Abstract
Background
Clinical cross‐reactivity between bony fish, cartilaginous fish, frog, and chicken muscle has previously been demonstrated in fish‐allergic patients. In indicative studies, two reports of anaphylaxis following the consumption of crocodile meat and IgE‐cross‐binding were linked to the major fish allergen parvalbumin (PV). This study investigates IgE‐binding proteins in crocodile meat with a focus on PV and their clinical relevance.
Methods
Proteins were extracted from muscle tissue of crocodile, three bony fish, and two cartilaginous fish. A cohort of fish‐allergic pediatric patients (n = 77) underwent allergen skin prick testing (SPT) to three fish preparations (n = 77) and crocodile (n = 12). IgE‐binding proteins were identified and quantified by SDS‐PAGE, mass spectrometric analyses, and immunoblotting using commercial and in‐house antibodies, as well as individual and pooled patients’ serum. PV isoforms were purified or recombinantly expressed before immunological analyses, including human mast cell degranulation assay.
Results
Of the tissues analyzed, PV was most abundant in heated crocodile preparation, triggering an SPT of ≥3 mm in 8 of 12 (67%) fish‐allergic patients. Seventy percent (31 of 44) of fish PV‐sensitized patients demonstrated IgE‐binding to crocodile PV. Crocodile β‐PV was the major IgE‐binding protein but 20‐fold less abundant than α‐PV. Cellular reactivity was demonstrated for β‐PV and epitopes predicted, explaining frequent IgE‐cross‐binding of β‐PVs. Both PV isoforms are now registered as the first reptile allergens with the WHO/IUIS (β‐PV as Cro p 1 and α‐PV as Cro p 2).
Conclusion
Fish‐allergic individuals may be at risk of an allergy to crocodile and should seek specialist advice before consuming crocodilian meat.
Keywords: allergy diagnosis and management, component‐resolved diagnostics, cross‐reactivity, fish allergy, food allergy, reptile, skin prick testing
Key Message.
Anaphylaxis to crocodile meat, a healthy alternative to fish, has been reported in pediatric food‐allergic sufferers, and IgE‐binding demonstrated among fish‐allergic individuals. This study suggests that fish‐allergic individuals are at risk of allergic reactions when consuming crocodilian meat due to high IgE‐cross‐binding and cell stimulation capability of parvalbumin, the major fish and crocodile allergen. Both crocodile β‐ and α‐parvalbumin (Cro p 1 and Cro p 2, respectively) are now the first reptile allergens registered with the WHO/IUIS. Fish‐allergic individuals should avoid crocodilian meat unless tolerance is confirmed or following consultation with their allergist. This study provides the foundation for corresponding diagnostic tools.
1. INTRODUCTION
Fish allergy is an often life‐long condition, which affects up to 3% of the general population, and frequently results in anaphylaxis. 1 , 2 Fish usually refers to bony fish (Osteichthyes), of which over 1,000 different species are consumed worldwide. Up to 95% of fish‐allergic individuals demonstrate IgE‐binding to the major allergen in bony fish muscle, β‐parvalbumin (PV). 3 The complexity of the food commodity “fish” poses a major challenge for diagnostics and management. 4 , 5 It is often recommended that fish‐allergic individuals avoid consuming all fish and fish products once diagnosed with an allergy to any fish species, which results in significant dietary restrictions. 6 However, the capacity of fish to trigger an allergic reaction is individual‐ and species‐specific. 7 , 8 , 9 Fish‐allergic individuals can demonstrate sensitization to a narrow range of fish, which may be difficult to predict because the current diagnostic capability is generally limited to a few well‐studied species. 10 , 11 Diagnostics and management are further complicated by a range of immunological and/or clinical cross‐reactivities reported to other related vertebrates such as frog and chicken (fish–chicken syndrome). 12 , 13 , 14
Tetrapods, including reptiles, evolved from early bony fish, which are closely related to cartilaginous fish (Chondrichthyes) such as sharks and rays. Our recent investigations suggest that some cartilaginous fish can be safely consumed by many bony fish‐allergic individuals as cartilaginous fish contain primarily α‐PV, which is considerably less allergenic. 15 , 16
In 2017, the first two cases of anaphylaxis after consuming reptilian meat (crocodile) were reported in one fish‐ and one chicken‐allergic pediatric patient, and linked to PV. 17 , 18 The exact crocodile species and IgE‐cross‐binding PV isoforms are unknown, and both children had tried crocodile meat as an alternative to fish/chicken, which was strictly avoided. In addition, IgE‐binding to crocodile proteins, including presumed PVs, was described for 20 of 27 fish‐allergic patients recently. 19 Crocodilians, including alligators and crocodiles, are the most consumed reptiles worldwide, and their meat is widely available in countries where they are farmed, but also throughout Europe. 20 However, the extent and molecular foundation of food allergy safety aspects of ingesting meat from crocodilians are under‐investigated. Initiated by indicative studies, we sought to characterize the IgE‐binding proteins in crocodile meat with a focus on PV and their clinical relevance.
