Abstract
Acute kidney injury (AKI) is one of the most common complications of liver transplantation (LT). We examined the impact of intraoperative management on risk for AKI following LT. In this retrospective observational study, we linked data from the electronic health record with standardized transplant outcomes. Our primary outcome was stage 2 or 3 AKI as defined by Kidney Disease Improving Global Outcomes guidelines within the first 7 days of LT. We used logistic regression models to test the hypothesis that the addition of intraoperative variables, including inotropic/vasopressor administration, transfusion requirements, and hemodynamic markers improves our ability to predict AKI following LT. We also examined the impact of postoperative AKI on mortality. Of the 598 adult primary LT recipients included in our study, 43% (n = 255) were diagnosed with AKI within the first 7 postoperative days. Several preoperative and intraoperative variables including (1) electrolyte/acid‐base balance disorder (International Classification of Diseases, Ninth Revision codes 253.6 or 276.x and International Classification of Diseases, Tenth Revision codes E22.2 or E87.x, where x is any digit; adjusted odds ratio [aOR], 1.917, 95% confidence interval [CI], 1.280–2.869; p = 0.002); (2) preoperative anemia (aOR, 2.612; 95% CI, 1.405–4.854; p = 0.002); (3) low serum albumin (aOR, 0.576; 95% CI, 0.410–0.808; p = 0.001), increased potassium value during reperfusion (aOR, 1.513; 95% CI, 1.103–2.077; p = 0.01), and lactate during reperfusion (aOR, 1.081; 95% CI, 1.003–1.166; p = 0.04) were associated with posttransplant AKI. New dialysis requirement within the first 7 days postoperatively predicted the posttransplant mortality. Our study identified significant association between several potentially modifiable variables with posttransplant AKI. The addition of intraoperative data did not improve overall model discrimination.
Abbreviations
- aHR
adjusted hazard ratio
- AKI
acute kidney injury
- aOR
adjusted odds ratio
- BMI
body mass index
- BUN
blood urea nitrogen
- CDC
Center for Disease Control and Prevention
- CI
confidence interval
- CMV
cytomegalovirus
- c‐statistic
concordance statistic
- DBD
donation after brain death
- DCD
donation after circulatory death
- EBV
Epstein‐Barr virus
- eGFR
estimated glomerular filtration rate
- FFP
fresh frozen plasma
- ICD‐9
International Classification of Diseases, Ninth Revision
- ICD‐10
International Classification of Diseases, Tenth Revision
- INR
international normalized ratio
- KDIGO
Kidney Disease Improving Global Outcomes
- LT
liver transplantation
- MAP
mean arterial pressure
- MDRD‐4
Modification of Diet in Renal Disease, 4 variable
- MELD
Model for End‐Stage Liver Disease
- MPOG
Multicenter Perioperative Outcomes Group
- OTIS
Organ Transplant Information System
- pRBC
packed red blood cells
- RRT
renal replacement therapy
- SD
standard deviation
INTRODUCTION
Acute kidney injury (AKI) is one of the most common complications following liver transplantation (LT), with more than half of all LT recipients demonstrating at least acute renal failure.[ 1 , 2 ] Posttransplant AKI is associated with longer stays in the intensive care unit,[ 3 ] increased graft rejection,[ 4 ] higher hospital costs,[ 3 ] and higher mortality[ 5 , 6 ] independent of pretransplant renal function.[ 7 ]
Previous studies have shown that donor factors and recipient preoperative factors increase the risk of AKI[ 8 , 9 ] and chronic kidney disease[ 10 , 11 ] following LT. A variety of preoperative and postoperative factors (eg, exposure to calcineurin inhibitors) have been linked to post‐LT AKI.[ 7 ] The long‐term impact of intraoperative events, such as acidosis, low hematocrit values, or duration of each transplant stage, and anesthesia factors, such as norepinephrine and blood transfusion (including red blood cells, plasma, and cryoprecipitate) on posttransplant AKI is not well studied.
Real‐time data capture within electronic medical records allows the opportunity to link intraoperative data[ 12 ] with postoperative outcomes, thus refining our understanding of the impact of the perioperative period. Given the time‐sensitive nature of the development of AKI, the identification of perioperative predictors of posttransplant renal dysfunction could allow the development of renal protection strategies directed at high‐risk patients, and the identification of intraoperative predictors may enable modification of intraoperative care to reduce the risk of renal injury in at risk patients.
The objective of this study is to identify modifiable risk factors associated with AKI following LT. Our hypothesis was that the addition of intraoperative variables, including inotropic/vasopressor administration, transfusion requirements, and hemodynamic markers, improves our ability to predict AKI following LT. In addition, intraoperative variables specifically curated at key stages of the transplant, such as reperfusion, might further improve our ability to predict AKI and provide insight into the mechanism of renal injury.
PATIENTS AND METHODS
Study design
For this retrospective observational study performed at our academic quaternary care center, we obtained institutional review board (HUM00153452) approval. This article was prepared in accordance with the standards set forth by the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.[ 13 ] Study methods including data collection, outcomes, and statistical analyses were established prospectively and presented at an institutional peer review committee on October 10, 2018, prior to data access.[ 14 ] No organs from executed prisoners were used.
Data collection
Our primary data sources were collected via combined queries of (1) the local University of Michigan Multicenter Perioperative Outcomes Group (MPOG) data set and (2) Michigan Medicine's Organ Transplant Information System (OTIS). The local MPOG data set collects information from the electronic perioperative anesthesia database (Centricity, General Electric Healthcare, Waukesha, WI) and electronic health record (Epic, Verona, WI). OTIS is an internal clinical database that tracks patients from waitlist enrollment through death and contains demographic, clinical, and donor variables. In addition, OTIS contains the information reported to the Scientific Registry of Transplant Recipients and tracks standardized outcomes, including (1) AKI, (2) postoperative dialysis, (3) mortality, (4) graft failure, and (5) retransplantation.
Inclusion and exclusion criteria
All primary, adult liver recipients who received transplants at Michigan Medicine between 2008 and 2018 were included in our study. Patients were excluded if they had preoperative stage 5 end‐stage renal disease, as defined by the Modification of Diet in Renal Disease–4 variable (MDRD‐4) equation or were on dialysis prior to transplant. If a patient had more than 1 documented LT during the time period, only their first transplant was included in analysis. Patients entered the cohort at the time of their surgery and continued for as long as followed in the OTIS database.
Primary outcome
Our primary outcome was stage 2 or 3 AKI as defined by Kidney Disease Improving Global Outcomes (KDIGO) guidelines, using the maximum measured serum creatinine within the first 7 days following transplantation compared with preoperative serum creatinine closest to the time of transplant. These guidelines specify stage 2 as 2.0 to 2.9 times baseline and stage 3 as 3.0 times baseline or ≥4.0 mg/dL or the initiation of renal replacement therapy (RRT).[ 15 ] RRT receipt within the 7 days was considered stage 3 regardless of the creatinine change. We did not consider urine output rate in our classification, as this information was not uniformly available within our database. Only considering KDIGO stage 2 or 3 AKI allowed us to model the most severe and drastic changes in kidney function.
Secondary outcome
The secondary outcome was survival, censored at the length of follow‐up in our database.
Covariates
In the MPOG database, data are stored, validated, and extracted for quality improvement and research purposes.[ 16 , 17 ] From the combined data set, we curated 107 covariates that were grouped as (1) demographic, (2) procedural, (3) etiology of liver failure, (4) donor/graft‐specific factors, (5) preoperative laboratory studies, and (6) intraoperative data (Table S1). Intraoperative measures included vasopressor/inotropic support, mean arterial pressure (MAP), resuscitation with blood products and fluids, and laboratory studies. Intraoperative variables were also classified according to the following stages of transplantation to enable additional phase‐specific modeling: (1) dissection, (2) anhepatic phase, and (3) reperfusion phase. Laboratory and vital sign values were quantified as a time‐weighted average over the entire window, assuming the most recent result as current, until a new value is documented. Medication and transfusion values were calculated as total administration during the phase of interest. Recipient comorbidities were curated from a combination of diagnostic codes, standardized entry in the history and physical evaluation perfomed preoperatively by the anesthesia provider, and free‐text search for relevant terminology. Diagnostic codes were grouped according to a previously validated approach.[ 18 ] Examples include (1) preoperative anemia (defined as iron deficiency or folate and B12 deficiencies)[ 19 ] and (2) preoperative electrolyte/acid‐base balance disorders (defined as syndrome of inappropriate antidiuretic hormone secretion or various electrolyte and acid/base disorders).[ 20 ] (Electrolyte/acid‐base balance disorders are based on the Elixhauser Comorbidity Index, which is positive if the patient has International Classification of Diseases, Ninth Revision [ICD‐9]/International Classification of Diseases, Tenth Revision [ICD‐10] diagnosis codes for syndrome of inappropriate antidiuretic hormone secretion [ICD‐9 253.6x and ICD‐10 E22.2x] or various electrolyte and acid/base disorders [ICD‐9 276 and 276.x and ICD‐10 E86.x, E87.x, E88.x], where x is any digit.) In addition, we calculated time‐weighted averages of physiologic measures taken throughout the LT process. Finally, we adjusted for preoperative (baseline) estimated glomerular filtration rate (eGFR) calculated using the MDRD‐4.[ 21 ]
Statistical analysis
Exploratory data analysis techniques, such as histograms, QQ‐Plots, box‐plots, scatterplots and basic descriptive (means, medians, interquartile ranges) were used to assess the distribution of dependent measures. This allowed us to identify the distribution of outcomes which in turn facilitated appropriate modeling strategies. In addition, these techniques also were used to explore the most informative transformations of the covariates, confounders, and relevant predictors considered in the analysis. Extreme values were identified and their removal from the analysis was determined. Missing patterns and rates were assessed. Descriptive statistics were compiled on LT patients developing and not developing postoperative AKI.
