Abstract
Apples are rich sources of selected micronutrients (e.g., iron, zinc, vitamins C and E) and polyphenols (e.g., procyanidins, phloridzin, 5′‐caffeoylquinic acid) that can help in mitigating micronutrient deficiencies (MNDs) and chronic diseases. This review provides an up‐to‐date overview of the significant bioactive compounds in apples together with their reported pharmacological actions against chronic diseases such as diabetes, cancer, and cardiovascular diseases. For consumers to fully gain these health benefits, it is important to ensure an all‐year‐round supply of highly nutritious and good‐quality apples. Therefore, after harvest, the physicochemical and nutritional quality attributes of apples are maintained by applying various postharvest treatments and hurdle techniques. The impact of these postharvest practices on the safety of apples during storage is also highlighted. This review emphasizes that advancements in postharvest management strategies that extend the storage life of apples should be optimized to better preserve the bioactive components crucial to daily dietary needs and this can help improve the overall health of consumers.
Keywords: health, hidden hunger, microbial safety, micronutrient deficiencies, polyphenol
1. INTRODUCTION
The consumption of apple (Malus domestica Borkh. Family: Rosaceae), which is one of the most grown fruits globally, has been encouraged since time immemorial due to the sheer abundance of nutrients and bioactive compounds present therein. The beneficial components in apples vary in the type and amount that can be found within the cultivars or varieties from different countries, regions, or continents; and differ in the composition in parts (fruit, flesh, peel, leaf, seed, or root) (Kalinowska et al., 2014). The availability of these nutrients and bioactives in apple makes it one of the most sought‐after fruits globally, with useful applications in the production of juices, beverages, wines, ciders, vinegar, and other food products of immense commercial value. The apple industry is estimated at be worth $10 billion globally (Candrawinata et al., 2013).
This huge global demand for the apple fruit has sparked interest in discovering newer ways of establishing constant supply with most of the research focus placed on extending shelf‐life, possibly, at the expense of its nutritive values. For example, over 65,700 articles on apples have been published in the past 50 years and indexed on the largest‐known, peer‐reviewed scientific database—Scopus®. Using the keywords; “postharvest” AND “treatment” AND “storage” AND “apples” during the literature search, the number of research outputs retrieved was 470 (Figure 1, viewed 14 May 2021). These articles explored and developed postharvest strategies for the storage of apples. Most of these articles (88.7%) were published in the last two decades, and out of the total, only 2.7%, 1.6%, and 1.2% within this subject area accounted for apple studies linked to medicine, veterinary, and nursing, respectively. Exceptional progress has been made in the development of postharvest tools at a commercial scale to maintain the freshness and safety of apples and other fresh produce (Mahajan et al., 2014). While the advances in this area of apple research are commendable and must be sustained, these are often applied in combination with appropriate storage management strategies to extend storage or shelf‐life with often a limited focus on bioactive components of apples.
FIGURE 1.
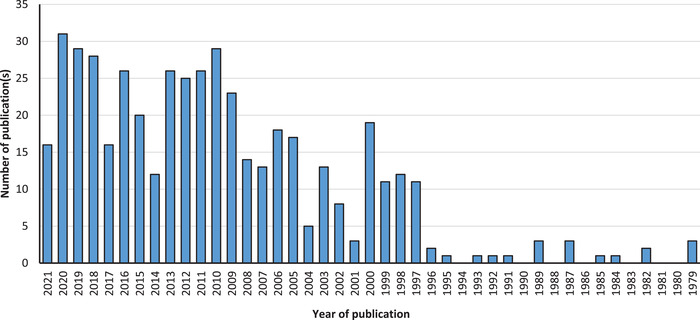
Summary of scopus search on the number of publications linked to “Postharvest treatment and storage of apples” based on the analysis of 470 documents. Source: Scopus, 2021
Micronutrient deficiencies (MNDs) “hidden hunger” is a major global challenge with an enormous impact on the health of the most vulnerable within the population. There is a heightened global consumer consciousness of the full benefits of dietary interventions of fruit and vegetables on human health and the impact of hidden hunger. Therefore, this article presents an overview of the bioactive compounds of apples and their pharmacological effects on chronic diseases. In addition, the article assessed the recent postharvest technological advances in the preservation of apples that are crucial in maintaining fruit quality during long‐term storage. Furthermore, this review presents future direction in apple research to ensure that the medicinal values can be fully harnessed and “an apple a day can truly keep the doctor away.”
2. BIOACTIVE COMPOUNDS IN APPLES
Essential or bioactive compounds in apples include macronutrients, vitamins, minerals, elements, flavanols, hydroxycinnamic acids, flavonols, dihydrochalcones, anthocyanins, and others. Numerous studies over the last three decades have shown that these compounds in apples exhibit significant biological effects on improving human health. Thus, the subsequent subsections discuss the role played by each of these compounds on consumer health.
2.1. Nutrients
The nutrients in apples include macronutrients (e.g., sugars, fibers, pectin, fat, protein), organic acids (e.g., malic acid), vitamins (e.g., C, E, B6), minerals (e.g., potassium, calcium, nitrogen, magnesium), and trace elements (e.g., zinc, iron, copper, manganese) (Skinner et al., 2018). These not only supply energy but also participate in many important processes in the body such as growth, bone health, immune functions, and so on. For example, malic, citric, and tartaric acids help the liver in the digestion processes in the body (Pal et al., 2020). The content and distribution of the sugars (mainly fructose and sucrose), together with the organic acids, are primarily responsible for the fruit's taste and appeal to consumers and possibly account for the major cultivar to cultivar or variety to variety differences (Ma et al., 2015). In terms of the dietary fiber content in apples, the fresh whole fruit was found to be composed of approximately between 2% and 3% total fiber. The insoluble fiber represents about 70% while the remainder is soluble, largely pectin (Li et al., 2002; Skinner et al., 2018). Diets rich in high fiber have been shown to confer immense health benefits such as gastrointestinal tract wellbeing and reduced mortality from cancers and cardiovascular diseases (Kim & Je, 2016; Myhrstad et al., 2020).
The vitamins and minerals in apples also provide essential micronutrients that aid the normal functions of biological and biochemical reactions in the body. For example, vitamins C and E in apples contribute to the total antioxidant potential widely attributed to the fruit by donating single hydrogen equivalents to free radicals rendering them stable and leading to their eventual detoxification. The vitamins also participate in the conversion of the oxidized forms of other antioxidants back to their active reduced states (Garcia‐Closas et al., 2004; Njus et al., 2020). Potassium and calcium minerals usually present in quantities of about 1.05–1.09 and 0.05–0.06 mg/g, respectively, in fresh apples are essential in promoting bone health and can reduce the risk of developing osteoporosis (Skinner et al., 2018; Yaegashi et al., 2008). Besides, the accumulation of calcium in apples also helps in the firming of the fruit and protection of its cell wall from fungal infections (Aghdam et al., 2012). The importance of the trace mineral, zinc, in normal body growth, development, immune system, and as a cofactor for many enzyme‐catalyzed metabolic reactions has been well reviewed in the literature (Roohani et al., 2013). With zinc deficiency still prevalent in children in low‐middle‐income countries (Gupta et al., 2020), the daily consumption of apples that contain about 3.6–4.4 µg zinc per 100 g fresh whole apple or 1400 µg zinc per 100 g apple pomace (Skinner et al., 2018) can go a long way in mitigating the deficiency. It was recently reported that the concentration of zinc in wild apples may be more than twice that obtained in cultivated apples indicating the impact of selection on zinc accumulation in the domestication process (Liao et al., 2017).
2.2. Polyphenols
Apples contain a variety of compounds with health‐promoting attributes but those belonging to the polyphenol class of phytochemicals are predominant and are particularly known for conferring antioxidant quality to the fruit. The polyphenol composition in the different parts of some common apple cultivars and antioxidant values are summarized in Table 1. Polyphenols, with thousands of member compounds, represent one of the most diverse groups of compounds. The basic polyphenols are structurally composed of phenols that are attached to carbon backbones (C1‐C6 to C3–C6 and C6‐C3–C6) or nitrogen. Some other polyphenol chemical structures are referred to as atypical since they exhibit complexity in their structures and thus are intricate to be assigned to a single class (Tsao, 2010).
TABLE 1.
Total polyphenol concentrations and antioxidant values of some common apple cultivars
| # Total polyphenol concentration (mg GAE/100 g) | *Antioxidant activity (mmol /100 g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Apple cultivars | Peel | Flesh/pulp | Seed | † Whole fruit | ORAC: TE | FRAP: Vit. C E | TEAC: TE | Referenc |
| ‘Granny Smith’ | 303.8 ± 5.9 | 31.2 ± 1.0 | NA | 120.0 ± 1.4 | 3.4 ± 0.7 | 0.9 ± 0.0 | 0.5 ± 0.0 | (Drogoudi & Pantelidis, 2011; Fu et al., 2011; Rautenbach & Venter, 2010; Ruiz‐Torralba et al., 2018) |
| ‘Starking’ | 6511.0 ± 360.0 | 720.0 ± 80.0 | 760.0 ± 87.0 | NA | 3.5 ± 0.7 | 0.7 ± 0.0 | 33.2 ± 1.1 b | (Almeida et al., 2017; Rautenbach & Venter, 2010; Valavanidis et al., 2009; Xu et al., 2016) |
| ‘Golden Delicious’ | 304.7 ± 3.7 | 128.3 ± 4.5 | 622.0 ± 0.5 | 197 ± 4.9 | 2.5 ± 0.9 | 0.6 ± 0.0 | 0.5 ± 0.0 | (Fu et al., 2011; Kschonsek et al., 2018; Rautenbach & Venter, 2010; Ruiz‐Torralba et al., 2018; Senica et al., 2019; Vieira et al., 2011) |
| ‘Jonagold’ | 416.9 ± 37.8 | 12.0 ± 1.0 | NA | 867.7 ± 105.5 | 11.2 ± 1.9 | 0.5 ± 0.0 | 12.4 ± 1.3 | (Groth et al., 2020; Kschonsek et al., 2018; Valavanidis et al., 2009; Wojdylo et al., 2008) |
| ‘Fuji’ | 499.2 ± 5.5 | 137.5 ± 3.6 | 820.0 ± 99.5 | 114.7 ± 0.0 | NA | NA | 35.7 ± 1.6 b | (Vieira et al., 2011; Wojdylo et al., 2008; Xu et al., 2016) |
| ‘Red delicious’ | 1187.0 ± 82.0 | 189.0 ± 0.3 | NA | 149.5 ± 9.1 | 3.9 ± 0.6 | 0.9 ± 0.0 | 0.5 ± 0.0 | (Fu et al., 2011; Henríquez et al., 2010; Rautenbach & Venter, 2010; Ruiz‐Torralba et al., 2018) |
| ‘Pink Lady’ | 580.0 ± 20.5 | 120.0 ± 0.5 | NA | 183.0 ± 0.7 | 22.2 ± 3.0 a | NA | NA | (Henríquez et al., 2010; Li et al., 2020) |
Note: Data are expressed as means (N = 3‐6) ± standard deviation or standard error;
Abbreviations: GAE – Gallic acid equivalents; TE – Trolox equivalents; Vit – Vitamin; NA – Not available.
Total polyphenol concentration was carried out on apple samples using the Folin–Ciocalteu method but different extraction protocols may have been employed.
*Antioxidant activity was conducted using the whole apple fruit extracts except when stated otherwise.