2. METHODS
2.1. In‐house crocodile and fish preparations
Muscle tissue was collected from saltwater crocodile (Crocodylus porosus), two bony fish, Asian seabass (Lates calcarifer) and Atlantic salmon (Salmo salar), and two cartilaginous fish, ghost shark (Callorhinchus milii) and bluespotted stingray (Neotrygon kuhlii). Proteins were extracted in phosphate‐buffered saline and skin prick test (SPT) preparations generated as described previously. 21 , 22
2.2. Fish‐allergic pediatric subjects
Seventy‐seven pediatric subjects with a history of IgE‐mediated symptoms after fish consumption (some after an open food challenge) were recruited (Table S1). All patients underwent SPT to two commercial fish preparations (tuna and salmon) and in‐house seabass preparation. Twelve fish‐allergic and four shellfish‐allergic (fish‐tolerating) individuals also underwent SPT using the heat‐treated crocodile preparation. Tuna (f40) and salmon (f41) sIgE levels were determined by ImmunoCAP (ThermoFisher).
Sera from two non‐atopic pediatric donors were used as negative controls. Ethics approval was obtained from the Sydney Children's Hospitals Network (LNR‐14/SCHN/185), and all parents gave written informed consents.
2.3. Purification and recombinant expression of PV
Crocodile and salmon PVs were purified by ammonium sulfate precipitation as described previously. 23 Some PV isoforms could not be separated by subsequent chromatography purification steps and were expressed in E. coli; Crocodile β‐ and α‐PV (https://www.ncbi.nlm.nih.gov/protein/XP_019397705 and XP_019400389, respectively, in the NCBI database (www.ncbi.nlm.nih.gov/protein)) and thornback ray (Raja clavata) α‐PV (P02630) as detailed in the supplement, and seabass β‐PV (AHW83198, Lat c 1 in the World Health Organization and the International Union of Immunological Societies (WHO/IUIS) database (www.allergen.org)) as described previously. 24
2.4. Molecular and immunological in vitro, and in silico analyses
The protein concentration for all extracts and purified PVs was estimated using the Pierce™ BCA Protein Assay kit (ThermoFisher) with bovine serum albumin as standard. Subsequently, all protein extracts were diluted to the same total protein concentration.
Gel‐electrophoresis, immunoblotting, and mass spectrometry analyses as well as degranulation assay and in silico analyses/predictions were performed as detailed in the supplement.
3. RESULTS
3.1. Patients’ characteristics and in vivo reactivity to crocodile meat
Seventy‐five of the 77 fish‐allergic patients (97%) had a positive SPT result of ≥3 mm to at least one of the three bony fish preparations (Table S1). Both tuna and salmon sIgE levels were elevated (>0.1 kU/L) in 36 of the 43 tested subjects (84%). It is unknown if any of the pediatric subjects have ever eaten crocodile meat.
Eight of 12 fish‐allergic subjects (67%) who underwent SPT to crocodile had an SPT wheal diameter of ≥3 mm, demonstrating in vivo skin reactivity (Figure 1). As atopic controls, four shellfish‐allergic individuals showed no skin reactivity on crocodile SPT (0 mm). In six subjects (50%), the wheal diameter was ≥5 mm, which is a higher threshold for a positive skin reaction suggested by Lessof et al. and Peters et al. 25 , 26 Notably, the median wheal diameter for crocodile (4.3 mm) was greater than for tuna (2.3 mm).
FIGURE 1.
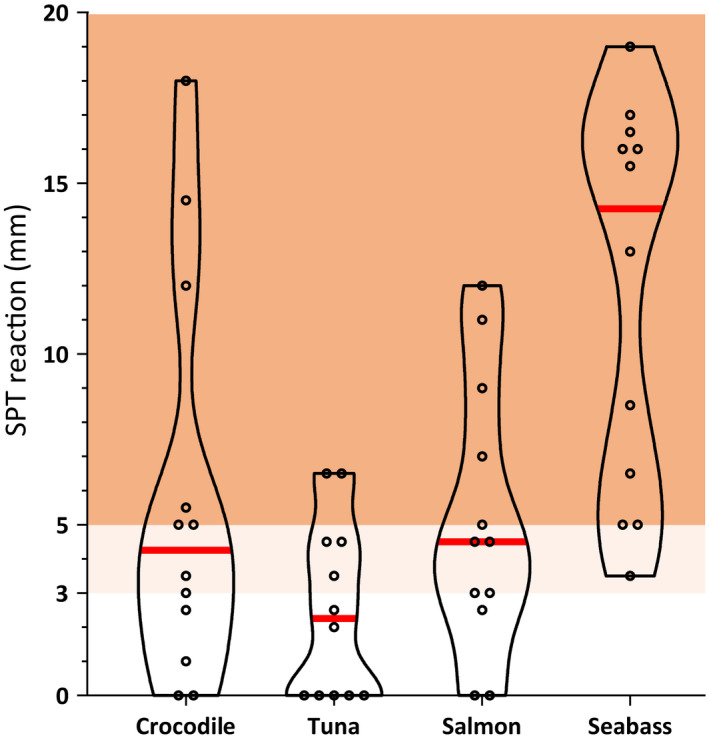
Skin prick test (SPT) reactions to preparations from crocodile and three bony fish by fish‐allergic individuals (n = 12; see Table S1). An SPT is considered positive if the wheal diameter is ≥3 mm or ≥5 mm (light and dark red areas or dark red area, respectively), depending on different clinical practice. The median for each preparation is indicated with a red line
3.2. Abundance and characteristics of crocodile PV isoforms
The SDS‐PAGE profiles of both raw and heated extracts as well as purified PV from crocodile are shown in Figure 2A. Multiple distinct bands were visible in the raw extract at 11, 13.5, 23–30, 35–48, 60, 80, and 250 kDa. In contrast, the 13.5 kDa band was the only distinct band with strong intensity in the heated extract and purified PV. Within the molecular weight range of PV (10–15 kDa), a second weak band at 11 kDa was observed.
