Abstract
Use of hypnotics is often associated with next‐morning residual effects and a higher risk of motor vehicle accidents. Measuring next‐morning effects on driving performance is therefore advised by regulatory agencies. Here, we examined driving performance following administration of daridorexant, a new dual orexin receptor antagonist developed to treat insomnia. Sixty healthy male and female subjects (50–79 years of age) were randomized in a placebo‐ and active‐controlled, four‐way cross‐over study. Each subject received evening administration of daridorexant 50 and 100 mg, zopiclone 7.5 mg, and placebo, in separate treatment phases of 4 days. Simulated driving performance was assessed after initial (day 2) and repeated dosing (day 5), 9 hours postdose. Standard deviation of the lateral position (SDLP) was the main outcome. On both days, with zopiclone, SDLP increased significantly compared with placebo, which confirmed sensitivity of the simulator. With daridorexant, on day 2, the placebo‐corrected mean (97.5% confidence interval) SDLP increased by 2.19 cm (0.46–3.93) and 4.43 cm (2.72–6.15) for 50 and 100 mg, respectively. On day 5, SDLP values for both daridorexant doses were significantly below the prespecified threshold of impairment (2.6 cm) and statistically not different from placebo. Daridorexant showed a lower self‐rated driving quality and higher effort compared to placebo on day 2 but not on day 5. In non‐insomnia subjects, daridorexant impaired simulated driving after initial but not after repeated dosing. Subjects should be cautioned about driving until they know how daridorexant affects them.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Daridorexant is a newly developed dual orexin receptor antagonist. Studies in patients with insomnia have not shown any next‐morning residual effects.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does daridorexant affect next‐morning driving performance in a simulator in healthy middle‐aged and elderly subjects?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Daridorexant impaired simulated driving performance after initial but not after repeated dosing. Results show that a state‐of‐the‐art driving simulation test, conducted under highly standardized conditions, is more sensitive to detect subtle drug effects on driving performance than the on‐the‐road test.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The high sensitivity of the driving simulator warrants its broader utilization in studying effects of central nervous system drugs.
Motor vehicle accidents (MVAs) are a major cause of injury and death in Europe and the United states. 1 , 2 An important underlying cause of MVAs after the use of some hypnotics are next‐morning residual central nervous system (CNS) drug effects causing sleepiness, reduced psychomotor functioning (e.g., eye‐hand coordination and reaction time), as well as diminished cognitive functioning (e.g., a decreased level of attention which might still be present the morning after using hypnotics). 3 The next‐morning effects on driving ability caused by sedative‐hypnotic drugs, such as GABA‐receptor agonists, have been previously described in detail, 4 , 5 , 6 , 7 demonstrating a significant increase in the standard deviation of the lateral position (SDLP). SDLP, a measure of sway of a motor vehicle along the lateral axis as primarily assessed in an on‐the‐road driving test in real traffic, is the main outcome variable of these studies, and considered the standard for assessing CNS drug effects on driving ability, as it has been used for more than 30 years. 8 , 9 , 10 The development of novel sleep drugs with limited or absent next‐morning residual CNS effects may help to reduce drug‐induced MVAs. 11 , 12
The new dual orexin receptor antagonist daridorexant (ACT‐541468) is being developed for the treatment of insomnia disorders. 13 , 14 , 15 The orexin system promotes wakefulness by integrating the influence of metabolism, circadian rhythm, and the need to sleep through efferent orexinergic axons projecting to the cerebral cortex and structures involving the brain stem and limbic system. 16 Daridorexant is a potent and selective compound that blocks the actions of the orexin neuropeptides at both orexin‐1 (OX1R) and orexin‐2 (OX2R) receptors, and has shown promising sleep‐promoting effects in phase II studies and in the recently published confirmatory phase III trials. 17 , 18 , 19 As a sleep‐promoting drug, its pharmacokinetic (PK) profile is favorable with rapid absorption (median time to maximum concentration of ~ 1 to 2 hours) and relatively rapid elimination from plasma with a terminal half‐life (t 1/2) of ~ 8 hours. 13 , 14 Next‐morning residual pharmacodynamic (PD) effects following evening administration of multiple doses of 25 mg daridorexant were previously explored in healthy subjects using a CNS test battery quantifying drug effects on alertness, sustained attention, (visuo)motor coordination, relevant neurocognitive domains, postural balance, and subjective experience. 14 , 15 No clinically relevant objective or subjective PD effects were present the morning after drug intake. Furthermore, in patients with insomnia, no next‐morning residual effects were observed with daridorexant 50 mg in phase II and phase III studies using subjective and objective assessments. 17 , 18 , 19
The US Food and Drug Administration (FDA) has issued a guidance on how to examine the untoward effects of new psychoactive drugs on driving ability. 11 A tiered approach is recommended, including pharmacological, toxicological, epidemiological, and clinical assessments. The latter includes driving studies, which have a higher specificity to assess impairment of driving than, for example, short‐lasting CNS test batteries that rather assess individual, albeit overlapping, functional CNS domains. To evaluate sustained vigilance and attention, the FDA requests monotonous driving (e.g., on a straight highway). Studies evaluating drug effects on driving ability can be performed on‐the‐road (real traffic), on closed‐loop tracks (e.g., on a landing strip), or in driving simulators. Each of these methods have their relative merits and disadvantages. 11 , 12 , 20 In view of the importance of this topic, a consensus protocol for assessing the impact of drugs on driving ability was established by an expert panel of the National Highway Traffic Safety Administration (part of the US Department of Transportation), in which both simulated, and on‐the‐road driving assessments are considered valid methods. 12
The current protocol was designed after consultation with the FDA and aimed at evaluating variables typically used to assess drug effects on driving ability (e.g., lane keeping, speed control, and self‐rated performance). 11 , 12 The primary objective was to assess the effects of daridorexant on objective driving performance as measured by the SDLP. The SDLP was examined using the custom‐built Green Dino driving simulator, which was used in previous studies to investigate potential effects of sedative drugs and alcohol. 21 , 22
METHODS
Subjects
The study was conducted between March and October 2019 at the Centre for Human Drug Research (CHDR) in Leiden, The Netherlands. The study was approved by the Medical Ethics Committee of Stichting Beoordeling Ethiek Biomedisch Onderzoek, Assen, The Netherlands, and was conducted according to the Dutch Act on Medical Research Involving Human Subjects, and in compliance with all International Council on Harmonisation‐Good Clinical Practice guidelines and the Declaration of Helsinki. This study was registered in the public registry of the Centrale Commissie Mensgebonden Onderzoek in the Netherlands (NL68520.056.19) and in clinicaltrials.gov (NCT03892902).
