Abstract
Objectives
To assess real‐world effectiveness, safety, and usage of erenumab in Canadian patients with episodic and chronic migraine with prior ineffective prophylactic treatments.
Background
In randomized controlled trials, erenumab demonstrated efficacy for migraine prevention in patients with ≤4 prior ineffective prophylactic migraine therapies. The “Migraine prevention with AimoviG: Informative Canadian real‐world study” (MAGIC) assessed real‐world effectiveness of erenumab in Canadian patients with migraine.
Methods
MAGIC was a prospective open‐label, observational study conducted in Canadian patients with chronic migraine (CM) and episodic migraine (EM) with two to six categories of prior ineffective prophylactic therapies. Participants were administered 70 mg or 140 mg erenumab monthly based on physician’s assessment. Migraine attacks were self‐assessed using an electronic diary and patient‐reported outcome questionnaires. The primary outcome was the proportion of subjects achieving ≥50% reduction in monthly migraine days (MMD) after the 3‐month treatment period.
Results
Among the 95 participants who mostly experienced two (54.7%) or three (32.6%) prior categories of ineffective prophylactic therapies and who initiated erenumab, treatment was generally safe and well tolerated; 89/95 (93.7%) participants initiated treatment with 140 mg erenumab. At week 12, 32/95 (33.7%) participants including 17/64 (26.6%) CM and 15/32 (48.4%) EM achieved ≥50% reduction in MMD while 30/86 (34.9%) participants including 19/55 (34.5%) CM and 11/31 (35.5%) EM achieved ≥50% reduction in MMD at week 24. Through patient‐reported outcome questionnaires, 62/95 (65.3%) and 45/86 (52.3%) participants reported improvement of their condition at weeks 12 and 24, respectively. Physicians observed improvement in the condition of 78/95 (82.1%) and 67/86 (77.9%) participants at weeks 12 and 24, respectively.
Conclusion
One‐third of patients with EM and CM achieved ≥50% MMD reduction after 3 months of erenumab treatment. This study provides real‐world evidence of erenumab effectiveness, safety, and usage for migraine prevention in adult Canadian patients with multiple prior ineffective prophylactic treatments.
Keywords: chronic migraine, effectiveness, episodic migraine, erenumab, real‐world
Abbreviations
- AE
adverse event
- CGI‐I
Clinical Global Impressions‐Improvement
- CGI‐S
Clinical Global Impressions‐Severity
- CM
chronic migraine
- EM
episodic migraine
- IQR
interquartile range
- MHD
monthly headache days
- MMD
monthly migraine days
- MSMD
monthly migraine‐specific medication treatment days
- MSQ
Migraine‐Specific Quality of Life Questionnaire
- P‐GIC
Patient’s Global Impression of Change
- PRO
patient‐reported outcome
- RCT
randomized controlled trial
- SAE
serious adverse event
- SD
standard deviation
INTRODUCTION
Migraine, a chronic neurological disorder affecting 1.04 billion individuals worldwide, is characterized by moderate or severe headache attacks and reversible associated symptoms such as photophobia, phonophobia, and nausea. 1 Patients with chronic and episodic migraine (CM and EM) are treated prophylactically with various drug classes, many of which are used off‐label, with limited clinical evidence. Thus, they have variable efficacy and substantial tolerability issues that often lead to discontinuation. 2
Erenumab is a first‐in‐class fully human monoclonal antibody targeting the calcitonin gene‐related peptide receptor. 3 , 4 , 5 , 6 Randomized controlled trials (RCTs) demonstrated a clinically meaningful reduction in monthly migraine days (MMD), monthly headache days (MHD), and monthly migraine‐specific medication treatment days (MSMD) in erenumab‐treated patients with migraine. 7 , 8 , 9 , 10 , 11 While erenumab RCTs included many patients with no prior ineffective prophylactic migraine treatments and included many others with fewer than two prior ineffective migraine prophylactic treatments, a recent randomized double‐blind placebo‐controlled study has shown efficacy for erenumab in patients with EM who had experienced inadequate efficacy of two to four migraine prophylactic drugs. 12 , 13 However, real‐world evidence of erenumab effectiveness, safety, and usage in Canada remains largely unreported. Here, we report the primary analysis and results of “Migraine prevention with AimoviG: Informative Canadian real‐world study” (MAGIC), a real‐world, prospective, open‐label, observational study conducted in Canadian patients with CM and EM, all of whom had experienced inadequate efficacy with at least two prophylactic treatment categories.
