Abstract
Head and neck squamous cell carcinomas (HNSCCs) are the sixth most common cancers worldwide. More than half of patients with HNSCC eventually experience disease recurrence and/or metastasis, which can threaten their long-term survival. HNSCCs located in the oral cavity and larynx are usually associated with tobacco and/or alcohol use, whereas human papillomavirus (HPV) infection, particularly HPV16 infection, is increasingly recognized as a cause of oropharyngeal HNSCC. Despite clinical, histologic, and molecular differences between HPV-positive and HPV-negative HNSCCs, current treatment approaches are the same. For recurrent disease, these strategies include chemotherapy, immunotherapy with PD-1-inhibitors, or a monoclonal antibody, cetuximab, that targets epidermal growth factor; these therapies can be administered either as single agents or in combination. However, these treatment strategies carry a high risk of toxic side effects; therefore, more effective and less toxic treatments are needed. The landscape of HNSCC therapy is changing significantly; numerous clinical trials are underway to test novel therapeutic options like adaptive cellular therapy, antibody-drug conjugates, new targeted therapy agents, novel immunotherapy combinations, and therapeutic vaccines. This review helps in understanding the various developments in HNSCC therapy and sheds light on the path ahead in terms of further research in this field.
Keywords: head and neck squamous cell carcinoma, human papillomavirus, novel targeted therapy, cetuximab, immune-checkpoint inhibitor
1. Introduction
Head and neck squamous cell carcinomas (HNSCCs) originate in the oral cavity, pharynx, larynx, and sinonasal track. Squamous cell carcinoma is the most common type of cancer in the head and neck region and the sixth leading cancer by incidence worldwide [1]. HNSCC accounts for about 5% of all cancers in the United States [2]. Many risk factors for HNSCC have been identified including exposure to carcinogens (such as tobacco, alcohol, betel nut, and air pollution [3]), infection with the oncoviruses Epstein–Barr virus or high-risk human papillomavirus (HPV) strains [4,5], and genetic factors. Several of these risk factors display geographical variations; for instance, betel nut chewing is most common in India, while exposure to carcinogenic air pollutants is more common in developing regions, such as India and China [6,7]. The incidence of HPV-driven HNSCC is increasing in Western countries, whereas Epstein–Barr virus-driven HNSCC is more common in developing countries in East Asia. One of the main genetic disorders that raises the risk of developing HNSCC is Fanconi anemia. This rare inherited disease is caused by mutations in one or more of the FANC family of DNA-repair genes. Individuals with Fanconi anemia have a 500- to 700-fold higher risk of developing HNSCC than the general population [8]. Other distinct factors that separate individuals with FA from the general population in terms of HNSCC include diagnosis at a young age (20–40 years old) and tumor localization in the oral cavity and tongue.
HPV-driven HNSCC occurs predominantly in the oropharynx; the primary oncogenic HPV strains are HPV16 (83–86% of HPV-positive HNSCCs), HPV33 (3.3–7.3%), HPV35 (2.2–4%), and HPV18 (<2%) [9,10,11,12]. In contrast, carcinogen exposure typically drives HPV-negative disease. HPV-positive HNSCC occurs in a younger patient population and has a more favorable prognosis than HPV-negative HNSCC. For patients with advanced-stage HPV-positive HNSCC, the 5-year survival rates are 75% to 80%, whereas fewer than 50% of patients with HPV-negative disease survive for 5 years [13]. Although HPV-positive and HPV-negative HNSCC distinctly differ genetically, they are treated in much the same way, an approach that produces significant morbidity [14].
In terms of the screening strategy for early-stage HNSCC, visual screening has been considered as a feasible, safe, and cost-effective option in the last few years amongst the high-risk group of tobacco, betel, and/or alcohol consumers [15].
In general, early-stage HNSCC is treated with surgery or radiation and has 5-year survival rates of approximately 70% to 90% [16]. Locally advanced disease requires multimodal treatment that combines surgery, radiation, and systemic treatment with platinum-based chemotherapy or anti-epidermal growth factor receptor (EGFR) targeted therapy with cetuximab [17,18,19].
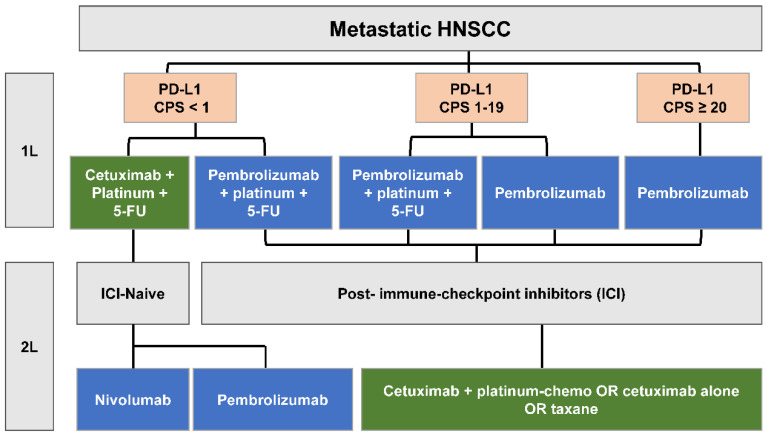
Local recurrence or the development of distant metastasis is common in HNSCC, affecting about 20% of patients treated for early-stage disease and 50% of those with locally advanced HNSCC [16]. The prognosis is poor for recurrent or metastatic (R/M) HNSCC, with a median duration of around 1 year of overall survival (OS) [17]. R/M HNSCC that is not treatable with surgical resection or definitive radiotherapy is treated with palliative systemic therapy that includes platinum-based chemotherapy, cetuximab, and/or immune checkpoint inhibitors (ICIs) with anti-programmed death 1 (PD-1) antibodies (Figure 1). Conventional therapy for locally advanced HNSCC often results in permanent impairments in chewing, swallowing, and tasting, along with a dry mouth, feeding tube dependence, and aspiration pneumonia [20,21]. These adverse events for survivors, coupled with the poor outcomes for R/M HNSCC, demonstrate the need for novel therapies with less toxicity and more efficacy. Several novel therapies—including molecular targeted therapies, antibody-drug conjugates, and immunotherapies—may be more selective, cause fewer adverse effects, and be more effective in the treatment of HNSCC. In our review, we describe recent developments in the understanding of the genomics and pathophysiology of HNSCC, assess progress in the management of HNSCC, and provide perspectives on future research and treatment directions.
Figure 1.
Standard-of-care treatment algorithm for metastatic HNSCC. 1L = first line, 2L = second line, CPS = combined positive score, 5-FU = 5-fluorouracil, ICI = immune-checkpoint inhibitor.
2. Genomics of HNSCC
Genomic studies in HNSCC have revealed frequent chromosomal changes, DNA copy number alterations, somatic mutations, and promoter methylation. Only a few of the mutations and chromosomal abnormalities driving HNSCC were known before the introduction of next-generation sequencing (NGS) [22,23]: TP53 and CDK2NA [24] mutations and amplification of 11q13, CCND1, and EGFR [25]. The first results of NGS studies in HNSCC [26,27] and The Cancer Genome Atlas (TCGA) [12] have offered a comprehensive understanding of the somatic genomic alterations driving HNSCC. In addition to previously known HNSCC-associated mutations in TP53, CDKN2A, and PIK3CA, these studies identified NOTCH1 as one of the most commonly mutated genes in HNSCC [28]. The topmost frequently mutated genes in HNSCC are TP53 (72%), CDKN2A (22%), FAT1 (23%), PIK3CA (21%), NOTCH1 (19%), KMT2D (MLL2) (18%), NSD1 (10%), CASP8 (9%), AJUBA (6%), and NFE2L2 (6%). HNSCCs infrequently carry RAS gene mutations; mutations in HRAS, at about 8%, are the most common in this group. Strikingly, NGS revealed that unlike many other solid tumors driven by mutations in oncogenes, HNSCCs are most often characterized by the loss of tumor-suppressor genes.
HPV-positive HNSCCs, however, typically lack mutations or alterations in TP53 and CDKN2A genes. Instead, these tumors more commonly have a PIK3CA genomic alteration with mutation (14%) and gene amplification (16%) [12], loss of TRAF3, and amplification of E2F1 [27]. NGS of 149 HPV-positive and 335 HPV-negative HNSCC/normal paired samples by Gillison et al. [29] confirmed PIK3CA mutations and identified mutations in ZNF750 and EP300 as candidate driver events in HPV-positive HNSCC. The outcome of the study was in line with earlier findings that showed TP53, CDKN2A, FAT1, CASP8, NOTCH1, and HRAS as the main mutations that drove cancer progression in HPV-negative HNSCC. Furthermore, a recent study also showed that high-risk HPV infections went together with mutations in PIK3CA, EP300, NF1, and RB1 in samples from benign tonsils, suggesting these mutations to be potential biomarkers to identify the cancer progression risk [30].
Substantial research has also confirmed the role of aberrant signaling pathway activation in HNSCC. For instance, EGFR is frequently expressed in 80% to 90% of HNSCC tumors, and its overexpression is associated with poor survival [31]. Other receptor tyrosine kinases, such as HER2 and MET, are also overexpressed in many HNSCCs and may contribute to resistance to EGFR-targeting drugs [14,32]. In addition, the PI3K-AKT-mTOR signaling pathway, which drives the development of many tumor types, is often altered in HNSCC [12].
3. Pathophysiology of HNSCC
3.1. HPV-Negative HNSCC
HPV-negative HNSCC is typically observed in patients with regular consumption of tobacco and alcohol [33,34]. The carcinogenic effects of tobacco and alcohol are attributable to the formation of DNA adducts, which dysregulate critical cellular processes [35]. DNA adducts can cause chromosomal mutation and instability, affecting cell homeostasis and other cellular mechanisms, which leads to cancer progression [35]. The carcinogenic effects of tobacco can also be enhanced by alcohol, which can facilitate the introduction of tobacco-related carcinogens in the mucosa [36,37]. In this way, the collaborative effect of both alcohol and tobacco contributes to the HNSCC carcinogenesis [38].
3.2. HPV-Positive HNSCC
HPV is a nonenveloped, circular, double-stranded DNA virus that causes approximately 70% of oropharyngeal squamous cell carcinoma in the United States [39]. HPV strains can be classified by their associated cancer risk into low- and high-risk groups. The high-risk strains include HPV16, HPV18, HPV26, HPV33, HPV35, and HPV59, along with seven others. HPV16 is the primary strain causing HNSCC in the oropharynx (83%) [11]. The E6 and E7 HPV oncoproteins degrade the tumor suppressor TP53 and retinoblastoma-associated protein (RB) on infection [40]. In turn, the inactivation of Rb function by E7 leads to an increase in p16INK4A (p16) levels. P16 expression is commonly used as a surrogate marker for HPV-related oropharyngeal tumors [24]. Tumors can only be sustained with persistent expression of the viral E6 and E7 oncoproteins.
4. Current Targeted Therapy for Head and Neck Cancer
4.1. EGFR Inhibitors
EGFR is overexpressed in ~80% of HNSCCs, leading to a poor prognosis [41,42]. Monoclonal antibodies (mAb), as well as TKI-based small molecules, can be used to target EGFR. Cetuximab, a chimeric mAb, is an FDA-approved targeted therapy for HNSCC that blocks EGFR signaling [14,43].
An early phase-III randomized trial in locally advanced HNSCC that compared radiation plus cetuximab to radiation alone showed a significantly better median duration of response (24.4 vs. 14.9 months, p = 0.005), progression-free survival (PFS) (17.1 vs. 12.4 months, p = 0.006), and OS (49 vs. 29.3 months, p = 0.006) in patients receiving cetuximab compared to those receiving radiation alone [44]. Yet, the addition of cetuximab led to increased rates of rash and infusion reactions compared to radiotherapy alone. Furthermore, two phase-III clinical trials found that, in patients with HPV-positive HNSCC, cetuximab was inferior to the combination of cisplatin with radiotherapy [45,46].
Single-agent cetuximab had low efficacy in R/M HNSCC, with overall response rates (ORRs) ranging from 10% to 13% [47]. The reason for the poor efficacy of cetuximab may be attributed to the ErbB protein and ligand aberrations and/or activation of other downstream signaling components [48]. Vermorken et al. [49] evaluated the effect of cetuximab alone on 103 HNSCC platinum-refractory patients. None of the patients showed a complete response (CR). The disease control rate (DCR) was 46% and only 13% of patients showed a partial response (PR).
In the EXTREME trial [49], R/M HNSCC patients were randomized to doublet chemotherapy (cisplatin or carboplatin plus 5-fluorouracil) vs. the same chemotherapy plus cetuximab for their first-line therapy. The median OS was 7.4 vs. 10.1 months, respectively (hazard ratio [HR] 0.80; p = 0.04). The median PFS was 3.3 vs. 5.6 months, respectively (HR 0.54; p < 0.001). The addition of cetuximab increased the response rate from 20% to 36% (p < 0.001). As expected, toxicity was worse in the chemotherapy plus cetuximab group, with more sepsis, anorexia, and skin and infusion-related reactions.
Panitumumab is a fully human immunoglobulin G2 (IgG2) EGFR mAb that has lower immunogenicity than cetuximab. The phase-III SPECTRUM trial of panitumumab combined with cisplatin and 5-fluorouracil as a front-line treatment for R/M HNSCC showed that the median PFS was longer in the group that received panitumumab (5.8 vs. 4.6 months, p = 0.0036). However, there was no significant difference in the median OS (11.1 vs. 9 months, p = 0.14). Several grade 3 or 4 adverse events were more common in the panitumumab group [50]. Zalutumumab is a high-affinity human IgG1 mAb to EGFR. A phase-III trial found no significant difference in the median OS between patients with R/M HNSCC who received zalutumumab plus the best supportive care and those who received just the best supportive care (6.7 vs. 5.2 months, p = 0.0648). Neither panitumumab nor zalutumumab is approved for the treatment of R/M HNSCC.
EGFR TKIs have also been studied in HNSCC. A phase-III study (LUX-Head & Neck 1) compared gefitinib to methotrexate for R/M HNSCC and found similar median OS durations (5.6 vs. 6.7 months) [51]. Given the lack of convincing evidence to support its use, gefitinib has not been further developed for HNSCC. In patients with platinum-resistant R/M HNSCC, afatinib (EGFR TKI) and cetuximab had similar response rates [52]. A phase-III trial comparing afatinib to methotrexate in patients with R/M HNSCC who had disease progression after platinum-based chemotherapy found median OS durations of 6.8 months with afatinib and 6 months with methotrexate (HR 0.96, p = 0.7) [53]. Although both LUX-Head & Neck 1 and 3 showed a modest PFS benefit with afatinib over methotrexate [54], this approach was abandoned owing to the lack of OS benefit and the development of more promising immunotherapies, to be discussed below.