FIGURE 2.
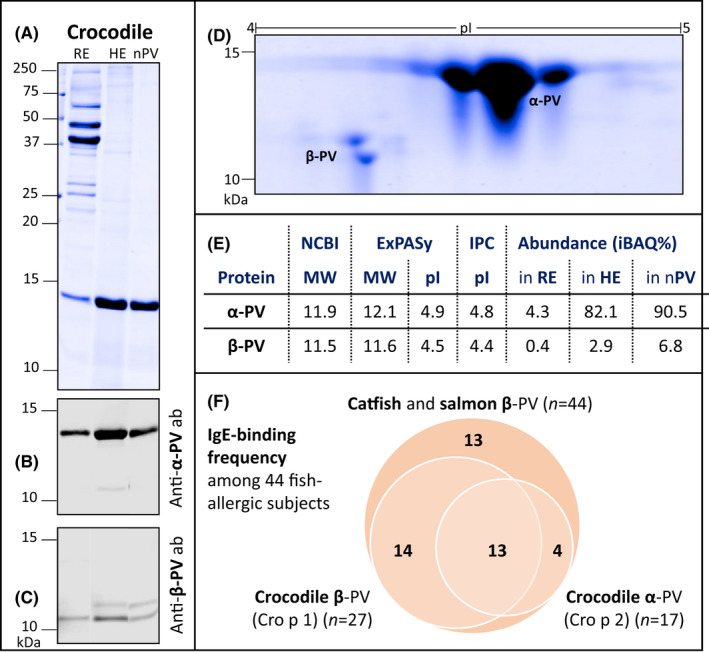
SDS‐PAGE profiles of crocodile raw (RE) and heated (HE) extracts and purified parvalbumin (nPV) (A), immunoblots with PV‐specific antibodies (ab) directed against α‐ (B) and β‐PV (C), and nPV separated by isoelectric focusing in 2‐D gel electrophoresis (D; No bands/signals visible above 15 kDa or below 10 kDa). PV isoforms were identified by mass spectrometric analyses and both molecular weight (MW) and isoelectric point (pI) were calculated (E). NCBI refers to www.ncbi.nlm.nih.gov, ExPASy to www.expasy.org, IPC to www.isoelectric.org and iBAQ% to the relative protein abundance. Frequency of IgE‐binding to crocodile α‐ and β‐parvalbumin (PV) among 44 fish‐allergic pediatric subjects with IgE‐binding to at least one PV from catfish and salmon (F) was investigated by IgE‐surfblotting (see Figure S1) and evaluated by densitometric analyses (see Table S2)
Subsequent immunoblotting suggested that the 13.5 kDa band consisted of α‐PV and the 11 kDa band of β‐PV. In the purified natural PV, the α‐PV band (13.5 kDa) was about 20‐fold more abundant compared with β‐PV and demonstrated a strong signal with the anti‐α‐PV antibody (Figure 2B). Using the anti‐β‐PV antibody, two signals at approximately 11 kDa but none at 13.5 kDa were visible (Figure 2C). The two predominant PV isoforms were further separated by their isoelectric point, demonstrating their difference in both molecular weight and isoelectric point (Figure 2D).
Mass spectrometric analyses identified the 13.5 kDa band as α‐PV (XP_019400389) and the 11 kDa band as β‐PV (XP_019400389) with a sequence coverage of 100% and 93%, respectively. Corresponding eight and six 2D‐gel spots constituted of the respective isoform. The predicted molecular weight was 11.9–12.1 kDa for α‐PV and 11.5–11.6 kDa for β‐PV (Figure 2E). In the purified crocodile PV preparation, 90.5% (iBAQ%) of proteins were α‐PV and 6.8% β‐PV, indicating a collective purity of over 95%, as also suggested by SDS‐PAGE (see nPV in Figure 2A). PV was the most abundant protein in the heated crocodile protein extract, which was further investigated for in vitro IgE‐binding as it corresponded to the preparation triggering in vivo skin reactivity (on SPT) and reflects consumption of heated crocodile meat.