METHODS
Protocol approvals and participant consents
An independent central ethics committee (Advarra) and local ethics committees approved the protocol. Between April 4, 2019, and April 3, 2020, 15 Canadian sites (Table S1) recruited participants who provided written informed consent. All procedures complied with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Participant overview and study design
MAGIC was a real‐world, observational, prospective, open‐label, two‐treatment, three‐period study of 70 mg and 140 mg erenumab administered monthly as per routine medical practice determined by the prescribing physician, independent of study participation. Patients aged 18 to 65 years with CM (≥15 MHD, of which ≥8 qualify as migraine days) and EM (<15 MHD) 14 were recruited in a 2:1 ratio. To be enrolled, patients must have previously experienced inadequate efficacy with two to six categories of prophylactic migraine therapies within 5 years prior to enrollment. The study planned to recruit 440 subjects but due to enrollment difficulties in part attributable to the 2020 COVID‐19 pandemic, enrollment was terminated at 131 participants.
Participants were enrolled at the screening visit (week ‐4) and completed daily migraine assessments on an eDiary application during a 28‐day screening period (Figure 1). As medical history could have been obtained through participant interviews, it may be subject to recall bias. To initiate erenumab therapy at the baseline visit (week 0), participants were required to have ≥6 MMD and demonstrate ≥80% eDiary compliance at the end of the screening period. All eligibility criteria are listed in the Supporting Methods. Erenumab‐treated participants completed the eDiary until treatment discontinuation or end of treatment period 1 (week 12), whichever occurred first. At week 12, the physician and participant decided whether to terminate or continue treatment for three additional months based on treatment response. If deemed adequate, participants continued erenumab treatment and completed the eDiary until the end of treatment period 2 (week 24).
FIGURE 1.
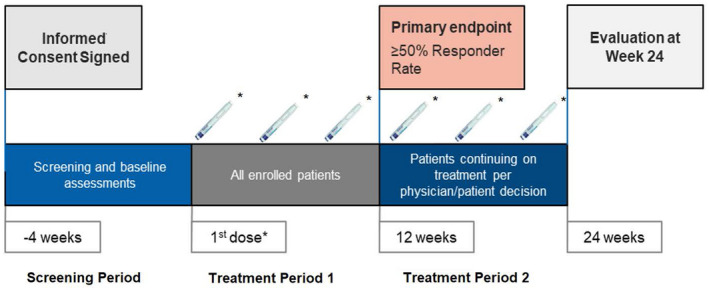
Study design. *70 mg or 140 mg erenumab decided at the prescribing physician’s discretion
Study objectives
MAGIC aimed to assess the real‐world effectiveness of 70 mg or 140 mg erenumab measured as ≥50% reduction in MMD at week 12 from baseline. The secondary objectives were to evaluate erenumab effectiveness at week 24 from baseline; change in MMD from baseline; patient‐reported outcomes (PROs) at weeks 12 and 24; and the Clinical Global Impressions‐Severity scale (CGI‐S) and the Clinical Global Impressions‐Improvement scale (CGI‐I) at weeks 12 and 24, respectively. Real‐world erenumab safety profile was also evaluated.
Assessment methods
Participant characteristics and medical history were obtained from patient medical charts. Daily assessments of migraine attacks and rescue medication use were self‐reported using the eDiary (see Supporting Methods). At baseline, and weeks 12 and 24, participants were invited to complete the Migraine‐Specific Quality of Life Questionnaire (MSQ), 15 while physicians were asked to complete the CGI‐S 16 scale. Overall change in patients’ condition was assessed by participants and physicians using the Patient Global Impression of Change (P‐GIC) 17 and CGI‐I 16 scales at weeks 12 and 24.
