Abstract
The global spread of avian influenza virus (AIV) of clade 2.3.4.4b since 2016 has caused severe losses in wild birds and poultry and has posed a risk for the infection of mammals including humans. The vaccination of poultry has been used to limit the spread of the virus and mitigate its socioeconomic impact. Here, we describe H5N8 epidemics in chickens, turkeys and ducks from different localities in Egypt from 2019 to 2021. About 41.7% (n = 88/211) flocks were tested positive by RT-qPCR for H5N8 viruses with prevalence rates of 45.1% (n = 65/144) and 34.3% (n = 23/67) in vaccinated and non-vaccinated flocks, respectively. A sequence analysis of the hemagglutinin and neuraminidase genes indicated not only the multiple introduction events of H5N8 viruses in Egypt but also the establishment of endemic viruses in commercial poultry in 2020/2021. The recent H5N8 viruses in poultry in Egypt are genetically distinct from the majority of licensed vaccines used in the field. Together, our findings indicate that poultry in Egypt is an endemic center for clade 2.3.4.4b in the Middle East. The efficiency of current vaccines should be regularly evaluated and updated to fully protect poultry flocks in Egypt against H5N8 viruses.
Keywords: avian influenza virus, H5N8, clade 2.3.4.4b, Egypt, poultry, vaccination failure
1. Introduction
Avian influenza viruses (AIV) belong to the family Orthomyxoviridae and infect a wide range of avian and mammalian species [1]. They are classified according to the antigenic properties of the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) into 16 HA and 9 NA subtypes, respectively. All AIV subtypes were isolated from wild birds, the natural reservoir, where the infection is usually asymptomatic, with few exceptions. In domestic birds, all AIVs are low pathogenic (LP), while some H5 and H7 subtypes can be highly pathogenic (HP) [2]. Since 1996, the HPAIV H5 subtype of the Goose/Guangdong lineage continues to cause severe economic losses in the poultry industry and pose a significant pandemic threat. The virus has been diversified into 10 HA phylogenetic clades (clades 0 to 9) and tens of suborder clades [3]. In 2014, clade 2.3.4.4a spread from Asia to Europe and North America, while in 2016 clade 2.3.4.4b spread from Asia to Europe and Africa [4,5]. In addition to the high mortality in domestic and wild birds, the virus succeeded to jump species barriers and infected mammals including humans, foxes and seals in several countries [6,7,8,9]. Therefore, it is paramount to efficiently control the virus in poultry to limit bird-to-human transmission [10].
In addition to the biosecurity measures and culling strategy, the mass vaccination of poultry is highly useful to protect poultry from AIV and prevent spillover to other mammals including humans [11]. Several AIV vaccines have been developed including inactivated whole virus vaccines and recombinant virus vector vaccines [12]. Experimental and field studies showed that the use of H5 or H7 vaccines, particularly those containing antigenically matched hemagglutinin similar to the field viruses, were effective at preventing morbidity and mortality in poultry, limiting virus replication, reducing viral loads in the environment, and interrupting poultry-to-poultry transmission [11,13,14,15]. Importantly, the effective vaccination of poultry against AIV (e.g., H7N9 in China) successfully eliminated human infection, emphasizing the importance of the active control of animal diseases in the one-health concept [16]. Similar to human influenza viruses, AIV vaccines should be regularly updated to fully protect poultry against exotic and newly introduced subtypes/serotypes [11,15]. The best example of the regular updating of AIV vaccine strains is China, where several AIV subtypes including H5/H7 AIV are endemic in poultry. Recently, an updated trivalent vaccine (H5-Re13, H5-Re14, and H7-Re4, of which the HA and NA genes originated from the newly detected H5N6 virus, H5N8 virus, and H7N9 virus, respectively) has been developed. Animal studies proved that the novel H5/H7 trivalent vaccine is immunogenic and could provide solid protection against viruses that are currently circulating in nature [17]. Outside China (e.g., in Egypt and Mexico), the use of outdated vaccines or mismatched vaccine strains conferred suboptimal protection to poultry against HPAIV [18,19].
In Egypt, three zoonotic AIVs have been detected in poultry including H5N1 (2005–2020), H9N2 (since 2013) and recently H5N8 clade 2.3.4.4b (since 2016) [20,21]. The latter virus was transmitted from wild birds, spread widely in commercial farms and replaced the endemic H5N1 in poultry [22]. Sequence analyses of H5N8 clade 2.3.4.4B in poultry in Egypt from 2016 to 2018 revealed multiple introductions via migratory birds along the Black Sea–Mediterranean and East African–West Asian migration flyways [23,24,25]. The location of Egypt on these two intersecting flyways of migratory birds from Asia and Europe represents a major threat for the introduction of AIV in poultry and its spread to neighboring countries [26,27]. It is worth mentioning that Egypt is the country with the highest confirmed human H5N1 infections worldwide [28], and clinical and subclinical H9N2 infections in humans have been reported [29]. Therefore, it is important to understand the epidemiology and evolution of these zoonotic AIVs in Egyptian poultry [20]. To mitigate the socioeconomic losses in the poultry industry, Egypt mainly embarked on the use of blanket H5/H9 vaccination campaigns, particularly in the commercial sector [30,31]. Nevertheless, H5N1 and to a lesser extent H9N2 viruses have been frequently isolated in vaccinated flocks [31]. The upsurge of H5N1 in vaccinated flocks in Egypt was associated with the evolution of antigenic drift variants, which acquired several mutations in the HA immunogenic epitopes under vaccination pressure, likely due to the use of antigenically mismatched vaccine strains [32,33]. For the recent H5N8 viruses, the prevalence of this virus in vaccinated flocks is not fully understood.
In this study, swab samples were collected from commercial chickens, ducks and turkey flocks with a history of respiratory signs and high morbidity and mortality rates. Virus detection, isolation and sequencing of the HA and NA of selected samples were performed. To understand the molecular epidemiology of H5N8 viruses in Egypt, we analyzed the HA/NA sequences from all H5N8 viruses in the GISAID from Eurasia and the Middle East since 2016.
