Abstract
Objectives
To evaluate the impact of aortic root abscess (ARA) on the postoperative outcomes of surgically managed infective endocarditis (IE) and to inform optimal surgical approach.
Methods
Between 2009 and 2020, 143 consecutive patients who underwent surgical management for aortic‐valve IE were included in a retrospective cohort study. Multivariable and propensity‐weighted analyses were used to adjust for demographic imbalances between those without (n = 93; NARA) and with an ARA (n = 50). Additionally, empirical subgroup analysis appraised the two most used surgical techniques; patch reconstruction (PR) and aortic root replacement (ARR).
Results
Demographic characteristics were similar between ARA and NARA except for logistic EuroSCORE, previous valve surgery, and multivalvular infection. In‐hospital mortality was 8% and 12% in NARA and ARA, respectively (p = .38), with mortality rates consistently nonsignificantly higher in ARA across all time periods. The overall reoperation rate was also higher in ARA (27% vs. 14%; p = .09) and ARA was shown to be associated with late reoperation (odds ratio [OR] = 2.74; 95% confidence interval [CI] = 1.18–6.36). Patients treated with an ARR showed a 16% increase in late mortality when compared with PR (40% vs. 24%; p = .27) and a 17% lower reoperation rate (14% vs. 31%; p = .24). Propensity‐weighted analysis identified ARR as a significant protective factor for reoperation (hazard ratio = 0.05; 95% CI = 0.01–0.34).
Conclusions
The presence of an ARA in aortic valve endocarditis was not associated with significantly higher early and late mortality but is linked with a higher reoperation rate at our institution. ARR in ARA is protective from reoperation so should be considered best practice in this setting.
Keywords: aortic root abscess, aortic root replacement, infective endocarditis, patch reconstruction
1. INTRODUCTION
Approximately half of all infective endocarditis (IE) patients are identified as high‐risk and undergo operative treatment. 1 The presence of a paravalvular abscess is a crucial indication for surgical management 2 due to its known association with in‐hospital mortality. 3 However, the etiology, sequelae, and management of patients with paravalvular abscesses occurring in different regions of the heart are distinct. 4 Aortic root abscesses (ARAs) are the most common type of paravalvular abscess, 5 , 6 , 7 previously described as a catastrophic complication of IE. If left untreated, ARA can cause severe valvular dysfunction, heart block, pseudoaneurysm formation, and obstruction to coronary blood flow. 8 , 9 However, the impact of an ARA on early and late postoperative outcomes is not well defined due to the fragmented evidence base of retrospective cohort studies and the emergence of modern surgical techniques. 5
The two primary goals for operative management of IE with or without an ARA are: (a) complete debridement of infective tissue and (b) reconstruction of cardiac morphology. 10 The choice between these approaches currently relies on the extent of infection, surgeon or institutional preference, and demographic factors. 9 In this study, we aim to establish the significance of ARA on postoperative outcomes and inform surgical decision‐making between the two most used techniques: patch reconstruction (PR; Figure 1) and aortic root replacement (ARR).
Figure 1.
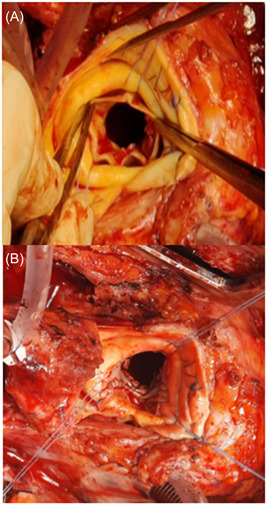
(A) Surgeon's view showing the ventricular surface of a bio‐prosthetic aortic valve studded with vegetations with florid endocarditis and root abscess. (B) Similar view after excision of the valve and repair of the abscess cavity with bovine pericardial patches in the noncoronary and left coronary sinuses.