(flesh + peel).
Symbols a and b refer to when pomace and seed apple samples, respectively, were used for the analysis.
In apples, polyphenol subclasses—flavanols, hydroxycinnamic acids, flavonols, dihydrochalcones, and anthocyanins are notable (Kschonsek et al., 2018; Rana & Bhushan, 2016; Wojdylo et al., 2008). These chemical structures (Figure 2) have been related to the multifaceted bioactivities of apples in many studies. Flavanol monomers (e.g., catechin and epicatechin) and oligomers—proanthocyanidins (e.g., procyanidins A and B)—are flavonoid polyphenols with the C6–C3–C6 structural motif and have their C3 heterocyclic (O‐containing) ring hydroxylated (Strat et al., 2016). In whole apples, the total flavanol concentration ranged from 24 to 105 mg/100 g, consisting majorly of about ≈1.3 mg/100 g catechin, 7.5–8.7 mg/100 g epicatechin, 11–14.6 mg/100 g procyanidin dimer, and 84.8 mg/100 g proanthocyanidin polymers (Bondonno et al., 2017).
FIGURE 2.
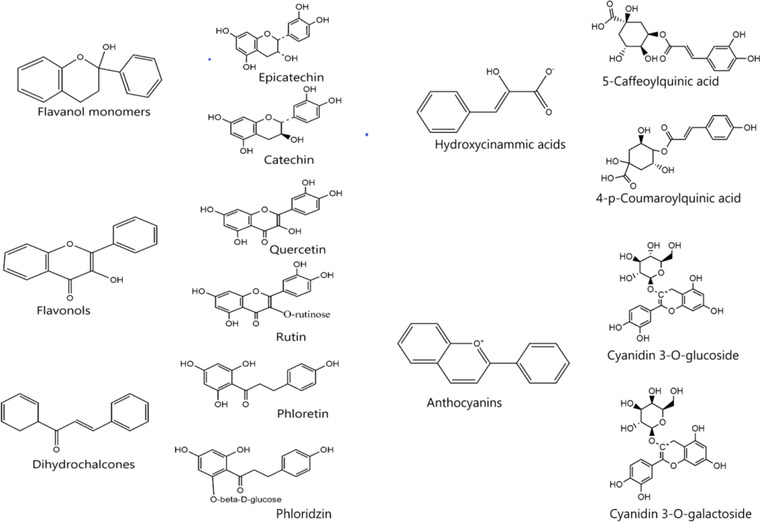
Chemical structures of polyphenols related to the multifaceted bioactivities of apples in many studies. Sources: Wojdylo et al. (2008), Rana and Bhushan (2016), and Kschonsek et al. (2018)
Furthermore, phenolic acids in apples are mainly represented in the form of hydroxycinnamic acids and the low‐occurring hydroxylbenzoic acids such as gentisic and syringic acid. Hydroxycinnamic acids are so‐named due to their derivation from cinnamic acid and are often esterified to quinic acids or glucose in foods (Kumar & Goel, 2019). Notable examples include 5‐caffeoylquinic acid, 4‐p‐coumaroylquinic acid, and 5‐p‐coumaroylquinic acid which are present in approximately 13.4, 2.3, and 1.1 mg/100 g ratio, respectively, in whole apples (Bondonno et al., 2017). Quercetin and its glycosides are the primary flavonols found in apples occurring in about 3.7–3.9 mg/100 g edible portion amounts and are typical of the 3‐hydroxyflavone structural backbone (D'Andrea, 2015). Quercetin glycoside, rutin, containing the disaccharide rutinose, is one of the most studied and pharmacologically active in apples (Latos‐Brozio & Masek, 2019).
Dihydrochalcones (notably, phloretin and phloridzin) structurally comprise the C6–C3–C6 backbone bicyclic flavonoids but without the heterocyclic C ring and the α–β double bond (Calliste et al., 2001). Phloretin and phloridzin are present in whole apple fruit at a concentration of 2.6 and 2.7 mg/100 g, respectively, with the latter often used as a marker to distinguish apples from other adulterants since it is mostly unique to the Rosaceae family (Bondonno et al., 2017). The presence of the dihydroxyacetophenone pharmacophore in phloretin also confers potent antioxidant qualities (Rana & Bhushan, 2016). Anthocyanins structures contain the anthocyanidin core bound to several glycosidic moieties at the C3, C5, or C7 positions. They are derivatives of 2‐phenylbenzopyrylium salts but with many attached hydroxyl and methoxy groups (Smeriglio et al., 2016) and may be responsible for the bright colors (red, purple) of apples. The peels of red‐fleshed apple varieties were reported to contain total anthocyanin content measured as cyanidin 3‐glucoside equivalents (29.5–175.8 mg/100 g fresh weight) that is more than the white‐fleshed varieties (1.4–30 mg/100 g fresh weight) and may also account for the higher antioxidant capacity values obtained in the study (Wang et al., 2015).
3. PHARMACOLOGICAL EFFECTS OF APPLE CONSTITUENTS AND POTENTIALS IN CHRONIC DISEASE PREVENTION
It is often said “an apple a day, keeps the doctor away” due to the numerous health benefits popularly associated with the ingestion of the fruit. However, these benefits have mostly been proven in in vitro, in vivo experiments as well as a few clinical trials. Many observational studies have also revealed positive correlations between apple intake and lower risk of developing many chronic diseases or dying from them (Bondonno et al., 2017).
Therefore, this section elucidates the major underlying biological effects that have been attributed to the compounds present in apples. The subsections primarily focus on the activities of these compounds in modulating oxidative stress, inflammation, and gastrointestinal tract (GIT) flora for health benefits. In addition, the subsection highlights the recent scientific evidence on the potential of apple supplementation against selected chronic diseases such as diabetes, obesity, cancer, and cardiovascular diseases.
3.1. Antioxidant properties of apples
Oxidative stress is implicated mostly in the development of many chronic diseases including diabetes, obesity, cancer, hypertension, and cardiovascular diseases (Egea et al., 2017). The occurrence of oxidative stress is a consequence of the inability of cells to regulate the production and utilization of cellular reactive oxygen (or nitrogen) species (ROS). The process of ROS generation—either endogenously in normal metabolism and in mitochondria or exogenously from exposure to xenobiotics and the environment—is usually tightly controlled by inherent cellular antioxidants that act by different mechanisms and at various stages to maintain the cellular redox homeostasis (Figure 3). This process involves enzymes, proteins, vitamins, carotenoids, polyphenols, and minerals as presented (Anwar et al., 2018; Nimse & Pal, 2015). Any compromise in the amount or actions of these antioxidants allows the uncontrolled overproduction of ROS, which leads to the onset or propagation of chronic diseases via many oxidative pathways. These activities have been reviewed comprehensively (Pisoschi et al., 2021; Zuo et al., 2019) and would not be the focus of this article. To summarize, three different steps of antioxidant actions in the regulation of ROS activities have been described in the literature. The first action involves preventing or suppressing the formation of excess ROS, for example, the superoxide dismutase (SOD) enzyme converts intracellular superoxide anions to hydrogen peroxide, which is in turn converted to water by catalase (CAT) or glutathione peroxidase (GPx) (Nimse & Pal, 2015). Second is the direct scavenging of excess ROS already produced thereby terminating oxidative chain propagations. Vitamins C and E and reduced glutathione are notable examples of antioxidants employing this mechanism (Oroian & Escriche, 2015). The third level of action involves the repairing mechanisms that ensure the removal of ROS‐mediated damaged cellular macromolecules by de novo antioxidants such as proteolytic and DNA‐repair enzymes (Oyenihi et al., 2014).
FIGURE 3.
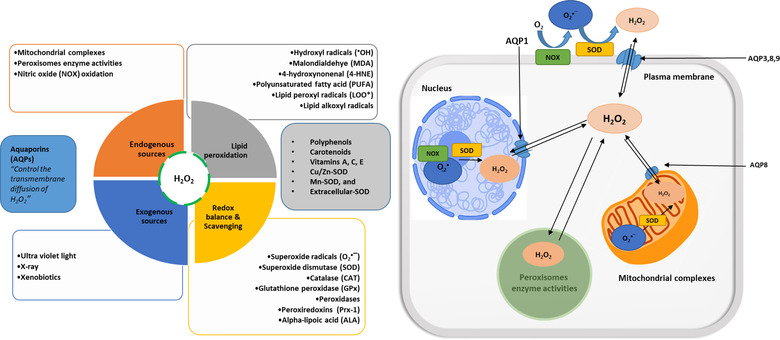
The annotation of sources, enzymes, and minerals (A), and cellular processes for the generation of reactive oxygen species (ROS) (B)
The supplementation with exogenous antioxidants in the attempt to complement the capacity of endogenous antioxidants and to compensate for any depletion in their amounts or functions is a widespread strategy to boost the overall cellular antioxidant defense system. Dietary intake through the consumption of fruits, vegetables, and herbs containing antioxidants (notably; polyphenols, carotenoids, and vitamins) remains invaluable sources for such exogenous supplementation. For example, the daily dietary intake of apples (2 ‘Fuji’ weighing ∼360 g) for 7 days was shown to increase SOD and GPx enzymes in the blood of hemodialysis patients (Giaretta et al., 2019). Apples contain high amounts of polyphenols and have been singled out as the major contributor to their antioxidant qualities due to positive correlations obtained between polyphenol concentrations and antioxidant values (Zhao et al., 2019). Polyphenols consist of diverse groups of antioxidants, which operate at different levels of antioxidant actions that are linked to their chemical structures. They possess aromatic rings, a highly conjugated system, and many free hydroxyl groups; features that are integral for the donation of electrons or hydrogen atoms to neutralize ROS and to chelate oxidative metals via different postulated mechanisms (Olszowy, 2019; Zhang & Tsao, 2016). Furthermore, polyphenol supplementation has been reported to also improve the activities of SOD, CAT, GPx enzymes through the induction of the nuclear factor erythroid‐related factor‐2 (Nrf2) upon signaling by ROS (Zhang & Tsao, 2016).
3.2. Modulation of the immune system/inflammation by apples
Inflammation and oxidative stress are inseparable partners since they exert their cellular effects through interconnected molecular pathways designed to propagate mutual signal cascades. However, excess activation of the two phenomena in concert can accelerate the development or exacerbation of chronic diseases as has been demonstrated in accelerated aging (Petersen & Smith, 2016), arthritis (Marchev et al., 2017), type 2 diabetes, obesity (Rains & Jain, 2011), cancer (Piotrowski et al., 2020), and cardiovascular diseases (Wenzel et al., 2017). Inflammatory cells, when signaled, mostly carry out their cellular functions through ROS‐mediated pathways. For example, macrophages have been shown to defend the cell from invaders via the generation of ROS (Ahmed et al., 2017). Chronic inflammation characterized by excess pro‐inflammatory cytokines, chemokines, and prostaglandins is facilitated through the continuous overproduction of oxidative stress as well as the inhibition of antioxidant defense systems; and is hindered upon the activation of the antioxidant transcription factor—Nrf2. In the same vein, oxidative stress increases inflammation by stimulating stress signals, notably extracellular signal‐regulated kinase (ERK), c‐Jun NH2‐terminal kinase (JNK), and p38 pathways, as well as activating NF‐κB (Ahmed et al., 2017). The two partners essentially feed off each other in a continuous cycle, hence the attenuation of oxidative stress may lead to the reduction in inflammatory processes and vice versa—a major target of many therapeutic interventions.
