Abstract
Objective
To validate the Clinical Frailty Scale (CFS) for prediction of 1‐year all‐cause mortality in the emergency department (ED) and compare its performance to the Emergency Severity Index (ESI).
Methods
Prospective cohort study at the ED of a tertiary care center in Northwestern Switzerland. All patients aged ≥65 years were included from March 18 to May 20, 2019, after informed consent. Frailty status was assessed using CFS, excluding level 9 (palliative). Acuity level was assessed using ESI. Both CFS and ESI were adjusted for age, sex and presenting condition in multivariable logistic regression. Prognostic performance was assessed for discrimination and calibration separately. Estimates were internally validated by Bootstrapping. Restricted mean survival time (RMST) was determined for all levels of CFS.
Results
In the final study population of 2191 patients, 1‐year all‐cause mortality was 17% (n = 372). RMST values ranged from 219 days for CFS 8 to 365 days for CFS 1. The adjusted CFS model had an area under receiver operating characteristic of 0.767 (95% confidence interval [CI]: 0.741–0.793), compared to 0.703 (95% CI: 0.673–0.732) for the adjusted ESI model.
Conclusion
The CFS predicts 1‐year all‐cause mortality for older ED patients and predicts survival time in a graded manner. The CFS is superior to the ESI when adjusted for age, sex, and presenting condition.
INTRODUCTION
Frailty is a state of increased vulnerability for poor resolution of homeostasis after a stressor event, so that frail individuals are at risk for adverse health outcomes and prolonged convalescence. 1 In the emergency department (ED), triage acuity predicts the outcomes of ED length of stay, hospitalization, intensive care unit (ICU) admission, and mortality within 30 days, 2 but may be ill‐suited for prediction of adverse outcomes beyond this time frame. In a large Danish study, for example, abnormal vital signs were strong risk factors for mortality within the first 2 days, but hereafter, the association declined with time from arrival most rapidly until day 7. 3
Longer term prognostication is likely more dependent on physiological reserve such as the degree of frailty rather than how ill or seriously injured the patient was at ED presentation. 4 Such prognostication is relevant as accurate predictions empower more informed shared decision‐making, 5 , 6 and should ideally encompass the period of acute illness or injury. Second, predictions about mortality guided by frailty measures enable physicians not usually invested in geriatrics to consider concepts essential to appropriate geriatric care. 7 , 8 Third, advancing an understanding of geriatric core concepts to the doorstep is essential because decisions in the ED sets the direction of many downstream actions, 9 , 10 and, for patients, an ED visit represents a “unique teachable moment” to consider goals of care. 11 Hence, this could contribute to a shift towards an integrated, patient‐centric approach that aligns with the vision of personalized medicine and geriatricized emergency care. 12 , 13 , 14 , 15
Frailty, as measured by the Clinical Frailty Scale (CFS) 16 has been validated for use in several ED settings, 17 , 18 , 19 and in other medical specialties, 20 , 21 , 22 , 23 and is one of the most commonly used frailty measures. The popularity of the CFS in acute settings is likely due to its practical applicability in routine clinical care. 24 , 25 , 26 It takes less than a minute to complete, 27 is simple and multidimensional, and is therefore feasible to be used at triage. 25 , 26
Frailty and triage acuity seem to be complementary measures, and if combined, a better understanding about complexity and vulnerability is provided. 15 , 28 , 29 We have previously shown that the CFS was superior to triage acuity (as measured by the Emergency Severity Index [ESI]) for prediction of 30‐day mortality, ICU admission, and hospitalization when corrected for age, sex, and presenting condition. 30 Furthermore, the CFS has been shown to be predictive for 1‐year‐mortality in a community dwelling population. 16 Other constructs that capture frailty in the hospital setting, Winograd's, Rockwood's, Donini's, and Schoevaerdts' index are well associated with 1‐year mortality, 31 but have inappropriately low diagnostic accuracy. 32 In these studies, measurements were obtained as part of a Comprehensive Geriatric Assessment on admitted patients up to 1 week after admission. Hence, prediction of 1‐year mortality with a construct that measure frailty has not yet been investigated prospectively in the ED setting using consecutive sampling.
OBJECTIVE
The primary objective of this study was to validate the CFS for predicting 1‐year all‐cause mortality in the ED setting and to compare its prognostic performance to that of the ESI when adjusted for chronological age, sex and presenting condition.
METHODS
Study design and setting
This was a prospective observational study with consecutive sampling conducted from March 18 to May 20, 2019, in the ED of the University Hospital Basel (UHBS), a tertiary care center which evaluates over 52,000 ED visits per year. Approximately two‐thirds of our ED population was raised in Northern or Central Europe. Most of the remaining population was raised in Mediterranean countries, Turkey, and Southeast Europe. 33
Participants
All patients aged 65 years and older were screened consecutively for inclusion. Patients who were unable to provide informed verbal consent due to treatment in the resuscitation bay or immediate transfer to the ICU were not screened for inclusion. In accordance with previous recommendations, we did not exclude patients with mild cognitive impairment. 34 Patients denying informed consent and patients unable to provide oral consent were not included. Ophthalmologic, pediatric and obstetric patients are treated at separate locations and were not screened for inclusion. A sample size estimation was performed for the validation study. As this study used the same population, we expected high mortality rates in this older population over the course of 1 year, ensuring that a sufficient number of events would be observed during the follow‐up period.
