Abstract
Objective
To examine changes in lung function over time in extremely prematurely born adolescents.
Working Hypothesis
Changes in lung function during adolescence would vary by ventilation mode immediately after birth.
Study Design
Longitudinal follow‐up study.
Patient Subject Selection
Participants from the United Kingdom Oscillation Study who were randomized at birth to high‐frequency oscillation (HFO) or conventional ventilation (CV) were assessed at 11–14 years (n = 319) and at 16–19 years (n = 159).
Methodology
Forced expiratory flow (FEF), forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and lung volumes including functional residual capacity (FRC) were reported as z‐scores. The diffusion capacity of the lungs for carbon monoxide (DLCO) was measured. Lung function trajectories were compared by mode of ventilation using mixed models. Changes in z‐scores were scaled to 5‐year average follow‐up.
Results
There were significant changes in the mean FEF75, FEF50, FEF25, FEV1, FVC, and DLCO z‐scores within the CV and HFO cohorts, but no significant differences in the changes between the two groups. The mean FRC z‐score increased in both groups, with an average change of greater than one z‐score. The mean FEV1/FVC z‐score increased significantly in the CV group, but not in the HFO group (difference in slopes: p = 0.02). Across the population, deterioration in lung function was associated with male sex, white ethnicity, lower gestational age at birth, postnatal corticosteroids, oxygen dependency at 36 weeks postmenstrual age, and lower birth weight, but not ventilation mode.
Conclusions
There was little evidence that the mode of ventilation affected changes in lung function over time.
Keywords: prematurity, ventilation mode, longitudinal changes, lung function
1. INTRODUCTION
Infants born extremely prematurely, that is before 29 weeks of gestation, suffer chronic respiratory morbidity 1 that persists into adult life. 2 , 3 , 4 The respiratory support these infants receive can be lifesaving but may be associated with lung damage leading to long‐term respiratory problems. 5 Bronchopulmonary dysplasia (BPD), birth weight z‐score, and sex are known predictors of future respiratory morbidity in preterm infants, but the reported effects are variable. 3 , 6 , 7 , 8 Few studies have presented longitudinal data pre and postpuberty on lung function changes within neonatal populations routinely exposed to antenatal steroids and postnatal surfactant. Earlier studies are conflicting as to whether conventional or high‐frequency oscillation are associated with better lung function at follow‐up. 9 , 10 , 11 , 12 , 13
The United Kingdom Oscillation Study (UKOS) found no significant difference in the risk of death or prevalence of BPD in infants born before 29 weeks of gestation who received HFO or conventional ventilation (CV) within 1 h of birth. 14 There were, however, beneficial effects of HFO on respiratory outcomes at 11–14 years of age, 15 which were not apparent at 16–19 years of age. 1 It is unclear whether these results were explained by catch up growth between 11 and 14 years and 16–19 years in those who had received CV, or whether the HFOV group's lung function had not improved as expected during puberty.
Our aims were to describe trends in lung function in UKOS participants between 11 and 14 years and 16–19 years and to determine which factors were associated with changes in lung function over time. In particular, we compared trends according to the allocated neonatal ventilation mode.
2. MATERIALS AND METHODS
Comprehensive lung function assessment was undertaken when the UKOS participants were 11–14 and 16–19 years of age. Full details of the respiratory assessments are reported elsewhere. 1 , 15 In brief, airway function was assessed by spirometry—forced expiratory flow at 75%, 50%, or 25% vital capacity (FEF75, FEF50, and FEF25, respectively), and forced expiratory flow at one second (FEV1) and lung volumes were assessed by measurements of functional residual capacity using the helium dilution technique and plethysmograph (FRCHe and FRCpleth, respectively) and forced volume vital capacity (FVC). The gas transfer was assessed by testing for the diffusing capacity of the lung for carbon monoxide (DLCO). All measurements are analyzed as z‐scores using published standards. 16 , 17 , 18 , 19 The same reference ranges were used at both time points and for the same individuals. The GLI Quanjer equations were used where available, however, the Rosenthal equations were used for FEF25 and FEF50 as Quanjer did not give reference equations for those values. Pubertal status was assessed by questionnaire when the participants were between 11 and 14 years of age. 15 Ethnic background was assessed by a self‐reported questionnaire and included the following options; white (British, Irish, Other), mixed (White and Black Caribbean, White and Black African, White and Asian, and Other), Asian (Indian, Pakistani, Bangladeshi, and Other), Black or Black British (Caribbean, African, and Other), and Chinese. These options were identical at each study point.