4.2. Farnesyltransferase Inhibitors
Of the three RAS genes (HRAS, KRAS, and NRAS), HRAS is the most commonly mutated in HNSCC [12]. Thus, the RAS-RAF pathway is a target of high therapeutic interest. Since proper trafficking and localization of RAS proteins require several posttranslational modifications, a potential strategy to inhibit oncogenic RAS activity is to disrupt these posttranslational modifications such as RAS prenylation through inhibition of farnesyltransferase.
In February 2021, the FDA designated farnesyltransferase inhibitor (FTI) tipifarnib, still under investigation, as a breakthrough therapy for the treatment of HRAS-mutant R/M HNSC based on the outcomes of the RUN-HN trial, especially for patients whose disease had progressed while being treated with platinum-based chemotherapy [55]. Tipifarnib is a first-in-class nonpeptidomimetic quinolinone that binds to and potently inhibits FT. The phase-II RUN-HN (NCT02383927) trial included 30 patients with R/M HRAS-mutant HNSCC treated with tipifarnib and demonstrated a 50% ORR in the 18 evaluable patients. In addition, tipifarnib showed a median PFS of 5.9 months, as compared to 2.8 months with the last prior line of therapy, and the median OS was 15.4 months with tipifarnib [56]. Currently, tipifarnib is being studied in a pivotal phase-II AIM-HN study (NCT03719690), which will further assess the ORR of patients with HRAS-mutant HNSCC treated with tipifarnib [56]. Another trial is currently evaluating the combination of tipifarnib and the PI3K inhibitor alpelisib in R/M HNSCC with HRAS and PIK3CA alterations (NCT04997902) (clinicaltrials.gov).
4.3. PI3K/AKT/mTOR Inhibitors for PI3K-Mutant HNSCC
The PI3K/AKT/mTOR signaling pathway is crucial for various cellular processes, including cell growth and division, metabolism, and migration, all of which can be dysregulated in cancer. This pathway is one of the most frequently activated ones in HNSCC, with activation in more than 90% of HNSCCs [57,58], including both HPV-positive and HPV-negative subsets [12]. Oncogenic activation of the PI3K pathway can be caused by EGFR activation, PI3K overexpression, gain-of-function mutations and/or amplifications in PIK3CA, loss-of-function (LOF) mutations in PTEN, or oncogenic alterations in AKT, TSC1/2, LKB1, or MTOR [57,59]. Therefore, use of PI3K/AKT/mTOR inhibitors is an appealing therapeutic strategy for HNSCCs regardless of their HPV status. Of the several PI3K pathway inhibitors in different stages of clinical development, only a few have been approved by the FDA for the treatment of hematological cancers (copanlisib, idelalisib, umbralisib, and duvelisib), renal cell carcinoma (temsirolimus and everolimus) [59], and metastatic breast cancer (alpelisib) [60,61].
Numerous preclinical studies in HNSCC xenografts with PIK3CA mutations demonstrated the susceptibility of these tumors to PI3K/AKT/mTOR inhibitors [62,63,64,65,66,67,68,69], supporting their clinical development. However, clinical trials with pan-PI3K and dual PI3K/mTOR inhibitors have demonstrated only modest response rates that were not consistently better in PIK3CA mutant vs. wild-type (wt) tumors. One patient with endometrial cancer with PIK3CA and PTEN mutations had a complete response (CR) to the pan-PI3K inhibitor copanlisib in a phase-I study [70]. This outcome led to the initiation of the biomarker-based phase-II MATCH trial (NCT02465060), which examined copanlisib treatment in patients with advanced refractory solid tumors and with mutations in PIK3CA and PTEN and loss of PTEN. Buparlisib (BKM120), another pan-PI3K inhibitor, showed limited antitumor activity in patients with platinum-refractory R/M HNSCC, with a disease control rate of 49% and an ORR of only 3% (NCT01527877). There was no significant difference between cohorts with PIK3CA-mutant and non-mutant tumors in PFS (1.7 months vs. 1.8 months) or OS (3.4 months vs. 5.8 months (NCT01737450)) [71,72].
Additionally, use of the dual PI3K/mTOR inhibitor apitolisib [73] in patients with advanced solid tumors demonstrated modest clinical activity. Of the 14 evaluable patients with PIK3CA mutations, three patients showed partial responses (PRs), eight had stable disease, and three had progressive disease. Of the 120 enrolled participants in the study, 15 HNSCC patients were included independent of their PIK3CA status, of which only three had a PR. Similar response rates were observed with the dual PI3K/mTOR inhibitors gedatolisib [74] and bimiralisib (PQR309) [75] in patients with various advanced-stage cancers. Gedatolisib was administered to 77 patients with solid tumors, including four patients with PIK3CA alterations, who had stable disease for more than 6 months [74]. Likewise, a phase-I trial with bimiralisib evaluated 28 patients with advanced solid tumors, two of whom had PIK3CA mutations. One of them had stable disease and the other experienced a 26% reduction in tumor volume on treatment with bimiralisib [75].
Despite promising preclinical evidence, limited clinical activity and drug-related toxicities have hindered the use of pan-PI3K and dual PI3K/mTOR inhibitors for most solid tumors, leading to the development of isoform-selective PI3K inhibitors. Several ongoing clinical trials are investigating whether PIK3CA-mutant tumors are sensitive to drugs that inhibit the class-I PI3K catalytic subunit α isoform. Dose-escalation studies with the PI3Kα inhibitors taselisib, TAK-117, and alpelisib in patients with advanced-stage solid tumors have shown promising clinical activity in those harboring PIK3CA alterations as compared to patients with wt PIK3CA [76,77,78]. Taselisib treatment showed an ORR of 36% in patients with PIK3CA mutations but 0% in patients without PIK3CA mutations [76]. However, TAK-117 displayed a PR rate of 7.5% of patients with PIK3CA-altered tumors [77]. The phase-I trial with alpelisib specifically enrolled patients with PIK3CA mutations and showed an ORR of 6% and stable disease rate of 52%. Moreover, a dose-expansion arm of this study included four patients with PIK3CA-wt tumors, but they had no clinical benefit with alpelisib treatment [78]. Overall, PI3K isoform-specific inhibitors have demonstrated high rates of stable disease in PIK3CA-altered cancers and are better tolerated than pan-PI3K and dual PI3K/mTOR inhibitors. In addition, PI3Kβ-, PI3Kγ-, and PI3Kδ-specific inhibitors are in clinical development at various stages for use either as single agents or in combinations with chemotherapy, targeted therapy, or radiation therapy in R/M HNSCC (Table 1).
Table 1.
Ongoing clinical trials with PI3K/AKT/mTOR inhibitors in HNSCC.
| Class | Drug | Patient Cohort | Biomarker | Phase | Clinical Trials | Intervention | Status |
|---|---|---|---|---|---|---|---|
| Pan-PI3K inhibitor | Buparlisib (BKM-120) | R/M HNSCC | None | III | NCT04338399 | Buparlisib + paclitaxel | Recruiting |
| LA-HNSCC | HPV-positive | I | NCT02113878 | Buparlisib with cisplatin + IMRT | Completed, Awaiting results | ||
| Copanlisib (BAY 80-6946) | R/M HNSCC | None | I | NCT03735628 | Copanlisib + nivolumab | Active, not recruiting | |
| R/M HNSCC | PIK3CA mutation, PTEN mutation/loss | II | NCT02465060 | Copanlisib | Recruiting | ||
| Isoform-specific PI3K inhibitor | Alpelisib (BYL-719) (PI3Kα) | LA-HNSCC | HPV-positive | II | NCT03601507 | Alpelisib | Recruiting |
| R/M HNSCC | HRAS overexpression, PIK3CA mutation and/or amplification | I/II | NCT04997902 | Tipifarnib + alpelisib | Recruiting | ||
| R/M HNSCC | PI3K pathway alterations | II | NCT03292250 | Alpelisib | Completed, awaiting results | ||
| R/M HNSCC | None | II | NCT02145312 | Alpelisib | Unknown | ||
| R/M HNSCC | None | I | NCT01822613 | Alpelisib + LJM716 | Completed, awaiting results | ||
| LA-HNSCC | None | I | NCT02282371 | Alpelisib with cetuximab + IMRT | Completed, awaiting results | ||
| LA-HNSCC | None | I | NCT02537223 | Alpelisib with cisplatin + IMRT | Completed, awaiting results | ||
| Duvelisib (VS-0145) (PI3K δ/γ) | R/M HNSCC | None | II | NCT05057247 | Duvelisib + docetaxel | Recruiting | |
| GSK2636771 (PI3K β) | R/M HNSCC | PTEN mutation/loss | II | NCT02465060 | GSK2636771 | Recruiting | |
| Parsaclisib (INCB050465) (PI3K β) | R/M HNSCC | None | I | NCT02646748 | Parsaclisib + pembrolizumab | Completed, awaiting results | |
| Serabelisib (INK-117) (PI3K α) | LA-HNSCC | PIK3CA mutation, KRAS mutation | I/II | NCT04073680 | Serabelisib + canagliflozin | Unknown | |
| Taselisib (GDC-0032) (PI3K α/δ/γ) | R/M HNSCC | PIK3CA mutation, PTEN mutation/loss | II | NCT02465060 | Taselisib | Recruiting | |
| Dual PI3K/mTOR inhibitor | Gedatolisib (PF-05212384) | R/M HNSCC | PI3K pathway alterations | I | NCT03065062 | Gedatolisib + palbociclib | Recruiting |
| AKT inhibitor | Ipatasertib (GDC-0068) | R/M HNSCC | AKT mutation | II | NCT02465060 | Ipatasertib | Recruiting |
| LA-HNSCC | None | I | NCT05172245 | Ipatasertib with cisplatin + RT | Recruiting | ||
| R/M HNSCC | None | II | NCT05172258 | Ipatasertib + pembrolizumab | Recruiting | ||
| Capivasertib (AZD5363) | R/M HNSCC | AKT mutation | II | NCT02465060 | Capivasertib | Recruiting |
AKT = AKT kinase; HPV = human papillomavirus; IMRT = intensity-modulated radiation therapy; mTOR = mammalian target of rapamycin; PI3K = phosphoinositide 3-kinase; LA-HNSCC = locally advanced head and neck squamous cell carcinoma; R/M HNSCC = recurrent and metastatic head and neck squamous cell carcinoma.
Although PI3Kα inhibitors demonstrated some clinical benefits in patients with PIK3CA mutations, they are insufficient to achieve a CR, and prolonged treatment results in therapy resistance. For this reason, development of this class of drugs as standalone modalities has not succeeded. In order to overcome therapeutic resistance and improve patient responses, PI3K pathway inhibitors are currently being evaluated for use in combination with other targeted therapies, chemotherapy, radiotherapy, and immunotherapies (Table 1). The addition of the pan-PI3K inhibitor PX-866 to docetaxel did not significantly improve the median PFS when compared to docetaxel alone in patients with R/M HNSCC (92 days; 95% CI, 46–119 vs. 82 days; 95% CI, 47–96; p = 0.42) or OS (263 days; 95% CI, 125–383 vs. 195 days; 95% CI, 121–NR; p = 0.62) [79]. Likewise, the combination of PX-866 with cetuximab had similar median PFS and OS durations when compared to cetuximab alone (PFS: 80 days in both groups; p = 0.48; OS: 211 days; 95% CI, 149–279 in the combination group vs. 256 days; 95% CI, 148–NR; p = 0.6) in the cetuximab-alone group. More importantly, patients harboring PIK3CA mutations did not have any response to combined PX-866 and cetuximab treatment [80]. The BERIL-1 phase-II trial in patients with platinum-refractory R/M HNSCC showed a modestly longer median PFS in patients receiving a combination of buparlisib and paclitaxel (4.6 months; 95% CI, 3.5–5.3) than in the paclitaxel and placebo group (3.5 months; 95% CI, 2.2–3.7; p = 0.011) (NCT01852292) [81]. A phase-III trial of this combination in R/M HNSCC is ongoing (NCT04338399). A phase-I trial with copanlisib and cetuximab (NCT02822482) in patients with R/M HNSCC in whom platinum or cetuximab therapy failed was stopped early due to unfavorable toxic effects in the combination arm [82]. Overall, these trials showed limited clinical efficacy and significant drug toxicity in the combination groups, emphasizing the need for better biomarkers of sensitivity.
However, in contrast to the results in R/M HNSCC, alpelisib in combination with drugs targeting the estrogen receptors has shown robust responses compared to single-agent drugs, leading to the FDA approval of alpelisib for the treatment of PIK3CA-mutant metastatic breast cancer [60,61]. Two phase-II clinical trials are currently evaluating the clinical efficacy of alpelisib as monotherapy in HPV-positive HNSCC (NCT03601507) and alpelisib in combination with the farnesyltransferase inhibitor tipifarnib in HRAS- and PIK3CA-mutant HNSCC (NCT04997902).
PIK3CA gene mutations are classified as canonical or noncanonical. Canonical mutations are the most common, occurring in one of three hotspot locations (E542, E545, and H1047) of the p110α subunit and leading to the activation of the PI3K pathway. Noncanonical mutations are rare, distributed throughout the p110α subunit, and may be activating or non-activating. Moreover, around 27% of the noncanonical mutations characterized in TCGA are unique to HNSCC and have not been identified in other cancers with PIK3CA alterations [83]. Alpelisib, in addition to being effective against canonical PIK3CA alterations, has shown efficacy in preclinical models with three frequently occurring noncanonical mutations [61,84]. Moreover, a recent study also reported a remarkable response (73% tumor shrinkage) in an HNSCC patient with a noncanonical activating PIK3CA mutation who received alpelisib monotherapy [83], underscoring the need to further understand the noncanonical PIK3CA mutation biology.
Despite ongoing clinical trials of PI3K inhibitors, their clinical translation has been challenging owing to their modest efficacy as single agents, their unfavorable toxicity profiles, the emergence of resistance, and the lack of a biomarker-selective approach for treatment. In retrospect, these disappointing clinical studies may have been predicted by preclinical studies that demonstrated that although PIK3CA-mutant HNSCC cell lines are more sensitive to PI3K inhibitors than PIK3CA-wt HNSCC cell lines [62,63], PI3K inhibition leads only to growth arrest, not cell death [85].
