Abstract
Background:
In human biomonitoring, blood is often used as a matrix to measure exposure to per- and polyfluoroalkyl substances (PFAS). Because the toxicokinetics of a substance (determining the steady-state blood concentration) may affect the toxic potency, the difference in toxicokinetics among PFAS has to be accounted for when blood concentrations are used in mixture risk assessment.
Objectives:
This research focuses on deriving relative potency factors (RPFs) at the blood serum level. These RPFs can be applied to PFAS concentrations in human blood, thereby facilitating mixture risk assessment with primary input from human biomonitoring studies.
Methods:
Toxicokinetic models are generated for 10 PFAS to estimate the internal exposure in the male rat at the blood serum level over time. By applying dose–response modeling, these internal exposures are used to derive quantitative internal RPFs based on liver effects.
Results:
Internal RPFs were successfully obtained for nine PFAS. Perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), perfluorononanoic acid (PFNA), perfluorododecanoic acid (PFDoDA), perfluorooctane sulfonic acid (PFOS), and hexafluoropropylene oxide-dimer acid (HFPO-DA, or GenX) were found to be more potent than perfluorooctanoic acid (PFOA) at the blood serum level in terms of relative liver weight increase, whereas perfluorobutane sulfonic acid (PFBS) and perfluorohexane sulfonic acid (PFHxS) were found to be less potent. The practical implementation of these internal RPFs is illustrated using the National Health and Nutrition Examination Survey (NHANES) biomonitoring data of 2017–2018.
Discussion:
It is recommended to assess the health risk resulting from exposure to PFAS as combined, aggregate exposure to the extent feasible. https://doi.org/10.1289/EHP10009
Introduction
As early as 1968, an unusually high nonionic fluorine fraction was noticed in human plasma.1 After subsequent isolation of an organic fluoride substance, traced down to resemble the structure of perfluorooctanoic acid (PFOA), researchers suggested that exposure to commercial products would be the cause of widespread contamination of human tissue by organic fluorocompounds from anthropomorphic origin.2 Years thereafter, human biomonitoring studies revealed high exposures to PFOA and perfluorooctane sulfonic acid (PFOS) in workers,3–5 and PFOA, PFOS, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PDUnDA), perfluorohexane sulfonic acid (PFHxS), N-methylperfluorooctanesulfonamidoacetic acid (MeFOSAA), and N-ethylperfluorooctanesulfonamidoacetic acid (EtFOSAA) in communities.6–10 Due to increasing awareness regarding the persistent, bioaccumulative, and toxic properties of perfluoroalkyl acids (PFAA),11,12 regulatory measures have been put in place and are under development to mitigate exposure to these chemicals at the European level [e.g., under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation and the Persistent Organic Pollutants (POPs) Regulation] and at the Mondial level (by inclusion in the annexes of the Stockholm and Rotterdam Conventions).13 However, to date, exposure to legacy perfluoroalkyl carboxylic acids (PFCA) and perfluoroalkyl sulfonic acids (PFSA) is still apparent.14–27
Due to the high bioaccumulation potential of legacy per- and polyfluoroalkyl substances (PFAS), they were substituted by substances with shorter carbon-chain length [e.g., perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), perfluorobutane sulfonic acid (PFBS), 6:2 fluorotelomer carboxylic acid (6:2 FTSA), 6:2 fluorotelomer carboxylic acid (6:2 FTCA)] and substances with other functional groups, like perfluoroether sulfonic and carboxylic acids [e.g., hexafluoropropylene oxide-dimer acid (HFPO-DA), hexafluoropropylene oxide-trimer acid (HFPO-TA), 6:2 chlorinated polyfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA), ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA), and Nafion by-product 2 (BP2)].28–30 These substances are presumed to be of less concern in comparison with their predecessors because of the more rapid elimination from the blood in experimental animal species.31 However, whereas elimination half-lives vary from hours to several days in the rat, mouse, and monkey, in humans it may take one to several months before a reduction of 50% of chemicals such as ADONA,32 PFBS,33,34 PFHxA,34–36 perfluoroheptanoic acid (PFHpA),34,36 or perfluoropentane sulfonic acid (PFPeS)34 in the blood is reached, leaving to question whether these alternatives truly are of no concern relative to their accumulation in humans, wildlife, and livestock.
The levels of PFBA, PFHxA, and PFBS in blood of the general population in Europe are nonetheless relatively low14,18–21,24,27; however, ADONA and HFPO-DA have not been screened for on a regular basis. Quantifiable plasma concentrations were reported in two targeted monitoring studies in young men in Germany (ADONA) and in employees of a perfluoropolymer production plant in the Netherlands (HFPO-DA),37–39 but two other studies did not find any evidence for exposure to ADONA.25,27 Studies in the United States (U.S.) revealed no quantifiable serum levels for HFPO-DA and no or very low HFPO-DA urine levels in the general population and in residents living in a hot-spot region.16,40,41 Exposure to BP2, perfluoro-3,5,7,9-tetraoxadecanoic acid (PFO4DA), HFPO-TA, and 6:2 Cl-PFESA was, however, recently discovered to be prominent in populations in Asia42,43 and the U.S.41
Members of the groups of PFCA and PFSA have been associated with hepatotoxicity, nephrotoxicity, hemotoxicity, reproductive toxicity, developmental toxicity, immunotoxicity, endocrine disruption (thyroid), and carcinogenicity in toxicological experimental animal models (in rats, mice, or monkeys), which correlate fairly well with findings in human epidemiological studies.44,45 The perfluoroether carboxylic acids ADONA and HFPO-DA illustrate a toxicity profile that is comparable to the PFCA and PFSA in experimental animal studies.46–49 However, for many of the emerging PFAS, little50–54 to no toxicity information is available, urging regulatory bodies to come up with advanced and novel high-throughput testing strategies to facilitate risk assessment or to focus on concern-driven exposure mitigation for PFAS as a group.13,55,56 Still, even for the PFAS for which considerable experimental data on toxicokinetics and toxicity are present, risk assessment proves challenging. For instance, the European Food Safety Authority (EFSA) could only establish a sum tolerable weekly intake (TWI) for four PFAS (PFOA, PFNA, PFHxS, PFOS) after it was asked by the European Commission to prepare a scientific opinion on the risks to human health related to the presence of PFAS in food.45
The aggregate exposure to PFAS stems from a large variety of different sources, such as diet (including drinking water),45,57–59 consumer products,60,61 and occupational sources,60 with diet being the predominant exposure pathway.45,62 Human biomonitoring is of added value in risk assessment because, apart from its importance as an early warning signal, it serves as an empirical measure for aggregate exposure.63 In experimental animal species, PFAA are readily absorbed from the gastrointestinal tract and are excreted unmetabolized.64–68 They deposit primarily in the blood, liver, and kidney,66,67,69–74 although for some of the substances (e.g., PFBA, HFPO-DA) partitioning into organs and tissues other than the liver and adipose tissue (e.g., kidney, spleen, brain, lung) remains largely unknown due to lack of data availability. The limited human data available for PFOA and PFOS on serum-to-liver partitioning suggest a substantially higher distribution of both compounds to the liver in comparison with distributions for rodents at comparable blood concentrations.75 Due to the deposition mainly in blood, liver, and kidney, blood is considered to be the most suitable matrix to evaluate exposure to these substances in human biomonitoring in comparison with matrices such as urine, hair, saliva, or exhaled air.76
Previously, we presented relative potency factors (RPFs) for a set of 23 PFAS to assess the cumulative risk on oral (external) exposure.77 This set of RPFs is useful when estimating the risk resulting from mixtures of PFAS in, for instance, drinking water or food samples. The methodology is based on the principle of dose addition, which assumes that chemicals contribute to a common adverse effect and behave as if they were a dilution of one another.78 Exposure of each substance is converted to equivalents of an index compound (PFOA in this case) by multiplying with an RPF. Subsequently, these equivalents are summed to provide the cumulative exposure. All further calculations can then be performed as equivalents of the index compound.
In human health risk assessment of persistent, bioaccumulating substances, it is important to consider body burdens or serum concentrations across time rather than external intake doses.75 We propose that specific, internal RPFs should be used when assessing the risk of exposure to multiple PFAS at the blood (serum or plasma) level. That is, kinetics determining the internal dose after (external) intake differ between PFAS, and therefore potencies based on external doses will not be the same as potencies based on internal doses. For example, based on external doses HFPO-DA has been shown to be less potent than PFOA in the male rat when looking at relative liver weight increase77,79 but was regarded to be more potent when these external concentrations were converted to blood serum concentrations.79 Considering the fact that HFPO-DA shows a lower degree of accumulation in male rats in comparison with PFOA,80,81 the differences in external potency between HFPO-DA and PFOA may at least partly be explained by differences in toxicokinetics. This topic will be further addressed in the “Discussion” section.
In the current study, we generated a toxicokinetic model for 10 PFAS to estimate the internal exposure in the male rat at the blood serum level over time. Subsequently, these internal exposures were used to derive internal RPFs for nine PFAS (including the index compound) based on relative liver weight increase in the male rat. We close by illustrating the use of these internal RPFs, having combined them with human biomonitoring data from the National Health and Nutrition Examination Survey (NHANES) 2017–2018 cycle.82
Materials and Methods
Conceptual Overview
Derivation of internal RPFs required various steps (Figure 1; steps 1–6). First, a toxicity database was compiled, which provided information on a common toxic adverse effect, whereby other factors influencing relative potency estimates were eliminated as much as possible (e.g., difference in exposure duration, species, sex, strain) (step 1). Subsequently, for each PFAS, a toxicokinetic model was obtained (step 2) and implemented (step 3). Correct implementation was confirmed by comparing the modeled serum concentration-time curves with the experimentally derived serum concentrations of single-dose studies. After that, the modeled serum concentration-time curves were verified against experimentally derived serum concentrations in repeated-dose studies (step 4). Then, implemented models were used to convert the external administered doses of the studies in the toxicity database into internal doses by modeling the exposure scenarios of the experimental animal studies (step 5). Finally, dose–response curves were fitted to the liver weight responses plotted against the internal doses (obtained in step 1) to be able to derive RPFs (step 6).
Figure 1.
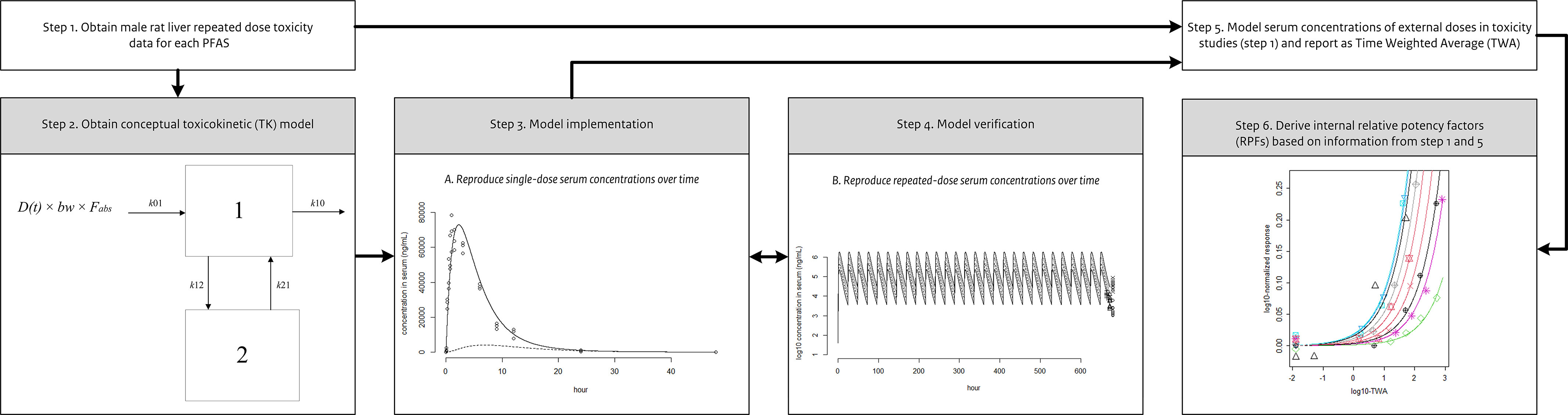
Conceptual overview of the methods applied to derive the internal relative potency factors (RPFs).