2. Materials and Methods
2.1. Virus Detection and Isolation
Tracheal and cloacal swabs were collected and submitted to the laboratory between November 2019 to March 2021. Samples (10 to 20 swabs from each flock) were collected from commercial poultry farms in different provinces in Egypt. Pooled samples were tested using RT-qPCR targeting the M gene of influenza A virus [34] and positive samples were further tested to detect the HA and NA subtypes [34] using a multiplex Real-Time RT-PCR applied biosystem 7500. Positive samples were inoculated in 10–12 day-old embryonated chicken eggs via the allantoic sac for one or two passages in a Class III Biosafety Cabinet. Allantoic fluids were tested using the hemagglutination test according to the OIE protocol.
2.2. Sequence of the HA and NA Genes
The HA and NA genes of the selected samples were amplified using one-step conventional RT-PCR according to the manufacturer instructions (SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase, Thermofisher, Waltham, MA, USA). Samples were purified from 1.5% gel using QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). Purified segments were subjected to sequencing using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) and universal forward and reverse primers as previously published [35,36]. Sequences were assembled and edited using Geneious Prime® 2021.0.1. We further compared the amino acid (aa) differences in HA of Egyptian H5N8 viruses, including the new sequences generated in this study as well as the European viruses from 2017 to 2021, to the sequence of the commercially available H5 vaccines in poultry in Egypt.
2.3. Phylogenetic Analysis
Nucleotide sequences of all-full or near-full HA (n = 2700) and NA (n = 1994) genes of H5N8 viruses from Asia, Africa, the Middle East (i.e., Iran, Iraq, Israel, Saudi Arabia) and Europe after a BLAST search in GISAID were downloaded. The date of data retrieval was 21 January 2022. The sequences, including 20 new HA and 17 new NA sequences generated in this study, were aligned using a multiple sequence alignment program (MAFFT). We first generated HA and NA phylogenetic trees for all HA/NA sequences by IQTree [37] and MrBayes implemented in Topali v2 [38]. Thereafter, MrBayes was used to determine the best nucleotide substitution model. Trees were generated after selecting 4 chains of 10,000,000 replicates and 25% buried-in parameters. Posterior probability values are shown on the main nodes. Deduced amino acid (aa) sequences were analyzed by Geneious Prime® 2021.0.1.
3. Results
3.1. Surveillance
The samples in this passive surveillance were submitted from flocks suffering from respiratory disorders and elevated morbidity or mortality and/or a reduction in egg production in layers’ and breeders’ birds. Samples from 211 commercial poultry flocks representing 67 non-vaccinated and 144 vaccinated flocks from different localities in Egypt were examined. About 41.7% (n = 88/211) of flocks were tested positive by RT-qPCR for H5N8 viruses. The prevalence in vaccinated flocks was higher than in non-vaccinated flocks, where 45.1% (n = 65/144) and 34.3% (n = 23/67) were positive, respectively. Epidemiological data for selected outbreaks are summarized in Table 1. We successfully isolated 20 viruses in embryonated chicken eggs and amplified the NA and/or HA by RT-PCR and subjected them to sequencing. These samples represented 15 chicken flocks (broiler, layers, breeders) with a capacity ranging from 500 to 35,000 chickens, 2 duck flocks with a capacity of 10,000 to 12,000 and 3 turkey flocks with a capacity of 5000 to 7000 turkeys. They were collected and submitted to the laboratory in 2019 (n = 8), 2020 (n = 10) and 2021 (n = 2). The age of the chickens ranged from 19 days to 40 weeks, ducks from 40 to 45 days and turkeys from 55 to 90 days. All flocks were vaccinated at least once except for three broiler chicken flocks that were not vaccinated. Two layer chicken and one broiler breeder flocks were vaccinated three and four times, respectively (Table 1). The sequences were submitted to the GISAID and assigned accession numbers EPI1999273 to EPI1999312 (Table 1).
Table 1.
H5N8 viruses isolated in this study.
| Virus | Date | Locality | Breed | Age | Number of Animals | Frequency of Vaccination | Accession Numbers | |
|---|---|---|---|---|---|---|---|---|
| HA | NA | |||||||
| A/duck/Egypt/Behera-AH1/2019 | October 2019 | Elbehera | Broiler | 34d | 18,000 | Once | EPI1999284 | EPI1999283 |
| A/turkey/Egypt/Alex-AH/2019 | October 2019 | Alexandria | Turkey | 55d | 6000 | Once | EPI1999276 | EPI1999275 |
| A/chicken/Egypt/Assuit-AH/2019 | November 2019 | Assuit | Broiler | 28d | 10,000 | Not | EPI1999303 | EPI1999302 |
| A/chicken/Egypt/Kafrelsheik-AH/2019 | November 2019 | Kafrelsheik | Broiler | 29d | 20,000 | Once | EPI1999290 | EPI1999289 |
| A/chicken/Egypt/Elmonufia-backyard-AH/2019 | December 2019 | Elmonufia | Local Breed | 44d | 500 | Not | EPI1999296 | EPI1999295 |
| A/turkey/Egypt/Cairo/AH/2019 | December 2019 | Cairo | Turkey | 60d | 7000 | Once | EPI1999274 | EPI1999273 |
| A/turkey/Egypt/Alex-AH1/2019 | December 2019 | Alexandria | Turkey | 90d | 5000 | Once | EPI1999279 | EPI1999277 |
| A/duck/Egypt/Elbehera-AH2/2019 | December 2019 | Elbehera | Duck | 40d | 10,000 | Once | EPI1999281 | EPI1999280 |
| A/chicken/Egypt/Cairo-HC11B-AH/2020 | January 2020 | Cairo | Broiler | 33d | 21,000 | Once | EPI1999299 | n.d. |
| A/duck/Egypt/Behera-HB2-AH/2020 | January 2020 | Elbehera | Duck | 45d | 12,000 | Once | EPI1999282 | n.d. |
| A/chicken/Egypt/Giza-AH/2020 | January 2020 | Giza | Layer | 24 wks | 20,000 | 3 times | EPI1999294 | EPI1999293 |
| A/chicken/Egypt/Alex-AH/2020 | January 2020 | Alexandria | Broiler | 31d | 17,000 | Once | EPI1999308 | EPI1999306 |
| A/chicken/Egypt/Elmonufia-AH/2020 | February 2020 | Elmonufia | Broiler | 26d | 18,000 | Once | EPI1999298 | EPI1999297 |
| A/chicken/Egypt/Giza-HG4L-AH/2020 | February 2020 | Giza | Broiler | 28d | 16,000 | Once | EPI1999292 | n.d. |
| A/chicken/Egypt/Sohag-AH/2020 | February 2020 | Sohag | Broiler | 32d | 15,000 | Not | EPI1999286 | EPI1999285 |
| A/chicken/Egypt/Qalyubia-layer-AH/2020 | March 2020 | Qalyubia | Layer | 32 wks | 35,000 | 3 times | EPI1999288 | EPI1999287 |
| A/chicken/Egypt/Alex-AH2/2020 | March 2020 | Alexandria | Broiler | 19d | 22,000 | Once | EPI1999310 | EPI1999309 |
| A/chicken/Egypt/Alex-AH1/2020 | May 2020 | Alexandria | Broiler | 29d | 15,000 | Once | EPI1999312 | EPI1999311 |
| A/chicken/Egypt/Behera-AH/2021 | February 2021 | Elbehera | Broiler | 33 | 12,000 | Once | EPI1999301 | EPI1999300 |
| A/chicken/Egypt/Alex-Breeder-AH/2021 | March 2021 | Alexandria | Broiler Breeder | 40 wks | 16,000 | 4 times | EPI1999305 | EPI1999304 |
Age: d = day, wks = weeks. n.d. = not done.
