewed are from a large tertiary hospital setting and therefore are affected by referral bias; more serious conditions may be overrepresented, as these patients are more likely to travel to a tertiary setting for care. Though these limitations exist, the paucity of data in the literature from Malawi makes this report a useful contribution.
Information about the spectrum of upper gastrointestinal disease in Malawi may be used to guide diagnosis and provision of care. Young, female patients presenting with dyspepsia are likely normal; endoscopy resources should instead be allocated to those most likely to have serious disease. In order to improve care in this setting, we are collecting more detailed demographic, past medical, and family history on patients presenting with esophageal cancers and varices to learn more about possible etiologic factors. Pathology resources to obtain confirmed histology on patients with suspected cancers are improving, with a Pathology Laboratory opening at KCH in July 2011. Finally, technical EGD resources to provide dilatation and stenting to patients with obstructive tumors and a therapeutic intervention for bleeding esophageal varices are being explored.
Acknowledgments
This work was supported by a grant from the Doris Duke Charitable Foundation to the University of North Carolina—Chapel Hill to fund Clinical Research Fellow Lindsey Wolf and by funding from the North Carolina Translational & Clinical Sciences Institute through the NIH Clinical and Translational Science Awards at the University of North Carolina—Chapel Hill.
Literature Cited
- 1.Ocama P, Kagimu MM, Odida M, et al. (2008) Factors associated with carcinoma of the oesophagus at Mulago Hospital, Uganda. Afr Health Sci, 8:80–84 [PMC free article] [PubMed] [Google Scholar]
- 2.Herszenyi L, Tulassay Z (2010) Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci, 14:249–258 [PubMed] [Google Scholar]
- 3.Li D, Dandara C, Parker MI (2010) The 341C/T polymorphism in the GSTP1 gene is associated with increased risk of oesophageal cancer. BMC Genet, 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kachala R (2010) Systematic review: epidemiology of oesophageal cancer in sub-Saharan Africa. Malawi Medical Journal, 22:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Tang L, Sun G, et al. (2006) Etiological study of esophageal squamous cell carcinoma in an endemic region: a population-based case control study in Huaian, China. BMC Cancer, 6:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang N, Wen D, Shan B, et al. (2011) Clustering and geographic variation of upper gastrointestinal cancers in a high-risk region of esophageal cancer in northern China. Asian Pac J Cancer Prev, 12:193–198 [PubMed] [Google Scholar]
- 7.Islami F, Kamangar F, Nasrollahzadeh D, et al. (2009) Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran - a review. Eur J Cancer, 45:3156–3165 [DOI] [PubMed] [Google Scholar]
- 8.Harries AD, Wirima JJ (1989) Upper gastrointestinal bleeding in Malawian adults and value of splenomegaly in predicting source of haemorrhage. East Afr Med J, 66:97–99 [PubMed] [Google Scholar]
- 9.Hansen DP, Daly DS (1978) A fibreendoscopic study of acute upper gastrointestinal hemorrhage in Nairobi, Kenya. Am J Trop Med Hyg, 27:197–200 [DOI] [PubMed] [Google Scholar]
- 10.Mudawi HM, Mahmoud AO, El Tahir MA, et al. (2010) Use of endoscopy in diagnosis and management of patients with dysphagia in an African setting. Dis Esophagus, 23:196–200 [DOI] [PubMed] [Google Scholar]
- 11.Mothes H, Chagaluka G, Chiwewe D, et al. (2009) Do patients in rural Malawi benefit from upper gastrointestinal endoscopy? Trop Doct, 39:73–76 [DOI] [PubMed] [Google Scholar]
- 12.Kelly P, Katema M, Amadi B, et al. (2008) Gastrointestinal pathology in the University Teaching Hospital, Lusaka, Zambia: review of endoscopic and pathology records. Trans R Soc Trop Med Hyg, 102:194–199 [DOI] [PubMed] [Google Scholar]