Apple polyphenols have been shown to exhibit these properties in vitro, in animal models, and in clinical trials of chronic inflammatory diseases (Farzaei et al., 2015). For instance, the amelioration of postprandial inflammation by the intake of ‘Gala’ apples was demonstrated recently to occur via the reduction of pro‐inflammatory cytokines; IFN‐γ, IL‐6, and TNF‐α (Liddle et al., 2021). Additionally, using the grass carp fish, Yang et al. (2021) reported the diminishing of the intestinal inflammatory response by the suppression of TLR4 signaling and transcription of IL‐1β, IL‐6, and TNF‐α genes following apple polyphenols supplementation.
3.3. Gastro‐intestinal tract (GIT) protection by apple consumption
Another major mechanism employed by apple constituents to improve human health is through their activities on the human gut microbiota. Apple proanthocyanidins and the pectin fiber have been particularly shown to interact with the gut microbiota, modulating its composition to achieve beneficial outcomes (Garcia‐Mazcorro et al., 2019; Koutsos et al., 2015). For example, the decrease in the Firmicutes/Bacteroidetes ratio accompanied by a rise in Akkermansia in the cecum was attributed to the intake of apple procyanidins and was shown to prevent obesity in mice (Masumoto et al., 2016). The anti‐inflammatory effects of apples related to the alleviation of metabolic syndrome are purportedly due to the modulation of the gut microbiota. In this regard, pectin was reported to stimulate the commensal bacteria, Faecalibacterium prausnitzii, in the human colon that possesses anti‐inflammatory actions (Chung et al., 2017; Garcia‐Mazcorro et al., 2019).
3.4. Apple consumption against type 2 diabetes and obesity
Type 2 diabetes (T2D), a chronic metabolic disease with a complex etiology and multifarious pathogenesis, is prevalent among adults with an estimated more than half a billion patients expected by 2045 (International Diabetes Federation, 2017). Although antidiabetic therapeutics are available with varying degrees of efficacy, diet and exercise have been proven to play major roles in the management of T2D (Magkos et al., 2020).
Apple supplementation in the diet of diabetic or obese rodents (Ogura et al., 2016; Tamura et al., 2020) and in vitro experiments (de Oliveira Raphaelli et al., 2019; D. Li et al., 2020) indicated an amelioration of the diabetic indices measured. These antidiabetic actions, mostly attributed to the polyphenol constituent, were reported to occur through the induction of beige adipocyte development in inguinal white adipose tissue. Other mechanisms include alleviation of insulin resistance and hyperglycemia, reduction of excess oxidative stress and inflammation, and inhibition of α‐glucosidase enzyme activity. In the analysis of three prospective longitudinal studies involving almost 200,000 total participants, higher consumption of whole apple, grape, or blueberry fruits was linked to a reduced risk of developing T2D (Muraki et al., 2013). With obesity, a major predisposing factor to the development of T2D, the ability of apples—a low glycemic index fruit, to decrease weight gain through its antioxidant and anti‐inflammatory activities, as well as actions on important signal transduction pathways has been highlighted (Asgary et al., 2018).
3.5. Apple supplementation to manage cancer
Cancer continues to present a great cause of health and economic burden to humans, occupying the second position in the global cause of mortality ranking and projected to overtake ischemic heart disease for the first position by 2060 (Mattiuzzi & Lippi, 2019). Polyphenol‐rich fruits or plant extracts have been suggested to help in the chemopreventive medicines niche due to their multifaceted medicinal modes of action targeted at preventing carcinogenesis (Oyenihi & Smith, 2019). The regular consumption of apples has shown beneficial effects on many types of cancer.
Apple polyphenols, flavonoids (Chang et al., 2018), phloretin (Lin et al., 2016), and polysaccharides (Y. H. Li et al., 2020) were reported to alleviate colorectal cancer endpoints. In the azoxymethane (rat carcinogenesis) model, apple anthocyanin—cyanidin‐3‐O‐galactoside—was recently demonstrated to reduce the appearance of the precancer indices assessed in the study (Bars‐Cortina et al., 2020). The purported modes of action against cancer include cell cycle arrest; inhibition of cell migration and invasion; apoptosis, anti‐angiogenesis; anti‐inflammatory; and antioxidant (Tu et al., 2017). Phloretin from apples may be an important anticancer phytochemical based on the results obtained in experiments on the breast (Wu et al., 2018), prostrate (Kim et al., 2020), cervical (Hsiao et al., 2019), lung, esophageal, gastric, and blood cancers (Choi, 2019).
3.6. Apple consumption for the prevention of cardiovascular diseases
Cardiovascular diseases (CVDs) including coronary heart disease, stroke, atherosclerosis, hypertension, cerebrovascular, and so on, remain the number one health challenge and cause of mortality globally and accounted for close to 18 million deaths in 2017 alone (Virani et al., 2020). This huge health burden, therefore, warrants urgent disease‐management approaches to lower the prevalence and mortality.
Dietary flavonoids were shown to exert an inverse relationship with CVD‐associated mortality in prospective cohort studies supporting the recommendation that the regular consumption of fruit and vegetables may help in reducing the risk of developing CVD (Kim & Je, 2017). The intake of apples decreased atherogenic cholesterol levels (Chai et al., 2012), enhanced endothelial function (Bondonno et al., 2018), and decreased the bone mass index (Gayer et al., 2019) in clinical trials. These collective research findings could be crucial in preventing CVD. It has been suggested that the modulation of the gut microbiota by apples should be considered a major link to its ability to reduce CVD risk markers (Koutsos et al., 2015).
4. ADVANCES IN HURDLE TECHNIQUES FOR APPLES
Various conventional hurdle techniques have been demonstrated over the years to maintain the quality of fresh fruit and retain the bioactive/nutritional compounds (Mahajan et al., 2014; Nyamende et al., 2021). Hurdle technologies ensure the freshness of the fruit, through microbial inactivation that occurs via a multiphase approach involving milder but synergistically/combinatorial effects of techniques (Ross et al., 2003). Techniques can be physical, chemical, or biological (Figure 4). The correct selection of a specific approach or combination of hurdle techniques can ensure microbial safety, stability, and quality (Pinela & Ferreira, 2017).
FIGURE 4.
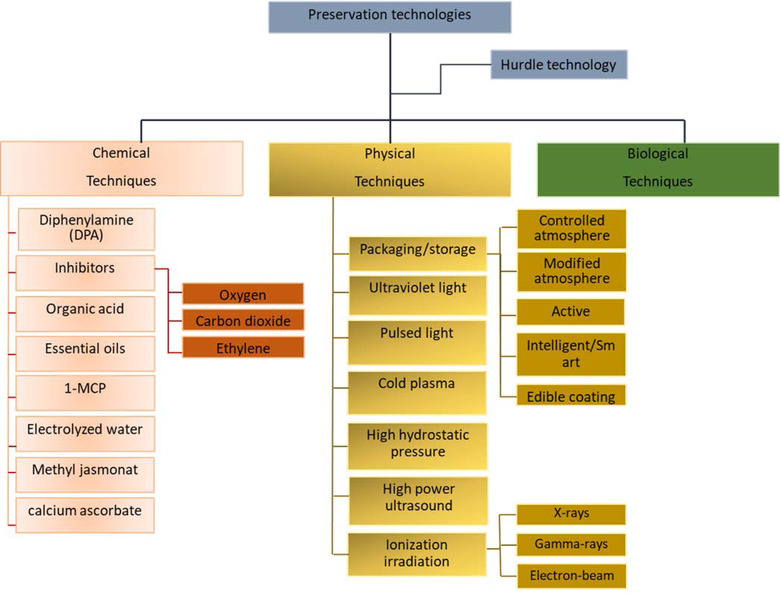
Technologies to extend the shelf‐life of fresh apple during postharvest
However, new hurdle techniques that often have an overarching focus on safety (microbial or chemical residue/toxins), due to public health and cross‐contamination concerns are emerging. Thus, this section discusses the advancement in hurdle technique for apples and highlights the unintended consequences on nutritional quality degradation during handling and storage.
4.1. Physical hurdles
Effective control and management of fresh apples along the supply chain using physical hurdle techniques, especially cold decontamination, is one of the most promising areas of approach (Pietrysiak et al., 2019). Cold storage temperature alone or in combination with other conventional techniques such as chemical and heat pretreatments have been used as a shelf‐life extension mechanism (Kabelitz et al., 2019; Ranjbar et al., 2018). Under low‐temperature storage, most of the biochemical processes that have detrimental effects on the fruit quality can be delayed, which enhances the storage life. However, not all fruits favor low‐temperature storage, and apple fruit is prone to chilling injury and browning during storage below the optimum temperature (Watkins & Liu, 2010).
Emerging knowledge in the area of packaging systems and nonthermal technologies for postharvest management of apple fruit has been reported to show beneficial effects in comparison to conventional approaches (Moreira et al., 2017). Controlled or modified atmosphere tools and active packaging systems (that can absorb or adsorb gases, moisture, and those that release bioactive compounds) and edible films/coating have been extensively reported as shelf‐life extension strategies for apples (da Rocha Neto et al., 2019; Khalifa et al., 2017; Mditshwa et al., 2017). These techniques have been used in combination with cold storage as a natural alternative to extend the shelf‐life of apples (Khalifa et al., 2017).
Furthermore, various nonthermal technologies such as pulsed electric field (PEF), irradiation, ozone, pulsed light (PL), ultra‐sound (US), short‐wave ultraviolet light (UV‐C), and high hydrostatic pressure (HHP) have been demonstrated to be effective decontaminants of spoilage and pathogenic microorganisms in apple fruit (Grimi et al., 2010; Manzocco et al., 2011; Moreira et al., 2015; Ribas‐Agustí et al., 2019; Salem & Moussa, 2014; Wu et al., 2012). However, in some cases, suboptimal or excessive use of these treatments alone can have detrimental effects on the quality of the apples (Table 2). Hence, it is recommended to combine these tools with dipping treatments using ascorbic acid, citric acid, and calcium chloride to minimize browning and for a better shelf‐life (Gómez et al., 2012; Salem & Moussa, 2014; Wu et al., 2012). Furthermore, the application of the edible coating in combination with the nonthermal techniques for extending the shelf‐life of fruits is emerging (Moreira et al., 2017; Moreira et al., 2015). It is also worth mentioning that the previously‐mentioned nonthermal technologies have limitation for an in‐depth treatment of opaque substances due to absorption and scattering of light and therefore, is only suitable to control surface microflora (Gómez et al., 2012; Manzocco et al., 2011).
TABLE 2.