2. MATERIALS AND METHODS
Our empirical study was registered locally and was granted institutional ethical approval. As no patient identifiable information was collected, informed consent for participation was waived. Data was refined from a local surgical database at the Bristol Heart Institute, capturing the details of consecutive surgically managed patients with active IE of the aortic valve from 2009 to 2020. One hundred forty‐three patients who underwent first‐time surgical management for active IE were included in the study. These patients were divided into two groups according to the absence (n = 93; Group NARA) or presence (n = 50; Group ARA) of an ARA. Within Group ARA, 15 patients received an ARR, 29 were treated with PR, and in six cases, the aortic annulus was repaired using Prolene suture. Follow‐up data, including date of death, reoperation, and adverse events, were assimilated on review of a local surgical database and each patient's electronic health record (mortality and reoperation follow‐up 100% complete).
Categorical variables are expressed as percentages, with all raw data tabulated. Continuous variables are expressed as mean values with their associated standard deviations. Univariable comparisons for categorical variables were performed using the chi‐squared test and the independent Student's t‐test for continuous variables. The Fisher's exact test was preferred when dichotomous variables had an expected count of <5 in >20% of cells. Statistical significance was defined as a probability of p < .05, using two‐tailed p values. All preoperative variables found to be significantly different between the Groups NARA and ARA were identified as potential confounding factors hence were included in all subsequent multivariable analyses. Additionally, “a priori” selection was used to include any independent predictors of in‐hospital mortality identified in previous research. We performed statistical analysis on the outcomes of all‐cause in‐hospital mortality, late mortality, and late reoperation (defined as any additional surgical procedure performed in the follow‐up period). Multivariable logistic regression and inverse propensity treatment weighting (IPTW) analysis were used to calculate an adjusted OR for in‐hospital mortality, defined according to the Society of Thoracic Surgeons definition (including all causes of death occurring during the same hospitalization in which the surgery was performed, even after 30 days). 11 , 12 Cox proportional hazard modeling was used to calculate the adjusted hazard ratio (HR) for late mortality and late reoperation (measured from the date of surgery to follow‐up). To graphically represent differences in survival from death and reoperation, survival curves were constructed using the Kaplan–Meier method. The log‐rank test was used to assess differences in survival curves.
For our subgroup analysis, Group ARA was divided into patients managed with an ARR (n = 15) versus PR (n = 29). IPTW analysis adjusted for variables that were statistically different between the two treatment groups on univariable analysis. Additionally, Kaplan–Meier survival curves were constructed, demonstrating differences in mortality and reoperation over time. All statistical analyses were performed on SPSS 26 (IBM Corp).
3. RESULTS
3.1. Preoperative variables
On univariate comparison, there were no statistically significant differences in medical history, baseline characteristics, or comorbidities between the treatment groups. However, Group ARA had a significantly lower proportion of patients with previous aortic valve replacement (AVR; 16% vs. 33%; p ≤ .01) and a higher rate of multivalvular infection (23% vs. 6%; p = .01). Additionally, the logistic EuroSCORE, was significantly higher in Group ARA (25.5 vs. 18.1; p = .04). Streptococcal infection was the most common infective agent (35%) but there were no significant differences in cultured microorganisms between Groups ARA and NARA (Table 1).
Table 1.