Gathering Toxicity Data (Step 1)
PFAS have been associated with disrupted hepatic metabolism, lipid accumulation in the liver, and adverse outcomes such as steatosis, necrosis, and hepatocellular tumor formation.77,83,84 Although one study suggests that some of these effects of PFAS on the liver involve the activation of the peroxisome proliferator-activated receptor-alpha () with uncertain relevance to human health,85 other studies suggest several other nuclear receptors [e.g., peroxisome proliferator–activated receptor (), constitutive androstane receptor (CAR)] and cytotoxicity may play a role in adverse effects on the liver,77 leading to the conclusion that the mechanism(s) underlying these adverse effects are still unclear and deserve further investigation.83 We consider the PFAS-induced hepatotoxic effects in rodents, including hypertrophy and liver weight increase, relevant for risk assessment and, thus, suitable for derivation of RPFs.77
A database of liver end points was previously created for 16 PFAS based on information retrieved from public literature.77 To ensure that the differences in potency would not be affected by differences in experimental setup, data were obtained from studies with the same species (rat), sex (male), and exposure route (oral) and comparable exposure duration (42–98 d).86–94 PFAS have mostly been studied under a subchronic exposure regimen in rats (in comparison with, for instance, mice) and therefore selection of this study protocol with rats resulted in the highest number of PFAS with toxicity data available. Chronic studies are available only for a limited number of PFAS (e.g., PFHxA,95 HFPO-DA,96 PFOS,97 PFOA98), and although there are also subacute studies available for quite a number of PFAS (for a recent overview see the studies cited in EFSA45 and ATSDR44), they are of lower preference due to the shorter exposure duration in comparison with subchronic studies. In general, male rats appear more sensitive to PFAS-induced liver toxicity in comparison with females and show more severe liver pathology in comparison with females at equipotent doses.94,97,99,100 These findings support the selection of male rat toxicity data for derivation of RPFs. The database may be found in Table S1.
In Bil et al.,77 we illustrated that derivation of oral external RPFs based on liver hypertrophy, absolute liver weight increase, and relative liver weight increase resulted in similar RPFs for absolute and relative liver weight and somewhat smaller RPFs for hepatic hypertrophy. Because the database for relative liver weight was most complete, the final external RPFs were based on this end point. For the same reason, relative liver weight was selected as the end point to derive internal RPFs. In addition, this allowed for direct comparison of internal and external RPFs.
Toxicokinetic Models and Equations (Step 2)
The public literature was screened for toxicokinetic data and models for the 16 PFAS included in the toxicity database, using search engine Google Scholar as well as screening the literature cited in several scientific reports and publications.44,45,79 For 10 PFAS [PFBA,68 PFHxA,81 PFOA,81 PFNA,101 PFDA,81 perfluorododecanoic acid (PFDoDA),73 PFBS,102 PFHxS,102 PFOS,102 HFPO-DA80], sufficient model parameter values were obtained from single-dose experiments. In most studies, multiple doses and exposure routes were tested. For concordance with the toxicity database, toxicokinetic studies with male rats exposed via the oral route and treated with a dose closest to the doses used in the oral repeated-dose toxicity studies, were selected.
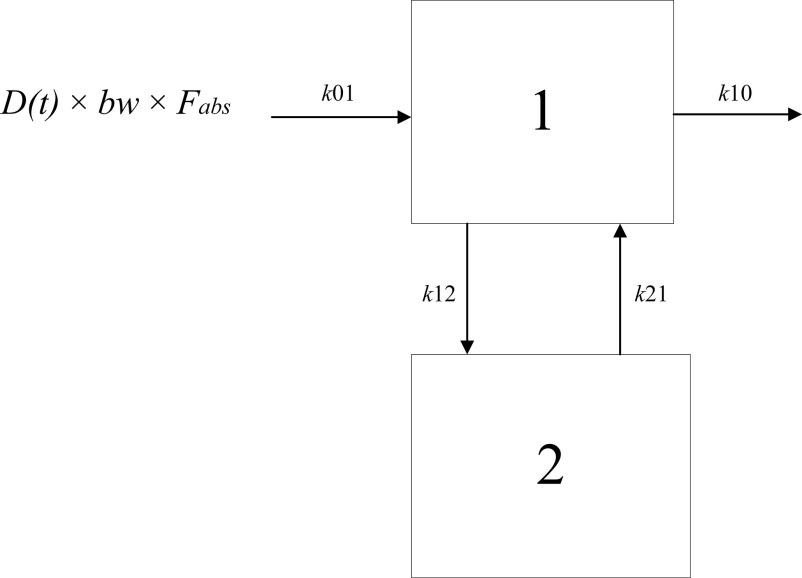
We implemented a compartmental model that incorporated mono- and biphasic processes (Figure 2). Compartments 1 and 2 represent the tissues with relatively fast and slow partitioning, respectively. The corresponding differential equations are:
| (1) |
| (2) |
| (3) |
where bolus dose of a PFAS (milligrams per kilogram body weight) at time point (hours), (kilograms), the fraction () of the amount of a PFAS in the gastrointestinal tract that will be absorbed over time (nanograms), the absorption rate constant from the gastrointestinal tract to the central compartment (Compartment 1) (1/unit time), C1 and concentration in Compartment 1 and 2 (nanograms per milliliter), the volume of distribution of Compartment 1 (milliliters), the elimination rate constant from Compartment 1 (1/unit time), and k12 and the transfer rate constants between compartments 1 and 2 (1/unit time).
Figure 2.
Schematic overview of the applied compartment model with first-order input (oral exposure). The amount A(t) of a PFAS (per- and polyfluoroalkyl substances) enters the gastrointestinal tract as the product of the externally administered dose D(t) and body weight (bw), after which a fraction () of the administered amount is absorbed (with rate constant k01) into the central compartment (Compartment 1). Elimination from the central compartment is characterized by the rate constant k10. Parameters k12 and k21 are transfer rate constants between Compartments 1 (central compartment) and 2 (peripheral compartment), which both are set to zero in case the kinetics of a PFAS are monophasic. Note: PFAS, per- and polyfluoroalkyl substances.
Model Implementation (Step 3)
To implement a kinetic model for each PFAS, values of the parameters mentioned in Equations 1, 2, and 3 were required. The publications on the single exposure toxicokinetic experiments provided most parameter values, i.e., on , , V1, k10 elimination half-life (in case of monophasic kinetics) and alpha and beta elimination half-lives (in case of biphasic kinetics), from which the other required parameters (k10, k01, k12, k21) were obtained using Equations 4–6 below.
The elimination rate (k10) was calculated from the k10 elimination half-life103 using Equation 4:
| (4) |
where k10 T1/2 is the known k10 elimination half-life.
The absorption rate (k01) was calculated from the 103,104 using Equation 5:
| (5) |
where k is either the known elimination rate (k10) in case of a one-compartment model or the known alpha rate (from Equation 4, where k10 T1/2 is substituted by T1/2) in case of a two-compartment model. This equation was solved numerically by varying k01.
Obtaining the absorption and elimination rates was done by using the relatively simple equations (Equations 4 and 5) above. The transfer rates between Compartment 1 and Compartment 2 (k12 and k21) were derived from the alpha rate, beta rate, and k10 by obtaining the roots of a more complex quadratic equation. The solution of this equation, and how k21 and k12 are derived from this, is reported in Supplemental Material, “Toxicokinetic model parameterization.”
Correct implementation of the compartment models was confirmed by visual inspection of the modeled serum concentration plotted together with the empirically obtained serum concentrations over time,68,73,81,101,102,105 and by evaluating the ratio between the modeled and reported area under the curve (AUC), maximum serum concentration (), and time at () values.
The original serum concentration data in Dzierlenga et al.81 and Huang et al.102 were retrieved from the Chemical in Effects Biological Systems (CEBS) database (https://manticore.niehs.nih.gov/cebssearch), and the study report of Gannon105 was obtained from the Health and Environmental Research Online (HERO) database (https://hero.epa.gov/). The data presented in the other publications were digitalized using DigitizeIt software (version 2.3.3; https://www.digitizeit.xyz/).
Verification (Step 4)
For seven PFAS (PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS), measured serum concentrations were available to verify the performance of the models to simulate the serum concentrations after repeated dosing. Verification was done by plotting modeled serum concentrations together with empirically obtained repeated-dose serum concentrations obtained after daily exposure for 28 d.99,100 These serum concentration data were retrieved from the CEBS database as well. Furthermore, the ratio between the modeled and reported mean serum concentration at the end of the study was evaluated.
Calculation of Internal Doses in the Serum for the Repeated-Dose Toxicity Studies (Step 5)
The exposure conditions of the experimental animal toxicity studies reported in Table S1 were implemented in the congener-specific kinetic models to convert the external administered doses under these conditions to internal doses, represented as time-weighted average (TWA) serum concentration in milligrams per milliliter. When available, the ratio between the modeled and reported mean serum concentration at the end of the study was also evaluated to verify the model predictions.
Because the maximum increase in relative liver weight by PFOA and PFOS is observed already after short-term exposure and does not change after longer exposure durations,88,91,106 the different subchronic exposure durations of the available studies were only considered to have a marginal impact on the liver response. For this reason, dose metrics depending strongly on the exposure duration, such as the AUC (in milligrams multiplied by hours per milliliter), were deemed unsuitable.
Dose–Response Analysis and Derivation of Internal RPFs (Step 6)
The RPF approach requires that a) chemicals contribute to a common effect, b) their dose–response curves are (approximately) parallel on log–dose scale, and c) chemicals do not interact.107 Parallelism ensures a constant dose factor between the curves at any effect level; thus the RPFs do not depend on the benchmark response, in case of continuous data such as relative liver weight.
Dose–response modeling and derivation of internal RPFs were performed in a manner similar to the procedure set out in Bil et al.77 In short, dose–response modeling was performed using PROAST software (version 70.2; https://www.rivm.nl/en/proast). A four-parameter exponential model,
| (6) |
was fitted to the (continuous) relative liver weight data plotted against the TWA obtained at step 5.
In a covariate analysis, parallel curves were fit to the data by applying the same shape parameters (maximum response parameter c and the steepness parameter d) in the four-parameter exponential model to all PFAS, but allowing the background (parameter a), the potency (parameter b), and the residual variance to be different between PFAS.108,109 A good description of the data of each PFAS by parallel curves was confirmed by visual inspection.
The RPF is defined as the ratio of two (equipotent) doses, for the index compound and for PFAS i, which both result in the same relative change in relative liver weight response (y):
| (7) |
When assuring the curves of the two PFAS are parallel, x/b in the exponential model (Equation 6) has the same value for both PFAS:
| (8) |
Combining Equations 7 and 8 gives Equation 9,
| (9) |
which shows that the RPF of each PFAS can be directly obtained from its potency parameter b and that of PFOA. Calculation of the RPFs and their 90% confidence intervals (CIs) based on potency parameter b was performed using PROAST software.
Calculating PFAS Mixture Exposure: Practical Implementation of Internal RPFs
The human biomonitoring data of NHANES82 were used to illustrate how to use internal RPFs in combination with a human biomonitoring data set. NHANES is designed to gather representative information for the U.S. general population. The National Center for Health Statistics (NCHS) Research Ethics Review Board reviewed and approved the study protocol. To participate in the survey, all respondents gave informed written consent, parents or guardians provided written permission for participants younger than 18 y, and children 12–17 y old provided assent.
In the 2017–2018 cycle, PFAS were measured in a random subsample of 1,929 individuals ages 12 and older. Characteristics of this subpopulation can be found on the NHANES website.82 Blood serum of each individual was screened for the quantitative detection of Me-FOSAA, PFHxA, PFOA (both linear and branched isomers), PFNA, PFDA, PFUnDA, PFHxS, PFHpS, PFOS (both linear and branched isomers), 6:2 Cl-PFESA, ADONA, and HFPO-DA. Lower limit of detection (LOD) for all substances was ). Additional information on the analytical methods, substance full names, and CAS numbers, and an overview of the data can be found in Table S2 and Figure S1, respectively.
To obtain PFOA equivalents (PEQs), PFAS serum concentrations of each participant were multiplied by the internal RPFs when both serum concentration data and internal RPFs were available. PEQs were summed to calculate the cumulative PFAS exposure per individual. NCHS recommends to use weights to account for unequal selection probabilities caused by the clustered design of NHANES and to account for oversampling certain demographic groups, if the sample would have to represent the U.S. general population.110 Because our goal was not to interpret the risk of the U.S. general population but rather to illustrate how to use internal RPFs in combination with a human biomonitoring data set, weighing was considered out of the scope of this paper.
Software
All calculations were performed in R (version 4.0.0, R Core Team). In addition, R packages were used for the kinetic models: deSolve (version 1.32),111 for dose–response analysis and deriving RPFs: PROAST software (version 70.2, RIVM, https://www.rivm.nl/en/proast), and for downloading and analyzing the NHANES data: nhanesA (version 0.6.5.3, Endres, https://CRAN.R-project.org/package=nhanesA).
Results
Model Parametrization (Step 2)
According to the authors of the original studies, the measured serum concentrations could be effectively described by either monophasic or biphasic kinetics.68,73,80,81,101,102 These toxicokinetic evaluations were implemented in our analysis (see Supplemental Materials, “Toxicokinetic model parameterization;” Tables S3–S4; Figures S2–S8). Only PFNA was regarded to be better described by monophasic kinetics rather than biphasic kinetics.
