Abstract
Background
We aimed at investigating the incidence, characteristics and outcome of ventilator‐associated pneumonia (VAP) in patients after cardiac arrest (CA) and its potential association with mild therapeutic hypothermia (MTH). We hypothesized, that MTH might increase the risk of VAP.
Methods
Prospective observational study including comatose adult patients after successful resuscitation from out‐of‐hospital or in‐hospital CA with presumed cardiac cause admitted to ICU and treated with MTH at 33°C for 24 h or normothermia (NT) with treatment of fever ≥38°C by pharmacological means. The primary outcome measure was the development of VAP. VAP diagnosis included mechanical ventilation >48 h combined with clinical and radiologic criteria. For a microbiologically confirmed VAP (mcVAP), a positive respiratory culture was required.
Results
About 23% of 171 patients developed VAP, 6% presented with mcVAP. VAP was associated with increased ICU‐LOS (9 (IQR 5–14) vs. 6 (IQR 3–9) days; p < .01), ventilator‐dependent days (6 (IQR 4–9) vs. 4 (IQR 2–7) days; p < .01) and duration of antibiotic treatment (9 (IQR 5–13) vs. 5 (IQR 2–9) days; p < .01), but not with mortality (OR 0.88 (95% CI: 0.43–1.81); p = .74). Patients treated with MTH (47%) presented higher VAP (30% vs. 17%; p = .04) and mcVAP rates (11% vs. 2%; p = .03). MTH was associated with VAP in multivariable logistic regression analysis with an OR of 2.67 (95% CI: 1.22–5.86); p = .01.
Conclusions
VAP appears to be a common complication in patients after CA, accompanied by more ventilator‐dependent days, prolonged antibiotic treatment, and ICU‐LOS. Treatment with MTH is significantly associated with development of VAP.
Keywords: cardiopulmonary resuscitation, mechanical ventilation, microbiology, mortality, neurological outcome, targeted temperature management, ventilator‐associated pneumonia
Editorial Comment.
With controlled post‐cardiac arrest and resuscitation intensive care, there are high risks for pulmonary complications. This analysis assessed ventilator‐associated pneumonia events in this case group at a single centre. In this sample, an association between this complication and controlled mild hypothermic treatment was demonstrated.
1. INTRODUCTION
Pneumonia is the most commonly observed complication in out‐of‐hospital cardiac arrest (OHCA) survivors admitted to intensive care unit (ICU). 1 In this cohort, it usually manifests as either early‐onset pneumonia or ventilator‐associated pneumonia (VAP). Reported incidences of early‐onset pneumonia range from 24% to 65% in the main studies focusing on pneumonia after OHCA. VAP as the most common infection in ventilated ICU patients is estimated to occur in 9%–27%. 2 , 3 The most recent study on OHCA survivors addressing the prevention of VAP revealed an incidence of 31%, 4 being lower than in previous cardiac arrest (CA) studies. 1 , 5 , 6 , 7 However, comparability is complicated by heterogeneous definitions of VAP, especially in CA patients under treatment with mild therapeutic hypothermia (MTH). 8 , 9
Hypothermia is known to reduce the general inflammatory response after return of spontaneous circulation (ROSC), but might also increase the susceptibility of the lungs to infections, 10 , 11 possibly resulting in a higher incidence of pneumonia. Several studies including OHCA patients treated with MTH vs. normothermia (NT) 12 , 13 , 14 and targeted temperature management at 33°C vs. 36°C 15 revealed no significant differences in the incidence of pneumonia. However, in another retrospective study, MTH was identified as the only risk factor in multivariate analysis for developing early‐onset pneumonia. Early‐onset pneumonia occurred in 65% of the patients in the first 3 days after successful CPR and was associated with increased length of mechanical ventilation and ICU stay, but not ICU mortality. 7
Little is known about the influence of MTH on the development of VAP, which is mainly determined by the interaction of the endotracheal tube and the presence of risk factors, virulence of involved pathogens and the competence of the host´s immune system. 16 We hypothesized that MTH might increase the risk of VAP on top of the compromised immune system of the critically ill patient.