Preoperative characteristics with univariate analysis
| Preoperative variable | Total (n = 143) | No ARA (n = 93) | ARA (n = 50) | p |
|---|---|---|---|---|
| Age (years) | 58.5 (SD ± 15.0) | 58.3 (SD ± 13.6) | 58.9 (SD ± 17.3) | .838 |
| Median – 60.6 | ||||
| Male | 78% (111/143) | 77% (72/93) | 78% (39/50) | .937 |
| BMI | 26.0 (SD ± 5.6) | 26.0 (SD ± 5.3) | 26.0 (SD ± 6.2) | .982 |
| Smoking | .254 | |||
| Never smoked | 46% (63/137) | 50% (45/90) | 38% (18/47) | |
| Ex‐smoker | 30% (41/137) | 30% (27/90) | 30% (14/47) | |
| Current smoker | 24% (33/137) | 20% (18/90) | 32% (15/47) | |
| Hypertension | 33% (46/141) | 35% (32/92) | 29% (14/49) | .454 |
| Neurological history | .108 | |||
| No history | 84% (116/138) | 82% (74/90) | 88% (42/48) | |
| Previous MI | 4% (6/139) | 4% (4/91) | 4% (4/48) | 1.000 |
| Rhythm | .166 | |||
| Sinus rhythm | 88% (124/141) | 91% (84/92) | 82% (40/49) | |
| Atrial fibrillation/flutter | 3% (4/141) | 3% (3/92) | 2% (1/49) | |
| Complete heart block/pacing | 6% (9/141) | 4% (3/92) | 10% (5/49) | |
| Cardiac surgical history | .410 | |||
| No previous surgery | 87% (120/138) | 90% (81/90) | 81% (39/48) | |
| Diabetes | 6% (9/143) | 5% (5/93) | 8% (4/50) | .720 |
| Creatinine | 120.4 (SD ± 69.1) | 115 (SD ± 56.6) | 130 (SD ± 87.4) | .274 |
| IVDU | 13% (12/92) | 11% (7/63) | 17% (5/29) | .417 |
| LVEF | .865 | |||
| Poor (<30%) | 9% (13/143) | 9% (8/93) | 10% (5/50) | |
| Organism grown | .227 | |||
| Culture negative | 32% (45/139) | 34% (32/93) | 26% (13/50) | .229 |
| Streptococci | 35% (49/139) | 34% (30/89) | 38% (19/50) | .611 |
| Staphylococci | 18% (25/139) | 18% (16/89) | 18% (9/50) | .997 |
| Enterococci | 6% (8/139) | 8% (7/89) | 2% (1/50) | .154 |
| Escherichia coli | 2% (3/139) | 1% (1/89) | 4% (2/50) | .263 |
| Operative urgency | .115 | |||
| Urgent | 58% (83/143) | 58% (54/92) | 58% (29/50) | |
| Emergency | 23% (33/143) | 18% (17/93) | 32% (16/50) | |
| Previous AVR | 19% (26/137) | 11% (10/89) | 33% (16/48) | .002 |
| Aortic explant | .004 | |||
| Native | 81% (111/137) | 89% (79/89) | 67% (32/48) | |
| Mechanical | 7% (10/137) | 6% (5/89) | 10% (5/48) | |
| Biological | 12% (16/137) | 6% (5/89) | 23% (11/48) | |
| Logistic EuroSCORE | 20.7 (SD ± 18.9) | 18.1 (SD ± 16.6) | 25.5 (SD ± 22.0) | .040 |
| Multivalvular infection | 17% (24/143) | 23% (21/93) | 6% (3/50) | .011 |
Note: Bold indicates statistical significance. Continuous variables are given as mean values (with associated standard deviation) unless stated otherwise. Categorical variables are given as percentages (with associated raw data).
Abbreviations: ARA, aortic root abscess; AVR, aortic valve replacement; BMI, body mass index; IVDU, intravenous drug user; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SD, standard deviation.
3.2. Operative variables
As the surgical approach is inherently different in patients with and without an ARA, there were several discrepancies in operative data. This included statistically significant differences in the type of aortic valve implant used (p = .02) and whether an aortic root operation was performed (p ≤.01; Table 2). Homograft and biological valve implants were preferred in Group ARA as they are known to have lower re‐infection rates compared with mechanical valves in cases of fulminant infection. 13 Additionally, a longer cumulative cross‐clamp time (118.7 ± 59.1 vs. 94.6 ± 42.9 min) and cumulative bypass time (168.1 ± 87.9 vs. 132.5 ± 64.9 min) in Group ARA reflects the increased complexity of ARA surgery.
Table 2.