Outcome
The primary endpoint was 1‐year all‐cause mortality. Data on all‐cause mortality at the 1‐year follow‐up were collected using a combination of electronic health records, official registries, insurance data, contact with patients, primary care physicians or patients' proxies as previously demonstrated. 4 Sufficient power for our analysis was targeted at an inclusion of 2100 patients, including 5% for missing data. 35 , 36
Predictors
All study team members took a 30‐min teaching session on the fundamental concept of frailty, visualized by a short explanatory video, and its assessment with the CFS as previously described. 30 A German version of the CFS was used for this purpose. 16 , 30 The CFS was assessed by a member of the study team, as soon as patients entered a treatment bay, immediately after evaluation by a triage nurse who assigned the ESI level. The study team consisted of 4th to 6th year (of 6 years) medical students. We have previously demonstrated a weighted Cohen's kappa of 0.74 (95% CI 0.64–0.85) between the study team and two experts, an Advanced Nurse Practitioner and an emergency physician, using a small, predefined subsample of the study population. 30 In a different study, interrater reliability of CFS assignments between ED nurses and an ED physician with expertise in geriatric emergency medicine was excellent. 18
Patient characteristics including demographics, ESI acuity level as assigned by triage nurses, 2 and presenting condition (medical vs. surgical) were retrieved from electronic health records. Medical patients are patients in whom conservative management is anticipated (e.g., dyspnea, localized weakness, chest pain); surgical patients are patients with injuries or disease who are anticipated to potentially require surgery (e.g., acute abdomen, femur fracture). The label “medical” versus “surgical” is assigned to patients upon ED arrival by the triage nurse; this serves to split these patients to dedicated teams. The ED is staffed with three teams of ED physicians (who see all patients), internal medicine residents on ED rotation (who see medical patients), and surgical residents on rotation (who see surgical patients). All patients are evaluated under the supervision of attending emergency physicians. 37 Importantly, the CFS levels assigned at ED presentation were not reassessed during the observation period of 365 days.
Data analysis
Categorical data are presented as counts with relative frequencies and continuous data as means and standard deviations (SDs). Patients who presented several times to the ED during the study period only had their first ED visit included in the analysis to avoid confounding. Observations with missing values were omitted in data analyses. All analyses were based on complete data only. The CFS independence from age, sex, and condition regarding prediction of mortality was assessed, using a Cox proportional hazards regression model adjusted for age (continuous), gender (binary), and presenting condition (binary: medical vs. surgical).
Patients of the groups “very fit” (CFS 1), “well” (CFS 2), “managing well” (CFS 3), “vulnerable” (CFS 4), “mildly frail” (CFS 5), “moderately frail” (CFS 6), “severely frail” (CFS7), and “very severely frail” (CFS 8) were monitored for a time interval of 365 days. Multivariable logistic regression models including age, sex, presenting condition, and either CFS or ESI, both categorical variables, were created. We collapsed CFS levels 1 and 2 to one group, as there were no events in the CFS level 1 group, making it unsuitable as reference level for statistical models. Patients with a CFS level 9 (“terminally ill”) were excluded as done previously. Otherwise, the terminal illness, a prerequisite of level 9, will confound the observed relationship between CFS and 1‐year mortality. Hazard ratios with 95% confidence intervals (CIs) were calculated for each predictor. The full‐factor CFS model was assessed regarding effect with a likelihood ratio test. The proportional hazards assumption for the covariates was tested using Schoenfeld's Test. Discriminative performance of each model was evaluated by calculating receiver operating characteristic (ROC) curves and the areas under the curve (AUROCs) with 95% CIs. We tested differences in AUROCs with the DeLong test. To assess calibration performance, calibration slope and intercept were extracted. Additionally, we calculated Brier scores to compare models. The predictive performance estimates were internally validated by bootstrapping as implemented in the package “rms” in R with 200 repetitions. 38
Restricted mean survival time (RMST) was calculated for all CFS levels over 365 days. 39 Survival curves were plotted for CFS levels 1 to level 8 to visualize 1‐year all‐cause mortality.
A p‐value of <0.05 was considered statistically significant. All statistical analyses were performed using R, version 4.0.2 (https://www.r‐project.org/). This study is presented in adherence with Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis reporting standards. 40
RESULTS
Patient characteristics
After screening of 7859 patients for inclusion, 550 patients aged 18 years and older denied verbal consent, resulting in a total number of 7309 included patients. Among these, 4850 patients were aged 64 years or younger, leaving 2459 consecutive patients aged 65 years and older for further analysis of whom 268 were re‐presentations. Thirty‐eight (1.7%) patients had missing data on CFS level, and 15 (0.7%) patients were lost to 1‐year follow up. The final study population included 2191 patients (Figure 1). Mean age (SD) was 78.9 years (8.4), 1124 (51.3%) were female, and 1281 (58.5%) received a medical diagnosis. 1040 (47.5%) patients were assigned a medium urgency triage level of ESI 3 (Table 1). The five presenting chief complaints dyspnea, abdominal pain, chest pain, dizziness, and back pain, based on a list of 36 predefined presenting chief complaints, 26 were the most commonly reported. Regarding patient characteristics (age, sex, triage level, and presenting condition), there does not appear to be a difference between the population of patients lost to follow‐up or between the population of patients with missing data on CFS level compared with patients analyzed (Of note, Table 1 depicts the overall population of 2191 patients aged 65 and older, for baseline demographics of the final study population, see Supporting Information Table S1).
FIGURE 1.