Selected nonthermal postharvest treatments principles, advantages, and limitations for shelf‐life extension of apple fruit during postharvest handling and storage
| Technologies | Principle | Advantages | Requirement(s)/Limitation(s) | References |
|---|---|---|---|---|
| Pulsed electric fields (PEF) |
High voltage electricity (20 – 80 kV cm−1) Mechanism of microbial inactivation is due to induced electrical breakdown of cell membranes when the transmembrane potential reaches approximately 1 V (electroporation) |
Short‐time treatment Can be used as sterilization and enzyme inactivation for longer shelf‐life Used to enhance bioactive compounds No side effects on the quality |
Degree of effectiveness depends on applied electric field intensity Treatment time Temperature Specific energy input Types of cells (cytoplasm and membrane) Cell size and shape Cell orientation in the electric field |
(Ribas‐Agustí et al., 2019) |
| Irradiation |
Dose varies between 0.2 and 0.6 kGy to 1 kGy The irradiation destroys the DNA of the cell and thus cannot function |
Nonthermal, minimum or no effect on flavor and nutrient Minimum dose is effective to control postharvest disease in apple |
Complete elimination depends on the dose | (Salem & Moussa, 2014 |
| Ultrasound |
Mechanical wave at a frequency exceeding the threshold of about 20 kHz Disrupts cells by the cavitation phenomenon Causes shear‐induced breakdown of cell walls Disruption and thinning of cell membranes and DNA damage via free radical production |
Nonthermal Minimum or no effect on flavor and nutrient |
Effectiveness depends on Wave frequency Power and treatment time High level of ultrasonic waves are needed to effectively kill all microorganisms Can adversely modifythe nutritional and sensory properties |
(Ferrario & Guerrero, 2016) |
| Pulsed light |
Involves short time pulses (100–400 µs) for an intense broad spectrum between 100 and1100 nm The lethal action attributed to dimmer formation, impairs DNA replication and subsequent cell division (photochemical effects) |
Very short treatment time to achieve desired results Microbial inactivation (1 µs–0.1 s) Minimum or no effect on flavor and nutrient |
Product surface should be smooth, clear, and without pores or grooves to avoid a shadow effect to light penetration Presence of particulate material, treatment time, distance of sample from the light source, number of lamps, orientation and design of lamps |
(Ferrario and Guerrero, 2016; Gómez et al., 2012) |
| Electromagnetic energy |
For the inactivation of microbialenzymes UV‐C light from 200 to 280 nm |
Easy to use Characterized by favorable cots of equipment, energy, and maintenance No residue and nontoxic |
Poor penetration Suitable for surface treatments |
(Manzocco et al., 2011) |
| Hyperbaric pressure | Up to 400–600 MPa |
Affects cell structure Makes the cell more permeable Increases diffusion Enhances the uptake of biologically active substances |
High pressure results in tissue softening and microstructure change due to cell damage | (Fernández‐Jalao et al., 2019; George et al., 2016) |
| High hydrostatic pressure (HPP) |
Uses water as a medium of transmit pressure (300 and 600 MPa) The mechanism is due to irreversible destruction of cellular structure (cell membrane/cell walls), resulting in permeability modification and functionality disruption Inactivates certain enzymes |
Has minimum effect on taste, flavor, texture, appearance, and nutritional quality | Effectiveness depends on the pressure, holding time and temperature applied | (Wu et al., 2012) |
| Cold plasma |
The species existing in the thermodynamic equilibrium are in thermal nonequilibrium state (e.g., glow discharges; electron temperature ≈ 10,000–100,000 K, heavier species temperature ≈ 300–1000 K |
Decontamination properties, effective against major food‐borne microbes such as, Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes |
Effectiveness depends on the output discharge, fruit exposure time, and the gas used. The high oxidative action of gases could affect the biochemical nature of the fruit. |
(Ukuku et al., 2019) |
| Electrolyzed water (EW) | Generated by the electrolysis of a diluted (0.5–1.0%) salt (NaCl) solution with the product of anode (+) and cathode (‐) solution. |
Effective in reducing or eliminating pathogenic microbes and their surrogates such as E. coli, L. innocua, and S. choleraesuis on apple slices. Total microorganism population size does not affect the effectiveness of EW Low adverse impact on the environment and possibly on human health |
Only surface sanitization/decontamination effect on whole apple fruit. | (Graça et al., 2017) |
The application of electromagnetic waves as decontamination is beneficial especially for internal defects that need a treatment, which can penetrate the depth of the fruit. In this aspect, the use of microwave (MW) could have potential by creating volumetric heating (Gamage et al., 2015). The potential of MW (100–120 Kj/kg) treatment as an alternative to chemical treatment during storage to control Bactrocera tryoni, Froggatt and Bactrocera jarvisi was demonstrated for ‘Granny Smith’ apples (Gamage et al., 2015). The authors further suggested that the application of mild heating along with other treatments (hot air) have more benefit than MW alone. Furthermore, during MW treatment of a whole fruit, uneven heating is unavoidable due to the fruit structure and thermal properties.
For a complete nonthermal technique, pulses UV (PUV) and UV‐C have been shown having great advantage with a short treatment duration (>10 s) (Ferrario & Guerrero, 2016). For maximum effectiveness of UV‐C treatment, factors such as the presence of dispersing reflector as well as ensuring the uniform exposure of fruit must be considered (Manzocco et al., 2011). Ultrasound is another emerging nonthermal postharvest treatment used to extend the shelf‐life of fresh apples (Ferrario & Guerrero, 2016). Ultrasound treatment is categorized as a nontoxic, safer, and environmentally friendly approach, but, its efficiency against microbial inactivation needs integration with other treatments such as chemical, heat, and pressure (Ferrario & Guerrero, 2016).
In addition to electromagnetic and sound energy in the postharvest shelf‐life extension on apples, the use of hyperbaric pressure also emerged as a potential alternative to chemical and thermal treatments (George et al., 2016). High‐pressure treatment is effective to eliminate pests on the fruit surface during postharvest processing of apples (Fernández‐Jalao et al., 2019). Table 2 presents the selected nonthermal postharvest treatments reported for shelf‐life extension of apple fruit during postharvest.
4.2. Natural compounds
Decontamination of apple fruit surface after harvest has been heavily dependent on fungicides or chlorinated water treatment. This practice has an impact on fruit quality and human health, and has resulted in the development of resistant strains (Li & Xiao, 2008). Essential oils (EOs) are one of the most promising plant metabolites, that are biologically safe, biodegradable in nature, and with a low risk to cause pathogen resistance (da Rocha Neto et al., 2019; Hu et al., 2017). Treatment of apples with EOs to prevent the growth of Penicillium expansum and Botrytis cinerea has been reported as an effective approach (Frankova et al., 2016).
Banani et al. (2018) reported significantly lower gray mold incidence and severity due to B. cinerea for “Red Fuji” apples treated with thyme and savory Eos, respectively. However, the efficacy of EOs depends on the natural resistance of the apple cultivar, length of storage, and method of application (dipping/spraying) (Lopez‐Reyes et al., 2013). The drawbacks of some EOs are their strong aroma and the volatility of active compounds, which might affect the organoleptic quality and storage life of the product (Ribeiro‐Santos et al., 2017). In this case, the combination of EOs with other postharvest treatments such as hot air (Frankova et al., 2016), as well as hurdle approach to maintaining a controlled‐release incorporated EOs with packaging (films/coating) is advantageous (Guerreiro et al., 2016). Decontamination using chemical or surface treatments for apple fruit can ensure the low or inhibited growth of microbes on the surface of the fruit; however, combining them with electromagnetic or sound and pressure treatments can ensure the inhibition of microbes on the pericarp and mesocarp of the fruit (Salem & Moussa, 2014).
4.3. Biological controls
Biological control is a promising alternative to chemical and heat treatments for a more naturally preserved apple fruit without detrimental effects on the quality (Dukare et al., 2019; Quaglia et al., 2011). Currently, available biological control agents for apple fruit postharvest decay management include various bacteria, yeast, and filamentous fungi (Castoria et al., 2005; Nadai et al., 2018). The application of microbial antagonists can be performed by pulverization or immersion in solution (Carmona‐Hernandez et al., 2019). Most of the microbial antagonists are naturally present on fruit and vegetable surfaces or they can be obtained from sources such as roots and soils (Droby et al., 2016; Dukare et al., 2019). The main mechanisms of antagonists against phytopathogens are by suppressing or interfering with the normal growth due to competition for space and nutrients, biofilms, parasitism, and by producing inhibitory metabolites (Dukare et al., 2019; Wallace et al., 2017).
Common types of pathogenic molds that affect the shelf‐life of apples include blue and grey mold and ring and brown rot caused by Penicillium expansum, Botrytis cinerea, Botryosphaeria dothidea, and Monilina functigena, respectively (Li & Xiao, 2008; Quaglia et al., 2011). However, P. expansum is the most aggressive (Mostafavi et al., 2012) and widely studied compared to the others inhibited by biocontrol agents so far (Czarnecka et al., 2019; Mari et al., 2012; Nadai et al., 2018; Quaglia et al., 2011; Vero et al., 2013; Wallace et al., 2017). In addition, P. expansum produces mycotoxins, such as patulin, that causes serious health problem for consumers (Nadai et al., 2018). The positive synergetic effect of various microbial antagonists such as Bacillus amyloliquefaciens PG12 (Chen et al., 2016), Paenibacillus polymyxa APEC136 and B. subtilis APEC170 (Kim et al., 2016) and B. subtilis 9407 (Fan et al., 2017) have been reported for apple fruit. More recently, the beneficial effects of other antagonistics including Starmerella bacillaris (Nadai et al., 2018), Debaryomyces hansenii, and Wickerhamomyces anomalus (Czarnecka et al., 2019), and various yeast isolates (Madbouly et al., 2020) have shown a beneficial effect as a biocontrol of postharvest pathogens of apple fruit. Table 3 presents the list of antagonists as a biocontrol against various molds that affect the shelf‐life of apples during postharvest.
TABLE 3.
Studies on different antagonistic and target microbes that affect the shelf‐life of apple during postharvest
| Cultivar(s) | Target microbes | Antagonists microbe(s) | Concentration(s) †, *, ** | Reference(s) |
|---|---|---|---|---|
| ‘Golden Delicious’ | Penicillium expansum | Trichoderma isolates | 1 × 108 † | (Quaglia et al., 2011) |
| ‘Gala’ and ‘Golden Delicious’ | P. expansum, B. cinerea, C. acutatum | Pullulans (L1 and L8) | 1 × 106 to 1 × 108 † | (Mari et al., 2012) |
| ‘Red Delicious’ and ‘Pink Lady’ | P. expansum, B. cinerea, C. acutatum | Cystofilobasidium infirmominiatum | 1 × 107 † | (Vero et al., 2013) |
| ‘Borkh’ | P. expansum | Pichia caribbica | 1 × 108 † | (Li et al., 2014) |
| ‘Fuji’ | Botryosphaeria dothidea | Bacillus amyloliquefaciens PG12 | 1 × 108 † | (Chen et al., 2016) |
| – | Fungal mycelia (C. gloesporioides, C. acutaum, B. dothidea) | Paenibacillus polyxa APEC136 and Bacillus subtilis Apec170 | 1 × 108 † | (Kim et al., 2016) |
| ‘Fuji’ | Bacillus subtilis 9407 | Botryosphaeria dothidea | 1 × 107 † | (Fan et al., 2017) |
| ‘McIntosh’ and ‘Spartan’ | P. expansum | Pseudomonas Fluorescens isolates 1–112, 2–28, 4–6 | 1 × 108 † | (Wallace et al., 2017) |
| ‘Golden Delicious’ | P. expansum | Saccharomyces/Bacillaris strains | 1 × 107 † | (Nadai et al., 2018) |
| ‘Ligol’ | M. functigena | Debaryomyces hansenii and Wickerhamomyces anomalus | 1 × 108 * | (Czarnecka et al., 2019) |
| ‘Golden Delicious’ | M. functigena | Schwanniomyces vanrijiae, Galactomyces geotrichum, Pichia kudriavzevii|) | 2 × 108 ** | (Madbouly et al., 2020) |
NB. † CFU/ml. *cells/ml. **spores/ml.