Statistical analysis
Baseline demographics and clinical data were reported for all participants as n (%) or mean (standard deviation [SD]) and median (interquartile range [IQR]), as appropriate. Categorical outcomes were reported as n (%) of all participants in the study at the timepoint of interest regardless of whether the data were missing or not. For outcomes for which change was reported (e.g., from baseline to week 12), descriptive statistics for each timepoint were reported only for participants with all available values. Missing data were not imputed, except for monthly prorating of variables (e.g., MMD; see Supporting Methods). In addition, all patients who could potentially complete the eDiary, including those for whom the data were missing, were included in the denominator for each calculation of response rate at week 12 and week 24, except those patients who discontinued or withdrew from the study beforehand. Therefore, patients with missing data at each timepoint were implicitly considered as not achieving the 50% response rate. Analyses were conducted using SAS® version 9.4 (SAS Institute). All presented analyses are part of the primary analysis of the MAGIC study dataset; they were preplanned and documented in the study statistical analysis plan, which was completed prior to data analysis.
RESULTS
Subject characteristics
Among 131 subjects recruited, one never registered their eDiary, 35 withdrew or failed screening, and 95 initiated erenumab therapy, 86 of whom continued treatment beyond week 12 (Figure 2). Participants were on average 41.4 years old at enrollment; 80.0% were female; and as expected, the majority were diagnosed with CM (67.4%). Most participants had previously experienced inadequate efficacy with two (54.7%) or three (32.6%) categories of prophylactic migraine therapies and one participant (1.1%) had a similar experience with more than four. Eighty‐nine (93.7%) subjects initiated erenumab at 140 mg. Table 1 lists additional patient characteristics.
FIGURE 2.
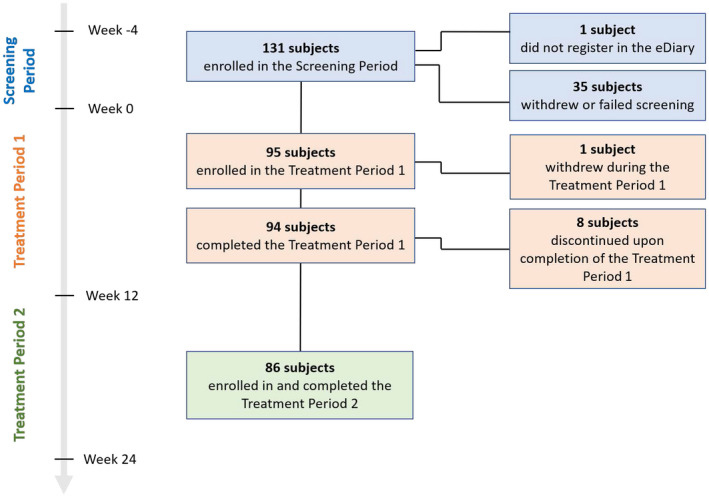
Subject disposition
TABLE 1.
Baseline characteristics
| Participants (N = 95) | |
|---|---|
| Sex, n (%) | |
| Female | 76 (80.0) |
| Male | 19 (20.0) |
| Age at enrollment (years) | |
| Mean (SD) | 41.4 (11.3) |
| Median (IQR) | 42.8 (32.1, 50.8) |
| Number missing | 0 |
| Age at migraine onset (years) | |
| Mean (SD) | 19.4 (10.7) |
| Median (IQR) | 17.4 (11.8, 24.5) |
| Number missing | 0 |
| Age at migraine diagnosis (years) | |
| Mean (SD) | 27.1 (10.9) |
| Median (IQR) | 26.5 (19.5, 33.8) |
| Number missing | 2 |
| Migraine type, n (%) | |
| Chronic migraine | 64 (67.4) |
| Episodic migraine | 31 (32.6) |
| Erenumab dose at initiation, n (%) | |
| 70 mg | 6 (6.3) |
| 140 mg | 89 (93.7) |
| Medication overuse during screening period, n (%) | |
| No | 27 (28.4) |
| Yes | 68 (71.6) |
| Migraine with aura, n (%) | |
| Never | 36 (37.9) |
| Rarely | 17 (17.9) |
| Unsure | 1 (1.1) |
| Yes—always | 9 (9.5) |
| Yes—sometimes | 32 (33.7) |
| Number of prior categories of prophylactic migraine therapies with inadequate efficacy, n (%) | |
| 2 | 52 (54.7) |
| 3 | 31 (32.6) |
| 4 | 11 (11.6) |
| 5 | 0 (0.0) |
| 6 | 1 (1.1) |
| Type of prior categories of prophylactic migraine therapies with inadequate efficacy, n (%) | |
| Divalproex sodium, sodium valproate | 3 (3.2) |
| Topiramate | 64 (67.4) |
| Beta blockers | 41 (43.2) |
| Tricyclic antidepressants and venlafaxine | 78 (82.1) |
| Flunarizine or verapamil | 12 (12.6) |
| Candesartan or lisinopril | 28 (29.5) |
| Pizotifen | 1 (1.1) |
| Botulinum toxin | 20 (21.1) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Primary outcome analysis
At week 12 from baseline, 32 participants (33.7%) experienced ≥50% reduction in MMD (Table 2).