The original UKOS trial was granted ethical approval by the South Thames Multicentre Research Ethics Committee. Subsequent follow‐up studies at 11–14 years and 16–19 years were approved by South West London National Research Ethics Service Committee and by The North East‐Tyne and Wear South Research Ethics Committee, respectively. Participants were invited to attend by letter, email, and phone call, and gave written consent to take part in this study.
2.1. Analysis
To investigate trends in lung function over time, we used random‐effects regression models (“mixed‐effects model”) as these allow for the longitudinal lung function measurements over time and also allow for the nonindependence among participants from multiple births. 20 These regression models allowed for incomplete follow‐up with the assumption that missing data points are “missing at random.” an assumption which was tested in sensitivity analyses described below. Ventilation group, age of young person at assessment, and the interaction between group and age were included in the model in the first instance as part of the unadjusted analyses. The adjusted analysis added in the young person's birth weight, gestational age, and whether surfactant was given as these were found to differ significantly between the two modes of ventilation at the 11–14‐year follow‐up. 21 The interaction terms in the unadjusted and adjusted analyses were assessed to determine if the trajectories of lung function differed between ventilation groups. Estimates of the yearly change in lung function with 95% confidence intervals (CIs) were calculated, along with the difference in rates of change. In keeping with the previous studies, we used FEF75 as the predefined primary outcome and have regarded the other lung function measures as secondary outcomes thus we have not adjusted these for multiple testing.
Maternal and child characteristics were compared for each lung function outcome where the child deteriorated versus maintained or improved in their lung function score over time. T tests, χ 2, or Fisher's exact tests were used to identify the characteristics associated with lung function deterioration.
The representativeness of those assessed at 16–19 years was determined by comparing the neonatal and maternal characteristics of participants with those not evaluated. Sensitivity analyses were undertaken where the model was further adjusted for factors that differed between participants and dropouts. The main model was fitted (i) using all participants and (ii) using only those with complete data to confirm validity of the “missing at random” assumption used in the statistical models. Analysis was conducted using the lme4 package in R (R Core Team, 2019).
3. RESULTS
3.1. Characteristics of the study sample
Three hundred and nineteen young people born before 29 completed weeks of gestational age had comprehensive lung function measurements at ages 11–4 years (160 received HFO); 159 were seen at 16–19 years (81 received HFO) (Figure 1). One hundred and forty‐eight young people were assessed at both time points (74 received HFO). At 11–14 years, the mean Tanner score for breast and genital development was three, and 5% of boys and 6% of girls had reached Tanner Stage 5 and complete puberty.
Figure 1.
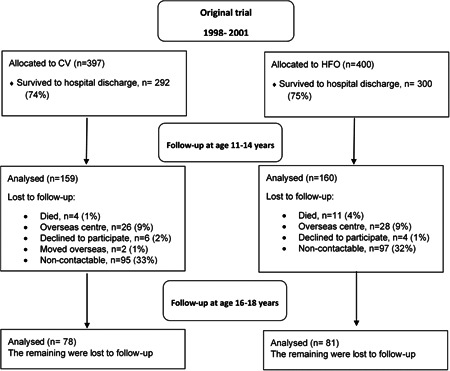
United Kingdom Oscillation Study CONSORT flow diagram. CV, conventional ventilation; high‐frequency oscillation
At the 11–14 year follow‐up, there were significant differences between CV and HFO groups in mean birth weight, gestational age at birth, and whether surfactant was given (Table 1), therefore those factors were used in the adjusted model in keeping with our previous study. 21 There were no other significant differences between the two ventilation groups in baseline maternal and neonatal characteristics or the characteristics of the children assessed at 16–19 years of age (Tables 1, 2). Participants differed from dropouts in terms of the mother's ethnic group, mean birthweight, gestational age, and whether the mother smoked during pregnancy (E‐Table S1), thus in a second sensitivity analysis, we included ethnic group and smoking during pregnancy as additional variables in the adjusted model. Further adjustment resulted in similar effect sizes and confidence intervals to the original model (E‐Table S2).