The starting parameters , , V1, k10 T1/2, T1/2, and T1/2 were used to obtain the other required parameters k10, k01, k12, k21 by using Equations 4 and 5, and Equations S7–S11 (Supplemental Material, “Toxicokinetic model parametrizations”). In some cases the k10 T1/2 (HFPO-DA), V1 (PFDoDA), or k10 T1/2 and V1 (PFNA) were not provided, and thus these values were obtained by optimizing the toxicokinetic models to the empirically obtained serum concentrations over time.73,101,105 Further details are provided in Supplemental Material, “Toxicokinetic model parameterization.”
The toxicokinetic model parameters listed in Table 1 were subsequently used to implement the toxicokinetic compartment models for PFBA, PFHxA, PFOA, PFNA, PFDA, PFDoDA, PFBS, PFHxS, PFOS, and HFPO-DA (Equations 1–3). When the kinetics of a PFAS could be explained by monophasic kinetics, the transfer rates k12 and k21 were set to zero.
Table 1.
Toxicokinetic model parameter values of per- and polyfluoroalkyl substances (PFAS).
| Compound | Study | Evaluation of TK model fit | Dose (mg/kg) | (−) | (h) | V1 (mL/kg) | T1/2 (h) | T1/2 (h) | k10 T1/2 (h) | k01 (1/h) | k10 (1/h) | k12 (1/h)i | k21 (1/h)i |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFBA | Chang et al.68 | Monophasica | 30 | 1.0b | 1.25a | 209a | — | — | 9.22a | 3.0a | 0.075h | — | — |
| PFHxA | Dzierlenga et al.81 | Biphasica | 160 | 1.0b | 0.890a | 348a | 1.46a | 13.7a | 1.63a | 2.2f | 0.43h | 0.039 | 0.056 |
| PFOA | Dzierlenga et al.81 | Monophasica | 12 | 1.0b | 6.37a | 154a | — | — | 258a | 0.92f | 0.0027h | — | — |
| PFNA | Tatum-Gibbs et al.101 | Monophasicc | 3 | 1.0b | — | 170j | — | — | — | 1.0b | 0.00025j | — | — |
| PFDA | Dzierlenga et al.81 | Biphasica | 10 | 1.0b | 9.06a | 228a | 123a | 995a | 478a | 0.50f | 0.0015i | 0.0021 | 0.0027 |
| PFDoDA | Kawabata et al.73 | Monophasica | 50 | 1.0a | 120a,g | 663d | — | — | 1,327a,g | 0.036f | 0.00052h | — | — |
| PFBS | Huang et al.102 | Biphasica | 20 | 1.0b | 2.18a | 148a | 2.37a | 5.36a | 2.73a | 0.68f | 0.25h | 0.018 | 0.15 |
| PFHxS | Huang et al.102 | Monophasica | 16 | 1.0b | 5.89a | 137a | — | — | 396a,g | 1.1f | 0.0018h | — | — |
| PFOS | Huang et al.102 | Biphasica | 2 | 1.0b | 14.3a | 280a | 74.4a,g | 972a,g | 478a,g | 0.24f | 0.0015h | 0.0040 | 0.0045 |
| HFPO-DA | Gannon et al.80,e | Biphasica | 10 | 1.0a | — | 142a | 2.8a | 72.2a | — | 3.3a | 0.24k | 0.0099 | 0.010 |
Note: —, no data; T1/2, alpha elimination half-life; T1/2, beta elimination half-life; HFPO-DA, hexafluoropropylene oxide-dimer acid; , fraction of the amount of a PFAS in the gastrointestinal tract that will be absorbed over time; k01, absorption rate constant from the gastrointestinal tract into Compartment 1; k10, elimination rate constant from Compartment 1; k10 T1/2, elimination half-life from Compartment 1; k12, transfer constant from Compartment 1 to Compartment 2; k21, transfer constant from Compartment 2 to Compartment 1; PFAS, per- and polyfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; TK, toxicokinetic; , time at maximum serum concentration; V1, volume of distribution of Compartment 1.
Value provided in the study from oral, single exposure of males at the dose provided in column “dose.”
Based on assumption in the original studies.
In the original publication the authors report that the data illustrated biphasic kinetics. See Supplementary Material, “Toxicokinetic model parameterization.”
Calculated in this study. See Supplemental Material, “Toxicokinetic model parameterization.”
Serum concentrations per individual animal over time reported in Gannon.105 Note that 12-h concentrations were assumed to be transposed in the original report. See also Supplementary Material, “Toxicokinetic model parameterization.”
Calculated according to Equation 5.
Provided in study in different unit (days).
Calculated according Equation 4.
Calculated according to method in Supplemental Material, “Toxicokinetic model parameterization.”
Optimized; note that study reports other values for V1 and k10. See remarks in Supplemental Material, “Toxicokinetic model parameterization.”
Optimized according to the method shown in Supplemental Material, “Toxicokinetic model parameterization.”
Model Implementation and Verification (Step 3 and 4)
For each model, the single-dose serum curve was plotted together with the original serum concentration data (Figures S9–S18). As plasma measures for HFPO-DA were performed, a 1:1 serum to plasma ratio was assumed according to the observations in Ehresman et al.112 Additionally, verification for repeated dose exposure was performed for seven PFAS (PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS) (Figures S19–S27). We show the study selection and model results for PFBS below for illustrative purposes.
Single-dose modeling verification.
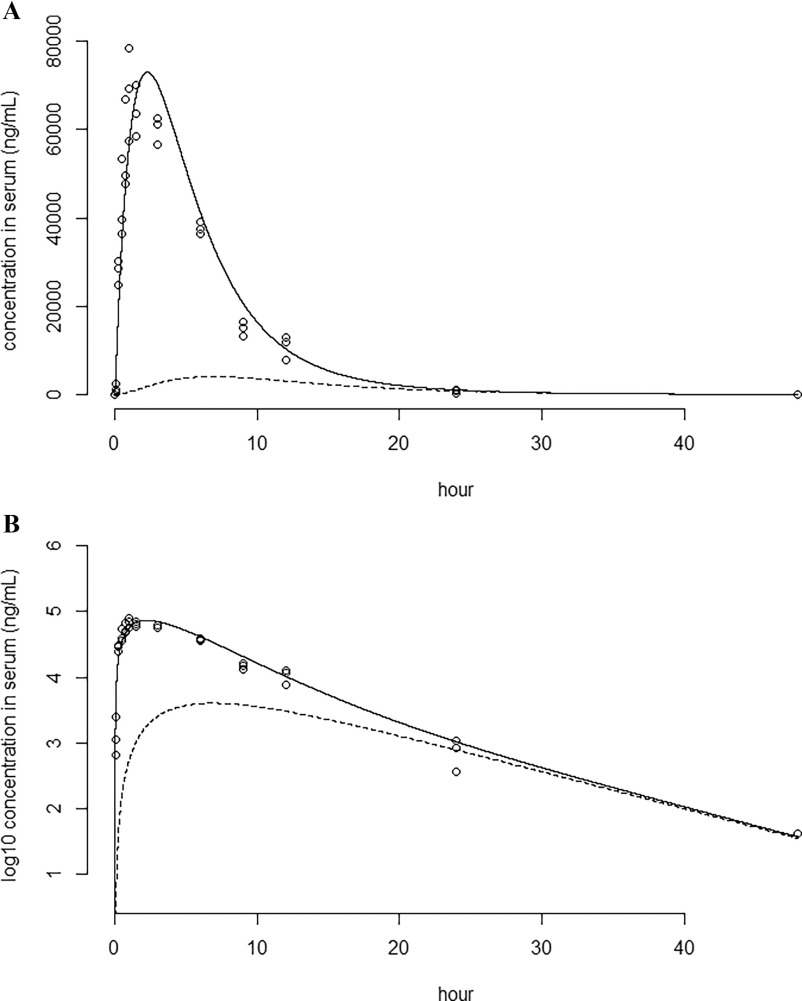
Because the repeated-dose study for PFBS in the toxicity database was dosed at 0, 30, 100, 300, and body weight/d, the dose was selected from the toxicokinetic experiments in Huang et al.102 (dosed at 4, 20, and ) because this dose was in the same range as the doses of the repeated-dose study. The same line of reasoning was used to select the doses for the other substances. To verify correct implementation, the single-dose experiments informing the parameter values were modeled. Table 2 shows the modeled AUC, , and in comparison with the values for these parameters reported in the original studies. The ratios between modeled and reported values range from 0.8 to 1.2.
Table 2.
Comparison of reported and modeled area under the curve (AUC), maximum serum concentration (), and time at maximum serum concentration () for the single-dose experiments.
| Compound | Study | AUC | (h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reported | Modeled | Ratio | Unitb | Reported | Modeled | Ratio | Unitb | Reported | Modeled | Ratio | ||
| PFBA | Chang et al.68 | 1,900 | 1,600 | 1.2 | 130 | 130 | 1.0 | µg/mL | 1.3 | 1.3 | 1.0 | |
| PFHxA | Dzierlenga et al.81 | 3.5 | 3.4 | 1.0 | 0.97 | 0.96 | 1.0 | mM | 0.89 | 0.91 | 1.0 | |
| PFOA | Dzierlenga et al.81 | 70 | 67 | 1.0 | 0.19 | 0.18 | 1.1 | mM | 6.4 | 6.4 | 1.0 | |
| PFNA | Tatum-Gibbs et al.101 | 15,000 | 18,000 | 0.8 | 22 | 18 | 1.2 | µg/mL | — | 8.8 | — | |
| PFDA | Dzierlenga et al.81 | 59 | 54 | 1.1 | 0.083 | 0.082 | 1.0 | mM | 9.1 | 11 | 0.8 | |
| PFDoDA | Kawabata et al.73 | — | 140 | — | 54 | 70 | 0.8 | µg/mL | 120 | 120 | 1.0 | |
| PFBS | Huang et al.102 | 1.8a | 1.8 | 1.0 | 0.25 | 0.24 | 1.0 | mM | 2.2 | 2.3 | 1.0 | |
| PFHxS | Huang et al.102 | 170a | 150 | 1.1 | 0.29 | 0.29 | 1.0 | mM | 5.9 | 5.9 | 1.0 | |
| PFOS | Huang et al.102 | 9.9a | 8.9 | 1.1 | 0.010 | 0.013 | 0.8 | mM | 14 | 17 | 0.8 | |
| HFPO-DA | Gannon et al.80 | — | 0.88 | — | — | 0.17 | — | mM | — | 0.86 | — | |
Note: —, no data; HFPO-DA, hexafluoropropylene oxide-dimer acid; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
AUC units in Huang et al.102 should be (C.R. Blystone, personal communication).
Units reported in the study are used.
Figure 3 shows the modeled single-dose serum concentration of PFBS over time, plotted together with the reported mean serum concentrations from Huang et al.102 Figures of the simulations of single-dose experiments for PFBA, PFHxA, PFOA, PFNA, PFDA, PFDoDA, PFHxS, PFOS, and HFPO-DA are included as Figures S9–S18.
Figure 3.
Perfluorobutane sulfonic acid (PFBS) serum concentration plotted against time (h) after an oral single dose of 20 mg/kg in male rats. Note: The solid line is the modeled concentration in the serum (nanograms per milliliter). The dashed line indicates the concentration in the peripheral compartment (nanograms per milliliter). Circles are the measured concentrations of the individual animals () retrieved from Huang et al.102 Figures are plotted on a linear (A) and (B) scale.
Repeated-dose modeling verification.
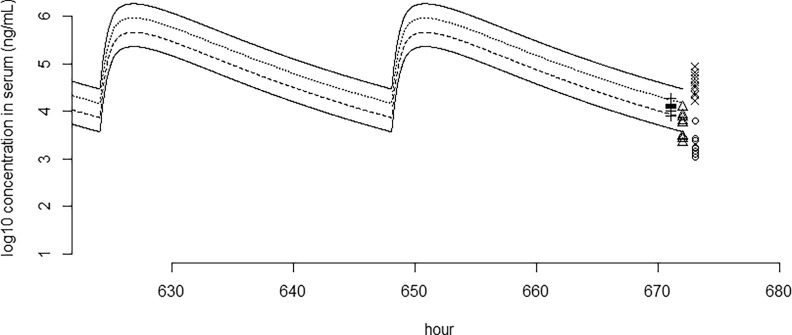
Figure 4 shows the modeled repeated-dose serum concentration of PFBS over time, plotted together with the mean measured serum concentrations shown in NTP99 at day 28. In Table 3 the modeled and reported serum concentrations are listed, showing that the model is able to predict the measured concentrations after 4 wk () of daily exposure, with ratios ranging from 0.5 to 5.5. Figures S19–S27 show the simulation of 28-d repeated-dose experiments for PFHxA, PFOA, PFNA, PFDA, PFHxS, and PFOS, in addition to that of PFBS.
Figure 4.
