Abstract
Background
Data suggest that hyaluronic acid (HA) fillers using VYC technology have a higher incidence of delayed‐onset nodule development at facial injection sites than earlier HA products.
Objective
To assess the incidence of delayed‐onset nodules with VYC products based on a single experienced injector.
Methods and Materials
Patients with delayed‐onset nodules after injections with VYC‐20L, VYC‐17.5L, and VYC‐15L were identified by retrospective chart review.
Results
Since 2010, 2139 patients received injections from the same physician with combinations of VYC‐20L (57.6% of patients; 2.4 syringes/patient), VYC‐17.5L (23.9%; 1.5), or VYC‐15L (18.5%; 1.5). Seven female patients (mean age, 62 years) developed delayed‐onset nodules for an overall incidence of 0.33%. A potential inflammatory trigger (reported by 6 patients) occurred 1–168 days prior to nodule development. Nodule biopsy in 1 patient confirmed a foreign‐body granuloma. The most effective treatment incorporated prednisone with or without hyaluronidase, and in 2 patients, nodules resolved spontaneously. The incidence of delayed‐onset nodules was not associated with injection technique or amount of product used.
Conclusion
VYC‐associated incidence of delayed‐onset nodules (0.33%) was lower than earlier estimates from previous studies. In the current analysis, VYC‐15L had a rate of delayed reactions comparable with non‐VYC products.
Keywords: aesthetic, complications, filler, safety, vycross
1. INTRODUCTION
Hyaluronic acid (HA) fillers are widely used in facial rejuvenation 1 , 2 and have been shown to be safe in clinical practice. 3 , 4 Filler‐related complications can be characterized in terms of their timing after injection. Early‐onset complications (mainly injection‐related side effects) occur shortly after injection, are usually mild in intensity, and resolve rapidly. 3 Delayed‐onset complications can occur weeks to months after the injection but are uncommon. The incidence of delayed‐onset hypersensitivity reactions to earlier nonanimal stabilized HA (NASHA) fillers has been estimated at 0.02%–0.4%. 3 , 5 However, there are reports of higher incidences of delayed‐onset nodule development with HA filler products using Vycross (VYC) technology (Allergan Aesthetics, an AbbVie company) compared with NASHA fillers, 6 , 7 , 8 ranging from 0.98% per patient 7 , 9 to 4.25%. 8
Evidence‐based studies to determine the definitive causes of delayed‐onset nodule formation related to VYC products are hampered by the low incidence of such events. One solution is additional prospective or retrospective studies on patient responses to VYC products to confirm the incidence and to identify clinical characteristics that may predispose patients to the occurrence of delayed‐onset nodules. In Canada, there are currently 5 available VYC products differentiated on the basis of their varying HA content, 1 , 10 , 11 , 12 3 of which are discussed in the current analysis: Juvéderm® Voluma® with lidocaine (VYC‐20L), Juvéderm® Volift® with lidocaine (VYC‐17.5L), and Juvéderm® Volbella® with lidocaine (VYC‐15L; Allergan Aesthetics, an AbbVie company). The objective of this retrospective analysis is to assess the incidence of delayed‐onset nodule formation with VYC products based on a single experienced injector.
2. METHODS
The author has been using VYC‐20L, VYC‐15L, and VYC‐17.5L since 2010, 2013, and 2014, respectively, for aesthetic procedures at a dermatology practice in Vancouver, BC, Canada. Filler injections were done using the author's standardized procedure, and patient data were entered in an electronic database. Injectable products were not diluted or mixed. Patients provided written informed consent prior to receiving filler injections.
Prior to treatment, patients were photographed without makeup, and facial skin was prepared for injection by washing twice with 4% chlorhexidine. 13 For lip treatments, a topical anesthetic (23% lidocaine, 7% tetracaine) was applied 30 min before injection. Injections were usually carried out with needles (27 g or 30 g) or cannulas (25 g), 38 mm or 50 mm in length. The choice of needle or cannula was based on the area to be injected, with cannula being the preferred method for soft tissue areas. Injections were performed in multiple planes depending on the requirement and product type. Patients were provided a written set of aftercare instructions: use cold packs for mild discomfort, gently cleanse skin, and do not engage in heavy exertion, put pressure on the face, wear makeup, or consume alcohol for 2 days.