The study population comprised 60 male and female middle‐aged and elderly subjects (30 subjects were 50–64 years and 30 subjects were 65–80 years in a 1:1 sex ratio). Subjects were healthy sleepers (i.e., no insomnia‐related medical history, or minor concomitant diseases such as hypertension were allowed), and had a driver’s license, and had driven at least 3000 km/year in the 2 years prior to study start. Each subject provided written informed consent before any screening procedures were performed.
Design
This was a randomized, placebo‐ and active‐controlled, four‐way cross‐over study. The study consisted of a medical screening (medical history, physical examination, blood chemistry and hematology, urinalysis, and electrocardiogram (ECG)) 28 to 3 days before first dosing. During screening, subjects performed a training drive to rule out simulator sickness and become familiar with the system. Middle‐aged and elderly eligible subjects were randomly assigned to one of the four treatment sequences using a Williams design. The four treatment periods were separated by a washout period of at least 1 week, and subjects were contacted for a safety follow‐up call 30–40 days after the last day of the last treatment period.
The duration of each treatment period was 5 days. Upon admission for each treatment period (day 1), an alcohol breath and urine drug test (including cannabinoids, cocaine, methadone, morphine, (meth)amphetamine, barbiturates, and 3,4‐methylenedioxy methamphetamine (ecstasy)) was performed. Furthermore, an ECG was recorded, vital signs were measured, and a physician reconfirmed suitability prior to dosing. Administration of study drug was performed between 10:00 pm and midnight, thereafter the subject was instructed to go to sleep. In the morning of day 2 and day 5 (i.e., 8 hours after administration of the study drug), subjects were woken up, a PK plasma sample was taken, followed by some time for toiletry and breakfast. At 9 hours postdose, subjects started a simulated drive, as described below.
Study drugs and dosing rationale
Daridorexant
Daridorexant was administered as the to‐be‐marketed film‐coated tablet formulation. A dose of 50 mg of daridorexant was selected for the following reasons: 50 mg was well‐tolerated in previous phase I and phase II trials and was the highest dose investigated in phase III. Therefore, it allowed the investigation of the potential effects of daridorexant on next‐morning driving performance at the highest therapeutic dose. A supratherapeutic dose of 100 mg daridorexant was included based on discussions with the FDA and recommendations in the applicable guidelines, to account for potentially higher exposure scenarios in subpopulations (e.g., caused by drug–drug interactions, or reduced clearance). 11 From a safety perspective, a dose of 100 mg in middle‐aged and elderly subjects was acceptable because in the first‐in‐human study, doses up to 200 mg had been safely administered to healthy subjects, 13 and in the completed phase II/III program a dose of 50 mg was safely administered in the evening without any signs of next‐morning residual effects. 17 , 18 , 19 Daridorexant‐matching tablets for oral administration with the same appearance and weight as daridorexant were used as placebo.
Zopiclone
Zopiclone, a nonbenzodiazepine hypnotic, which preferentially binds to the GABAA‐α1 receptor subunit, is used for the treatment of insomnia. 23 The PKs of zopiclone are similar to those of daridorexant in that, upon oral administration, zopiclone is rapidly absorbed, with maximum plasma concentration (Cmax) reached within 1–2 hours and the t 1/2 ranges from 3.5 to 6.5 hours. 23 Zopiclone was chosen as an active reference based on its proven ability to confirm assay sensitivity in multiple previous driving studies (on‐the‐road and simulator). 24 , 25 , 26 A dose of 7.5 mg zopiclone (over‐encapsulated tablets to maintain blinding) was chosen as this dose was used safely in previous driving studies. 24 Zopiclone was administered on day 1 and day 4 only, whereas on day 2 and day 3, matching placebo was administered to reduce the risk of potential development of tolerance as advocated in the FDA guidelines. 11
Driving assessments
Simulated driving was executed in a simulator provided by Green Dino BV, Groningen, The Netherlands. The simulator consisted of a fully equipped car module, including a steering wheel, clutch, gas, and brake pedals, manual transmission stick, and direction indicator. 21 , 22 The pedal controls were linked to a computer that simulated the environment and traffic. The driving scenario was projected on three 24‐inch LCD monitors positioned side‐by‐side. The sounds of the car and other traffic were played from a soundbar below the computer screens. The custom‐built scenario included 60 minutes of simulated drive on a dual lane highway, similar to the classic on‐the‐road test. 8 , 9 , 10 As in the on‐the‐road test, participants were instructed to keep the speed at 95 km/hour and to stay on the right lane as much as possible (except to take over slower cars or trucks), and to otherwise behave on the road as they would while driving a real car.
Primary outcome of the study was the SDLP measured in cm. This variable has been widely used in the characterization of drug effects on driving. The SDLP is a measure of stability of keeping a steady position on the road or in the lane (i.e., a measure of “swerving” of the car). 8 , 9 , 10 In addition, other driving variables were evaluated (i.e., speed control (mean speed in km/h and SD of mean speed)), whereas subjective assessment of driving performance was investigated immediately after the driving test. Subjects indicated the perceived quality of their own driving performance on a 15 cm visual analog scale (VAS), ranging from “I drove exceptionally poorly” to “I drove exceptionally well.” The mental effort required to complete the driving test was subjectively assessed on a separate VAS, scored from “absolutely no effort” to “extreme effort,” as described earlier. 10 , 27
Safety evaluations
During the treatment periods, subjects were monitored at prespecified timepoints by assessment of adverse events (AEs), clinical laboratory tests (hematology, biochemistry, and urinalysis), physical examination, supine vital signs, and ECG. On the last day of the last treatment period (i.e., end of study), safety assessments were repeated. Four weeks after the end of study, subjects were contacted via telephone to confirm the status of any ongoing AEs.