Operative characteristics with univariate analysis
| Operative variable | Total (n = 143) | No ARA (n = 93) | ARA (n = 50) | p |
|---|---|---|---|---|
| Aortic valve implant type | .019 | |||
| Mechanical | 28% (39/139) | 29% (26/89) | 26% (13/50) | |
| Biological | 67% (93/139) | 70% (62/89) | 62% (31/50) | |
| Homograft | 5% (7/139) | 1% (1/89) | 12% (6/50) | |
| Aortic valve ring size | 22.8 (SD ± 2.5) | 22.8 (SD ± 2.6) | 22.8 (SD ± 2.5) | .796 |
| Aortic root operation | <.001 | |||
| No root operation | 55% (79/143) | 85% (79/93) | 0% (0/50) | |
| 6.0 Prolene sutures | 4% (6/143) | 0% (0/93) | 12% (6/50) | |
| PR | 23% (33/143) | 3% (4/93) | 54% (29/50) | |
| ARR | 15% (22/143) | 5% (7/93) | 30% (15/50) | |
| Cumulative bypass time (min) | 144.5 (SD ± 75.0) | 132.5 (SD ± 64.9) | 168.1 (SD ± 87.9) | .011 |
| Cumulative cross clamp (min) | 102.7 (SD ± 50.0) | 94.6 (SD ± 42.9) | 118.7 (SD ± 59.1) | .009 |
Note: Bold indicates statistical significance. Continuous variables are given as mean values (with associated standard deviation) unless stated otherwise. Categorical variables are given as percentages (with associated raw data).
Abbreviations: ARA, aortic root abscess; ARR, aortic root replacement; PR, patch reconstruction.
3.3. Postoperative outcomes
The presence of an ARA was associated with several differences in postoperative outcomes. Importantly, mortality rates were consistently higher in Group ARA across all time periods, including in‐hospital (12% vs. 8%; p = .38), 1‐year (20% vs. 11%; p = .13), and late mortality (30% vs. 19%; p = .15; Table 3). Additionally, the reoperation rate was markedly higher in Group ARA (27% vs. 14% p = .085), driven by a high incidence of structural deterioration in this group (18% vs. 3%).
Table 3.
Postoperative outcomes with univariate analysis
| Postoperative variable | Total (n = 143) | No ARA (n = 93) | ARA (n = 50) | p |
|---|---|---|---|---|
| Stroke | 7% (7/101) | 8% (5/65) | 6% (2/36) | .686 |
| Dialysis | 9% (9/102) | 5% (3/65) | 16% (6/37) | .069 |
| Deep sternal wound infection | 2% (2/85) | 0% (0/57) | 8% (2/28) | .106 |
| Late reoperation | 19% (26/139) | 14% (13/90) | 27% (13/49) | .081 |
| Reoperation | .035 | |||
| No reoperation | 81% (112/139) | 84% (76/90) | 73% (36/49) | |
| Reoperation for structural deterioration | 9% (12/139) | 3% (3/90) | 18% (9/49) | |
| In‐hospital mortality | 9% (13/143) | 8% (7/93) | 12% (6/50) | .375 |
| Late mortality | 23% (33/143) | 19% (18/93) | 30% (15/50) | .150 |
| Follow‐up (months) | Range: 0–142 | 49.6 (SD ± 41.1) | 47.0 (SD ± 40.1) | .717 |
| Median: 43.0 |
Note: Bold indicates statistical significance. Continuous variables are given as mean values (with associated standard deviation) unless stated otherwise. Categorical variables are given as percentages (with associated raw data).
Abbreviation: ARA, aortic root abscess.
3.3.1. Aortic root abscess versus no aortic root abscess
Unadjusted data suggested an inconclusive trend toward increased risk of in‐hospital mortality given the presence of an ARA (odds ratio [OR] = 1.68; 95% confidence interval [CI] = 0.53–5.29). Additionally, our multivariable logistic regression model gave no further insight into this relationship (Table 4). However, it was notable that on propensity‐weighted statistical adjustment, there was a subtle shift toward the null value (OR = 1.29; 95% CI = 0.38–4.37).
Table 4.