3.3. Differential IgE‐binding capacity of crocodile β‐ and α‐PV
Based on our previous study, 14–49% of the 77 fish‐allergic subjects have IgE binding to PV from catfish and salmon, depending on the isoform. 22 A total of 57% of subjects (n = 77) showed IgE‐binding to at least one PV isoform. Among these 44 subjects, 31 (70%) demonstrated IgE‐binding to crocodile PV, 13 (30%) to both α‐ (13.5 kDa) and β‐ (11 kDa), 14 (32%) only to β‐, and four (9%) only to α‐PV (Figure 2F; see Figure S1 and Table S2 for corresponding immunoblot analyses). The signal to β‐PV was up to 500‐fold stronger compared with α‐PV. Among the 33 subjects with no IgE‐binding to catfish or salmon PV, four subjects (12, 39, 57, and 77) showed IgE‐binding to crocodile PV. Among all 77 fish‐allergic subjects, eight (10%) showed IgE‐binding to a 25 kDa band (myosin light chain and dimeric PV), three (4%) each to a 35 kDa (tropomyosin) and 65 kDa band, and seven (9%) to other bands. No IgE‐binding to heated crocodile extract was observed using serum from four shellfish‐allergic (fish‐tolerant) and two non‐atopic control individuals. Subsequently, the allergen names Cro p 1 and Cro p 2 were assigned for crocodile β‐ and α‐PV, respectively, and registered with the WHO/IUIS (see Figure S2 for their SDS‐PAGE profiles).
3.4. Crocodile PV induces degranulation in human mast cells
Using serum from a fish‐allergic subject, both Cro p 1 and the positive control, seabass PV (Lat c 1), induced β‐hexosaminidase release, indicating degranulation (Figure S3). The highest release was observed after treatment with 1 μg/ml PV, which was higher with Lat c 1 as compared with Cro p 1 (16.8% versus 3.4% of total). Cro p 2 was unable to produce any significant degranulation above background (0.5%).
3.5. Differential protein and PV composition in fish and crocodile extracts
Three β‐ and two α‐PV isoforms as well as corresponding heated and raw protein extracts were analyzed for their protein profile (Figure 3A). Up to three PV bands were identified by immunoblotting and mass spectrometric analyses for each species at 10–15 kDa. Monoclonal antibody PARV‐19, raised against α‐PV but known to also recognize different β‐PVs, 27 detected one PV band each for crocodile, seabass, and shark (Figure 3B). The in‐house antibody, raised against β‐PV, detected PV bands from all species except ray (Figure 3C). β‐PV isoforms were predominant in bony fish (seabass and salmon) and α‐PV isoforms in cartilaginous fish (shark and ray), whereas crocodile preparations contained considerable amounts of both β‐PV and α‐PV.
FIGURE 3.
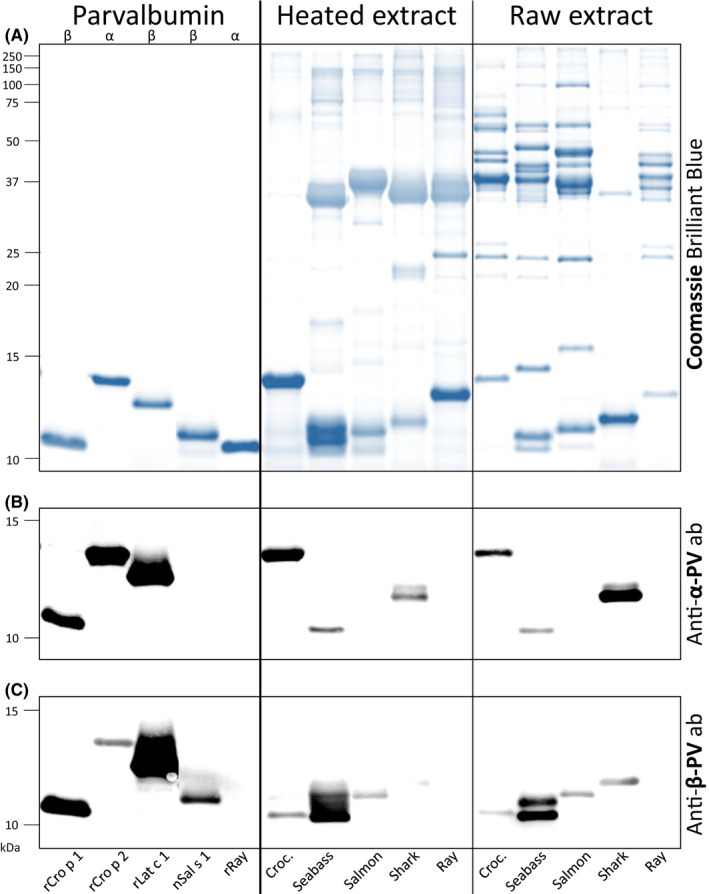
SDS‐PAGE protein profiles (A) of recombinant (r) and natural (n) purified parvalbumin (PV) isoforms with corresponding heated and raw extracts from the muscle tissue of crocodile, two bony fish (seabass and salmon) and two cartilaginous fish (shark and ray), and immunoblots with parvalbumin‐specific antibodies (ab; B and C). Note: Croc., crocodile
3.6. Differential IgE‐binding capacity of fish and crocodile
Sera from six subjects were investigated for IgE‐binding to the above‐mentioned purified PV, heated and raw extracts. The strongest signals were observed to monomeric β‐PV from crocodile (Cro p 1), seabass (Lat c 1), and salmon (Sal s 1) using a serum pool (Figure 4A). Cro p 1 showed a very weak and no signal in the heated and raw extract, respectively, in accordance with its low abundance in these extracts. The strongest IgE‐binding to a non‐parvalbumin containing band was to tropomyosin in the heated salmon extract at 37 kDa.