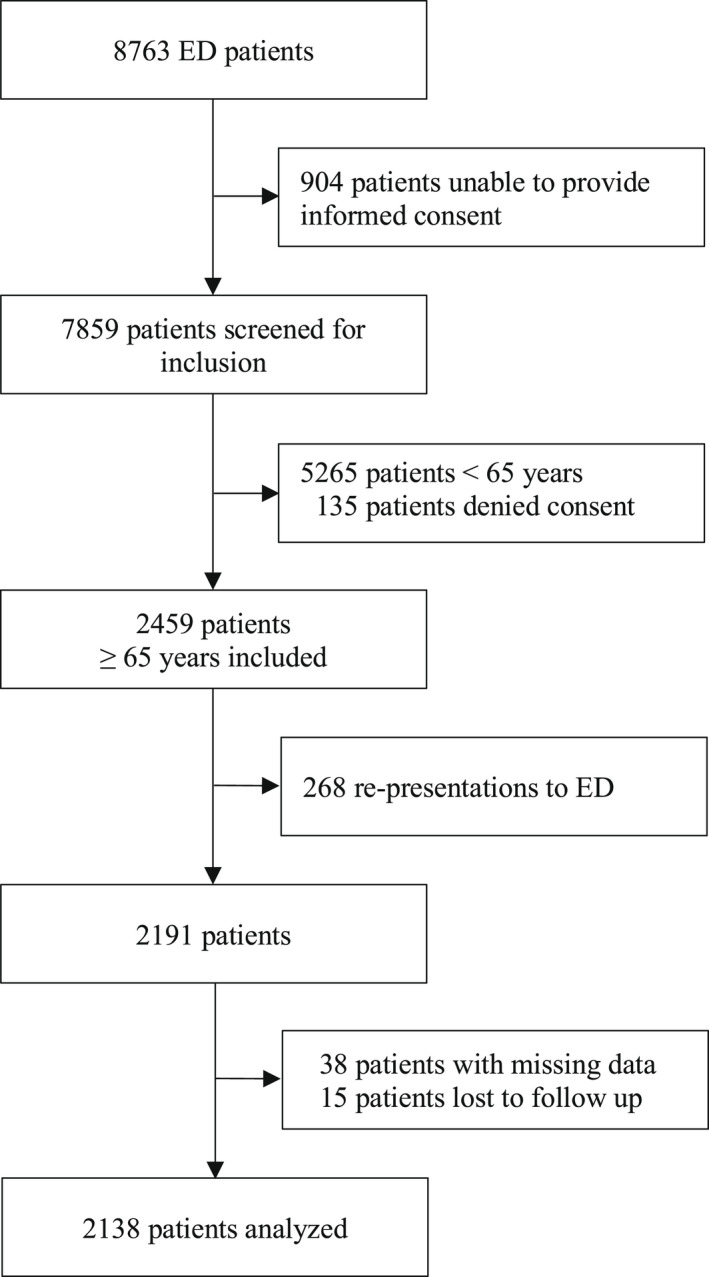
The chart displays recruitment and follow‐up procedure of consecutive ED patients aged 65 and older
TABLE 1.
Patient characteristics of patients alive and patients dead after 1 year
|
All (N = 2191) |
Alive at 1 year (N = 1796) |
Dead at 1 year (N = 380) |
Lost to followup (N = 15) |
|
|---|---|---|---|---|
| Age, mean (SD) | 78.9 (8.42) | 78 (8.13) | 82.9 (8.62) | |
| Female gender, No (%) | 1124 (51.3) | 939 (52.3) | 176 (46.3) | 9 (60) |
| Medical patients, No (%) | 1281 (58.5) | 1,013 (56.4) | 259 (68.2) | 9 (60) |
| ESI level, No (%) | ||||
| 1 | 95 (4.3) | 54 (3) | 41 (10.8) | 0 (0) |
| 2 | 799 (36.5) | 654 (36.4) | 139 (36.6) | 6 (40) |
| 3 | 1040 (47.5) | 852 (47.4) | 181 (47.6) | 7 (46.7) |
| 4 | 238 (10.9) | 218 (12.1) | 18 (4.7) | 2 (13.3) |
| 5 | 19 (0.9) | 18 (1) | 1 (0.3) | 0 (0) |
| CFS level, No (%) | ||||
| 1 | 35 (1.6) | 35 (1.9) | 0 (0) | 0 (0) |
| 2 | 319 (14.6) | 309 (17.2) | 7 (1.8) | 3 (20) |
| 3 | 623 (28.4) | 561 (31.2) | 55 (14.5) | 7 (46.7) |
| 4 | 398 (18.2) | 338 (18.8) | 56 (14.7) | 4 (26.7) |
| 5 | 270 (12.3) | 205 (11.4) | 65 (17.1) | 0 (0) |
| 6 | 215 (9.8) | 150 (8.4) | 64 (16.8) | 1 (6.7) |
| 7 | 167 (7.6) | 109 (6.1) | 58 (15.3) | 0 (0) |
| 8 | 113 (5.2) | 52 (2.9) | 61 (16.1) | 0 (0) |
| 9 | 13 (0.6) | 7 (0.4) | 6 (1.6) | 0 (0) |
| Missing, No (%) | 38 (1.7) | 30 (1.7) | 8 (2.1) | 0 (0) |
| Chief Complaints, no (%) | ||||
| Dyspnea | 157 (7.2) | 118 (6.7) | 39 (10.6) | 0 (0) |
| Abdominal pain | 110 (5.0) | 90 (5.1) | 20 (5.4) | 1 (6.7) |
| Chest pain | 110 (5.0) | 96 (5.4) | 14 (8.8) | 2 (13.3) |
| Dizziness | 92 (4.2) | 85 (4.8) | 7 (1.9) | 0 (0) |
| Fatigue | 87 (4.0) | 59 (3.3) | 28 (7.6) | 0 (0) |
15 patients were lost to follow‐up as depicted in the last column.
Abbreviations: CFS, clinical frailty scale; ESI; emergency severity index; SD, standard deviation
Of note, 8 out of 380 patients that died were not assigned a CFS level; i.e. 372 patients (17%) of the final study population (n=2191) died.