4.4. PI3K Inhibitors for NOTCH1-Mutant HNSCC
NOTCH1 is one of the most frequently mutated genes in HNSCC [12]. Functional characterization has confirmed structural predictions that most of the NOTCH1 mutations in HNSCC are LOF mutations, supporting a tumor-suppressor function for NOTCH1 in HNSCC [86]. The occurrence of these mutations may predict a response to ICI and PI3K inhibitors [86]. Previous research from our group used an unbiased pharmacogenomics approach and identified a remarkable correlation between NOTCH1 LOF mutations and sensitivity to PI3K inhibitors in HNSCC cell lines [87]. Testing of a panel of seven different PI3K pathway inhibitors in 59 HNSCC cell lines showed that the NOTCH1 mutation correlated with inhibitor sensitivity. PI3K inhibition led to more apoptosis in NOTCH1-mutant than in NOTCH1-wt HNSCC cell lines in vitro. Similarly, NOTCH1-mutant HNSCC xenografts treated with PI3K pathway inhibitors demonstrated elevated cell death and significant tumor volume reduction in vivo [87].
This promising preclinical evidence was bolstered by findings from two independent studies reporting that vulnerability to PI3K inhibition was conferred by NOTCH1 mutations. PX-866 led to significant tumor reduction in two NOTCH1-mutant HNSCC patient-derived xenograft models [63]. Activation of the NOTCH signaling pathway in breast cancers conferred resistance to PI3K/mTOR inhibitors [88]. One patient with heavily pretreated R/M HNSCC with a LOF NOTCH1 mutation was enrolled in a phase-I study of bimiralisib and had a PR (85% reduction in target lesion) that was sustained for 36 weeks [89]. This observation was later tested in a phase-II trial (NCT03740100) [90] that showed modest single-agent activity of bimiralisib in NOTCH1-mutant HNSCC. Patients treated with bimiralisib had better outcomes (ORR: 17%, OS: 7 months, and PFS: 5 months) than historical control patients treated with standard therapies (ORR: 5.8%, OS: 5.1 months, and PFS: 2.7 months). Overall, these studies support NOTCH1 LOF mutations as a predictive biomarker for the sensitivity to PI3K pathway inhibitors in HNSCC.
Mechanistic studies revealed a differential protein expression profile between NOTCH1-mutant and -wt HNSCC cells when treated with PI3K inhibitors. PDK1, a downstream signaling molecule in the PI3K pathway [87], and Aurora kinase B were exclusively and significantly downregulated in NOTCH1-mutant HNSCC on PI3K inhibition [91,92]. As a result, depleting cellular levels of NOTCH1 [87], PDK1 [87], or Aurora kinase B [93] in NOTCH1-wt HNSCC cells sensitized them to PI3K/AKT/mTOR inhibition, leading to cell death. Since PI3K pathway inhibitors have modest single-agent activity, these mechanistic studies may identify effective combinatorial approaches that are superior to monotherapy and may also overcome the innate and acquired resistance that may develop with the use of targeted therapy.
4.5. Aurora Kinase Inhibitors
Aurora kinases are serine-threonine kinases that play an important role in cell-cycle regulation. These kinases help in the regulation of cell division, most importantly promoting the entry into mitosis, centrosome maturation, microtubule spindle assembly, and completion of cytokinesis. Overexpression of the Aurora kinases induces aneuploidy and genomic instability, which are frequently observed in many tumors [94]. McMillan’s group was the first to demonstrate that HPV-transformed cells were sensitive to the Aurora A kinase inhibitor alisertib [95]. Using HPV-transformed cells, they showed that alisertib led to mitotic delay, polyploidy, and apoptosis in vitro and decreased tumor size in vivo [96,97,98]. In addition to HPV status, our in vitro studies [99] have shown that protein levels of Rb predict the response of squamous cancer cells to Aurora kinase inhibitors. Manipulating Rb protein expression altered the sensitivity to Aurora kinase inhibitors in HNSCC and other cancer types [100,101,102]. In addition, we observed that inhibition of Rb upregulated mitotic checkpoint complex (MCC) genes and resulted in chromosomal instability and prolonged mitosis. Our recent study has demonstrated that the combination of depletion of the MCC protein TRIP13 with low alisertib concentrations selectively enhanced cell death in HPV-positive, but not HPV-negative, HNSCC cells.
Substantial preclinical data targeted toward Aurora kinases have resulted in the development of multiple small molecule inhibitors. Drugs that are being tested in clinical trials are the pan-Aurora kinase drugs AMG 900, ilorasertib (ABT348), and danusertib (PHA-739,358); the Aurora kinase A- and B-targeted drugs AT-9283 and TT00420; the Aurora kinase B-targeted drugs barasertib (AZD1152) and chiauranib; the Aurora kinase A-specific drugs alisertib (MLN8237) and ENMD2076. Alisertib is being widely evaluated in various tumor indications, with seven ongoing early-phase clinical trials including a phase-I/II trial in Rb-deficient HPV-positive HNSCC in combination with a PD-1-inhibitor (NCT04555837).
4.6. FGFR Inhibitors
The FGFR family consists of four transmembrane receptor tyrosine kinases, FGFR1–4, which are activated by 18 fibroblast growth factor (FGF) ligands [103]. FGFR1–3 are commonly amplified and overexpressed in HNSCC [104,105], with 10% to 17% of HNSCC tumors having recurrent FGFR1 amplifications [106,107]. FGFR3-activating mutations are present in 11% of HPV-positive oropharyngeal SCCs (OPSCCs) [29,108]. FGFR fusions are also found to be strong predictors of a response; however, the frequency of FGFR3-TACC3 fusions is lower in HNSCC patients (0.7%) [12].
FGFR inhibition is effective for some cancers with FGFR alterations. The FGFR-TKI erdafitinib was approved for patients with advanced urothelial cancers harboring specific FGFR genomic alterations [109]. Dumbrava et al. [110] showed a CR to an FGFR inhibitor in a patient with R/M HNSCC with FGF amplifications. A small phase-I study (n = 10) showed a disease control rate of 80% with the FGFR TKI rogaratinib [111]. Several ongoing clinical studies with a variety of FGFR inhibitors in cancers with FGFR genomic alterations are open to HNSCC patients. The outcomes of these trials will provide insights into the clinical efficacy of FGFR inhibition in HNSCC.
4.7. Epigenetic Targeted Inhibitors
Certain epigenetic modifications—that is, changes in gene expression that do not involve DNA sequence changes—play a crucial role in cancer development and the interaction between tumor cells and their microenvironment [112]. At present, nine FDA-approved agents, representing four epigenetic targets (DNMT, HDAC, IDH, and EZH2), are used for the treatment of a variety of cancers, and several drugs that target epigenetic mechanisms are currently in clinical trials [113]. Many studies have reported that HPV-negative HNSCC has lower DNA methylation levels than HPV-positive HNSCC, which harbors distinctly hypermethylated genomes [114]. Thus, researchers expect that HPV-positive HNSCC will have a more robust response to epigenetic targeted therapy.
Rodriguez et al. [115] investigated the combination of the HDAC inhibitor vorinostat with the PD-1-inhibitor pembrolizumab in patients with PD-L1-positive, immunotherapy-naive R/M HNSCC. This trial found higher response rates (32%) in the combination-treated cohort than in the control (20%) group treated with single-agent anti-PD-1 mAbs. These results point to a need for further clinical investigation in a phase-II study. A patient cohort preselected for PD-L1 expression would also be needed in further assessments of the combination treatment.
Regarding DNMT inhibitors, a phase-I trial of the use of the DNMT inhibitor azacytidine as neoadjuvant monotherapy, involving a phase-IB trial of decitabine monotherapy, is currently underway in patients with HPV-positive HNSCC. In addition, the trial is also examining R/M HNSCC patients’ use of decitabine in combination with PD-L1 inhibitor durvalumab, regardless of HPV status. Results from this study in patients with ICI refractory HNSCC are pending (NCT03019003).
4.8. VEGF Inhibitors
Vascular endothelial growth factor (VEGF) inhibitors are used to block angiogenesis in several solid tumors, such as colorectal, renal cell, ovarian, gastric, and thyroid cancers [116]. FDA-approved bevacizumab is a VEGF-targeted mAb used to treat numerous cancers, either as a single agent or in combination with chemotherapy or radiotherapy [116]. In vitro data in HNSCC showed decreased VEGF secretion [117], reduced tumor growth, and enhanced cancer cell death when bevacizumab was used with radiotherapy [118]. In a phase-III trial in R/M HNSCC, adding bevacizumab to platinum-based chemotherapy significantly improved both the PFS (p = 0.0014) and ORR (p = 0.016), although no significant improvement in OS was found [119]. Moreover, the bevacizumab-chemotherapy combination was associated with significantly more treatment-related grade 3–5 bleeding events (6.7% vs. 0.5%; p < 0.001) and treatment-related deaths (9.3% vs. 3.2%; p = 0.022) than chemotherapy alone [120].
Studies of VEGFR inhibitors in R/M HNSCC showed modest effects. Vandetanib, a TKI that inhibits both EGFR and VEGFR, resulted in an ORR of 13% (PR in 2/15 patients) when combined with docetaxel in platinum-resistant R/M HNSCC [121]. The VEGFR TKIs sorafenib and sunitinib were both well-tolerated but also had only modest therapeutic effects [122,123]. Given their modest activity and considerable toxicity, most VEGFR inhibitor development has been halted for R/M HNSCC, with the exception of lenvatinib, which is being tested in combination with immunotherapy (described below).
4.9. IAP Inhibitors
Cellular inhibitor of apoptosis (cIAPs) and X-linked IAP (XIAP) proteins are both negative regulators of caspase-mediated apoptosis, and additionally, XIAPs also regulates mitochondria-mediated apoptosis [124]. A potent, orally active, small-molecule IAP inhibitor, Debio 1143 (AT-406, SM-406, xevinapant), may promote apoptosis in tumor cells by blocking both XIAP and cIAPs via restoration of caspase activity [125]. Sun and colleagues investigated the efficacy of Debio 1143 in a phase-II study evaluating 96 patients who received chemoradiotherapy (high-dose cisplatin and concurrent radiotherapy) with or without Debio 1143 (NCT02022098) [125]. The median PFS in the group that received chemoradiotherapy alone was 16.9 months; the median PFS was not reached in the group that also received Debio 1143 (p = 0.0069). At 2 years, the chemoradiotherapy + Debio 1143 group had a PFS rate of 72%, while the chemoradiotherapy-only group had a PFS rate of 41% (p = 0.0026). In addition, the chemoradiotherapy + Debio 1143 combination had a favorable safety profile at 2 to 3 years of follow-up [126]. This was the first treatment regimen to have better efficacy than chemoradiotherapy in a randomized trial. In February 2020, Debio 1143 was designated as a breakthrough therapy by the FDA for the treatment, in combination with chemoradiotherapy, of patients with locally advanced HNSCC that were previously untreated and unresectable. A phase-III trial of Debio 1143 is currently ongoing in combination with chemoradiotherapy and is expected to enroll about 700 patients (NCT04459715). These data suggest that inhibition of IAPs is yet another novel and promising approach for patients with high-risk locally advanced HNSCC. However, all relevant current trials are focused on locally advanced HNSCC, with none in the R/M HNSCC setting.
4.10. STAT3 Inhibitors
Several lines of evidence support a role for a signal transducer and activator of transcription 3 (STAT3) in HNSCC progression and survival [127]. Activated STAT3, defined as phosphorylated or nuclear STAT3, is found in 37% to 75% of HNSCC tumors and associated with a more advanced disease stage and poor survival [128,129,130]. Likewise, there are increased levels of phosphorylated STAT3 in most HNSCC cell lines [131]. HNSCC preclinical models depend on constitutively activated STAT3 for proliferation and survival [129,132,133]. The small-molecule STAT3 inhibitor TTI-101 inhibited anchorage-dependent and -independent growth of multiple human HNSCC cell lines in vitro and reduced tumor growth of radiation-resistant human HNSCC xenografts in vivo [131].
In addition to its direct effects on cancer cells, STAT3 also contributes to tumor vascularization and cancer immune evasion [134] by inhibiting the maturation of dendritic cells [135,136,137,138] and stimulating immunosuppressive cells in the tumor microenvironment, including myeloid-derived suppressor cells [139,140,141], M2 macrophages [135,142,143], T-helper 17 cells [144,145,146], and regulatory T cells [135]. The combination of the antisense STAT3 oligonucleotide danvatirsen with α-PD-L1 therapy (durvalumab) in HNSCC patients improved response rates over those in historic controls treated with α-PD-L1 therapy alone [147]. This result suggests that STAT3 inhibition may synergize with ICI in R/M HNSCC. Inhibiting STAT3 may also reduce immune-related adverse events [148].
However, inhibiting STAT3 in patients has been a challenge. The small-molecule STAT3 inhibitor TTI-101 is currently undergoing phase-I testing. No currently recruiting trials are investigating the most advanced of the STAT3 antisense oligonucleotides, danvatirsen (IONIS-STAT3-2.5Rx, AZD9150). The recent development of proteolysis-targeting chimera (PROTAC) drugs offers a promising future strategy for specifically inhibiting STAT3 in humans [127].
4.11. Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) are complex targeted agents that are composed of an antibody attached to a cytotoxic drug. This has shown promise in the treatment of certain cancers, including acute leukemia, breast cancer, cervical cancer, and Hodgkin’s lymphoma. Since they are delivered locally, ADCs are expected to be more effective and safer compared to standard chemotherapy [149]. ADCs are targeted to tissue factors expressed on tumor cell surfaces so that they can deliver a toxic payload to these cells [150]. Tisotumab vedotin is an ADC consisting of a human mAb that binds to tissue factor-011 and a microtubule-disrupting agent, monomethyl auristatin E. The FDA has granted accelerated approval to tisotumab vedotin for the treatment of adults with R/M cervical cancer that has progressed during or after chemotherapy. The drug was approved based on data points from the innovaTV 204 trial in a phase-II setting (NCT03438396), where 101 patients were treated and demonstrated an ORR of 24% (95% CI, 15.9–33.3%), with a CRR of 7% and median duration of response of 8.3 months (95% CI, 4.2 months–NR) [151]. Preliminary data from the phase-II innovaTV 207 trial (NCT03485209), which enrolled HNSCC patients who experienced disease progression after treatment with platinum chemotherapy and an ICI, demonstrated antitumor activity and a manageable safety profile. Patients receiving tisotumab vedotin alone had an ORR of 16%, median PFS of 4.2 months, and median OS of 9.4 months [152].
5. Immunotherapy for Head and Neck Cancer
Immunotherapy can be an effective treatment option for HNSCC given the nature of enhanced mutation in tumor cells, which leads to immune cell infiltration [153,154]. The responses of ICIs are dependent on tumor-derived T-cells [155]. The FDA approved the use of the PD-1 mAb nivolumab for the treatment of platinum-resistant R/M HNSCC in 2016 and the use of pembrolizumab as a frontline treatment for HNSCC in 2019, markedly altering the landscape of standard HNSCC therapy and engendering a major shift toward immunotherapy as a focus of future therapy development.