Patients who had not received pretransplant dialysis were analyzed for development of AKI and subsequent mortality. Mortality was determined from the LT database. First, patients who developed AKI were compared with patients without AKI using Fisher's exact and chi‐square tests for categorical variables and an independent t test for continuous variables. Heavily skewed variables, such as transfusion quantities and norepinephrine doses, were log transformed for inclusion in the regressions. Missing data were imputed via the method of multiple imputation using fully conditional specification and predictive mean matching. To determine the independent associations with AKI, we used logistic regressions with forward selection using the likelihood ratio.
Mortality was analyzed using Cox proportional hazards models. First, the proportionality assumption was confirmed with Schoenfeld residuals. Then all variables were entered using forward selection. For both the logistic regression and Cox proportional hazards models, the multiple imputation models were combined with Rubin's rules. Variables with p < 0.05 and 95% confidence intervals (CIs) that excluded 1 were deemed statistically significant. No adjustment was made for multiple models. Discriminations of the logistic regression models were assessed with the concordance statistic (c‐statistic), which was calculated separately for each model, and the pooled results and standard errors were calculated after logistic transform using the method of DeBray. Pooled results were then back transformed for presentation.[ 22 ]
Power
Power analysis and sample size determination were done for 2 correlated proportions with a range between 10% and 20% dropout. Parameters used for this analysis were determined based on previous knowledge. We assumed the incidence of AKI to be 40% and that mild hypotension was associated with an increased adjusted odds ratio (aOR) of AKI of 1.34 (95% CI, 1.16–1.56). In addition, we assumed a 2‐sided test with α = 0.05 and an intracluster correlation of 0.5. Results from this analysis indicated that a sample ranging between 185 and 600 would provide 85% power to test our research questions. Power analysis was performed with PASS 2021 software (NCSS Statistical Software, Kaysville, UT, USA). In addition, simulation studies show that for different intraclass correlations structures a sample size ranging between 150 to 500 will provide power ranging between 80% and 90% to test the significance of parameters.[ 23 ] Although the study was powered to detect the significance of individual parameters, the study may be underpowered to find a difference in discrimination between the preoperative (models 1 and 2) and preoperative and intraoperative (models 3 and 4) models.
Models
Preoperative recipient‐specific model (Model 1)
We first created a model that assessed association between our primary outcome, AKI, and a variety of patient‐specific variables that would be known preoperatively (model 1). Variables included in each model can be found in Table S1.
Preoperative and donor‐specific model (Model 2)
The next model incorporated donor‐specific variables, including donor age, donor sex, donor cause of death, and graft ischemic time.
Phase‐specific model: Reperfusion (Model 3)
To test our hypothesis that variables specific to each phase of transplantation may have a previously undetected association that could further improve AKI prediction, we created a model using intraoperative data censored to the period following reperfusion. Additional variables in this model included transfusions and norepinephrine administration given during reperfusion and laboratory values, including lactate, potassium, and ionized calcium measured during reperfusion.
Model with data at case completion (Model 4)
Model 4 was composed of full data known at case completion, including case duration, total transfusion requirements, and cumulative dosage of norepinephrine.
Mortality (Models 5 and 6)
Model 5 comprised data known by the end of the operation plus AKI and receipt or not of dialysis. We also performed a nonpredetermined analysis to assess the relationship between the subset of patients with stage 3 AKI needing dialysis within 7 days following transplantation and mortality (model 6). All statistical analyses were performed in SPSS 27.0 (IBM, Armonk, NY).
Comparison of model discrimination
Model discrimination was quantified using the area under the receiver operator characteristic curve (c‐statistic). Comparison between the discrimination of preoperative models (models 1 and 2) and preoperative and intraoperative models (models 3 and 4) was done using the Hanley and McNeil method to calculate the z score for each of the values. We then combined the z scores with Rubin’s rules and calculated the 2‐tailed p value.[ 24 ]
RESULTS
Cohort characteristics and univariate associations
Of the 598 adult primary LT recipients included in our study, 43% (n = 255) were diagnosed with AKI within the first 7 postoperative days, and 149 (25%) had KDIGO stage 2 and 106 (18%) stage 4. Of the patients, 66.1% (n = 395) were men, and the median age at the time of transplant was 54 years (standard deviation [SD], 11 years). A total of 80.6% of the patients (n = 482) identified as White, and 7.5% (n = 45) identified as Black. Median body mass index (BMI) was 29.2 kg/m2 (SD, 6.1 kg/m2). Patients had a median baseline eGFR of 78.7 mL/min/1.73 m2 (SD, 41) and Model for End‐Stage Liver Disease (MELD) of 19 (SD, 8). In addition, 11% (n = 63) had preoperative anemia, 61% (n = 365) had a preoperative electrolyte/acid‐base balance disorder, and 35% (n = 208) had preexisting cardiac arrhythmia.
Patients subsequently developing AKI were more likely to have a higher BMI (30.3 kg/m2 [SD, 6.6 kg/m2] compared with 28.4 kg/m2 [SD, 5.5 kg/m2]; p < 0.001), preexisting anemia (14.9% vs. 7.3%; p = 0.003), cardiac arrhythmia (41.2% vs. 30.0%; p = 0.006), and fluid/electrolyte disorder (70.2% vs. 54.2%; p < 0.001). Somewhat surprisingly, patients developing AKI following LT also had a higher baseline eGFR (87.3 mL/min/1.73 m2 [SD, 42.9 mL/min/1.73 m2] compared with 72.4 mL/min/1.73 m2 [SD, 37.7 mL/min/1.73 m2]; p < 0.001) and lower MELD scores (18 [SD, 7] compared with 20 [SD, 9]; p < 0.001). Intraoperatively, patients developing AKI required larger volume transfusion with red cells (10.7 units [SD, 17.1 units] vs. 8.2 units [SD, 14.5 units]; p < 0.001) and plasma (13.7 units [SD, 17.6 units] vs. 11.0 units [SD, 13.6 units]; p < 0.001). Characteristics for our full cohort, as well as the univariate descriptive differences between the AKI and non‐AKI cohorts are presented in Tables 1, 2, 3.
TABLE 1.