Patients were evaluated and treated as needed over time and returned to the clinic for follow‐up assessment and/or for reported complications. Patients who reported delayed‐onset nodules, defined as nodules developing at least 4 weeks after HA injection, returned to the clinic for assessment and management and were seen regularly until nodule resolution. Information on nodule location, date of first appearance, potential immunologic triggers (e.g., dental procedures, vaccine administration, and viral illness), and information about subsequent nodule treatment, and the status was recorded in each patient's chart. If patients had nodules associated with more than one product, they were included in the count for each associated product. Nodule status was categorized as “resolved” or “ongoing” at the time of retrospective chart review (October 31, 2019).
The incidence of delayed‐onset nodule development for each VYC product was expressed in terms of the total number of patients treated and the number of syringes used. Timelines of nodule development were recorded as the time between onset of the nodule and the date of the last VYC product injection in the same facial area. Treatment response, expressed as time to resolution, was measured as the time (in days) between observation and complete resolution of the nodule.
2.1. Histology
A biopsy on a delayed‐onset nodule was performed in 1 patient, after obtaining written informed consent. The nodule was fixed with formalin, embedded in paraffin, and stained with hematoxylin and eosin or Alcian blue before histologic examination.
3. RESULTS
A total of 2139 patients received VYC filler injections since 2010 with the majority of patients receiving VYC‐20L (see Table 1 for treatment details). Delayed‐onset nodules were reported in 7 patients (overall incidence, 0.33%), with the highest incidence observed with VYC‐20L at 0.49% per patient (Table 1). All patients who developed nodules were Caucasian. Two of the 7 patients developed nodules associated with more than 1 VYC product (patient 1: VYC‐17.5L and VYC‐20L; patient 6: VYC‐15L and VYC‐17.5L). All 7 patients with delayed‐onset nodules were female (mean age, 62 years; range, 55–70 years). The median total number of syringes for patients who developed nodules was 6 (range, 2–22 syringes). These injections were administered over a period spanning 1 session to repeated injections covering 5 years.
TABLE 1.
Incidence of delayed‐onset nodules with VYC products (N = 2139 patients) a
| VYC‐20L | VYC‐17.5L | VYC‐15L | |
|---|---|---|---|
| Total no. of patients, n (%) | 1232 (57.6) | 512 (23.9) | 395 (18.5) |
| Total no. of syringes | 2971 | 792 | 585 |
| Total no. of syringes per patient | 2.4 | 1.5 | 1.5 |
| Volume of filler injected (ml) | |||
| Mean | 2.0 | 1.7 | 1.0 |
| Range | 1–6 | 1–3 | NA b |
| No. of patients with nodules | 6 | 2 c | 1 |
| Nodule incidence per patient (%) | 0.49 | 0.39 | 0.25 |
| Nodule incidence per syringe (%) | 0.20 | 0.25 | 0.17 |
| Time to nodule formation (week) | |||
| Mean | 46 | 36 | 17 |
| Range | 11–81 | 17–55 | NA d |
Patient status as of December 2019.
Patient treated with VYC‐15L received a total of 1 ml.
Two patients had nodules associated with 2 different products. One patient had nodules associated with both VYC‐17.5L and VYC‐15L injections, and another had nodules associated with VYC‐17.5L and VYC‐20L injections.
Only 1 patient had nodules associated with VYC‐15L injection.
VYC treatment history/number of treatments and the timing of nodule development for each of the 7 patients who developed nodules are summarized in Figure 1, and the nodule locations for each patient are shown on the facial image in Figure 2. With the exception of patient 5, all other patients received filler treatments utilizing combinations of VYC products at various times prior to the onset of nodules. However, delayed‐onset nodules did not develop at every injection site or with every VYC product injected. On examination, all nodules were found to be subcutaneous, nontender, and non‐erythematous. A typical presentation of multiple nodules on 1 patient is shown in Figure 3. A biopsy of a nodule revealed evidence of foreign‐body granuloma in proximity to HA (Figure 4).
