Abstract
Aim:
Guidance on post-cardiac arrest prognostication is largely based on data from out-of-hospital cardiac arrest (OHCA), despite clear differences between the OHCA and in-hospital cardiac arrest (IHCA) populations. Early prediction of mortality after IHCA would be useful to help make decisions about post-arrest care. We evaluated the ability of lactate and need for vasopressors after IHCA to predict hospital mortality.
Methods:
Single center retrospective observational study of adult IHCA patients who achieved sustained return of spontaneous circulation (ROSC), required mechanical ventilation peri-arrest and had a lactate checked within 2 h after ROSC. We evaluated the association of post-ROSC lactate and need for vasopressors with mortality using multivariate logistic regression.
Results:
A total of 364 patients were included. Patients who received vasopressors within 3 h after ROSC had significantly higher mortality compared to patients who did not receive vasopressors (58% vs. 43%, p = 0.03). Elevated lactate level was associated with mortality (44% if lactate <5 mmol/L, 58% if lactate 5–10 mmol/L, and 73% if lactate >10 mmol/L, p < 0.01). A multivariable model with lactate group and post-ROSC vasopressor use as predictors demonstrated moderate discrimination (AUC 0.64 [95%CI:0.59–0.70]). Including other variables, the most parsimonious model included lactate, age, body mass index, race, and history of arrhythmia, cancer and/or liver disease (AUC 0.70 [95% CI: 0.64–0.75]).
Conclusion:
Post-ROSC lactate and need for vasopressors may be helpful in stratifying mortality risk in patients requiring mechanical ventilation after IHCA.
Keywords: IHCA (in hospital cardiac arrest), OHCA (out of hospital cardiac arrest), ROSC (return of spontaneous circulation), Lactate, Vasopressors
Introduction
Approximately 292,000 adults suffer an in-hospital cardiac arrest (IHCA) each year in the United States. The incidence of IHCA is increasing over time, and although improving, the mortality remains extremely high.1,2 The high prevalence and mortality of cardiac arrest has made development of new treatments and prediction tools a high priority. However, the bulk of research informing treatment and prognostication has been done with a focus on out of hospital cardiac arrest (OHCA), which differs from IHCA in patient and arrest characteristics as well as outcomes.3–8
Early post-arrest prediction of hospital mortality would be useful to both clinicians and families to aid in medical decision making for cardiac arrest patients who survive the initial arrest but remain critically ill. Current prognostication guidelines focus almost exclusively on neuroprognostication since neurologic injury is the primary cause of death in OHCA patients who survive the acute event. However, neurologic injury is a much less common cause of death after IHCA, while shock and comorbid withdrawal of care are more prevalent.8,9 These findings identify a need for the development of prognostication tools focused on IHCA.
Elevation in lactate is a natural candidate to serve as a tool for IHCA prognostication. Lactate elevation occurs under ischemic conditions and is known to be associated with mortality after OHCA as well as in sepsis and other forms of critical illness.10,11 Patients with post-cardiac arrest syndrome, a result of ischemia-reperfusion injury similar physiologically to sepsis, frequently experience shock and elevated lactate. Prior investigators have found that post-ROSC elevation in lactate and the need for vasopressors are highly predictive of mortality after OHCA, but whether these are similarly predictive in patients after IHCA is unknown.5,10,11
We conducted the following study to investigate whether post-arrest lactate and need for vasopressors, both alone and when combined with other peri-arrest and patient variables, were associated with hospital mortality in patients obtaining ROSC but remaining critically ill after IHCA.
Methods
Population
This was a retrospective single-center observational study of IHCA events occurring at a tertiary care center in the United States between January 2008 and December 2018. Patients were selected from a prospectively-collected database of IHCA events. All events that elicit a “Code Blue” emergency response are included in the database. Data collected prospectively includes patient demographics, cardiac arrest data (including initial rhythm and downtime, among others), and outcomes.
Patients who achieved sustained return of spontaneous circulation (ROSC) (evidence of a palpable pulse or a measurable blood pressure for >20 min.) following an IHCA event, were >18 years old, had a lactate measured within 2 h of ROSC, and were intubated within one hour after ROSC or prior to arrest were included. The patient population was limited to those requiring mechanical ventilation after arrest in order to focus on those who remain critically-ill after ROSC, as this is the patient group for whom prognostication is most challenging.