5.1. PD-1/PD-L1 Inhibitors
PD-1 is an immune-response suppressor primarily expressed on immune cells such as T and B lymphocytes, dendritic cells, and myeloid cells. Binding with the ligands, PD-L1 or PD-L2 activates PD-1, and prolonged PD-1 activation impairs and exhausts the immune response. HNSCC cells, among many other types of cancer cells, express PD-L1 [156,157]. PD-L1 expression inhibits the anticancer responses of tumor-infiltrating lymphocytes (TILs) and allows tumor cells to evade immune surveillance.
In the phase-IB KEYNOTE-012 study, patients with R/M HNSCC who received pembrolizumab had an ORR of 18% and 6-month PFS rate of 23% [158,159,160]. In the single-arm, phase-II KEYNOTE-055 study, patients with R/M HNSCC that was resistant to both platinum agents and cetuximab received pembrolizumab. The ORR was 16%, median PFS was 2.1 months, and median OS was 8 months [161].
The CheckMate 141 randomized phase-III trial compared nivolumab to the standard of care in patients with R/M HNSCC who had disease progression within 6 months of receiving platinum-based chemotherapy [162]. The ORR was 5.8% for standard care and 13.3% for nivolumab. The primary endpoint, OS, was significantly better in the nivolumab group than in the standard-care group (7.7 months vs. 5.1 months, p = 0.01) [163]. These results made nivolumab a standard treatment option for platinum-resistant R/M HNSCC. At the 2-year follow-up, CheckMate 141 showed a sustained OS advantage in patients treated with nivolumab compared to those treated with standard care (16.9% vs. 6%) [163].
KEYNOTE-048 was a randomized, phase-III trial for previously untreated R/M HNSCC (n = 882) with three arms: pembrolizumab alone, pembrolizumab plus chemotherapy (5-fluorouracil and a platinum-based agent), and chemotherapy plus cetuximab. Outcomes were reported for the total study population and for groups with PD-L1 combined positive scores (CPS) of 20 or higher and 1 or higher. Pembrolizumab alone was compared to chemotherapy plus cetuximab. Pembrolizumab plus chemotherapy was compared to chemotherapy plus cetuximab.
In the total study population, patients treated with pembrolizumab plus chemotherapy had a longer median OS than patients treated with the prior standard of care, chemotherapy plus cetuximab (13 vs. 10.7 months; p = 0.034). The median OS for the pembrolizumab-alone group was longer than that for the chemotherapy plus cetuximab group in both the CPS ≥ 20 (14.7 vs. 11 months; p = 0.004) and CPS ≥ 1 (13.6 vs. 10.4 months; p = 0.001) subgroups [164]. Based on KEYNOTE-048, pembrolizumab was approved by the FDA for use as the first-line therapy (in combination with chemotherapy) in all patients with R/M HNSCC. It was also approved as a single agent in those with CPS ≥ 1.
A recent post-hoc analysis compared the CPS <1 (n = 128) and CPS 1–19 (n = 373) subgroups [165]. In the CPS < 1 subgroup, the median OS duration of patients treated with pembrolizumab was shorter than in patients treated with chemotherapy plus cetuximab (7.9 vs. 11.3 months; HR, 1.51; 95% CI, 0.96 to 2.37). In the CPS 1–19 subgroup, however, the median OS was slightly longer in the pembrolizumab group (10.8 vs. 10.1 months; HR, 0.86; 95% CI, 0.66 to 1.12). The median OS duration for the group with CPS < 1 receiving pembrolizumab plus chemotherapy was 10.7 months, as compared to 11.3 months in the group receiving chemotherapy plus cetuximab (HR, 1.21; 95% CI, 0.76 to 1.94). In the group with CPS 1–19, the median OS was 12.7 months for the pembrolizumab plus chemotherapy group and 9.9 months for the chemotherapy plus cetuximab group (HR, 0.71; 95% CI, 0.54 to 0.94).
Despite the clear benefits of ICI, only a subset of patients with R/M HNSCC benefitted from it. Expression of PD-L1 is the best-studied predictive biomarker for PD-1/PD-L1 inhibitors. The number of PD-L1-positive cells (including tumor cells, lymphocytes, and macrophages) compared to the total number of viable tumor cells, referred to as the CPS value, when known in the HNSCC setting, allows for a better response to immunotherapy compared to analyzing PD-L1 expression alone [166]. Around 50% to 60% of HNSCC tumor cells express PD-L1 (i.e., the total positive score) [167], but when infiltrating immune cells are included in the measurement (i.e., CPS), the percentage of PD-L1-positive cells increases to 85%. Subgroup analysis of KEYNOTE-048 patients confirmed better responses to ICI with increasing CPS scores.
There are several other biomarkers that have been studied but not fully validated yet, which can contribute toward better response prediction to anti-PD1 therapy, for example, expression of PD-L2 [168]. KEYNOTE-012 data revealed that PD-L2 protein expression helped in predicting the response to anti-PD-1 therapy irrespective of expression of PD-L1 [169]. Another example of a biomarker helping in predicting the response to anti-PD1 therapy can be attributed to the HPV status, as well. In the KEYNOTE-012 trial, patients with PD-L1-positive/HPV-positive disease had a better ORR than PD-L1-positive/HPV-negative disease, with 25% and 14% ORR, respectively. In this case, from the overall patient pool, 62% patients had a HPV-negative status, whereas 38% were HPV-positive [158]. Subsequently, another trial (KEYNOTE-048) showed that HPV-positive patients displayed better results for combination therapy involving pembrolizumab plus chemotherapy, thereby enabling such patients to potentially receive combination therapy [170]. A number of trials are currently underway to evaluate several different combinations using ICI, therapeutic vaccines, co-stimulatory agonists, and targeted and cytotoxic agents (Table 2).
Table 2.
Ongoing clinical trials evaluating novel immunotherapies in patients with recurrent/metastatic HNSCC.
| Novel Immunotherapies in Combination with PD-1/PD-L1 Inhibitors, and Other Novel Checkpoint Inhibitor/Immunotherapies | |||||
|---|---|---|---|---|---|
| Drug(s) | Study Phase | Clinical Trials | Study Name | Intervention | HPV Status |
| Lenvatinib | III | NCT04199104 | LEAP-10 | Pembrolizumab vs. pembrolizumab + lenvatinib | HPV-positive |
| Bempegaldesleukin | II/III | NCT04969861 | PROPEL-36 | Bempegaldesleukin + pembrolizumab | |
| Nivolumab + ipilimumab | III | NCT03700905 | IMSTAR-HN | Nivolumab + ipilimumab vs. surgery + RT | HPV-negative |
| Nivolumab | III | NCT03576417 | NIVOSTOP | Nivolumab + RT + cisplatin vs. RT + cisplatin | unknown |
| Nivolumab + ipilimumab | III | NCT02741570 | CheckMate 651 | Nivolumab + ipilimumab vs. SOC (EXTREME regimen) | HPV-positive |
| Abemaciclib | I/II | NCT03655444 | Abemaciclib + nivolumab | ||
| Ramucirumab | I/II | NCT03650764 | Ramucirumab + pembrolizumab | unknown | |
| Duvelisib | I/II | NCT04193293 | Duvelisib + pembrolizumab | unknown | |
| Intratumoral MK-1454 | II | NCT04220866 | Intratumoral MK-1454 + pembrolizumab vs. pembrolizumab | unknown | |
| Eftilagimod alpha | II | NCT04811027 | TACTI-003 | Eftilagimod alpha + pembrolizumab vs. pembrolizumab | HPV-positive |
| BNT113 | II | NCT04534205 | AHEAD-MERIT | BNT113 + pembrolizumab vs. pembrolizumab | HPV-positive |
| PDS0101 (HPV E6/E7 vaccine) | II | NCT04260126 | VERSATILE002 | Pembrolizumab + PDS0101 (HPV E6/E7 vaccine) | HPV-positive |
| Pepinemab | I/II | NCT04815720 | KEYNOTE B84 | Pepinemab + pembrolizumab | |
| Atezolizumab | II | NCT03818061 | ATHENA | Atezolizumab + bevacizumab | HPV-positive |
| Avelumab | I | NCT03498378 | Avelumab + Palbociclib + cetuximab | unknown | |
| Alisertib | I | NCT04555837 | Alisertib + pembrolizumab | HPV-positive | |
| Cemiplimab | II | NCT04831450 | Maintenance cemiplimab (anti-PD1) | ||
| Other Novel Checkpoint Inhibitors/Immunotherapy | |||||
| Tiragolumab | II | NCT04665843 | SKYSCRAPER-09 | Tiragolumab + atezolizumab vs. atezolizumab | HPV-positive |
| Relatlimab | II | NCT04326257 | Nivolumab + relatlimab vs. nivolumab + ipilimumab | unknown | |
| Monalizumab | III | NCT04590963 | INTERLINK-1 | Monalizumab + cetuximab vs. cetuximab | |
| Epacadostat | I/II | NCT02327078 | ECHO-204 | Epacadostat + nivolumab | unknown |
| Enoblituzumab | I | NCT02475213 | MGA271 | Enoblituzumab + pembrolizumab | unknown |
| IMA201 | I | NCT03247309 | IMA201 (TCR-engineered in solid tumors, ACTengine) | ||
| KITE-439 | I | NCT03912831 | KITE-439 (E7 T-cell receptor + cyclophosphamide + fludarabine) | HPV-positive | |
| Autologous TILs | II | NCT03083873 | Autologous TILs | HPV-positive | |
HPV, human papillomavirus; RT, radiotherapy; SOC, standard of care; TCR, T-cell receptor; TIL, tumor-infiltrating lymphocyte.
5.2. Vaccines
HPV-positive HNSCC contains E6 and E7 oncoproteins that are recognized as non-self-antigens required to maintain the malignant phenotype [171]. HPV-targeted therapeutic vaccines work against HPV infection by inducing a T-cell response. There are FDA-approved HPV vaccines available in the market to protect from high-risk HPV infection and cancer caused by them [172], but these vaccines do not treat established cancer.
E6/E7 antigen-targeting vaccines demonstrated efficacy in patients with HPV-induced cervical intraepithelial neoplasia and are now being evaluated in an R/M HNSCC setting [173]. However, vaccines targeting HPV16 have, by themselves, failed to treat recurrent advanced HPV-positive cancers. Therefore, combinations of ICI with therapeutic HPV vaccines are being tested for recurrent advanced HPV-positive cancers. A phase-IB/II trial (NCT03260023) of an HPV16 E6/E7 and IL-2 vaccine (TG4001) plus a PD-L1 inhibitor in patients with R/M HPV16-positive cancers that did not respond to available standard treatments demonstrated an ORR of 23.5% [174]. The vaccine-immunotherapy combination induced an HPV16 E6/E7-specific T-cell response and increased the number of TILs in the tumor microenvironment.
A combination of a synthetic long-peptide HPV16 vaccine (ISA101) with nivolumab, in a phase-II study, showed promising data with a 33% ORR and median OS duration of 17.5 months (NCT02426892) in patients with HPV16-positive R/M HNSCC [175]. In addition, two more phase-II trials of ISA101 are underway, one with utomilumab, a CD137 agonist, for patients with incurable HPV16-positive OPSCC (NCT03258008) [176], and the other with cemiplimab in R/M HPV16-positive OPSCC.
ADXS11-001 (axalimogene filolisbac) is a live-attenuated vaccine encoding an HPV16 E7 oncoprotein. It is currently in phase-II trials evaluating the HPV-specific T-cell response and safety in a neoadjuvant setting in HPV-positive OPSCC (NCT02002182). DPX-E7 is a synthetic peptide-based vaccine targeting HPV16 E711-19, which is under investigation in an open-label phase-IB/II trial. This trial, the results of which have not yet been released, includes HLA-A*02-01 patients with HPV16-associated OPSCC, anal cancer, and cervical cancer (NCT02865135).
Studies have shown that the E6 oncoprotein regulates telomerase reverse transcriptase (TERT) gene transcriptional activation, leading to its overexpression on cancer cell surfaces and thereby cell proliferation. A vaccine containing telomerase-derived cancer peptides, UCPVax, has been designed to activate CD4+ T-helper cells against telomerase-expressing cells. It is currently in a phase-II VolATIL trial (NCT03946358) in combination with atezolizumab in locally advanced or metastatic HPV-positive patients.
Nucleic acid-derived vaccines, such as DNA and RNA vaccines, are easier to synthesize and develop compared to peptide vaccines. MEDI0457, a DNA vaccine, was evaluated in a phase-IB/II trial enrolling 22 patients with locally advanced HPV16/HPV18-positive HNSCC. The tumor regression rate was 50%, and an increase in T-effector cells could overcome HPV-driven tumor immune evasion. Currently, MEDI0457 is being evaluated in combination with the PD-L1 inhibitor durvalumab in a phase-IB/IIA study (NCT03162224) of 35 patients with HPV-associated R/M HNSCC. BNT113 is an RNA lipoplex-based mRNA vaccine encoding HPV16 E6 and E7 that is being evaluated in combination with pembrolizumab in HPV-positive and PD-L1-expressing HNSCC (NCT04534205). In the future, HPV-directed therapeutic vaccines may move beyond targeting E6 and E7 to include the viral E1, E2, E4, E5, and L1 proteins [177].
In addition to HPV vaccines, recent advances in NGS technologies have led to better identification of neoantigens, thereby boosting the development of cancer vaccine research and strategies. Cancer vaccination enhances tumor-specific CD4+ and CD8+ T cells, which eliminates tumor cells without affecting the normal cells. Currently, two neoantigen vaccine trials are ongoing. First, there is a combination of personalized cancer vaccination with an anti-PD-1 mAb in a phase-IB setting, focused on advanced HNSCC patients. The tumor-derived neoantigens would be specific to an individual and tumor (NCT03568058) in this study. Second, there is a phase-I trial with HNSCC patients with progressive disease after anti-PD-1 or anti-PD-L1 treatment. The patients in the trial have been termed as requiring a “personalized and adjusted neoantigen peptide vaccine” (PANDA-VAC); they will be vaccinated with six neoantigens alongside treatment with pembrolizumab (NCT04266730).
5.3. Adoptive Cellular Therapy
Chimeric antigen receptor (CAR)-T cell therapy is a popular treatment option for hematologic malignancies [178]. CAR-T cells use activated cytotoxic T lymphocytes that are primed to patient-specific antigens to cause tumor cell death. There have been several CAR-T-based cell therapy trials against different antigens for HNSCC. For example, T4 immunotherapy, a CAR-T cell therapy primed to antigens for the ErbB family (EGFR, HER2-4), which is highly upregulated in HNSCC [179], was studied in a dose-escalation phase-I study for locally advanced or recurrent HNSCC. The CAR-T cell doses were increased from 1 × 107 to 10 × 107 T4+ T cells in different patient cohorts. After 6 weeks, patients who received the highest dose of 10 × 107 T4+ T cells displayed stable disease. The overall disease control rate was 69%, despite the patients having had rapidly progressing tumors on trial entry [179].