FIGURE 1.

Vycross (VYC) filler treatment and nodule development timelines for patients who developed delayed‐onset nodules. VYC product injection points (volumes injected are shown in parentheses), timing of potential triggering events for nodule formation (note that there was no recorded triggering event for patient 7), and whether nodules were resolved or ongoing (as of October 31, 2019) are indicated. Patient 1 (aged 70 years) had the most intensive treatment pattern, with 4 injection sessions of each VYC product between September 2014 and June 2019; areas injected included the lips and tear troughs (VYC‐15L), marionette lines (VYC‐17.5L), and the jaws, cheeks, chins, and temples (VYC‐17.5L and VYC‐20L). One nodule each from patients 3 and 4 resolved without any treatment. *VYC‐20L injection; ^VYC‐17.5L injection; #VYC‐15L injection. †Injection was by cannula. Circles indicate timing of nodule formation, wherein each color represents the last VYC product injected in the same area as the nodule (red, VYC‐20L; blue, VYC‐17.5L; green, VYC‐15L). Each colored circle represents a separate reaction site for delayed‐onset nodule formation. ¶Since November 2013, patient 4 had extensive VYC product injections with no additional delayed‐onset nodule development. Pot, potential
FIGURE 2.
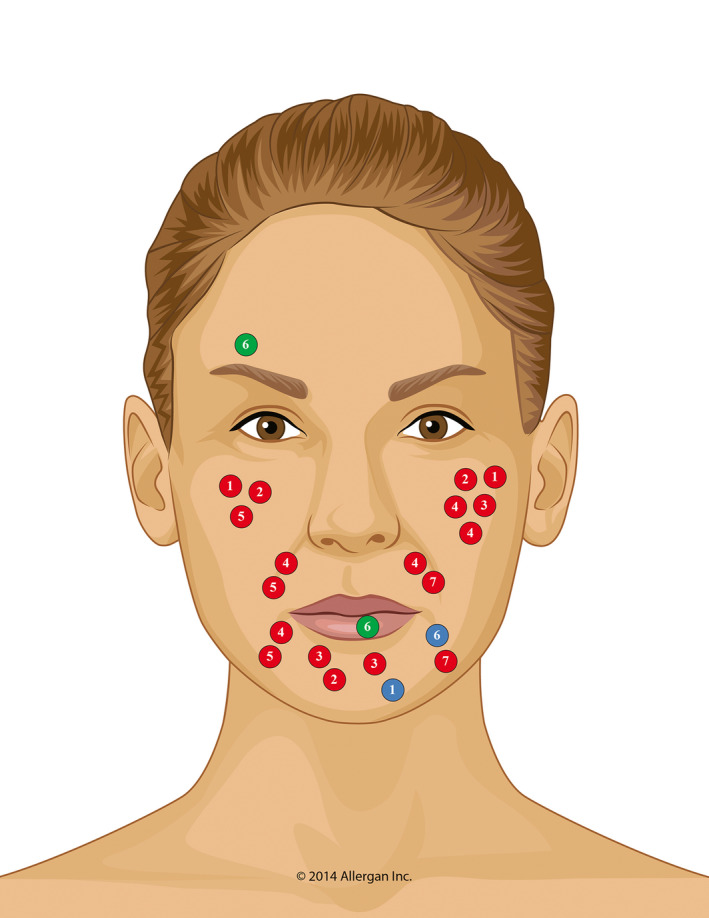
Location of delayed‐onset nodules. Circles represent the location of nodules, with the different colors indicating the last VYC product injected in the same location as the subsequent nodule (red, VYC‐20L; blue, VYC‐17.5L; green, VYC‐15L); numbers within circles are patient IDs (see Figure 1). Most nodules were associated with VYC‐20L injections. In the 6 patients with delayed‐onset nodules related to VYC‐20L, there were a total of 18 nodules, which were often located on the cheeks (~50% of nodules). In contrast, there were 2 patients with a single nodule each related to VYC‐17.5L and 1 patient with 2 nodules related to VYC‐15L
FIGURE 3.