Main results
All‐cause mortality was 17% (372 deaths) within 365 days. Table 1 demonstrates the mortality rate stratified by CFS level.
The effect of the factor “CFS group” was estimated in a Cox regression model and tested using a likelihood ratio test (Χ 2 = 175.43; p ≤ 0.001). Survival curves for all CFS levels, except level 9, are shown in Figure 2. Higher frailty levels were associated with higher hazard ratios (see Table 2). Results of the Schoenfeld test were only significant for the independent variable “CFS group,” indicating that proportionality for the hazard ratios of the CFS groups cannot be assumed (Χ 2 = 38.48; p ≤ 0.001). The RMST is 365 days for the CFS level 1, while declining or remaining constant for every upgrade in CFS level down to 219 days in CFS level 8 (Table 2).
FIGURE 2.
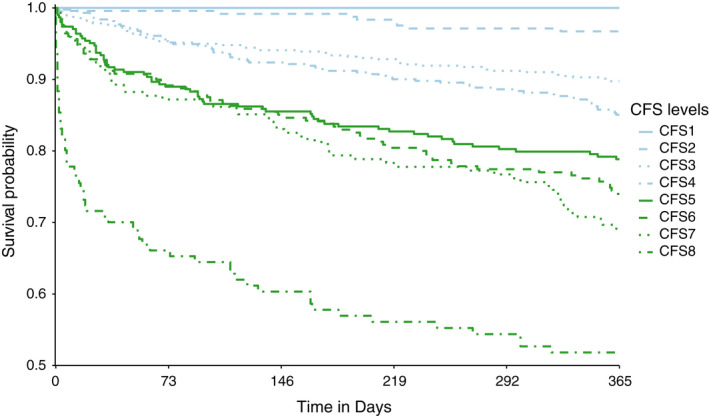
Survival curves for the populations of each CFS level, except level 9 (“terminally ill”), is shown. CFS levels 1 and 2 were included here, in contrast to how these values were excluded in the logistic regression model (Table 2), as this graph is a descriptive representation. The graph was cropped to 0.5 on the y‐axis
TABLE 2.
Odds ratios and RMST for 1‐yr mortality
| 1‐Yr mortality | ||||
|---|---|---|---|---|
| OR | CI | RMST | SE (RMST) | |
| Age, (yr) | 1.04 | 1.03–1.06 | — | — |
| Female gender | 0.66 | 0.54–0.82 | — | — |
| Medical condition | 1.28 | 1.03–1.58 | — | — |
| CFS level | ||||
| 1 (reference) | — | — | 365 | 0.00 |
| 2 (reference) | — | — | 359 | 1.80 |
| 3 | 3.22 | 1.59–6.52 | 342 | 3.05 |
| 4 | 4.72 | 2.33–9.56 | 336 | 4.33 |
| 5 | 7.18 | 3.53–14.59 | 311 | 7.37 |
| 6 | 8.90 | 4.37–18.11 | 305 | 8.62 |
| 7 | 10.76 | 5.28–21.94 | 298 | 10.24 |
| 8 | 22.93 | 11.30–46.54 | 219 | 16.84 |
Note: The CFS group “fit/well” (CFS level 1 and 2) was defined as reference category for the ORs. The RMSTs were calculated for each CFS group of the cox proportional hazards model. Medians and corresponding CIs for each RMST could statistically not be estimated, as too few events occurred in each CFS group.
Abbreviation: SE, standard error.
The ROC curves of the multivariable logistic models with CFS group and ESI level (Figure 3) visualize models' discriminatory performance for 1‐year all‐cause mortality. The CFS model yielded an AUROC of 0.767 (95% CI 0.741–0.793), while the ESI model (both models adjusted for age, sex, and presenting condition) yielded an AUROC of 0.703 (95% CI 0.673–0.732), with a significant DeLong's test comparing the AUCs (z = 5.69; p‐value <0.001). Bootstrapped estimates of calibration slope and intercept yielded 0.960 and −0.047, respectively, for the CFS model, while calibration slope and intercept were 0.973 and −0.029 for the ESI model respectively. Additionally, the Hosmer‐Lemeshow test was non‐significant for both models, confirming acceptable fit. A lower Brier score of 0.126 for the CFS model compared to 0.134 for the ESI model in the same population indicates overall better predictive performance of the CFS model (Supporting Information Table S2).
FIGURE 3.
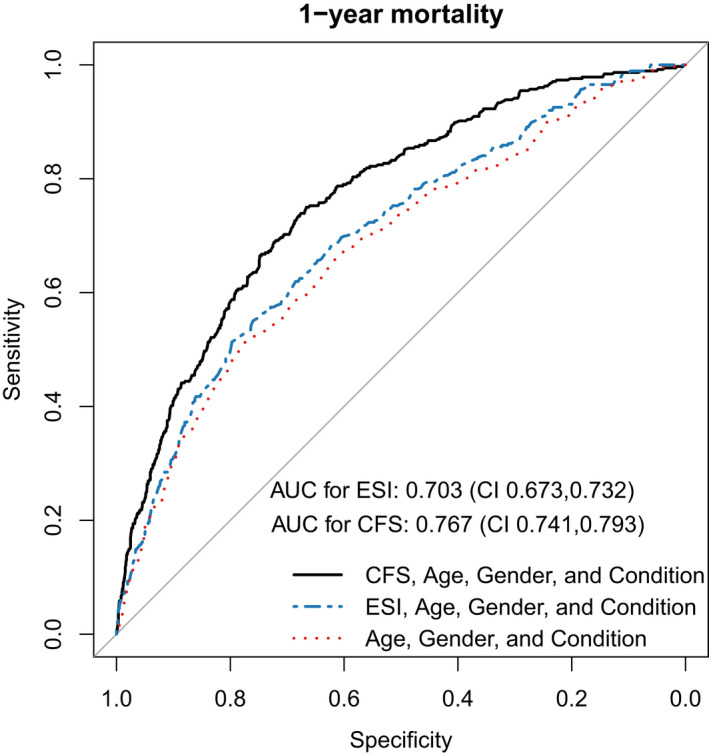
Comparison of the three adjusted logistic regression models for prediction of 1‐year mortality, featuring CFS groups or ESI levels, and Null model. The AUROC of the CFS model was 0.767 (95% CI 0.741–0.793) and of the ESI model was 0.703 (95% CI 0.673–0.732). The calibration slope measured 0.959 in the CFS model and 0.973 in the ESI model
Odds ratios (ORs) for the different predictors in the CFS model are shown in Table 2.