TABLE 2.
Treatment effectiveness
| Screening period baseline (N = 95) | Week 12 (n = 95) | Week 24 (n = 86) | |
|---|---|---|---|
| ≥50% reduction in MMD, n (%) | |||
| No | – | 54 (56.8) | 43 (50.0) |
| Yes | – | 32 (33.7) | 30 (34.9) |
| Number missing | – | 9 (9.5) | 13 (15.1) |
| MMD | |||
| Mean (SD) | 15.7 (6.1) | 10.4 (7.7) | 9.7 (6.7) |
| Median (IQR) | 14.0 (11.2, 19.0) | 9.0 (4.0, 14.6) | 8.52 (4.7, 12.3) |
| Number missing, n (%) | 0 | 9 (9.5) | 13 (15.1) |
| MMD changes from baseline | |||
| Mean (SD) b | – | −4.9 (5.8) | −5.7 (6.1) |
| Median (IQR) | – | −4.6 (−8.0, −1.0) | −5.0 (−9.3, −1.8) |
| Number missing, n (%) | – | 9 (9.5) | 13 (15.1) |
| P‐GIC, n (%) | |||
| 1‐Very much improved | – | 11 (11.6) | 15 (17.4) |
| 2‐Much improved | – | 28 (29.5) | 20 (23.3) |
| 3‐Minimally improved | – | 23 (24.2) | 10 (11.6) |
| 4‐No change | – | 7 (7.4) | 5 (5.8) |
| 5‐Minimally worse | – | 2 (2.1) | 1 (1.2) |
| 6‐Much worse | – | 1 (1.1) | 0 (0.0) |
| Number missing | – | 23 (24.2) | 35 (40.7) |
| CGI‐S a , n (%) | |||
| 0‐Not assessed | 1 (1.1) | 5 (5.3) | 3 (3.5) |
| 1‐Normal, not at all ill | 28 (29.5) | 23 (24.2) | 21 (24.4) |
| 2‐Borderline mentally ill | 1 (1.1) | 16 (16.8) | 19 (22.1) |
| 3‐Mildly ill | 15 (15.8) | 22 (23.2) | 18 (20.9) |
| 4‐Moderately ill | 29 (30.5) | 23 (24.2) | 16 (18.6) |
| 5‐Markedly ill | 12 (12.6) | 3 (3.2) | 2 (2.3) |
| 6‐Severely ill | 7 (7.4) | 1 (1.1) | 1 (1.2) |
| 7‐Among the most extremely ill patients | 2 (2.1) | 1 (1.1) | 0 (0.0) |
| Number missing | 0 (0.0) | 1 (1.1) | 6 (7.0) |
| CGI‐I, n (%) | |||
| 0‐Not assessed | – | 2 (2.1) | 2 (2.3) |
| 1‐Very much improved | – | 24 (25.3) | 27 (31.4) |
| 2‐Much improved | – | 37 (38.9) | 25 (29.1) |
| 3‐Minimally improved | – | 17 (17.9) | 15 (17.4) |
| 4‐No change | – | 14 (14.7) | 9 (10.5) |
| 5‐Minimally worse | – | 0 (0.0) | 2 (2.3) |
| Number missing | – | 1 (1.1) | 6 (7.0) |
Abbreviations: CGI‐I, Clinical Global Impressions‐Improvement; CGI‐S, Clinical Global Impressions‐Severity; IQR, interquartile range; MMD, monthly migraine days; P‐GIC, Patient Global Impression of Change; SD, standard deviation.