In addition to T4 CAR-T cell therapy, recent research has focused on developing effective HPV-targeted T-cell receptors (TCRs). This therapeutic approach involves introducing a tumor antigen to alter or modify the genetics of T-cells. The genetically altered T-cells express receptors targeting a restricted epitope (HLA-A*02:01) of E6 TCR T cells. This has been investigated for treatment of patients with metastatic HPV16-positive HNSCC [180]. Initial results from a phase-I/II trial carried out in a pool of 12 patients with various metastatic, HPV16-positive, HLA-A*02:01-positive cancers showed that two patients had partial responses to this therapy. E6 TCR memory T cells were observed in these two patients at a high percentage after one month of treatment and ~7% after 10 months of treatment. However, no levels of E6 TCR memory T cells could be observed in patients who did not respond to the therapy [181,182]. Other ongoing TCR therapy trials include: E7 TCR treatment in HLA-A*02:01-positive patients with relapsed/refractory HPV16-positive cancers (KITE-439, NCT03912831); HPV-E6-specific TCR T-cell transfer in HPV-positive HNSCC (NCT03578406); autologous TIL infusion (LN-145/LN-145-S1) followed by IL-2 administration in patients with R/M HNSCC (NCT03083873).
5.4. Novel Immunotherapies
Apart from PD-1, other T-cell exhaustion markers are also targets for ICI and are upregulated in HPV-positive HNSCC, such as cytotoxic T-lymphocyte protein 4 (CTLA4), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), and lymphocyte activation gene 3 protein (LAG3) [183,184].
Potential ICI resistance mechanisms in R/M HNSCC are diverse, and many molecular targeted agents are being tested in combination with ICIs. Potential combinations of ICI with Aurora kinase and STAT3 inhibitors are described above. Additional ICI-containing combinations are also being investigated based on the success of this strategy in other solid tumors. HPV-specific immunotherapies are further under development. The summary below is not exhaustive but focuses on the most promising or clinically advanced combinations.
Recently, a multi-kinase inhibitor against VEGFR1-3, lenvatinib, was tested with pembrolizumab and showed a 46% ORR in patients with advanced solid tumors [185]. Chen et al. [186] reported an ORR of 28.6% with a median OS of 6.2 months in a small cohort (n = 14) of patients with immunotherapy-refractory HNSCC. Two ongoing phase-II trials are testing combinations of lenvatinib with pembrolizumab for heavily pretreated R/M HNSCC patients. In addition, a study of a combination of pembrolizumab with the VEGFR2 inhibitor cabozantinib for patients with PD-L1-positive, CPS >1 R/M HNSCC resulted in an impressive ORR of 45% and an overall clinical benefit rate of 90%. The 1-year OS and PFS rates were 68% and 52%, respectively [187].
Previous studies have shown that PI3K pathway inhibition modulates the tumor microenvironment and has a direct effect on immune cells that could be used to improve patient responses [188]. Tumor hypoxia has been reported to be a resistance mechanism in R/M HNSCC patients treated with a PD-1 blockade. Preclinical studies in PD-1-inhibitor-resistant mouse models showed resistant tumors had high oxidative metabolism that led to increased intratumoral hypoxia and decreased CD8+ T cells [189]. Moreover, PI3K inhibitors induced a decrease in tumor hypoxia specifically in head and neck tumor xenograft models [190]. Therefore, the addition of PI3K inhibitors to ICI therapy could improve responses in patients who otherwise would not benefit from ICI therapy alone. However, combining PI3K inhibitors with ICI has been challenging as PI3K inhibitors may alter antitumor immunity or lead to immune-related adverse events. This obstacle could be overcome by adopting a modified treatment regimen with intermittent, rather than daily, dosing of PI3K inhibitors [191,192]. Notably, a phase-I/II trial with copanlisib combined with nivolumab and/or ipilimumab is ongoing in patients with advanced solid cancers who have genomic alterations in PIK3CA and PTEN (NCT04317105).
Two phase-II trials investigated the combination of cetuximab with either pembrolizumab or nivolumab [193,194]. The combination containing pembrolizumab showed promising results, with an ORR of 45% and median OS of 18 months in patients with platinum-refractory tumors who received no prior anti-EGFR therapy or immunotherapy. The nivolumab + cetuximab combination showed an ORR of 22% in a population with a high proportion of patients who had previously received cetuximab or ICI treatment [194]. Based on these data and the clear unmet need for therapy options in ICI-resistant R/M HNSCC, the combination of cetuximab and pembrolizumab is now included in the National Comprehensive Cancer Network guidelines [195].
EAGLE was a randomized phase-III study that compared the anti-PD-L1 mAb durvalumab plus the CTLA4 inhibitor tremelimumab to chemotherapy in R/M HNSCC. Durvalumab did not improve survival, either as a single agent or in combination with tremelimumab. Moreover, the HPV biomarker status did not predict a response to the combination [196]. CheckMate 651 was a phase-III trial comparing nivolumab plus ipilimumab with chemotherapy plus cetuximab as a frontline therapy in platinum-eligible patients with R/M HNSCC. The study did not meet the primary endpoint, but there were some positive observations in terms of OS for a small set of patients who had tumors that expressed PD-L1 with a CPS ≥ 20 [197]. Thus, current data do not support a role for CTLA4 inhibition in HNSCC.
A couple of other mAb combination-based treatments with PD-1/PD-L1 inhibitors are also currently under investigation, e.g., tiragolumab, humanized anti-TIGIT mAb, and relatimab, an anti-LAG3 mAb. Combination therapy involving tiragolumab and atezolizumab (PD-L1 inhibitor) is currently being evaluated as a frontline treatment for PD-L1-positive R/M HNSCC in the SKYSCRAPER-09 trial (NCT04665843). Along similar lines, a combination of relatlimab and nivolumab is also under trial for R/M HNSCC treatment (NCT04326257).
HPV16 oncoproteins in HPV-positive HNSCC have led to development of HPV-specific immunotherapies. One such example is a fusion protein, CUE-101, which selectively binds and activates HPV16 E7-specific CD8+ T cells, as shown in preclinical studies [198]. It is currently being evaluated in a dose-escalation and expansion phase-I study with or without a PD-1-inhibitor as a frontline treatment for patients with HPV16-positive R/M HNSCC (NCT03978689). Initial data showed increased tumor-infiltrating T cells (CD3+ GZMB+) in tumor tissue post-CUE-101 administration in one patient, and out of 33 patients, eight demonstrated no disease progression for 12 weeks or more [199].
In addition to activation of antigen-specific T cells, a newly developed treatment modality called near-infrared photoimmunotherapy (NIR-PIT) is currently being studied. It uses a mAb that is attached to IRDye700DX (IR700), a photo-absorbing dye, and activated by near-infrared light [200]. This causes the cells to die by swift swelling, blebbing, and rupturing, allowing the internal cell contents to come into contact with the extracellular compartment, leading to a strong immune response. A 50% ORR in previously inoperable HNSCC patients was observed in a phase-I/II trial of cetuximab with NIR-PIT (NCT02422979) [201]. NIR-PIT was approved as a treatment regimen for recurrent HNSCC in Japan given its high efficacy and low adverse event rate. A phase-III trial involving NIR-PIT is currently ongoing (active since 2018) for HNSCC patients with recurrent characteristics who have experienced treatment failure or tumor progression during or after at least two lines of therapy.
Additional approaches that are under investigation but beyond the scope of this review include oncolytic viruses, inhibitors of B7-H3 (enoblituzumab), inhibitors of IDO1 (epacadostat), NKG2A inhibitors (monalizumab), and definitive therapy for the treatment of oligometastatic disease.
6. Conclusions
The management of R/M HNSCC is rapidly evolving. Although current approaches to systemic therapies are limited to chemotherapy, anti-PD-1 immunotherapy, and cetuximab, multiple new approaches are currently under development and investigation. Progress in understanding the genomic landscape and tumor microenvironment of HNSCC has helped in delivering personalized and effective treatment approaches. For example, HRAS-mutant HNSCC responds to the farnesyltransferase inhibitor tipifarnib. Other promising targeted therapies include PI3K inhibitors for NOTCH1-mutant tumors and Aurora kinase inhibitors for Rb-deficient, HPV-positive HNSCCs. With recent approval of the ADC tisotumab vedotin for cervical cancer and promising phase-II results in HNSCC, it is highly possible that tisotumab vedotin will represent another option in R/M HNSCC. Recent advances in ICI have improved outcomes in both HPV-positive and -negative HNSCC. Advancements in data and research around molecular structures and immunological features have equipped us to differentiate between HPV-positive and -negative HNSCC and enabled us to create targeted therapeutic approaches as well as personalized medicine beyond ICI. Amongst these new opportunities, the most promising are NIR-PIT for localized recurrence; novel fusion proteins; anti-HPV therapeutic vaccines; HPV-specific adoptive T-cell therapies for HPV-positive R/M HNSCC; the combination of targeted therapies with ICIs. Future therapies for R/M HNSCC are likely to be directed toward specific patient subpopulations based on a better understanding of cancer biology.
Acknowledgments
We thank Amy Ninetto, ELS at The University of Texas MD Anderson Cancer Center for critical editing of the manuscript.
Author Contributions
S.G., P.A.S. and F.M.J. wrote the manuscript; S.G., P.A.S. and F.M.J. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Faye M. Johnson received research funding from Takeda Pharmaceutical Company, Viracta Therapeutics, and Trovagene. The other authors have no conflicts of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shibata H., Zhou L., Xu N., Egloff A.M., Uppaluri R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021;112:978–988. doi: 10.1111/cas.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison M.L. Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: Implications for clinical research in head and neck cancers. J. Clin. Oncol. 2006;24:5623–5625. doi: 10.1200/JCO.2006.07.1829. [DOI] [PubMed] [Google Scholar]
- 4.Marur S., Forastiere A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016;91:386–396. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Karbalaie Niya M.H., Safarnezhad Tameshkel F., Keyvani H., Esghaei M., Panahi M., Zamani F., Tabibzadeh A. Epstein-Barr virus molecular epidemiology and variants identification in head and neck squamous cell carcinoma. Eur. J. Cancer Prev. 2020;29:523–530. doi: 10.1097/CEJ.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 6.Wong I.C., Ng Y.K., Lui V.W. Cancers of the lung, head and neck on the rise: Perspectives on the genotoxicity of air pollution. Chin. J. Cancer. 2014;33:476–480. doi: 10.5732/cjc.014.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra A., Meherotra R. Head and neck cancer: Global burden and regional trends in India. Asian Pac. J. Cancer Prev. 2014;15:537–550. doi: 10.7314/APJCP.2014.15.2.537. [DOI] [PubMed] [Google Scholar]
- 8.Velleuer E., Dietrich R. Fanconi anemia: Young patients at high risk for squamous cell carcinoma. Mol. Cell. Pediatr. 2014;1:9. doi: 10.1186/s40348-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaud D.S., Langevin S.M., Eliot M., Nelson H.H., Pawlita M., McClean M.D., Kelsey K.T. High-risk HPV types and head and neck cancer. Int. J. Cancer. 2014;135:1653–1661. doi: 10.1002/ijc.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellsague X., Alemany L., Quer M., Halec G., Quiros B., Tous S., Clavero O., Alos L., Biegner T., Szafarowski T., et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 12.The Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan C., Issaeva N., Yarbrough W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck. 2018;3:12. doi: 10.1186/s41199-018-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R., Ramadas K., Thomas G., Muwonge R., Thara S., Mathew B., Rajan B., Trivandrum Oral Cancer Screening Study Group Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 16.Fasano M., Della Corte C.M., Viscardi G., Di Liello R., Paragliola F., Sparano F., Iacovino M.L., Castrichino A., Doria F., Sica A., et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol. 2021;13:1758835920949418. doi: 10.1177/1758835920949418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argiris A., Harrington K.J., Tahara M., Schulten J., Chomette P., Ferreira Castro A., Licitra L. Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laramore G.E., Scott C.B., al-Sarraf M., Haselow R.E., Ervin T.J., Wheeler R., Jacobs J.R., Schuller D.E., Gahbauer R.A., Schwade J.G., et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: Report on Intergroup Study 0034. Int. J. Radiat. Oncol. Biol. Phys. 1992;23:705–713. doi: 10.1016/0360-3016(92)90642-U. [DOI] [PubMed] [Google Scholar]
- 19.Bachaud J.M., Cohen-Jonathan E., Alzieu C., David J.M., Serrano E., Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1996;36:999–1004. doi: 10.1016/S0360-3016(96)00430-0. [DOI] [PubMed] [Google Scholar]