DISCUSSION
We performed a prospective validation of the CFS in a sample of consecutive ED patients aged 65 and older for predicting 1‐year all‐cause mortality. We found the CFS to be an independent predictor for 1‐year all‐cause mortality after correcting for age, sex, and medical condition and that each increase in CFS level is associated with decreased survival times. In addition, the CFS displays superior discriminatory performance compared to the ESI when adjusted for age, sex, and presenting condition.
We found the CFS to be superior to the ESI in accurately discriminating between those who would experience the outcome and those who would not over 1 year. This is in line with our previous findings for 30 day‐follow‐up. Previous studies investigating prognostic performance of the CFS produced survival curves that largely confirm the separation observed in this study at 1 year in both the acute care setting and the primary care setting. 2 , 41 , 42 However, the CFS in these studies was collapsed into different subgroups rendering it difficult to compare results directly. For clinical purposes, we believe that the use of RMST in this study could potentially facilitate a better comprehension of prognosis in conversations with patients, an aspect that is of particular importance in the emergency setting. 11
Very few observations were lost to follow‐up during this study, allowing for a complete case analysis and omitting imputation procedures often necessary in prognostic research. This also enables us to consider a high number of events per predictor variable, increasing the validity of our findings.
Measures of illness acuity should guide immediate actions by predicting high urgency outcomes (e.g., ICU‐admission within a short timespan). Frailty describes the ability to recover after acute illness. 1 Measures of frailty should demonstrate the ability to provide graded risk estimates to more meaningful patient‐centered modulation of treatment plans for low urgency outcomes in the long term. We show that the CFS does indeed perform well in a 1‐year time‐horizon, stratifying the population in distinguishable subsets with worsening outcomes for each CFS level increase and may, thus, be a valuable addition to the ESI for emergency clinicians. Imagine two 80‐year‐old patients (A and B) with suspected pneumonia both assigned an ESI level of 3. The needs of patient A living with mild frailty might be very different from the very severely frail patient B who spends most of her time in bed and might be in her last year of life.
The CFS has been reported to be predictive in a variety of clinical scenarios, such as cardiopulmonary resuscitation and trauma. 43 , 44 In some scenarios, however, the CFS may be of limited use compared to estimates of illness acuity, but this is controversial. 45 For example, CFS did not predict mortality well in patients admitted with COVID‐19 in one study, and illness acuity as measured using Early Warning Score outperformed CFS in a similar study. 46 Both studies suggest the presence of a protective interaction term between CFS and the presence of coronavirus disease 2019 (COVID‐19) that may be attributed to immune senescence in older more frail adults. However, these findings are likely biased by selection as they are restricted to patients who were ultimately admitted after their initial evaluation in the ED and at least 65 years old. Other studies did find that frailty was associated with adverse outcomes in COVID‐19 that worsened with increasing frailty. 42 , 47
To our surprise, we found separation on the survival plot for CFS level 8 compared to lower levels. A CFS of 7 or above has been considered a range that indicated futility of resuscitation attempts previously, and subjects with CFS levels 6 to 9 have been grouped when examining outcomes after cardiopulmonary resuscitation. Given the considerable separation we observe in Figure 2 between CFS levels 5 to 7 and level 8, despite excluding patients treated in the resuscitation bay, grouping levels 6 to 9 seems inappropriate because of the significant differences in survival‐rates. Hence, outcome‐incidences should be presented without grouping in future studies of the CFS. Furthermore, surviving cardiac resuscitation is often also related to other adverse clinical outcomes and more knowledge is needed on the ability to recover after cardiopulmonary resuscitation depending on the degree of frailty.
LIMITATIONS
This was a single‐center study, which might limit generalizability, and the sample may not be representative of the general ED population. The sampled population came from the Northwestern part of Switzerland, which has a well‐established primary care infrastructure and we expect 7% to be nursing home‐residents based on previous studies from this site on a similar population. Many older patients are referred to the ED after initial evaluation by their primary care physician, which may lead to the inclusion of a relatively high percentage of frail and possibly acutely ill patients. However, we found the observed 1‐year mortality in this study to be lower than what is being reported on the general population in the acute care setting elsewhere. This may imply that the observed ability to accurately predict individual risk will be impacted in other settings and suggests an underestimation of 1‐year mortality compared to the general population. 48 However, this limitation likely applies to most areas of prospective research where procuring verbal consent is essential, by which those who are most ill are filtered out. Furthermore, the older population will often experience temporary cognitive impairment due to the acute illness itself, 7 , 49 which may put measures of acute illness at further disadvantage to frailty models for study designs that require consent. We included patients with minor cognitive impairment, which we expect will have reduced the observed differences in overall mortality to the general ED population. Studies used for service evaluation may be better suited despite severe limitations of this design. To comprehend the possible impact on prognostic performance, studies are needed that compare the case‐mix using these two different types of sampling in one population. Using assessments of frailty at arrival to predict adverse outcomes has been likened with “predicting speed at traffic lights” in that frailty may be progressing at different paces and patient preferences and social networks may affect the estimate of prognosis more than expected. Also, frailty likely varies over time getting both better and worse, although improvements in degree of frailty may not be associated with improvements in prognosis. Hence, our observations may be confounded by variability in frailty and patient preferences that we cannot account for. However, comprehension of 1‐year‐mortality risk may promote addressing long‐term problems often neglected or presumed to be handled in the primary sector after discharge. 50 In addition, the CIs of the OR for prediction of 1‐year mortality were rather wide for both models. As there is no overlap of the CFS model's and the ESI model's CIs, the hypothesis of the CFS model's superiority remains stable nevertheless.