We collected data on pre-arrest diagnoses and interventions, post-arrest laboratory results, and post-arrest clinical status and interventions in the initial hours after arrest. We also verified the intra-arrest characteristics that were collected prospectively. Specific variables collected were pre-arrest vasopressor use, pre-arrest mechanical ventilation, location of arrest (emergency department, intensive care unit, general floor, procedural areas and other (radiology department and dialysis units)), total downtime (no-flow and low-flow times), initial rhythm, lactate level within two hours after ROSC, and need for vasopressors within three hours after ROSC. We excluded patients with no lactate checked within 2 h after ROSC, those who did not require mechanical ventilation after ROSC, non-index arrests, patients whose index arrest was in the out-of-hospital setting, and those with insufficient information in the medical record to satisfy inclusion criteria.
Pre-arrest diagnoses were obtained by the authors through chart review by extracting the most recent primary diagnosis prior to arrest. Diagnoses were divided into 5 categories: sepsis, cardiac disease (myocardial infarction, primary arrhythmia, cardiogenic shock, post-cardiac surgery, and congestive heart failure), respiratory disease (pulmonary embolism, pneumonia, COPD or other causes of acute respiratory failure), acute bleeding, and other (including but not limited to renal failure, decompensated liver failure, electrolyte disturbances, and drug overdose/side effect). For patients with more than one diagnosis, the primary diagnosis was selected by consensus after discussion between the first and senior author.
Statistical analysis
Baseline characteristics and arrest variables are presented with descriptive statistics. Continuous variables are presented as means and standard deviations or medians and interquartile ranges (IQR), as appropriate, depending on the normality of the data. Differences between groups were tested using Student’s t-test or Wilcoxon’s rank-sum test. Categorical data are presented as counts and proportions with the differences between groups tested using Chi-square or Fisher’s exact test.
The primary outcome for this study was hospital mortality. Univariate models were performed to assess the association between hospital mortality and covariates selected a priori, including post-ROSC lactate level (categorized into three groups: <5 mmol/L, 5–10 mmol/L, or > 10 mmol/L), receipt of vasopressors in the first 3 h after ROSC, age, downtime, in-hospital cardiac arrest location, pre-arrest diagnosis, initial rhythm, pre-arrest vasopressor use, and pre-arrest invasive mechanical ventilation. Lactate was evaluated as a categorical variable both for simplicity and based on prior literature suggesting the categorical approach was as predictive as using lactate as a continuous variable.10
For the primary analysis, multivariable logistic regression was performed with categorical lactate level and receipt of vasopressors as the independent variables and hospital mortality as the dependent variable. In the secondary analysis, all variables with a p-value less than 0.25 in univariate analysis were included in the model. The most parsimonious model was then developed using backward selection. Non-normal continuous variables were log transformed prior to evaluation for the final model.
An estimate of effect size and variability was reported as an odds ratio (OR) and 95% confidence intervals (CI). The discriminatory power of each model was assessed using the area under the receiver operating characteristic curve (AUC). Observations with missing data were omitted from the analyses. All analyses were two-sided with a significance level of 0.05, and were performed using STATA (version 14.2).
Results
We evaluated 541 patients between 2008 and 2018 who achieved sustained ROSC following IHCA. After exclusions as noted in Fig. 1, 364 patients were included in the analysis. Baseline and arrest characteristics are presented in Table 1, Nonsurvivors were more likely to have a history of liver disease, arrhythmia or cancer at baseline. Although race as a variable differed between survivors and nonsurvivors, this appeared to be driven by the category of “unknown/not reported,” which was more common in nonsurvivors. There was no significant difference in pre-arrest mechanical ventilation, pre-arrest vasopressor use, or initial rhythm between survivors and nonsurvivors.