Table 1.
Maternal and neonatal characteristics of the children according to ventilation group.
| 11–14 years | 16–19 years | |||||
|---|---|---|---|---|---|---|
| CV | HFOV | p Value | CV | HFO | p Value | |
| N | 159 | 160 | 78 | 81 | ||
| Male (%) | 85 (53) | 77 (48) | 0.409 | 38 (49) | 39 (48) | >0.999 |
| Mother's ethnic group (%) = white | 142 (90) | 143 (89) | 0.920 | 68 (87) | 71 (88) | 0.329 |
| Black | 11 (7) | 10 (6) | 9 (12) | 6 (7) | ||
| Other | 5 (3) | 7 (4) | 1 (1) | 4 (5) | ||
| Birthweight (g) (mean (SD)) | 923.1 (206.0) | 866.5 (208.9) | 0.016 | 921.3 (226.2) | 867.5 (207.1) | 0.119 |
| Birthweight z‐score (mean (SD)) | −0.6 (1.0) | −0.6 (1.0) | 0.521 | −0.6 (1.1) | −0.6 (1.0) | 0.740 |
| Gestational age, weeks (mean (SD)) | 27.0 (1.2) | 26.7 (1.4) | 0.014 | 27.1 (1.3) | 26.7 (1.5) | 0.079 |
| Gestational group = 26‐28 week (%) | 129 (81) | 108 (68) | 0.008 | 60 (77) | 51 (63) | 0.081 |
| Multiple birth (%) | 39 (25) | 37 (23) | 0.871 | 13 (17) | 16 (20) | 0.765 |
| Surfactant given (%) | 158 (99) | 152 (95) | 0.036 | 78 (100) | 76 (94) | 0.059 |
| Mother smoked during pregnancy (%) | 31 (21) | 38 (26) | 0.408 | 16 (22) | 14 (18) | 0.743 |
| Systemic steroids given before extubation (%) | 36 (23) | 48 (31) | 0.161 | 20 (26) | 29 (36) | 0.224 |
| Oxygen dependency at 36 weeks postmenstrual age (%) | 95 (60) | 88 (55) | 0.457 | 42 (54) | 44 (54) | >0.999 |
| Oxygen dependency at 28 days (%) | 131 (82) | 131 (82) | >0.999 | 59 (76) | 63 (78) | 0.896 |
| Oxygen‐dependent at discharge (%) | 34 (22) | 37 (23) | 0.858 | 18 (24) | 19 (23) | >0.999 |
Note: The data are presented as the mean (SD) or number (%) unless specified.
Abbreviation: CV, conventional ventilation; HFO, high‐frequency oscillation; SD, standard deviation.
Table 2.
Characteristics of the children according to ventilation group and time.
| CV | HFO | p Value | CV | HFO | p Value | |
|---|---|---|---|---|---|---|
| n | 159 | 160 | 78 | 81 | ||
| Age (years) | 12.5 (0.6) | 12.5 (0.6) | 0.660 | 17.9 (0.8) | 17.9 (0.7) | 0.936 |
| Weight (kg) | 44.4 (12.5) | 44.9 (11.5) | 0.709 | 63.6 (16.4) | 62.0 (15.7) | 0.535 |
| Height (cm) | 152.7 (9.2) | 151.5 (8.2) | 0.264 | 167.8 (8.6) | 166.3 (9.4) | 0.302 |
| BMI | 18.8 (3.7) | 19.4 (3.8) | 0.190 | 22.4 (4.7) | 22.3 (4.8) | 0.881 |
Note: The data are presented as the mean (SD) or number (%) unless specified.
Abbreviation: CV, conventional ventilation; HFO, high‐frequency oscillation; SD, standard deviation.
3.2. Change in lung function z‐score over time
The plots of lung function over time (Figures 2 and SE1) and the adjusted model (Table 3) show the mean rates of changes in the FEF, FEV1, FVC, DLCO, and FRC results, which were similar in the two ventilation groups. There was no evidence for a change in FEF75 z‐score (primary outcome in the 11–14 year follow‐up) with age in either group nor was their evidence for a difference between the groups (Table 3). Results were virtually identical when only participants with complete data were included (E‐Table S3). The mean FEF50 and FEF25 z‐scores improved with age in both ventilation groups with FEF50 increasing by 0.04 z‐scores per additional year of age for both groups and FEF25 increasing by 0.07 z‐scores per year in the CV group and 0.06 z‐scores in the HFO group. The mean forced expiratory flow z‐scores were below expected reference values with mean values at 19 years of age at −0.8 or lower for both groups (Figure 2, Tables 2 and 4).