Characteristics of LT patients developing stage 2 or 3 AKI: recipient and donor factors
| Variable | Level | All data (n = 598) | No KDIGO Stage 2 or 3 (n = 343) | KDIGO Stage 2 or 3 (n = 255) | p value | |||
|---|---|---|---|---|---|---|---|---|
| Mean, n | SD, Percentage | Mean, n | SD, Percentage | Mean, n | SD, Percentage | Chi‐square test, t test | ||
| Age, years | 54 | 11.0 | 54 | 11 | 52 | 11 | 0.06 | |
| BMI, kg/m2 | 29.2 | 6.1 | 28.4 | 5.5 | 30.3 | 6.6 | <0.001 | |
| Sex | Female | 203 | 33.9 | 117 | 34.1 | 86 | 33.7 | 0.92 |
| Male | 395 | 66.1 | 226 | 65.9 | 169 | 66.3 | ||
| Race | White/Caucasian (3) | 482 | 80.6 | 278 | 81.0 | 204 | 80.0 | 0.65 |
| Black (reference) | 45 | 7.5 | 25 | 7.3 | 20 | 7.8 | ||
| Other (Not White/Caucasian or Black) (1) | 19 | 3.2 | 13 | 3.8 | 6 | 2.4 | ||
| Unknown (2) | 52 | 8.7 | 27 | 7.9 | 25 | 9.8 | ||
| Liver failure etiology | Hepatitis B virus | 18 | 3.0 | 9 | 2.6 | 9 | 3.5 | 0.52 |
| Hepatitis C virus | 183 | 30.6 | 99 | 28.9 | 84 | 32.9 | 0.29 | |
| Hepatocellular carcinoma | 170 | 28.4 | 96 | 28.0 | 74 | 29.0 | 0.78 | |
| Nonalcoholic steatohepatitis | 84 | 14.0 | 43 | 12.5 | 41 | 16.1 | 0.22 | |
| Cryptogenic cirrhosis | 52 | 8.7 | 29 | 8.5 | 23 | 9.0 | 0.81 | |
| Primary sclerosing cholangitis | 62 | 10.4 | 36 | 10.5 | 26 | 10.2 | 0.91 | |
| Alpha‐1‐antitrypsin deficiency | 19 | 3.2 | 12 | 3.5 | 7 | 2.7 | 0.60 | |
| Fulminant liver failure | 25 | 4.2 | 19 | 5.5 | 6 | 2.4 | 0.05 | |
| Alcohol‐related cirrhosis | 127 | 21.2 | 74 | 21.6 | 53 | 20.8 | 0.82 | |
| Biliary Etiology | 33 | 5.5 | 22 | 6.4 | 11 | 4.3 | 0.27 | |
| Autoimmune hepatitis | 24 | 4.0 | 16 | 4.7 | 8 | 3.1 | 0.35 | |
| Donor factors | Age, years | 40 | 16.0 | 40 | 16 | 40 | 15 | 0.67 |
| Donor sex | Female | 226 | 37.8 | 132 | 38.5 | 94 | 36.9 | 0.53 |
| Male | 367 | 61.4 | 207 | 60.3 | 160 | 62.7 | ||
| CDC high‐risk donor | 91 | 15.2 | 47 | 13.7 | 44 | 17.3 | 0.23 | |
| DBD | 536 | 89.6 | 315 | 91.8 | 221 | 86.7 | 0.11 | |
| DCD | 40 | 6.7 | 19 | 5.5 | 21 | 8.2 | ||
| Graft ischemic times | Warm ischemia, minutes | 31 | 9.0 | 31 | 9 | 32 | 9 | 0.24 |
| Cold ischemia, minutes | 364 | 170.0 | 371 | 180 | 355 | 156 | 0.26 | |
| Total ischemia, minutes | 391 | 153.0 | 394 | 149 | 387 | 157 | 0.60 | |
| Donor CMV status | Positive | 345 | 57.7 | 195 | 56.9 | 150 | 58.8 | 0.35 |
| Negative | 249 | 41.6 | 147 | 42.9 | 102 | 40.0 | ||
| Unknown | 4 | 0.7 | 1 | 0.3 | 3 | 1.2 | ||
| Donor EBV status | Positive | 401 | 67.1 | 228 | 66.5 | 173 | 67.8 | 0.54 |
| Negative | 33 | 5.5 | 22 | 6.4 | 11 | 4.3 | ||
| Unknown | 164 | 27.4 | 93 | 27.1 | 71 | 27.8 | ||
| Donor cause of death | Anoxia | 124 | 20.7 | 71 | 20.7 | 53 | 20.8 | 0.20 |
| Asphyxiation | 19 | 3.2 | 12 | 3.5 | 7 | 2.7 | ||
| Blunt injury | 2 | 0.3 | 1 | 0.3 | 1 | 0.4 | ||
| Cardiovascular | 23 | 3.8 | 15 | 4.4 | 8 | 3.1 | ||
| Cerebrovascular | 174 | 29.1 | 108 | 31.5 | 66 | 25.9 | ||
| Central nervous system tumor | 1 | 0.2 | 1 | 0.3 | 0 | 0.0 | ||
| Drowning | 3 | 0.5 | 0 | 0.0 | 3 | 1.2 | ||
| Drug intoxication | 25 | 4.2 | 12 | 3.5 | 13 | 5.1 | ||
| Electrical | 1 | 0.2 | 0 | 0.0 | 1 | 0.4 | ||
| Gunshot/stab | 47 | 7.9 | 29 | 8.5 | 18 | 7.1 | ||
| Intracranial hemorrhage/stroke | 34 | 5.7 | 16 | 4.7 | 18 | 7.1 | ||
| Motor vehicle accident | 30 | 5.0 | 16 | 4.7 | 14 | 5.5 | ||
| Other (Not White/Caucasian or Black) | 9 | 1.5 | 5 | 1.5 | 4 | 1.6 | ||
| Seizure | 1 | 0.2 | 0 | 0.0 | 1 | 0.4 | ||
| Donor cause of death (categorical) | Trauma | 164 | 27.4 | 97 | 28.3 | 67 | 26.3 | 0.80 |
| Anoxia/asphyxiation | 143 | 23.9 | 83 | 24.2 | 60 | 23.5 | 0.94 | |
| Stroke | 208 | 34.8 | 124 | 36.2 | 84 | 32.9 | 0.63 | |
| Drug intoxication | 25 | 4.2 | 12 | 3.5 | 13 | 5.1 | 0.29 | |
| Cardiovascular | 23 | 3.8 | 15 | 4.4 | 8 | 3.1 | 0.49 | |
| Anemia (iron deficiency) | 63 | 10.5 | 25 | 7.3 | 38 | 14.9 | 0.00 | |
| Cardiac arrhythmia | 208 | 34.8 | 103 | 30.0 | 105 | 41.2 | 0.01 | |
| Valvular diseases of the heart | 46 | 7.7 | 24 | 7.0 | 22 | 8.6 | 0.48 | |
| Chronic obstructive pulmonary disease | 93 | 15.6 | 58 | 16.9 | 35 | 13.7 | 0.27 | |
| Fluid and electrolyte disorders | 365 | 61.0 | 186 | 54.2 | 179 | 70.2 | <0.001 | |
| Diabetes mellitus (recipient) | None | 379 | 63.4 | 215 | 62.7 | 164 | 64.3 | 0.96 |
| Uncomplicated | 175 | 29.3 | 101 | 29.4 | 74 | 29.0 | ||
| Complicated | 29 | 4.8 | 17 | 5.0 | 12 | 4.7 | ||
| Missing/unknown | 15 | 2.5 | 10 | 2.9 | 5 | 2.0 | ||
| Hypertension (recipient) | None | 304 | 50.8 | 179 | 52.2 | 125 | 49.0 | 0.63 |
| Uncomplicated | 195 | 32.6 | 109 | 31.8 | 86 | 33.7 | ||
| Complicated | 84 | 14.0 | 45 | 13.1 | 39 | 15.3 | ||
| Missing/unknown | 15 | 2.5 | 10 | 2.9 | 5 | 2.0 | ||
| Hypothyroidism | 87 | 14.5 | 50 | 14.6 | 37 | 14.5 | ||
| Neurologic disorders | 73 | 12.2 | 38 | 11.1 | 35 | 13.7 | 0.35 | |
| Peripheral vascular disorders | 37 | 6.2 | 16 | 4.7 | 21 | 8.2 | 0.08 | |
| Pulmonary circulation disorders | 39 | 6.5 | 18 | 5.2 | 21 | 8.2 | 0.15 | |
| Unexpected or unanticipated weight loss | 132 | 22.1 | 63 | 18.4 | 69 | 27.1 | 0.01 | |
| Other comorbidities | Cerebrovascular disease | 6 | 1.0 | 3 | 0.9 | 3 | 1.2 | 0.71 |
| Ischemic heart disease | 19 | 3.2 | 13 | 3.8 | 6 | 2.4 | 0.32 | |
| Snoring | 184 | 30.8 | 97 | 28.3 | 87 | 34.1 | 0.10 | |
| Recipient CMV status | Positive | 332 | 55.5 | 200 | 58.3 | 132 | 51.8 | 0.12 |
| Negative | 259 | 43.3 | 137 | 39.9 | 122 | 47.8 | ||
| Equivocal | 5 | 0.8 | 4 | 1.2 | 1 | 0.4 | ||
| Recipient EBV status | Positive | 556 | 93.0 | 317 | 92.4 | 239 | 93.7 | 0.15 |
| Negative | 26 | 4.3 | 15 | 4.4 | 11 | 4.3 | ||
| Equivocal | 2 | 0.3 | 0 | 0.0 | 1 | 0.4 | ||
| Outcomes | 30‐day mortality | 23 | 3.8 | 14 | 4.1 | 9 | 3.5 | 0.73 |
| 90‐day mortality | 31 | 5.2 | 16 | 4.7 | 15 | 5.9 | 0.51 | |
| 1‐year mortality | 54 | 9.0 | 30 | 8.7 | 24 | 9.4 | 0.78 | |
| 3‐year mortality | 78 | 13.0 | 35 | 10.2 | 43 | 16.9 | 0.02 | |
TABLE 2.