We included only the covariables age, sex, and condition (medical versus surgical) for our regression models because these parameters were routinely available at ED presentation. We do not have information on comorbidities, like the Charlson ‐ Comorbidity Index (CCI). 51 While the CFS is a tool for the measurement of “frailty,” the CCI is a tool to measure “comorbidity.” There may be some overlap between these parameters; however, they should be considered as different. 52 Furthermore, the CCI score was not available for our study patients at ED arrival. The CCI may not be properly used in the emergency setting, since diagnoses in inpatients’ discharge reports are more carefully maintained than those of outpatients' discharge reports. ED diagnoses lists may be incomplete due to the short average ED length of stay of approximately 5 h, possibly leading to a distortion of data concerning comorbidity. In addition, the CCI was derived in 1987. 51 Therefore, the selection of comorbidities as well as the weights representing the effect magnitude likely require updating. 53 Importantly, the timing of outcome prediction in this study is at arrival and on undifferentiated patients. At this time point, few predictors are available, and the “price” of additional predictors, such as a detailed history to determine specific phenotypes, is high in terms of time consumption and resources that may be better spent after differentiation. This is also indicated in a recent retrospective study from the United Kingdom, where CCI was only available on those who were eventually admitted. Of note, that study found that the CFS was a strong independent predictor after adjusting for CCI, illness severity, age, and sex. 25 In addition, we did not include health literacy, nutritional status, depression, or dementia because we did not seek to upgrade the CFS or the ESI models, but rather compare these given covariates that are readily available in the acute care setting on arrival.
Furthermore, mortality might not be the ideal outcome as it is not the only health care outcome important to patients, 54 especially if they have exceeded their life expectancy. However, as an approximation, a 79‐year‐old Swiss has an average life expectancy of over 10 years. Demographically (and emotionally), most of our patients were not close to death, even 95 year‐old Swiss having an average life expectancy of years (“survivors effect”). As our study was not designed to correct for individual theoretical life expectancies, we are not able to present such numbers. However, great care should be taken when assuming the utilitarian perspective that time added to life at old age is worth less than when added at a younger age.
We do not have complete data on the number of hospitalizations and the number of ICU admissions over 1 year, which are other relevant outcomes. Hospitalization and ICU admission rates would have to be collected from several hospitals in the Basel area, for which we lack ethical approval. Second, profound interactions among these co‐variates (CFS level, number of hospitalizations, number of ICU admission, number of institutionalizations) are to be expected when included in one single model. This would likely reduce transportability and clinical utility of the model considerably. The purpose of this work was not to develop yet another model for prediction, which is unlikely to be validated, 5 but rather compare acuity to frailty at arrival with an intentional focus on validation of existing models.
The CFS requires raters assess the baseline state, a feat that may be difficult in the acute care setting. 8 Even though we did not administer a “criterion‐standard” frailty test in this study, 55 we previously found good agreement with an expert panel for the CFS ratings performed on this cohort, despite raters being senior medical students. 30 Additionally, the CFS displayed excellent reliability, regardless of clinical experience even after brief online training in a similar setting. 23 Hence, we expect limited bias for the CFS measurements compared to routinely collected ESI. However, we acknowledge that, ideally, to better assess real‐world clinical relevance, ratings would have been performed by both research assistants and clinicians.
It is well established that it is both stressful and difficult to make predictions in the clinical context, 56 and that illness acuity cannot stand alone when assessing prognosis. 57 Whereas life expectancy can be accurately calculated for a population, it is more difficult to determine for individuals. This study shows that it is possible to make surprisingly accurate estimates of the risk of death for up to 1 year based on knowledge available at arrival to the ED. In patients living with malignant conditions, 1‐year or even extended survival is likely to be discussed, whereas in patients living with frailty and carrying comparably serious outcomes, prognosis is less often considered or recognized by care givers. Awareness of and acceptance around a likely 1‐year prognosis may create a paradigm shift for emergency physicians to use a disease‐oriented approach to solve the immediate problem and to engage an integrated, individually tailored model of care. 12 , 13 , 14 , 58 Our study provides a foundation to justify intervention studies evaluating impact and unintended consequences of accelerating or altering ED processes resulting from CFS +/− ESI screening.
CONCLUSIONS
The CFS predicts 1‐year all‐cause mortality for older ED patients and predicts survival time in a graded manner for each CFS level. As a predictor of 1‐year mortality, the CFS is superior to the ESI when adjusted for age, sex, and presenting condition.
CONFLICT OF INTEREST
M.R. reports no conflict of interest. S.K.N. reports no conflict of interest. M.B. reports no conflict of interest. T.K. reports no conflict of interest. T.D. reports no conflict of interest. C.R.C. is contracted collaborator with the Geriatric ED Collaborative, Geriatric Emergency Care Applied Research (GEAR) Network, and ACEP Geriatric ED Accreditation Board of Governors. RB reports no conflicts of interest. CHN reports no conflicts of interest.