Figure 2.
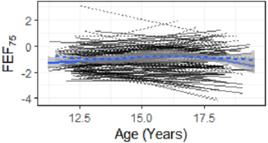
Individual trajectories for FEV75 by mode of ventilation. FEV, forced expiratory volume [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
the mean change in lung function per year (slope) a by ventilation group, along with the difference in slopes (with 95% CI).
| Unadjusted analyses | Adjusted for child's birthweight, gestational age, and surfactant given | |||||||
|---|---|---|---|---|---|---|---|---|
| CV | HFO |
Difference HFO‐CV |
p Value for the difference | CV | HFO |
Difference HFO‐CV |
p Value for the difference | |
| FEF75 z‐score | 0.02 | 0.00 | 0.02 (−0.04 to 0.08) | 0.56 | 0.01 | 0.00 | 0.02 (−0.04 to 0.08) | 0.59 |
| FEF50 z‐score | 0.04 | 0.04 | 0.01 (−0.04 to 0.06) | 0.72 | 0.04 | 0.04 | 0.01 (−0.04 to 0.05) | 0.76 |
| FEF25 z‐score | 0.07 | 0.06 | 0.01 (−0.04 to 0.07) | 0.57 | 0.07 | 0.06 | 0.01 (−0.04 to 0.06) | 0.61 |
| FEV1 z‐score | −0.02 | −0.04 | 0.02 (−0.04 to 0.07) | 0.48 | −0.03 | −0.04 | 0.02 (−0.04 to 0.07) | 0.53 |
| FVC z‐score | 0.03 | 0.05 | −0.01 (−0.07 to 0.05) | 0.68 | 0.03 | 0.04 | −0.01 (−0.07 to 0.04) | 0.65 |
| FEV1/FVC z‐score | 0.09 | −0.02 | 0.10 (0.02–0.18) | 0.02 | 0.08 | −0.01 | 0.10 (0.02–0.18) | 0.02 |
| DLCO z‐score | −0.02 | −0.05 | 0.02 (−0.04 to 0.09) | 0.49 | −0.02 | −0.04 | 0.02 (−0.05 to 0.09) | 0.57 |
| FRCpleth z‐score | 0.12 | 0.15 | −0.04 (−0.10 to 0.03) | 0.26 | 0.12 | 0.15 | −0.03 (−0.10 to 0.03) | 0.32 |
| FRCHe z‐score | 0.13 | 0.17 | −0.04 (−0.12 to 0.04) | 0.30 | 0.13 | 0.17 | −0.04 (−0.12 to 0.04) | 0.30 |
Abbreviations: CI, confidence interval; CV, conventional ventilation; DLCO, diffusion capacity of the lungs for carbon monoxide; FEF, forced expiratory flow; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FRC, functional residual capacity; HFO, high‐frequency oscillation.
Mean change per year in lung function z‐score is expressed as the number of standard deviations from the random effects regression model using all available data (319 at age 11–14 and 159 at age 16–19 years).
Table 4.
Predicted mean values a of lung function from 11 to 19 years of age.
| CV | HFO | |||||
|---|---|---|---|---|---|---|
| 11 years | 19 years |
Difference (19–11 years) |
11 years | 19 years |
Difference (19–11 years) |
|
| FEF75 z‐score | −1.53 | −1.41 | 0.12 | −1.25 | −1.26 | −0.01 |
| FEF50 z‐score | −1.78 | −1.44 | 0.34 | −1.48 | −1.20 | 0.28 |
| FEF25 z‐score | −1.52 | −0.95 | 0.57 | −1.27 | −0.80 | 0.47 |
| FEV1 z‐score | −1.23 | −1.44 | −0.21 | −0.91 | −1.26 | −0.35 |
| FVC z‐score | −0.45 | −0.20 | 0.25 | −0.37 | −0.01 | 0.36 |
| FEV1/FVC z‐score | −2.43 | −1.77 | 0.66 | −1.73 | −1.85 | −0.12 |
| DLCO z‐score | −1.32 | −1.52 | −0.20 | −1.02 | −1.38 | −0.36 |
| FRCpleth z‐score | −0.43 | 0.52 | 0.95 | −0.61 | 0.61 | 1.21 |
| FRCHe z‐score | −0.56 | 0.48 | 1.04 | −0.75 | 0.62 | 1.37 |
Abbreviations: CI, confidence interval; CV, conventional ventilation; DLCO, diffusion capacity of the lungs for carbon monoxide; FEF, forced expiratory flow; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FRC, functional residual capacity; HFO, high‐frequency oscillation.