CGI‐S was used to assess the severity of psychopathology related to migraine.
Changes from baseline were calculated only for patients where both the data at baseline and other timepoints (12 and 24 weeks) were available.
Erenumab safety
Erenumab was generally safe and well tolerated. Overall, 34 adverse events (AEs) were reported in 23 participants (24.0%). Most AEs (88.2%) were assessed as mild and no action was taken for 67.7% of AEs. Eight AEs related to constipation and an additional mild case of rectal hemorrhage, which was classified as a serious AE (SAE), were considered to have a suspected causal relationship with erenumab. In addition, no AEs related to cardiac or vascular disorders were reported in this study despite the fact that 7.0% of the patients enrolled had pre‐existing cardiovascular comorbidities, such as atrial fibrillation, supraventricular tachycardia, and hypertension (Table S2). A detailed summary of AEs/SAEs is provided in Tables 3 and S3.
TABLE 3.
Summary of adverse events
| All AEs | SAEs | |||
|---|---|---|---|---|
| Participants (N = 96) a | Events (n = 34) | Participants (N = 96) a | Events (n = 1) | |
| Any AE, n (%) | ||||
| Yes | 23 (24.0) | 34 (100.0) | 1 (1.0) | 1 (100.0) |
| Status, n (%) | ||||
| Ended | 12 (12.5) | 19 (55.9) | 1 (1.0) | 1 (100.0) |
| Ongoing | 11 (11.5) | 15 (44.1) | 0 (0.0) | 0 (0.0) |
| Severity, n (%) | ||||
| Mild | 20 (20.8) | 30 (88.2) | 1 (1.0) | 1 (100.0) |
| Moderate | 3 (3.1) | 3 (8.8) | 0 (0.0) | 0 (0.0) |
| Number of missing | 1 (1.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Action taken with erenumab drug, n (%) | ||||
| Concomitant drug taken | 4 (4.2) | 7 (20.6) | 1 (1.0) | 1 (100.0) |
| Erenumab permanently discontinued due to this adverse event | 3 (3.1) | 3 (8.8) | 0 (0.0) | 0 (0.0) |
| No action taken | 16 (16.7) | 23 (67.7) | 0 (0.0) | 0 (0.0) |
| Non‐drug therapy given | 1 (1.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| What was the outcome of the subject/adverse event, n (%) | ||||
| Completely recovered | 11 (11.5) | 18 (52.9) | 1 (1.0) | 1 (100.0) |
| Condition improving | 7 (7.3) | 7 (20.6) | 0 (0.0) | 0 (0.0) |
| Condition still present and unchanged | 7 (7.3) | 9 (26.5) | 0 (0.0) | 0 (0.0) |
| Subject recovered with sequelae, n (%) | ||||
| No | 9 (9.4) | 15 (83.3) | 1 (1.0) | 1 (100.0) |
| Yes | 2 (2.1) | 2 (11.1) | 0 (0.0) | 0 (0.0) |
| Number of missing | 1 (1.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| Assessment of causality to erenumab, n (%) | ||||
| Not suspected | 9 (9.4) | 12 (35.3) | 0 (0.0) | 0 (0.0) |
| Suspected | 15 (15.6) | 22 (64.7) | 1 (1.0) | 1 (100.0) |
| AE seriousness assessment, n (%) | ||||
| Death | 0 (0.0) | – | 0 (0.0) | – |
| Life threatening | 0 (0.0) | – | 0 (0.0) | – |
| Involved or prolonged inpatient hospitalization | 0 (0.0) | – | 0 (0.0) | – |
| Involved persistent or significant disability or incapacity | 0 (0.0) | – | 0 (0.0) | – |
| Other seriousness criteria | 1 (1.0) b | – | 1 (1.0) b | – |
| Congenital anomaly/birth defect | 0 (0.0) | – | 0 (0.0) | – |
| Other significant medical events | 0 (0.0) | – | 0 (0.0) | – |
Abbreviations: AE, adverse event; SAE, serious adverse event.