Representative images from patient 6 showing non‐inflammatory nodules in 3 locations 17 weeks after VYC‐15L injections in the lips and forehead (arrows on left and right images, respectively), and VYC‐17.5L injections in the marionette lines (arrow on center image). Nodules appeared 4 weeks after a tooth extraction and resolved following treatment with hyaluronidase and prednisone (see Figure 1 and Table 2 for additional details on this patient)
FIGURE 4.
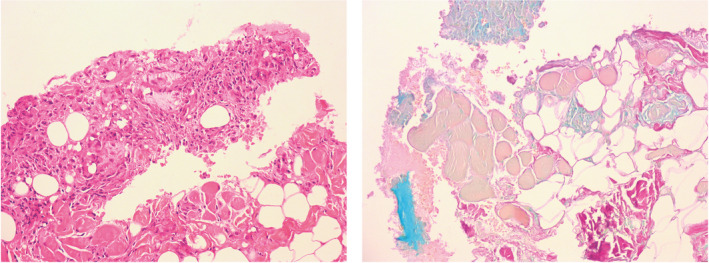
Representative photomicrographs from patient 4’s nodule biopsy. Left image: Hematoxylin and eosin (H and E) staining showing granuloma in relation to hyaluronic acid (HA) filler product VYC‐20L (100× magnification). Right image: Alcian blue staining showing HA (blue) with 1 focus adjacent to granulomatous inflammation (100× magnification). See Figure 1 and Table 2 for additional details on this patient
In 6 of the patients, delayed‐onset nodules developed between September and December. With the exception of patient 7, all other patients reported a potential inflammatory trigger prior to the development of nodules, which were mostly related to dental procedures, including dental cleaning (n = 3; patients 1, 2, and 3), dental implants (n = 1; patient 4), gum irritation related to a dental brace (n = 1; patient 5), and tooth extraction (n = 1; patient 6). One patient who underwent dental cleaning also had a CT angiogram (patient 3). For 1 patient (patient 7), no known triggering event was recorded. The time between the presumed inflammatory trigger and subsequent nodule development was highly variable, ranging from 1 to 168 days (Figure 1). No infections or other immune triggers were identified in this population.
All 7 patients were followed‐up until nodule resolution; treatment management is summarized in Table 2. One nodule each from patients 3 and 4 resolved spontaneously without any treatment. For the remaining patients who responded to therapy, mean time to resolution was 79 days (range, 33–138 days). Prednisone (25–50 mg/day for 1 or 2 weeks) and intralesional hyaluronidase (15–150 IU) were major components in the treatment plans. Treatment incorporating prednisone with or without hyaluronidase had a shorter mean resolution time compared with therapy that included an antibiotic (45 days with prednisone ±hyaluronidase [patients 1, 2, and 6] vs. 84 days for therapy incorporating an antibiotic [patient 4]). To date, 5 of the 7 patients have reported no further incidents since the last reporting. The 2 remaining patients were lost to follow‐up.
TABLE 2.
Management of delayed‐onset nodules
| Patient ID | Management | Time to resolution (days) |
|---|---|---|
| 1 |
Prednisone: 50 mg/day × 1week ILK: 5 mg |
Ongoing as of October 31, 2019 (36 days since nodule reported) |
| 2 |
Prednisone: 50 mg/day × 1week Prednisone: 25 mg/day × 5 days |
33 |
| 3 | No treatment | 1 |
| 4 |
Initial nodules: Minocycline: 100 mg/day × 2 month Hyal: 3 treatments over 20 weeks (150 IU, 75 IU, 45 IU) Secondary nodules: No treatment |
84 26 a |
| 5 |
Hyal: 1 treatment (22.5 IU) ILK: 6.5 mg |
71 & 98 b |
| 6 |
Hyal: 6 treatments over 45 days (60 IU, 75 IU, 135 IU, 120 IU, 30 IU, 15 IU) Prednisone: 30 mg/day × 1 week Diphenhydramine/cetirizine |
87 |
| 7 | Hyal: 3 treatments over 7 weeks (60 IU, 30 IU, 15 IU) | 138 |
Abbreviations: Hyal, hyaluronidase; ILK, intralesional Kenalog; IU, International Unit.