Adjusted for child's birthweight, gestational age, and surfactant given from the random effects regression model using all available data (319 at age 11–14 and 159 at age 16–19 years).
Mean FRC increased with age for both groups; the CV group had a mean increase of 0.12 z‐score per year regarding FRCpleth and 0.13 z‐score regarding FRCHe, while in the HFO group FRCpleth increased by 0.15 z‐score and FRCHe by 0.17 z‐score (Table 3). This is equivalent to a predicted increase in mean z‐score of between 0.95 and 1.37 from 11 to 19 years of age for both groups (Table 4).
The slope of the mean trajectory for FEV1/FVC z‐score with increasing age differed significantly by mode of ventilation (interaction test: p = 0.02; Table 3). The mean ratio increased by 0.08 z‐score per year for the CV group compared to a decrease of −0.01 z score per year in the HFO group. Despite the changes in slopes, the mean z‐scores in both groups remained low with predicted values less than −1.7 z‐scores at 19 years of age (Table 4).
3.3. Factors associated with deterioration in lung function
Those whose lung function scores decreased from 11 to 14 to 16–19 years of age tended to be male (FEF75), born to mothers of white ethnicity (FEF75, FEV1, FVC, and DLCO), have lower mean gestational age (FEF50), lower mean birthweight (FEF50, FEV1, and FRCpleth), received postnatal corticosteroids (FEF75 and FEV1), and were oxygen dependent at 36 weeks postmenstrual age (FEV1; E‐Table S4). Mode of ventilation was not associated with deterioration in lung function with the exception of FEV1/FVC (0.10; 95% CI 0.02–0.18, p = 0.02) favoring those conventionally ventilated.
4. DISCUSSION
We demonstrated overall no significant difference in the change in lung function between 11 and 14 and 16–19 years according to neonatal mode of ventilation. Only the FEV1/FVC results showed significantly different trajectories for CV and HFO, with the HFO group's mean z‐scores remaining similar over time, whereas the CV group's mean z‐scores increased by 0.08 z‐scores per year. The CV group had similar mean FEV1/FVC results to the HFO group at 16–19 years, this may be an indication of “catch up growth.”
There was a decrease in small airway lung function, which is consistent with the deterioration previously reported in a subsample of UKOS young people measured at 1 year and 11–14 years. 22 Small airway function was measured by spirometry and impulse oscillometry. Forced expiratory flow at 75% of the FVC (FEF75) has been suggested to be a noneffort dependent, sensitive marker of small airway disease. 23 , 24 Airways resistance at 5 Hz has been shown to be nonvolitional marker of small airways. 25
FEF75 was pre‐specified as the primary outcome in the UKOS trial follow‐up at ages 11–14 and 16–19 years as described in our primary publications at those time points. 1 , 15 Hence we followed that plan for these analyses for consistency. FEF75 has been suggested to be a noneffort dependent, a sensitive marker of small airway disease 23 , 24 and the small airways are an area of concern in prematurity‐related lung disease. It is accepted that the new Quanjer reference values for FEV1 have to some extent, negated the need for additional measurements of small airways disease within a symptomatic population (those with cystic fibrosis and asthma) beyond FEV1, 26 however, it remains an accurate measure of small airways disease. We felt it was important to maintain consistency and report our primary outcome, but do report all spirometry results (including FEV1) using the most up‐to‐date reference ranges.
There have been three recent longitudinal studies reporting lung function in adults born prematurely. 3 , 27 , 28 All three showed a mismatch in lung function trajectories concerning airways and lung volumes, in concordance with our reported data. Furthermore, airways (FEV1) were affected to a larger degree. In one study, 27 however, the mean FEV1/FVC was shown to fall in contrast to the results of our study. In that study, only 40% of infants received postnatal surfactant compared to 90% in our study population. It should also be noted however that in our study, only those who were conventionally ventilated showed an improvement in mean FEV1/FVC.