PK assessments
At predose (trough) and 8 hours postdose, 4 mL blood samples were collected by venipuncture to measure residual plasma concentration of daridorexant using a validated liquid chromatography coupled to tandem mass spectrometry assay with a lower limit of quantification of 0.500 ng/mL, as described elsewhere. 13 The inter‐batch precision for daridorexant was ≤ 6.0% and the accuracy −1.4% to 3.6%.
Statistics
Sample size
Sample size estimations were carried out via simulations using SAS version 9.4 (SAS Institute, Cary, NC) and were based on data obtained from a previous 4‐way cross‐over study performed with the Green Dino driving simulator. 21 In this study, an average within‐subject increase in SDLP of 2.6 cm (compared with placebo) and an SD of the SDLP of 4.01 cm were observed with blood alcohol concentrations of 0.05% (legal threshold in the Netherlands and many European countries), which is considered clinically relevant. 24 , 25 , 26 With a 2‐sided type 1 error of 2.5% and an SD of 4.01 cm, a sample size of 60 subjects provides > 80% power to demonstrate that daridorexant 50 mg does not increase SDLP by ≥ 2.6 cm compared with placebo on day 2 and day 5 (if the true mean difference is 0 cm). Both day 2 and day 5 assay sensitivity tests were powered at > 90%.
Statistical methodology
The study was based on a hierarchical testing approach (Figure S1 ) which consisted of assay sensitivity testing, followed by testing the effect of daridorexant on driving performance. SDLP was analyzed based on least square means (LSM) differences from placebo with a mixed model for repeated measurements with treatment, study day, period, sex, and age group (50–64 and 65–80 years) as fixed effects and subject as random effect. An unstructured covariance matrix was used to account for correlation between repeated measurements from the same subject.
Statistical testing strategy
First, assay sensitivity was determined on day 2 and day 5 (Figure S1 ) by comparing the mean difference of SDLP between zopiclone and placebo.
According to the testing strategy (Figure S1 ), the null hypothesis of both assay sensitivity tests was formulated such that the mean difference in SDLP of zopiclone vs. placebo on day 2 and day 5 was ≤ 0 cm. Both assay sensitivity tests needed to be rejected in a sequential order at an alpha‐level of 5%.
The primary end point (the effect of daridorexant 50 and 100 mg on SDLP) was investigated with the null hypothesis formulated such that the mean difference of daridorexant compared with placebo in SDLP on day 2 and day 5 was > 2.6 cm at an alpha‐level of 2.5% (i.e., the null hypothesis was rejected if the upper limit of the 97.5% confidence interval (CI) of the mean SDLP was below 2.6 cm).
SDLP was also analyzed by symmetry to evaluate whether there was a difference in the number of subjects with an increase or decrease in SDLP, as described in literature. 28 , 29 In short, an improvement was defined as a decrease of SDLP > 2.6 cm compared with placebo and an impairment was defined as an increase of SDLP > 2.6 cm compared with placebo. Subjects with changes within these thresholds were designated neutral. The number of impaired and improved subjects was identified, and the two frequencies were compared using McNemar’s test. 29 The P values for the two proportions of subjects with impairment and improvement were calculated using an exact binomial test.
Exposure‐effect relationships were explored by linear regression of SDLP values vs. residual plasma concentrations.
RESULTS
Demographics and subject disposition
A total of 30 middle‐aged and 30 elderly (1:1 male to female ratio) subjects were randomized to one of the treatment sequences and 58 completed all treatments. Subject characteristics are presented in Table 1 . One subject discontinued the study due to an AE (dislocated shoulder due to a fall, not related to the study drug) during placebo treatment and one subject discontinued the study due to a positive alcohol breath test during treatment with daridorexant 50 mg. None of the discontinued subjects was excluded from the analysis set. Six individual driving assessments (out of a total of 480 possible drives) in 6 subjects were excluded from the analysis as they were performed more than 1 hour after the scheduled timepoint.
Table 1.
Subject characteristics
| Middle‐aged (n = 30) | Elderly (n = 30) | Overall (n = 60) | |
|---|---|---|---|
| Age, years | 58.5 (4.4) | 70.7 (3.5) | 64.6 (7.3) |
| Weight, kg | 75.8 (12.8) | 77.6 (12.6) | 76.7 (12.6) |
| Height, cm | 171.5 (10.9) | 172.3 (10.2) | 171.9 (10.5) |
| BMI, kg/m2 | 25.7 (2.6) | 26.1 (3.2) | 25.9 (2.9) |
Numbers represent mean (SD).
BMI, body mass index.
Sensitivity of the driving simulator
With zopiclone treatment, on day 2, LSM difference (95% CI) to placebo was 4.75 cm (3.23–6.26) with P < 0.0001, and on day 5 the LSM difference (95% CI) to placebo was 2.37 cm (1.19–3.56) with P < 0.0001 (Figure S2 , Table S1 ). These results confirmed sensitivity of the simulator to measure changes in SDLP after administration of zopiclone.
Primary driving end point
On day 2, LSM (97.5% CI) differences to placebo were 2.19 cm (0.46–3.93) and 4.43 cm (2.72–6.15) for daridorexant 50 and 100 mg, respectively (Figure 1 , Table 2 ; i.e., the upper bound of the CIs exceeded the threshold of 2.6 cm). Therefore, the null hypothesis was not rejected. After repeated dosing on day 5, LSM (97.5% CI) differences to placebo for 50 and 100 mg daridorexant were 0.26 cm (−1.08 to 1.59; P < 0.0001) and 0.94 cm (−0.40 to 2.27; P = 0.0027), respectively (i.e., the upper bound of the CIs for both doses of daridorexant did not exceed the threshold of 2.6 cm). Therefore, on day 5, both null hypotheses were rejected (Table 2 ).
Figure 1.