Modeled perfluorobutane sulfonic acid (PFBS) serum concentrations over time upon daily dosing together with 28-d serum concentrations (symbols) in male rats () as reported in NTP,99 plotted on -scale. Only the last of the experiment are shown. Note: Lowest solid line and circles ; dashed line and triangles ; dotted line and plusses ; upper solid line and crosses . To distinguish measured points, they have been shifted slightly.
Table 3.
External dose and modeled and reported concentrations at the end of the study for each of the doses in 28-d repeated-dose toxicity studies for male rats specifically.
| Compound | Reference | External dose (mg/kg body weight/d) | Concentration at end of the study (mg/mL) | ||
|---|---|---|---|---|---|
| Modeled | Reported mean | Ratio | |||
| PFBS | NTP99 | 62.6 | 3.7 | 2.2 | 1.3 |
| 125 | 7.4 | 5.3 | 1.4 | ||
| 250 | 15 | 12 | 1.3 | ||
| 500 | 30 | 43 | 0.7 | ||
| PFHxS | NTP99 | 0.625 | 71 | 66 | 1.1 |
| 1.25 | 140 | 92 | 1.5 | ||
| 2.5 | 280 | 130 | 2.2 | ||
| 5 | 570 | 160 | 3.6 | ||
| 10 | 1,100 | 200 | 5.5 | ||
| PFOS | NTP99 | 0.312 | 13 | 24 | 0.5 |
| 0.625 | 26 | 52 | 0.5 | ||
| 1.25 | 52 | 94 | 0.6 | ||
| 2.5 | 100 | 170 | 0.6 | ||
| 5 | 210 | 320 | 0.7 | ||
| PFHxA | NTP100 | 62.6 | 1 | 0.38 | 2.6 |
| 125 | 2 | 0.50 | 4.0 | ||
| 250 | 4 | 1.3 | 3.1 | ||
| 500 | 8.1 | 3.4 | 2.4 | ||
| 1,000 | 16 | 11 | 1.5 | ||
| PFOA | NTP100 | 0.625 | 48 | 51 | 0.9 |
| 1.25 | 96 | 73 | 1.3 | ||
| 2.5 | 190 | 95 | 2.0 | ||
| 5 | 380 | 110 | 3.5 | ||
| 10 | 770 | 150 | 5.1 | ||
| PFNA | NTP100 | 0.625 | 95 | 57 | 1.7 |
| 1.25 | 190 | 160 | 1.2 | ||
| 2.5 | 380 | 380 | 1.0 | ||
| 5 | 760 | 360 | 2.1 | ||
| PFDA | NTP100 | 0.156 | 9 | 8.5 | 1.1 |
| 0.312 | 18 | 23 | 0.8 | ||
| 0.625 | 36 | 43 | 0.8 | ||
| 1.25 | 72 | 100 | 0.7 | ||
| 2.5 | 140 | 260 | 0.5 | ||
Note: NTP, National Toxicology Program; PFBS, perfluorobutane sulfonic acid; PFDA, perfluorodecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Calculation of Internal Doses in the Serum for the Repeated-Dose Toxicity Studies (Step 5)
The internal doses were calculated for each of the doses in the repeated-dose toxicity studies as presented in Table S1. Please note that no subchronic toxicity study for PFDA was available, hampering further calculation of an internal RPF for this substance with the toxicokinetic model available. Modeled TWA and serum concentration at the end of the study are provided for each substance in Table 4, together with the serum concentration measured at the end of the dosing experiments, when available. The ratios between modeled and reported serum concentrations at the end of the study ranged from 0.2 to 7.0. The modeled TWA serum concentration was used as dose metric for dose–response modeling and RPF derivation.
Table 4.
External dose, modeled TWA (time-weighted average) serum concentration, and modeled and reported concentrations at the end of the study for each of the doses in subchronic repeated-dose toxicity studies for male rats specifically.
| Substance | Reference | External dose (mg/kg body weight/d) | Modeled TWA (mg/mL) | Concentration at end of the study (mg/mL) | ||
|---|---|---|---|---|---|---|
| Modeled | Reported mean | Ratio | ||||
| PFBS | Lieder et al.86 | 30 | 0.016 | 0.0018 | — | — |
| 100 | 0.053 | 0.0059 | — | — | ||
| 300 | 0.16 | 0.018 | — | — | ||
| 1,000 | 3.7 | 0.059 | — | — | ||
| PFHxS | Butenhoff et al.87 | 0.3 | 0.024 | 0.042 | 0.044 | 1.0 |
| 1 | 0.080 | 0.14 | 0.089 | 1.6 | ||
| 3 | 0.24 | 0.42 | 0.13 | 3.2 | ||
| 10 | 0.80 | 1.4 | 0.20 | 7.0 | ||
| PFOS | Seacat et al.88 | 0.03 | 0.0015 | 0.0025 | 0.0040 | 0.6 |
| 0.13 | 0.0065 | 0.011 | 0.017 | 0.6 | ||
| 0.34 | 0.017 | 0.029 | 0.044 | 0.7 | ||
| 1.33 | 0.066 | 0.11 | 0.15 | 0.7 | ||
| PFBA | Butenhoff et al.89 | 1.2 | 0.0028 | 0.0012 | 0.0061 | 0.2 |
| 6 | 0.014 | 0.0061 | 0.014 | 0.4 | ||
| 30 | 0.071 | 0.030 | 0.052 | 0.6 | ||
| PFHxA | Loveless et al.90 | 20 | 0.0017 | 0.00032 | — | — |
| 100 | 0.0083 | 0.0016 | — | — | ||
| 500 | 0.041 | 0.0081 | — | — | ||
| PFOA | Perkins et al.91 | 0.06 | 0.0047 | 0.0059 | 0.0071 | 0.8 |
| 0.64 | 0.050 | 0.062 | 0.041 | 1.5 | ||
| 1.94 | 0.15 | 0.19 | 0.070 | 2.7 | ||
| 6.5 | 0.51 | 0.63 | 0.14 | 4.5 | ||
| PFNA | Mertens et al.92 | 0.025 | 0.0044 | 0.010 | — | — |
| 0.125 | 0.022 | 0.051 | — | — | ||
| 0.6 | 0.11 | 0.25 | — | — | ||
| PFDoDA | Kato et al.93 | 0.1 | 0.0019 | 0.0049 | — | — |
| 0.5 | 0.0096 | 0.024 | — | — | ||
| 2.5 | 0.048 | 0.12 | — | — | ||
| HFPO-DA | Haas94 | 0.1 | 0.000051 | 0.000069 | — | — |
| 10 | 0.0051 | 0.00069 | — | — | ||
| 100 | 0.051 | 0.0069 | — | — | ||
Note: —, no data; HFPO-DA, hexafluoropropylene oxide-dimer acid; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Dose–Response Analysis and Derivation of Internal RPFs (Step 6)
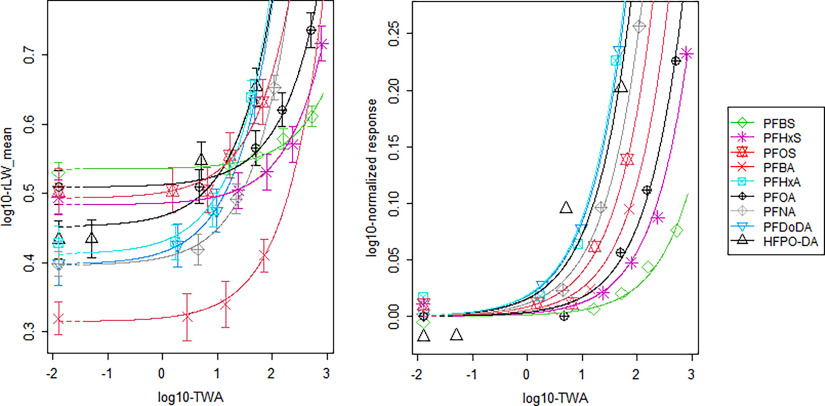
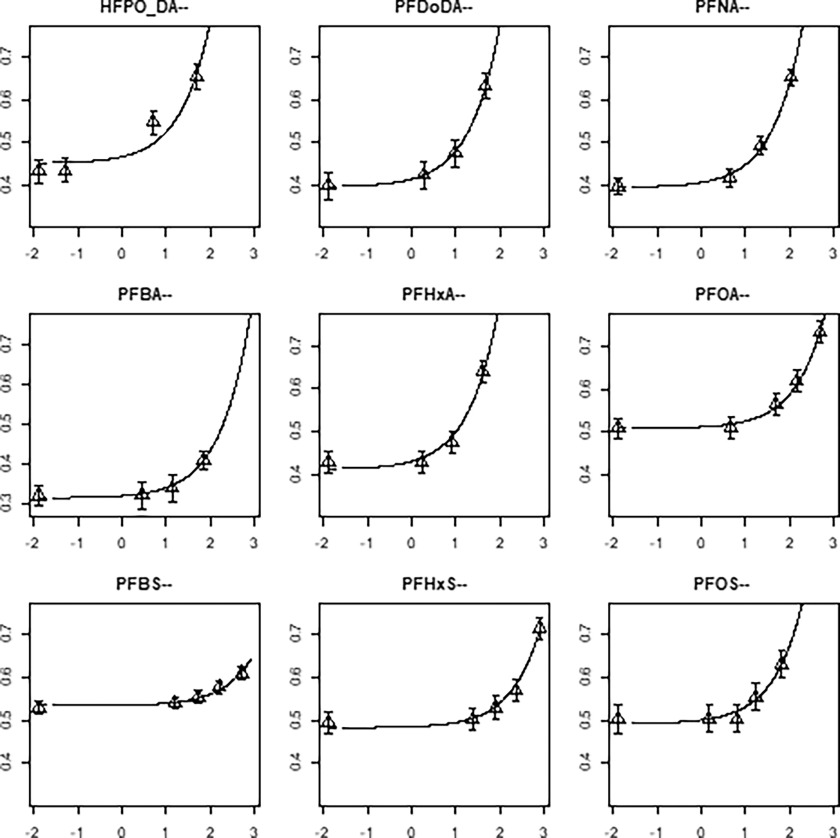
Dose–response curves were fit to the data using an exponential model with compound as a covariate on the background parameter (a), the potency parameter (b), and the residual variance (Equation 6). In this analysis the parameters c and d, determining the shape of the curves, were the same for each PFAS. This approach resulted in dose–response curves which have a study-specific background (Figure 5, left panel) and potency (Figure 5, right panel) but have the same shape—i.e., they are parallel on the log-dose scale. In Figure 6 the curves from Figure 5 are plotted separately to confirm that curves with the same shape provide a good description of the measured data of all PFAS. Only one data point for HFPO-DA could not be described by the model, because the confidence interval was slightly next to the dose–response curve. However, this deviation was considered marginal, and HFPO-DA was included for further derivation of the RPFs.
Figure 5.
Dose–response curves for mean relative liver weight increase (g/100 g body weight) plotted against the internal dose (expressed as TWA in milligrams per milliliter) on a -scale for nine PFAS, obtained by fitting the exponential model with a study-specific (modeled) background response (left plot) and normalized to the background response (right plot). Note: Figures represent geometric mean response for each dose group, as derived from the summary data (Table S1). For clarity, the whiskers indicating the 95% confidence intervals of the means are not plotted in the right plot. HFPO-DA, hexafluoropropylene oxide-dimer acid; PFAS, per- and polyfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; rLW, relative liver weight; TWA, time-weighted average.
Figure 6.
Individual dose–response curves for relative liver weight increase (g/100 g body weight) (y-axis) plotted against the internal dose (expressed as TWA in milligrams per milliliter) (x-axis) on a -scale for nine PFAS, obtained by simultaneously fitting the exponential model to confirm the same curve shape is applicable to all PFAS. Note: Triangles and whiskers are the geometric mean relative liver weights and their 95% confidence intervals, as derived from the summary data reported in Table S1. HFPO-DA, hexafluoropropylene oxide-dimer acid; PFAS, per- and polyfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; TWA, time-weighted average.