Characteristics of LT patients developing Stage 2 or 3 AKI: Baseline laboratory values
| Variable | Level | All data (n = 598) | No KDIGO Stage 2 or 3 (n = 343) | KDIGO Stage 2 or 3 (n = 255) | p value | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t test | ||
| Baseline | Serum creatinine (mg/dL) | 1.2 | 0.7 | 1.3 | 0.7 | 1.0 | 0.6 | <0.001 |
| eGFR, mL/minute/1.37 m2 | 78.7 | 40.6 | 72.4 | 37.7 | 87.3 | 42.9 | <0.001 | |
| Bilirubin (mg/dL) | 8.1 | 9.1 | 8.6 | 9.5 | 7.3 | 8.3 | 0.09 | |
| BUN (mg/dL) | 22.0 | 15.0 | 24.7 | 17.6 | 18.4 | 11.1 | <0.001 | |
| White blood cell count (K/uL) | 6.1 | 3.3 | 6.2 | 3.5 | 5.9 | 3.0 | 0.33 | |
| Hematocrit (%) | 31.4 | 6.2 | 31.3 | 6.3 | 31.6 | 6.1 | 0.64 | |
| Platelets (%) | 97.8 | 71.2 | 102 | 75 | 92 | 65 | 0.07 | |
| Sodium | 136 | 5.0 | 136 | 5 | 135 | 5 | 0.10 | |
| Albumin (g/dL) | 3 | 0.6 | 3.1 | 0.6 | 2.9 | 0.6 | <0.001 | |
| Fibrinogen (mg/dL) | 222 | 131 | 224 | 130 | 220 | 132 | 0.73 | |
| INR | 1.6 | 0 | 1.7 | 0.7 | 1.6 | 0.5 | 0.05 | |
| MELD score | 19 | 8 | 20 | 9 | 18 | 7 | 0.01 | |
| MELD (laboratory) | 19 | 8 | 20 | 9 | 18 | 7 | 0.01 | |
| MELD (with exception points added) | 23 | 6 | 24 | 6 | 22 | 6 | <0.001 | |
| MAP (prior to reperfusion), mm Hg | 73 | 11 | 72 | 11 | 73 | 11 | 0.44 | |
TABLE 3.
Characteristics of LT patients developing Stage 2 or 3 AKI: Intraoperative details (by phase of transplantation)
| Variable | Level | All data (n = 598) | No KDIGO Stage 2 or 3 (n = 343) | KDIGO Stage 2 or 3 (n = 255) | p value | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t test | ||
| Intraoperative data | Transfusion pRBC, units | 2 | 3 | 2 | 3 | 2 | 3 | 1.00 |
| Dissection phase | Transfusion FFP, units | 3 | 4 | 3 | 4 | 3 | 5 | 0.80 |
| Transfusion cryoprecipitate (5‐packs) | 0 | 2 | 0 | 2 | 0 | 1 | 0.86 | |
| Hematocrit (%) | 28.9 | 5.7 | 28.9 | 5.9 | 28.8 | 5.4 | 0.84 | |
| Glucose (mg/dL) | 119 | 34 | 119 | 30 | 120 | 39 | 0.80 | |
| Lactate (mmol/L) | 2.1 | 1.2 | 2.1 | 1.2 | 2.1 | 1.2 | 0.85 | |
| pH | 7.4 | 0.1 | 7.4 | 0.1 | 7.4 | 0.1 | 0.61 | |
| Ionized calcium (mmol/L) | 1.16 | 0.80 | 1.22 | 1.03 | 1.06 | 0.12 | 0.04 | |
| Sodium (mmol/L) | 136 | 5 | 136 | 5 | 136 | 5 | 0.27 | |
| Potassium (mmol/L) | 3.9 | 0.5 | 3.9 | 0.5 | 3.9 | 0.5 | 0.83 | |
| Norepinephrine, μg | 141 | 386 | 135 | 394 | 148 | 376 | 0.70 | |
| Anhepatic | Transfusion pRBC, units | 2 | 5 | 2 | 5 | 2 | 4 | 0.93 |
| Transfusion FFP, units | 3 | 4 | 2 | 5 | 3 | 4 | 0.75 | |
| Transfusion cryoprecipitate (5‐packs) | 0 | 1 | 0 | 1 | 0 | 0 | 0.49 | |
| Hematocrit | 26.4 | 5.0 | 26.4 | 5.5 | 26.3 | 4.7 | 0.69 | |
| Glucose | 145 | 46 | 146 | 45 | 144 | 47 | 0.50 | |
| Lactate | 4.1 | 1.9 | 4.1 | 1.9 | 4.2 | 1.9 | 0.54 | |
| pH | 7.35 | 0.10 | 7.35 | 0.12 | 7.36 | 0.06 | 0.45 | |
| Ionized calcium | 1.08 | 0.19 | 1.08 | 0.19 | 1.09 | 0.20 | 0.55 | |
| Sodium | 137 | 6 | 137 | 5 | 136 | 7 | 0.17 | |
| Potassium | 4.0 | 0.6 | 4.0 | 0.7 | 3.9 | 0.6 | 0.21 | |
| Norepinephrine, μg | 104 | 241 | 99 | 241 | 110 | 241 | 0.57 | |
| Immediate reperfusion | Norepinephrine, μg | 6 | 14 | 6 | 14 | 6 | 14 | 0.74 |
| MAP, mm Hg | 55 | 14 | 55 | 14 | 56 | 14 | 0.45 | |
| Reperfusion | Transfusion pRBC, units | 5 | 10 | 4 | 9 | 6 | 12 | 0.02 |
| Transfusion FFP, units | 6 | 10 | 5 | 8 | 7 | 12 | 0.01 | |
| Transfusion cryoprecipitate (5‐packs) | 2 | 5 | 2 | 4 | 3 | 5 | 0.05 | |
| Hematocrit | 23.4 | 4.9 | 23.8 | 5.7 | 23.0 | 3.6 | 0.05 | |
| Glucose | 204 | 54 | 201 | 54 | 208 | 53 | 0.13 | |
| Lactate | 5.1 | 2.5 | 4.9 | 2.4 | 5.4 | 2.5 | 0.01 | |
| pH | 7.34 | 0.06 | 7.34 | 0.07 | 7.34 | 0.06 | 0.91 | |
| Ionized calcium | 1.88 | 2.41 | 1.87 | 2.27 | 1.89 | 2.59 | 0.94 | |
| Sodium | 138 | 5 | 138 | 5 | 138 | 5 | 0.95 | |
| Potassium | 3.8 | 0.6 | 3.8 | 0.6 | 3.9 | 0.6 | 0.02 | |
| Norepinephrine, μg | 623 | 1227 | 514 | 1157 | 770 | 1303 | 0.01 | |
| Total intraoperative results | Transfusion pRBC, units | 9 | 16 | 8 | 14 | 10 | 17 | <0.001 |
| Transfusion FFP, units | 12 | 16 | 11 | 14 | 14 | 18 | <0.001 | |
| Hematocrit | 26.5 | 4.3 | 26.6 | 4.5 | 26.3 | 4.0 | 0.33 | |
| Glucose | 156 | 38 | 154 | 37 | 158 | 40 | 0.13 | |
| Lactate | 3.5 | 1.7 | 3.4 | 1.7 | 3.6 | 1.7 | 0.04 | |
| pH | 7.36 | 0.06 | 7.36 | 0.06 | 7.36 | 0.07 | 0.90 | |
| Ionized calcium | 1.79 | 2.43 | 1.80 | 2.31 | 1.78 | 2.60 | 0.95 | |
| Sodium | 137 | 5 | 137 | 5 | 137 | 5 | 0.92 | |
| Potassium | 3.9 | 0.5 | 3.9 | 0.5 | 3.9 | 0.5 | 0.41 | |
We found that 3.8% (n = 23) patients died within 30 days of transplant, 9.0% (n = 54) died within 1 year, and 13.0% (n = 78) died within 3 years. Additional details can be found in Table 4.
TABLE 4.