FIGURE 4.
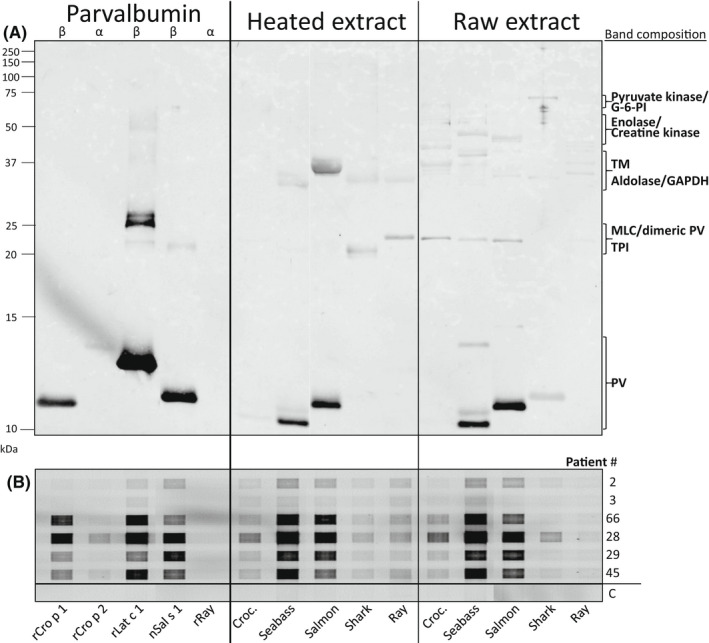
IgE‐immunoblot of recombinant (r) and natural (n) purified parvalbumin (PV) isoforms with corresponding heated and raw extracts from the muscle tissue of crocodile, two bony fish (seabass and salmon) and two cartilaginous fish (shark and ray) using a serum pool of six fish‐allergic pediatric subjects (A). The composition of protein bands corresponding to signals in the IgE‐immunoblot is based on mass spectrometric analyses and molecular weight. Individual IgE‐binding was evaluated by grid immunoblotting (B). Serum from a fish‐tolerating individual was used as negative control (C). Patient # refers to Table S1. Note: Croc., crocodile; G‐6‐PI, glucose‐6‐phosphate isomerase; TM, tropomyosin; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; MLC, myosin light chain; TPI, triosephosphate isomerase
Individual IgE‐binding capacity was evaluated by grid immunoblotting, confirming high IgE‐binding capacity for β‐ but not α‐PV isoforms also under non‐denaturing conditions (Figure 4B). Seabass and salmon β‐PV exhibited the strongest IgE‐binding for four and two subjects, respectively. IgE‐binding to crocodile β‐PV had a signal intensity of 34–75% and 31–173% compared with seabass and salmon β‐PV, respectively. Half of the subjects (3 of 6) showed weak IgE‐binding to crocodile α‐PV; 4‐ to 26‐fold weaker than the corresponding signal to any β‐PV. No IgE‐binding to ray α‐PV was observed.
3.7. Sequence comparisons
High amino acid sequence conservation (95%–100% sequence identity) among crocodilian α‐ and β‐PVs (see Figure S4) suggests that our findings for saltwater crocodile also translate to known PV isoforms from highly consumed alligator and other crocodile species. Phylogenetic analyses demonstrated two clusters, one for crocodile and cartilaginous fish α‐PV, and one for crocodile and bony fish β‐PV (Figure S5). The overall sequence identity of crocodile β‐PV with three here investigated and two commonly consumed European bony fish β‐PV is 58%–72%, which is 49%–65% with α‐PV from crocodile and two cartilaginous fish; crocodile α‐PV’s identity with fish β‐PVs is only 50%–61% (Table S3). Figure 5A shows the corresponding sequence alignment. Mapping of identical residues onto the structural model of Cro p 1 (Figure 5B and C) indicate only small surface patches of identical residues, which mainly cluster around calcium‐binding sites and could be recognized by cross‐reactive antibodies.
FIGURE 5.