- 20.Mott F.E., Sacks R., Johnson F., Hutcheson K.A., Gallagher N., Varghese S., Zaveri J. Subjective functional outcomes in oropharyngeal cancer treated with induction chemotherapy using the MD Anderson Symptom Inventory (MDASI) Laryngoscope Investig. Otolaryngol. 2020;5:1104–1109. doi: 10.1002/lio2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDowell L., Rischin D., Gough K., Henson C. Health-Related Quality of Life, Psychosocial Distress and Unmet Needs in Older Patients With Head and Neck Cancer. Front. Oncol. 2022;12:834068. doi: 10.3389/fonc.2022.834068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessri M., Farah C.S. Harnessing massively parallel sequencing in personalized head and neck oncology. J. Dent. Res. 2014;93:437–444. doi: 10.1177/0022034514524783. [DOI] [PubMed] [Google Scholar]
- 23.Jessri M., Farah C.S. Next generation sequencing and its application in deciphering head and neck cancer. Oral Oncol. 2014;50:247–253. doi: 10.1016/j.oraloncology.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 25.Berenson J.R., Yang J., Mickel R.A. Frequent amplification of the bcl-1 locus in head and neck squamous cell carcinomas. Oncogene. 1989;4:1111–1116. [PubMed] [Google Scholar]
- 26.Agrawal N., Frederick M.J., Pickering C.R., Bettegowda C., Chang K., Li R.J., Fakhry C., Xie T.X., Zhang J., Wang J., et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stransky N., Egloff A.M., Tward A.D., Kostic A.D., Cibulskis K., Sivachenko A., Kryukov G.V., Lawrence M.S., Sougnez C., McKenna A., et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loyo M., Li R.J., Bettegowda C., Pickering C.R., Frederick M.J., Myers J.N., Agrawal N. Lessons learned from next-generation sequencing in head and neck cancer. Head Neck. 2013;35:454–463. doi: 10.1002/hed.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillison M.L., Akagi K., Xiao W., Jiang B., Pickard R.K.L., Li J., Swanson B.J., Agrawal A.D., Zucker M., Stache-Crain B., et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 2019;29:1–17. doi: 10.1101/gr.241141.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilmarinen T., Munne P., Hagstrom J., Haglund C., Auvinen E., Virtanen E.I., Haesevoets A., Speel E.J.M., Aaltonen L.M. Prevalence of high-risk human papillomavirus infection and cancer gene mutations in nonmalignant tonsils. Oral Oncol. 2017;73:77–82. doi: 10.1016/j.oraloncology.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Rubin Grandis J., Melhem M.F., Gooding W.E., Day R., Holst V.A., Wagener M.M., Drenning S.D., Tweardy D.J. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl. Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 32.Madoz-Gurpide J., Zazo S., Chamizo C., Casado V., Carames C., Gavin E., Cristobal I., Garcia-Foncillas J., Rojo F. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J. Transl. Med. 2015;13:282. doi: 10.1186/s12967-015-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marur S., D’Souza G., Westra W.H., Forastiere A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha P., Logan H.L., Mendenhall W.M. Human papillomavirus, smoking, and head and neck cancer. Am. J. Otolaryngol. 2012;33:130–136. doi: 10.1016/j.amjoto.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khariwala S.S., Ma B., Ruszczak C., Carmella S.G., Lindgren B., Hatsukami D.K., Hecht S.S., Stepanov I. High Level of Tobacco Carcinogen-Derived DNA Damage in Oral Cells Is an Independent Predictor of Oral/Head and Neck Cancer Risk in Smokers. Cancer Prev. Res. 2017;10:507–513. doi: 10.1158/1940-6207.CAPR-17-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis. 2008;29:1467–1474. doi: 10.1093/carcin/bgn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillison M.L. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29:779–792. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 38.Di Credico G., Polesel J., Dal Maso L., Pauli F., Torelli N., Luce D., Radoi L., Matsuo K., Serraino D., Brennan P., et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer. 2020;123:1456–1463. doi: 10.1038/s41416-020-01031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahal B.A., Catalano P.J., Haddad R.I., Hanna G.J., Kass J.I., Schoenfeld J.D., Tishler R.B., Margalit D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2019;28:1660–1667. doi: 10.1158/1055-9965.EPI-19-0038. [DOI] [PubMed] [Google Scholar]
- 40.Dyson N., Howley P.M., Munger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 41.Beck T.N., Golemis E.A. Genomic insights into head and neck cancer. Cancers Head Neck. 2016;1:1. doi: 10.1186/s41199-016-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandis J.R., Tweardy D.J. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 43.Azoury S.C., Gilmore R.C., Shukla V. Molecularly targeted agents and immunotherapy for the treatment of head and neck squamous cell cancer (HNSCC) Discov. Med. 2016;21:507–516. [PubMed] [Google Scholar]
- 44.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., Jones C.U., Sur R., Raben D., Jassem J., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 45.Gillison M.L., Trotti A.M., Harris J., Eisbruch A., Harari P.M., Adelstein D.J., Jordan R.C.K., Zhao W., Sturgis E.M., Burtness B., et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehanna H., Robinson M., Hartley A., Kong A., Foran B., Fulton-Lieuw T., Dalby M., Mistry P., Sen M., O’Toole L., et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermorken J.B., Herbst R.S., Leon X., Amellal N., Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–2719. doi: 10.1002/cncr.23442. [DOI] [PubMed] [Google Scholar]
- 48.Yamaoka T., Ohba M., Ohmori T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017;18:2420. doi: 10.3390/ijms18112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.-R., Cupissol D., et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 50.Vermorken J.B., Stohlmacher-Williams J., Davidenko I., Licitra L., Winquist E., Villanueva C., Foa P., Rottey S., Skladowski K., Tahara M., et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 51.Stewart J.S., Cohen E.E., Licitra L., Van Herpen C.M., Khorprasert C., Soulieres D., Vodvarka P., Rischin D., Garin A.M., Hirsch F.R., et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 52.Seiwert T.Y., Fayette J., Cupissol D., Del Campo J.M., Clement P.M., Hitt R., Degardin M., Zhang W., Blackman A., Ehrnrooth E., et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014;25:1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machiels J.P., Haddad R.I., Fayette J., Licitra L.F., Tahara M., Vermorken J.B., Clement P.M., Gauler T., Cupissol D., Grau J.J., et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 54.Guo Y., Ahn M.J., Chan A., Wang C.H., Kang J.H., Kim S.B., Bello M., Arora R.S., Zhang Q., He X., et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019;30:1831–1839. doi: 10.1093/annonc/mdz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho A.L., Hanna G.J., Scholz C.R., Gualberto A., Park S.H. Preliminary activity of tipifarnib in tumors of the head and neck, salivary gland and urothelial tract with HRAS mutations. J. Clin. Oncol. 2020;38:6504. doi: 10.1200/JCO.2020.38.15_suppl.6504. [DOI] [Google Scholar]
- 56.Ho A.L., Brana I., Haddad R., Bauman J., Bible K., Oosting S., Wong D.J., Ahn M.J., Boni V., Even C., et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma With HRAS Mutations. J. Clin. Oncol. 2021;39:1856–1864. doi: 10.1200/JCO.20.02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marquard F.E., Jucker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020;172:113729. doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- 58.Jung K., Kang H., Mehra R. Targeting phosphoinositide 3-kinase (PI3K) in head and neck squamous cell carcinoma (HNSCC) Cancers Head Neck. 2018;3:3. doi: 10.1186/s41199-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janku F., Yap T.A., Meric-Bernstam F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018;15:273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 60.Juric D., Janku F., Rodon J., Burris H.A., Mayer I.A., Schuler M., Seggewiss-Bernhardt R., Gil-Martin M., Middleton M.R., Baselga J., et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2019;5:e184475. doi: 10.1001/jamaoncol.2018.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., Iwata H., Conte P., Mayer I.A., Kaufman B., et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 62.Lui V.W., Hedberg M.L., Li H., Vangara B.S., Pendleton K., Zeng Y., Lu Y., Zhang Q., Du Y., Gilbert B.R., et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keysar S.B., Astling D.P., Anderson R.T., Vogler B.W., Bowles D.W., Morton J.J., Paylor J.J., Glogowska M.J., Le P.N., Eagles-Soukup J.R., et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol. Oncol. 2013;7:776–790. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizrachi A., Shamay Y., Shah J., Brook S., Soong J., Rajasekhar V.K., Humm J.L., Healey J.H., Powell S.N., Baselga J., et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat. Commun. 2017;8:14292. doi: 10.1038/ncomms14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keam B., Kim S., Ahn Y.O., Kim T.M., Lee S.H., Kim D.W., Heo D.S. In vitro anticancer activity of PI3K alpha selective inhibitor BYL719 in head and neck cancer. Anticancer Res. 2015;35:175–182. [PubMed] [Google Scholar]
- 66.Fritsch C., Huang A., Chatenay-Rivauday C., Schnell C., Reddy A., Liu M., Kauffmann A., Guthy D., Erdmann D., De Pover A., et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol. Cancer Ther. 2014;13:1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 67.Liu N., Rowley B.R., Bull C.O., Schneider C., Haegebarth A., Schatz C.A., Fracasso P.R., Wilkie D.P., Hentemann M., Wilhelm S.M., et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 68.Amornphimoltham P., Patel V., Sodhi A., Nikitakis N.G., Sauk J.J., Sausville E.A., Molinolo A.A., Gutkind J.S. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 69.Liu D., Hou P., Liu Z., Wu G., Xing M. Genetic alterations in the phosphoinositide 3-kinase/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mammalian target of rapamycin. Cancer Res. 2009;69:7311–7319. doi: 10.1158/0008-5472.CAN-09-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patnaik A., Appleman L.J., Tolcher A.W., Papadopoulos K.P., Beeram M., Rasco D.W., Weiss G.J., Sachdev J.C., Chadha M., Fulk M., et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016;27:1928–1940. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim H.R., Kang H.N., Yun M.R., Ju K.Y., Choi J.W., Jung D.M., Pyo K.H., Hong M.H., Ahn M.J., Sun J.M., et al. Mouse-human co-clinical trials demonstrate superior anti-tumour effects of buparlisib (BKM120) and cetuximab combination in squamous cell carcinoma of head and neck. Br. J. Cancer. 2020;123:1720–1729. doi: 10.1038/s41416-020-01074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fayette J., Digue L., Segura-Ferlay C., Treilleux I., Wang Q., Lefebvre G., Daste A., Even C., Thaunat S.C., Guyennon A., et al. Buparlisib (BKM120) in refractory head and neck squamous cell carcinoma harbouring or not a PI3KCA mutation: A phase II multicenter trial. Ann. Oncol. 2019;30:v455. doi: 10.1093/annonc/mdz252.012. [DOI] [Google Scholar]
- 73.Dolly S.O., Wagner A.J., Bendell J.C., Kindler H.L., Krug L.M., Seiwert T.Y., Zauderer M.G., Lolkema M.P., Apt D., Yeh R.F., et al. Phase I Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016;22:2874–2884. doi: 10.1158/1078-0432.CCR-15-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shapiro G.I., Bell-McGuinn K.M., Molina J.R., Bendell J., Spicer J., Kwak E.L., Pandya S.S., Millham R., Borzillo G., Pierce K.J., et al. First-in-Human Study of PF-05212384 (PKI-587), a Small-Molecule, Intravenous, Dual Inhibitor of PI3K and mTOR in Patients with Advanced Cancer. Clin. Cancer Res. 2015;21:1888–1895. doi: 10.1158/1078-0432.CCR-14-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wicki A., Brown N., Xyrafas A., Bize V., Hawle H., Berardi S., Cmiljanovic N., Cmiljanovic V., Stumm M., Dimitrijevic S., et al. First-in human, phase 1, dose-escalation pharmacokinetic and pharmacodynamic study of the oral dual PI3K and mTORC1/2 inhibitor PQR309 in patients with advanced solid tumors (SAKK 67/13) Eur. J. Cancer. 2018;96:6–16. doi: 10.1016/j.ejca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Juric D., Krop I., Ramanathan R.K., Wilson T.R., Ware J.A., Sanabria Bohorquez S.M., Savage H.M., Sampath D., Salphati L., Lin R.S., et al. Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors. Cancer Discov. 2017;7:704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juric D., de Bono J.S., LoRusso P.M., Nemunaitis J., Heath E.I., Kwak E.L., Macarulla Mercade T., Geuna E., Jose de Miguel-Luken M., Patel C., et al. A First-in-Human, Phase I, Dose-Escalation Study of TAK-117, a Selective PI3Kalpha Isoform Inhibitor, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2017;23:5015–5023. doi: 10.1158/1078-0432.CCR-16-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juric D., Rodon J., Tabernero J., Janku F., Burris H.A., Schellens J.H.M., Middleton M.R., Berlin J., Schuler M., Gil-Martin M., et al. Phosphatidylinositol 3-Kinase alpha-Selective Inhibition with Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J. Clin. Oncol. 2018;36:1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jimeno A., Bauman J.E., Weissman C., Adkins D., Schnadig I., Beauregard P., Bowles D.W., Spira A., Levy B., Seetharamu N., et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol. 2015;51:383–388. doi: 10.1016/j.oraloncology.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jimeno A., Shirai K., Choi M., Laskin J., Kochenderfer M., Spira A., Cline-Burkhardt V., Winquist E., Hausman D., Walker L., et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann. Oncol. 2015;26:556–561. doi: 10.1093/annonc/mdu574. [DOI] [PubMed] [Google Scholar]