Unadjusted OR figures for in‐hospital mortality alongside Logistic regression and propensity‐weighted analysis
| Unadjusted OR | Logistic regression | Propensity‐weighted regression | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| ARA | 1.68 (0.53–5.29) | .379 | 2.07 (0.42–10.18) | .371 | 1.29 (0.38–4.37) | .677 |
| Logistic EuroSCORE | 1.05 (1.02–1.08) | <.001 | 1.05 (1.02–1.09) | .001 | ||
| Previous AVR | 1.38 (0.35–5.42) | .642 | 0.64 (0.10–3.99) | .629 | ||
| Surgery pre‐2016 | 2.56 (0.79–8.26) | .116 | 2.14 (0.53–8.56) | .284 | ||
| Multivalvular Infection | 5.33 (1.61–17.68) | .006 | 12.98 (2.55–65.98) | .002 | ||
| Staphylococcal infection | 1.47 (0.38–5.79) | .579 | 0.82 (0.14–5.02) | .834 | ||
| NYHA Class ≥ 3 | 1.12 (0.55–2.28) | .764 | 0.72 (0.16–3.13) | .658 | ||
Note: Bold indicates statistical significance.
Abbreviations: ARA, aortic root abscess; AVR, aortic valve replacement; CI, confidence interval; NYHA, New York Heart Association; OR, odds ratio.
Although our Kaplan–Meier curve demonstrated a consistently higher mortality rate in Group ARA, this association was not statistically significant on cox regression statistical modeling (OR = 1.67; 95% CI = 0.77–3.53; p = .19) or on review of the log‐rank test (p = .17; Figure 2A). Nevertheless, we were able to identify logistic EuroSCORE (OR = 1.05; 95% CI = 1.02–1.08; p ≤ .01) and multivalvular infection (OR = 5.33; 95% CI = 1.61–17.68; p = .02) as risk factors for both in‐hospital and late mortality (Table 5). Additionally, the presence of an ARA was shown to be strongly associated with late reoperation (OR = 2.74; 95% CI = 1.18–6.36; p = .02), the only identifiable independent reoperation in our empirical analysis (Table 5). Further, though the log‐rank test did not reach clear‐cut statistical significance (p = .06), Figure 2B depicts the strong association between ARA and high reoperation rates.
Figure 2.
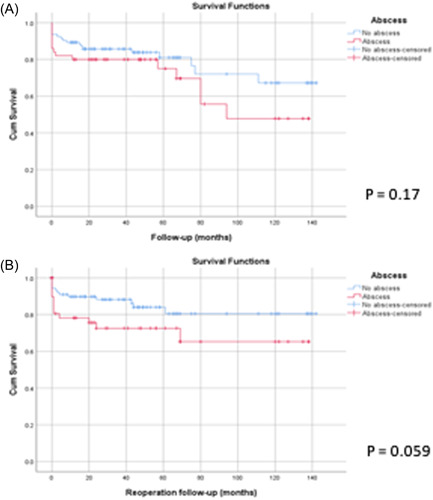
Kaplan–Meier curves show (A) survival from death and (B) survival from reoperation in patients with and without an ARA. ARA, aortic root abscess.
Table 5.
Cox proportional hazard analysis for late mortality and reoperation
| Late mortality | Late reoperation | |||
|---|---|---|---|---|
| RR (95% CI) | p | RR (95% CI) | p | |
| ARA | 1.65 (0.77–3.53) | .185 | 2.74 (1.18–6.36) | .019 |
| logistic EuroSCORE | 1.04 (1.02–1.05) | <.001 | 1.02 (<1.00–1.04) | .096 |
| Previous AVR | 0.43 (0.16–1.19) | .105 | 0.37 (0.10–1.33) | .128 |
| Multivalvular Infection | 2.80 (1.19–6.58) | .018 | 1.96 (0.70–5.52) | .203 |
| Surgery pre‐2016 | 1.82 (0.79–4.18) | .158 | 0.58 (0.24–1.38) | .218 |
Note: Bold indicates statistical significance.