This patient had a recurrence of nodules after initial nodules resolved; the secondary nodules resolved spontaneously (see Figure 1).
Following VYC‐20L injection, patient initially had a nodule on left marionette line and later nodules developed on right nasolabial folds and right cheek; nodules had a common resolution date but different detection dates.
4. DISCUSSION
Prior studies on the incidence of delayed‐onset nodule development with VYC fillers were based on data from sites with multiple injectors, different injection techniques and methods of skin preparation, dilution of HA fillers prior to injection, and a range of facial areas injected. 6 , 7 , 9 These variables have been suggested as potential factors affecting delayed‐onset nodule formation 7 , 9 , 14 , 15 and the apparent variation in reported incidence. 7 , 16 , 17 The present analysis is based on a large patient cohort (N = 2139) receiving aesthetic therapy with 3 VYC products from the same physician at a single site using standard HA preparatory and injection techniques, thus minimizing the impact of external variables on HA filler‐related delayed‐onset nodule development. 9 , 16 , 17 Over an assessment period of 10 years, only 7 patients were identified as having delayed‐onset nodules, an overall incidence of 0.33%. Although patient numbers are small, incidence of nodule development was higher with VYC‐20L (0.49%) compared with VYC‐17.5L (0.39%) and VYC‐15L (0.25%).
Findings from prior studies with VYC fillers are shown in Table 3. 6 , 8 , 9 , 18 The incidence rate determined in the present analysis for VYC products (0.33%) is lower than other published estimates, 6 , 8 , 9 but is similar to incidence rates for other HA fillers (<0.4%). 3 , 5 For VYC‐20L, 1 analysis 9 conducted over a 9‐year period estimated a 0.98% incidence, whereas another study 18 reported no events that could be characterized as delayed‐onset nodules over a 2‐year follow‐up. Although others have noted a relatively high incidence of delayed nodules related to VYC‐15L (1.0%–4.25%), 9 , 11 the observed rate in this current study (0.25%) was comparable with that reported for non‐VYC products. For VYC‐17.5L, there is little information on the risk of long‐term complications. In an 18‐month study, Dayan et al. 19 assessed outcomes in 123 patients after initial and repeat treatments of nasolabial folds with VYC‐17.5L. No specific data on defined delayed‐onset nodule development were provided, although 3 patients had long‐term adverse events (weeks to months after injection), 1 of which was described as a moderate skin mass that resolved after treatment with triamcinolone cream. 3 , 5 In the current analysis, 2 patients had delayed‐onset nodules related to VYC‐17.5L injection, thus providing new insight into delayed‐onset nodule formation for this HA filler.
TABLE 3.
Incidence of VYC‐associated delayed‐onset nodule formation in published studies
| Study | VYC filler | Number of patients treated | Number of patients with nodules (Number With Immune Trigger) | Incidence per patient, % | Mean time to nodule onset, weeks (range) |
|---|---|---|---|---|---|
| Sadeghpour et al. 6 | VYC‐20L | 315 | 0 | 0 | 0 |
| VYC‐17.5L | 219 | 0 | 0 | 0 | |
| VYC‐15L | 495 | 5 (1) | 1.0 | 35.8 (20–54) | |
| Artzi et al. 8 | VYC‐15L | 400 | 17 (0) | 4.25 | 8.4 (5–12) |
| Humphrey et al. 9 | VYC‐20L | 4500 | 44 (15) | 0.98 | 16* (4–52) |
| Few et al. 18 | VYC‐20L | 235 | 0 | 0 | 0 |
Median time to nodule onset.
The mechanisms leading to delayed‐onset nodule development are unclear for all HA‐related fillers, including VYC products. The VYC fillers investigated in the current analysis combine high‐ and lower‐molecular weight (>600 Kda) HA. 2 , 6 High‐molecular weight HA has an anti‐inflammatory effect on the immune system, but there is evidence that low‐molecular weight HA (<200 Kda) is proinflammatory and can activate the immune system when contaminated with bacterial protein. 6 , 9 , 20 , 21 , 22 Thus, this suggested mechanism would not explain the observed higher incidence of delayed‐onset nodules with VYC products reported by others.