Fig. 1 –
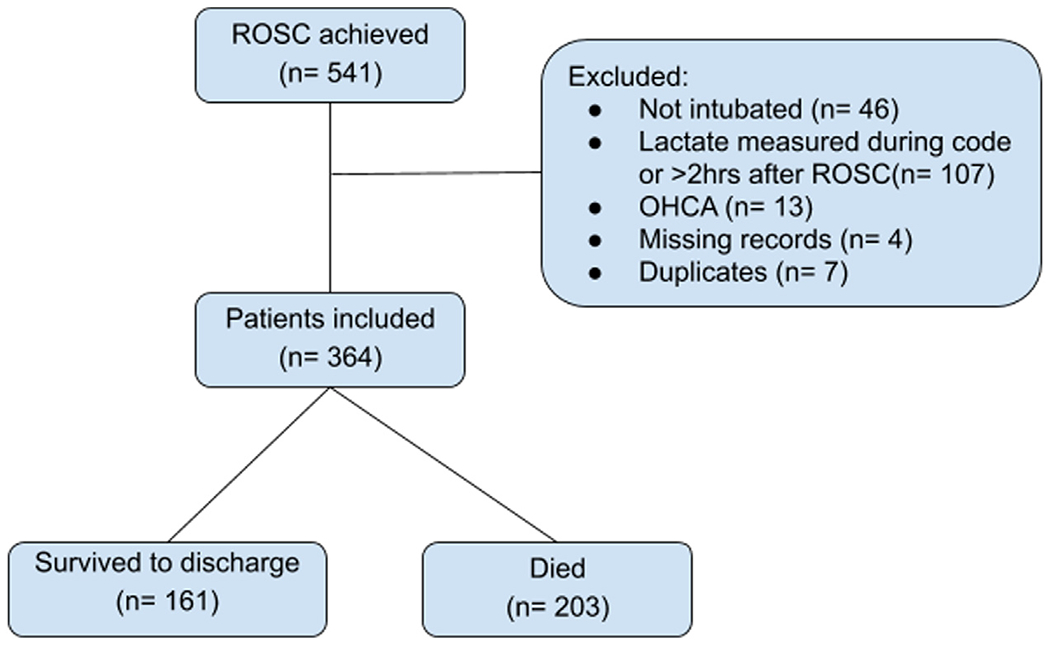
Flow chart of screened and included patients.
ROSC = return of spontaneous circulation. OHCA = out of hospital cardiac arrest.
Table 1 –
Baseline characteristics of patients stratified by survival to discharge status.
| Non-survivor (n = 203) | Survivor (n = 161) | P-value | |
|---|---|---|---|
| Age, years—median (IQR) | 72 (60, 81) | 68 (57, 77) | 0.06 |
| Body mass index, kg/m2—median (IQR) | 27.1 (22.9, 32.1) | 28.3 (24.9, 31.1) | 0.13 |
| Male, n (%) | 128 (63.1) | 105 (65.2) | 0.67 |
| Race, n (%) | 0.045 | ||
| - White | 121 (59.6) | 104 (64.6) | |
| - Black/African American | 30 (14.89) | 31 (19.3) | |
| - Other | 18 (8.9) | 15 (9.3) | |
| - Unknown/not reported | 34 (16.8) | 11 (6.8) | |
| Past medical history, n (%) | |||
| - Coronary artery disease | 71 (35.0) | 53 (32.9) | 0.74 |
| - Cancer | 54 (26.6) | 27 (16.8) | 0.03 |
| - Congestive heart failure | 60 (29.6) | 37 (23.0) | 0.19 |
| - Arrhythmia | 39 (19.2) | 14 (8.7) | <0.01 |
| - Chronic obstructive pulmonary disease | 33 (16.3) | 21 (13.0) | 0.46 |
| - Dementia/Alzheimer | 10 (4.9) | 9 (5.6) | 0.82 |
| - Diabetes | 80 (39.4) | 66 (41.0) | 0.76 |
| - Alcohol use disorder | 24 (11.8) | 14 (8.7) | 0.39 |
| - Hypertension | 130 (64.0) | 103 (64.0) | 0.99 |
| - Hyperlipidemia | 76 (37.4) | 59 (36.6) | 0.91 |
| - Liver disease | 23 (11.3) | 6 (3.7) | 0.01 |
| - Prior arrest | 10 (4.9) | 5 (3.1) | 0.44 |
| Pre-arrest variables: | |||
| Pre-arrest vasopressor use, n (%) | 60 (29.6) | 48 (29.8) | 0.96 |
| Pre-arrest invasive mechanical ventilation, n (%) | 59 (29.1) | 48 (29.8) | 0.88 |
| Pre-arrest diagnosis, n (%) | 0.18 | ||
| - Sepsis | 27 (13.3) | 30 (18.6) | |
| - Cardiac related reasons | 49 (24.1) | 51 (31.7) | |
| - Acute bleeding | 25 (12.3) | 16 (9.9) | |
| - Respiratory related reasons | 60 (29.6) | 36 (22.4) | |
| - Other | 42 (20.7) | 28 (17.4) | |
| Intra-arrest variables: | |||
| In hospital cardiac arrest location, n (%) | 0.08 | ||
| - ICU | 73 (36.0) | 53 (32.9) | |