The successful application of biocontrol agents mostly involves a complex interaction between the host, pathogen, antagonists, and environment (Li et al., 2013; Nunes, 2012). According to the previously mentioned studies, the inhibition/biocontrol efficacy of the antagonistic yeasts, pathogenic fungus, or bacteria could be depending on the pH, relative humidity, prolonged cultivation of microorganisms, or successful colonization (biofilm formation and swarming motility). Furthermore, biocontrol efficacy of yeast can be influenced by oxidative stress and incorporating an antioxidant such as ascorbic acid can induce stress tolerance and biocontrol efficacy of antagonistic yeast (Li et al., 2014). In addition, the effectiveness of antagonistic yeast (P. guilliemondii) against P. expansum, B. cinerea, and C. gloesporioides was improved by combining it with hot water (30°C) treatment for ‘Red Fuji’ apple (Zhao & Yin, 2018).
The development of biocontrol, however, is a tedious process requiring a detailed knowledge of several disease factors such as pathogen, host, disease epidemiology, constitutive or induced host resistance, and the environmental condition (Droby et al., 2016; Nunes, 2012). Though the application of EOs and biocontrols have been proven as effective methods to control the postharvest pathogens of apples, however, at higher concentrations, and depending on the application methods or frequency of treatments, phytotoxic tendencies have been reported (Lopez‐Reyes et al., 2013). Therefore, cautious advancements are required to avoid the determinate effects of the hurdle approach on the quality of the fruit.
5. CONCLUSION
The value of apple fruit and the role of its bioactive constituents in the delivery of essential micro‐ and phyto‐nutrients to humans cannot be overemphasized. The multitargeted and multilevel pharmacological actions of its vitamins, minerals, and polyphenols especially against chronic diseases could indeed “keep the doctor away.” Thus, research strategies that prioritize the medicinal value of apples from the farm gate to consumer plate should be actively promoted. Therefore, as efforts to maintain fresh fruit safety and control biohazards heighten in the wake of the global COVID‐19 pandemic, it is important to balance these activities with access to nutritional fruit. Advancements in the application of hurdle techniques such as the use of cold plasma, electrolyzed water, nano‐bubbles, high pressure, ultrasounds, and superheated steam/vapor for apples should include nutritional quality considerations. Further research is needed on the impact of these emerging tools on bioactive compounds of fresh apples during storage and the entire supply chain.
AUTHOR CONTRIBUTIONS
Ayodeji B. Oyenihi: Conceptualization; Formal analysis; writing – original draft. Zinash A. Belay: Conceptualization; Formal analysis; writing – original draft. Asanda Mditshwa: Conceptualization; Formal analysis; writing – original draft. Oluwafemi J. Caleb: Conceptualization; Formal analysis; writing – original draft.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors are highly grateful to the National Research Foundation (NRF) and the Agricultural Research Council (ARC) of South Africa for research grant (Grant No.: 116272, 119192) and support, respectively. Ayodeji B Oyenihi is thankful to the Innovation Fellowship awarded by the Cape Peninsula University of Technology, South Africa. Zinash A Belay is grateful to the NRF Freestanding Post‐Doctoral Fellowship (Grant No.: 120729) for financial support.
Oyenihi, A. B. , Belay, Z. A. , Mditshwa, A. , & Caleb, O. J. (2022). “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. Journal of Food Science, 87, 2291–2309. 10.1111/1750-3841.16155
Contributor Information
Ayodeji B. Oyenihi, Email: oyenihia@cput.ac.za.
Oluwafemi J. Caleb, Email: caleboj@sun.ac.za.
REFERENCES
- Aghdam, M. S. , Hassanpouraghdam, M. B. , Paliyath, G. , & Farmani, B. (2012). The language of calcium in postharvest life of fruits, vegetables and flowers. Scientia Horticulturae, 144, 102–115. 10.1016/j.scienta.2012.07.007 [DOI] [Google Scholar]
- Ahmed, S. M. , Luo, L. , Namani, A. , Wang, X. J. , & Tang, X. (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease, 1863(2), 585–597. 10.1016/j.bbadis.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Almeida, D. P. F. , Gião, M. S. , Pintado, M. , & Gomes, M. H. (2017). Bioactive phytochemicals in apple cultivars from the Portuguese protected geographical indication “Maçã de Alcobaça”: Basis for market segmentation. International Journal of Food Properties, 20(10), 2206–2214. 10.1080/10942912.2016.1233431 [DOI] [Google Scholar]
- Anwar, H. , Hussain, G. , & Mustafa, I. (2018). Antioxidants from natural sources. In Antioxidants in foods and its applications, (pp. 1–27). 10.5772/intechopen.75961 [DOI] [Google Scholar]
- Asgary, S. , Rastqar, A. , & Keshvari, M. (2018). Weight loss associated with consumption of apples: A review. Journal of the American College of Nutrition, 37(7), 627–639. 10.1080/07315724.2018.1447411 [DOI] [PubMed] [Google Scholar]
- Banani, H. , Olivieri, L. , Santoro, K. , Garibaldi, A. , Gullino, M. L. , & Spadaro, D. (2018). Thyme and savory essential oil efficacy and induction of resistance against Botrytis cinerea through priming of defense responses in apple. Foods, 7(2), 11. 10.3390/foods7020011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bars‐Cortina, D. , Martinez‐Bardaji, A. , Macia, A. , Motilva, M. J. , & Pinol‐Felis, C. (2020). Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. Journal of Nutritional Biochemistry, 83, 108418. 10.1016/j.jnutbio.2020.108418 [DOI] [PubMed] [Google Scholar]
- Bondonno, N. P. , Bondonno, C. P. , Blekkenhorst, L. C. , Considine, M. J. , Maghzal, G. , Stocker, R. , & Croft, K. D. (2018). Flavonoid‐rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Molecular Nutrition & Food Research, 62(3), 1700674. 10.1002/mnfr.201700674 [DOI] [PubMed] [Google Scholar]
- Bondonno, N. P. , Bondonno, C. P. , Ward, N. C. , Hodgson, J. M. , & Croft, K. D. (2017). The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends in Food Science & Technology, 69, 243–256. 10.1016/j.tifs.2017.04.012 [DOI] [Google Scholar]
- Calliste, C. A. , Le Bail, J. C. , Trouillas, P. , Pouget, C. , Habrioux, G. , Chulia, A. J. , & Duroux, J. L. (2001). Chalcones: Structural requirements for antioxidant, estrogenic and antiproliferative activities. Anticancer Research, 21(6A), 3949–3956. https://www.ncbi.nlm.nih.gov/pubmed/11911276 [PubMed] [Google Scholar]
- Candrawinata, V. I. , Golding, J. B. , Roach, P. D. , & Stathopoulos, C. E. (2013). From apple to juice—The fate of polyphenolic compounds. Food Reviews International, 29(3), 276–293. 10.1080/87559129.2013.790049 [DOI] [Google Scholar]
- Carmona‐Hernandez, S. , Reyes‐Pérez, J. J. , Chiquito‐Contreras, R. G. , Rincon‐Enriquez, G. , Cerdan‐Cabrera, C. R. , & Hernandez‐Montiel, L. G. (2019). Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy, 9(3), 121. 10.3390/agronomy9030121 [DOI] [Google Scholar]
- Castoria, R. , Morena, V. , Caputo, L. , Panfili, G. , De Curtis, F. , & De Cicco, V. (2005). Effect of the biocontrol yeast Rhodotorula glutinis strain LS11 on patulin accumulation in stored apples. Phytopathology, 95(11), 1271–1278. 10.1094/PHYTO-95-1271 [DOI] [PubMed] [Google Scholar]
- Chai, S. C. , Hooshmand, S. , Saadat, R. L. , Payton, M. E. , Brummel‐Smith, K. , & Arjmandi, B. H. (2012). Daily apple versus dried plum: Impact on cardiovascular disease risk factors in postmenopausal women. The Journal of the Academy of Nutrition and Dietetics, 112(8), 1158–1168. 10.1016/j.jand.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Chang, H. , Lei, L. , Zhou, Y. , Ye, F. , & Zhao, G. (2018). Dietary flavonoids and the risk of colorectal cancer: An updated meta‐analysis of epidemiological studies. Nutrients, 10(7), 10.3390/nu10070950 [DOI] [PMC free article] [PubMed]
- Chen, X. , Zhang, Y. , Fu, X. , Li, Y. , & Wang, Q. (2016). Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biology and Technology, 115, 113–121. 10.1016/j.postharvbio.2015.12.021 [DOI] [Google Scholar]
- Choi, B. Y. (2019). Biochemical basis of anti‐cancer‐effects of phloretin—A natural dihydrochalcone. Molecules (Basel, Switzerland), 24(>2). 10.3390/molecules24020278 [DOI] [PMC free article] [PubMed]
- Chung, W. S. F. , Meijerink, M. , Zeuner, B. , Holck, J. , Louis, P. , Meyer, A. S. , Wells, J. M. , Flint, H. J. , & Duncan, S. H. (2017). Prebiotic potential of pectin and pectic oligosaccharides to promote anti‐inflammatory commensal bacteria in the human colon. Fems Microbiology Ecology, 93(11). 10.1093/femsec/fix127 [DOI] [PubMed]
- Czarnecka, M. , Żarowska, B. , Połomska, X. , Restuccia, C. , & Cirvilleri, G. (2019). Role of biocontrol yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in plants' defence mechanisms against Monilinia fructicola in apple fruits. Food microbiology, 83, 1–8. 10.1016/j.fm.2019.04.004 [DOI] [PubMed] [Google Scholar]
- D'Andrea, G. (2015). Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia, 106, 256–271. 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- da Rocha Neto, A. C. , Beaudry, R. , Maraschin, M. , Di Piero, R. M. , & Almenar, E. (2019). Double‐bottom antimicrobial packaging for apple shelf‐life extension. Food Chemistry, 279, 379–388. 10.1016/j.foodchem.2018.12.021 [DOI] [PubMed] [Google Scholar]
- de Oliveira Raphaelli, C. , Dos Santos Pereira, E. , Camargo, T. M. , Vinholes, J. , Rombaldi, C. V. , Vizzotto, M. , & Nora, L. (2019). Apple phenolic extracts strongly inhibit alpha‐glucosidase activity. Plant Foods for Human Nutrition, 74(3), 430–435. 10.1007/s11130-019-00757-3 [DOI] [PubMed] [Google Scholar]
- Droby, S. , Wisniewski, M. , Teixidó, N. , Spadaro, D. , & Jijakli, M. H. (2016). The science, development, and commercialization of postharvest biocontrol products. Postharvest Biology and Technology, 122, 22–29. 10.1016/j.postharvbio.2016.04.006 [DOI] [Google Scholar]
- Drogoudi, P. D. , & Pantelidis, G. (2011). Effects of position on canopy and harvest time on fruit physico‐chemical and antioxidant properties in different apple cultivars. Scientia Horticulturae, 129(4), 752–760. 10.1016/j.scienta.2011.05.036 [DOI] [Google Scholar]