One subject received erenumab before the end of the screening period and was therefore not eligible for this study. However, this subject was included in the safety analyses.
One mild case of rectal hemorrhage was reported as an SAE.
Secondary outcome analyses
MMD, MHD, and monthly acute MSMD
At week 12, 26.6% of participants with CM (n = 17) and 48.4% of those with EM (n = 15) achieved ≥50% reduction in MMD. At week 24, 34.5% with CM (n = 19) and 35.5% with EM (n = 11) achieved ≥50% reduction in MMD. Table S4 describes the change in MMD from baseline and ≥50% reduction in MMD stratified by erenumab dose and number of previous prophylactic migraine therapy categories with inadequate efficacy. On average, there was a reduction of 4.9 (SD 5.8) MMDs at week 12 and 5.7 (SD 6.1) MMDs at week 24 from baseline (15.7 [SD 6.1] MMDs; Table 2). Subjects achieved a mean MHD reduction of 4.9 (SD 5.8) and 6.1 (SD 6.1) at weeks 12 and 24, respectively. While 71.6% of the participants met the criteria for acute medication overuse at erenumab initiation, monthly acute MSMD decreased on average by 2.7 days (SD 4.0) and 2.7 days (SD 3.6) at weeks 12 and 24, respectively (Table S5).
PROs
As described in Table 2, 62 (65.3%) and 45 (52.3%) participants reported improvement using the P‐GIC self‐assessment score at weeks 12 and 24, respectively. MSQ‐reported scores aligned with these observations (Table S6). In both instances, however, missing PRO responses increased at follow‐up timepoints.
Physician assessments of migraine severity and improvement
Physicians assessed 50 (52.6%) subjects as moderately to extremely ill at baseline. Twenty‐eight (29.5%) and 19 (22.1%) participants were assessed as moderately to extremely ill at weeks 12 and 24, respectively (Table 2). Assessing patient improvement using CGI‐I, physicians perceived improvement from baseline in the condition of 78 (82.1%) and 67 (77.9%) participants at weeks 12 and 24, respectively (Table 2).
DISCUSSION
Current therapeutic migraine management includes off‐label use of various preventive medication classes. Pivotal trials suggest that erenumab is an option for patients with difficult‐to‐treat migraine. 12 , 18 , 19 , 20 MAGIC aimed to assess the real‐world effectiveness, safety, and usage of erenumab in Canadian patients with CM and EM who previously experienced inadequate efficacy with two to six categories of migraine prophylactic therapies.
The reduction in MMD and monthly acute MSMD observed in erenumab‐treated subjects in MAGIC aligned with observations from RCTs. 9 , 12 The proportion of participants with EM experiencing ≥50% MMD reduction at week 12 (48.4%) was similar to what was observed in the STRIVE trial (41.3% in the 70 mg group and 48.1% in the 140 mg group) 8 and higher than that reported in the LIBERTY trial (30.0% of patients with EM treated with 140 mg erenumab). 12 The proportion of participants with CM experiencing ≥50% MMD reduction at week 12 in MAGIC (26.6%) differed from what was observed in a previous RCT (41.0%). 9 Differences in study design may explain the observed discrepancy in effectiveness. Importantly, both participants and physicians reported improvement in ≥50% of treated subjects over the treatment period. This finding aligns with a recent real‐life case series in which 50% of erenumab users reported ≥50% reduction in migraine frequency after 12 months of treatment. 21
While MAGIC allowed the enrollment of real‐world patients who previously experienced inadequate efficacy with two to six categories of migraine prophylactic therapies, most participants had only had such occurrences with two or three categories of prophylactic migraine therapies. These observations contrast with another recent real‐world report in which the participants had experienced inadequate effectiveness with, on average, 4.8 categories of prophylactic therapies. 22 Differences in study designs and eligibility criteria used in the two studies may explain the discrepancy in the baseline characteristics of enrolled patients. In fact, while MAGIC included 32.6% of patients with EM, patients with EM represented 5.9% of screened patients and none of the patients included in the primary analysis group presented by Robblee et al. 22 Furthermore, Robblee and colleagues also enrolled patients with more diverse conditions, such as hemiplegic and posttraumatic migraine, which were excluded in MAGIC. Despite these differences, the results from MAGIC and Robblee et al.’s study provide complementary data supporting the real‐world effectiveness of erenumab for patients with EM and CM and diverse clinical characteristics at treatment initiation.