| - ED | 35 (17.2) | 31 (19.3) | |
| - Floor | 65 (32.0) | 38 (23.6) | |
| - Procedural area | 24 (11.8) | 26 (16.1) | |
| - Other | 6 (3.0) | 13 (8.1) | |
| Initial Rhythm, n (%) | 0.80 | ||
| - Shockable (VT/VF) | 40 (19.7) | 33 (20.8) | |
| - Non-shockable (PEA/Asystole) | 163 (80.3) | 126 (79.2) | |
| Witnessed, n (%) | 191 (94.6) | 155 (96.9) | 0.32 |
| Total Downtime, minutes—median (IQR) | 10 (517) | 7 (414) | <0.01 |
| Post-arrest variables: | |||
| Initial lactate (within 2 h after ROSC), mmol/L | <0.01 | ||
| - Median (IQR) | 7.2 (4.4, 10.1) | 5.1 (3.1, 7.5) | |
| - <5 | 59 (29.1) | 75 (46.6) | |
| - 5–10 | 93 (45.8) | 67 (41.6) | |
| - >10 | 51 (25.1) | 19 (11.8) | |
| Vasopressors post-ROSC, n (%) | 178 (87.7) | 128 (79.5) | 0.03 |
| Target temperature management, n (%) | 78 (38.6) | 47 (29.2) | 0.06 |
| Code status after arrest, n (%) | <0.01 | ||
| - Full code | 20 (9.9) | 137 (5.1) | |
| - DNR/DNI | 37 (18.2) | 19 (11.8) | |
| - CMO | 146 (71.9) | 5 (3.1) | |
| Length of hospital stay post-arrest, days—median (IQR) | 3 (1, 7) | 16 (10, 26) | <0.01 |
IQR = interquartile range. ICU = intensive care unit. ED = emergency department. VT = ventricular tachycardia. VF = ventricular fibrillation. PEA = pulseless electrical activity. ROSC = return of spontaneous circulation. DNR = Do Not Resuscitate. DNI = Do Not Intubate. CMO = Comfort Measures Only (hospice). Variables with missing data include BMI (13 patients), initial rhythm (2 patients), total downtime (2 patients) and length of hospital stay (1 patient).
Hospital mortality rates by lactate level and vasopressor use are presented in Fig. 2. Mortality increased with increase in lactate (44% if lactate <5 mmol/L, 58% if lactate 5–10 mmol/L, and 73% if lactate >10 mmol/L, p < 0.01) and was higher when post-arrest vasopressor use was required (58% if requiring vasopressors after ROSC vs. 43% if not, p = 0.03). The combination of lactate category and vasopressor use was able to differentiate patients into groups ranging in mortality from 33% to 75%.