- Dukare, A. S. , Paul, S. , Nambi, V. E. , Gupta, R. K. , Singh, R. , Sharma, K. , & Vishwakarma, R. K. (2019). Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Critical Reviews in Food Science and Nutrition, 59(9), 1498–1513. 10.1080/10408398.2017.1417235 [DOI] [PubMed] [Google Scholar]
- Egea, J. , Fabregat, I. , Frapart, Y. M. , Ghezzi, P. , Gorlach, A. , Kietzmann, T. , & Daiber, A. (2017). European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU‐ROS). Redox Biology, 13, 94–162. 10.1016/j.redox.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, H. , Ru, J. , Zhang, Y. , Wang, Q. , & Li, Y. (2017). Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiological Research, 199, 89–97. 10.1016/j.micres.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Farzaei, M. H. , Rahimi, R. , & Abdollahi, M. (2015). The role of dietary polyphenols in the management of inflammatory bowel disease. Current Pharmaceutical Biotechnology, 16(3), 196–210. 10.2174/1389201016666150118131704 [DOI] [PubMed] [Google Scholar]
- Fernández‐Jalao, I. , Sánchez‐Moreno, C. , & De Ancos, B. (2019). Effect of high‐pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innovative Food Science & Emerging Technologies, 51, 20–31. 10.1016/j.ifset.2018.06.002 [DOI] [Google Scholar]
- Ferrario, M. , & Guerrero, S. (2016). Effect of a continuous flow‐through pulsed light system combined with ultrasound on microbial survivability, color and sensory shelf life of apple juice. Innovative Food Science & Emerging Technologies, 34, 214–224. 10.1016/j.ifset.2016.02.002 [DOI] [Google Scholar]
- Frankova, A. , Smid, J. , Bernardos, A. , Finkousova, A. , Marsik, P. , Novotny, D. , & Kloucek, P. (2016). The antifungal activity of essential oils in combination with warm air flow against postharvest phytopathogenic fungi in apples. Food Control, 68, 62–68. 10.1016/j.foodcont.2016.03.024 [DOI] [Google Scholar]
- Fu, L. , Xu, B.‐T. , Xu, X.‐R. , Gan, R.‐Y. , Zhang, Y. , Xia, E.‐Q. , & Li, H.‐B. (2011). Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry, 129(2), 345–350. 10.1016/j.foodchem.2011.04.079 [DOI] [PubMed] [Google Scholar]
- Gamage, T. , Sanguansri, P. , Swiergon, P. , Eelkema, M. , Wyatt, P. , Leach, P. , Alexander, D. L. J. , & Knoerzer, K. (2015). Continuous combined microwave and hot air treatment of apples for fruit fly (Bactrocera tryoni and B. jarvisi) disinfestation. Innovative Food Science & Emerging Technologies, 29, 261–270. 10.1016/j.ifset.2015.02.009 [DOI] [Google Scholar]
- Garcia‐Closas, R. , Berenguer, A. , Tormo, M. J. , Quiros, S. M. , Navarro, J. R. C. , & Gonzalez, A. (2004). Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. British Journal of Nutrition, 91(6), 1005–1011. 10.1079/bjn20041130 [DOI] [PubMed] [Google Scholar]
- Garcia‐Mazcorro, J. F. , Pedreschi, R. , Yuan, J. , Kawas, J. R. , Chew, B. , Dowd, S. E. , & Noratto, G. (2019). Apple consumption is associated with a distinctive microbiota, proteomics and metabolomics profile in the gut of Dawley Sprague rats fed a high‐fat diet. Plos One, 14(3), e0212586. 10.1371/journal.pone.0212586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayer, B. A. , Avendano, E. E. , Edelson, E. , Nirmala, N. , Johnson, E. J. , & Raman, G. (2019). Effects of intake of apples, pears, or their products on cardiometabolic risk factors and clinical outcomes: A systematic review and meta‐analysis. Current Developments in Nutrition, 3(10), nzz109. 10.1093/cdn/nzz109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, J. M. , Selvan, T. S. , & Rastogi, N. K. (2016). High‐pressure‐assisted infusion of bioactive compounds in apple slices. Innovative Food Science & Emerging Technologies, 33, 100–107. 10.1016/j.ifset.2015.11.010 [DOI] [Google Scholar]
- Giaretta, A. G. , Schulz, M. , Silveira, T. T. , de Oliveira, M. V. , Patrício, M. J. , Gonzaga, L. V. , Fett, R. , da Silva, E. L. , & Wazlawik, E. (2019). Apple intake improves antioxidant parameters in hemodialysis patients without affecting serum potassium levels. Nutrition Research, 64, 56–63. 10.1016/j.nutres.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Gómez, P. L. , García‐Loredo, A. , Nieto, A. , Salvatori, D. M. , Guerrero, S. , & Alzamora, S. M. (2012). Effect of pulsed light combined with an antibrowning pretreatment on quality of fresh cut apple. Innovative Food Science & Emerging Technologies, 16, 102–112. 10.1016/j.ifset.2012.05.011 [DOI] [Google Scholar]
- Graça, A. , Santo, D. , Quintas, C. , & Nunes, C. (2017). Growth of Escherichia coli, Salmonella enterica and Listeria spp., and their inactivation using ultraviolet energy and electrolyzed water, on ‘Rocha'fresh‐cut pears. Food Control, 77, 41–49. 10.1016/j.foodcont.2017.01.017 [DOI] [Google Scholar]
- Grimi, N. , Mamouni, F. , Lebovka, N. , Vorobiev, E. , & Vaxelaire, J. (2010). Acoustic impulse response in apple tissues treated by pulsed electric field. Biosystems Engineering, 105(2), 266–272. 10.1016/j.biosystemseng.2009.11.005 [DOI] [Google Scholar]
- Groth, S. , Budke, C. , Neugart, S. , Ackermann, S. , Kappenstein, F. S. , Daum, D. , & Rohn, S. (2020). Influence of a selenium biofortification on antioxidant properties and phenolic compounds of apples (Malus domestica). Antioxidants (Basel), 9(2). 10.3390/antiox9020187 [DOI] [PMC free article] [PubMed]
- Guerreiro, A. C. , Gago, C. M. , Faleiro, M. L. , Miguel, M. G. , & Antunes, M. D. (2016). Edible coatings enriched with essential oils for extending the shelf‐life of ‘Bravo de Esmolfe’ fresh‐cut apples. International Journal of Food Science & Technology, 51(1), 87–95. 10.1111/ijfs.12949 [DOI] [Google Scholar]
- Gupta, S. , Brazier, A. K. M. , & Lowe, N. M. (2020). Zinc deficiency in low‐ and middle‐income countries: Prevalence and approaches for mitigation. Journal of Human Nutrition and Dietetics, 33(5), 624–643. 10.1111/jhn.12791 [DOI] [PubMed] [Google Scholar]
- Henríquez, C. , Almonacid, S. , Chiffelle, I. , Valenzuela, T. , Araya, M. , Cabezas, L. , & Speisky, H. (2010). Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chilean Journal of Agricultural Research, 70(4), 523–536. 10.4067/S0718-58392010000400001 [DOI] [Google Scholar]
- Hsiao, Y. H. , Hsieh, M. J. , Yang, S. F. , Chen, S. P. , Tsai, W. C. , & Chen, P. N. (2019). Phloretin suppresses metastasis by targeting protease and inhibits cancer stemness and angiogenesis in human cervical cancer cells. Phytomedicine, 62, 152964. 10.1016/j.phymed.2019.152964 [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Zhang, J. , Kong, W. , Zhao, G. , & Yang, M. (2017). Mechanisms of antifungal and anti‐aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus . Food Chemistry, 220, 1–8. 10.1016/j.foodchem.2016.09.179 [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation . (2017). IDF diabetes atlas (8th ed.). International Diabetes Federation. [Google Scholar]
- Kabelitz, T. , Schmidt, B. , Herppich, W. B. , & Hassenberg, K. (2019). Effects of hot water dipping on apple heat transfer and post‐harvest fruit quality. LWT—Food Science and Technology, 108, 416–420. 10.1016/j.lwt.2019.03.067 [DOI] [Google Scholar]
- Kalinowska, M. , Bielawska, A. , Lewandowska‐Siwkiewicz, H. , Priebe, W. , & Lewandowski, W. (2014). Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiology and Biochemistry, 84, 169–188. 10.1016/j.plaphy.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Khalifa, I. , Barakat, H. , El‐Mansy, H. A. , & Soliman, S. A. (2017). Preserving apple (Malus domestica var. Anna) fruit bioactive substances using olive wastes extract‐chitosan film coating. Information Processing in Agriculture, 4(1), 90–99. 10.1016/j.inpa.2016.11.001 [DOI] [Google Scholar]
- Kim, U. , Kim, C. Y. , Lee, J. M. , Oh, H. , Ryu, B. , Kim, J. , & Park, J. H. (2020). Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathology & Oncology Research, 26(2), 977–984. 10.1007/s12253-019-00643-y [DOI] [PubMed] [Google Scholar]
- Kim, Y. , & Je, Y. (2016). Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta‐analysis of prospective cohort studies. Archives of Cardiovascular Diseases, 109(1), 39–54. 10.1016/j.acvd.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Kim, Y. , & Je, Y. (2017). Flavonoid intake and mortality from cardiovascular disease and all causes: A meta‐analysis of prospective cohort studies. Clinical Nutrition Espen, 20, 68–77. 10.1016/j.clnesp.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Kim, Y. S. , Balaraju, K. , & Jeon, Y. (2016). Effects of rhizobacteria Paenibacillus polymyxa APEC136 and Bacillus subtilis APEC170 on biocontrol of postharvest pathogens of apple fruits. Journal of Zhejiang University—SCIENCE B, 17(12), 931–940. 10.1631/jzus.B1600117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsos, A. , Tuohy, K. M. , & Lovegrove, J. A. (2015). Apples and cardiovascular health—Is the gut microbiota a core consideration? Nutrients, 7(6), 3959–3998. 10.3390/nu7063959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kschonsek, J. , Wolfram, T. , Stockl, A. , & Bohm, V. (2018). Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants (Basel), 7(1). 10.3390/antiox7010020 [DOI] [PMC free article] [PubMed]
- Kumar, N. , & Goel, N. (2019). Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst), 24, e00370. 10.1016/j.btre.2019.e00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latos‐Brozio, M. , & Masek, A. (2019). Structure–activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chemistry & Biodiversity, 16(12), e1900426. 10.1002/cbdv.201900426 [DOI] [PubMed] [Google Scholar]
- Li, B. W. , Andrews, K. W. , & Pehrsson, P. R. (2002). Individual sugars, soluble, and insoluble dietary fiber contents of 70 high consumption foods. Journal of Food Composition and Analysis, 15(6), 715–723. 10.1006/jfca.2002.1096 [DOI] [Google Scholar]
- Li, C. , Zhang, H. , Yang, Q. , Komla, M. G. , Zhang, X. , & Zhu, S. (2014). Ascorbic acid enhances oxidative stress tolerance and biological control efficacy of Pichia caribbica against postharvest blue mold decay of apples. Journal of Agricultural and Food Chemistry, 62(30), 7612–7621. 10.1021/jf501984n [DOI] [PubMed] [Google Scholar]