3.2. HA Phylogenetic Analysis
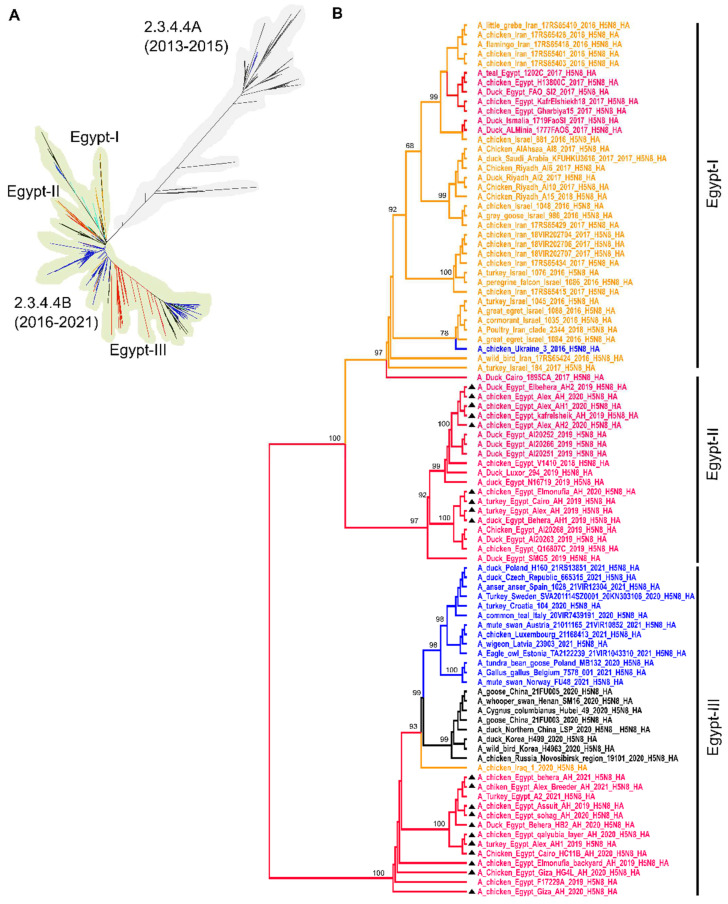
The analyzed sequences of the HA from Egypt represented viruses from 2016 to 2021 including twenty new HA sequences in this study. The phylogenetic analysis indicated that the Egyptian viruses of domestic birds are allocated in three major phylogroups (Figure 1A), and a few Egyptian viruses from wild birds from 2016 (n = 6) are sporadically found in different groups. Phylogroup-I and II are two daughter clusters and distinct from phylogroup-III. Phylogroup-I includes a large proportion of H5N8 viruses from wild and domestic birds in Egypt and the Middle East (i.e., Iran, Israel and Saudi Arabia) in 2016–2018. This group indicated the parallel and multiple dispersals of diverse H5N8 viruses in the Middle East and Africa in 2016–2018. They were not detected after 2018. Phylogroup-II contains only Egyptian viruses, including nine new sequences generated in this study, from domestic birds (i.e., chickens, ducks and turkeys) from 2017 to 2020. Phylogroup-III contains viruses from poultry in Egypt from 2017 to 2021 including 11 new sequences generated in this study as well as published sequences from Europe, Iraq, the Russian Federation and Asia (in 2020 and 2021) (Figure 1B).
Figure 1.
Phylogenetic relatedness of the HA of Egyptian H5N8 to Eurasian viruses. Nucleotide sequences of all-full or near-full HA (n = 2700) genes of H5N8 viruses from Asia, Africa, the Middle East and Europe were retrieved on 21 January 2022 and aligned using MAFFT, and the tree was generated by IQTree. The tree shows clade 2.3.4.4a mainly circulated in wild birds and rarely in poultry from 2013 to 2015, and clade 2.3.4.4B viruses from 2016 to 2021 (A). The phylogenetic tree of the Egyptian H5N8 viruses in clade 2.3.4.4B, including new sequences generated in this study (marked in black triangles), and selected sequences from other countries was generated by MrBayes implemented in Topali v.2 using the GTR + G model. Trees were generated after selecting 4 chains of 10,000,000 replicates and 25% buried-in parameters. Posterior probability values are shown on the main nodes (B). Egyptian H5N8 sequences are shown in red, H5N8 sequences from the Middle East are shown in orange, sequences from Europe are shown in blue and Asian viruses are in black. Branches depicted in cyan in panel A refer to sequences from other African countries.