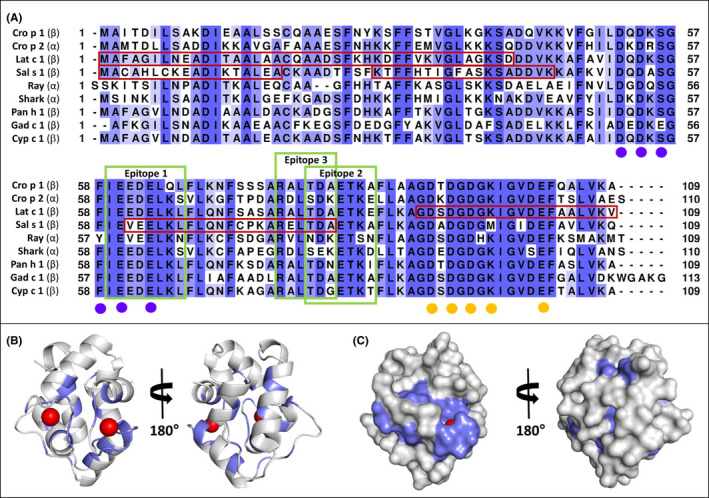
Sequence alignment of β‐parvalbumin (PV) from crocodile (Cro p 1) with crocodile α‐PV (Cro p 2) and fish‐derived β‐ and α‐PV isoforms (A; see Table S3 for information on sequence identities and similarities). Reported IgE‐binding regions for seabass β‐PV (Lat c 1) and salmon β‐PV (Sal s 1) are boxed (dark red) (see Table S4 for sequence identities in these regions). Three IgE‐binding epitopes (green boxes) were predicted in silico, which could explain observed IgE‐binding to multiple β‐PVs but not α‐PVs (refer to supporting information, including Table S5, for details of epitope predictions). Residues coordinating calcium ions are marked using purple and orange circles. Cartoon (B) and molecular surface (C) representations of Cro p 1 model with residues that are identical among proteins in the sequence alignment highlighted in slate. Calcium ions are shown as red spheres
4. DISCUSSION
Based on these findings, fish‐allergic individuals who are sensitized to the major allergen PV appear to be at risk of an allergic reaction upon consumption of meat from saltwater crocodile, and most likely other crocodilians. Seventy percent of the fish PV‐sensitized subjects demonstrated IgE‐binding to heat‐stable crocodile PV; 62% to β‐, 39% to α‐PV, and 30% to both (n = 44). Over half (67%) of the 12 fish‐allergic subjects who underwent crocodile meat skin testing had a positive in vivo SPT of ≥3 mm. Saltwater crocodile β‐PV and α‐PV were further characterized, purified from the natural source, generated recombinantly, and registered as the first reptile allergens with the WHO/IUIS as Cro p 1 and Cro p 2, respectively.
As a result of this study, we established that β‐PV is the major IgE‐binding allergen, not only in bony fish, but also in crocodile. Two distinct crocodile PV isoforms demonstrated different IgE‐binding capacities. While α‐PV (Cro p 2) was the most abundant isoform, the β‐isoform (Cro p 1) displayed more frequent and stronger recognition by IgE antibodies, probably because of higher similarity including sequence identity to fish β‐PVs, which are known primary sensitizers. Cellular reactivity, evident by human mast cell degranulation, was confirmed for the β‐isoform using serum from one subject, which should be expanded in future studies. Oral food challenges are required to further evaluate the likelihood and severity of allergic reactions upon exposure to crocodile meat. Two clinical reports of anaphylaxis to crocodile meat suggested that (α‐)PV was the likely cause of the allergic reactions. 17 , 18 Haroun‐Díaz et al. described IgE‐binding to 12 and 15 kDa bands, presumably constituting β‐ and α‐PV, by 20 and 18 fish‐allergic patients, respectively (n = 27). Similar to this and our observations in crocodile, edible frog and bullfrog β‐PV showed more frequent IgE‐binding compared with α‐PV in fish‐allergic patients. 13 , 14 However, α‐PV has been identified as the protein causing anaphylaxis after ingesting fried frog legs in a patient who demonstrated no IgE‐binding to β‐PV. 28 Another case report identified a patient with IgE‐mediated symptoms after consuming turtle meat, which was assumed to be the primary sensitizer through inhalation of cooking fumes. 29 PV was identified as the only IgE‐binding protein but not further characterized.
Amino acid sequence comparisons of over 4,000 vertebrate PVs suggest the possibility of high cross‐binding among β‐PV not only from fish and crocodilians but also from other ingested vertebrates such as snakes, lizards, turtles, and frogs (data not shown). Detailed in vitro, in silico and structure analyses should further investigate the species‐ and individual‐specific differential IgE‐binding capacity of β‐ and α‐PV. In vitro identification and characterization of epitopes could help to better understand and predict bony fish‐allergic individual's (non‐)tolerance to meat from crocodilians and other vertebrates, including cartilaginous fish. In particular, sustainably sourced rays could be a safe alternative for many fish‐allergic individuals, which requires further investigations.
Meat from amphibians and reptiles is often considered “exotic” and consumption can be region‐specific and associated with cultural occasions or traveling. 30 Crocodilians are the closest living relatives of birds, which can be primary sensitizers of food allergies or, in rare cases, also cause clinical cross‐reactivity in fish‐allergic individuals (fish‐chicken syndrome). 12
In summary, fish‐allergic individuals may be at risk of serious allergic reactions upon consumption of crocodilian meat due to high IgE‐cross‐binding of crocodile β‐PV, which we term the “fish‐crocodile syndrome.” We propose that fish‐allergic individuals should avoid the consumption of crocodilian meat unless tolerance is confirmed or following consultation with their allergist. Further research should assist with improving the accuracy of determining the clinical relevance of novel allergens, utilizing improved component‐resolved diagnostics and in vivo targeted SPT, which will allow for reduced unexpected allergic reactions and the need for oral food challenges. This study provides the molecular and clinical foundation for implementing crocodile PV in diagnostic tools.
CONFLICTS OF INTERESTS
All authors declare that they have no conflicts of interests regarding the work presented in this manuscript.