Internal RPFs
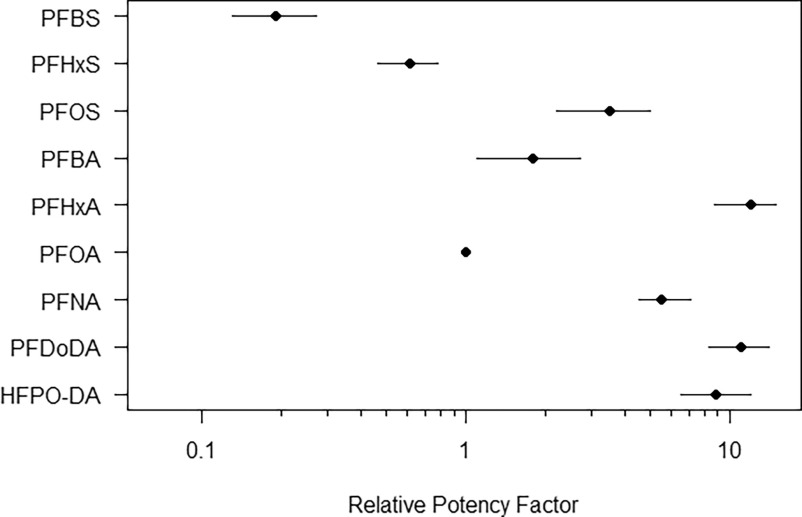
The RPF analysis indicates that, based on TWA blood concentrations, the range in potencies relative to PFOA varies between 0.2 and 10 (Table 5). PFBA, PFHxA, PFNA, PFDoDA, PFOS, and HFPO-DA are more potent than PFOA, whereas PFBS and PFHxS are less potent in comparison with PFOA in terms of relative liver weight increase. Because dose–response modeling was used for RPF derivation, the uncertainty of the underlying toxicity data could be taken into account. The 90% CI ranges of the RPFs are illustrated in Figure 7 and are provided as numerical values in Table S5.
Table 5.
Internal relative potency factors (RPFs) for PFAS based on time-weighted average (TWA) serum concentrations expressed against relative liver weight in the male rat obtained from Subchronic repeated-dose toxicity studies. RPFs were derived by simultaneous fitting of parallel curves and expressing the relative potency of each PFAS against the index compound (PFOA) that received an RPF of 1.
| Compound | Internal RPF |
|---|---|
| PFBS | 0.2 |
| PFHxS | 0.6 |
| PFOS | 3 |
| PFBA | 2 |
| PFHxA | 10 |
| PFOA | 1 |
| PFNA | 5 |
| PFDoDA | 10 |
| HFPO-DA | 9 |
Note: HFPO-DA, hexafluoropropylene oxide-dimer acid; PFAS, per- and polyfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Figure 7.
The internal relative potencies of nine PFAS based on relative liver weight (circles), plotted on a log10-scale. The horizontal lines indicate the 90% confidence interval ranges. Perfluorooctanoic acid (PFOA) was used as index compound (i.e., relative potency factor ). Note: HFPO-DA, hexafluoropropylene oxide-dimer acid; PFAS, per- and polyfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDoDA, perfluorododecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Calculating PFAS Mixture Exposure: Practical Implementation of Internal RPFs
NHANES 2017–2018 human biomonitoring measurements.
In NHANES 2017–2018, several of the PFAS measured were detected in a large fraction of the population, with exception of PFHxA, ADONA, and HFPO-DA, which were detected above LOD in nine, zero, and one individuals, respectively. Median exposure values of PFOA (linear), PFNA, PFHxS, PFOS (linear and branched isomers) were highest, although exposure to PFDA, PFUnDA, PFHpS, Me-FOSAA, and 6:2 Cl-PFESA was also prominent in the sampled population. A summary box plot of the measured serum concentrations is provided in Figure S1.
Cumulative exposure of PFAS in the NHANES sample expressed as sum of PFOA equivalents (PEQs).
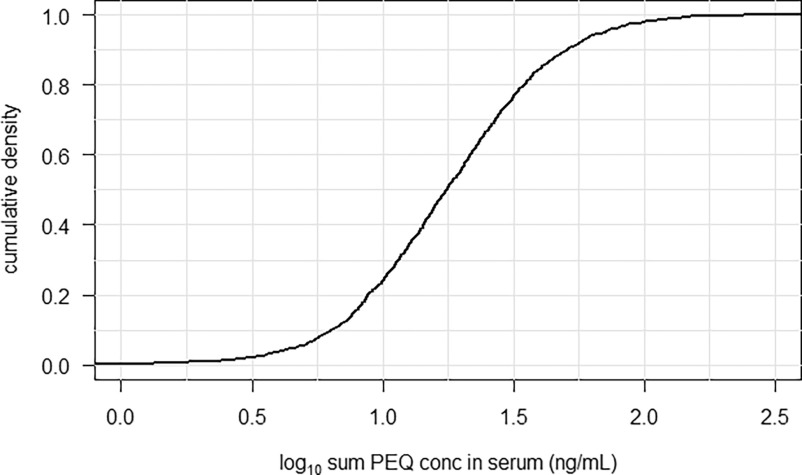
Exposure to six PFAS (PFHxA, PFOA, PFNA, PFHxS, PFOS, HFPO-DA) for each participant in the NHANES study population was converted to PEQs by combining serum concentrations (above LOD) with internal RPFs. For the other PFAS (ADONA, PFDA, PFHpS, Me-FOSAA, and 6:2 Cl-PFESA), no internal RPFs are available, so these PFAS could not be included in the assessment of the cumulative PEQ exposure. A cumulative density plot of the sum PEQ in the NHANES study population is presented in Figure 8. The density plot may be found in Figure S28. The black line represents cumulative exposure as sum PEQ (nanograms per milliliter) of all PFAS from which internal RPFs were derived. Furthermore, for each individual the contribution of each PFAS to the individual’s total PEQ concentration was derived. Exposure to PFOS contributes highest to the sum PEQ (Figure S29). Together, linear and branched PFOS contributed approximately 80% to the sum PEQ on average.
Figure 8.
Cumulative density plot of the sum PFOA Equivalent (PEQ) concentration in serum (ng/mL) from the 2017–2018 National Health and Nutrition Examination Survey (NHANES) study population, ages 12 and older ().82 Note: The black line represents the sum PEQ of all PFAS [perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), perfluoroocane sulfonic acid (PFOS), hexafluoropropylene oxide-dimer acid (HFPO-DA)] of which internal relative potency factors were derived.
Discussion
In the current paper, we present a methodology based on internal RPFs, which allowed us to perform mixture risk assessment for PFAS based on serum concentration data. The compartment models were capable of predicting the serum concentrations for single-dose exposure with ratios between modeled and reported values ranging from 0.8 to 1.2 and the repeated-dose experimental data with ratios between modeled and reported values ranging from 0.2 to 7.0. We subsequently succeeded in modeling internal doses for 10 PFAS in the male rat by using these compartment models. These internal doses were used to calculate nine internal RPFs based on liver effects in the male rat. Because PFAS contribute to common adverse health effects (such as liver effects and immune effects), it is strongly recommended to assess the risk of PFAS as combined, cumulative, exposure.45,56 In the current study, quantitative internal RPFs are provided for as many PFAS as currently achievable to facilitate a group approach for PFAS risk assessment.
Previously, Gomis et al.79 employed toxicokinetic modeling to qualitatively rank the potencies of six PFAS based on external dose, serum concentration (AUC), and target organ concentration (AUC). The ranking of PFAS in the current paper from most potent to least potent is different from that based on serum concentrations in Gomis et al.79 This difference in approach likely has a methodological foundation. Whereas we both used animal toxicity data and based our analysis on the same end point (i.e., liver toxicity in the male rat), Gomis et al.79 used a different kinetic model concept to convert from external to internal dose, used AUCs instead of TWAs, and used no adverse observed effect levels (NOAELs) instead of dose–response analysis. Nevertheless, both studies point in the same direction: Relative potencies for PFAS are, overall, more comparable at the blood level than based on the oral external dose, and hence, toxicokinetic differences among PFAS significantly affect potency. Specifically, the range of internal RPFs for the PFAS in our study ranged from 0.2 to 10, whereas the external RPFs for the same PFAS ranged from 0.001 to 10.77 This observation is also supported by the findings of Vogs et al.,113 who report that recalculation of external effect concentrations to internal effect concentrations in zebrafish reduced the difference in potency between PFAS considerably.
As a general remark, our analysis did not allow us to take into account the uncertainty of the underlying toxicokinetic data, which is captured by the ratio difference in measured and modeled serum concentrations, and hence this uncertainty is not reflected in the CIs of the RPFs that are reported in Table S5. The largest ratio differences between measured and modeled point estimate serum concentrations at the end of the study ranged from 0.2 to 7.0 (Table 4). Based on the toxicokinetic information currently available for the PFAS that we could derive RPFs for, we are not able to reduce this uncertainty. We used calibrated models based on the best toxicokinetic data currently available: the single-dose experiments that include multiple measurements over time. The repeated-dose studies did not allow for derivation of toxicokinetic model parameters due to absence of information or a too-limited number of measurements taken over time. In that regard, all internal RPFs, but especially the internal RPF for PFDoDA, should be interpreted with caution. For this substance, there was only one high-dose toxicokinetic study available,114 and the difference with the dosing regimen in the subchronic study was substantial (toxicokinetic study body weight/d; top-dose toxicity study body weight/d). This circumstance forced us to make more crude assumptions in comparison with the analyses of the other substances (see Supplementary Material, “Toxicokinetic model parameterization”) because we were unable to verify the toxicokinetic model for repeated-dose exposure of PFDoDA with empirical data because data were lacking. As a consequence, we have reservations as to whether the model is able to predict the internal concentration at the dosing range of the toxicity study and consequently whether our analysis correctly reflects the internal RPF of PFDoDA.
Although the adverse effects resulting from PFAS exposure manifest at the target site, relative potencies based on serum concentrations are required for risk assessment based on human biomonitoring data. This requirement is because exposure to PFAS is usually assessed based on blood measurements in human biomonitoring studies.76 Also, Health-Based Guidance Values (HBGVs) are available on the level of the serum concentration.44,45 To estimate the cumulative risk in a study population, the sum PEQ distribution should be compared to such an HBGV expressed on serum concentration level based on toxicity data for PFOA itself44 or based on toxicity data of the sum of PFAS where PFOA is the main contributor.45 In using RPFs, it is assumed that the relative toxicities are the same in humans and in male rats. Potential interspecies differences are not corrected for in derivation of the RPFs but in derivation of the HBGV. Thus, when using the RPF methodology, researchers assume that the same interspecies factor for possible remaining differences in toxicokinetics (e.g., serum-to-liver partitioning) and toxicodynamics, based on information of the index compound, is also applicable to the other PFAS.
For some PFAS with relatively short elimination half-lives, single blood samples represent exposure as a snapshot in time, which is surrounded by uncertainty of intake and elimination processes. As our analysis illustrates, the and values in male rats may differ considerably for substances with a high elimination rate (see Figure 4). To observe potential background exposure of PFAS in humans that is missed by looking at blood solely, paired urine–blood samples could be taken for substances with shorter elimination half-lives16,45 to verify whether blood concentrations provide a proper estimate of the internal exposure. Moreover, researchers should be aware of compounds that may be metabolized in the body, such as mono-, di-, and tripolyfluoroalkyl phosphate esters (PAPs),115 fluorotelomer alcohols (FTOHs),36,115–117 perfluoroalkyl sulfonamidoethanols (FOSEs),118,119 perfluoroalkyl sulfonamides (FOSAs),118,119 and perfluoroalkyl phosphinic acids (PFPiA).120 Here as well, it is important to take account of the exposure metric used. For intake doses, it is most convenient to use the RPF of the parent compound, under the assumption that the metabolization pathway between species is similar. Conversely, at the internal level, it is important to consider the RPFs for the stable intermediate and final metabolites, particularly for those that bioaccumulate. Because the concentrations of PFAA in the human blood are generally high in comparison with their precursors,115 exposure to these substances may be taken into account at least to some extent.
We acknowledge that the proposed method is subject to further refinement, but we are of the opinion that the actual risk can be estimated more accurately when potency differences between PFAS are taken into account and when several PFAS can be included in the risk calculation,121 which is in line with the goal to account for cumulative exposure to substances.122 To further align the RPF methodology with the existing HBGVs for PFAS, it is essential to study the relative potencies for effects other than liver toxicity in the male rat. This applies to the external as well as the internal RPFs. Discussion of this issue was already touched on in Bil et al.,77 where it was noted that it would be relevant to validate whether RPFs based on data for other end points, sex, species, and life stages would result in comparable estimates of relative potencies and whether liver toxicity RPFs, which will probably be the largest set of in vivo RPFs, may be a proxy of the differences in overall toxicity among PFAS. One potential database for further study would be that of developmental toxicity studies with mice.123–136 Models may also be established for female mice, because toxicokinetic information for model implementation is readily available in the public literature.64,65,67,68,71,80,137 This approach would allow modeling of systemic internal exposure and derivation of internal RPFs for developmental toxicity. For human (epidemiological) data sets, it has not been illustrated yet whether it is feasible to obtain a relevant set of internal RPFs. Currently, efforts are being made to investigate whether it is possible to derive internal RPFs based on human data sets for parameters indicative of immune functioning. These analyses will be supplemented by internal RPF analyses for immune-relevant parameters from animal toxicity studies. This development is particularly relevant in the European context, because the point of departure for the EFSA TWI is based on immunosuppression in children.138
Due to the absence of internal RPFs, exposure to PFDA, PFUnDA, PFHpS, Me-FOSAA, and 6:2 Cl-PFESA measured in the NHANES study could not be included in the example calculation, which consequently underestimates cumulative exposure and hence underestimates the cumulative risk. Furthermore, it is not unlikely that the general population is exposed to an unknown fraction of PFAS, because an increasing proportion of unknown total organofluorine has been observed in several human biomonitoring studies in recent years.27,139 Wishing to have a better understanding of the risk associated with cumulative exposure to PFAS, we encourage researchers to focus on identifying the unknown fraction of PFAS in human blood and to subsequently investigate the toxicokinetics and toxicity of these substances. This investigation could be done by performing in vivo animal experiments, but researchers may also employ other sources, such as in vitro studies and accompanying quantitative in vitro to in vivo extrapolation (QIVIVE) methods,140,141 or human epidemiological studies. Such data would allow researchers to extend the number of internal RPFs and to verify the animal toxicity data-derived RPFs with alternative resources.