- 13.Fedail SS, Araba BM, Homeida MM, et al. (1983) Upper gastrointestinal fibreoptic endoscopy experience in the Sudan. Analysis of 2500 endoscopies. Lancet, 2:897–899 [DOI] [PubMed] [Google Scholar]
- 14.Agbakwuru EA, Fatusi AO, Ndububa DA, et al. (2006) Pattern and validity of clinical diagnosis of upper gastrointestinal diseases in south-west Nigeria. Afr Health Sci, 6:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Surveillance Epidemiology and End Results Stat Fact Sheets: Esophagus. http://seer.cancer.gov/statfacts/html/esoph.html. Accessed January 18, 2011. [Google Scholar]
- 16.Abdulkareem FB, Onyekwere CA, Awolola NA, et al. (2008) A clinicopathologic review of oesophageal carcinoma in Lagos. Nig Q J Hosp Med, 18:53–56 [DOI] [PubMed] [Google Scholar]
- 17.Aduful H, Naaeder S, Darko R, et al. (2007) Upper gastrointestinal endoscopy at the Korle Bu teaching hospital, Accra, Ghana. Ghana Med J, 41:12–16 [PMC free article] [PubMed] [Google Scholar]
- 18.Hassab MA (1998) Gastro-esophageal decongestion and splenectomy GEDS (Hassab), in the management of bleeding varices. Review of literature. Int Surg, 83:38–41 [PubMed] [Google Scholar]
- 19.Lodenyo H, Rana F, Mutuma GZ, et al. (2005) Patterns of upper gastrointestinal diseases based on endoscopy in the period 1998-2001. Afr J Health Sci, 12:49–54 [DOI] [PubMed] [Google Scholar]
- 20.Taye M, Kassa E, Mengesha B, et al. (2004) Upper gastrointestinal endoscopy: a review of 10,000 cases. Ethiop Med J, 42:97–107 [PubMed] [Google Scholar]
- 21.Missalek W, Jones F, Mmuni K, et al. (1991) Value of fibreoptic oesophago-gastro-duodenoscopy: experience with 4000 procedures at Kilimanjaro Christian Medical Centre, Moshi, Tanzania. Trop Doct, 21:165–168 [DOI] [PubMed] [Google Scholar]
- 22.Kefenie H (1983) Oesophagogastroduodenoscopies: a review of 720 cases. Ethiop Med J, 21:95–99 [PubMed] [Google Scholar]
- 23.Tsega E (1981) Analysis of fibreoptic gastroduodenoscopy in 1084 Ethiopians. Trop Geogr Med, 33:149–154 [PubMed] [Google Scholar]
- 24.Wankya BM, Shah MV, Gitau W, et al. (1979) Upper gastrointestinal endoscopic experience at Kenyatta National Hospital. East Afr Med J, 56:675–680 [PubMed] [Google Scholar]
- 25.Tsega E (1977) Fibre-optic upper-gastrointestinal endoscopy on 300 patients. Ethiop Med J, 15:49–53 [PubMed] [Google Scholar]
- 26.Olokoba AB, Olokoba LB, Jimoh AA, et al. (2009) Upper gastrointestinal tract endoscopy indications in northern Nigeria. J Coll Physicians Surg Pak, 19:327–328 [PubMed] [Google Scholar]
- 27.Onyekwere CA, Hameed H, Anomneze EE, et al. (2008) Upper gastrointestinal endoscopy findings in Nigerians: a review of 170 cases in Lagos. Niger Postgrad Med J, 15:126–129 [PubMed] [Google Scholar]
- 28.Danbauchi SS, Keshinro IB, Abdu-Gusau K (1999) Fifteen years of upper gastrointestinal endoscopy in Zaria (1978 - 1993). Afr J Med Med Sci, 28:87–90 [PubMed] [Google Scholar]
- 29.Andrew PJ, Dixon RA, Iya D, et al. (1995) Upper gastrointestinal endoscopy in an urban hospital in northern Nigeria: association of presenting features with endoscopic findings. Trop Doct, 25:9–11 [DOI] [PubMed] [Google Scholar]
- 30.Kodjoh N, Hountondji A, Addra B (1991) [The contribution of endoscopy in the diagnosis of esophago-gastro-duodenal disorders in a tropical milieu. Experience in Benin with 930 examinations]. Ann Gastroenterol Hepatol (Paris), 27:261–267 [PubMed] [Google Scholar]
- 31.Malu AO, Wali SS, Kazmi R, et al. (1990) Upper gastrointestinal endoscopy in Zaria, northern Nigeria. West Afr J Med, 9:279–284 [PubMed] [Google Scholar]
- 32.Adesola AO, Olumide F, Popoola AO, et al. (1978) Endoscopy in upper gastro-intestinal disease in Nigerians. Niger Med J, 8:69–73 [PubMed] [Google Scholar]
- 33.Lewis EA, Aderoju EA, Ayoola EA, et al. (1978) Experience with upper gastrointestinal endoscopy in Ibadan. Niger Med J, 8:420–424 [PubMed] [Google Scholar]