- Li, D. , Yang, Y. , Sun, L. , Fang, Z. , Chen, L. , Zhao, P. , Wang, Z. C. , & Guo, Y. (2020). Effect of young apple (Malus domestica Borkh. cv. Red Fuji) polyphenols on alleviating insulin resistance. Food Bioscience, 36, 100637. 10.1016/j.fbio.2020.100637 [DOI] [Google Scholar]
- Li, H. , & Xiao, C. (2008). Characterization of fludioxonil‐resistant and pyrimethanil‐resistant phenotypes of Penicillium expansum from apple. Phytopathology, 98(4), 427–435. 10.1094/PHYTO-98-4-0427 [DOI] [PubMed] [Google Scholar]
- Li, W. , Yang, R. , Ying, D. , Yu, J. , Sanguansri, L. , & Augustin, M. A. (2020). Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Industrial Crops and Products, 147, 112250. 10.1016/j.indcrop.2020.112250 [DOI] [Google Scholar]
- Li, Y. , Han, L.‐R. , Zhang, Y. , Fu, X. , Chen, X. , Zhang, L. , Mei, R. , & Wang, Q. (2013). Biological control of apple ring rot on fruit by Bacillus amyloliquefaciens 9001. The Plant Pathology Journal, 29(2), 168. 10.5423/PPJ.SI.08.2012.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. H. , Wang, S. , Sun, Y. , Xu, W. Q. , Zheng, H. N. , Wang, Y. , & Mei, Q. B. (2020). Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydrate Polymers, 230, 115726. 10.1016/j.carbpol.2019.115726 [DOI] [PubMed] [Google Scholar]
- Liao, L. , Fang, T. , Ma, B. , Deng, X. , Zhao, L. , & Han, Y. (2017). Assessment of calcium and zinc accumulation in cultivated and wild apples. Journal of the Science of Food and Agriculture, 97(12), 4258–4263. 10.1002/jsfa.8289 [DOI] [PubMed] [Google Scholar]
- Liddle, D. M. , Lois, X. , Ward, E. , Cox, L. C. , Wright, A. J. , & Robinson, L. E. (2021). Apple consumption reduces markers of postprandial inflammation following a high fat meal in overweight and obese adults: A randomized, crossover trial. Food & Function, 12(14), 6348–6362. 10.1039/d1fo00392e [DOI] [PubMed] [Google Scholar]
- Lin, S. T. , Tu, S. H. , Yang, P. S. , Hsu, S. P. , Lee, W. H. , Ho, C. T. , Wu, C. H. , Lai, Y. H. , Chen, M. Y. , & Chen, L. C. (2016). Apple polyphenol phloretin inhibits colorectal cancer cell growth via inhibition of the type 2 glucose transporter and activation of p53‐mediated signaling. Journal of Agricultural and Food Chemistry, 64(36), 6826–6837. 10.1021/acs.jafc.6b02861 [DOI] [PubMed] [Google Scholar]
- Lopez‐Reyes, J. G. , Spadaro, D. , Prelle, A. , Garibaldi, A. , & Gullino, M. L. (2013). Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. Journal of Food Protection, 76(4), 631–639. 10.4315/0362-028X.JFP-12-342 [DOI] [PubMed] [Google Scholar]
- Ma, B. , Chen, J. , Zheng, H. , Fang, T. , Ogutu, C. , Li, S. , Han, Y. , & Wu, B. (2015). Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chemistry, 172, 86–91. 10.1016/j.foodchem.2014.09.032 [DOI] [PubMed] [Google Scholar]
- Madbouly, A. K. , Elyousr, K. A. A. , & Ismail, I. M. (2020). Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biological Control, 144, 104239. 10.1016/j.biocontrol.2020.104239 [DOI] [Google Scholar]
- Magkos, F. , Hjorth, M. F. , & Astrup, A. (2020). Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nature reviews Endocrinology, 16(10), 545–555. 10.1038/s41574-020-0381-5 [DOI] [PubMed] [Google Scholar]
- Mahajan, P. V. , Caleb, O. J. , Singh, Z. , Watkins, C. B. , & Geyer, M. (2014). Postharvest treatments of fresh produce. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 372(2017), 20130309. 10.1098/rsta.2013.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzocco, L. , Da Pieve, S. , Bertolini, A. , Bartolomeoli, I. , Maifreni, M. , Vianello, A. , & Nicoli, M. C. (2011). Surface decontamination of fresh‐cut apple by UV‐C light exposure: Effects on structure, colour and sensory properties. Postharvest Biology and Technology, 61(2–3), 165–171. 10.1016/j.postharvbio.2011.03.003 [DOI] [Google Scholar]
- Marchev, A. S. , Dimitrova, P. A. , Burns, A. J. , Kostov, R. V. , Dinkova‐Kostova, A. T. , & Georgiev, M. I. (2017). Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Annals of the New York Academy of Sciences, 1401(1), 114–135. 10.1111/nyas.13407 [DOI] [PubMed] [Google Scholar]
- Mari, M. , Martini, C. , Spadoni, A. , Rouissi, W. , & Bertolini, P. (2012). Biocontrol of apple postharvest decay by Aureobasidium pullulans . Postharvest Biology and Technology, 73, 56–62. 10.1016/j.postharvbio.2012.05.014 [DOI] [Google Scholar]
- Masumoto, S. , Terao, A. , Yamamoto, Y. , Mukai, T. , Miura, T. , & Shoji, T. (2016). Non‐absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Science Reports, 6, 31208. 10.1038/srep31208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzi, C. , & Lippi, G. (2019). Current cancer epidemiology. Journal of Epidemiology and Global Health, 9(4), 217–222. 10.2991/jegh.k.191008.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mditshwa, A. , Fawole, O. A. , Vries, F. , van der Merwe, K. , Crouch, E. , & Opara, U. L. (2017). Minimum exposure period for dynamic controlled atmospheres to control superficial scald in ‘Granny Smith'apples for long distance supply chains. Postharvest Biology and Technology, 127, 27–34. 10.1016/j.postharvbio.2016.12.009 [DOI] [Google Scholar]
- Moreira, M. R. , Álvarez, M. V. , Martín‐Belloso, O. , & Soliva‐Fortuny, R. (2017). Effects of pulsed light treatments and pectin edible coatings on the quality of fresh‐cut apples: A hurdle technology approach. Journal of the Science of Food and Agriculture, 97(1), 261–268. 10.1002/jsfa.7723 [DOI] [PubMed] [Google Scholar]
- Moreira, M. R. , Tomadoni, B. , Martín‐Belloso, O. , & Soliva‐Fortuny, R. (2015). Preservation of fresh‐cut apple quality attributes by pulsed light in combination with gellan gum‐based prebiotic edible coatings. LWT—Food Science and Technology, 64(2), 1130–1137. 10.1016/j.lwt.2015.07.002 [DOI] [Google Scholar]
- Mostafavi, H. A. , Mirmajlessi, S. M. , Mirjalili, S. M. , Fathollahi, H. , & Askari, H. (2012). Gamma radiation effects on physico‐chemical parameters of apple fruit during commercial post‐harvest preservation. Radiation Physics and Chemistry, 81(6), 666–671. 10.1016/j.radphyschem.2012.02.015 [DOI] [Google Scholar]
- Muraki, I. , Imamura, F. , Manson, J. E. , Hu, F. B. , Willett, W. C. , van Dam, R. M. , & Sun, Q. (2013). Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. Bmj, 347, f5001. 10.1136/bmj.f5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrstad, M. C. W. , Tunsjo, H. , Charnock, C. , & Telle‐Hansen, V. H. (2020). Dietary fiber, gut microbiota, and metabolic regulation‐current status in human randomized trials. Nutrients, 12(3). 10.3390/nu12030859 [DOI] [PMC free article] [PubMed]
- Nadai, C. , Fernandes Lemos, W. J. , Favaron, F. , Giacomini, A. , & Corich, V. (2018). Biocontrol activity of Starmerella bacillaris yeast against blue mold disease on apple fruit and its effect on cider fermentation. Plos One, 13(9), e0204350. 10.1371/journal.pone.0204350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse, S. B. , & Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. RSC Advances, 5(35), 27986–28006. 10.1039/c4ra13315c [DOI] [Google Scholar]
- Njus, D. , Kelley, P. M. , Tu, Y. J. , & Schlegel, H. B. (2020). Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radical Biology and Medicine, 159, 37–43. 10.1016/j.freeradbiomed.2020.07.013 [DOI] [PubMed] [Google Scholar]
- Nunes, C. A. (2012). Biological control of postharvest diseases of fruit. European Journal of Plant Pathology, 133(1), 181–196. 10.1007/s10658-011-9919-7 [DOI] [Google Scholar]
- Nyamende, N. E. , Domtchouang, F. R. , Belay, Z. A. , Keyser, Z. , Oyenihi, A. , & Caleb, O. J. (2021). Alternative postharvest pre‐treatment strategies for quality and microbial safety of ‘Granny Smith’ apple. Heliyon, 7(5), e07104. 10.1016/j.heliyon.2021.e07104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, K. , Ogura, M. , Shoji, T. , Sato, Y. , Tahara, Y. , Yamano, G. , …, & Tatsuoka, H. (2016). Oral administration of apple procyanidins ameliorates insulin resistance via suppression of pro‐inflammatory cytokine expression in liver of diabetic ob/ob mice. Journal of Agricultural and Food Chemistry, 64(46), 8857–8865. 10.1021/acs.jafc.6b03424 [DOI] [PubMed] [Google Scholar]
- Olszowy, M. (2019). What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiology and Biochemistry, 144, 135–143. 10.1016/j.plaphy.2019.09.039 [DOI] [PubMed] [Google Scholar]
- Oroian, M. , & Escriche, I. (2015). Antioxidants: Characterization, natural sources, extraction and analysis. Food Research International, 74, 10–36. 10.1016/j.foodres.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Oyenihi, A. B. , Brooks, N. L. , Oguntibeju, O. O. , & Aboua, Y. (2014). Antioxidant‐rich natural products and diabetes mellitus. In Antioxidant‐rich natural products and human health intech, Chapter 14, 317–345. 10.5772/57192 [DOI]
- Oyenihi, A. B. , & Smith, C. (2019). Are polyphenol antioxidants at the root of medicinal plant anti‐cancer success? Journal of Ethnopharmacology, 229, 54–72. 10.1016/j.jep.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Pal, R. S. , Pal, Y. , Wal, A. , & Wal, P. (2020). Herbal detoxifiers: An eminent need of today. Current Nutrition & Food Science, 16(4), 424–432. 10.2174/1573401315666190311153200 [DOI] [Google Scholar]
- Petersen, K. S. , & Smith, C. (2016). Ageing‐associated oxidative stress and inflammation are alleviated by products from grapes. Oxidative Medicine and Cellular Longevity, 2016, 6236309. 10.1155/2016/6236309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrysiak, E. , Smith, S. , & Ganjyal, G. M. (2019). Food safety interventions to control Listeria monocytogenes in the fresh apple packing industry: A review. Comprehensive Reviews in Food Science and Food Safety, 18(6), 1705–1726. 10.1111/1541-4337.12496 [DOI] [PubMed] [Google Scholar]
- Pinela, J. , & Ferreira, I. C. (2017). Nonthermal physical technologies to decontaminate and extend the shelf‐life of fruits and vegetables: Trends aiming at quality and safety. Critical Reviews in Food Science and Nutrition, 57(10), 2095–2111. 10.1080/10408398.2015.1046547 [DOI] [PubMed] [Google Scholar]