Previous studies also suggest that delayed‐onset nodules may be related to filler preparation and injection. 8 , 9 , 16 Humphrey et al. 9 found that patients with delayed‐onset nodules received a higher cumulative VYC‐20L dose (5.0 ml) than those without nodule development (0.5–1.5 ml lower cumulative volume), suggesting that this difference increased the risk of nodule development. However, in the current analysis, there was no evidence to suggest a relationship between injected volume and nodule development because all the patients with delayed‐onset nodules received a wide range of dosing, similar to patients who did not develop delayed‐onset nodules.
It is suggested that the majority of delayed‐onset nodules in response to HA filler injections have an inflammatory and immune‐mediated origin, although there is considerable evidence that the etiology is multifactorial. 6 , 7 , 8 , 16 , 23 Several studies have associated development of delayed‐onset inflammatory nodules with formation of a foreign‐body granuloma. 16 , 24 , 25 , 26 In the current analysis, a foreign‐body granuloma was identified in a biopsied nodule, lending some support to previous studies. Lemperle et al. 24 postulated that foreign‐body granulomas are nonallergic in origin based on the observation that testing granuloma patients with the same filler material several years after the initial reaction did not lead to formation of new granulomas at the test site; their conclusion was that a foreign‐body granuloma is not a result of a late type IV allergic granulomatosis reaction. 24 A similar observation was made in the current analysis; when patient 3 with delayed‐onset nodules after VYC‐20L injections was subsequently challenged in a skin test with VYC‐20L, there was no type IV reaction, suggesting that the nodules were not the result of an allergic‐type reaction (data not shown). However, Turkmani et al. 14 suggest that hypersensitivity tests have a limited sensitivity and that a negative result does not necessarily exclude a hypersensitivity reaction. Although there is evidence that delayed‐onset nodule development is due, at least in part, to formation of foreign‐body granulomas, its pathogenesis requires further investigation.
Previous studies suggest that immune responses may trigger inflammatory events. 6 , 7 , 9 , 14 , 15 , 20 In the current analysis, 86% of patients had an identifiable triggering event (mostly related to dental procedures) and similar events have been identified in 20% 6 –39% 7 of patients. Seasonal variation in nodule development was also observed, with the majority of patients (71%) developing nodules between September and December, supporting similar observations in other studies and suggesting that viral infections more common at this time of year may provide an inflammatory/immunological trigger. 7 , 9 , 14 Turkmani et al. 14 reported a delayed hypersensitivity reaction to different brands of HA fillers (including VYC products) after an influenza‐like illness. Nodule development occurred 3–5 days after the illness but 5.1 months (range, 2–10 months) from the HA filler injection. 14 Delayed inflammatory reactions to HA fillers after exposure to the COVID‐19 spike protein and vaccine, including erythema, edema, and nodules, have also been recently reported. 27 , 28 Based on these observations, clinicians are recommended to perform proper skin preparation prior to HA injections to avoid infection and to advise patients to delay filler treatment if areas of inflammation/infection are present. Because dental procedures appeared to provoke a reaction in several patients in the current study, refraining from dental procedures before and after filler injection for about 2 weeks may also be recommended. Ultimately, the underlying cause of delayed‐onset nodules remains incompletely understood and it is difficult to postulate what properties of fillers trigger nodule development in some patients. 14 Fortunately, as indicated by our data, nodule development seems rare and nodules that do form can resolve spontaneously or with treatment.
In the present analysis, the time required to resolve nodules was highly variable. Similar to other studies, some nodules resolved spontaneously without any treatment, while others required substantial treatment. 6 , 7 , 9 An effective component of successful treatment appeared to be systemic or intralesional corticosteroid with or without hyaluronidase, 8 , 9 , 29 reflecting the inflammatory origin of delayed‐onset nodules. Hyaluronidase has also been recognized as an important component of therapy based on the premise that this treatment removes the HA filler, eliminating the source of the inflammatory reaction. 14 , 30 Artzi et al. 8 found that broad‐spectrum antibiotics with intralesional hyaluronidase was the most effective treatment in patients with VYC‐15L‐related nodules. Humphrey et al. 9 also found that oral antibiotics were a component of successful nodule treatment in at least some of their patients. It is important to note that nodules resolved either spontaneously or with treatment for all 7 patients in this study.