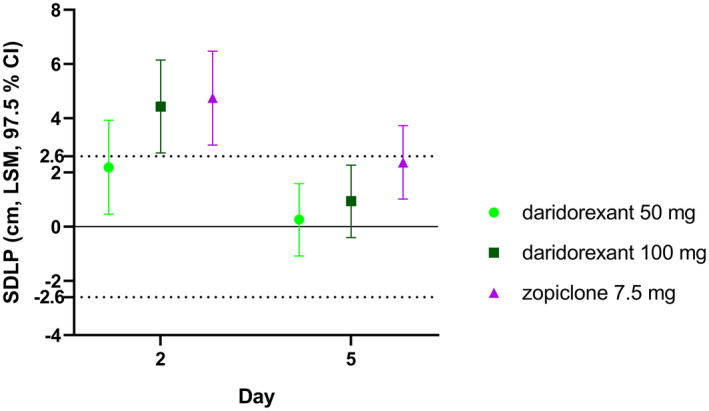
Primary driving end point analysis. LSM estimates [cm] of placebo‐corrected SDLP for daridorexant 50 and 100 mg, and zopiclone on day 2 and day 5 (n = 56–59). CI, confidence interval; LSM, least square mean; SDLP, standard deviation of the lateral position.
Table 2.
LSM estimates (cm) of SDLP and difference to placebo for daridorexant 50 and 100 mg, zopiclone, and placebo on day 2 and day 5
| Treatment | n | LSM (97.5% CI) | Difference to placebo (97.5% CI) | P value for threshold 2.6 cm |
|---|---|---|---|---|
| Day 2 | ||||
| Daridorexant 50 mg | 57 | 39.58 (37.21–41.96) | 2.19 (0.46–3.93) | 0.2991 |
| Daridorexant 100 mg | 59 | 41.82 (39.46–44.19) | 4.43 (2.72–6.15) | 0.9917 |
| Zopiclone 7.5 mg | 57 | 42.13 (39.76–44.51) | 4.75 (3.01–6.48) | 0.9972 |
| Placebo | 58 | 37.39 (35.02–39.76) | ||
| Day 5 | ||||
| Daridorexant 50 mg | 59 | 36.82 (34.58–39.06) | 0.26 (−1.08–1.59) | < 0.0001* |
| Daridorexant 100 mg | 59 | 37.50 (35.26–39.74) | 0.94 (−0.40–2.27) | 0.0027* |
| Zopiclone 7.5 mg | 56 | 38.94 (36.68–39.74) | 2.37 (1.02–3.73) | 0.3533 |
| Placebo | 58 | 36.56 (34.32–38.81) |
CI, confidence interval; LSM, least square mean; SDLP, standard deviation of the lateral position.
P values (one‐sided) are based on the mean difference of SDLP daridorexant/zopiclone treatment –placebo ≥ 2.6 cm.
*Statistically significant. Zopiclone data are included for complete display of the data.
Drug effects on SDLP (i.e., LSM differences to placebo) were further investigated by age group (middle‐aged and elderly) and sex, with results showing similar patterns as compared with the overall study population (Figures S3 , S4 ). No period or carry‐over effects were detected (i.e., a “learning effect” could be excluded).
Symmetry analysis
With zopiclone treatment, significantly more subjects (62.5%) showed impaired driving compared with improved (5.4%) driving on day 2 (P < 0.0001), as well as on day 5 (50.0% impaired vs. 12.5% improved, P = 0.0005). For daridorexant, on day 2, a significant difference in the number of impaired vs. improved subjects was found: 43.6% impaired vs. 14.5% improved (P = 0.0070) and 65.5% impaired vs. 6.9% improved (P < 0.0001), for daridorexant 50 and 100 mg, respectively (Figure 2 , Table 3 ). On day 5, results show that daridorexant treatment was statistically not different from placebo: 25.9% of subjects showed impaired vs. 27.6% improved and 24.1% impaired vs. 12.1% improved performance with 50 and 100 mg, respectively. The symmetry analysis by age and sex showed similar patterns compared with the overall study population.
Figure 2.
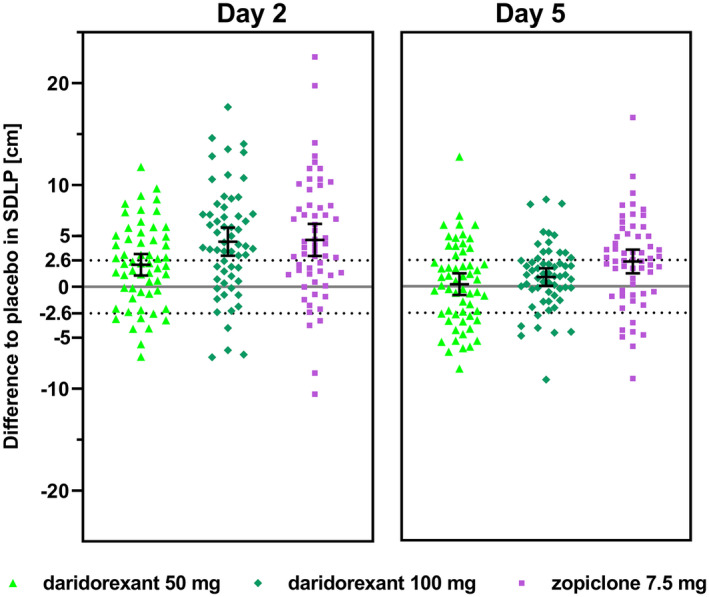
Symmetry analysis of daridorexant 50 mg, daridorexant 100 mg, and zopiclone (n = 56–59). Placebo‐subtracted individual values are presented together with mean and their 95% CIs from mixed model of repeated measurements. The dashed line (2.6 cm) represents the mean effect (=impairment) observed with alcohol using the same simulator. Values < −2.6 cm represent an improvement. Values between the dashed lines are neutral. CI, confidence interval; SDLP, standard deviation of the lateral position.
Table 3.