AUTHOR CONTRIBUTIONS
R.B. acquired funding for the study. C.H.N. conceived the study; C.H.N. and R.B. designed the trial and supervised the conduct of the trial and data collection. T.K. and T.D. undertook and supervised recruitment of patients. M.R., S.K.N., C.R.C., M.B., C.H.N., and T.K. analyzed the data. M.R., S.K.N., C.R.C., and C.H.N. interpreted the data, including quality control. M.R., S.K.N., and C.H.N. drafted the manuscript, and all authors contributed substantially to its revision. C.H.N. takes responsibility for the paper as a whole.
ETHICS APPROVAL
The study was approved by a local ethics committee (www.eknz.ch, 236/13) and registered on Clinical Trials Registry (NCT03892551).
Supporting information
Figure S1
Table S1
Table S2
ACKNOWLEDGMENTS
We thank Gilles Dutilh, PhD, Clinical Trial Unit for statistical advice. We thank John Kellett MD for helpful discussions on prognostication.
Rueegg M, Nissen SK, Brabrand M, et al. The clinical frailty scale predicts 1‐year mortality in emergency department patients aged 65 years and older. Acad Emerg Med. 2022;29:572‐580. doi: 10.1111/acem.14460
Supervising Editor: Dr. Ula Hwang
Funding information
Scientific funds from the University Hospital of Basel. Open access funding provided by Universitat Basel.[Correction added on 22 May 2022, after first online publication : CSAL Funding statement has been added.]
A related article appears on page 678.
REFERENCES
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grossmann FF, Nickel CH, Christ M, Schneider K, Spirig R, Bingisser R. Transporting clinical tools to new settings: cultural adaptation and validation of the Emergency Severity Index in German. Ann Emerg Med. 2011;57:257‐264. [DOI] [PubMed] [Google Scholar]
- 3. Bech CN, Brabrand M, Mikkelsen S, Lassen A. Risk factors associated with short term mortality changes over time, after arrival to the emergency department. Scand J Trauma Resusc Emerg Med. 2018;26:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nickel CH, Kellett J, Nieves Ortega R, et al. A simple prognostic score predicts one‐year mortality of alert and calm emergency department patients: a prospective two‐center observational study. Int J Clin Pract. 2020;74:e13481. [DOI] [PubMed] [Google Scholar]
- 5. Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogan TM, Richmond NL, Carpenter CR, et al. Shared decision making to improve the emergency care of older adults: a research agenda. Acad Emerg Med. 2016;23:1386‐1393. [DOI] [PubMed] [Google Scholar]
- 7. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept: (see editorial comments by Dr. William Hazzard on pp 794–796). J Am Geriatr Soc. 2007;55:780‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mooijaart SP, Nickel CH, Conroy SP, et al. A European Research Agenda for Geriatric Emergency Medicine: a modified Delphi study. Eur Geriatr Med. 2021;12:413‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carpenter CR, Mooijaart SP. Geriatric screeners 2.0: time for a paradigm shift in emergency department vulnerability research. J Am Geriatr Soc. 2020;68:1402‐1405. [DOI] [PubMed] [Google Scholar]
- 11. Ouchi K, George N, Schuur JD, et al. Goals‐of‐care conversations for older adults with serious illness in the emergency department: challenges and opportunities. Ann Emerg Med. 2019;74:276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179‐185. [DOI] [PubMed] [Google Scholar]
- 13. Carpenter CR, Platts‐Mills TF. Evolving prehospital, emergency department, and “inpatient” management models for geriatric emergencies. Clin Geriatr Med. 2013;29:31‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of Emergency Physicians; American Geriatrics Society, Emergency Nurses Association, Society for Academic Emergency Medicine, Geriatric Emergency Department Guidelines Task Force . Geriatric emergency department guidelines. Ann Emerg Med. 2014;63:e7‐e25. [DOI] [PubMed] [Google Scholar]
- 15. Hogervorst VM, Buurman BM, De Jonghe A, et al. Emergency department management of older people living with frailty: a guide for emergency practitioners. Emerg Med J. 2021;38:724‐729. [DOI] [PubMed] [Google Scholar]
- 16. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serina P, Lo AX, Kocherginsky M, et al. The clinical frailty scale and health services use for older adults in the emergency department. J Am Geriatr Soc. 2021;69(3):837‐839. [DOI] [PubMed] [Google Scholar]
- 18. Lo AX, Heinemann AW, Gray E, et al. Inter‐rater reliability of clinical frailty scores for older patients in the emergency department. Acad Emerg Med. 2021;28:110‐113. [DOI] [PubMed] [Google Scholar]
- 19. Ringer T, Thompson C, McLeod S, Melady D. Inter‐rater agreement between self‐rated and staff‐rated clinical frailty scale scores in older emergency department patients: a prospective observational study. Acad Emerg Med. 2020;27:419‐422. [DOI] [PubMed] [Google Scholar]
- 20. Wallis SJ, Wall J, Biram RW, Romero‐Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943‐949. [DOI] [PubMed] [Google Scholar]
- 21. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187:799‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nissen SK, Fournaise A, Lauridsen JT, et al. Cross‐sectoral inter‐rater reliability of the clinical frailty scale—a Danish translation and validation study. BMC Geriatr. 2020;20:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis ET, Dent E, Alkhouri H, et al. Which frailty scale for patients admitted via emergency department? A cohort study. Arch Gerontol Geriatr. 2019;80:104‐114. [DOI] [PubMed] [Google Scholar]
- 25. Elliott A, Taub N, Banerjee J, et al. Does the clinical frailty scale at triage predict outcomes from emergency care for older people? Ann Emerg Med. 2021;77:620‐627. [DOI] [PubMed] [Google Scholar]
- 26. Elliott A, Phelps K, Regen E, Conroy SP. Identifying frailty in the Emergency Department‐feasibility study. Age Ageing. 2017;46:840‐845. [DOI] [PubMed] [Google Scholar]