Although emissions of PFAS in Europe may be expected to diminish due to several policy initiatives, such as the planned restriction for nonessential uses of PFAS under REACH, exposure to the bulk of PFAS already emitted to the environment may continue, due to the persistence of these substances and their tendency to remain in the water phase. Consequently, development of mixture-based risk assessment tools for PFAS will remain an important topic in the future and deserves the necessary attention.
Conclusion
Human biomonitoring is of added value in risk assessment because it provides an empirical measure for aggregate exposure to multiple chemicals and thereby reflects the body burden. Consequently, risk assessment based on such information transcends the compartmentalized regulatory frameworks, specific uses, and isolated exposure routes for which risk assessments are historically performed. In the current study, we present a methodology that enables researchers to assess the risk of exposure to multiple PFAS with primary input from human biomonitoring studies. Internal RPFs were derived based on systemic internal exposure in the male rat resulting in liver effects. PFBA, PFHxA, PFNA, PFDoDA, PFOS, and HFPO-DA were found to be more potent than PFOA at the serum level, whereas PFBS and PFHxS were found to be less potent. This work highlights the importance of using the correct set of RPFs, because potency changes when based on internal systemic exposure in comparison with external exposure. Data availability was a restricting factor in derivation of internal RPFs. To better understand the cumulative risk associated with human exposure to PFAS, it is essential to identify the PFAS in the unknown organofluorine fraction in human blood and to obtain toxicokinetic and toxicity data for more PFAS.
Supplementary Material
Acknowledgments
This work was supported by the European Union (EU) Horizon 2020 research and innovation program under Grant Agreement No. 733032 HBM4EU (www.HBM4EU.eu) and received cofunding from the authors’ organization.
HBM4EU represents a joint effort of 30 countries, the European Environment Agency, and the European Commission, cofunded by Horizon 2020. The main aim of the initiative is to coordinate and advance human biomonitoring in Europe. HBM4EU provides evidence of the actual exposure of citizens to chemicals and the possible health effects to support policy-making. The project involves collaboration between several Commission services, EU agencies, national representatives, stakeholders, and scientists, demonstrating how research funding can build bridges between the research and policy worlds. The authors would like to thank E. Verbruggen, and M. Mengelers for critically reading a draft version of the manuscript.
References
- 1.Taves DR. 1968. Evidence that there are two forms of fluoride in human serum. Nature 217(5133):1050–1051, PMID: , 10.1038/2171050b0. [DOI] [PubMed] [Google Scholar]
- 2.Guy W, Taves DR, Brey W Jr. 1976. Organic fluorocompounds in human plasma: prevalence and characterization. Biochemistry Involving Carbon-Fluorine Bonds 117–134, 10.1021/bk-1976-0028.ch007 [accessed 3 January 2021]. [DOI] [Google Scholar]
- 3.Ubel F, Sorenson S, Roach D. 1980. Health status of plant workers exposed to fluorochemicals–a preliminary report. Am Ind Hyg Assoc J 41(8):584–589, PMID: , 10.1080/15298668091425310. [DOI] [PubMed] [Google Scholar]
- 4.Olsen GW, Burris JM, Burlew MM, Mandel JH. 2000. Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers. Drug Chem Toxicol 23(4):603–620, PMID: , 10.1081/dct-100101973. [DOI] [PubMed] [Google Scholar]
- 5.Olsen GW, Burris JM, Burlew MM, Mandel JH. 2003. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med 45(3):260–270, PMID: , 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 6.Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, et al. 2003. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspect 111(16):1892–1901, PMID: , 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, et al. 2004. The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. J Occup Health 46(2):141–147, PMID: , 10.1539/joh.46.141. [DOI] [PubMed] [Google Scholar]
- 8.Olsen GW, Huang HY, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH. 2005. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect 113(5):539–545, PMID: , 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. 2004. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol 38(13):3698–3704, PMID: , 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- 10.Kuklenyik Z, Needham LL, Calafat AM. 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem 77(18):6085–6091, PMID: , 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 11.Renner R. 2001. Growing concern over perfluorinated chemicals. Environ Sci Technol 35(7):154A–160A, PMID: , 10.1021/es012317k. [DOI] [PubMed] [Google Scholar]
- 12.Giesy JP, Kannan K. 2002. Peer reviewed: perfluorochemical surfactants in the environment. Environ Sci Technol 36(7):146A–152A, PMID: , 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- 13.European Commission. 2020. Commission Staff Working Document. Poly- and perfluoroalkyl substances (PFAS). Accompanying the document ‘Communication from the European Commission to the European Parlement, the Council, the European Economic and Social Committee and the Committee of the Regions Chemicals Strategy for Sustainability Towards a Toxic-Free Environment’. https://ec.europa.eu/environment/pdf/chemicals/2020/10/SWD_PFAS.pdf [accessed 3 January 2021].
- 14.Sochorova L, Hanzlikova L, Cerna M, et al. 2017. Perfluorinated alkylated substances and brominated flame retardants in serum of the Czech adult population. Int J Hyg Environ Health 220(2 pt A):235–243, PMID: , 10.1016/j.ijheh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Toms LML, Bräunig J, Vijayasarathy S, Phillips S, Hobson P, Aylward LL, et al. 2019. Per- and polyfluoroalkyl substances (PFAS) in Australia: current levels and estimated population reference values for selected compounds. Int J Hyg Environ Health 222(3):387–394, PMID: , 10.1016/j.ijheh.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong L-Y. 2019. Legacy and alternative per-and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 131:105048, PMID: , 10.1016/j.envint.2019.105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu CH, Riker CD, Lu SE, Fan ZT. 2020. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016–2018. Int J Hyg Environ Health 223(1):34–44. Jan, PMID: , 10.1016/j.ijheh.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, et al. 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int 110:149–159, PMID: , 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Ledda C, La Torre G, Cinà D, Paravizzini G, Vitale E, Pavone P, et al. 2018. Serum concentrations of perfluorinated compounds among children living in Sicily (Italy). Toxicol Lett 298:186–193, PMID: , 10.1016/j.toxlet.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Heffernan AL, Cunningham TK, Drage DS, Aylward LL, Thompson K, Vijayasarathy S, et al. 2018. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health 221(7):1068–1075, PMID: , 10.1016/j.ijheh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Pitter G, Da Re F, Canova C, Barbieri G, Zare Jeddi M, Daprà F, et al. 2020. Serum levels of Perfluoroalkyl Substances (PFAS) in adolescents and young adults exposed to contaminated drinking water in the Veneto Region, Italy: a cross-sectional study based on a health surveillance program. Environ Health Perspect 128(2):27007, PMID: , 10.1289/EHP5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colles A, Bruckers L, Den Hond E, Govarts E, Morrens B, Schettgen T, et al. 2020. Perfluorinated substances in the Flemish population (Belgium): Levels and determinants of variability in exposure. Chemosphere 242:125250, PMID: , 10.1016/j.chemosphere.2019.125250. [DOI] [PubMed] [Google Scholar]
- 23.Pirard C, Dufour P, Charlier C. 2020. Background contamination of perfluoralkyl substances in a Belgian general population. Toxicol Lett 333:13–21, PMID: , 10.1016/j.toxlet.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Duffek A, Conrad A, Kolossa-Gehring M, Lange R, Rucic E, Schulte C, et al. 2020. Per- and polyfluoroalkyl substances in blood plasma – Results of the German Environmental Survey for children and adolescents 2014–2017 (GerES V). Int J Hyg Environ Health 228:113549, PMID: , 10.1016/j.ijheh.2020.113549. [DOI] [PubMed] [Google Scholar]
- 25.Gockener B, Weber T, Rudel H, Bucking M, Kolossa-Gehring M. 2020. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ Int 145:106123, PMID: , 10.1016/j.envint.2020.106123. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Barregard L, Xu Y, Scott K, Pineda D, Lindh CH, et al. 2020. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health 19(1):1–11, PMID: , 10.1186/s12940-020-00588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miaz LT, Plassmann MM, Gyllenhammar I, Bignert A, Sandblom O, Lignell S, et al. 2020. Temporal trends of suspect-and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017. Environ Sci Process Impacts 22(4):1071–1083, PMID: , 10.1039/c9em00502a. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chang W, Wang L, Zhang Y, Zhang Y, Wang M, et al. 2019. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol Environ Saf 182:109402, PMID: , 10.1016/j.ecoenv.2019.109402. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins ZR, Sun M, DeWitt JC, Knappe DR. 2018. Recently detected drinking water contaminants: GenX and other per‐and polyfluoroalkyl ether acids. J Am Water Works Assoc 110(7):13–28, 10.1002/awwa.1073. [DOI] [Google Scholar]
- 30.Wang Z, Cousins IT, Scheringer M, Hungerbuehler K. 2015. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int 75:172–179, PMID: , 10.1016/j.envint.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Ritter SK. 2010. Fluorochemicals go short. Chem Eng News Archive 88(5):12–17, 10.1021/cen-v088n005.p012. [DOI] [Google Scholar]
- 32.EFSA (European Food Safety Authority). 2011. Scientific opinion on the safety evaluation of the substance, 3H‐perfluoro‐3‐[(3‐methoxy‐propoxy) propanoic acid], ammonium salt, CAS No. 958445‐44‐8, for use in food contact materials. EFSA J 9(6):2182, 10.2903/j.efsa.2011.2182. [DOI] [Google Scholar]
- 33.Olsen GW, Chang S-C, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, et al. 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256(1–2):65–74, PMID: , 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Fletcher T, Pineda D, Lindh CH, Nilsson C, Glynn A, et al. 2020. Serum half-lives for short- and long-chain perfluoroalkyl acids after ceasing exposure from drinking water contaminated by firefighting foam. Environ Health Perspect 128(7):77004, PMID: , 10.1289/EHP6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93(10):2419–2425, PMID: , 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 36.Russell MH, Himmelstein MW, Buck RC. 2015. Inhalation and oral toxicokinetics of 6:2 FTOH and its metabolites in mammals. Chemosphere 120:328–335, PMID: , 10.1016/j.chemosphere.2014.07.092. [DOI] [PubMed] [Google Scholar]
- 37.Fromme H, Wockner M, Roscher E, Volkel W. 2017. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. Int J Hyg Environ Health 220(2 pt B):455–460, PMID: , 10.1016/j.ijheh.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 38.ECHA (European Chemical Agency). 2021. Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate. https://echa.europa.eu/nl/registration-dossier/-/registered-dossier/2679/7/11/6/?documentUUID=14523516-270d-4653-93d7-338345468d2e [accessed 15 March 2021].
- 39.Charles River Laboratories. 2017. DuPont-C30031_516655: Determination of HFPO-DA in EDTA human plasma samples. Wilmington, DE: Chemours. https://echa.europa.eu/nl/registration-dossier/-/registered-dossier/2679/7/11/6 [accessed 15 March 2021]. [Google Scholar]
- 40.Pritchett JR, Rinsky JL, Dittman B, Christensen A, Langley R, Moore Z, et al. 2019. Notes from the field: targeted biomonitoring for GenX and other per-and polyfluoroalkyl substances following detection of drinking water contamination—North Carolina, 2018. Mmwr Morb Mortal Wkly Rep 68(29):647–648, PMID: , 10.15585/mmwr.mm6829a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotlarz N, McCord J, Collier D, Lea CS, Strynar M, Lindstrom AB, et al. 2020. Measurement of novel, drinking water-associated PFAS in blood from adults and children in Wilmington, North Carolina. Environ Health Perspect 128(7):77005, PMID: , 10.1289/EHP6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awad R, Zhou Y, Nyberg E, Namazkar S, Yongning W, Xiao Q, et al. 2020. Emerging per-and polyfluoroalkyl substances (PFAS) in human milk from Sweden and China. Environ Sci Process Impacts 22(10):2023–2030, PMID: , 10.1039/d0em00077a. [DOI] [PubMed] [Google Scholar]
- 43.Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Guo Y, et al. 2017. First report on the occurrence and bioaccumulation of hexafluoropropylene oxide trimer acid: an emerging concern. Environ Sci Technol 51(17):9553–9560, PMID: , 10.1021/acs.est.7b02259. [DOI] [PubMed] [Google Scholar]
- 44.Agency for Toxic Substances and Disease Registry. 2021. Toxicological Profile for Perfluoroalkyls, https://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf [accessed 7 July 2021]. [PubMed]
- 45.EFSA. 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):e06223, PMID: , 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ECHA. 2019. Member State Committee Support Document for Identification of 2,3,3,3-Tetrafluoro-2-(Heptafluoropropoxy)Proprionic Acid, Its Salts and Its Acyl Halides (Covering Any of Their Individual Isomers and Combinations thereof) as Substances of Very High Concern because of Their Hazardous Properties which Cause Probable Serious Effects to Human Health and the Environment which Give Rise to an Equivalent Concern to Those of CMR and PBT/vPvB Substances (Article 57F). https://echa.europa.eu/documents/10162/53fa6a5b-e95f-3128-ea9d-fa27f43b18bc [accessed 3 January 2021].