Furthermore, the data from MAGIC contribute additional insights on the real‐world effectiveness of erenumab in patients with migraine who primarily have CM and who have previously experienced inadequate efficacy with at least two prophylactic drugs. These patients are therefore likely to have more severe disease than those enrolled in the Reuter et al. erenumab RCT, which only included patients with EM. 12 MAGIC participants are also likely to be more severely affected than those in pivotal trials because of the broader selection criteria that allowed inclusion of patients with CM experiencing continuous pain, as well as patients with several comorbidities, such as psychiatric disorders and cardiovascular diseases.
As anticipated from a systematic review and meta‐analysis of RCTs, 23 erenumab was generally safe. Safety findings from MAGIC align with the safety and tolerability of erenumab described in prior RCTs. 9 , 12
Because MAGIC was a real‐world observational study, some limitations inherent to this type of study were observed. For instance, the proportion of missing daily assessments increased as the study progressed, possibly due to respondent fatigue, eDiary technical issues, or a decrease in the user’s desire to share their experience. However, statistics for each timepoint were reported only for participants with all available values. In addition, the use of an eDiary may have biased the enrolled population toward individuals with higher‐than‐average socioeconomic status and proficiency using electronic devices.
Data from this study are mainly generalizable to Canada and representative of real‐world treatment.
CONCLUSION
The results of the real‐world Canadian MAGIC study suggest that, when administered in patients with CM and EM who experienced repeated ineffective prophylactic migraine therapy, erenumab treatment was safe and resulted in ≥50% MMD reduction in 33.7% of participants. The current findings appear to confirm and complement the data obtained from pivotal clinical trials of erenumab and recent real‐world studies.
CONFLICT OF INTEREST
Novartis Pharmaceuticals Canada Inc. funded the study. The sponsor was involved in the design, interpretation, and reporting of study results. AA and GSP were full‐time employees of IQVIA Solutions Canada Inc. at the time of the study. JKM and JM are full‐time employees of IQVIA Solutions Canada Inc. DR, AF, and NB are full‐time employees of Novartis Pharmaceuticals Canada Inc. WJB has served on medical advisory boards or received speaker honoraria from Allergan, Novartis Pharmaceuticals Inc., Weber and Weber, Teva, Eli Lilly, and Lundbeck. SS has received speaker honoraria from Abbvie, Miravo, Novartis Pharmaceuticals Inc., Teva, Eli Lilly, and Lundbeck. RG has served on medical advisory boards or received speaker honoraria from Allergan, Novartis Pharmaceuticals Inc., Teva, Eli Lilly, and Lundbeck. EL, JG, and SC have received consultation fees from Novartis Pharmaceuticals Inc.
AUTHOR CONTRIBUTIONS
Study concept and design: Werner J. Becker, Sian Spacey, Elizabeth Leroux, Rose Giammarco, Jonathan Gladstone, Suzanne Christie, Arash Akaberi, G. Sarah Power, Jagdeep K. Minhas, Johanna Mancini, Driss Rochdi, Ayca Filiz, Natacha Bastien. Acquisition of data: Rose Giammarco, Suzanne Christie. Analysis and interpretation of data: Werner J. Becker, Sian Spacey, Elizabeth Leroux, Rose Giammarco, Jonathan Gladstone, Suzanne Christie, Arash Akaberi, G. Sarah Power, Jagdeep K. Minhas, Johanna Mancini, Driss Rochdi, Ayca Filiz, Natacha Bastien. Drafting of the manuscript: Werner J. Becker, Arash Akaberi, G. Sarah Power, Jagdeep K. Minhas, Johanna Mancini, Driss Rochdi, Ayca Filiz, Natacha Bastien. Revising for intellectual content: Werner J. Becker, Sian Spacey, Elizabeth Leroux, Rose Giammarco, Jonathan Gladstone, Suzanne Christie, Arash Akaberi, G. Sarah Power, Jagdeep K. Minhas, Johanna Mancini, Driss Rochdi, Ayca Filiz, Natacha Bastien. Final approval of the completed manuscript: Werner J. Becker, Sian Spacey, Elizabeth Leroux, Rose Giammarco, Jonathan Gladstone, Suzanne Christie, Arash Akaberi, G. Sarah Power, Jagdeep K. Minhas, Johanna Mancini, Driss Rochdi, Ayca Filiz, Natacha Bastien.