- 27. McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg. 2020;271:283‐289. [DOI] [PubMed] [Google Scholar]
- 28. Mowbray F, Brousseau AA, Mercier E, Melady D, Émond M, Costa AP. Examining the relationship between triage acuity and frailty to inform the care of older emergency department patients: findings from a large Canadian multisite cohort study. CJEM. 2020;22:74‐81. [DOI] [PubMed] [Google Scholar]
- 29. Blomaard LC, Speksnijder C, Lucke JA, et al. Geriatric screening, triage urgency, and 30‐day mortality in older emergency department patients. J Am Geriatr Soc. 2020;68:1755‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaeppeli T, Rueegg M, Dreher‐Hummel T, et al. Validation of the clinical frailty scale for prediction of thirty‐day mortality in the Emergency Department. Ann Emerg Med. 2020. [DOI] [PubMed] [Google Scholar]
- 31. Drame M, Novella JL, Jolly D, et al. Rapid cognitive decline, one‐year institutional admission and one‐year mortality: analysis of the ability to predict and inter‐tool agreement of four validated clinical frailty indexes in the safes cohort. J Nutr Health Aging. 2011;15:699‐705. [DOI] [PubMed] [Google Scholar]
- 32. Carpenter CR, Shelton E, Fowler S, et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta‐analysis. Acad Emerg Med. 2015;22:1‐21. [DOI] [PubMed] [Google Scholar]
- 33. Jauslin AS, Schultze L, Knuchel D, Simon NR, Nickel CH, Bingisser R. Disparities in emergency department access, resource allocation, and outcomes between migrants and the local population. Swiss Med Wkly. 2021;151:w30070. [DOI] [PubMed] [Google Scholar]
- 34. Prusaczyk B, Cherney SM, Carpenter CR, DuBois JM. Informed consent to research with cognitively impaired adults: transdisciplinary challenges and opportunities. Clin Gerontol. 2017;40:63‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503‐1510. [DOI] [PubMed] [Google Scholar]
- 36. van Smeden M, de Groot JA, Moons KG, et al. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med Res Methodol. 2016;16:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Möckel M, Nickel CH, Behringer W, Backus B. Status of physician education in emergency medicine in four European countries: no primary specialty yet. Eur J Emerg Med. 2021;28:257‐259. [DOI] [PubMed] [Google Scholar]
- 38. Harrell FE Jr. R package ‘rms’. 6.2–0 ed2021.
- 39. Royston P, Parmar MKB. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time‐to‐event outcome. BMC Med Res Methodol. 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) the TRIPOD statement. Circulation. 2015;131:211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020;49:1071‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hewitt J, Carter B, Vilches‐Moraga A, et al. The effect of frailty on survival in patients with COVID‐19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444‐e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braude P, Carter B, Parry F, et al. Predicting 1 year mortality after traumatic injury using the Clinical Frailty Scale. J Am Geriatr Soc;n/a. [DOI] [PubMed] [Google Scholar]
- 44. Mowbray FI, Manlongat D, Correia RH, et al. Prognostic association of frailty with post‐arrest outcomes following cardiac arrest: a systematic review and meta‐analysis. Resuscitation. 2021;167:242‐250. [DOI] [PubMed] [Google Scholar]
- 45. Cosco TD, Best J, Davis D, et al. What is the relationship between validated frailty scores and mortality for adults with COVID‐19 in acute hospital care? A systematic review. Age Ageing. 2021;50:608‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Owen RK, Conroy SP, Taub N, et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID‐19 infection: a retrospective observational study using electronic health records. Age Ageing. 2021;50:307‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simon NR, Jauslin AS, Rueegg M, et al. Association of frailty with adverse outcomes in patients with suspected COVID‐19 infection. J Clin Med. 2021;10:2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Debray TP, Damen JA, Snell KI, et al. A guide to systematic review and meta‐analysis of prediction model performance. BMJ. 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
- 49. Carpenter CR, Hammouda N, Linton EA, et al. Delirium prevention, detection, and treatment in emergency medicine settings: a Geriatric Emergency Care Applied Research (GEAR) network scoping review and consensus statement. Acad Emerg Med. 2021;28:19‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keeble E, Roberts HC, Williams CD, Van Oppen J, Conroy SP. Outcomes of hospital admissions among frail older people: a 2‐year cohort study. Br J Gen Pract. 2019;69:e555‐e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 52. Arendts G, Burkett E, Hullick C, Carpenter CR, Nagaraj G, Visvanathan R. Frailty, thy name is. Emerg Med Australas. 2017;29:712‐716. [DOI] [PubMed] [Google Scholar]
- 53. Fernando DT, Berecki‐Gisolf J, Newstead S, Ansari Z. The Australian injury comorbidity index to predict mortality. Ann Emerg Med. 2020;75:339‐353. [DOI] [PubMed] [Google Scholar]
- 54. Kellett J. Death is not the only healthcare outcome important to patients. Eur J Intern Med. 2016;32:e11‐e12. [DOI] [PubMed] [Google Scholar]
- 55. Sutton JL, Gould RL, Daley S, et al. Psychometric properties of multicomponent tools designed to assess frailty in older adults: a systematic review. BMC Geriatr. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christakis NA, Iwashyna TJ. Attitude and self‐reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389‐2395. [DOI] [PubMed] [Google Scholar]
- 57. Kellett J. Prognostication—the lost skill of medicine. Eur J Intern Med. 2008;19:155‐164. [DOI] [PubMed] [Google Scholar]
- 58. Krumholz HM. Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2