Abbreviations: ARA, aortic root abscess; AVR, aortic valve replacement; CI, confidence interval; RR, risk ratio.
3.3.2. Patch reconstruction versus aortic root replacement
Preoperative data with univariable analysis were tabulated (Table S1). In our subgroup analysis, we found that Group PR was composed of a greater proportion of males (89% vs. 60%; p = .03) while group ARR had a higher incidence of previous AVR (64% vs. 24%; p = .006) and staphylococcal infection (40% vs. 10%; p = .04; Table S1). There were also several notable differences in the unadjusted postoperative outcomes for patients treated with PR versus ARR (Table S3). Patients treated with an ARR showed a 16% increase in late mortality when compared with PR (40% vs. 24%; p = .27) and a 17% lower reoperation rate (14% vs. 31%; p = .24) (Table S2). Figure 3 demonstrates that ARRs have a stronger association with lower reoperation rates (p = .255) than higher mortality (p = .442). However, both associations failed to reach statistical significance with a moderate likelihood that these relationships are due to random chance. Nevertheless, on propensity‐weighted analysis, ARR was identified as a significant protective factor for reoperation (HR = 0.05; 95% CI = 0.01–0.34) while the same statistical model yielded a poorly defined relationship between surgical technique and late mortality (HR = 2.60; 95% CI = 0.39–17.29; Table S3).
Figure 3.
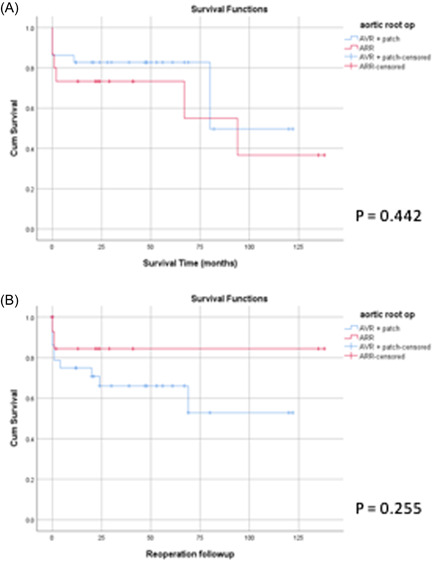
Kaplan–Meier curves show (A) survival from death and (B) survival from reoperation for ARA patients treated with an ARR versus PR. ARA, aortic root abscess; PR, patch reconstruction.
4. DISCUSSION
In our institution, we found that ARA was not associated with a clear‐cut increased risk of in‐hospital mortality or late mortality. This may suggest that modern standards of care have improved the survival rates for endocarditis complicated by ARA. However, ARA remains to be a large contributor to the surgical workload (responsible for one third of aortic valve endocarditis surgeries). 14 , 15 , 16 , 17
A salient finding from our postoperative analysis was a 13% higher reoperation rate in patients with an ARA (Table 3). Evidence of this relationship was strengthened by our Cox proportional hazard analysis, which identified ARA as an independent risk factor for reoperation (HR = 2.74; 95% CI = 1.18–6.36; p = .12; Table 5). However, this finding was not consistent with other centers. 15 , 17 Differences in institutional preference for ARR versus PR may explain this finding as preceding reports have suggested that ARR accomplishes more comprehensive removal of infected tissue and reconstruction of cardiac morphology compared with PR and so can achieve lower rates of reinfection and graft deterioration. 18 , 19 Therefore, our high reoperation rate may reflect our institutional practice of performing ARR in one third of ARA cases.
Subgroup analysis of group ARR versus group PR provides further insight into this association. Patients who received ARR were associated with a 17% lower risk of late reoperation compared with PR, and our propensity‐weighted analysis provided additional evidence to suggest that ARR is associated with a lower reoperation rate compared with PR (RR = 0.05; 95% CI = 0.01–0.34). Our results coincide with the Chen et al. 8 meta‐analysis which also showed that patients treated with an ARR were at a lower risk of reoperation at 1‐year follow‐up. Therefore, there is growing evidence to suggest that ARR is associated with advantageous reoperation rates, a hypothesis that is also intuitive and backed by well‐established surgical principles.
