To the Editor,
The clinical features of hyper IgE syndrome (HIES) have been clearly highlighted. The syndrome is a complex immunodeficiency characterized by recurrent staphylococcal skin and lung infections, chronic mucocutaneous candidiasis and atopic dermatitis‐like eczema, associated with hyper IgE and often high eosinophil levels. Other features are scoliosis, osteoporosis, primary teeth retention and joint hyperextensibility. 1 The autosomal dominant form is caused by STAT3 dominant‐negative mutations, while the autosomal recessive forms are caused by ZNF341, DOCK8, PGM3 or CARD11 biallelic mutation.
Several studies have shown that the STAT3 protein is involved in signal transduction of a broad number of cytokines such as IL‐6, IL‐10 and IL‐21. Impairment of IL‐6 signalling causes a reduction in Th17 polarization and is responsible for the chronic mucocutaneous candidiasis, 2 while defective IL‐10 and IL‐21 signalling leads to an imbalance towards IL‐4 production and Th2 polarization possibly linked to the susceptibility to pathogens controlled by Th1 lymphocytes.
Dupilumab is a fully human monoclonal antibody targeting the alpha‐subunit of IL‐4 receptor, inhibiting the IL‐4 and IL‐13 signalling, that are key cytokines in the development of atopic dermatitis. In 2020, FDA approved the use of dupilumab for the treatment of atopic dermatitis in patients over 6 years of age.
Herein, we report the successful treatment of a 17‐year‐old patient affected by STAT3‐HIES with a severe atopic‐like dermatitis with a recovery of Th1 polarization.
Since the very first months of life, the patient presented with recurrent bronchospasms and severe atopic dermatitis with frequent Staphylococcal superinfections, later followed by allergy to several oral and inhalant allergens. From 5 years of life, he developed recurrent cutaneous cold abscesses and frequent upper airway infections. His laboratory work‐up was characterized by a hyper IgE (>5000), hypereosinophilia (>20%) and multiple sensitization to oral allergens (assessed by serum‐specific IgE). He was treated with topical and oral corticosteroids, antihistaminergics and oral cyclosporine A (5 mg/kg), with a partial benefit on the cutaneous manifestations.
The patient presented for the first time to our attention when he was 16 years old because of severe pruritic eczematous lesions with signs of superinfection (Figure 1A) (SCORAD 45/103, DLQI 8/30), scoliosis, joint hyperextensibility, arched palate and a retained tooth. The IgE levels were >5000 and eosinophils 20%, while lymphocytic proliferation test and neutrophil oxidative index were normal. An autosomal dominant HIES was suspected, and an NGS panel for primary immune deficiencies revealed a de novo heterozygous STAT3 mutation (c.1150T>C; p.F384L, Figure 1B) already reported in previous patients with STAT3‐HIES. 1 An increase in Th2 lymphocytes (CD4+IL‐4+IFNg−) and a decrease in Th1 (CD4+IL‐4‐IFNg+) lymphocytes were detected, while Th17 (CD4+IL17+IFNg−) were in the lower range compared with controls, even if they resulted comparable to Th17 levels reported in the literature in STAT3‐HIES (Figure 1D). 2
FIGURE 1.
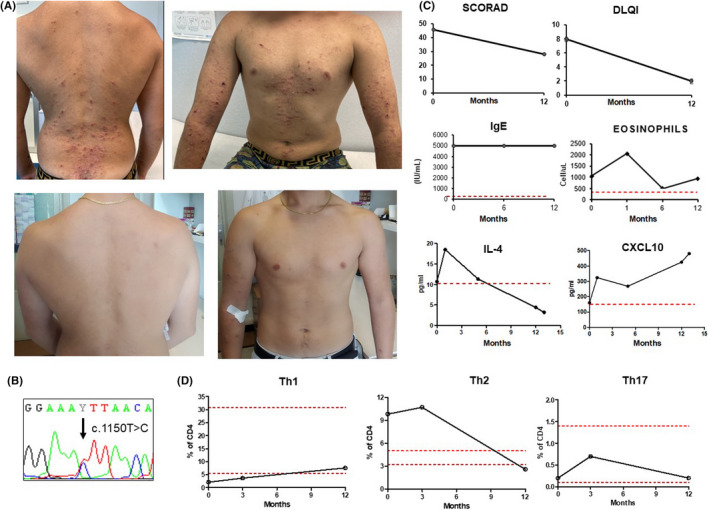
(A) Cutaneous lesions before (upper pictures) and after (lower pictures) treatment with dupilumab; (B) Sanger sequencing; (C) Modification of SCORAD, DLQI, IgE, eosinophils, IL‐4 and CXCL1 before and after 12 months of treatment; (D) Modification of Th1, Th2 and Th17 lymphocytes before and after 12 months of treatment
The inefficacy of previous treatments prompted the choice of an anti‐IL‐4/IL‐13 therapy with Dupilumab in addition to topical therapies with barrier creams and steroids. The first injection of 600 mg, followed by 300 mg every other week, induced a progressive clearing of skin lesions. After two injections, a cold abscessus developed following a traumatic lesion of the knee. The lesion spontaneously healed without need for further treatment. An evident therapeutic efficacy was observed after the 3rd injection. At follow‐up after 12 months, the SCORAD (28/103) and DLQI (2/30) showed a significant clinical improvement. Laboratory examinations showed an initial increase in the eosinophil count, as observed in the previous report in atopic dermatitis treated with dupilumab and a subsequent normalization, while IgE remained elevated (Figure 1C). After 10 months, a decrease in Th2 lymphocytes was evident, together with an increase in Th1 lymphocytes (Figure 1D). These changes were associated with a reduction in IL‐4 and an increase in CXCL10 (Figure 1C) with no clear pattern of modification of other cytokines (Figure 2).