3.3. NA Phylogenetic Analysis
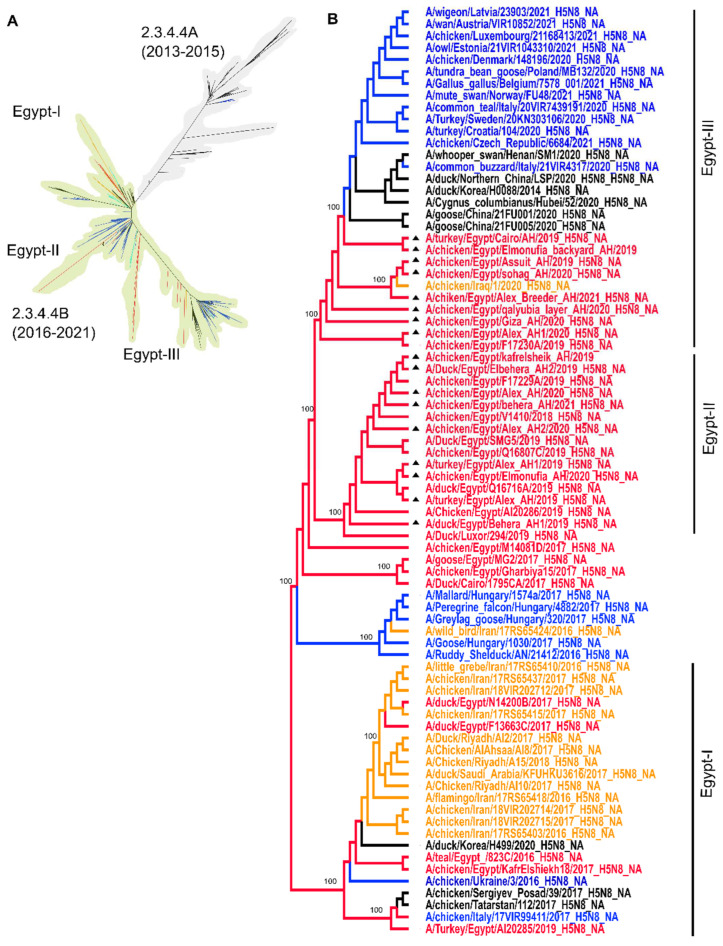
The phylogroups of the NA of H5N8 in Egypt and the Middle East were relatively similar to those of the HA, although the available number of NA sequences was less than that of the HA (Figure 2A). Similar to the HA phylogeny, the NA sequences in phylogroup-I represent a few Egyptian viruses from wild birds and poultry in 2016–2019 along with viruses from the Middle East and Eurasia. Phylogroup-II contains only recent Egyptian viruses from 2019/2021, including nine new sequences generated in this study, clustered together representing the endemic strains in poultry. Phylogroup-III is a daughter cluster to phylogroup-II and is formed of Egyptian H5N8 viruses from 2019/2021 including eight new sequences generated in this study and viruses from Eurasia and a contemporary virus from chickens in Iraq (Figure 2B).
Figure 2.
Phylogenetic relatedness of the NA of Egyptian H5N8 to Eurasian viruses. Nucleotide sequences of all-full or near-full NA (n = 1994) genes of H5N8 viruses from Asia, Africa, the Middle East and Europe were retrieved on 21 January 2022 and aligned using MAFFT and the tree was generated by IQTree (A). The phylogenetic tree of the Egyptian H5N8 viruses, including new sequences generated in this study (marked in black triangles), and selected sequences from other countries was generated by MrBayes implemented in Topali v.2 using the GTR + G model. Trees were generated after selecting 4 chains of 10,000,000 replicates and 25% buried-in parameters. Posterior probability values are shown on the main nodes (B). Egyptian H5N8 sequences are shown in red, H5N8 sequences from the Middle East are shown in orange, sequences from Europe are shown in blue and Asian viruses are in black. Branches depicted in cyan in panel A refer to sequences from other African countries.
3.4. Comparison of HA Protein with Commercially Licensed H5 Vaccines in Poultry in Egypt
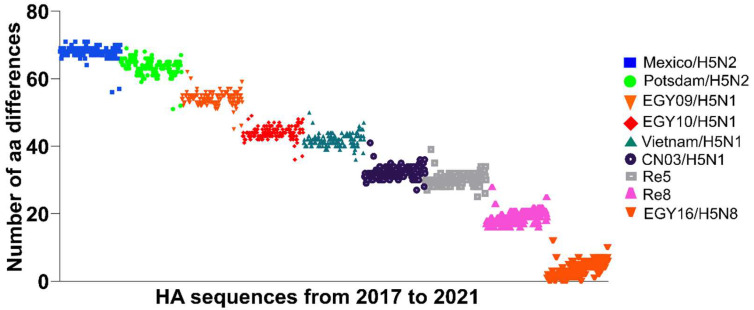
We compared the identity and number of amino acid differences in the HA of Egyptian viruses and commercially available vaccines in Egypt (Table 2). The results showed that the Egyptian H5N8 viruses share a low genetic identity (75.3–92.0%) compared to vaccines based on the historic H5N2 viruses from Potsdam/1986 and Mexico/1994 (Table 2). In contrast to the H5N1 viruses in 2006–2008, these vaccines are not commonly used to protect against the current H5N8 viruses in poultry in Egypt. Conversely, a higher genetic identity was observed compared to clade 2.2 (88.7–96.4%). The highest identity was observed with clade 2.3.4.4 from China (Re8) and wild birds in Egypt in 2016 (EGY16/H5N8) (94.9–100%) (Table 2) and therefore they are preferred in the field over other vaccines.
Table 2.
Genetic identity of Egyptian H5N8 viruses to commercially available H5 vaccines currently used in poultry in Egypt.
| No. | Vaccine Seed Virus | Subtype | Abbreviation | Clade/Lineage | Accession Numbers (aa) | Company | AA Identity to Egyptian H5N8 (Min–Max) |
|---|---|---|---|---|---|---|---|
| 1 | A/chicken/Mexico/232/1994 | H5N2 | Mexico/H5N2 | North American | AAR88841 | Ceva, Mexico | 75.3–90.1 |
| 2 | A/duck/Potsdam/1402-6/1986 | H5N2 | Potsdam/H5N2 | Eurasian | ABI84497 | Intervet, The Netherlands | 87.2–92.0 |
| 3 | A/chicken/Egypt/18-H/2009 | H5N1 | EGY09/H5N1 | 2.2.1.1 | ADG28676 | Harbin Veterinary Research Institute, China | 88.7–94.2 |
| 4 | A/duck/Egypt/M2583D /2010 | H5N1 | EGY10/H5N1 | 2.2.1.1 | AEP37317 | ME-VAC, Egypt | 90.8–96.4 |
| 5 | A/chicken/Vietnam/C58/2004 | H5N1 | Vietnam/H5N1 | 1 | AAW80718.1 | Zoetis, USA | 90.9–96.7 |
| 6 | A/duck/China/E319-2/2003 | H5N1 | CN03/H5N1 | 2.3.2 | AAR99628 | Boehringer Ingelheim, Germany | 92.5–98.4 |
| 7 | A/duck/Anhui/1/2006 | H5N1 | Re5 | 2.3.4 | ADG59091 | QYH, China | 92.9–98.4 |
| 8 | A/chicken/Guizhou/4/2013 | H5N1 | Re8 | 2.3.4.4 | EPI675769 | Merial, USA & QYH, China | 94.9–97.1 |
| 9 | A/green-winged teal/Egypt/877/2016 | H5N8 | EGY16/H5N8 | 2.3.4.4b | ART29489 | ME-VAC, Egypt | 97.8–100 |
The identity was calculated for the mature HA protein after the sequence of the signal peptide. For Mexican H5N2, only HA1 sequence is available.Nevertheless, our analysis indicated a temporal trend for the increased number of aa differences, particularly against clade 2.3.4.4 based vaccines (Figure 3).