- 47.Conley JM, Lambright CS, Evans N, McCord J, Strynar MJ, Hill D, et al. 2021. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ Int 146:106204, PMID: , 10.1016/j.envint.2020.106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, et al. 2019. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect 127(3):37008, PMID: , 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon SC. 2011. Toxicological evaluation of ammonium 4,8-dioxa-3H-perfluorononanoate, a new emulsifier to replace ammonium perfluorooctanoate in fluoropolymer manufacturing. Regul Toxicol Pharmacol 59(1):64–80, PMID: , 10.1016/j.yrtph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Lang JR, Strynar MJ, Lindstrom AB, Farthing A, Huang H, Schmid J, et al. 2020. Toxicity of Balb-c mice exposed to recently identified 1, 1, 2, 2-tetrafluoro-2-[1, 1, 1, 2, 3, 3-hexafluoro-3-(1, 1, 2, 2-tetrafluoroethoxy) propan-2-yl] oxyethane-1-sulfonic acid (PFESA-BP2). Toxicology 441:152529, PMID: , 10.1016/j.tox.2020.152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Li H, Yao J, Guo H, Zhang H, Guo Y, et al. 2021. Chronic exposure to PFO4DA and PFO5DoDA, two perfluoroalkyl ether carboxylic acids (PFECAs), suppresses hepatic stress signals and disturbs glucose and lipid metabolism in male mice. J Hazard Mater 411:124963, PMID: , 10.1016/j.jhazmat.2020.124963. [DOI] [PubMed] [Google Scholar]
- 52.Guo H, Wang J, Yao J, Sun S, Sheng N, Zhang X, et al. 2019. Comparative hepatotoxicity of novel PFOA alternatives (perfluoropolyether carboxylic acids) on male mice. Environ Sci Technol 53(7):3929–3937, PMID: , 10.1021/acs.est.9b00148. [DOI] [PubMed] [Google Scholar]
- 53.Sheng N, Pan Y, Guo Y, Sun Y, Dai J. 2018. Hepatotoxic effects of hexafluoropropylene oxide trimer acid (HFPO-TA), A novel perfluorooctanoic acid (PFOA) alternative, on mice. Environ Sci Technol 52(14):8005–8015, PMID: , 10.1021/acs.est.8b01714. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Zhou X, Sheng N, Cui R, Cui Q, Guo H, et al. 2018. Subchronic hepatotoxicity effects of 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA), a novel perfluorooctanesulfonate (PFOS) alternative, on adult male mice. Environ Sci Technol 52(21):12809–12818, PMID: , 10.1021/acs.est.8b04368. [DOI] [PubMed] [Google Scholar]
- 55.Patlewicz G, Richard AM, Williams AJ, Grulke CM, Sams R, Lambert J, et al. 2019. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing. Environ Health Perspect 127(1):14501, PMID: , 10.1289/EHP4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cousins IT, DeWitt JC, Glüge J, Goldenman G, Herzke D, Lohmann R, et al. 2020. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ Sci Process Impacts 22(7):1444–1460, PMID: , 10.1039/d0em00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandsma SH, Koekkoek JC, van Velzen MJM, de Boer J. 2019. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands. Chemosphere 220:493–500, PMID: , 10.1016/j.chemosphere.2018.12.135. [DOI] [PubMed] [Google Scholar]
- 58.Costello MCS, Lee LS. 2020. Sources, fate, and plant uptake in agricultural systems of per-and polyfluoroalkyl substances. Curr Pollution Rep 1–21, 10.1007/s40726-020-00168-y. [DOI] [Google Scholar]
- 59.Zafeiraki E, Gebbink WA, Hoogenboom RLAP, Kotterman M, Kwadijk C, Dassenakis E, et al. 2019. Occurrence of perfluoroalkyl substances (PFASs) in a large number of wild and farmed aquatic animals collected in the Netherlands. Chemosphere 232:415–423, PMID: , 10.1016/j.chemosphere.2019.05.200. [DOI] [PubMed] [Google Scholar]
- 60.Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. 2020. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22(12):2345–2373, PMID: , 10.1039/d0em00291g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultes L, Vestergren R, Volkova K, Westberg E, Jacobson T, Benskin JP. 2018. Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure. Environ Sci Process Impacts 20(12):1680–1690, PMID: , 10.1039/c8em00368h. [DOI] [PubMed] [Google Scholar]
- 62.Poothong S, Papadopoulou E, Padilla-Sánchez JA, Thomsen C, Haug LS. 2020. Multiple pathways of human exposure to poly-and perfluoroalkyl substances (PFASs): from external exposure to human blood. Environ Int 134:105244, PMID: , 10.1016/j.envint.2019.105244. [DOI] [PubMed] [Google Scholar]
- 63.Louro H, Heinälä M, Bessems J, Buekers J, Vermeire T, Woutersen M, et al. 2019. Human biomonitoring in health risk assessment in Europe: current practices and recommendations for the future. Int J Hyg Environ Health 222(5):727–737, PMID: , 10.1016/j.ijheh.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Fujii Y, Niisoe T, Harada KH, Uemoto S, Ogura Y, Takenaka K, et al. 2015. Toxicokinetics of perfluoroalkyl carboxylic acids with different carbon chain lengths in mice and humans. J Occup Health 57(1):1–12, PMID: , 10.1539/joh.14-0136-OA. [DOI] [PubMed] [Google Scholar]
- 65.Sundström M, Chang S-C, Noker PE, Gorman GS, Hart JA, Ehresman DJ, et al. 2012. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reprod Toxicol 33(4):441–451, PMID: , 10.1016/j.reprotox.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Kemper RA. 2003. Perfluorooctanoic Acid: Toxicokinetics in the Rat. DuPont-7473. US-EPA Administrative Record AR-226.1499. Newark, DE: E.I. du Pont de Nemours and Company. [Google Scholar]
- 67.Chang S-C, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, et al. 2012. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod Toxicol 33(4):428–440, PMID: , 10.1016/j.reprotox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Chang S-C, Das K, Ehresman DJ, Ellefson ME, Gorman GS, Hart JA, et al. 2008. Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol Sci 104(1):40–53, PMID: , 10.1093/toxsci/kfn057. [DOI] [PubMed] [Google Scholar]
- 69.Ylinen M, Auriola S. 1990. Tissue distribution and elimination of perfluorodecanoic acid in the rat after single intraperitoneal administration. Pharmacol Toxicol 66(1):45–48, PMID: , 10.1111/j.1600-0773.1990.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 70.Ylinen M, Kojo A, Hanhijarvi H, Peura P. 1990. Disposition of perfluorooctanoic acid in the rat after single and subchronic administration. Bull Environ Contam Toxicol 44(1):46–53, PMID: , 10.1007/BF01702360. [DOI] [PubMed] [Google Scholar]
- 71.Gannon SA, Johnson T, Nabb DL, Serex TL, Buck RC, Loveless SE. 2011. Absorption, distribution, metabolism, and excretion of [1-14C]-perfluorohexanoate ([14C]-PFHx) in rats and mice. Toxicology 283(1):55–62, PMID: , 10.1016/j.tox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Iwabuchi K, Senzaki N, Mazawa D, Sato I, Hara M, Ueda F, et al. 2017. Tissue toxicokinetics of perfluoro compounds with single and chronic low doses in male rats. J Toxicol Sci 42(3):301–317, PMID: , 10.2131/jts.42.301. [DOI] [PubMed] [Google Scholar]
- 73.Kawabata K, Tamaki S, Kokubo E, Kobayashi Y, Shinohara T, Sakai A, et al. 2017. Disposition of perfluorododecanoic acid in male rats after oral administration. Fundam Toxicol Sci 4(4):179–186, 10.2131/fts.4.179. [DOI] [Google Scholar]
- 74.Kim SJ, Heo SH, Lee DS, Hwang IG, Lee YB, Cho HY. 2016. Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food Chem Toxicol 97:243–255, PMID: , 10.1016/j.fct.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 75.Fragki S, Dirven H, Fletcher T, Grasl-Kraupp B, Bjerve Gützkow K, Hoogenboom R, et al. 2021. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not? Crit Rev Toxicol 51(2):141–164, PMID: , 10.1080/10408444.2021.1888073. [DOI] [PubMed] [Google Scholar]
- 76.Vorkamp K, Castaño A, Antignac J-P, Boada LD, Cequier E, Covaci A, et al. 2021. Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ Int 146:106082, PMID: , 10.1016/j.envint.2020.106082. [DOI] [PubMed] [Google Scholar]
- 77.Bil W, Zeilmaker M, Fragki S, Lijzen J, Verbruggen E, Bokkers B. 2021. Risk assessment of per- and polyfluoroalkyl substance mixtures: a relative potency factor approach. Environ Toxicol Chem 40(3):859–870, PMID: , 10.1002/etc.4835. [DOI] [PubMed] [Google Scholar]
- 78.EFSA. 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J 17(3):e05634, PMID: , 10.2903/j.efsa.2019.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomis MI, Vestergren R, Borg D, Cousins IT. 2018. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int 113:1–9, PMID: , 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 80.Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, et al. 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340:1–9, PMID: , 10.1016/j.tox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Eifrid MA, Gibbs ST, et al. 2020. Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd: Sprague Dawley SD rats following intravenous or gavage administration. Xenobiotica 50(6):722–732, PMID: , 10.1080/00498254.2019.1683776. [DOI] [PubMed] [Google Scholar]
- 82.CDC (U.S. Centers for Disease and Control Prevention). 2017. National Health and Nutrition Examination Survey Data. Hyattsville, MD: Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017 [accessed 22 February 2021]. [Google Scholar]
- 83.Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. 2021. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40(3):606–630, PMID: , 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirk AB, Michelsen-Correa S, Rosen C, Martin CF, Blumberg B. 2021. PFAS and potential adverse effects on bone and adipose tissue through interactions with PPARγ. Endocrinology 162(12): bqab194, PMID: , 10.1210/endocr/bqab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corton JC, Peters JM, Klaunig JE. 2018. The PPARα-dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Arch Toxicol 92(1):83–119, PMID: , 10.1007/s00204-017-2094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lieder PH, York RG, Hakes DC, Chang S-C, Butenhoff JL. 2009. A two-generation oral gavage reproduction study with potassium perfluorobutanesulfonate (K+ PFBS) in Sprague Dawley rats. Toxicology 259(1–2):33–45, PMID: , 10.1016/j.tox.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 87.Butenhoff JL, Chang S-C, Ehresman DJ, York RG. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol 27(3–4):331–341, PMID: , 10.1016/j.reprotox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, et al. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183(1–3):117–131, PMID: , 10.1016/S0300-483X(02)00511-5. [DOI] [PubMed] [Google Scholar]
- 89.Butenhoff JL, Bjork JA, Chang S-C, Ehresman DJ, Parker GA, Das K, et al. 2012. Toxicological evaluation of ammonium perfluorobutyrate in rats: twenty-eight-day and ninety-day oral gavage studies. Reprod Toxicol 33(4):513–530, PMID: , 10.1016/j.reprotox.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Loveless SE, Slezak B, Serex T, Lewis J, Mukerji P, O’Connor JC, et al. 2009. Toxicological evaluation of sodium perfluorohexanoate. Toxicology 264(1–2):32–44, PMID: , 10.1016/j.tox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Perkins RG, Butenhoff JL, Kennedy GL Jr., Palazzolo MJ. 2004. 13‐Week dietary toxicity study of ammonium perfluorooctanoate (APFO) in male rats. Drug Chem Toxicol 27(4):361–378, PMID: , 10.1081/dct-200039773. [DOI] [PubMed] [Google Scholar]
- 92.Mertens JJWM, Sved DW, Marit GB, Myers NR, Stetson PL, Murphy SR, et al. 2010. Subchronic toxicity of S-111-S-WB in Sprague Dawley rats. Int J Toxicol 29(4):358–371, PMID: , 10.1177/1091581810370372. [DOI] [PubMed] [Google Scholar]
- 93.Kato H, Fujii S, Takahashi M, Matsumoto M, Hirata-Koizumi M, Ono A, et al. 2015. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environ Toxicol 30(11):1244–1263, PMID: , 10.1002/tox.21996. [DOI] [PubMed] [Google Scholar]
- 94.Haas MC. 2009. Memo Report HFPO Dimer Acid Ammonium Salt, A 90-day Oral (Gavage) Toxicicty Study of H-28548 in Rats with a 28-Day Recovery. DuPont Report No. 17751-1026. Newark, DE: DuPont Haskell Global Centers.https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/4222142 [accessed 4 May 2018]. [Google Scholar]
- 95.Klaunig JE, Shinohara M, Iwai H, Chengelis CP, Kirkpatrick JB, Wang Z, et al. 2015. Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley rats. Toxicol Pathol 43(2):209–220, PMID: , 10.1177/0192623314530532. [DOI] [PubMed] [Google Scholar]
- 96.Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2, 3, 3, 3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague-Dawley rats. Toxicol Rep 2:939–949, PMID: , 10.1016/j.toxrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Butenhoff JL, Chang S-C, Olsen GW, Thomford PJ. 2012. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology 293(1–3):1–15, PMID: , 10.1016/j.tox.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 98.NTP (National Toxocology Program). 2020. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Perfluorooctanoic Acid (CASRN 335-67-1) Administered in Feed to Sprague Dawley (Hsd: Sprague Dawley® SD®) Rats.https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr598_508.pdf?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=tr598 [accessed 3 May 2021] [DOI] [PMC free article] [PubMed]
- 99.NTP. 2019. NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Sulfonates (Perfluorobutane Sulfonic Acid, Perfluorohexane Sulfonate Potassium Salt, and Perfluorooctane Sulfonic Acid) Administered by Gavage to Sprague Dawley (Hsd: Sprague Dawley SD) Rats. NTP Toxicity Report Series, 10.22427/NTP-TOX-96. [DOI]
- 100.NTP. 2019. NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Carboxylates (Perfluorohexanoic Acid, Perfluorooctanoic Acid, Perfluorononanoic Acid, and Perfluorodecanoic Acid) Administered by Gavage to Sprague Dawley (Hsd: Sprague Dawley SD) Rats. NTP Toxicity Report Series, 10.22427/NTP-TOX-97. [DOI]
- 101.Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB, et al. 2011. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology 281(1–3):48–55, PMID: , 10.1016/j.tox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 102.Huang MC, Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Eifrid MA, et al. 2019. Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd: sprague Dawley SD rats after intravenous and gavage administration. Toxicol Rep 6:645–655, PMID: , 10.1016/j.toxrep.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jamhekar S, Breen P. 2012. Chapter 6: Extravascular routes of drug administration. In: Basic Pharmacokinetics. 2nd ed. https://www.pharmpress.com/files/docs/Basic%20Pharmacokinetics%20sample.pdf [accessed 15 October 2020].