Supporting information
Supplementary Material
Becker WJ, Spacey S, Leroux E, et al. A real‐world, observational study of erenumab for migraine prevention in Canadian patients. Headache. 2022;62:522–529. doi: 10.1111/head.14291
REFERENCES
- 1. Dodick DW. Migraine. Lancet. 2018;391:1315‐1330. [DOI] [PubMed] [Google Scholar]
- 2. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS‐II). Headache. 2013;53:644‐655. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Yue TL, Barone FC, et al. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc Natl Acad Sci U S A. 1995;92:11480‐11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmermann U, Fischer JA, Frei K, Fischer AH, Reinscheid RK, Muff R. Identification of adrenomedullin receptors in cultured rat astrocytes and in neuroblastboma x glioma hybrid cells (NG108‐15). Brain Res. 1996;724:238‐245. [DOI] [PubMed] [Google Scholar]
- 5. Durham PL. Calcitonin gene‐related peptide (CGRP) and migraine. Headache. 2006;46(suppl 1):S3‐S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi L, Lehto SG, Zhu DXD, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther. 2016;356:223‐231. [DOI] [PubMed] [Google Scholar]
- 7. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026‐1037. [DOI] [PubMed] [Google Scholar]
- 8. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 9. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16:425‐434. [DOI] [PubMed] [Google Scholar]
- 10. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol. 2016;15:382‐390. [DOI] [PubMed] [Google Scholar]
- 11. Broessner G, Reuter U, Bonner JH, et al. The spectrum of response to erenumab in patients with episodic migraine and subgroup analysis of patients achieving >/=50%, >/=75%, and 100% response. Headache. 2020;60:2026‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reuter U, Goadsby PJ, Lanteri‐Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two‐to‐four previous preventive treatments were unsuccessful: a randomised, double‐blind, placebo‐controlled, phase 3b study. Lancet. 2018;392:2280‐2287. [DOI] [PubMed] [Google Scholar]
- 13. Ashina M, Tepper S, Brandes JL, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double‐blind, placebo‐controlled study. Cephalalgia. 2018;38:1611‐1621. [DOI] [PubMed] [Google Scholar]
- 14. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 15. Jhingran P, Osterhaus JT, Miller DW, Lee JT, Kirchdoerfer L. Development and validation of the Migraine‐Specific Quality of Life Questionnaire. Headache. 1998;38:295‐302. [DOI] [PubMed] [Google Scholar]
- 16. Guy W. ECDEU Assessment Manual for Psychopharmacology. U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 17. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27:26‐35. [DOI] [PubMed] [Google Scholar]
- 18. Stewart WF, Wood C, Reed ML, Roy J, Lipton RB, AMPP Advisory Group . Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170‐1178. [DOI] [PubMed] [Google Scholar]
- 19. Queiroz LP, Peres M, Piovesan EJ, et al. A nationwide population‐based study of migraine in Brazil. Cephalalgia. 2009;29:642‐649. [DOI] [PubMed] [Google Scholar]
- 20. Kim BK, Chung YK, Kim JM, Lee KS, Chu MK. Prevalence, clinical characteristics and disability of migraine and probable migraine: a nationwide population‐based survey in Korea. Cephalalgia. 2013;33:1106‐1116. [DOI] [PubMed] [Google Scholar]
- 21. Eghtesadi M, Leroux E, Pagé G. Real‐life response to erenumab in a therapy‐resistant case series of migraine patients from the Province of Québec, Eastern Canada. Clin Drug Investig. 2021;41:733‐739. [DOI] [PubMed] [Google Scholar]
- 22. Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real‐world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60:2014‐2025. [DOI] [PubMed] [Google Scholar]
- 23. Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine: a systematic review and meta‐analysis of randomized clinical trials. Medicine (Baltimore). 2019;98:e18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