Figure 3.
Amino acid differences of Egyptian H5N8 from 2017 to 2021 and licensed H5 vaccine strains in poultry in Egypt. Amino acid sequences of Egyptian H5N8 from 2017 to 2021 were retrieved from GISAID. All sequences including those generated in this study were aligned against different vaccine strains. Number of amino acid differences compared to the vaccine strain. Number of amino acid (aa) differences are shown in the y-axis. Each dot represents one HA sequence and sequences are arranged from 2017 to 2021. The figure was generated by GraphPad Prism 9.0.0 and was further edited using Inkscape 0.92. For the abbreviations of the vaccine strains, refer to Table 2.
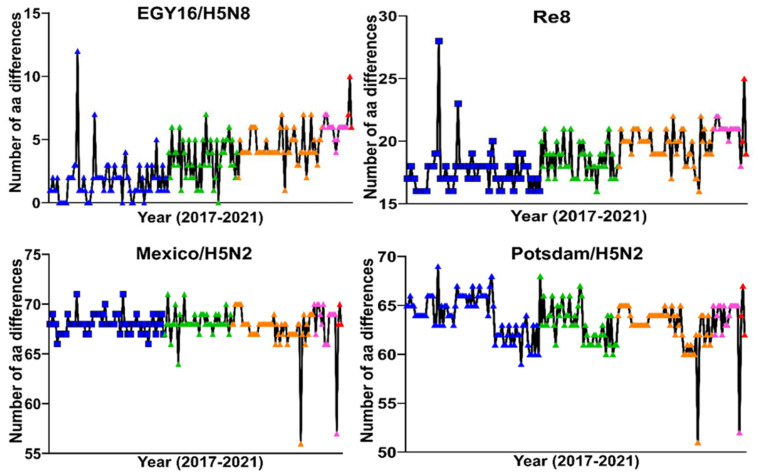
The Egyptian H5N8 viruses isolated in 2020/2021 possessed a higher number of aa differences compared to their ancestors in comparison to clade 2.3.4.4 vaccines, indicating a continuous genetic drift from the vaccine strains (Figure 4).
Figure 4.
Number of amino acid differences of Egyptian H5N8 from 2017 to 2021 compared to selected vaccine strains. HA sequences of viruses isolated from 2017 (blue), 2018 (green), 2019 (orange), 2020 (pink) and 2021 (red) were compared to vaccine strains. Clade 2.3.4.4 EGY16/H5N8 (upper left) and Re8 (upper right) are commonly used in poultry in Egypt, while historic H5N2 virus-based vaccines (lower panels) are less used. Although clade 2.3.4.4 viruses are closely related to the Egyptian viruses, there is an increasing number of aa differences in the Egyptian H5N8 viruses particularly from 2020/2021 compared to the H5N2 vaccines. For the abbreviations of the vaccine strains, refer to Table 2.
Compared to the vaccine strain from the local Egyptian H5N8 strain (A/green-winged teal/Egypt/877/2016), the HA protein of the new viruses generated in this study in phylogroup Egypt-II possessed R72N/S (n = 9/9) in addition to S94R (n = 4/9), R169Q (n = 4/9), T188I (n = 4/9), V522A (n = 2/9) and V532M (n = 2/9) (Table 3). Similarly, new viruses generated in this study in phylogroup Egypt-III possessed T140A (n = 10/11), N236D (n = 8/11), V522A (n = 11/11) and V532M (n = 10/11). R169Q and T188I were rarely observed in this group (Table 3). All Egyptian viruses generated in this study possessed E268G. These mutations were also enriched in comparison to other vaccines, e.g., Re8 vaccine (data not shown). We further analyzed the prevalence of these mutations in the European H5N8 viruses from 2017 to 2021 (n = 1169) (Supplementary Table S1). The prevalence of N/S72, R94, Q169 and I188 in these viruses was ≤0.6% and a higher prevalence for A140 (48.2%), D236 (48.1%), G268 (93.7%), A522 (48.3%) and M532 (50.2%) was observed. These mutations are predicted to be in (residues 72, 140) or adjacent to (residues 94, 169, 188) the HA immunogenic epitopes [39] (Supplementary Table S1).
Table 3.
Amino acid differences in Egyptian H5N8 viruses compared to the vaccines strain.