Our analysis did not show a significant association between ARR and postoperative mortality. This is in agreement with the aforementioned meta‐analysis, 8 which reported that, although positively correlated, ARR was not a significant risk factor for increased early mortality (OR = 1.30; 95% CI = 0.84–2.00; p = .66). 8 In our institution, group ARR was not associated with an increased risk of mortality even despite a higher logistic EuroSCORE, a finding similar to the report by Leontyev et al. 20 Therefore, evidence of subtle trends of ARR‐associated mortality in observational studies is likely to be confounded by the higher risk population on which they were performed. 20 , 21 This consideration does not explain lower reoperation rates observed in patients treated by ARR, so the disparity in reoperation rates can still be attributed to the operation itself. Therefore, we may be able to recommend a lower threshold for ARR in cases with less extensive damage to the aortic root to reduce reoperation rates for ARA patients safely. This change in practice could constitute a step forward in precise surgical decision‐making for IE patients, bringing the management of this disease into the realm of evidence‐based medicine. Nevertheless, more research is required in this area to establish the equity of ARR and PR in the treatment of ARA.
Our single‐center experience has highlighted the limitations inherent to retrospective observational studies. The sensitivity of logistic regression and Cox proportional hazard models are weakened when used on cohorts of patients with a low frequency of expected events and a high number of covariates relative to sample size. This could be overcome with the emerging use of merged databases. Additionally, we used logistic EuroSCORE, which has now been replaced by EuroSCORE II.
5. CONCLUSION
The causal relationship between ARA and mortality remains uncertain and may be more subtle than previously thought due to the influence of confounding factors, which were not adequately addressed by preceding reports. We have also shown that the ARR technique is associated with an advantageous postoperative profile compared with PR. Clearly, more research is needed in this area with larger numbers but based on our findings and other published literature; ARR could be recommended as the best practice treatment for IE complicated by ARA.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Our study was granted institutional approval and did not require International Review Board.
AUTHORS CONTRIBUTIONS
Hunaid A. Vohra and William M. Harris conceived the project idea and designed the analysis. William M. Harris collected the data with the help of Shubhra Sinha. William M. Harris performed the analysis under the supervision of Shubhra Sinha and Hunaid A. Vohra. William M. Harris wrote the manuscript with input from Shubhra Sinha, Massimo Caputo, Gianni D. Angelini, Eltayeb M. Ahmed, Cha Rajakaruna, Umberto Benedetto, and Hunaid A. Vohra. All authors reviewed the results and approved the final version of the manuscript.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
This study was funded/supported* by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
Harris WM, Sinha S, Caputo M, et al. Surgical outcomes and optimal approach to treatment of aortic valve endocarditis with aortic root abscess. J Card Surg. 2022;37:1917‐1925. 10.1111/jocs.16464
Accepted for oral presentation at the SCTS Annual Meeting on May 8−10, 2022.