Fig. 2 –
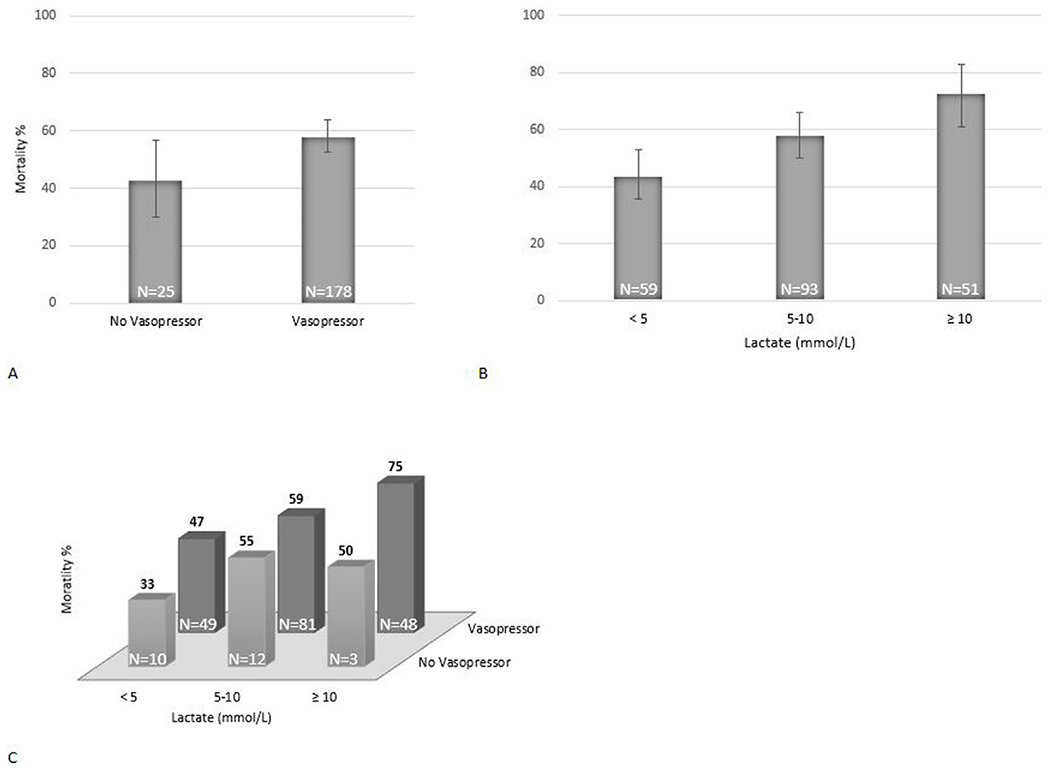
Mortality by lactate and vasopressor categories. Vertical lines in Fig. 2A and 2B represent 95% confidence intervals.
The AUC for lactate alone as a predictor of hospital mortality was 0.643 (95% CI: 0.586–0.700); for post-ROSC need for vasopressors alone, it was 0.541 (95% CI: 0.502–0.579). Combining lactate and post-ROSC vasopressor requirement, the AUC for mortality was 0.642 (95% CI: 0.585–0.698). After inclusion of all candidate variables (p < 0.25 in univariable analysis) and using backward selection to select the most parsimonious model, the following predictors were included: lactate, age, body mass index (BMI), race, and past medical history of cancer, arrhythmia, and liver disease. Using this multivariable model, the AUC for hospital mortality was 0.702 (95% CI: 0.648–0.755). (Table 2, Fig. 3)
Table 2 –
Results of final mutivariable model after backwards selection.
| Odds ratio (95% CI) | P-value | AUC (95% CI) | |
|---|---|---|---|
| Baseline lactate (logged) | 2.34 (1.58—3.44) | <0.001 | AUC for model: 0.702 (95% CI: 0.648—0.755) |
| Age (logged) | 2.83 (1.05—7.59) | 0.040 | |
| BMI (logged) | 0.60 (0.22—1.63) | 0.320 | |
| Race group | |||
| White | reference | ||
| Black/African American | 0.94 (0.51—1.72) | 0.835 | |
| Other | 1.07 (0.48—2.41) | 0.862 | |
| Unknown/not reported | 3.21 (1.44—7.16) | 0.004 | |
| PMH of cancer | 1.80 (1.02—3.17) | 0.041 | |
| PMH of arrhythmia | 2.20 (1.09—7.16) | 0.027 | |
| PMH of liver disease | 3.42 (1.25—9.38) | 0.017 |
AUC = area under the receiver operator curve. BMI = body mass index. PMH = past medical history.