Pubertal status was assessed at 11–14 years and 90% of participants had reached Tanner Stage 2 suggesting they had entered puberty. 29 , 30 It usually takes 4–5 years to complete puberty once they pass Tanner Stage 2. 31 We reassessed the young people on average when they were 5 years older, anticipating the majority of participants would have completed puberty. Puberty is a period of lung development characterized by rapid growth of the airways and lung volumes. 32 , 33 This growth occurs dysynaptically, that is at different times. 32 , 33 , 34 In our study population, mean FEV1 decreased by up to 0.35 z‐scores between the two‐time points, while mean FVC increased. This would suggest that the airway growth in our population was affected to a higher degree than the lung volume growth, which was conserved. Given that the pubertal growth phase of the lungs is the last positive effector of lung function, it is possible that prematurely born infants would be at risk of adult lung disease due to this disruption. This mismatch in the growth of the airways and lung volumes was seen in a study of 297 infants born before 29 weeks compared to 260 infants born at term. 4 In that study, whilst mean FEV1 and mean FVC z‐scores decreased in those born preterm, the changes in mean FEV1 were more statistically significant. The effect was more pronounced in those who had developed BPD compared with those had not. The poorer lung function overall in that study may reflect that only 73% of the infants were exposed to antenatal steroids and 40% received surfactant. In contrast in the UK and Ireland EPICure study of individuals born before 26 weeks of gestation between 11 and 19 years, mean FEV1 values decreased over time but were similar in those with and without neonatal BPD and term‐born controls. 3 The disparity in lung development was, however, not shown in a smaller study of 35 preterm infants assessed at 10 s and 18 years, which highlighted that airway and lung volume z‐scores tracked through puberty. 35 In that study, however, in addition to lower rates of antenatal steroid exposure (44%) and postnatal surfactant (49%), the young people were born at a later gestational age that is up to 31 weeks of gestation.
We observed an association between a deterioration in FEF75 and male sex, white ethnicity, and postnatal corticosteroid use. Male sex and postnatal corticosteroid use are known to be associated with poor respiratory outcomes. 36 , 37 , 38 Caution is needed when interpreting the ethnicity results as, although this could represent survivor bias as other studies have highlighted, 39 , 40 the majority of UKOS participants were white.
Our study has strengths and some limitations. We believe it is the first to quantify the effects of HFO versus CV on lung growth trajectories of young people born extremely prematurely. There was incomplete follow‐up and so we compared baseline and neonatal characteristics in those included with those not and adjusted statistically for the few differences observed. We also compared the analyses using all participants assuming data were missing at random with those with complete data and found no differences in effect sizes and p values. These comparisons provided assurance of the robustness of our findings. While factors other than the “neonatal” mode of ventilation could exert influences on respiratory outcomes, we accounted for this by using multivariable analyses and by using z‐scores standardized for height, age, and sex. Since the completion of the study, further reference ranges have been suggested by the GLI for lung volumes and DLCO. For consistency, we have continued to use the reference equations used in other publications relating to the UKOS cohort. 1 , 15
In conclusion, there was little evidence that the mode of ventilation affected changes in lung function over time in these young people born very prematurely. Despite some improvements in the mean FEF, FEV1/FVC, and FRC over time, “normal” respiratory function was not achieved, which may result in an increased susceptibility to COPD.
AUTHOR CONTRIBUTIONS
Alessandra Bisquera: Formal analysis (equal); writing—original draft (lead); writing—review & editing (equal). Christopher Harris: Data curation (equal); formal analysis (equal); writing—review & editing (equal). Alan Lunt: Investigation (equal); supervision (equal); writing—review & editing (equal). Sanja Zivanovic: Investigation (equal); writing—review & editing (equal). Neil Marlow: Funding acquisition (equal); writing—review & editing (equal). Sandy Calvert: Funding acquisition (equal); writing—review & editing (equal). Anne Greenough: Conceptualization (equal); funding acquisition (equal); methodology (equal); project administration (equal); supervision (equal); writing—review & editing (equal). Janet L Peacock: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); supervision (equal); writing—review & editing (equal).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
E‐Figure 1: Individual trajectories for lung function measures by mode of ventilation, with less (smoothed) curves by ventilation group (solid line is CV, dotted line is HFOV).