There are some limitations to the current analysis. Patient outcomes were based on retrospective chart review with the assumption that all relevant data had been consistently captured. In addition, exact timing of nodule development and potential triggering events were based on patient recall, which may not have been reliable. Finally, delayed‐onset nodule incidence rates, particularly for VYC‐17.5L and VYC‐15L, were based on a small number of observed cases. Because incidence of delayed‐onset nodule formation was based on patient self‐reports (similar to previous studies), there may have been patients with delayed‐onset nodules that were not captured in the analysis. Nevertheless, the low incidence rates emphasize that VYC‐associated delayed‐onset nodule development is a relatively uncommon complication and demonstrate the need for additional long‐term studies to validate incidence rates, define pathogenesis, and characterize patients at increased risk for these events.
5. CONCLUSIONS
In the current analysis, the overall incidence of delayed‐onset nodule development with VYC HA filler products was 0.33%, which is lower than published estimates. Incidence rates were highest with VYC‐20L (0.49%), the most frequently used filler, then VYC‐17.5L (0.39%). The incidence rate of VYC‐15L (0.25%) was comparable with previous reports for non‐VYC products. Corticosteroids (prednisone) and hyaluronidase were important components of successful treatment, and nodule resolution occurred over variable time frames. The data suggest that a standardized injection protocol may be associated with the lower incidence of VYC product delayed‐onset nodule development observed in the current analysis, that an inflammatory trigger may play a significant role in subsequent nodule development, and that reactive nodule treatment strategies are associated with successful resolution in affected patients. Importantly, all delayed‐onset nodules resolved completely, either spontaneously or with treatment.
CONFLICT OF INTEREST
None disclosed.
AUTHOR CONTRIBUTION
JKR collected data, analyzed data, and wrote the manuscript. Editorial support for writing the manuscript was funded by an unrestricted educational grant provided by Allergan Aesthetics, an AbbVie Company, Irvine, California.
ETHICAL APPROVAL
No ethics review board approval was utilized for this retrospective chart review.
ACKNOWLEDGEMENT
The author thanks Debra Payne at Allergan Aesthetics, an AbbVie company, for reviewing the manuscript for scientific accuracy, and Dr. Misha Zarbafian for data collection.
Rivers JK. Incidence and treatment of delayed‐onset nodules after VYC filler injections to 2139 patients at a single Canadian clinic. J Cosmet Dermatol. 2022;21:2379–2386. doi: 10.1111/jocd.15013
DATA AVAILABILITY STATEMENT
The data are available on request from the author.