| Phylogroup | Virus/aa Position (H5 Numbering) * | 72 | 94 | 140 | 169 | 188 | 236 | 268 | 522 | 532 |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine strain | A/green-winged teal/Egypt/877/2016 | R | S | T | R | T | N | E | V | V |
| Egypt-II | A/Duck/Egypt/Elbehera_AH2/2019 | N | . | . | . | . | . | G | . | . |
| A/chicken/Egypt/kafrelsheik_AH/2019 | N | . | . | . | I | . | G | . | . | |
| A/chicken/Egypt/Alex_AH1/2020 | N | . | . | . | I | . | G | A | . | |
| A/chicken/Egypt/Alex_AH/2020 | N | . | . | . | I | . | G | A | M | |
| A/chicken/Egypt/Alex_AH2/2020 | N | . | . | . | I | . | G | . | . | |
| A/chicken/Egypt/Elmonufia_AH/2020 | S | R | . | Q | . | . | G | . | M | |
| A/turkey/Egypt/Cairo/AH/2019 | S | R | . | Q | . | . | G | . | . | |
| A/turkey/Egypt/Alex_AH/2019 | S | R | . | Q | . | . | G | . | . | |
| A/duck/Egypt/Behera_AH1/2019 | S | R | . | Q | . | . | G | . | . | |
| Egypt-III | A/chicken/Egypt/behera-AH/2021 | . | . | A | Q | . | . | G | A | . |
| A/chiken/Egypt/Alex-Breeder-AH/2021 | . | . | A | Q | . | . | G | A | M | |
| A/chicken/Egypt/Giza-AH/2020 | . | . | A | . | . | D | G | A | M | |
| A/chicken/Egypt/Elmonufia-backyard-AH/2019 | . | . | A | . | . | D | G | A | M | |
| A/chicken/Egypt/qalyubia-layer-AH/2020 | . | . | A | . | N | D | G | A | M | |
| A/chicken/Egypt/sohag-AH/2020 | . | . | A | . | . | D | G | A | M | |
| A/chicken/Egypt/Assuit-AH/2019 | . | . | A | . | . | D | G | A | M | |
| A/Duck/Egypt/Behera-HB2-AH/2020 | . | . | A | . | . | D | G | A | M | |
| A/Chicken/Egypt/Giza-HG4L-AH/2020 | . | . | . | . | . | D | G | A | M | |
| A/Chicken/Egypt/Cairo-HC11B-AH/20 | . | . | A | . | . | D | G | A | M | |
| A/turkey/Egypt/Alex-AH1/2019 | . | . | A | . | . | . | G | A | M |
* Residue numbering is based on the sequence of the mature H5 HA protein. Dots indicate residues identical to the vaccine strain.
4. Discussion
The panzootic HPAIV H5N8 clade 2.3.4.4 devastated the poultry industry in Asia, Europe, North America and Africa [4,5]. Therefore, vaccination against H5N8 has been successfully implemented in several countries to limit the tremendous economic losses in the poultry industry [40,41]. The vaccination of poultry against HPAIV is highly useful to reduce morbidity, mortality, virus excretion and bird-to-bird transmission. However, mismatched vaccines or improper vaccination may accelerate virus evolution and may lead to the escape from vaccine-induced antibodies [19,32,42]. In China, the regular update of AIV vaccines was efficiently successful at mitigating the economic losses in poultry and reducing the public health threat caused by AIV [11,16,17,40]. Outside China, little is known about the prevalence and genetic properties of H5N8 viruses in vaccinated poultry. An experimental study described the inefficiency of non-clade 2.3.4.4 H5 vaccines to prevent morbidity, mortality or shedding in chickens that were experimentally infected with an Egyptian H5N8 virus isolated from wild birds in 2016 [41]. However, the prevalence of HPAIV H5N8 in vaccinated flocks in Egypt is largely unknown, which has been described in this short communication paper.
Our surveillance showed a high prevalence rate of H5N8 in vaccinated flocks, which received the vaccine up to four times. Vaccinal breaks can be attributed to factors related to the vaccination process (e.g., improper vaccination, vaccination coverage), the bird (e.g., age, species, immune suppression) or the vaccine strain (e.g., seed virus, antigen mass, storage and transport conditions) [42]. One of the major limitations of our passive surveillance is the lack of data on the levels of antibodies after vaccination or the cross-reactivity between the viruses isolated in this study and vaccine-induced antibodies. However, the high prevalence of AIV H5N8 in vaccinated poultry, the increased distinction of circulating 2020/2021 viruses from vaccine strains and the selection of mutations in the HA immunogenic epitopes (i.e., R72N/S, T140A) highlight the need to revise the efficiency of the currently licensed H5 vaccines in Egypt. Furthermore, our sequence analyses confirmed previous findings of multiple introductions of H5N8 from Eurasia into Egypt via wild birds in 2016–2019 [23,24,25,36,43] and revealed the establishment of a new sub-cluster in 2020/2021. These results indicate the importance of poultry in Egypt in the global epidemiology of H5N8 clade 2.3.4.4b along the migration flyways and as an endemic hotspot for H5N8 in the Middle East. The impact of mutations in viruses of phylogroups Egypt-II and Egypt-III compared to the extinct phylogroup Egypt-I on biological fitness remains to be investigated. Last but not least, among the reasons for the endemicity of H5N1 clade 2.2.1 in poultry in Egypt since 2006 were the poor biosecurity measures, low vaccination coverage, improper vaccination, the use of outdated vaccine strains (e.g., H5N2 from 1973), the absence of an efficient monitoring system, inadequate training of field technicians and the lack of periodical evaluation and updating of AIV vaccines [30,31,44]. To control H5N8 in poultry in Egypt, these factors should be seriously considered.
Together, HPAIV H5N8 was detected in 45.1% of H5-vaccinated poultry flocks in Egypt in 2019/2021. The sequence analysis indicated three phylogroups representing endemic H5N8 viruses and multiple introductions into Egyptian poultry from Eurasia. The efficacy of current vaccines should be evaluated and biosecurity measures should be improved. Poultry in Egypt is vulnerable to the frequent introduction of H5N8 viruses from Eurasia and a hotspot for H5N8 in the Middle East.
Acknowledgments
The authors thank all veterinarians and farmers who submitted the samples to the laboratory of the Faculty of Veterinary Medicine, Damanhour University, Egypt. Colleagues in the National Reference Laboratory for Quality Control on Poultry Production (NLQP) are thanked for their help in sequencing the isolates. Laboratories submitted sequences to GISAID and used for the analysis in this are thanked.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14071431/s1, Table S1: Prevalence of HA mutations in the European H5N8 viruses from 2017 to 2021.