FIGURE 2.
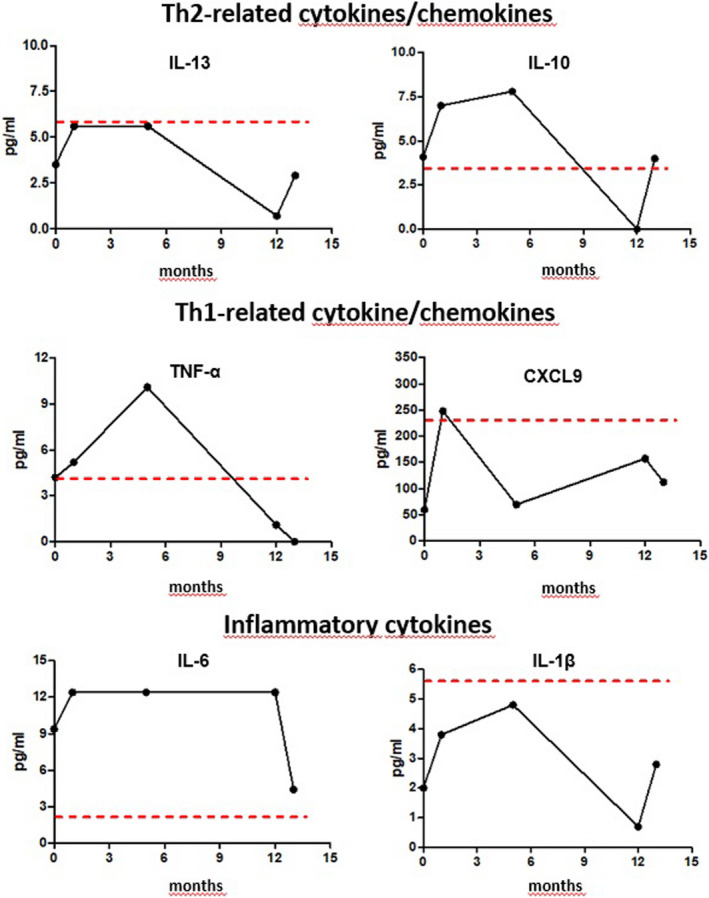
Behaviour of IL‐13, IL‐10, CXCL9, TNFα, IL‐6 and IL‐1β before and after 1, 5, 12 and 13 months of dupilumab. Cytokine/chemokine profiling: The BD CBA assays for human soluble protein (BD Bioscience 558264) was used to assess plasma cytokine/chemokines according to the manufacturer's protocols. Data were analysed with the FCAP Array software. Flow cytometry evaluation of lymphocytes was performed on 50 µl of whole blood. Cells were stained with membrane markers CD3, CD4 and CD8, fixed and permeabilized (Cytofix/Cytoperm [BD]); T lymphocyte polarization was defined in CD3+CD4+CD8− cells by intracellular expression of IFN‐g for Th1, IL‐4 for TH‐2 and IL‐17 for Th17
In the literature, an excellent clinical response to dupilumab has been described in 6 cases of STAT3‐HIES, 3 , 4 , 5 , 6 with evidence of amelioration of cutaneous lesions and IgE levels. 3 , 4 In one case, eosinophilic esophagitis also resolved. 4
Our report suggests that IL‐4/IL‐13 blockade correlates with a reversion of the Th1/Th2 unbalance observed in STAT3‐HIES at lymphocyte level. This evidence corroborates the role of an altered lymphocyte polarization in the genesis of the main clinical features that characterize this syndrome. In fact, not only the eczema resolved, but also the diffuse skin infections were promptly cleared by the treatment with a stable efficacy in time. IL‐4 has been shown to inhibit Th1 and Th17 differentiation 7 and to influence keratinocytes, diminishing the expression of fibronectin and thus impairing wound healing. 8 Considering the role of Th1 in controlling Staphylococcal infections, we speculate that IL‐4/IL‐13 blockade in STAT3‐HIES could not only improve the atopic manifestation of the syndrome but also have a positive role in preventing skin infections. Moreover, our case represents the longer follow‐up reported so far (at present 18 months), underlying the safety of dupilumab use in STAT3‐HIES.