Symmetry analysis of daridorexant 50 mg and 100 mg, and zopiclone vs. placebo
| Contrast | Day |
Impaired n (%) |
Improved n (%) |
Neutral n (%) |
McNemar χ2 | P value |
|---|---|---|---|---|---|---|
| Zopiclone vs. placebo |
2 5 |
35 (62.5) 28 (50.0) |
3 (5.4) 7 (12.5) |
18 (32.1) 21 (37.5) |
26.95 12.60 |
< 0.0001 0.0005 |
| Daridorexant 50 mg vs. placebo |
2 5 |
24 (43.6) 15 (25.9) |
8 (14.5) 16 (27.6) |
23 (41.8) 27 (46.6) |
8.00 0.03 |
0.0070 1.00 |
| Daridorexant 100 mg vs. placebo |
2 5 |
38 (65.5) 14 (24.1) |
4 (6.9) 7 (12.1) |
16 (27.6) 37 (63.8) |
27.52 2.33 |
< 0.0001 0.19 |
Impaired subject = subject with increase in SDLP from corresponding treatment over placebo > 2.6 cm; Improved subject = subject with decrease in SDLP from corresponding treatment over placebo < −2.6 cm.
SDLP, standard deviation of the lateral position.
Speed control
Neither mean speed nor the SD of the mean speed revealed a clear effect of any treatment (Table S2 ).
Subjective driving performance
Subjects self‐rated their driving quality significantly lower on day 2 after administration of either dose of daridorexant compared with placebo (Figure 3a ). After administration of zopiclone, subjects did not rate the quality of driving different from placebo. On day 5, none of the active treatments showed a significant difference to placebo.
Figure 3.
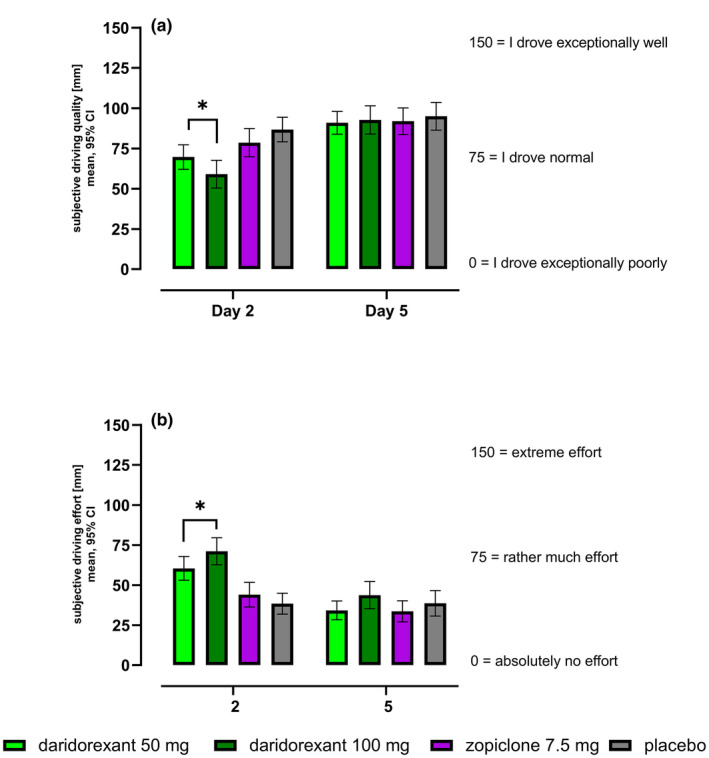
VAS subjective driving quality (a) and effort (b) in mm: absolute values (mean, 95% CI) for each treatment on day 2 and day 5 (n = 56–59). CI, confidence interval; VAS, visual analog scale, arithmetic mean is presented. *Statistically significant (difference to placebo).
Results showed a significantly higher perceived effort required to complete the driving task on day 2 for both daridorexant 50 and 100 mg (Figure 3b ), whereas results for zopiclone were similar to placebo. On day 5, none of the active treatments showed a significant difference to placebo.
Safety evaluation
No serious AEs were reported during the study. All reported AEs were mild, except for one severe AE (shoulder dislocation) during placebo treatment which led to study discontinuation. Ninety‐three percent of the subjects reported at least one AE and, overall, more AEs were reported on active treatment compared with placebo. The most frequently reported treatment‐emergent AEs (≥ 10 subjects in at least 1 treatment period) were somnolence, headache, and fatigue. To place the reported AEs in perspective with driving performance, analysis of the safety data focused on the AEs reported on the driving test days (i.e., on day 2 and day 5), CNS‐related AEs of somnolence, headache, and fatigue were compared. On day 2, more subjects reported somnolence, headache, and fatigue following administration of daridorexant 50 and 100 mg compared with zopiclone (Table 4 ). On day 5, the number of AEs reported was very low and similar across all four treatments (i.e., a large decrease in the number of reported occurrences of somnolence, headache, and fatigue was found between day 2 and day 5 for all treatments). Other safety variables (laboratory values, vital signs, and ECG) were unremarkable.
Table 4.
Cumulative number of most frequently reported adverse events on day 2 and day 5 per treatment
| Placebo (n = 59) | Zopiclone 7.5 mg (n = 58) | Daridorexant 50 mg (n = 59) | Daridorexant 100 mg (n = 59) | |||||
|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | |
| Somnolence | 5 | 2 | 18 | 3 | 20 | 1 | 25 | 1 |
| Headache | 10 | 0 | 4 | 0 | 12 | 3 | 11 | 0 |
| Fatigue | 1 | 1 | 3 | 0 | 8 | 0 | 9 | 1 |
Presented are adverse events reported in ≥ 10 subjects in at least one treatment period.
Pharmacokinetics
Following administration of daridorexant 50 and 100 mg, mean (95% CI) plasma concentrations were 424 (380–467) and 797 ng/mL (713–882) in the morning of day 2, respectively. On day 5, a slight increase was observed with mean (95% CI) plasma concentrations of 498 (441–556) and 967 ng/mL (876–1058), respectively. The observed plasma concentrations were similar in middle‐aged and elderly subjects and in male and female subjects, consistent with the known daridorexant profile from previous studies. 14 , 15
Exposure‐effect relationships
Relative to placebo, the SDLP increments and daridorexant plasma concentrations showed a weak trend for correlation (Figure S5 ; i.e., SDLP increased minimally with increasing plasma concentrations after initial dosing on day 2), with a less pronounced trend for correlation after repeated dosing on day 5. There was no clear influence of age or sex on the correlation of SDLP (differences to placebo) and plasma concentrations of daridorexant.