Patient outcomes after LT (n = 598)
| Stage | n | Percentage | Stage | Total | Percentage |
|---|---|---|---|---|---|
| (A) Outcome: KDIGO | |||||
| 0 | 227 | 38.0 | 0 or 1 | 343 | 57.4 |
| 1 | 116 | 19.4 | |||
| 2 | 149 | 24.9 | 2 or 3 | 255 | 42.6 |
| 3 | 106 | 17.7 | |||
| Total | Percentage | AKI (Stage 2 or 3) | |||||
|---|---|---|---|---|---|---|---|
| Yes (n = 255) | Percentage | No (n = 343) | Percentage | p value (chi‐square test) | |||
| (B) Outcome: mortality | |||||||
| 30 days | 23 | 3.8 | 9 | 3.5 | 14 | 4.1 | 0.12 |
| 90 days | 31 | 5.2 | 15 | 5.9 | 16 | 4.7 | 0.44 |
| 1 year | 54 | 9.0 | 24 | 9.4 | 30 | 8.7 | 0.08 |
| 3 years | 78 | 13.0 | 43 | 16.9 | 35 | 10.2 | 0.02 |
Model composed of recipient‐specific, preoperative variables (Model 1)
In an adjusted multivariate logistic model, several preoperative variables, including (1) BMI (aOR, 1.077; 95% CI, 1.044–1.112; p < 0.001), (2) electrolyte/acid‐base balance disorder (aOR, 2.040; 95% CI, 1.374–3.030; p < 0.001), (3) preoperative anemia (aOR, 2.985; 95% CI, 1.623–5.489; p < 0.001), and (4) cardiac arrhythmia (aOR, 1.818; 95% CI, 1.240–2.666; p = 0.002) were associated with post‐LT AKI. (Cardiac arrhythmias are based on the Elixhauser Comorbidity Index, which is positive if the patient has ICD‐9/ICD‐10 diagnosis codes for mechanical complication of pacemaker/defibrillator [ICD‐9 996.01, 996.04; ICD‐10 T82.1x], atrioventricular block and various dysrhythmias [ICD‐9 426.0x, 426.7x, 426.9x, 426.1, 426.0, 426.2, 426.3, 427.x except 427.5; ICD‐10: I441.x, I442.x, I443.x, I45.6x, I45.9x, I47, I48, I49, I47.x, I48.x, I49.x], tachycardia, bradycardia unspecified [ICD‐9 785.0x; ICD‐10: R00.0x, R00.1x, R00.8x]; or defibrillator, pacemaker, cardiac device [ICD‐9 V45.0x, V53.3x; ICD‐10: Z45.0x, Z95.0x], where x is any digit.)
In addition, the preoperative laboratory data, including (1) lower serum albumin (aOR, 0.611; 95% CI, 0.431–0.865; p = 0.01), (2) lower blood urea nitrogen (BUN; aOR, 0.963; 95% CI, 0.944–0.982; p < 0.001), (3) lower international normalized ratio (INR; aOR, 0.606; 95% CI, 0.420–0.875; p = 0.01), and (4) higher eGFR (aOR, 1.007; 95% CI, 1.001–1.013; p = 0.03), were also associated with AKI. Full results of the multivariate logistic regression are provided in Table 5. Model 1 had good discrimination (c‐statistic, 0.741 ± 0.026). (The following is the interpretation of the c‐statistic: 0.5 or less for a poor model, more than 0.7 for a good model, more than 0.8 for a strong model, and 1.0 for a perfect model.)
TABLE 5.
Multivariate logistic regression results in Models 1 to 4
| aOR | 95% CI | p value | ||
|---|---|---|---|---|
| Model 1 | ||||
| Patient demographics | BMI | 1.077 | 1.044–1.112 | <0.001 |
| Comorbidities | Fluid or electrolyte disorders | 2.040 | 1.374–3.030 | <0.001 |
| Iron‐deficiency anemia | 2.985 | 1.623–5.489 | <0.001 | |
| Cardiac arrhythmia | 1.818 | 1.240–2.666 | 0.00 | |
| Preoperative laboratory values | Albumin | 0.611 | 0.431–0.865 | 0.01 |
| Sodium | 0.965 | 0.928–1.003 | 0.07 | |
| BUN | 0.963 | 0.944–0.982 | <0.001 | |
| INR | 0.606 | 0.420–0.875 | 0.01 | |
| eGFR, MDRD‐4 | 1.007 | 1.001–1.013 | 0.03 | |
| Model 2 | ||||
| Patient demographics | BMI | 1.077 | 1.044–1.112 | <0.001 |
| Comorbidities | Fluid or electrolyte disorders | 2.040 | 1.374–3.030 | <0.001 |
| Iron‐deficiency anemia | 2.985 | 1.623–5.489 | <0.001 | |
| Cardiac arrhythmia | 1.818 | 1.240–2.666 | 0.00 | |
| Preoperative laboratory values | Albumin | 0.611 | 0.431–0.865 | 0.01 |
| Sodium | 0.965 | 0.928–1.003 | 0.07 | |
| BUN | 0.963 | 0.944–0.982 | <0.001 | |
| INR | 0.606 | 0.420–0.875 | 0.01 | |
| eGFR, MDRD‐4 | 1.007 | 1.001–1.013 | 0.03 | |
| Model 3 | ||||
| Patient demographics | Age | 0.979 | 0.962–0.995 | 0.01 |
| BMI | 1.079 | 1.044–1.114 | <0.001 | |
| Comorbidities | Fluid or electrolyte disorders | 1.917 | 1.280–2.869 | 0.00 |
| Iron‐deficiency anemia | 2.612 | 1.405–4.854 | 0.00 | |
| Cardiac arrhythmia | 1.629 | 1.101–2.410 | 0.02 | |
| Unexpected weight loss | 1.596 | 1.006–2.532 | 0.05 | |
| Preoperative laboratory values | Albumin | 0.576 | 0.410–0.808 | 0.00 |
| BUN | 0.952 | 0.935–0.969 | <0.001 | |
| INR | 0.469 | 0.321–0.685 | <0.001 | |
| eGFR, MDRD‐4 | 1.007 | 1.001–1.013 | 0.03 | |
| Reperfusion variables | Reperfusion potassium | 1.513 | 1.103–2.077 | 0.01 |
| Reperfusion lactate | 1.081 | 1.003–1.166 | 0.04 | |
| Model 4 | ||||
| Patient demographics | Age | 0.979 | 0.962–0.995 | 0.01 |
| BMI | 1.079 | 1.044–1.114 | <0.001 | |
| Comorbidities | Fluid or electrolyte disorders | 1.917 | 1.280–2.869 | 0.00 |
| Iron‐deficiency anemia | 2.612 | 1.405–4.854 | 0.00 | |
| Cardiac arrhythmia | 1.629 | 1.101–2.410 | 0.15 | |
| Unexpected weight loss | 1.596 | 1.006–2.532 | 0.05 | |
| Preoperative laboratory values | Albumin | 0.576 | 0.410–0.808 | 0.00 |
| BUN | 0.952 | 0.935–0.969 | <0.001 | |
| INR | 0.469 | 0.321–0.685 | <0.001 | |
| eGFR, MDRD‐4 | 1.007 | 1.001–1.013 | 0.03 | |
| Reperfusion variables | Reperfusion potassium, mmol/L | 1.513 | 1.103–2.077 | 0.01 |
| Reperfusion lactate, mmol/L | 1.081 | 1.003–1.166 | 0.04 |
Model 1, preoperative variables: AKI after LT (c‐statistic = 0.741 ± 0.026); model 2, preoperative variables plus donor factors (c‐statistic = 0.741 ± 0.026); model 3, preoperative variables, donor factors, and intraoperative variables: reperfusion (c‐statistic = 0.759 ± 0.032); model 4, complete end of case data (c‐statistic = 0.759 ± 0.032).
Model composed of preoperative recipient and donor variables (Model 2)
We incorporated additional donor‐specific factors, including (1) donor age, (2) donor sex, (3) Center for Disease Control and Prevention (CDC) high‐risk donor, (4) donation after circulatory death (DCD)/donation after brain death (DBD), (5) graft ischemia times (warm, cold, total), (6) donor cytomegalovirus (CMV)/Epstein‐Barr virus (EBV) status, and (7) donor cause of death into the original model. Notably, none of these additional variables were selected for inclusion with Model 2 (Table 5), and the discrimination remained unchanged (c‐statistic, 0.741 ± 0.026). A calibration plot for model 2 showing the proportion of patients with AKI for each quintile of risk is shown in Figure S1A.
Phase‐specific modeling—Reperfusion phase (Model 3)
Next we created a model that examined the contribution from individual phases of LT. Based on univariate analysis (Table 4), we selected reperfusion phase as the phase of transplantation that provided the most informative, phase‐specific data. Model 3 includes laboratory studies, MAP, and norepinephrine administration from the reperfusion phase. Notably, reperfusion potassium (aOR, 1.513; 95% CI, 1.103–2.077; p = 0.01) and reperfusion lactate (aOR, 1.081; 95% CI, 1.003–1.166; p = 0.04) were the only reperfusion phase‐specific variables included in model 3. In addition, lower age (aOR, 0.979; 95% CI, 0.962–0.995; p = 0.01) and unexpected preoperative weight loss (aOR, 1.596; 95% CI, 1.006–2.532; p = 0.05) now became significant. Model discrimination increased to 0.759 ± 0.032. A calibration plot for model 3 showing the proportion of patients with AKI for each quintile of risk is shown in Figure S1B.