- 81.Soulieres D., Faivre S., Mesia R., Remenar E., Li S.H., Karpenko A., Dechaphunkul A., Ochsenreither S., Kiss L.A., Lin J.C., et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323–335. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 82.Marret G., Isambert N., Rezai K., Gal J., Saada-Bouzid E., Rolland F., Chausson M., Borcoman E., Alt M., Klijanienko J., et al. Phase I trial of copanlisib, a selective PI3K inhibitor, in combination with cetuximab in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Investig. New Drugs. 2021;39:1641–1648. doi: 10.1007/s10637-021-01152-z. [DOI] [PubMed] [Google Scholar]
- 83.Jin N., Keam B., Cho J., Lee M.J., Kim H.R., Torosyan H., Jura N., Ng P.K., Mills G.B., Li H., et al. Therapeutic implications of activating noncanonical PIK3CA mutations in head and neck squamous cell carcinoma. J. Clin. Investig. 2021;131:e150335. doi: 10.1172/JCI150335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dogruluk T., Tsang Y.H., Espitia M., Chen F., Chen T., Chong Z., Appadurai V., Dogruluk A., Eterovic A.K., Bonnen P.E., et al. Identification of Variant-Specific Functions of PIK3CA by Rapid Phenotyping of Rare Mutations. Cancer Res. 2015;75:5341–5354. doi: 10.1158/0008-5472.CAN-15-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazumdar T., Byers L.A., Ng P.K., Mills G.B., Peng S., Diao L., Fan Y.H., Stemke-Hale K., Heymach J.V., Myers J.N., et al. A comprehensive evaluation of biomarkers predictive of response to PI3K inhibitors and of resistance mechanisms in head and neck squamous cell carcinoma. Mol. Cancer Ther. 2014;13:2738–2750. doi: 10.1158/1535-7163.MCT-13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah P.A., Huang C., Li Q., Kazi S.A., Byers L.A., Wang J., Johnson F.M., Frederick M.J. NOTCH1 Signaling in Head and Neck Squamous Cell Carcinoma. Cells. 2020;9:2677. doi: 10.3390/cells9122677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sambandam V., Frederick M.J., Shen L., Tong P., Rao X., Peng S., Singh R., Mazumdar T., Huang C., Li Q., et al. PDK1 Mediates NOTCH1-Mutated Head and Neck Squamous Carcinoma Vulnerability to Therapeutic PI3K/mTOR Inhibition. Clin. Cancer Res. 2019;25:3329–3340. doi: 10.1158/1078-0432.CCR-18-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muellner M.K., Uras I.Z., Gapp B.V., Kerzendorfer C., Smida M., Lechtermann H., Craig-Mueller N., Colinge J., Duernberger G., Nijman S.M. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat. Chem. Biol. 2011;7:787–793. doi: 10.1038/nchembio.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janku F., Johnson F.M., Opyrchal M., Dowlati A., Hierro C., Forester M., Blagden S.P., Wicki A., Schmitz D., Adjei A.A. Abstract B109: Oral dual PI3K/mTOR inhibitor bimiralisib demonstrates tolerability and a signal of activity in head and neck squamous cell cancer with NOTCH1 loss-of-function mutation. Mol. Cancer Ther. 2019;18((Suppl. S12)):B109. doi: 10.1158/1535-7163.TARG-19-B109. [DOI] [Google Scholar]
- 90.Johnson F.M., Janku F., Lee J.J., Schmitz D., Streefkerk H., Frederick M. Single-arm study of bimiralisib in head and neck squamous cell carcinoma (HNSCC) patients (pts) harboring NOTCH1 loss of function (LOF) mutations. J. Clin. Oncol. 2020;38:TPS6590. doi: 10.1200/JCO.2020.38.15_suppl.TPS6590. [DOI] [Google Scholar]
- 91.Sambandam V., Shen L., Tong P., Peng S., Mazumdar T., Singh R., Pickering C.R., Myers J.N., Wang J., Frederick M., et al. Abstract 2977: PI3K/mTOR pathway inhibition induces Aurora B mediated cell death inNOTCH1mutant head and neck squamous (HNSCC) cells. Cancer Res. 2018;78((Suppl. S13)):2977. doi: 10.1158/1538-7445.AM2018-2977. [DOI] [Google Scholar]
- 92.Sambandam V., Mazumdar T., Shen L., Zhao H., Peng S., Wang J., Johnson F.M. Aurora kinases mediate resistance to PI3K inhibition in head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. 2020;106:1184. doi: 10.1016/j.ijrobp.2019.11.117. [DOI] [Google Scholar]
- 93.Shah P.A., Fernandez A.M., Sambandam V., Zhao H., Mazumdar T., Shen L., Wang J., Johnson F.M. Aurora kinase B expression shields HNSCC from PI3K inhibition-induced apoptosis through downstream mediators AKT and PDK1; Proceedings of the AACR Annual Meeting 2022; New Orleans, LA, USA. 8–13 April 2022. [Google Scholar]
- 94.Lin Y.S., Su L.J., Yu C.T., Wong F.H., Yeh H.H., Chen S.L., Wu J.C., Lin W.J., Shiue Y.L., Liu H.S., et al. Gene expression profiles of the aurora family kinases. Gene Expr. 2006;13:15–26. doi: 10.3727/000000006783991962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gabrielli B., Bokhari F., Ranall M.V., Oo Z.Y., Stevenson A.J., Wang W., Murrell M., Shaikh M., Fallaha S., Clarke D., et al. Aurora A Is Critical for Survival in HPV-Transformed Cervical Cancer. Mol. Cancer Ther. 2015;14:2753–2761. doi: 10.1158/1535-7163.MCT-15-0506. [DOI] [PubMed] [Google Scholar]
- 96.Shaikh M.H., Idris A., Johnson N.W., Fallaha S., Clarke D.T.W., Martin D., Morgan I.M., Gabrielli B., McMillan N.A.J. Aurora kinases are a novel therapeutic target for HPV-positive head and neck cancers. Oral Oncol. 2018;86:105–112. doi: 10.1016/j.oraloncology.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Martin D., Fallaha S., Proctor M., Stevenson A., Perrin L., McMillan N., Gabrielli B. Inhibition of Aurora A and Aurora B Is Required for the Sensitivity of HPV-Driven Cervical Cancers to Aurora Kinase Inhibitors. Mol. Cancer Ther. 2017;16:1934–1941. doi: 10.1158/1535-7163.MCT-17-0159. [DOI] [PubMed] [Google Scholar]
- 98.Tayyar Y., Idris A., Vidimce J., Ferreira D.A., McMillan N.A. Alpelisib and radiotherapy treatment enhances Alisertib-mediated cervical cancer tumor killing. Am. J. Cancer Res. 2021;11:3240–3251. [PMC free article] [PubMed] [Google Scholar]
- 99.Ghosh S., Mazumdar T., Xu W., Powell R.T., Stephan C., Shen L., Pickering C.R., Wang J., Johnson F.M. Rb deficient HPV+ HNSCC experienced enhanced sensitivity to aurora kinase inhibitors by altering the balance of MAD2 and TRIP13 levels; Proceedings of the AACR Annual Meeting 2022; New Orleans, LA, USA. 8–13 April 2022. [Google Scholar]
- 100.Gong X., Du J., Parsons S.H., Merzoug F.F., Webster Y., Iversen P.W., Chio L.C., Van Horn R.D., Lin X., Blosser W., et al. Aurora A Kinase Inhibition Is Synthetic Lethal with Loss of the RB1 Tumor Suppressor Gene. Cancer Discov. 2019;9:248–263. doi: 10.1158/2159-8290.CD-18-0469. [DOI] [PubMed] [Google Scholar]
- 101.Oser M.G., Fonseca R., Chakraborty A.A., Brough R., Spektor A., Jennings R.B., Flaifel A., Novak J.S., Gulati A., Buss E., et al. Cells Lacking the RB1 Tumor Suppressor Gene Are Hyperdependent on Aurora B Kinase for Survival. Cancer Discov. 2019;9:230–247. doi: 10.1158/2159-8290.CD-18-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyu J., Yang E.J., Zhang B., Wu C., Pardeshi L., Shi C., Mou P.K., Liu Y., Tan K., Shim J.S. Synthetic lethality of RB1 and aurora A is driven by stathmin-mediated disruption of microtubule dynamics. Nat. Commun. 2020;11:5105. doi: 10.1038/s41467-020-18872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turner N., Grose R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 104.Freier K., Schwaenen C., Sticht C., Flechtenmacher C., Muhling J., Hofele C., Radlwimmer B., Lichter P., Joos S. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC) Oral Oncol. 2007;43:60–66. doi: 10.1016/j.oraloncology.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Wheeler S.E., Shi H., Lin F., Dasari S., Bednash J., Thorne S., Watkins S., Joshi R., Thomas S.M. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head Neck. 2014;36:385–392. doi: 10.1002/hed.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koole K., Clausen M.J., van Es R.J., van Kempen P.M., Melchers L.J., Koole R., Langendijk J.A., van Diest P.J., Roodenburg J.L., Schuuring E., et al. FGFR Family Members Protein Expression as Prognostic Markers in Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Mol. Diagn. Ther. 2016;20:363–374. doi: 10.1007/s40291-016-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goke F., Bode M., Franzen A., Kirsten R., Goltz D., Goke A., Sharma R., Boehm D., Vogel W., Wagner P., et al. Fibroblast growth factor receptor 1 amplification is a common event in squamous cell carcinoma of the head and neck. Mod. Pathol. 2013;26:1298–1306. doi: 10.1038/modpathol.2013.58. [DOI] [PubMed] [Google Scholar]
- 108.Symer D.E., Akagi K., Geiger H.M., Song Y., Li G., Emde A.K., Xiao W., Jiang B., Corvelo A., Toussaint N.C., et al. Diverse tumorigenic consequences of human papillomavirus integration in primary oropharyngeal cancers. Genome Res. 2022;32:55–70. doi: 10.1101/gr.275911.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bednova O., Leyton J.V. Targeted Molecular Therapeutics for Bladder Cancer-A New Option beyond the Mixed Fortunes of Immune Checkpoint Inhibitors? Int. J. Mol. Sci. 2020;21:7268. doi: 10.3390/ijms21197268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dumbrava E.I., Alfattal R., Miller V.A., Tsimberidou A.M. Complete Response to a Fibroblast Growth Factor Receptor Inhibitor in a Patient with Head and Neck Squamous Cell Carcinoma Harboring FGF Amplifications. JCO Precis. Oncol. 2018;2:1–7. doi: 10.1200/PO.18.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schuler M., Cho B.C., Sayehli C.M., Navarro A., Soo R.A., Richly H., Cassier P.A., Tai D., Penel N., Nogova L., et al. Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1454–1466. doi: 10.1016/S1470-2045(19)30412-7. [DOI] [PubMed] [Google Scholar]
- 112.Jones P.A., Baylin S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 113.Nepali K., Liou J.P. Recent developments in epigenetic cancer therapeutics: Clinical advancement and emerging trends. J. Biomed. Sci. 2021;28:27. doi: 10.1186/s12929-021-00721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakagawa T., Kurokawa T., Mima M., Imamoto S., Mizokami H., Kondo S., Okamoto Y., Misawa K., Hanazawa T., Kaneda A. DNA Methylation and HPV-Associated Head and Neck Cancer. Microorganisms. 2021;9:801. doi: 10.3390/microorganisms9040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodriguez C.P., Wu Q.V., Voutsinas J., Fromm J.R., Jiang X., Pillarisetty V.G., Lee S.M., Santana-Davila R., Goulart B., Baik C.S., et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020;26:837–845. doi: 10.1158/1078-0432.CCR-19-2214. [DOI] [PubMed] [Google Scholar]
- 116.De Aguiar R.B., de Moraes J.Z. Exploring the Immunological Mechanisms Underlying the Anti-vascular Endothelial Growth Factor Activity in Tumors. Front. Immunol. 2019;10:1023. doi: 10.3389/fimmu.2019.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heydar H., Mansouri K., Norooznezhad M., Norooznezhad F., Mohamadnia A., Bahrami N. Bevacizumab Inhibits Angiogenic Cytokines in Head and Neck Squamous Cell Carcinoma: From Gene to the Protein. Int. J. Hematol. Oncol. Stem Cell Res. 2018;12:136–141. [PMC free article] [PubMed] [Google Scholar]
- 118.Hoang T., Huang S., Armstrong E., Eickhoff J.C., Harari P.M. Enhancement of radiation response with bevacizumab. J. Exp. Clin. Cancer Res. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Argiris A., Li S., Savvides P., Ohr J.P., Gilbert J., Levine M.A., Chakravarti A., Haigentz M., Jr., Saba N.F., Ikpeazu C.V., et al. Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019;37:3266–3274. doi: 10.1200/JCO.19.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hyytiäinen A., Wahbi W., Väyrynen O., Saarilahti K., Karihtala P., Salo T., Al-Samadi A. Angiogenesis Inhibitors for Head and Neck Squamous Cell Carcinoma Treatment: Is There Still Hope? Front. Oncol. 2021;11:683570. doi: 10.3389/fonc.2021.683570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Limaye S., Riley S., Zhao S., O’Neill A., Posner M., Adkins D., Jaffa Z., Clark J., Haddad R. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN) Oral Oncol. 2013;49:835–841. doi: 10.1016/j.oraloncology.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Lalami Y., Garcia C., Flamen P., Ameye L., Paesmans M., Awada A. Phase II trial evaluating the efficacy of sorafenib (BAY 43-9006) and correlating early fluorodeoxyglucose positron emission tomography-CT response to outcome in patients with recurrent and/or metastatic head and neck cancer. Head Neck. 2016;38:347–354. doi: 10.1002/hed.23898. [DOI] [PubMed] [Google Scholar]
- 123.Xue C., Huang Y., Huang P.Y., Yu Q.T., Pan J.J., Liu L.Z., Song X.Q., Lin S.J., Wu J.X., Zhang J.W., et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann. Oncol. 2013;24:1055–1061. doi: 10.1093/annonc/mds581. [DOI] [PubMed] [Google Scholar]
- 124.Gomez-Roca C., Even C., Le Tourneau C., Baste N., Delord J.P., Sarini J., Vergez S., Temam S., Hoffmann C., Rochaix P., et al. Exploratory window-of-opportunity trial to investigate the tumor pharmacokinetics/pharmacodynamics of the IAP antagonist Debio 1143 in patients with head and neck cancer. Clin. Transl. Sci. 2022;15:55–62. doi: 10.1111/cts.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X.S., Tao Y., Le Tourneau C., Pointreau Y., Sire C., Kaminsky M.C., Coutte A., Alfonsi M., Boisselier P., Martin L., et al. Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: A double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 2020;21:1173–1187. doi: 10.1016/S1470-2045(20)30327-2. [DOI] [PubMed] [Google Scholar]
- 126.Debiopharm Novel IAP Inhibitor Shows Significant OS Benefit for High-Risk Head and Neck Cancer. In targetedonc.com. 2020. [(accessed on 16 June 2022)]. Available online: https://www.targetedonc.com/view/novel-iap-inhibitor-shows-significant-os-benefit-for-high-risk-head-and-neck-cancer.