AUTHOR CONTRIBUTION
Thimo Ruethers: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Project administration (equal); Software (lead); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (lead). Roni Nugraha: Conceptualization (supporting); Formal analysis (supporting); Methodology (supporting); Software (supporting); Visualization (supporting); Writing – review & editing (supporting). Aya C. Taki: Conceptualization (supporting); Methodology (supporting); Project administration (supporting); Supervision (supporting); Writing – review & editing (supporting). Andrea O'Malley: Investigation (supporting); Visualization (supporting); Writing – review & editing (supporting). Shaymaviswanathan Karnaneedi: Investigation (supporting); Writing – review & editing (supporting). Stephanie Zang: Formal analysis (supporting); Investigation (supporting); Visualization (supporting). A. Brenda Kapingidza: Investigation (supporting); Writing – review & editing (supporting). Sam Mehr: Conceptualization (supporting); Investigation (supporting); Methodology (supporting); Resources (equal); Writing – review & editing (supporting). Sandip D. Kamath: Methodology (supporting); Resources (supporting); Supervision (supporting); Validation (supporting); Writing – review & editing (supporting). Maksymilian Chruszcz: Conceptualization (supporting); Funding acquisition (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Supervision (equal); Validation (supporting); Visualization (supporting); Writing – review & editing (supporting). Graham Mackay: Conceptualization (supporting); Funding acquisition (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Supervision (equal); Validation (supporting); Writing – review & editing (supporting). Dianne E. Campbell: Conceptualization (supporting); Funding acquisition (equal); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Supervision (equal); Writing – review & editing (supporting). Andreas L. Lopata: Conceptualization (equal); Funding acquisition (lead); Methodology (equal); Project administration (equal); Resources (lead); Supervision (lead); Validation (equal); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the Melbourne Mass Spectrometry and Proteomics Facility of The Bio21 Molecular Science and Biotechnology Institute at The University of Melbourne, Australia, for the support of mass spectrometry analysis, Dr Michael F. Sharp for generating recombinant seabass parvalbumin, Ms Trúc T. Cao for intellectual input and the support with laboratory procedures, and Dr Lirui Sun for the assistance with densitometric analyses.
Ruethers T, Nugraha R, Taki AC, et al. The first reptilian allergen and major allergen for fish‐allergic patients: Crocodile β‐parvalbumin. Pediatr Allergy Immunol. 2022;33:e13781. doi: 10.1111/pai.13781
Editor: Alexandra Santos
Funding information
Funding for this research was provided by the National Health and the Medical Research Council Australia (NHMRC; project grant GNT1086656 to AL and DC). TR and SK are PhD full‐time scholars of the Centre for Food and Allergy Research, Australia. AO, ABK, and MC were partially supported by the R01AI077653 grant from the National Institute of Allergy and Infectious Diseases, USA. SDK is an NHMRC Peter Doherty Early Career Research Fellow (GNT1124143). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of any funding bodies.
Contributor Information
Thimo Ruethers, Email: thimo.ruethers@my.jcu.edu.au.
Andreas L. Lopata, Email: andreas.lopata@jcu.edu.au.
REFERENCES
- 1. Sharp MF, Lopata AL. Fish allergy: in review. Clin Rev Allergy Immunol. 2014;46:258‐271. [DOI] [PubMed] [Google Scholar]
- 2. Tsabouri S, Triga M, Makris M, Kalogeromitros D, Church MK, Priftis KN. Fish and shellfish allergy in children: review of a persistent food allergy. Pediatr Allergy Immunol. 2012;23:608‐615. [DOI] [PubMed] [Google Scholar]
- 3. Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1‐250. [DOI] [PubMed] [Google Scholar]
- 4. Ruethers T, Taki AC, Johnston EB, et al. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28‐57. [DOI] [PubMed] [Google Scholar]
- 5. Kalic T, Radauer C, Lopata AL, Breiteneder H, Hafner C. Fish allergy around the world—precise diagnosis to facilitate patient management. Front Allergy. 2021;2:732178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis CM, Gupta RS, Aktas ON, Diaz V, Kamath SD, Lopata AL. Clinical management of seafood allergy. J Allergy Clin Immunol Pract. 2020;8:37‐44. [DOI] [PubMed] [Google Scholar]
- 7. Xepapadaki P, Christopoulou G, Stavroulakis G, et al. Natural history of IgE‐mediated fish allergy in children. J Allergy Clin Immunol Pract. 2021;9:3147‐3156.e5. [DOI] [PubMed] [Google Scholar]
- 8. Sørensen M, Kuehn A, Mills ENC, et al. Cross‐reactivity in fish allergy: a double‐blind, placebo‐controlled food‐challenge trial. J Allergy Clin Immunol. 2017;140:1170‐1172. [DOI] [PubMed] [Google Scholar]