REFERENCES
- 1. Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69(3):325‐344. [DOI] [PubMed] [Google Scholar]
- 2. David TE, Regesta T, Gavra G, Armstrong S, Maganti MD. Surgical treatment of paravalvular abscess: long‐term results. Eur J Cardiothorac Surg. 2007;31(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 3. Varela Barca L, Navas Elorza E, Fernández‐Hidalgo N, et al. Correction to: Prognostic factors of mortality after surgery in infective endocarditis: systematic review and meta‐analysis. Infection. 2019;47(6):897. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner FJ, Omari BO, Robertson JM, Nelson RJ, Pandya A, Milliken JC. Annular abscesses in surgical endocarditis: anatomic, clinical, and operative features. Ann Thorac Surg. 2000;70(2):442‐447. [DOI] [PubMed] [Google Scholar]
- 5. Naqvi TZ, Boyatt J, Siegel RJ. Predictors of mortality in paravalvular abscess. J Am Soc Echocardiogr. 2005;18(12):1404‐1408. [DOI] [PubMed] [Google Scholar]
- 6. Graupner C, Vilacosta I, SanRomán J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002;39(7):1204‐1211. [DOI] [PubMed] [Google Scholar]
- 7. Aguado JM, González‐Vílchez F, Martín‐Durán R, Arjona R, Vázquez de Prada JA. Perivalvular abscesses associated with endocarditis. Clinical features and diagnostic accuracy of two‐dimensional echocardiography. Chest. 1993;104(1):88‐93. [DOI] [PubMed] [Google Scholar]
- 8. Chen GJ, Lo WC, Tseng HW, Pan SC, Chen YS, Chang SC. Outcome of surgical intervention for aortic root abscess: a meta‐analysis. Eur J Cardiothorac Surg. 2018;53(4):807‐814. [DOI] [PubMed] [Google Scholar]
- 9. Lee S, Chang BC, Park HK. Surgical experience with infective endocarditis and aortic root abscess. Yonsei Med J. 2014;55(5):1253‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jamil M, Sultan I, Gleason TG, et al. Infective endocarditis: trends, surgical outcomes, and controversies. J Thorac Dis. 2019;11(11):4875‐4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Overman DM, Jacobs JP, Prager RL, et al. Report from the Society of Thoracic Surgeons National Database Workforce: clarifying the definition of operative mortality. World J Pediatr Congenit Heart Surg. 2013;4(1):10‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krumholz HM, Brindis RG, Brush JE, et al. Standards for statistical models used for public reporting of health outcomes: an American Heart Association Scientific Statement from the Quality of Care and Outcomes Research Interdisciplinary Writing Group: cosponsored by the Council on Epidemiology and Prevention and the Stroke Council. Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113(3):456‐462. [DOI] [PubMed] [Google Scholar]
- 13. Savage EB, Saha‐Chaudhuri P, Asher CR, Brennan JM, Gammie JS. Outcomes and prosthesis choice for active aortic valve infective endocarditis: analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2014;98(3):806‐814. [DOI] [PubMed] [Google Scholar]
- 14. Yoshioka D, Toda K, Yokoyama JY, et al. Recent surgical results for active endocarditis complicated with perivalvular abscess. Circ J. 2017;81(11):1721‐1729. [DOI] [PubMed] [Google Scholar]
- 15. Said SM, Abdelsattar ZM, Schaff HV, et al. Outcomes of surgery for infective endocarditis: a single‐centre experience of 801 patients. Eur J Cardiothorac Surg. 2018;53(2):435‐439. [DOI] [PubMed] [Google Scholar]
- 16. Mahmoud K, Hammouda T, Kandil H, Mashaal M. Prevalence and predictors of aortic root abscess among patients with left‐sided infective endocarditis: a cross‐sectional comparative study. Egypt Heart J. 2020;72(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sha ZJ, Liu YH, Yang B, et al. Root abscess in the setting of infectious endocarditis: short‐ and long‐term outcomes. J Thorac Cardiovasc Surg. 2020;45:2800‐2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okita Y, Franciosi G, Matsuki O, Robles A, Ross DN. Early and late results of aortic root replacement with antibiotic‐sterilized aortic homograft. J Thorac Cardiovasc Surg. 1988;95(4):696‐704. [PubMed] [Google Scholar]
- 20. Leontyev S, Davierwala PM, Krögh G, et al. Early and late outcomes of complex aortic root surgery in patients with aortic root abscesses. Eur J Cardiothorac Surg. 2016;49(2):447‐454. Discussion 54‐5. [DOI] [PubMed] [Google Scholar]
- 21. Yankah AC, Pasic M, Klose H, Siniawski H, Weng Y, Hetzer R. Homograft reconstruction of the aortic root for endocarditis with periannular abscess: a 17‐year study. Eur J Cardiothorac Surg. 2005;28(1):69‐75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