DISCUSSION
Investigation of driving performance based on SDLP, using a monotonous highway scenario with a long test duration of 1 hour, is recognized as an important element of the safety evaluation of new CNS‐active drugs. 10 , 11 , 12 In this study, the next‐morning residual effects of single and multiple evening administrations of 50 and 100 mg daridorexant on simulated driving were assessed in 60 healthy male and female middle‐aged and elderly subjects. Study treatments were administered on 4 consecutive evenings, and simulated driving was performed in the morning on day 2 and day 5, at 9 hours postdose. The main outcome, the SDLP, was evaluated by comparing the mean daridorexant‐placebo difference to a predefined threshold of 2.6 cm. This threshold had been established in a previous study by evaluating subjects that drove under the influence of alcohol at the legal limit (i.e., a blood level of 0.05%). 21
The present study was designed according to applicable guidances. 11 For safety reasons, a driving simulator was chosen because there was no experience regarding potential next‐morning residual effects of the required supratherapeutic dose of daridorexant 100 mg.
To demonstrate validity of the study (i.e., the sensitivity of the simulator to detect drug effects), zopiclone effects on SDLP were assessed. Zopiclone, a nonbenzodiazepine hypnotic, is known to elicit reliable and repeatable increases of SDLP, 24 comparable with the effect of blood alcohol levels of 0.05–0.08%. 30 These next‐morning effects have led to the advice not to drive within 12 hours of administration of zopiclone. 23 Both on day 2 and day 5, mean SDLP values observed with zopiclone were significantly higher compared with placebo, such that sensitivity of the simulator and study validity could be confirmed.
Results of the primary analysis for daridorexant showed that the placebo‐corrected SDLP mean values increased dose‐dependently on day 2, whereas on day 5, after repeated dosing, SDLP with daridorexant 50 and 100 mg was significantly below the threshold (i.e., statistically not different from placebo).
Results of the primary end point analysis were supported by the analysis of the SDLP based on symmetry. As expected, based on the observed SDLP values, on day 2, more subjects showed impaired than improved driving following both daridorexant doses, although on day 5, slightly more subjects showed improved (16) than impaired (15) driving performance on 50 mg, whereas many were in the neutral category (27).
Driving performance on all three active treatments improved on day 5 vs. day 2, whereas SDLP on placebo only minimally changed. A similar “first‐dose” effect on next‐morning driving performance has been reported in studies with the dual orexin receptor antagonist suvorexant. 25 , 31 The authors suggested that a potential explanation for the observed changes between initial and repeated dosing could be that the study population developed behavioral strategies to compensate for unwanted drug effects. Behavioral adaptation to drug effects has been shown in subjects exposed to repeated drinking sessions in which they were able to adapt their motor function (eye‐hand coordination) during weekly tests. 32 An explanation of the disappearance of the effects after repeated dosing could also be development of tolerance to unwanted effects. Such acute desensitization has previously been described after a single dose of zolpidem. 33
Subjective driving assessments with daridorexant, for which subjects were asked after each driving session about their perception of their driving quality and the effort required to complete the test, showed a concordant pattern of results with the objective variables: daridorexant 50 and 100 mg showed lower self‐rated driving quality and higher self‐rated effort than placebo on day 2, whereas on day 5 results were similar to placebo.
Interestingly, results of the subjective driving quality and effort with zopiclone are in marked contrast to the corresponding objective SDLP values. Subjects rated their driving quality and the required effort as similar to placebo, in strong discordance with the actual objective driving performance based on SDLP, which was significantly worse than placebo. The apparent lack of accurate judgment of one’s own driving performance associated with CNS drugs (such as alcohol, zaleplon, zolpidem, and alprazolam) has been described previously. 10 , 27 Based on these self‐rated results, subjects were better able to judge their own driving performance when treated with daridorexant compared with zopiclone (Figure 3 ), which represents an important safety aspect, because being aware of adverse drug effects and recognizing that driving is impaired are essential for taking appropriate countermeasures. 27 Overall, the effects on driving performance were similar across subgroups for age and sex.
An important aspect of this study is the chosen population. The simulated driving test shows a PD effect of daridorexant in healthy sleepers who do not benefit from the drug. In these subjects with a normally functioning orexin system, it is not surprising that blocking orexin induces changes in vigilance. It remains to be established whether the effects observed in this study can be generalized to patients with insomnia who may have an upregulated orexin system and would benefit from daridorexant. 24 , 34 Results from daridorexant phase II and III studies support this hypothesis, based on the absence of excessive morning sleepiness, decreased daytime sleepiness, and improved daytime functioning observed in patients with insomnia. 17 , 18 , 19 In addition, the incidence of somnolence reported in phase II and III studies was considerably lower compared with that reported in the driving study, highlighting the differences between healthy sleepers and subjects with insomnia.