- 127.Shiah J.V., Grandis J.R., Johnson D.E. Targeting STAT3 with Proteolysis Targeting Chimeras and Next-Generation Antisense Oligonucleotides. Mol. Cancer Ther. 2021;20:219–228. doi: 10.1158/1535-7163.MCT-20-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shah N.G., Trivedi T.I., Tankshali R.A., Goswami J.A., Jetly D.H., Kobawala T.P., Shukla S.N., Shah P.M., Verma R.J. Stat3 expression in oral squamous cell carcinoma: Association with clinicopathological parameters and survival. Int. J. Biol. Markers. 2006;21:175–183. doi: 10.1177/172460080602100307. [DOI] [PubMed] [Google Scholar]
- 129.Masuda M., Suzui M., Yasumatu R., Nakashima T., Kuratomi Y., Azuma K., Tomita K., Komiyama S., Weinstein I.B. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 130.Lee T.L., Yeh J., Van Waes C., Chen Z. Epigenetic modification of SOCS-1 differentially regulates STAT3 activation in response to interleukin-6 receptor and epidermal growth factor receptor signaling through JAK and/or MEK in head and neck squamous cell carcinomas. Mol. Cancer Ther. 2006;5:8–19. doi: 10.1158/1535-7163.MCT-05-0069. [DOI] [PubMed] [Google Scholar]
- 131.Bharadwaj U., Eckols T.K., Xu X., Kasembeli M.M., Chen Y., Adachi M., Song Y., Mo Q., Lai S.Y., Tweardy D.J. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget. 2016;7:26307–26330. doi: 10.18632/oncotarget.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grandis J.R., Drenning S.D., Chakraborty A., Zhou M.Y., Zeng Q., Pitt A.S., Tweardy D.J. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth In vitro. J. Clin. Investig. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grandis J.R., Drenning S.D., Zeng Q., Watkins S.C., Melhem M.F., Endo S., Johnson D.E., Huang L., He Y., Kim J.D. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc. Natl. Acad. Sci. USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasquez-Dunddel D., Pan F., Zeng Q., Gorbounov M., Albesiano E., Fu J., Blosser R.L., Tam A.J., Bruno T., Zhang H., et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su Y.L., Banerjee S., White S.V., Kortylewski M. STAT3 in Tumor-Associated Myeloid Cells: Multitasking to Disrupt Immunity. Int. J. Mol. Sci. 2018;19:1803. doi: 10.3390/ijms19061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hammerich L., Tacke F. Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis. World J. Gastrointest. Pathophysiol. 2015;6:43–50. doi: 10.4291/wjgp.v6.i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kortylewski M., Kujawski M., Wang T., Wei S., Zhang S., Pilon-Thomas S., Niu G., Kay H., Mule J., Kerr W.G., et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 138.Huynh J., Chand A., Gough D., Ernst M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer. 2019;19:82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 139.Marvel D., Gabrilovich D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng P., Corzo C.A., Luetteke N., Yu B., Nagaraj S., Bui M.M., Ortiz M., Nacken W., Sorg C., Vogl T., et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Greten T.F., Wang X.W., Korangy F. Current concepts of immune based treatments for patients with HCC: From basic science to novel treatment approaches. Gut. 2015;64:842–848. doi: 10.1136/gutjnl-2014-307990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giurisato E., Xu Q., Lonardi S., Telfer B., Russo I., Pearson A., Finegan K.G., Wang W., Wang J., Gray N.S., et al. Myeloid ERK5 deficiency suppresses tumor growth by blocking protumor macrophage polarization via STAT3 inhibition. Proc. Natl. Acad. Sci. USA. 2018;115:E2801–E2810. doi: 10.1073/pnas.1707929115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J., Liao D., Chen C., Liu Y., Chuang T.H., Xiang R., Markowitz D., Reisfeld R.A., Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 144.Stepkowski S.M., Chen W., Ross J.A., Nagy Z.S., Kirken R.A. STAT3: An important regulator of multiple cytokine functions. Transplantation. 2008;85:1372–1377. doi: 10.1097/TP.0b013e3181739d25. [DOI] [PubMed] [Google Scholar]
- 145.Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.-W., Kanno Y., Powrie F., et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailey S.R., Nelson M.H., Himes R.A., Li Z., Mehrotra S., Paulos C.M. Th17 cells in cancer: The ultimate identity crisis. Front. Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cohen E.E., Harrington K.J., Hong D., Mesia R., Brana I., Perez Segura P., Wise-Draper T.M., Scott M.L., Mitchell P.D., Mugundu G.M., et al. 1044O A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Ann. Oncol. 2018;29((Suppl. S8)):viii372–viii399. doi: 10.1093/annonc/mdy287. [DOI] [Google Scholar]
- 148.Johnson D.E., O’Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chau C.H., Steeg P.S., Figg W.D. Antibody-drug conjugates for cancer. Lancet. 2019;394:793–804. doi: 10.1016/S0140-6736(19)31774-X. [DOI] [PubMed] [Google Scholar]
- 150.Markham A. Tisotumab Vedotin: First Approval. Drugs. 2021;81:2141–2147. doi: 10.1007/s40265-021-01633-8. [DOI] [PubMed] [Google Scholar]
- 151.Coleman R.L., Lorusso D., Gennigens C., Gonzalez-Martin A., Randall L., Cibula D., Lund B., Woelber L., Pignata S., Forget F., et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- 152.Hong D.S., Birnbaum A., Steuer C., Taylor M., George T.J., Lacy J., Wang B., Beca F., Nicacio L., Soumaoro I., et al. Efficacy and Safety of Tisotumab Vedotin in Patients with Head and Neck Squamous Cell Carcinoma: Results From a Phase II Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2022;112:e10–e11. doi: 10.1016/j.ijrobp.2021.12.028. [DOI] [Google Scholar]
- 153.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mandal R., Senbabaoglu Y., Desrichard A., Havel J.J., Dalin M.G., Riaz N., Lee K.W., Ganly I., Hakimi A.A., Chan T.A., et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J., et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qiao X.-w., Jiang J., Pang X., Huang M.-c., Tang Y.-j., Liang X.-h., Tang Y.-l. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020;11:1721. doi: 10.3389/fimmu.2020.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C., et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 159.Mehra R., Seiwert T.Y., Gupta S., Weiss J., Gluck I., Eder J.P., Burtness B., Tahara M., Keam B., Kang H., et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chow L.Q.M., Haddad R., Gupta S., Mahipal A., Mehra R., Tahara M., Berger R., Eder J.P., Burtness B., Lee S.H., et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016;34:3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bauml J., Seiwert T.Y., Pfister D.G., Worden F., Liu S.V., Gilbert J., Saba N.F., Weiss J., Wirth L., Sukari A., et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J. Clin. Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K.J., Kasper S., Vokes E.E., Even C., et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-Year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 165.Burtness B., Rischin D., Greil R., Soulieres D., Tahara M., de Castro G., Jr., Psyrri A., Brana I., Baste N., Neupane P., et al. Pembrolizumab Alone or With Chemotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma in KEYNOTE-048: Subgroup Analysis by Programmed Death Ligand-1 Combined Positive Score. J. Clin. Oncol. 2022;40:2321–2332. doi: 10.1200/JCO.21.02198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.-T., Lee N.Y., Leidner R., Lewis R.L., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rischin D., Harrington K.J., Greil R., Soulieres D., Tahara M., Castro G.d., Psyrri A., Baste N., Neupane P.C., Bratland A., et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) J. Clin. Oncol. 2019;37:6000. doi: 10.1200/JCO.2019.37.15_suppl.6000. [DOI] [Google Scholar]
- 168.Economopoulou P., de Bree R., Kotsantis I., Psyrri A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019;9:827. doi: 10.3389/fonc.2019.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Solinas C., Aiello M., Rozali E., Lambertini M., Willard-Gallo K., Migliori E. Programmed cell death-ligand 2: A neglected but important target in the immune response to cancer? Transl. Oncol. 2020;13:100811. doi: 10.1016/j.tranon.2020.100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burtness B., Goldwasser M.A., Flood W., Mattar B., Forastiere A.A., Eastern Cooperative Oncology G. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 171.Peng S., Ferrall L., Gaillard S., Wang C., Chi W.Y., Huang C.H., Roden R.B.S., Wu T.C., Chang Y.N., Hung C.F. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio. 2021;12:e03224-20. doi: 10.1128/mBio.03224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou J.Z., Jou J., Cohen E. Vaccine Strategies for Human Papillomavirus-Associated Head and Neck Cancers. Cancers. 2021;14:33. doi: 10.3390/cancers14010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang C., Dickie J., Sutavani R.V., Pointer C., Thomas G.J., Savelyeva N. Targeting Head and Neck Cancer by Vaccination. Front. Immunol. 2018;9:830. doi: 10.3389/fimmu.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tourneau J.-P., Delord P., Cassier D., Loirat A., Tavernaro B., Bastien K., Bendjama C.L. 1210P—Phase Ib/II trial of TG4001 (Tipapkinogene sovacivec), a therapeutic HPV-vaccine, and Avelumab in patients with recurrent/metastatic (R/M) HPV-16+ cancers. Ann. Oncol. 2019;30:494–495. doi: 10.1093/annonc/mdz253.036. [DOI] [Google Scholar]
- 175.Massarelli E., William W., Johnson F., Kies M., Ferrarotto R., Guo M., Feng L., Lee J.J., Tran H., Kim Y.U., et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chester C., Sanmamed M.F., Wang J., Melero I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 177.Bhatt K.H., Neller M.A., Srihari S., Crooks P., Lekieffre L., Aftab B.T., Liu H., Smith C., Kenny L., Porceddu S., et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J. Exp. Med. 2020;217:e20200389. doi: 10.1084/jem.20200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fournier C., Martin F., Zitvogel L., Kroemer G., Galluzzi L., Apetoh L. Trial Watch: Adoptively transferred cells for anticancer immunotherapy. Oncoimmunology. 2017;6:e1363139. doi: 10.1080/2162402X.2017.1363139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Papa S., Adami A., Metoudi M., Achkova D., Schalkwyk M.v., Pereira A.P., Bosshard-Carter L., Whilding L., Stegen S.v.d., Davies D., et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC) J. Clin. Oncol. 2018;36:3046. doi: 10.1200/JCO.2018.36.15_suppl.3046. [DOI] [Google Scholar]
- 180.Draper L.M., Kwong M.L., Gros A., Stevanovic S., Tran E., Kerkar S., Raffeld M., Rosenberg S.A., Hinrichs C.S. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin. Cancer Res. 2015;21:4431–4439. doi: 10.1158/1078-0432.CCR-14-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hinrichs C.S., Doran S.L., Stevanovic S., Adhikary S., Mojadidi M., Kwong M.L., Faquin W.C., Feldman S., Somerville R., Sherry R.M., et al. A phase I/II clinical trial of E6 T-cell receptor gene therapy for human papillomavirus (HPV)-associated epithelial cancers. J. Clin. Oncol. 2017;35:3009. doi: 10.1200/JCO.2017.35.15_suppl.3009. [DOI] [Google Scholar]
- 182.Doran S.L., Stevanovic S., Adhikary S., Gartner J.J., Jia L., Kwong M.L.M., Faquin W.C., Hewitt S.M., Sherry R.M., Yang J.C., et al. T-Cell Receptor Gene Therapy for Human Papillomavirus-Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019;37:2759–2768. doi: 10.1200/JCO.18.02424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gameiro S.F., Ghasemi F., Barrett J.W., Koropatnick J., Nichols A.C., Mymryk J.S., Maleki Vareki S. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV-counterparts that has implications for immunotherapy. Oncoimmunology. 2018;7:e1498439. doi: 10.1080/2162402X.2018.1498439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Le X., Dang M., Hegde V.L., Jiang B., Slay R., Xiao W., Akagi K., Fresquez J., Marcelo K.L., Luo Q., et al. TIGIT as a therapeutic target of HPV-positive head and neck squamous cell carcinomas. medRxiv. 2021 doi: 10.1101/2021.12.02.21266776. [DOI] [Google Scholar]
- 185.Taylor M.H., Lee C.H., Makker V., Rasco D., Dutcus C.E., Wu J., Stepan D.E., Shumaker R.C., Motzer R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020;38:1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen T.H., Chang P.M., Yang M.H. Combination of pembrolizumab and lenvatinib is a potential treatment option for heavily pretreated recurrent and metastatic head and neck cancer. J. Chin. Med. Assoc. 2021;84:361–367. doi: 10.1097/JCMA.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 187.Saba N.F., Ekpenyong A., McCook-Veal A., Patel M., Schmitt N.C., Stokes W.A., Bates J.E., Rudra S., Abousaud M.I., Muzaffar J., et al. A phase II trial of pembrolizumab and cabozantinib in patients (pts) with recurrent metastatic head and neck squamous cell carcinoma (RMHNSCC); Proceedings of the ASCO 2022; Chicago, IL, USA. 3–7 June 2022. [Google Scholar]
- 188.Caforio M., de Billy E., De Angelis B., Iacovelli S., Quintarelli C., Paganelli V., Folgiero V. PI3K/Akt Pathway: The Indestructible Role of a Vintage Target as a Support to the Most Recent Immunotherapeutic Approaches. Cancers. 2021;13:4040. doi: 10.3390/cancers13164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zandberg D.P., Menk A.V., Velez M., Normolle D., DePeaux K., Liu A., Ferris R.L., Delgoffe G.M. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J. Immunother. Cancer. 2021;9:e002088. doi: 10.1136/jitc-2020-002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cerniglia G.J., Dey S., Gallagher-Colombo S.M., Daurio N.A., Tuttle S., Busch T.M., Lin A., Sun R., Esipova T.V., Vinogradov S.A., et al. The PI3K/Akt Pathway Regulates Oxygen Metabolism via Pyruvate Dehydrogenase (PDH)-E1alpha Phosphorylation. Mol. Cancer Ther. 2015;14:1928–1938. doi: 10.1158/1535-7163.MCT-14-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Qi Z., Xu Z., Zhang L., Zou Y., Li J., Yan W., Li C., Liu N., Wu H. Overcoming resistance to immune checkpoint therapy in PTEN-null prostate cancer by intermittent anti-PI3Kalpha/beta/delta treatment. Nat. Commun. 2022;13:182. doi: 10.1038/s41467-021-27833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eschweiler S., Ramirez-Suastegui C., Li Y., King E., Chudley L., Thomas J., Wood O., von Witzleben A., Jeffrey D., McCann K., et al. Intermittent PI3Kdelta inhibition sustains anti-tumour immunity and curbs irAEs. Nature. 2022;605:741–746. doi: 10.1038/s41586-022-04685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sacco A.G., Chen R., Worden F.P., Wong D.J.L., Adkins D., Swiecicki P., Chai-Ho W., Oppelt P., Ghosh D., Bykowski J., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chung C.H., Bonomi M., Steuer C.E., Li J., Bhateja P., Johnson M., Masannat J., Song F., Hernandez-Prera J.C., Wenig B.M., et al. Concurrent Cetuximab and Nivolumab as a Second-Line or beyond Treatment of Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results of Phase I/II Study. Cancers. 2021;13:1180. doi: 10.3390/cancers13051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.NCCN Guidelines for Head and Neck Cancers, Version 2.2022. 2022. [(accessed on 26 April 2022)]. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437.
- 196.Ferris R.L., Haddad R., Even C., Tahara M., Dvorkin M., Ciuleanu T.E., Clement P.M., Mesia R., Kutukova S., Zholudeva L., et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020;31:942–950. doi: 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 197.BMS Bristol Myers Squibb Provides Update on CheckMate-651 Trial Evaluating Opdivo (nivolumab) Plus Yervoy (ipilimumab) Versus EXTREME Regimen as First-Line Treatment for Squamous Cell Carcinoma of the Head and Neck. 2021. [(accessed on 16 June 2022)]. Available online: https://www.ono-pharma.com/news/20210719.html.
- 198.Quayle S.N., Girgis N., Thapa D.R., Merazga Z., Kemp M.M., Histed A., Zhao F., Moreta M., Ruthardt P., Hulot S., et al. CUE-101, a Novel E7-pHLA-IL2-Fc Fusion Protein, Enhances Tumor Antigen-Specific T-Cell Activation for the Treatment of HPV16-Driven Malignancies. Clin. Cancer Res. 2020;26:1953–1964. doi: 10.1158/1078-0432.CCR-19-3354. [DOI] [PubMed] [Google Scholar]
- 199.Chung C., Dimitrios Colevas A., Gibson M., Adkins D., Sukari A., Wirth L., Burtness B., Bauman J., Rodriguez C., Worden F., et al. 438 A phase 1 trial of CUE-101, a novel HPV16 E7-pHLA-IL2-Fc fusion protein, alone and in combination with pembrolizumab in patients with recurrent/metastatic HPV16+ head and neck cancer. J. Immunother Cancer. 2021;9:A468. doi: 10.1136/jitc-2021-SITC2021.438. [DOI] [Google Scholar]
- 200.Mitsunaga M., Ogawa M., Kosaka N., Rosenblum L.T., Choyke P.L., Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cognetti D.M., Johnson J.M., Curry J.M., Kochuparambil S.T., McDonald D., Mott F., Fidler M.J., Stenson K., Vasan N.R., Razaq M.A., et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck. 2021;43:3875–3887. doi: 10.1002/hed.26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.