ACKNOWLEDGEMENT
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy\'s and St Thomas\' NHS Foundation Trust and King\'s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We thank Mrs. Deirdre Gibbons for secretarial assistance. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Bisquera A, Harris C, Lunt A, et al. Longitudinal changes in lung function in very prematurely born young people receiving high‐frequency oscillation or conventional ventilation from birth. Pediatric Pulmonology. 2022;57:1489‐1496. 10.1002/ppul.25918
Professors Peacock and Greenough are joint senior authors.
DATA AVAILABILITY STATEMENT
Data will be made available on request.
REFERENCES
- 1. Harris C, Bisqura A, Lunt A, Peacock JL, Greenough A. Outcomes of the neonatal trial of high‐frequency oscillation at 16 to 19 years. N Engl J Med. 2020;383:689‐691. [DOI] [PubMed] [Google Scholar]
- 2. Caskey S, Gough A, Rowan S, et al. Structural and functional lung impairment in adult survivors of bronchopulmonary dysplasia. Ann Am Thorac Soc. 2016;13:1262‐1270. [DOI] [PubMed] [Google Scholar]
- 3. Hurst JR, Beckmann J, Ni Y, et al. Respiratory and cardiovascular outcomes in survivors of extremely preterm birth at 19 years. Am J Respir Crit Care Med. 2020;202:422‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doyle LW, Irving L, Haikerwal A, Lee K, Ranganathan S, Cheong J. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax. 2019;74:1147‐1153. [DOI] [PubMed] [Google Scholar]
- 5. Carvalho CG, Silveira RC, Procianoy RS. Ventilator‐induced lung injury in preterm infants. Rev Bras Ter Intensiva. 2013;25:319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bentsen MH, Markestad T, Oymar K, Halvorsen T. Lung function at term in extremely preterm‐born infants: a regional prospective cohort study. BMJ Open. 2017;7:e016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenough A, Limb E, Marston L, Marlow N, Calvert S, Peacock J. Risk factors for respiratory morbidity in infancy after very premature birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F320‐F323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cousins M, Hart K, Gallacher D, Palomino MA, Kotecha S. Long‐term respiratory outcomes following preterm birth. Revista Médica Clínica Las Condes. 2018;29:87‐97. [Google Scholar]
- 9. HiFi Study Group . High‐frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants: assessment of pulmonary function at 9 months of corrected age. J Pediatr. 1990;116:933‐941. [DOI] [PubMed] [Google Scholar]
- 10. Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;3:CD000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moriette G, Paris‐Llado J, Walti H, et al. Prospective randomized multicenter comparison of high‐frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics. 2001;107:363‐372. [DOI] [PubMed] [Google Scholar]
- 12. Hofhuis W, Huysman M, van der Wiel EC, et al. Worsening of V'maxFRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high‐frequency oscillation ventilation. Am J Respir Crit Care Med. 2002;166:1539‐1443. [DOI] [PubMed] [Google Scholar]
- 13. Courtney SE, Durand DJ, Asselin JM, Eichenwald EC, Stark AR. Pro/con clinical debate: High‐frequency oscillatory ventilation is better than conventional ventilation for premature infants. Crit Care. 2003;7:423‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson AH, Peacock JL, Greenough A, et al. United Kingdom Oscillation Study Group High‐frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;347:633‐642. [DOI] [PubMed] [Google Scholar]
- 15. Zivanovic S, Peacock J, Alcazar‐Paris M, et al. Late outcomes of a randomized trial of high‐frequency oscillation in neonates. N Engl J Med. 2014;370:1121‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kadzmarski M. Transient reference values for impulse oscillometry for children aged 3‐18 years. Pediatr Pulmonol. 2008;43:1193‐1197. [DOI] [PubMed] [Google Scholar]
- 17. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenthal M, Bain SH, Cramer D, et al. Lung function in white children aged 4 to 19 years: I‐‐Spirometry. Thorax. 1993;48:794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II‐‐Single breath analysis and plethysmography. Thorax. 1993;48:803‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauzet O, Wright KC, Marston L, Brocklehurst P, Peacock JL. Modelling the hierarchical structure in datasets with very small clusters: a simulation study to explore the effect of the proportion of clusters when the outcome is continuous. Stat Med. 2013;32:1429‐1438. [DOI] [PubMed] [Google Scholar]