Full data, case completion model (Model 4)
Model 4 included the full available data at case completion. This included data from all 3 phases of transplantation as well as cumulative case totals (eg, norepinephrine dose during dissection, norepinephrine dose during anhepatic phase, norepinephrine dose during reperfusion, and total norepinephrine administered intraoperatively). For laboratory studies, time‐weighted averages (using preoperative values from case initiation until the first intraoperative value was obtained) were calculated for each phase of transplantation and again for the total case duration. Notably, the addition of full case data did not alter the model selected or improve discrimination when compared with the model comprised entirely of preoperative and reperfusion phase data (Table 5).
Comparison of model discrimination
There was no difference in discrimination between the 2 preoperative models (model 1, recipient‐specific; and model 2, recipient and donor characteristics; c‐statistic, 0.741 ± 0.026). Furthermore, there was no statistically significant difference in discrimination between the 2 preoperative and intraoperative models (model 3, reperfusion phase; and model 4, full data, case completion; c‐statistic, 0.759 ± 0.032). The addition of intraoperative details also did not significantly improve model discrimination (comparing the c‐statistic of models 2 and 3 using the method of Hanley and McNeil[ 24 ]; p = 0.10).
Association with mortality
Next, we determined the independent associations between factors and mortality using Cox proportional hazards models. The first mortality model (model 5) included our primary outcome (KDIGO stage 2 or 3 AKI) as a covariate in addition to other factors specified previously. In this model, we found patients with hepatocellular carcinoma (adjusted hazard ratio [aHR], 1.611; 95% CI, 1.075–2.414; p = 0.02), longer warm ischemia time in minutes (aHR, 1.025; 95% CI, 1.006–1.045; p = 0.01), logarithm of total fresh frozen plasma (FFP) administered (aHR, 3.412; 95% CI, 1.959–5.942; p < 0.001), lower reperfusion ionized calcium (aHR, 0.835; 95% CI, 0.707–0.987; p = 0.04), and more acidic reperfusion pH (per 0.10 point change; aHR, 0.469; 95% CI, 0.367–0.601; p < 0.001) had a greater hazard for mortality. Notably, posttransplant AKI was not associated with mortality (Table 6).
TABLE 6.
Cox proportional hazards ratio models associated with mortality in Models 5 and 6
| aHR | 95% CI | p value | ||
|---|---|---|---|---|
| Model 5 | ||||
| Patient demographics | Black/African American | Reference | ||
| Race | White | 1.010 | 0.498–2.045 | 0.98 |
| Unknown | 3.058 | 1.314–7.117 | 0.01 | |
| Other | 3.388 | 1.159–9.902 | 0.03 | |
| Comorbidities | Pulmonary/circulation disorders | 1.731 | 0.863–3.472 | 0.12 |
| Hepatocellular carcinoma | 1.611 | 1.075–2.414 | 0.02 | |
| Preoperative laboratory values | Platelets | 1.005 | 1.003–1.007 | <0.001 |
| eGFR, MDRD‐4 | 1.008 | 1.003–1.013 | 0.00 | |
| Donor factors | CMV positive | 1.431 | 0.964–2.124 | 0.08 |
| EBV positive | 0.702 | 0.352–1.399 | 0.29 | |
| Warm ischemia time, minutes | 1.025 | 1.006–1.045 | 0.01 | |
| Intraoperative details | log(cryoprecipitate total) | 2.345 | 0.865–6.359 | 0.09 |
| log(FFP total) | 3.412 | 1.959–5.942 | <0.001 | |
| Reperfusion ionized calcium | 0.835 | 0.707–0.987 | 0.04 | |
| Reperfusion pH (scale 10) | 0.469 | 0.367–0.601 | <0.001 | |
| Outcomes | KDIGO stage 2 or 3 | 1.202 | 0.818–1.767 | 0.35 |
| Model 6 | ||||
| Patient demographics | Black/African American | Reference | ||
| Race | White | 0.926 | 0.455–1.884 | 0.83 |
| Unknown | 2.962 | 1.286–6.822 | 0.01 | |
| Other | 3.138 | 1.066–9.232 | 0.04 | |
| Comorbidities | Pulmonary/circulation disorders | 1.453 | 0.714–2.957 | 0.30 |
| Hepatocellular carcinoma | 1.72 | 1.138–2.599 | 0.01 | |
| Preoperative laboratory values | Platelets | 1.005 | 1.003–1.007 | <0.001 |
| eGFR, MDRD‐4 | 1.009 | 1.004–1.014 | <0.001 | |
| Donor factors | CMV positive | 1.523 | 1.021–2.271 | 0.04 |
| EBV positive | 0.758 | 0.399–1.44 | 0.38 | |
| Warm ischemia time, minutes | 1.025 | 1.005–1.045 | 0.02 | |
| Intraoperative details | log(cryoprecipitate total) | 2.115 | 0.742–6.024 | 0.16 |
| log(FFP total) | 3.152 | 1.801–5.517 | <0.001 | |
| Reperfusion ionized calcium | 0.830 | 0.696–0.991 | 0.04 | |
| Reperfusion pH (scale 10) | 0.499 | 0.385–0.647 | <0.001 | |
| Outcomes | Dialysis by day 7 | 3.324 | 1.603–6.894 | 0.001 |
In model 5, the primary outcome KDIGO 2 or 3 is included. In model 6, RRT is included.
In model 6, receipt of dialysis was a strong independent predictor of mortality (aOR, 3.324; 95% CI, 1.603–6.894; p = 0.001; Table 6). A Kaplan‐Meier curve showing survival in the population requiring dialysis compared with not requiring dialysis is shown in Figure 1.
FIGURE 1.
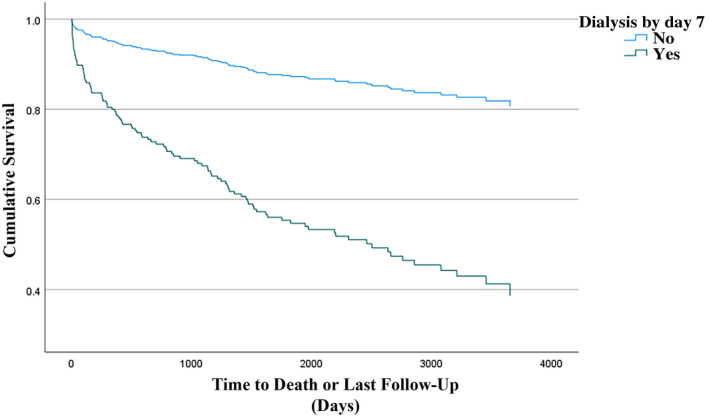
Kaplan‐Meier curve estimate of mortality as a function of time for patients requiring dialysis within 7 days after transplantation
DISCUSSION
Using robust, validated observational databases, we report an overall AKI incidence of 43% within the first 7 days following LT. We identify several demographics and comorbidities including BMI, preexisting electrolyte/acid‐base balance disorder, anemia, and cardiac arrhythmias that are associated with the primary outcome. In addition, preoperative studies including serum albumin, BUN, and INR informed the risk of AKI.
Our study builds on prior studies to integrate data from the electronic health record and intraoperative record with standard, reportable transplantation outcomes. We leveraged this capability to study associations through the intraoperative course. Our results show that increased potassium and lactate following graft reperfusion improve the prediction of patients susceptible to postoperative AKI. Furthermore, the addition of full case data does not improve model discrimination.
Concordance with previous studies
Our observed incidence of 43% is consistent with previous studies when accounting for differences in defining AKI (≥KDIGO stage 1 vs. ≥stage 2) and more stringent exclusion of patients with baseline renal dysfunction.[ 25 , 26 , 27 ] Although it is known that men are more at risk for end‐stage liver disease, there is not an agreed consensus of the impact sex has on developing AKI requiring RRT.[ 28 , 29 ] We found a consistent relationship between larger BMI and AKI, which agrees with previous studies.[ 25 , 30 , 31 ] We found no relationship between female sex and AKI, which agrees with some studies[ 32 , 33 ] but contrasts with another study placing female patients at increased risk.[ 25 ] The influence of preoperative risk factors such as anemia and electrolyte/acid‐base balance disorders have been found in previous studies.[ 7 , 9 , 34 ]
Our finding that preoperative hypoalbuminemia is associated with postoperative AKI agrees with previous findings in the LT population[ 35 ] as well as other surgical populations.[ 36 , 37 , 38 , 39 ] Other preoperative variables, including hyponatremia[ 40 ] and etiology of liver failure,[ 25 ] have been shown to influence postoperative AKI; however, these were not found to be significant in our study. This could be secondary to differences in patient population or, potentially, results from a redundancy in preoperative variables, leading to the selection of some variables and nonselection of others by our methodology. Specifically, our methodology selected preoperative diagnosis of electrolyte/acid‐base balance disorders as a significant risk factor, which also includes the ICD‐10 code for hyponatremia (E87.1), suggesting that it is electrolyte disorders in general not just 1 type (hyponatremia) that is associated with AKI.