Another important methodological aspect of this study is the use of a highly sensitive driving simulator to evaluate SDLP. Other CNS‐active drugs were examined in on‐the‐road driving studies conducted in real traffic with subjects accompanied in the car by an investigator and a driving instructor with access to dual control pedals. If a subject displayed inadequate control of the car during on‐the‐road driving, the instructor could abort the drive, whereas in the simulator, deviations from the lateral position were not corrected. 8 , 9 , 10 , 24 , 25 , 26 The chosen simulator appears to be very sensitive to steering maneuvers by the driver and, in addition, the gravitational feedback resulting from changes of the lane position during real driving is not present, resulting in weaker signaling of subtle changes of the position. Similarly, the alertness‐raising stimulus created by real traffic is missing in the simulator, which makes the 1 hour long simulated drive on a highway even more monotonous, and, consequently, the task of maintaining a steady lane position more difficult compared with real driving. It has been shown previously that simulated driving may pose higher demands in keeping a steady position compared with on‐the‐road driving tests, indicated by the approximately two‐fold increased SDLP with placebo and zopiclone in the simulator. 21 , 35 Recently, the simulator used in this study has also shown sensitivity to the effects of the selective orexin receptor antagonist seltorexant. 36
When comparing the study with conventional on‐the‐road studies, it is worth highlighting the new concept of including a supratherapeutic dose, with 100 mg daridorexant in the current study. The FDA’s rationale to include a supratherapeutic dose in the update of the driving guidance is to account for potentially increased drug exposure due to drug–drug interactions, or in patients with specific genetic traits or other characteristics (e.g., reduced clearance). 11 For safety reasons, this new paradigm may favor the conduct of future driving studies in a simulator.
It appears that the methodology of the driving simulator used in this study is better able to detect subtle effects on driving performance than the rather crude on‐the‐road test, making it difficult to indirectly compare the results with former studies.
Plasma levels of daridorexant determined 8 hours postdose in the morning of days 2 and 5 were in line with data from previous studies, and no obvious correlation between plasma levels and SDLP was present. 13 , 14 , 15 A poor correlation between plasma concentrations of psychoactive drugs and SDLP has been shown previously. 37 In contrast, blood levels of alcohol do show a correlation with SDLP. 21 , 38
Overall, daridorexant was well‐tolerated. The distribution of the AEs was remarkable. Most AEs were reported on day 2 (i.e., after the first dose of study treatment in each period, i.e., out of 47 subjects reporting somnolence, 43 subjects reported this AE on day 2; Table 4 ). The high frequency of subjects reporting somnolence after initial vs. repeated dosing was consistent with the results of the objective (SDLP) and subjective (VAS driving quality and effort) variables, in which an impairing effect of daridorexant on driving performance was observed after initial dosing. The observed association between driving results and AE reporting was not present with zopiclone: subjects did not self‐report impaired driving quality or increased driving efforts on either day, and despite the absence of somnolence on day 5, with zopiclone treatment subjects continued to show impaired driving performance, contrary to subjects administered daridorexant 50 or 100 mg.
This study demonstrated next‐morning residual PD effects in healthy sleepers following initial exposure to daridorexant, which seem to disappear after repeated dosing. As it cannot be excluded that the drug is used on an as‐needed basis, patients taking daridorexant should be cautioned about driving until they know how daridorexant affects them. 39
The high sensitivity of the driving simulator warrants its broader use in studying (residual) effects of CNS drugs.
FUNDING
The study was funded by Idorsia Pharmaceuticals Ltd.
CONFLICT OF INTEREST
C.M., C.V., M.M., and J.D. are employees and stockholders of Idorsia Pharmaceuticals Ltd. S.B., G.E.J., and R.G.Z. are employees of CHDR that received financial compensation for the conduct of the study.
AUTHOR CONTRIBUTIONS
C.M. and S.B. wrote the manuscript. C.M., C.V., M.M., and J.D. designed the research. S.B., G.J., and R.Z. performed the research. C.M. and M.M. analyzed the data. M.M. contributed analytical tools.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S2
ACKNOWLEDGMENTS
The authors would like to thank Adam F. Cohen for his input on the study design. Furthermore, we would like to thank at Idorsia Pharmaceuticals Ltd. Racheal Rowles who coordinated the study, Giancarlo Sabattini who was responsible for bioanalysis, and Alexandre Mathis who managed data processing. Peggy de Mesmaeker was responsible for on‐site monitoring. In addition, we would like to thank Esther Davidse and her team for providing invaluable support during execution of the driving assessments. Last, we would like to thank the nursing staff at CHDR and the participants for their contribution to the study.
- 1. European Commission, Directorate General for Transport . Annual accident report <https://ec.europa.eu/transport/road_safety/sites/default/files/pdf/statistics/dacota/asr2018.pdf> (2018). Accessed May 12, 2021.
- 2. U.S. Department of Transportation . Traffic safety facts 2017. A compilation of motor vehicle crash data <https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/812806> (2017). Accessed May 12, 2021.
- 3. Buscemi, N. et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta‐analysis of RCTs. J. Gen. Intern. Med. 22, 1335–1350 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daurat, A. et al. Lorazepam impairs highway driving performance more than heavy alcohol consumption. Accid. Anal. Prev. 60, 31–34 (2013). [DOI] [PubMed] [Google Scholar]
- 5. Jongen, S. , Vuurman, E. , Ramaekers, J.G. & Vermeeren, A. Comparing the effects of oxazepam and diazepam in actual highway driving and neurocognitive test performance: a validation study. Psychopharmacology 235, 1283–1294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Sluiszen, N. et al. Driving performance and neurocognitive skills of long‐term users of benzodiazepine anxiolytics and hypnotics. Hum. Psychopharmacol. 34, e2715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois, S. , Bedard, M. & Weaver, B. The impact of benzodiazepines on safe driving. Traffic Inj. Prev. 9, 404–413 (2008). [DOI] [PubMed] [Google Scholar]
- 8. O'Hanlon, J.F. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br. J. Clin. Pharmacol. 18, 121–129 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Hanlon, J.F. , Haak, T.W. , Blaauw, G.J. & Riemersma, J.B. Diazepam impairs lateral position control in highway driving. Science 217, 79–81 (1982). [DOI] [PubMed] [Google Scholar]
- 10. Verster, J.C. & Roth, T. Standard operation procedures for conducting the on‐the‐road driving test, and measurement of the standard deviation of lateral position (SDLP). Int. J. Gen. Med. 4, 359–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . Guidance for industry: evaluating drug effects on the ability to operate a motor vehicle <https://www.fda.gov/media/90670/download> (2017). Accessed May 12, 2021.