- Piotrowski, I. , Kulcenty, K. , & Suchorska, W. (2020). Interplay between inflammation and cancer. Reports of Practical Oncology & Radiotherapy, 25(3), 422–427. 10.1016/j.rpor.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoschi, A. M. , Pop, A. , Iordache, F. , Stanca, L. , Predoi, G. , & Serban, A. I. (2021). Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. European Journal of Medicinal Chemistry, 209, 112891. 10.1016/j.ejmech.2020.112891 [DOI] [PubMed] [Google Scholar]
- Quaglia, M. , Ederli, L. , Pasqualini, S. , & Zazzerini, A. (2011). Biological control agents and chemical inducers of resistance for postharvest control of Penicillium expansum Link on apple fruit. Postharvest Biology and Technology, 59(3), 307–315. 10.1016/j.postharvbio.2010.09.007 [DOI] [Google Scholar]
- Rains, J. L. , & Jain, S. K. (2011). Oxidative stress, insulin signaling, and diabetes. Free Radical Biology & Medicine, 50(5), 567–575. 10.1016/j.freeradbiomed.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana, S. , & Bhushan, S. (2016). Apple phenolics as nutraceuticals: Assessment, analysis and application. Journal of Food Science & Technology, 53(0022–1155 (Print)), 1727–1738. 10.1007/s13197-015-2093-8 [DOI] [PMC free article] [PubMed]
- Ranjbar, S. , Rahemi, M. , & Ramezanian, A. (2018). Comparison of nano‐calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Scientia Horticulturae, 240, 57–64. 10.1016/j.scienta.2018.05.035 [DOI] [Google Scholar]
- Rautenbach, F. , & Venter, I. (2010). Hydrophilic and lipophilic antioxidant capacity of commonly consumed South African fruits, vegetables, grains, legumes, fats/oils and beverages. Journal of Food Composition and Analysis, 23(7), 753–761. 10.1016/j.jfca.2010.03.018 [DOI] [Google Scholar]
- Ribas‐Agustí, A. , Martín‐Belloso, O. , Soliva‐Fortuny, R. , & Elez‐Martínez, P. (2019). Enhancing hydroxycinnamic acids and flavan‐3‐ol contents by pulsed electric fields without affecting quality attributes of apple. Food Research International, 121, 433–440. 10.1016/j.foodres.2018.11.057 [DOI] [PubMed] [Google Scholar]
- Ribeiro‐Santos, R. , Andrade, M. , de Melo, N. R. , & Sanches‐Silva, A. (2017). Use of essential oils in active food packaging: Recent advances and future trends. Trends in Food Science & Technology, 61, 132–140. 10.1016/j.tifs.2016.11.021 [DOI] [Google Scholar]
- Roohani, N. , Hurrell, R. , Kelishadi, R. , & Schulin, R. (2013). Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences, 18(2), 144–157. https://www.ncbi.nlm.nih.gov/pubmed/23914218 [PMC free article] [PubMed] [Google Scholar]
- Ross, A. I. , Griffiths, M. W. , Mittal, G. S. , & Deeth, H. C. (2003). Combining nonthermal technologies to control foodborne microorganisms. International Journal of Food Microbiology, 89(2–3), 125–138. 10.1016/s0168-1605(03)00161-2 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Torralba, A. , Guerra‐Hernández, E. J. , & García‐Villanova, B. (2018). Antioxidant capacity, polyphenol content and contribution to dietary intake of 52 fruits sold in Spain. CyTA—Journal of Food, 16(1), 1131–1138. 10.1080/19476337.2018.1517828 [DOI] [Google Scholar]
- Salem, E. , & Moussa, Z. (2014). Improvement shelf‐life extension of apple by prestorage thermal treatment, CaCl2 and gamma irradiation. Arab Journal of Nuclear Science and Applications, 47(1), 181–188. [Google Scholar]
- Scopus (2021). https://www.scopus.com/term/analyzer.uri?sid=11a917a9e22aea7ad06947dc4eae505e&origin=resultslist&src=s&s=TITLE‐ABS‐KEY%28Postharvest+treatment+and+storage+of+apples%29+AND+PUBYEAR+%3e+1970&sort=plf‐f&sdt=b&sot=b&sl=77&count=470&analyzeResults=Analyze+results&txGid=bca57e05ddb653feed77baf07c59c9ef. Date Accessed: May 14 2021
- Senica, M. , Stampar, F. , Veberic, R. , & Mikulic‐Petkovsek, M. (2019). Cyanogenic glycosides and phenolics in apple seeds and their changes during long term storage. Scientia Horticulturae, 255, 30–36. 10.1016/j.scienta.2019.05.022 [DOI] [Google Scholar]
- Skinner, R. C. , Gigliotti, J. C. , Ku, K. M. , & Tou, J. C. (2018). A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutrition Reviews, 76(12), 893–909. 10.1093/nutrit/nuy033 [DOI] [PubMed] [Google Scholar]
- Smeriglio, A. , Barreca, D. , Bellocco, E. , & Trombetta, D. (2016). Chemistry, pharmacology and health benefits of anthocyanins. Phytotherapy Research, 30(8), 1265–1286. 10.1002/ptr.5642 [DOI] [PubMed] [Google Scholar]
- Strat, K. M. , Rowley, T. J. , Smithson, A. T. , Tessem, J. S. , Hulver, M. W. , Liu, D. , Davy, B. M. , Davy, K. P. , & Neilson, A. P. (2016). Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. The Journal of Nutritional Biochemistry, 35, 1–21. 10.1016/j.jnutbio.2015.12.008 [DOI] [PubMed] [Google Scholar]
- Tamura, Y. , Tomiya, S. , Takegaki, J. , Kouzaki, K. , Tsutaki, A. , & Nakazato, K. (2020). Apple polyphenols induce browning of white adipose tissue. Journal of Nutritional Biochemistry, 77, 108299. 10.1016/j.jnutbio.2019.108299 [DOI] [PubMed] [Google Scholar]
- Tsao, R. (2010). Chemistry and biochemistry of dietary polyphenols. Nutrients, 2(12), 1231–1246. 10.3390/nu2121231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, S. H. , Chen, L. C. , & Ho, Y. S. (2017). An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. Journal of Food and Drug Analysis., 25(1), 119–124. 10.1016/j.jfda.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukuku, D. O. , Niemira, B. A. , & Ukanalis, J. (2019). Nisin‐based antimircobial combination with cold plasma treatment inactivate Listeria monocytogenes on Granny Smith apples. LWT, 104, 120–127. 10.1016/j.lwt.2018.12.049 [DOI] [Google Scholar]
- Valavanidis, A. , Vlachogianni, T. , Psomas, A. , Zovoili, A. , & Siatis, V. (2009). Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. International Journal of Food Science & Technology, 44(6), 1167–1175. 10.1111/j.1365-2621.2009.01937.x [DOI] [Google Scholar]
- Vieira, F. G. K. , Borges, G. D. S. C. , Copetti, C. , Di Pietro, P. F. , Nunes, E. d. C. , & Fett, R. (2011). Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Scientia Horticulturae, 128(3), 261–266. 10.1016/j.scienta.2011.01.032 [DOI] [Google Scholar]
- Vero, S. , Garmendia, G. , González, M. B. , Bentancur, O. , & Wisniewski, M. (2013). Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Research, 13(2), 189–199. 10.1111/1567-1364.12021 [DOI] [PubMed] [Google Scholar]
- Virani, S. S. , Alonso, A. , Benjamin, E. J. , Bittencourt, M. S. , Callaway, C. W. , Carson, A. P. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , Delling, F. N. , Djousse, L. , Elkind, M. S. V. , Ferguson, J. F. , Fornage, M. , Khan, S. S. , Kissela, B. M. , Knutson, K. L. , Kwan, T. W. , Lackland, D. T. , …, & Stroke Statistics, S. (2020). Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation, 141(9), e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Wallace, R. L. , Hirkala, D. L. , & Nelson, L. M. (2017). Postharvest biological control of blue mold of apple by Pseudomonas fluorescens during commercial storage and potential modes of action. Postharvest Biology and Technology, 133, 1–11. 10.1016/j.postharvbio.2017.07.003 [DOI] [Google Scholar]
- Wang, X. , Li, C. , Liang, D. , Zou, Y. , Li, P. , & Ma, F. (2015). Phenolic compounds and antioxidant activity in red‐fleshed apples. Journal of Functional Foods, 18, 1086–1094. 10.1016/j.jff.2014.06.013 [DOI] [Google Scholar]
- Watkins, C. , & Liu, F. (2010). Temperature and carbon dioxide interactions on quality of controlled atmosphere‐stored ‘Empire'apples. Hortscience, 45(11), 1708–1712. 10.21273/HORTSCI.45.11.1708 [DOI] [Google Scholar]
- Wenzel, P. , Kossmann, S. , Münzel, T. , & Daiber, A. (2017). Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox‐derived reactive oxygen and nitrogen species. Free Radical Biology and Medicine, 109, 48–60. 10.1016/j.freeradbiomed.2017.01.027 [DOI] [PubMed] [Google Scholar]
- Wojdylo, A. , Oszmianski, J. , & Laskowski, P. (2008). Polyphenolic compounds and antioxidant activity of new and old apple varieties. Journal of Agricultural and Food Chemistry, 56(15), 6520–6530. 10.1021/jf800510j [DOI] [PubMed] [Google Scholar]
- Wu, K. H. , Ho, C. T. , Chen, Z. F. , Chen, L. C. , Whang‐Peng, J. , Lin, T. N. , & Ho, Y. S. (2018). The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J Food Drug Anal, 26(1), 221–231. 10.1016/j.jfda.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , Zhang, M. , & Wang, S. (2012). Effects of high pressure argon treatments on the quality of fresh‐cut apples at cold storage. Food Control, 23(1), 120–127. 10.1016/j.foodcont.2011.06.021 [DOI] [Google Scholar]
- Xu, Y. , Fan, M. , Ran, J. , Zhang, T. , Sun, H. , Dong, M. , Zhang, Z. , & Zheng, H. (2016). Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi Journal of Biological Sciences, 23(3), 379–388. 10.1016/j.sjbs.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaegashi, Y. , Onoda, T. , Tanno, K. , Kuribayashi, T. , Sakata, K. , & Orimo, H. (2008). Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. European Journal of Epidemiology, 23(3), 219–225. 10.1007/s10654-008-9225-7 [DOI] [PubMed] [Google Scholar]
- Yang, G. , Yu, R. , Geng, S. , Xiong, L. , Yan, Q. , Kumar, V. , Wen, C. , & Peng, M. (2021). Apple polyphenols modulates the antioxidant defense response and attenuates inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture, 534, 736284. 10.1016/j.aquaculture.2020.736284 [DOI] [Google Scholar]
- Zhang, H. , & Tsao, R. (2016). Dietary polyphenols, oxidative stress and antioxidant and anti‐inflammatory effects. Current Opinion in Food Science, 8, 33–42. 10.1016/j.cofs.2016.02.002 [DOI] [Google Scholar]
- Zhao, T. , Sun, L. , Wang, Z. , Nisar, T. , Gong, T. , Li, D. , Niu, P. , & Guo, Y. (2019). The antioxidant property and α‐amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT, 111, 252–259. 10.1016/j.lwt.2019.05.006 [DOI] [Google Scholar]
- Zhao, Y. , & Yin, J. (2018). Effects of Pichia guilliermondii and hot air treatment on the postharvest preservation of red Fuji apple quality attributes. Journal of Food Protection, 81(2), 186–194. 10.4315/0362-028X.JFP-17-244 [DOI] [PubMed] [Google Scholar]
- Zuo, L. , Prather, E. R. , Stetskiv, M. , Garrison, D. E. , Meade, J. R. , Peace, T. I. , & Zhou, T. (2019). Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. International Journal of Molecular Sciences, 20(18). 10.3390/ijms20184472 [DOI] [PMC free article] [PubMed]