Although MELD score has been previously shown to predict AKI after LT,[ 9 , 32 , 33 ] other studies have failed to replicate this association.[ 25 , 41 ] Our study did not find any association between MELD score and AKI. An additional surprising finding was that patients with a higher baseline eGFR have a higher susceptibility for posttransplant AKI. This conflicts with prior studies showing higher serum creatinine (lower eGFR) to be associated with posttransplant AKI.[ 2 , 42 , 43 , 44 ] This interesting result could result from (1) covariates that were unable to be quantified in our data (such as graft mismatch), (2) inefficiencies associated with eGFR equation accuracy in patients with cirrhosis, (3) unaccounted operative factors such as procedural or technical complexity (eg, with vascular clamping for anastomosis), or (3) physiologic etiology (BUN is also lower in the AKI, perhaps secondary to dilutional effect from ascites).[ 45 ] Finally, Black patients were not more likely to develop AKI following LT, which builds on an earlier study showing no difference in early hemodialysis based on race, but lower discontinuation of dialysis in Black transplant patients.[ 46 ]
A variety of donor factors have been shown to be associated with posttransplant AKI, including donation after cardiac death,[ 47 ] ischemia time,[ 48 , 49 ] high‐risk grafts,[ 32 ] and older donor age.[ 32 ] Our study failed to show any association between donor‐specific variables and posttransplant AKI. This may be the result of improved institutional effort in matching high‐risk donors with recipients at lower risk for complications such as AKI and lower risk donors with higher risk recipients.
Our study builds on prior work incorporating intraoperative variables into the prediction model. Prior studies have characterized the effect of major classes of intraoperative variables, including blood transfusion,[ 25 , 49 ] hemodynamic variables (most notably, intraoperative hypotension),[ 25 , 50 , 51 , 52 ] vasopressor/inotropic support,[ 25 , 51 , 52 ] surgical technique,[ 25 ] laboratory values,[ 12 , 25 , 52 ] and hypovolemia.[ 26 , 30 ] Our study failed to show an association between intraoperative MAP or intraoperative transfusion (packed red blood cells [pRBC], FFP, or cryoprecipitate) with the primary outcome. We did, however, find an association between higher lactate and potassium levels, but not norepinephrine doses and AKI. Further study is needed to determine if potassium and lactate levels may act as intermediate variables, mediating the effects of blood transfusions and blood pressure. Further study is also needed to determine if factors such as hypotension, which seems to fluctuate earlier, may be an earlier marker than potassium and lactate, which would be lagging indicators. The kidney excretes potassium and metabolizes lactate. Rises in potassium and lactate may be an early marker of renal dysfunction and ischemia. This might allow for an early intervention that reverses renal ischemia and prevents dysfunction from progressing to AKI. Further study is needed to better understand the possible protective association of INR with AKI. One potential explanation is that correction of INR leads to resuscitation with a high volume of FFP, which may lead to a better volume reserve that protects against the decreased organ perfusion associated with large hemorrhages.
Posttransplant AKI was not an independent risk for mortality. This contrasts with prior studies showing a strong association.[ 27 , 48 ] This could be because the study was powered to detect the secondary outcome of mortality, which occurred with much lower frequency than the primary outcome. However, as we found that receipt of dialysis within 7 days after transplant was associated with late mortality, it may be that AKI with recovery of renal function has little effect on mortality, whereas AKI that persists is associated with mortality. This is similar to a study in cardiac surgery patients where AKI, defined by KDIGO, was not associated with late mortality when discharge renal function was included in the model[ 53 ] or, as the subanalysis in patients requiring dialysis within 7 days suggests, that the effect may be driven by a smaller subset of all posttransplant patients with AKI.
Strengths and weaknesses
The limitations of our study include the retrospective design from a single center that limits the causality. In addition, this cross‐sectional study only allows us to look at associations at isolated time points, limiting how much we can truly infer from the results. However, given the extreme strength of associations, stage‐specific prediction models based on these findings would elucidate specific intraoperative changes needed to prevent AKIs. This risk‐prediction model could be trained with multicenter data, allowing it to be widely applicable and implemented into current anesthesia management best practices. Although we had accurate and complete urine output during the intraoperative period, we did not have urine output consistently documented for 7 postoperative days. Therefore, our primary outcome did not include urine output in the diagnostic criteria despite inclusion in the KDIGO definition. Another limitation is that surgical technique (classical bicaval anastomosis vs. piggyback) was not collected as part of our standardized reporting metrics. Future studies will control for this covariate through the language processing of operative reports. A final limitation is that our objective was to stratify the risk for postoperative AKI at the time of transplantation completion. Model discrimination might be improved by incorporating postoperative data, specifically administration of nephrotoxic immunosuppressants,[ 54 ] subsequent development of sepsis,[ 41 ] and early allograft dysfunction[ 55 ]; however, this was beyond the scope of our objective.
Conclusions
In this retrospective observational study performed at an academic quaternary care center, we found a 43% incidence of AKI within the first 7 days following LT and receipt of dialysis within 7 days of LT was associated with increased mortality. Our study demonstrated that most AKI discrimination (0.741 ± 0.026 compared with 0.759 ± 0.032 for full model) can be obtained with preoperative factors. The addition of intraoperative data did not improve overall model discrimination, although the study may have been underpowered to detect this change. Even if adequately powered, the overall improvement in discrimination by adding intraoperative data is minimal. This may suggest that by the time the intraoperative data are collected, renal injury has already mostly occurred. These high‐risk patients would be ideal for prospective studies to prevent AKI. In addition, intraoperative intermediate outcomes of potassium and lactate levels, potentially indicative of early renal ischemia or dysfunction, contribute a small component of overall AKI risk. Future studies on intraoperative and postoperative management may assess whether early intervention can mitigate this risk.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Fig S1
Table S1
ACKNOWLEDGMENTS
The authors acknowledge Mikele Garrett, BBA, MBA (Department of Anesthesiology, University of Michigan Medical School), Basanthi Krishnan, BS, MBA (Organ Transplant Information System, University of Michigan Medical School), and Arina Bierdz (Data Office for Clinical and Translational Research, University of Michigan) for their contributions in data acquisition and electronic search query programming for this project.
Berkowitz RJ, Engoren MC, Mentz G, Sharma P, Kumar SS, Davis R, et al. Intraoperative risk factors of acute kidney injury after liver transplantation. Liver Transpl. 2022;28:1207–1223. 10.1002/lt.26417
Funding information
All work and partial funding is attributed to the Division of Transplantation Surgery, Department of Surgery, Michigan Medicine, Ann Arbor, MI, and the Department of Anesthesiology, University of Michigan Medical School (Ann Arbor, MI). The project was supported in part by a Foundation for Anesthesia Education and Research–Mentored Research Training Grant to Nicholas J. Douville.