Author Contributions
Conceptualization, A.H.S., H.S.A.E.-H. and E.M.A.; methodology, A.H.S., A.R.E., A.M.A., H.A.S., A.A.I., H.S.A.E.-H. and E.M.A.; software, A.H.S. and E.M.A.; validation, A.H.S. and E.M.A.; formal analysis, A.H.S. and E.M.A.; investigation, A.H.S., A.R.E., A.M.A., H.A.S., A.A.I., H.S.A.E.-H. and E.M.A.; resources, A.H.S.; data curation, A.H.S. and E.M.A.; writing—original draft preparation, A.H.S. and E.M.A.; writing—review and editing, A.H.S. and E.M.A.; visualization, A.H.S. and E.M.A.; supervision, A.H.S. and E.M.A.; project administration, A.H.S.; funding acquisition, A.H.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Collection of samples from animals was approved by the Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Damanhour University, Damanhour 22511, Egypt and according to the guidelines of the World Health Organization for Animal Health OIE.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences generated in this study are deposited in the GISAID and assigned accession numbers: EPI1999273 to EPI1999312.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was funded by IFT corporation.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaplan B.S., Webby R.J. The avian and mammalian host range of highly pathogenic avian H5N1 influenza. Virus Res. 2013;178:3–11. doi: 10.1016/j.virusres.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander D.J. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 3.Smith G.J., Donis R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Respir. Viruses. 2015;9:271–276. doi: 10.1111/irv.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antigua K.J.C., Choi W.S., Baek Y.H., Song M.S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms. 2019;7:156. doi: 10.3390/microorganisms7060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D.H., Bertran K., Kwon J.H., Swayne D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017;18:269–280. doi: 10.4142/jvs.2017.18.S1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd T., Banyard A.C., Lean F.Z.X., Byrne A.M.P., Fullick E., Whittard E., Mollett B.C., Bexton S., Swinson V., Macrelli M., et al. Encephalitis and Death in Wild Mammals at a Rehabilitation Center after Infection with Highly Pathogenic Avian Influenza A(H5N8) Virus, United Kingdom. Emerg. Infect. Dis. 2021;27:2856–2863. doi: 10.3201/eid2711.211225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyankova O.G., Susloparov I.M., Moiseeva A.A., Kolosova N.P., Onkhonova G.S., Danilenko A.V., Vakalova E.V., Shendo G.L., Nekeshina N.N., Noskova L.N., et al. Isolation of clade 2.3.4.4b A(H5N8), a highly pathogenic avian influenza virus, from a worker during an outbreak on a poultry farm, Russia, December 2020. Eurosurveillance. 2021;26:2100439. doi: 10.2807/1560-7917.ES.2021.26.24.2100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin D.L., Siebert U., Lakemeyer J., Grilo M., Pawliczka I., Wu N.H., Valentin-Weigand P., Haas L., Herrler G. Highly Pathogenic Avian Influenza A(H5N8) Virus in Gray Seals, Baltic Sea. Emerg. Infect. Dis. 2019;25:2295–2298. doi: 10.3201/eid2512.181472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver I., Roberts J., Brown C.S., Byrne A.M., Mellon D., Hansen R., Banyard A.C., James J., Donati M., Porter R., et al. A case of avian influenza A(H5N1) in England, January 2022. Eurosurveillance. 2022;27:2200061. doi: 10.2807/1560-7917.ES.2022.27.5.2200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z., Lu Y., Short K.R., Lu J. One health insights to prevent the next HxNy viral outbreak: Learning from the epidemiology of H7N9. BMC Infect. Dis. 2019;19:138. doi: 10.1186/s12879-019-3752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H. H5N1 avian influenza in China. Sci. China C Life Sci. 2009;52:419–427. doi: 10.1007/s11427-009-0068-6. [DOI] [PubMed] [Google Scholar]
- 12.Li C., Bu Z., Chen H. Avian influenza vaccines against H5N1 ‘bird flu’. Trends Biotechnol. 2014;32:147–156. doi: 10.1016/j.tibtech.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Jadhao S.J., Lee C.W., Sylte M., Suarez D.L. Comparative efficacy of North American and antigenically matched reverse genetics derived H5N9 DIVA marker vaccines against highly pathogenic Asian H5N1 avian influenza viruses in chickens. Vaccine. 2009;27:6247–6260. doi: 10.1016/j.vaccine.2009.07.110. [DOI] [PubMed] [Google Scholar]
- 14.Shi J., Deng G., Ma S., Zeng X., Yin X., Li M., Zhang B., Cui P., Chen Y., Yang H., et al. Rapid Evolution of H7N9 Highly Pathogenic Viruses that Emerged in China in 2017. Cell Host Microbe. 2018;24:558–568. doi: 10.1016/j.chom.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swayne D.E., Kapczynski D.R. Animal Influenza. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. Vaccines and vaccination for avian influenza in poultry; pp. 378–434. [Google Scholar]
- 16.Zeng X., Tian G., Shi J., Deng G., Li C., Chen H. Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci. China Life Sci. 2018;61:1465–1473. doi: 10.1007/s11427-018-9420-1. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X., Chen X., Ma S., Wu J., Bao H., Pan S., Liu Y., Deng G., Shi J., Chen P., et al. Protective efficacy of an H5/H7 trivalent inactivated vaccine produced from Re-11, Re-12, and H7-Re2 strains against challenge with different H5 and H7 viruses in chickens. J. Integr. Agric. 2020;19:2294–2300. doi: 10.1016/S2095-3119(20)63301-9. [DOI] [Google Scholar]
- 18.Hafez M.H., Arafa A., Abdelwhab E.M., Selim A., Khoulosy S.G., Hassan M.K., Aly M.M. Avian influenza H5N1 virus infections in vaccinated commercial and backyard poultry in Egypt. Poult. Sci. 2010;89:1609–1613. doi: 10.3382/ps.2010-00708. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.-W., Senne D.A., Suarez D.L. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J. Virol. 2004;78:8372–8381. doi: 10.1128/JVI.78.15.8372-8381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S.H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses. 2018;10:121. doi: 10.3390/v10030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan K.E., El-Kady M.F., EL-Sawah A.A.A., Luttermann C., Parvin R., Shany S., Beer M., Harder T. Respiratory disease due to mixed viral infections in poultry flocks in Egypt between 2017 and 2018: Upsurge of highly pathogenic avian influenza virus subtype H5N8 since 2018. Transbound. Emerg. Dis. 2021;68:21–36. doi: 10.1111/tbed.13281. [DOI] [PubMed] [Google Scholar]
- 22.Amer F., Li R., Rabie N., El-Husseiny M.H., Yehia N., Hagag N.M., Samy M., Selim A., Hassan M.K., Hassan W.M.M., et al. Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8. Viruses. 2021;13:1565. doi: 10.3390/v13081565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salaheldin A.H., El-Hamid A., Elbestawy A.R., Veits J., Hafez H.M., Mettenleiter T.C., Abdelwhab E.M. Multiple Introductions of Influenza A(H5N8) Virus into Poultry, Egypt, 2017. Emerg. Infect. Dis. 2018;24:943–946. doi: 10.3201/eid2405.171935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yehia N., Naguib M.M., Li R., Hagag N., El-Husseiny M., Mosaad Z., Nour A., Rabea N., Hasan W.M., Hassan M.K., et al. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3.4.4b causing outbreaks in wild birds and poultry in Egypt. Infect. Genet. Evol. 2018;58:56–65. doi: 10.1016/j.meegid.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Hassan K.E., Saad N., Abozeid H.H., Shany S., El-Kady M.F., Arafa A., El-Sawah A.A.A., Pfaff F., Hafez H.M., Beer M., et al. Genotyping and reassortment analysis of highly pathogenic avian influenza viruses H5N8 and H5N2 from Egypt reveals successive annual replacement of genotypes. Infect. Genet. Evol. 2020;84:104375. doi: 10.1016/j.meegid.2020.104375. [DOI] [PubMed] [Google Scholar]
- 26.Kammon A., Heidari A., Dayhum A., Eldaghayes I., Sharif M., Monne I., Cattoli G., Asheg A., Farhat M., Kraim E. Characterization of Avian Influenza and Newcastle Disease Viruses from Poultry in Libya. Avian Dis. 2015;59:422–430. doi: 10.1637/11068-032215-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 27.Salaheldin A.H., Veits J., Abd El-Hamid H.S., Harder T.C., Devrishov D., Mettenleiter T.C., Hafez H.M., Abdelwhab E.M. Isolation and genetic characterization of a novel 2.2.1.2a H5N1 virus from a vaccinated meat-turkeys flock in Egypt. Virol. J. 2017;14:48. doi: 10.1186/s12985-017-0697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2021, 15 April 2021. [(accessed on 16 March 2022)]. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2021-15-april-2021.