Still, several areas remain to be understood in this syndrome. In fact, in all the described cases, IgE remained extremely high despite the brilliant clinical efficacy. 3 , 4 , 5 Up to now, the underlying mechanism responsible for IgE increase in STAT3‐HIES remains unknown. Our and other's observation suggest that a STAT3‐dependent IL‐4/IL‐13 independent mechanism could be implicated. A possible example might be represented by the defective IFNγ production demonstrated in STAT3‐HIES, being IFNγ known to inhibit IgE production. 9 Irrespective of disease mechanism, this observation suggests a limited role of IgE in STAT3‐HIES manifestations with a consequent implication for drug choice. Furthermore, Th17 cells do not appear to be stably affected by IL‐4/IL‐13 pathway blockade despite the known role of IL‐4 signalling in inhibiting Th17 polarization. 10 Obviously, both observations will require independent confirmations to draw reliable conclusions.
In conclusion, the treatment with dupilumab in STAT3‐HIES may achieve a satisfactory clinical remission and immunological restore of physiological T‐cell polarization, thus allowing the patient to recover a better quality of life.
INFORMED CONSENT
Informed consent was obtained from the patient.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13770.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Genova within the CRUI‐CARE Agreement. [Correction added on 11‐May‐2022, after first online publication: CRUI‐CARE funding statement has been added.]
Editor: Fabio Candotti
REFERENCES
- 1. Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper‐IgE syndrome. N Engl J Med. 2007;357(16):1608‐1619. [DOI] [PubMed] [Google Scholar]
- 2. Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper‐IgE syndrome. Nature. 2008;452(7188):773‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sogkas G, Hirsch S, Jablonka A, Witte T, Schmidt RE, Atschekzei F. Dupilumab to treat severe atopic dermatitis in autosomal dominant hyper‐IgE syndrome. Clin Immunol. 2020;215:108452. [DOI] [PubMed] [Google Scholar]
- 4. Dixit C, Thatayatikom A, Pappa H, Knutsen AP. Treatment of severe atopic dermatitis and eosinophilic esophagitis with dupilumab in a 14‐year‐old boy with autosomal dominant hyper‐IgE syndrome. J Allergy Clin Immunol Pract. 2021;9(11):4167‐4169. [DOI] [PubMed] [Google Scholar]
- 5. Su CJ, Tseng HC. Treatment efficacy of dupilumab in a hyper‐immunoglobulin E syndrome patient with severe atopic dermatitis. JAAD Case Rep. 2021;9(11):60‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staudacher O, Krüger R, Kölsch U, et al. Relieving job: Dupilumab in autosomal dominant STAT3 hyper‐IgE syndrome. J Allergy Clin Immunol Pract. 2022;10:349‐351.e1. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell RE, Hassan M, Burton BR, et al. IL‐4 enhances IL‐10 production in Th1 cells: implications for Th1 and Th2 regulation. Sci Rep. 2017;7(1):11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serezani A, Bozdogan G, Sehra S, et al. IL‐4 impairs wound healing potential in the skin by repressing fibronectin expression. J Allergy Clin Immunol. 2017;139(1):142‐151.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avery DT, Ma CS, Bryant VL, et al. STAT3 is required for IL‐21‐induced secretion of IgE from human naive B cells. Blood. 2008;112(5):1784‐1793. [DOI] [PubMed] [Google Scholar]
- 10. Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL‐4 during Th17 maturation. J Immunol. 2011;187(9):4440‐4450. [DOI] [PMC free article] [PubMed] [Google Scholar]