- 104.Certara. 2021. Phoenix Assistance Library, WNL Classic Models, WNL Classic Model Calculations, Pharmacokinetic Models, Model 3. https://onlinehelp.certara.com/phoenix/8.2/index.html#t=topics%2Fpkmodelcalc.htm%23TOC_Model_3bc-3&rhtocid=_35_3_4_2 [accessed 3 January 2021].
- 105.Gannon SA. 2008. Memo Report HFPO Dimer Acid Ammonium Salt, Biopersistence and Pharmacokinetic Screen in the Rat, DuPont Report No. 24281. Wilmington, DE: E.I. du Pont de Nemours and Company. https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/4221044 [accessed 15 April 2019].
- 106.Elcombe CR, Elcombe BM, Foster JR, Chang S-C, Ehresman DJ, Butenhoff JL. 2012. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR. Toxicology 293(1–3):16–29, PMID: , 10.1016/j.tox.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 107.Bosgra S, van der Voet H, Boon PE, Slob W. 2009. An integrated probabilistic framework for cumulative risk assessment of common mechanism chemicals in food: an example with organophosphorus pesticides. Regul Toxicol Pharmacol 54(2):124–133, PMID: , 10.1016/j.yrtph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Slob W. 2002. Dose-response modeling of continuous endpoints. Toxicol Sci 66(2):298–312, PMID: , 10.1093/toxsci/66.2.298. [DOI] [PubMed] [Google Scholar]
- 109.Slob W, Setzer RW. 2014. Shape and steepness of toxicological dose–response relationships of continuous endpoints. Crit Rev Toxicol 44(3):270–297, PMID: , 10.3109/10408444.2013.853726. [DOI] [PubMed] [Google Scholar]
- 110.Chen T, Clark J, Riddles M, Mohadjer L, Fakhouri T. 2020. National Health and Nutrition Examination Survey, 2015−2018: sample design and estimation procedures. Vital Health Stat 2(184):1–35, PMID: . [PubMed] [Google Scholar]
- 111.Soetaert K, Petzoldt T, Setzer RW. 2010. Solving differential equations in R: package deSolve. J Stat Soft 33(9):1–25, 10.18637/jss.v033.i09. [DOI] [Google Scholar]
- 112.Ehresman DJ, Froehlich JW, Olsen GW, Chang S-C, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 103(2):176–184, PMID: , 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 113.Vogs C, Johanson G, Näslund M, Wulff S, Sjödin M, Hellstrandh M, et al. 2019. Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio). Environ Sci Technol 53(7):3898–3907, PMID: , 10.1021/acs.est.8b07188. [DOI] [PubMed] [Google Scholar]
- 114.Kawabata K, Matsuzaki H, Nukui S, Okazaki M, Sakai A, Kawashima Y, et al. 2017. Perfluorododecanoic acid induces cognitive deficit in adult rats. Toxicol Sci 157(2):421–428, PMID: , 10.1093/toxsci/kfx058. [DOI] [PubMed] [Google Scholar]
- 115.Dagnino S, Strynar MJ, McMahen RL, Lau CS, Ball C, Garantziotis S, et al. 2016. Identification of biomarkers of exposure to FTOHs and PAPs in humans using a targeted and nontargeted analysis approach. Environ Sci Technol 50(18):10216–10225, PMID: , 10.1021/acs.est.6b01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang MC, Robinson VG, Waidyanatha S, Dzierlenga AL, DeVito MJ, Eifrid MA, et al. 2019. Toxicokinetics of 8:2 fluorotelomer alcohol (8:2-FTOH) in male and female Hsd: Sprague Dawley SD rats after intravenous and gavage administration. Toxicol Rep 6:924–932, PMID: , 10.1016/j.toxrep.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nilsson H, Karrman A, Rotander A, van Bavel B, Lindstrom G, Westberg H. 2013. Biotransformation of fluorotelomer compound to perfluorocarboxylates in humans. Environ Int 51:8–12, PMID: , 10.1016/j.envint.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Xu L, Krenitsky DM, Seacat AM, Butenhoff JL, Anders M. 2004. Biotransformation of N-ethyl-N-(2-hydroxyethyl) perfluorooctanesulfonamide by rat liver microsomes, cytosol, and slices and by expressed rat and human cytochromes P450. Chem Res Toxicol 17(6):767–775, PMID: , 10.1021/tx034222x. [DOI] [PubMed] [Google Scholar]
- 119.Xie W, Wu Q, Kania-Korwel I, Tharappel JC, Telu S, Coleman MC, et al. 2009. Subacute exposure to N-ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats. Arch Toxicol 83(10):909–924, PMID: , 10.1007/s00204-009-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joudan S, Yeung LWY, Mabury SA. 2017. Biological cleavage of the C-P bond in perfluoroalkyl phosphinic acids in male Sprague-Dawley rats and the formation of persistent and reactive metabolites. Environ Health Perspect 125(11):117001, PMID: , 10.1289/EHP1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.RIVM. 2021. Memorandum on the implementation of the EFSA sum TWI of PFASs. https://www.rivm.nl/sites/default/files/2021-06/Memorandum%20on%20implementation%20of%20the%20EFSA%20sum%20TWI%20of%20PFASs.pdf [accessed 29 May 2021].
- 122.European Union. 2020. Recast of the EU Directive on the Quality of Water Intended for Human Consumption. https://data.consilium.europa.eu/doc/document/ST-6230-2020-INIT/en/pdf [accessed 29 May 2021].
- 123.Das KP, Grey BE, Zehr RD, Wood CR, Butenhoff JL, Chang S-C, et al. 2008. Effects of perfluorobutyrate exposure during pregnancy in the mouse. Toxicol Sci 105(1):173–181, PMID: , 10.1093/toxsci/kfn099. [DOI] [PubMed] [Google Scholar]
- 124.Iwai H, Hoberman AM. 2014. Oral (gavage) combined developmental and perinatal/postnatal reproduction toxicity study of ammonium salt of perfluorinated hexanoic acid in mice. Int J Toxicol 33(3):219–237, PMID: , 10.1177/1091581814529449. [DOI] [PubMed] [Google Scholar]
- 125.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90(2):510–518, PMID: , 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 126.Das KP, Grey BE, Rosen MB, Wood CR, Tatum-Gibbs KR, Zehr RD, et al. 2015. Developmental toxicity of perfluorononanoic acid in mice. Reprod Toxicol 51:133–144, PMID: , 10.1016/j.reprotox.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 127.Harris MW, Birnbaum LS. 1989. Developmental toxicity of perfluorodecanoic acid in C57BL/6N mice. Fundam Appl Toxicol (3):442–448, PMID: , 10.1016/0272-0590(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 128.Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L, et al. 2017. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicol Sci 155(2):409–419, PMID: , 10.1093/toxsci/kfw219. [DOI] [PubMed] [Google Scholar]
- 129.Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, et al. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 74(2):369–381, PMID: , 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- 130.Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, et al. 2020. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect 128(2):27006, PMID: , 10.1289/EHP6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cope HA, Blake BE, Love C, McCord J, Elmore SA, Harvey JB, et al. 2021. Latent, sex-specific metabolic health effects in CD-1 mouse offspring exposed to PFOA or HFPO-DA (GenX) during gestation. Emerg Contam 7:219–235, PMID: , 10.1016/j.emcon.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu L-L, Chen Y-K, Zhang Q-Y, Chen L-J, Zhang K-K, Li J-H, et al. 2022. Gestational exposure to GenX induces hepatic alterations by the gut–liver axis in maternal mice: a similar mechanism as PFOA. Sci Total Environ 820:153281, PMID: , 10.1016/j.scitotenv.2022.153281. [DOI] [PubMed] [Google Scholar]
- 133.Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, et al. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci 74(2):382–392, PMID: , 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- 134.Yahia D, El-Nasser MA, Abedel-Latif M, Tsukuba C, Yoshida M, Sato I, et al. 2010. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. J Toxicol Sci 35(4):527–533, PMID: , 10.2131/jts.35.527. [DOI] [PubMed] [Google Scholar]
- 135.Yahia D, Tsukuba C, Yoshida M, Sato I, Tsuda S. 2008. Neonatal death of mice treated with perfluorooctane sulfonate. J Toxicol Sci 33(2):219–226, PMID: , 10.2131/jts.33.219. [DOI] [PubMed] [Google Scholar]
- 136.Wolf CJ, Zehr RD, Schmid JE, Lau C, Abbott BD. 2010. Developmental effects of perfluorononanoic acid in the mouse are dependent on peroxisome proliferator-activated receptor-alpha. PPAR Res 2010:1–11, PMID: , 10.1155/2010/282896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lau C, Rumpler J, Das KP, Wood CR, Schmid JE, Strynar MJ, et al. 2020. Pharmacokinetic profile of Perfluorobutane Sulfonate and activation of hepatic nuclear receptor target genes in mice. Toxicology 441:152522, PMID: , 10.1016/j.tox.2020.152522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abraham K, Mielke H, Fromme H, Völkel W, Menzel J, Peiser M, et al. 2020. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch Toxicol 94(6):2131–2147, PMID: , 10.1007/s00204-020-02715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yeung LW, Mabury SA. 2016. Are humans exposed to increasing amounts of unidentified organofluorine. Environ Chem 13(1):102–110, 10.1071/EN15041. [DOI] [Google Scholar]
- 140.Reardon AJ, Rowan-Carroll A, Ferguson SS, Leingartner K, Gagné R, Kuo N, et al. 2020. High-throughput transcriptomics and benchmark concentration modeling for potency ranking of per-and polyfluoroalkyl substances (PFAS) in exposed human liver cell spheroids. bioRxiv Preprint posted online October 21, 2020. 10.1101/2020.10.20.347328. [DOI] [Google Scholar]
- 141.Rowan-Carroll A, Readon A, Leingartner K, Gagné R, Williams A, Kuo N, et al. 2020. High-throughput transcriptomic evaluation of per- and polyfluoroalkyl substances (PFAS) in primary human liver spheroids to inform read-across. bioRxiv. Preprint posted online October 15, 2020. 10.1101/2020.10.15.341362. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.