- 29.Gomaa M.R., Kayed A.S., Elabd M.A., Zeid D.A., Zaki S.A., El Rifay A.S., Sherif L.S., McKenzie P.P., Webster R.G., Webby R.J., et al. Avian influenza A(H5N1) and A(H9N2) seroprevalence and risk factors for infection among Egyptians: A prospective, controlled seroepidemiological study. J. Infect. Dis. 2015;211:1399–1407. doi: 10.1093/infdis/jiu529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelwhab E.M., Hafez H.M. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: Epidemiology and control challenges. Epidemiol. Infect. 2011;139:647–657. doi: 10.1017/S0950268810003122. [DOI] [PubMed] [Google Scholar]
- 31.Abdelwhab E.M., Hassan M.K., Abdel-Moneim A.S., Naguib M.M., Mostafa A., Hussein I.T.M., Arafa A., Erfan A.M., Kilany W.H., Agour M.G., et al. Introduction and enzootic of A/H5N1 in Egypt: Virus evolution, pathogenicity and vaccine efficacy ten years on. Infect. Genet. Evol. 2016;40:80–90. doi: 10.1016/j.meegid.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Cattoli G., Milani A., Temperton N., Zecchin B., Buratin A., Molesti E., Aly M.M., Arafa A., Capua I. Antigenic drift in H5N1 avian influenza virus in poultry is driven by mutations in major antigenic sites of the hemagglutinin molecule analogous to those for human influenza virus. J. Virol. 2011;85:8718–8724. doi: 10.1128/JVI.02403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cattoli G., Fusaro A., Monne I., Coven F., Joannis T., El-Hamid H.S., Hussein A.A., Cornelius C., Amarin N.M., Mancin M., et al. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine. 2011;29:9368–9375. doi: 10.1016/j.vaccine.2011.09.127. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann B., Hoffmann D., Henritzi D., Beer M., Harder T.C. Riems influenza a typing array (RITA): An RT-qPCR-based low density array for subtyping avian and mammalian influenza a viruses. Sci. Rep. 2016;6:27211. doi: 10.1038/srep27211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann E., Stech J., Guan Y., Webster R.G., Perez D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 36.Tarek M., Naguib M.M., Arafa A.S., Tantawy L.A., Selim K.M., Talaat S., Sultan H.A. Epidemiology, Genetic Characterization, and Pathogenesis of Avian Influenza H5N8 Viruses Circulating in Northern and Southern Parts of Egypt, 2017–2019. Animals. 2021;11:2208. doi: 10.3390/ani11082208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne I., Lindner D., Bayer M., Husmeier D., McGuire G., Marshall D.F., Wright F. TOPALi v2: A rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics. 2009;25:126–127. doi: 10.1093/bioinformatics/btn575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duvvuri V.R., Duvvuri B., Cuff W.R., Wu G.E., Wu J. Role of positive selection pressure on the evolution of H5N1 hemagglutinin. Genom. Proteom. Bioinform. 2009;7:47–56. doi: 10.1016/S1672-0229(08)60032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui P., Zeng X., Li X., Li Y., Shi J., Zhao C., Qu Z., Wang Y., Guo J., Gu W., et al. Genetic and biological characteristics of the globally circulating H5N8 avian influenza viruses and the protective efficacy offered by the poultry vaccine currently used in China. Sci. China Life Sci. 2022;65:795–808. doi: 10.1007/s11427-021-2025-y. [DOI] [PubMed] [Google Scholar]
- 41.Kandeil A., Sabir J.S.M., Abdelaal A., Mattar E.H., El-Taweel A.N., Sabir M.J., Khalil A.A., Webby R., Kayali G., Ali M.A. Efficacy of commercial vaccines against newly emerging avian influenza H5N8 virus in Egypt. Sci. Rep. 2018;8:9697. doi: 10.1038/s41598-018-28057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swayne D.E., Spackman E., Pantin-Jackwood M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird-agricultural interface. Ecohealth. 2014;11:94–108. doi: 10.1007/s10393-013-0861-3. [DOI] [PubMed] [Google Scholar]
- 43.Yehia N., Hassan W.M.M., Sedeek A., Elhusseiny M.H. Genetic variability of avian influenza virus subtype H5N8 in Egypt in 2017 and 2018. Arch. Virol. 2020;165:1357–1366. doi: 10.1007/s00705-020-04621-7. [DOI] [PubMed] [Google Scholar]
- 44.Peyre M., Samaha H., Makonnen Y.J., Saad A., Abd-Elnabi A., Galal S., Ettel T., Dauphin G., Lubroth J., Roger F., et al. Avian influenza vaccination in Egypt: Limitations of the current strategy. J. Mol. Genet. Med. 2009;3:198–204. doi: 10.4172/1747-0862.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences generated in this study are deposited in the GISAID and assigned accession numbers: EPI1999273 to EPI1999312.