- 12. Kay, G. & Logan, B.K. Drugged driving expert panel report: a consensus protocol for assessing the potential of drugs to impair driving <https://www.nhtsa.gov/sites/nhtsa.dot.gov/files/811438.pdf> (2011). Accessed May 24, 2021. [PubMed]
- 13. Muehlan, C. , Heuberger, J. , Juif, P.‐E. , Croft, M. , van Gerven, J. & Dingemanse, J. Accelerated development of the dual orexin receptor antagonist ACT‐541468: integration of a microtracer in a first‐in‐human study. Clin. Pharmacol. Ther. 104, 1022–1029 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Muehlan, C. , Brooks, S. , Zuiker, R. , van Gerven, J. & Dingemanse, J. Multiple‐dose clinical pharmacology of ACT‐541468, a novel dual orexin receptor antagonist, following repeated‐dose morning and evening administration. Eur. Neuropsychopharmacol. 29, 847–857 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Muehlan, C. , Boehler, M. , Brooks, S. , Zuiker, R. , van Gerven, J. & Dingemanse, J. Clinical pharmacology of the dual orexin receptor antagonist ACT‐541468 in elderly subjects: Exploration of pharmacokinetics, pharmacodynamics and tolerability following single‐dose morning and repeated‐dose evening administration. J. Psychopharmacol. 34, 326–335 (2019). [DOI] [PubMed] [Google Scholar]
- 16. Sakurai, T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181 (2007). [DOI] [PubMed] [Google Scholar]
- 17. Dauvilliers, Y. et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann. Neurol. 87, 347–356 (2020). [DOI] [PubMed] [Google Scholar]
- 18. Zammit, G. , Dauvilliers, Y. , Pain, S. , Sebök Kinter, D. , Mansour, Y. & Kunz, D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology 94, 22–32 (2020). [DOI] [PubMed] [Google Scholar]
- 19. Mignot, E. et al. Sleep and daytime effects of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double‐blind, 3‐month, placebo‐controlled, phase 3 trials. Lancet Neurol. 21, 125–139 (2022). [DOI] [PubMed] [Google Scholar]
- 20. Anund, A. & Kircher, K. Advantages and disadvantages of different methods to evaluate sleepiness warning systems <https://www.diva‐portal.org/smash/get/diva2:675399/FULLTEXT02.pdf> (2009). Accessed May 24, 2021.
- 21. Huizinga, C.R. et al. Evaluation of simulated driving in comparison to laboratory‐based tests to assess the pharmacodynamics of alprazolam and alcohol. J. Psychopharmacol. 33, 791–800 (2019). [DOI] [PubMed] [Google Scholar]
- 22. Jacobs, M. , Hart, E.P. , Mejia Miranda, Y. , Groeneveld, G.J. , van Gerven, J.M.A. & Roos, R.A.C. Predictors of simulated driving performance in Huntington’s disease. Park. Relat. Disord. 60, 64–69 (2019). [DOI] [PubMed] [Google Scholar]
- 23. Wadworth, A.N. & McTavish, D. Zopiclone. A review of its pharmacological properties and therapeutic efficacy as an hypnotic. Drugs Aging 3, 441–459 (1993). [DOI] [PubMed] [Google Scholar]
- 24. Verster, J.C. , Spence, D.W. , Shahid, A. , Pandi‐Perumal, S.R. & Roth, T. Zopiclone as positive control in studies examining the residual effects of hypnotic drugs on driving ability. Curr. Drug. Saf. 6, 209–218 (2011). [DOI] [PubMed] [Google Scholar]
- 25. Vermeeren, A. et al. On‐the‐road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non‐elderly healthy volunteers. Sleep 38, 1803–1813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vermeeren, A. et al. On‐the‐road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep 42, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verster, J.C. & Roth, T. Drivers can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology 219, 775–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laska, E. , Meisner, M. & Wanderling, J. A maximally selected test of symmetry about zero. Statist. Med. 31, 3178–3191 (2012). [DOI] [PubMed] [Google Scholar]
- 29. Simen, A.A. et al. A randomized, crossover, placebo‐controlled clinical trial to assess the sensitivity of the CRCDS Mini‐Sim to the next‐day residual effects of zopiclone. Ther. Adv. Drug Saf. 6, 86–97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leufkens, T.R. & Vermeeren, A. Zopiclone’s residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo‐controlled studies. Clin. Ther. 36, 141–150 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Vermeeren, A. et al. On‐the‐road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology 233, 3341–3351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vogel‐Sprott, M. Is behavioral tolerance learned? Alcohol Health Res. World 21, 161–168 (1997). [PMC free article] [PubMed] [Google Scholar]
- 33. De Haas, S.L. , Schoemaker, R.C. , Van Gerven, J.M.A. , Hoever, P. , Cohen, A.F. & Dingemanse, J. Pharmacokinetics, pharmacodynamics and the pharmacokinetic/pharmacodynamic relationship of zolpidem in healthy subjects. J. Psychopharmacol. 24, 1619–1629 (2010). [DOI] [PubMed] [Google Scholar]
- 34. Riemann, D. et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med. Rev. 14, 19–31 (2010). [DOI] [PubMed] [Google Scholar]
- 35. Helland, A. et al. Comparison of driving simulator performance with real driving after alcohol intake: a randomised, single blind, placebo‐controlled, cross‐over trial. Accid. Anal. Prev. 53, 9–16 (2013). [DOI] [PubMed] [Google Scholar]
- 36. Brooks, S. et al. Acute effects of seltorexant, a selective orexin‐2 antagonist (JNJ‐ 42847922), on driving after bedtime administration. Sleep 44(Suppl. 2), A136 (2021). [Google Scholar]
- 37. Verster, J.C. & Roth, T. Blood drug concentrations of benzodiazepines correlate poorly with actual driving impairment. Sleep Med. Rev. 17, 153–159 (2013). [DOI] [PubMed] [Google Scholar]
- 38. Mets, M.A. et al. Effects of alcohol on highway driving in the STISIM driving simulator. Hum. Psychopharmacol. 26, 434–439 (2011). [DOI] [PubMed] [Google Scholar]
- 39. US Food and Drug Administration . Daridorexant highlights of prescribing information <https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214985s000lbl.pdf> (2022). Accessed March 14, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S2