- 9. Leung ASY, Leung NYH, Wai CYY, et al. Characteristics of Chinese fish‐allergic patients: findings from double‐blind placebo‐controlled food challenges. J Allergy Clin Immunol Pract. 2020;8:2098‐2100.e8. [DOI] [PubMed] [Google Scholar]
- 10. Mourad AA, Bahna SL. Fish‐allergic patients may be able to eat fish. Expert Rev Clin Immunol. 2015;11:419‐430. [DOI] [PubMed] [Google Scholar]
- 11. Kuehn A, Swoboda I, Arumugam K, Hilger C, Hentges F. Fish allergens at a glance: variable allergenicity of parvalbumins, the major fish allergens. Front Immunol. 2014;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuehn A, Codreanu‐Morel F, Lehners‐Weber C, et al. Cross‐reactivity to fish and chicken meat ‐ a new clinical syndrome. Allergy. 2016;71:1772‐1781. [DOI] [PubMed] [Google Scholar]
- 13. Hilger C, Thill L, Grigioni F, et al. IgE antibodies of fish allergic patients cross‐react with frog parvalbumin. Allergy. 2004;59:653‐660. [DOI] [PubMed] [Google Scholar]
- 14. Hamada Y, Nagashima Y, Shiomi K. Reactivity of serum immunoglobulin E to bullfrog Rana catesbeiana parvalbumins in fish‐allergic patients. Fish Sci. 2004;70:1137‐1143. [Google Scholar]
- 15. Kalic T, Morel‐Codreanu F, Radauer C, et al. Patients allergic to fish tolerate ray based on the low allergenicity of its parvalbumin. J Allergy Clin Immunol Pract. 2019;7(500–508):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephen JN, Sharp MF, Ruethers T, Taki A, Campbell DE, Lopata AL. Allergenicity of bony and cartilaginous fish ‐ molecular and immunological properties. Clin Exp Allergy. 2017;47:300‐312. [DOI] [PubMed] [Google Scholar]
- 17. Ballardini N, Nopp A, Hamsten C, et al. Anaphylactic reactions to novel foods: case report of a child with severe crocodile meat allergy. Pediatrics. 2017;139:e20161404. [DOI] [PubMed] [Google Scholar]
- 18. Haroun‐Díaz E, Blanca‐López N, Vázquez de la Torre M, et al. Severe anaphylaxis due to crocodile‐meat allergy exhibiting wide cross‐reactivity with fish allergens. J Allergy Clin Immunol Pract. 2018;6:669‐670.e1. [DOI] [PubMed] [Google Scholar]
- 19. Haroun‐Díaz E, Blanca‐López N, Martín‐Pedraza L, et al. Sensitization profile to related animal proteins (crocodile, frog, and chicken) among fish‐allergic patients. J Investig Allergol Clin Immunol. 2021;32:9. [DOI] [PubMed] [Google Scholar]
- 20. Klein G, Andreoletti O, Budka H, et al. Public health risks involved in the human consumption of reptile meat scientific opinion of the panel on biological hazards. EFSA J. 2007;578:1‐55. doi: 10.2903/j.efsa.2007.578 [DOI] [Google Scholar]
- 21. Ruethers T, Taki AC, Khangurha J, et al. Commercial fish ELISA kits have a limited capacity to detect different fish species and their products. J Sci Food Agric. 2020;100:4353‐4363. [DOI] [PubMed] [Google Scholar]
- 22. Ruethers T, Taki AC, Karnaneedi S, et al. Expanding the allergen repertoire of salmon and catfish. Allergy. 2021;76:1443‐1453. [DOI] [PubMed] [Google Scholar]
- 23. Ruethers T, Raith M, Sharp MF, et al. Characterization of Ras k 1 a novel major allergen in Indian mackerel and identification of parvalbumin as the major fish allergen in 33 Asia‐Pacific fish species. Clin Exp Allergy. 2018;48:452‐463. [DOI] [PubMed] [Google Scholar]
- 24. Sharp MF, Kamath SD, Koeberl M, et al. Differential IgE binding to isoallergens from Asian seabass (Lates calcarifer) in children and adults. Mol Immunol. 2014;62:77‐85. [DOI] [PubMed] [Google Scholar]
- 25. Lessof MH, Buisseret PD, Merrett J, Merrett TG, Wraith DG. Assessing the value of skin prick tests. Clin Exp Allergy. 1980;10:115‐120. [DOI] [PubMed] [Google Scholar]
- 26. Peters RL, Allen KJ, Dharmage SC, et al. Skin prick test responses and allergen‐specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132:874‐880. [DOI] [PubMed] [Google Scholar]
- 27. Saptarshi SR, Sharp MF, Kamath SD, Lopata AL. Antibody reactivity to the major fish allergen parvalbumin is determined by isoforms and impact of thermal processing. Food Chem. 2014;148:321‐328. [DOI] [PubMed] [Google Scholar]
- 28. Hilger C, Grigioni F, Thill L, Mertens L, Hentges F. Severe IgE‐mediated anaphylaxis following consumption of fried frog legs: definition of alpha‐parvalbumin as the allergen in cause. Allergy. 2002;57:1053‐1058. [DOI] [PubMed] [Google Scholar]
- 29. Jo R, Ito T, Egusa C, et al. A case of immediate type of food allergy due to parvalbumin from soft‐shelled turtle (Trionychidae) occurring in the working environment. J Eur Acad Dermatol Venereol. 2016;30:1419‐1420. [DOI] [PubMed] [Google Scholar]
- 30. Cawthorn D‐M, Hoffman LC. Controversial cuisine: a global account of the demand, supply and acceptance of “unconventional” and “exotic” meats. Meat Sci. 2016;120:19‐36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