REFERENCES
- 1. Hotta TA. The expanding market of Health Canada‐approved hyaluronic acid‐injectable dermal fillers. Plast Surg Nurs. 2017;37(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 2. Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 Suppl):139S‐148S. [DOI] [PubMed] [Google Scholar]
- 3. Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their management. J Dermatol Surg. 2016;20(2):100‐106. [Google Scholar]
- 4. Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open. 2019;7(6):e2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman PM, Mafong EA, Kauvar AN, Geronemus RG. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28(6):491‐494. [DOI] [PubMed] [Google Scholar]
- 6. Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, Dover JS, Kaminer MS. Delayed‐onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085‐1094. [DOI] [PubMed] [Google Scholar]
- 7. Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed‐onset nodules secondary to a smooth cohesive 20 mg/ml hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929‐939. [DOI] [PubMed] [Google Scholar]
- 8. Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid‐based gel. Dermatol Surg. 2016;42(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 9. Humphrey S, Jones DH, Carruthers JD, et al. Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20‐mg/ml hyaluronic acid filler in 4500 patients. J Am Acad Dermatol. 2020;83(1):86‐95. [DOI] [PubMed] [Google Scholar]
- 10. Government of Canada . Active licence listing by company: Allergan. 2020. https://health‐products.canada.ca/mdall‐limh/information.do?companyId_idCompanie=112685&lang=eng Accessed December 9, 2021
- 11. Ogilvie P, Benouaiche L, Philipp‐Dormston WG, et al. VYC‐25L hyaluronic acid injectable gel is safe and effective for long‐term restoration and creation of volume of the lower face. Aesthet Surg J. 2020;40(9):NP499‐NP510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogilvie P, Thulesen J, Leys C, et al. Expert consensus on injection technique and area‐specific recommendations for the hyaluronic acid dermal filler VYC‐12L to treat fine cutaneous lines. Clin Cosmet Investig Dermatol. 2020;13:267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shive M, Hou Z, Zachary C, Cohen J, Rivers JK. The use of chlorhexidine as a skin preparation on the head and neck: a systematic review of ocular and ototoxicity. Dermatol Surg. 2021;47(1):34‐37. [DOI] [PubMed] [Google Scholar]
- 14. Turkmani MG, De Boulle K, Philipp‐Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza‐like illness. Clin Cosmet Investig Dermatol. 2019;12:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King M, Bassett S, Davies E, King S. Management of delayed onset nodules. J Clin Aesthet Dermatol. 2016;9(11):E1‐E5. [PMC free article] [PubMed] [Google Scholar]
- 16. Graivier MH, Bass LM, Lorenc ZP, Fitzgerald R, Goldberg DJ, Lemperle G. Differentiating nonpermanent injectable fillers: prevention and treatment of filler complications. Aesthet Surg J. 2018;38(suppl_1):S29‐S40. [DOI] [PubMed] [Google Scholar]
- 17. Glogau RG, Kane MA. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol Surg. 2008;34(Suppl 1):S105‐S109. [DOI] [PubMed] [Google Scholar]
- 18. Few J, Cox SE, Paradkar‐Mitragotri D, Murphy DK. A multicenter, single‐blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient‐reported outcomes at 2 years. Aesthet Surg J. 2015;35(5):589‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dayan S, Maas CS, Grimes PE, et al. Safety and effectiveness of VYC‐17.5L for long‐term correction of nasolabial folds. Aesthet Surg J. 2020;40(7):767‐777. [DOI] [PubMed] [Google Scholar]
- 20. Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vistejnova L, Safrankova B, Nesporova K, et al. Low molecular weight hyaluronan mediated CD44 dependent induction of IL‐6 and chemokines in human dermal fibroblasts potentiates innate immune response. Cytokine. 2014;70(2):97‐103. [DOI] [PubMed] [Google Scholar]
- 22. Hee C, Nakab L, Messina D. In vitro inflammatory and immune response to uncrosslinked hyaluronic acid (HA) and HA fillers [abstract 7809]. J Am Acad Dermatol. 2018;79(3 suppl 1):AB164. [Google Scholar]
- 23. Alijotas‐Reig J, Fernández‐Figueras MT, Puig L. Inflammatory, immune‐mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241‐258. [DOI] [PubMed] [Google Scholar]
- 24. Lemperle G, Gauthier‐Hazan N, Wolters M, Eisemann‐Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible Causes. Plast Reconstr Surg. 2009;123(6):1842‐1863. [DOI] [PubMed] [Google Scholar]
- 25. Bhojani‐Lynch T. Late‐onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2017;5(12):e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42(2):232‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munavalli GG, Guthridge R, Knutsen‐Larson S, Brodsky A, Matthew E, Landau M. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2022;314(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michon A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID‐19 vaccination ‐ A case report. J Cosmet Dermatol. 2021;20(9):2684‐2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philipp‐Dormston WG, Goodman GJ, De Boulle K, et al. Global approaches to the prevention and management of delayed‐onset adverse reactions with hyaluronic acid‐based fillers. Plast Reconstr Surg Glob Open. 2020;8:e2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Signorini M, Liew S, Sundaram H, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers—Evidence‐ and opinion‐based review and consensus recommendations. Plast Reconstr Surg. 2016;137(6):961e‐971e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on request from the author.