SEE EDITORIAL ON PAGE 1131
REFERENCES
- 1. Lima EQ, Zanetta DMT, Castro I, Massarollo PCB, Mies S, Machado MM, et al. Risk factors for development of acute renal failure after liver transplantation. Ren Fail. 2003;25:553–60. [DOI] [PubMed] [Google Scholar]
- 2. Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, et al. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123–9. [PubMed] [Google Scholar]
- 3. Wyatt CM, Arons RR. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. 2004;78:1351–5. [DOI] [PubMed] [Google Scholar]
- 4. Markmann JF, Markmann JW, Markmann DA, Bacquerizo A, Singer J, Holt CD, et al. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation. 2001;72:1113–22. [DOI] [PubMed] [Google Scholar]
- 5. Contreras G, Garces G, Quartin AA, Cely C, LaGatta MA, Barreto GA, et al. An epidemiologic study of early renal replacement therapy after orthotopic liver transplantation. J Am Soc Nephrol. 2002;13:228–33. [DOI] [PubMed] [Google Scholar]
- 6. Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–9. [DOI] [PubMed] [Google Scholar]
- 7. Durand F, Francoz C, Asrani SK, Khemichian S, Pham TA, Sung RS, et al. Acute kidney injury after liver transplantation. Transplantation. 2018;102:1636–49. [DOI] [PubMed] [Google Scholar]
- 8. Barreto AGC, Daher EF, Junior GBS, Garcia JHP, Magalhães CBA, Lima JMC, et al. Risk factors for acute kidney injury and 30‐day mortality after liver transplantation. Ann Hepatol. 2015;14:688–94. [PubMed] [Google Scholar]
- 9. Romano TG, Schmidtbauer I, Silva FMDQ, Pompilio CE, D'Albuquerque LAC, Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS One. 2013;8:e64089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD‐based allocation on end‐stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma P, Bari K. Chronic kidney disease and related long‐term complications after liver transplantation. Adv Chronic Kidney Dis. 2015;22:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoo S, Lee H‐J, Lee H, Ryu H‐G. Association between perioperative hyperglycemia or glucose variability and postoperative acute kidney injury after liver transplantation: a retrospective observational study. Anest Analg. 2017;124:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–94. [DOI] [PubMed] [Google Scholar]
- 14. University of Michigan Anesthesia Clinical Research Committee . [cited 2021 July 19]. Available from: https://anes.med.umich.edu/research/acrc.html
- 15. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group . Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colquhoun DA, Shanks AM, Kapeles SR, Shah N, Saager L, Vaughn MT, et al. Considerations for integration of perioperative electronic health records across institutions for research and quality improvement: the approach taken by the multicenter perioperative outcomes group. Anesth Analg. 2020;130:1133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douville NJ, Jewell ES, Duggal N, Blank R, Kheterpal S, Engoren MC, et al. Association of intraoperative ventilator management with postoperative oxygenation, pulmonary complications, and mortality. Anesth Analg. 2019;130:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 19. Multicenter Perioperative Outcomes Group . Comorbidity‐deficiency anemia. [cited 2021 Feb 18]. Available from: https://phenotypes.mpog.org/Comorbidity%20‐%20Deficiency%20Anemia [Google Scholar]
- 20. Multicenter Perioperative Outcomes Group . Comorbidity‐fluid/electrolyte disorders. [cited 2021 Feb 18]. Available from: https://phenotypes.mpog.org/Comorbidity%20‐%20Fluid!Electrolyte%20Disorders [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 22. Debray TPA, Damen JAAG, Snell KIE, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta‐analysis of prediction model performance. BMJ. 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
- 23. Jung S‐H, Ahn C. Sample size estimation for GEE method for comparing slopes in repeated measurements data. Stat Med. 2009;22:1305–15. [DOI] [PubMed] [Google Scholar]
- 24. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 25. Hilmi IA, Damian D, Al‐Khafaji A, Planinsic R, Boucek C, Sakai T, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919–26. [DOI] [PubMed] [Google Scholar]
- 26. Angeli P, Bezinover D, Biancofiore G, Bienholz A, Findlay J, Paugam Burtz C, et al. Acute kidney injury in liver transplant candidates: a position paper on behalf of the Liver Intensive Care Group of Europe. Minerva Anestesiol. 2017;83:88–101. [DOI] [PubMed] [Google Scholar]
- 27. Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta‐analysis. J Clin Med Res. 2019;8:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the risk of AKI following cardio‐thoracic surgery: a meta‐analysis. Clin J Am Soc Nephrol. 2016;11:2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018;19:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim WH, Lee H‐C, Lim L, Ryu H‐G, Jung C‐W. Intraoperative oliguria with decreased SvO2 predicts acute kidney injury after living donor liver transplantation. J Clin Med Res. 2018;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, et al. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int. 2013;26:842–52. [DOI] [PubMed] [Google Scholar]
- 32. Leithead JA, Rajoriya N, Gunson BK, Muiesan P, Ferguson JW. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol. 2014;60:1180–6. [DOI] [PubMed] [Google Scholar]
- 33. Klaus F, Keitel da Silva C, Meinerz G, Carvalho LM, Goldani JC, Cantisani G, et al. Acute kidney injury after liver transplantation: incidence and mortality. Transplant Proc. 2014;46:1819–21. [DOI] [PubMed] [Google Scholar]
- 34. Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, et al. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539–48. [DOI] [PubMed] [Google Scholar]
- 35. Sang B‐H, Bang J‐Y, Song J‐G, Hwang G‐S. Hypoalbuminemia within two postoperative days is an independent risk factor for acute kidney injury following living donor liver transplantation: a propensity score analysis of 998 consecutive patients. Crit Care Med. 2015;43:2552–61. [DOI] [PubMed] [Google Scholar]
- 36. Wiedermann CJ, Wiedermann W, Joannidis M. Causal relationship between hypoalbuminemia and acute kidney injury. World J Nephrol. 2017;6:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee E‐H, Baek S‐H, Chin J‐H, Choi D‐K, Son H‐J, Kim W‐J, et al. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off‐pump coronary artery bypass surgery. Intensive Care Med. 2012;38:1478–86. [DOI] [PubMed] [Google Scholar]
- 38. Kim K, Bang J‐Y, Kim S‐O, Kim S, Kim JU, Song J‐G. Association of preoperative hypoalbuminemia with postoperative acute kidney injury in patients undergoing brain tumor surgery: a retrospective study. J Neurosurg. 2018;128:1115–22. [DOI] [PubMed] [Google Scholar]
- 39. Shin K‐H, Han S‐B. Early postoperative hypoalbuminemia is a risk factor for postoperative acute kidney injury following hip fracture surgery. Injury. 2018;49:1572–6. [DOI] [PubMed] [Google Scholar]
- 40. Cimen S, Guler S, Ayloo S, Molinari M. Implications of hyponatremia in liver transplantation. J Clin Med Res. 2014;4:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cabezuelo JB, Ramírez P, Ríos A, Acosta F, Torres D, Sansano T, et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073–80. [DOI] [PubMed] [Google Scholar]
- 42. Lebrón Gallardo M, Herrera Gutierrez ME, Seller Pérez G, Curiel Balsera E, Fernández Ortega JF, Quesada GG. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004;10:1379–85. [DOI] [PubMed] [Google Scholar]
- 43. Alvares‐da‐Silva MR, Waechter FL, Francisconi CF, Barros E, Thomé F, Traiber C, et al. Risk factors for postoperative acute renal failure at a new orthotopic liver transplantation program. Transplant Proc. 1999;31:3050–2. [DOI] [PubMed] [Google Scholar]
- 44. Rossi AP, Vella JP. Acute kidney disease after liver and heart transplantation. Transplantation. 2016;100:506–14. [DOI] [PubMed] [Google Scholar]
- 45. Beben T, Rifkin DE. GFR estimating equations and liver disease. Adv Chronic Kidney Dis. 2015;22:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alvarado M, Schaubel DE, Reddy KR, Bittermann T. Black race is associated with higher rates of early‐onset end‐stage renal disease and increased mortality following liver transplantation. Liver Transpl. 2021;27:1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leithead JA, Tariciotti L, Gunson B, Holt A, Isaac J, Mirza DF, et al. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am J Transplant. 2012;12:965–75. [DOI] [PubMed] [Google Scholar]
- 48. Tokodai K, Lannsjö C, Kjaernet F, Romano A, Januszkiewicz A, Ericzon B‐G, et al. Association of post‐reperfusion syndrome and ischemia‐reperfusion injury with acute kidney injury after liver transplantation. Acta Anaesthesiol Scand. 2020;64:742–50. [DOI] [PubMed] [Google Scholar]
- 49. Zongyi Y, Baifeng L, Funian Z, Hao L, Xin W. Risk factors of acute kidney injury after orthotopic liver transplantation in China. Sci Rep. 2017;7:41555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mizota T, Hamada M, Matsukawa S, Seo H, Tanaka T, Segawa H. Relationship between intraoperative hypotension and acute kidney injury after living donor liver transplantation: a retrospective analysis. J Cardiothorac Vasc Anesth. 2017;31:582–9. [DOI] [PubMed] [Google Scholar]
- 51. Xu X, Ling Q, Wei Q, Wu J, Gao F, He Z‐L, et al. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:259–63. [PubMed] [Google Scholar]
- 52. Lee H‐C, Yoon SB, Yan S‐M, Kim WH, Ryu HG, Jung C‐W, et al. Prediction of acute kidney injury after liver transplantation: machine learning approaches vs. logistic regression model. J Clin Med Res. 2018;7:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Engoren M, Habib RH, Arslanian‐Engoren C, Kheterpal S, Schwann TA. The effect of acute kidney injury and discharge creatinine level on mortality following cardiac surgery*. Crit Care Med. 2014;42:2069–74. [DOI] [PubMed] [Google Scholar]
- 54. Kong Y, Wang D, Shang Y, Liang W, Ling X, Guo Z, et al. Calcineurin‐inhibitor minimization in liver transplant patients with calcineurin‐inhibitor‐related renal dysfunction: a meta‐analysis. PLoS One. 2011;6:e24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wadei HM, Lee DD, Croome KP, Mai ML, Golan E, Brotman R, et al. Early allograft dysfunction after liver transplantation is associated with short‐ and long‐term kidney function impairment. Am J Transplant. 2016;16:850–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