Fig. 3 –
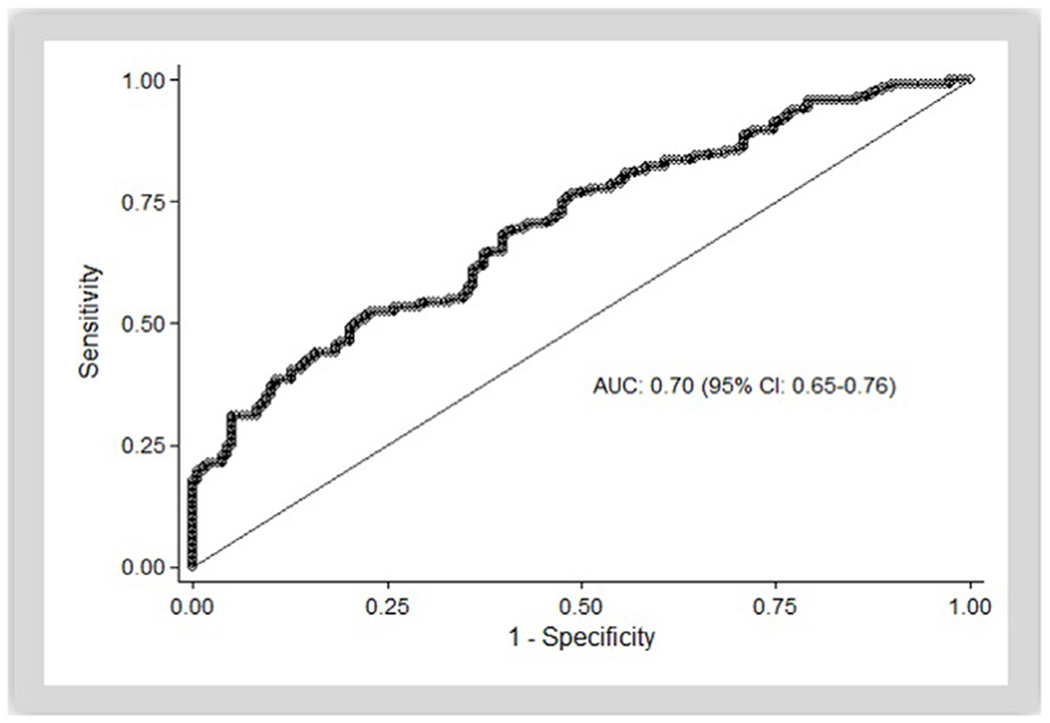
Area under the curve for full model using backwards selection, including lactate, age, body mass index (BMI), race, and past medical history of cancer, arrhythmia, and liver disease.
Of the nonsurvivors, 35% were deemed to have died of withdrawal of care due to neurologic injury, and another 35% from withdrawal of support due to comorbid disease. The remaining patients died of recurrent cardiac arrest (13%), refractory shock (13%) or refractory respiratory failure (4%).
Discussion
In this study, we aimed to test if a previously reported model for predicting mortality after OHCA is useful for IHCA patients who require mechanical ventilation after ROSC. Our results show that lactate level and need for vasopressor support were helpful in separating high and low mortality risk groups. The AUC for lactate and post-ROSC vasopressor use indicated a moderate ability to predict mortality, with elevation in lactate appearing to be the stronger predictive variable. The AUC improved with the addition of age, BMI, past medical history significant for cancer, arrhythmia, or liver disease and race to the model, while initial rhythm, pre-arrest vasopressors and pre-arrest mechanical ventilation were not associated with mortality in this cohort.
There is a well-known association between lactate and mortality in critically ill patients. Given this association and the ease with which lactate measurements can be obtained, the importance of lactate measurement in the monitoring and resuscitation of patients with sepsis and other forms of shock is well-established.12 Although lactate measured during ongoing CPR has been shown to predict mortality after IHCA in one study,13 most of the evidence in post-arrest patients has been derived from OHCA 5–7 An additional study found that a lower lactate level at 0, 12, and 24 h, as well as greater clearance of lactate over the first 12 h was associated with better survival and good neurological outcomes after OHCA.10
Previous work aimed at developing scoring systems to predict mortality after IHCA has been limited. Ebell et al.14 used pre-arrest clinical variables to build a score (GO-FAR score) to predict mortality after IHCA. This score was developed with the goal of informing physician-family discussions regarding resuscitation/code status before a cardiac arrest occurs, and was not meant to be used for mortality prediction after ROSC. Chan et al.15 conducted a study based on data from the American Heart Association’s Get-With-The-Guidelines-Resuscitation registry, a nationwide registry of IHCA events. In this study, the investigators utilized pre-arrest and intra-arrest variables to predict mortality. They did not include post-ROSC variables. Pre-arrest vasopressor requirement and initial rhythm were associated with mortality in that work, while there was no association in the present study. Although reasons for this difference are not clear, by limiting our population to those requiring mechanical ventilation after ROSC and those with a lactate checked within two hours, we may have selected for a more critically-ill population, which may have decreased the predictive value of pre-arrest vasopressor requirement and initial rhythm. Over 80% of our entire cohort was on vasopressors after ROSC, and this variable is not reported in the study by Chan et al., making comparison difficult.