Therefore, we aimed to investigate the incidence, characteristics and outcome of VAP in a population of comatose patients after successful resuscitation from OHCA and in‐hospital cardiac arrest (IHCA) and the potential association of VAP development with MTH treatment.
2. METHODS
This prospective observational single center trial consecutively included adult (age ≥18 years) comatose patients (with a score <8 on the Glasgow Coma Scale) after successful cardiopulmonary resuscitation (CPR) from CA (OHCA or IHCA) with presumed cardiac cause admitted to the medical ICU of the University Hospital of Innsbruck from September 2008 to November 2014. The presence of trauma as noncardiogenic cause of CA as well as life expectancy of <24 h as determined by the treating physicians was considered as exclusion criteria.
The primary outcome measure was the incidence of VAP in CA arrest patients treated with NT or MTH, secondary outcome measures included neurological outcome, mortality, ICU‐length of stay (LOS), and ventilator‐dependent days.
To diagnose a VAP, we used a standardized approach relying on clinical, radiologic, and microbiologic criteria and patients had to be ventilated for more than 48 h, according to the ATS/IDSA guidelines from 2005 2 and their update in 2016. 17 Clinical criteria were documented fever or hypothermia (with fever defined as a core body temperature ≥38°C and hypothermia <35°C, mostly measured by the urinary bladder catheter), an abnormal total peripheral white‐cell count (>10,000 mm3 or >15% immature neutrophils [bands], regardless of white‐cell count, or leukopenia with a white‐cell count <4500 mm3), new‐onset purulent sputum or change in the character of sputum, plus at least one of the following two features: auscultatory findings of rales on pulmonary examination or evidence of pulmonary consolidation, or acute changes made in the ventilator support system to enhance oxygenation, as determined by arterial blood gas analysis, or a worsening ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FIO2 ≤ 240 mm Hg (32 kPa)). Radiographic criteria were the presence of a new or progressive and persistent infiltrate characteristic of bacterial pneumonia or new consolidation in the chest X‐ray on 2 consecutive days.
2.1. Microbiologic criteria
A positive respiratory culture was required to diagnose a confirmed and definite pneumonia. The semiquantitative culture of distal pulmonary secretion samples was obtained by endotracheal aspirate or bronchoalveolar lavage based on clinical indication. The specified thresholds to be considered clinically significant amounts as determined by standard culture were moderate (+++) and heavy (++++).
If no bacteriological sample was available, the diagnosis was retained if the previous signs were present in association with purulent endotracheal aspirates and hypoxemia (PaO2/FIO2 ≤ 240 mm Hg (32 kPa)) not explained by pulmonary embolism, edema, or atelectasis.
Patients meeting all three types of criteria including a positive respiratory culture developed a “microbiologically confirmed VAP” (mcVAP).
Additionally, we analyzed if patients had an infiltrate in the initial chest X‐ray at admission.
Antibiotics were given according to the local protocol. Treating physicians made all decisions regarding antibiotics as guided treatment on a clinical indication. Initial antibiotic treatment and its duration on ICU were documented for each patient.
C‐reactive protein (CRP) [mg/dl], procalcitonin (PCT) [µg/L], and leucocyte count [G/L] were determined daily in 24 h intervals until 192 h. We also analyzed the maximum level within 192 h.
Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores as well as the use of vasopressors were documented at the day of ICU admission. Additionally, serum lactate [mg/dl] (Roche) as an established marker for circulatory shock was measured at admission.