- 21. Greenough A, Peacock J, Zivanovic S, et al. United Kingdom Oscillation Study: long‐term outcomes of a randomised trial of two modes of neonatal ventilation. Health Technol Assess. 2014;18:1‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo J, Zivanovic S, Lunt A, et al. Longitudinal assessment of lung function in extremely prematurely born children. Pediatr Pulmonol. 2018;53:324‐331. [DOI] [PubMed] [Google Scholar]
- 23. Gelb AF, Zamel N. Simplified diagnosis of small‐airway obstruction. N Engl J Med. 1973;288:395‐398. [DOI] [PubMed] [Google Scholar]
- 24. Bakker EM, Borsboom GJ, van der Wiel‐Kooij EC, Caudri D, Rosenfeld M, Tiddens HA. Small airway involvement in cystic fibrosis lung disease: routine spirometry as an early and sensitive marker. Pediatr Pulmonol. 2013;48:1081‐1088. [DOI] [PubMed] [Google Scholar]
- 25. Postma DS, Brightling C, Baldi S, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7:402‐416. [DOI] [PubMed] [Google Scholar]
- 26. Lukic KZ, Coates AL. Does the FEF25‐75 or the FEF75 have any value in assessing lung disease in children with cystic fibrosis or asthma? Pediatr Pulmonol. 2015;50:863‐868. [DOI] [PubMed] [Google Scholar]
- 27. Doyle LW, Adams AM, Robertson C, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/low‐birthweight survivors born in the surfactant era. Thorax. 2017;72:712‐719. [DOI] [PubMed] [Google Scholar]
- 28. Simpson SJ, Turkovic L, Wilson AC, et al. Lung function trajectories throughout childhood in survivors of very preterm birth: longitudinal cohort study. Lancet Child Adolesc Health. 2018;2:350‐359. [DOI] [PubMed] [Google Scholar]
- 29. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child. 1966;41:454‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966;41:613‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys' and girls' timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 2011;47:1389‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quanjer PH, Stanojevic S, Stocks J, et al. Changes in the FEV₁/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J. 2010;36:1391‐1399. [DOI] [PubMed] [Google Scholar]
- 33. Smith J, Emerson SR, Kurti SP, Gandhi K, Harms CA. Lung volume and expiratory flow rates from pre‐ to post‐puberty. Eur J Appl Physiol. 2015;115:1645‐1652. [DOI] [PubMed] [Google Scholar]
- 34. Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma‐chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med. 2017;196:39‐46. [DOI] [PubMed] [Google Scholar]
- 35. Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid‐childhood to adulthood. Thorax. 2013;68:767‐776. [DOI] [PubMed] [Google Scholar]
- 36. Harris C, Crichton S, Zivanovic S, et al. Effect of dexamethasone exposure on the neonatal unit on the school age lung function of children born very prematurely. PLoS One. 2018;13:e0200243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71:305‐310. [DOI] [PubMed] [Google Scholar]
- 38. Harris C, Zivanovic S, Lunt A, et al. Lung function and respiratory outcomes in teenage boys and girls born very prematurely. Pediatr Pulmonol. 2020;55:682‐689. [DOI] [PubMed] [Google Scholar]
- 39. Khan SQ, Berrington de Gonzalez A, Best AF, et al. Infant and youth mortality trends by race/ethnicity and cause of death in the United States. JAMA Pediatr. 2018;172:e183317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Opondo C, Jayaweera H, Hollowell J, Li Y, Kurinczuk JJ, Quigley MA. Variations in neonatal mortality, infant mortality, preterm birth and birth weight in England and Wales according to ethnicity and maternal country or region of birth: an analysis of linked national data from 2006 to 2012. J Epidemiol Community Health. 2020;74:336‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
E‐Figure 1: Individual trajectories for lung function measures by mode of ventilation, with less (smoothed) curves by ventilation group (solid line is CV, dotted line is HFOV).
Data Availability Statement
Data will be made available on request.