In a retrospective study looking at patients with ROSC after OHCA, Cocchi et al.16 found an AUC for mortality of 0.82 using lactate and post-ROSC vasopressor requirement as predictors. The same group repeated a similar larger study more recently, finding an AUC of 0.73 with a similar model.5 Applying this model to our IHCA population, the AUC was 0.64, indicating a moderate ability to discriminate between survivors and nonsurvivors. This AUC appears lower than that found in either of the OHCA cohorts, although confidence intervals from the 2019 work and the present study overlap. Presence or absence of severe elevation in lactate and need for vasopressors did appear to differentiate between patients with high (>75%) and relatively low (33%) mortality in our cohort, which may be useful for clinicians.
Many possibilities for why post-arrest lactate and vasopressor need might be somewhat less predictive of outcome in our IHCA cohort exist. Aside from the cardiac arrest setting (OHCA vs IHCA), the need for mechanical ventilation was not a requirement for inclusion in the study by Cocchi et al., and a lower percentage of the overall cohort required vasopressors after ROSC (only 63% of patients included, compared to 84% in this study). We restricted our population to those requiring mechanical ventilation after ROSC in order to focus on the patients for whom prognostication is most challenging, but lactate and need for vasopressors may have appeared more predictive of outcome if we included those less-sick patients not requiring mechanical ventilation after ROSC. As the vast majority of our patients were on vasopressors after ROSC, our ability to assess the predictive value of vasopressor requirement for mortality may have been limited. The higher percentage requiring vasopressors may also reflect different etiologies of arrest (e.g. more sepsis) in the IHCA population. In addition, downtimes in IHCA are known to be shorter on average than in OHCA, and this is clearly seen when comparing median downtimes in the present study to those in the prior work (median low-flow time of 10 and 21 min in survivors and nonsurvivors in OHCA cohort, compared to 7 and 10 min in the present study).16 In our cohort, median downtime also differed by only a few minutes between survivors and nonsurvivors, and the range in lactates was also smaller, again likely contributing to the difficulty in using these variables to accurately predict mortality except at the extremes of high and low risk.
The other variables in our final model were age, liver disease, arrhythmia, and cancer all associated with worse survival. Race was also associated with survival, but when looked at in more detail only “unknown/not reported” race bore this association. Other studies have looked at IHCA outcomes by race and found that survival is worse in patients identified as black than in those identified as white.17 Work by the same group has suggested that much of this difference is due to black patients often receiving care at hospitals where IHCA outcomes are worse and overall access to care may be more limited. Fortunately this disparity in IHCA outcomes has been found to be decreasing over time.18 In the present study, the association of race with mortality was driven by the group listed as “unknown/not reported,” with other categories not seeming to have any such association. Race at our institution is self-reported, suggesting that unknown/not reported race may have been a marker of some other predictor of poor outcome, such as limited prior access to medical care or arrival to the hospital at an illness level precluding a patient being able to provide a history.
Finally, our results highlight once again some potential limitations of utilizing data from the OHCA population to inform decision making for IHCA patients. More research on this complex and heterogeneous disease process is needed.
The strength of any conclusions from this study is limited by it being from a single center, with many data points collected retrospectively. Numbers of patients not requiring vasopressors were small (only 16% of the cohort), and numbers were very small in some stratification groups, limiting the strength of any conclusions that can be drawn for those categories (e.g. lactate >10 and no vasopressor requirement included only 3 patients). We were also unable to account for some details on the variables considered, such as total dose of vasopressors, which may impact prognosis. Similarly, although we were able to ascertain which patients had a code status change to “do not resuscitate” or “comfort measures only” after the initial arrest and resuscitation, due to the retrospective design we were unable to assess how long after arrest these changes were made. Therefore whether such a code status change could have affected prognosis, or was made in recognition of impending death, is not certain.
Conclusion
Post-ROSC lactate and need for vasopressor support may help differentiate between patients with higher vs lower hospital mortality. These variables, although useful, appear to be less predictive after IHCA than after OHCA.
Conflicts of interest and funding support
None of the authors have any conflicts of interest to report. Dr. Berg is supported by a research grant from the NHLBI (K23 HL128814). Dr. Donnino’s effort was supported, in part, by grants from the National Heart, Lung and Blood Institute (K24HL127101 and R01HL136705).
Footnotes
CRediT authorship contribution statement
Mahmoud S. Issa: Conceptualization, Data curation, Validation, Project administration, Writing - original draft, Writing - review & editing. Anne V. Grossestreuer: Conceptualization, Methodology, Supervision, Formal analysis, Writing - review & editing. Het Patel: Data curation, Writing - review & editing. Lethu Ntshinga: Data curation, Writing - review & editing. Amin Coker: Data curation, Writing - review & editing. Tuyen Yankama: Methodology, Formal analysis, Writing - review & editing. Michael W. Donnino: Conceptualization, Methodology, Supervision, Visualization, Writing - review & editing. Katherine M. Berg: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019;12:e005580, doi: 10.1161/CIRCOUTCOMES.119.005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med 2011;39:2401–6, doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moskowitz A, Holmberg MJ, Donnino MW, Berg KM. In-hospital cardiac arrest: are we overlooking a key distinction? Curr Opin Crit Care 2018;24:151–7, doi: 10.1097/MCC.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA 2019;321:1200–10, doi: 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocchi MN, Salciccioli J, Yankama T, et al. Predicting outcome after out-of-hospital cardiac arrest: lactate, need for vasopressors, and cytochrome c. J Intensive Care Med 2019, doi: 10.1177/0885066619873315 885066619873315. [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Cariou A, Mourvillier B, et al. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: the OHCA score. Eur Heart J 2006;27:2840–5, doi: 10.1093/eurheartj/ehl335. [DOI] [PubMed] [Google Scholar]
- 7.Williams TA, Martin R, Celenza A, et al. Use of serum lactate levels to predict survival for patients with out-of-hospital cardiac arrest: a cohort study. Emerg Med Australas 2016;28:171–8, doi: 10.1111/1742-6723.12560. [DOI] [PubMed] [Google Scholar]
- 8.Witten L, Gardner R, Holmberg MJ, et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation 2019;136:93–9, doi: 10.1016/j.resuscitation.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004;30:2126–8, doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 10.Donnino MW, Andersen LW, Giberson T, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med 2014;42:1804–11, doi: 10.1097/CCM.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dell’Anna AM, Sandroni C, Lamanna I, et al. Prognostic implications of blood lactate concentrations after cardiac arrest: a retrospective study. Ann Intensive Care 2017;7:101, doi: 10.1186/s13613-017-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 2013;3:12, doi: 10.1186/2110-5820-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C-H, Huang C-H, Chang W-T, et al. Monitoring of serum lactate level during cardiopulmonary resuscitation in adult in-hospital cardiac arrest. Crit Care 2015;19:344, doi: 10.1186/s13054-015-1058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebell MH, Jang W, Shen Y, Geocadin RG, Get With the Guidelines—Resuscitation Investigators. Development and validation of the Good Outcome Following Attempted Resuscitation (GO-FAR) score to predict neurologically intact survival after in-hospital cardiopulmonary resuscitation. JAMA Intern Med 2013;173:1872–8, doi: 10.1001/jamainternmed.2013.10037. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Spertus JA, Krumholz HM, et al. A validated prediction tool for initial survivors of in-hospital cardiac arrest. Arch Intern Med 2012;172:947–53, doi: 10.1001/archinternmed.2012.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocchi MN, Miller J, Hunziker S, et al. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol 2011;77:1063–71. [PubMed] [Google Scholar]
- 17.Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA 2009;302:1195–201, doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph L, Chan PS, Bradley SM, et al. Temporal Changes in the racial gap in survival after in-hospital cardiac arrest. JAMA Cardiol 2017;2:976–84, doi: 10.1001/jamacardio.2017.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]