CA data such as the rate of bystander resuscitation, time to return of spontaneous circulation and first monitored rhythm were collected from the emergency or heart alarm protocol according to the Utstein Style. 18 Furthermore, it was documented if patients underwent MTH, which was part of our standardized treatment strategy for comatose survivors of CA based on current knowledge at study initiation. 19 Since 2010 recommendations were published for including patients with a nonshockable rhythm with a low level of evidence additionally to ventricular fibrillation cardiac arrest patients with a high level of evidence. 20 , 21 We adhered to following protocol during the whole study period:
MTH was routinely applied to comatose patients with an initially shockable rhythm that had received advanced life support within 15 min and showed a return of spontaneous circulation within 60 min after collapse and with an initially nonshockable rhythm, if the event was observed and time to return of spontaneous circulation less than 25 min. Patients who underwent MTH were maintained at a target core body temperature of 32–34°C for 24 h using an intravascular cooling device (Thermogard XP, Zoll®, Intravascular Temperature Management Systems) and then gradually rewarmed with a rate of 0.2–0.4°C/h. After the intervention, patients received temperature management (for fever control ≥38°C) until 72 h after admission. Core body temperature was measured in the urinary bladder using a Foley catheter.
Patients not undergoing MTH received temperature management by pharmacological measures (for fever control ≥38°C) over 72 h. 22 In patients treated with MTH, we assessed the duration of hypothermia (in hours), in all patients we documented ventilator‐dependent days and the ICU – LOS (in days).
Sedation was mandated in the MTH group until rewarming. When 36.5°C were reached, sedation was stopped. Further sedation was dependent on clinical needs. In the NT group, sedation was used if necessary and discontinued as soon as possible. Duration and type of sedation were documented for each patient.
To assess neurological outcome, the Cerebral Performance Categories (CPC) Scale was used. 23 , 24 To receive binary outcome parameters, patients were classified into two groups according to their neurological outcomes: ‘good outcome’ (CPC 1 and 2) and ‘poor outcome’ (CPC 3–5). The five categories of CPC have been described in detail elsewhere, briefly they comprise: (1) Good cerebral performance; (2) Moderate cerebral disability; (3) Severe cerebral disability; (4) Coma/vegetative state; and (5) Brain death. 23
The assessment of neurological outcome was performed by one physician immediately before discharge from hospital (including also secondary or tertiary hospitals or rehab hospitals) to home in survivors following a standardized protocol. The CPC of patients transferred from ward (and not directly from ICU) were determined in person or per telephone interview with the patient him/herself, his/her family members, and the treating physician. Mortality was equally determined at discharge from hospital to home.
The study protocol was approved by the Ethics Committee of the Medical University of Innsbruck (protocol number UN3493 272/4.31). Written informed consent was obtained from next of kin or retrospectively from patients who recovered.
2.2. Statistical analysis
Categorical data are given as counts and percentages, continuous data as medians and interquartile ranges. Normal distribution of continuous data was checked by the Kolmogorov–Smirnov test. For univariate comparison of variables regarding neurological outcome and MTH being not normally distributed, we used the Mann– Whitney U test for continuous and the Fisher´s Exact test for categorical data.
Logistic regression models were used to assess the influence of certain parameters on the development of VAP and outcome. In multivariable analysis, we adjusted for the SOFA score, which already includes clinical relevant covariates. To assess the magnitude of a likely bias, such as guarantee‐time or immortal time bias, we additionally performed logistic regression analysis at two different time points designated during the follow‐up period. We analyzed only those subjects who had survived until the landmark time and were on mechanical ventilation eligible for VAP diagnosis. 25 The first landmark time was set at 48 h when patients already could have had a competing event before diagnosis of VAP (death or extubation <48 h) and the second at 7 days when the time of mechanical ventilation was already considered as prolonged.
Values with a p < .05 were considered as statistically significant. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp was used to analyze data.
3. RESULTS
3.1. Patient characteristics
Of 173 consecutive CA patients, 2 patients were excluded from the population due to missing values (n = 1: missing data of respirator settings, n = 1: missing data of CPR). Hundred and seventy‐one patients were included in the final analysis (Figure 1).
FIGURE 1.
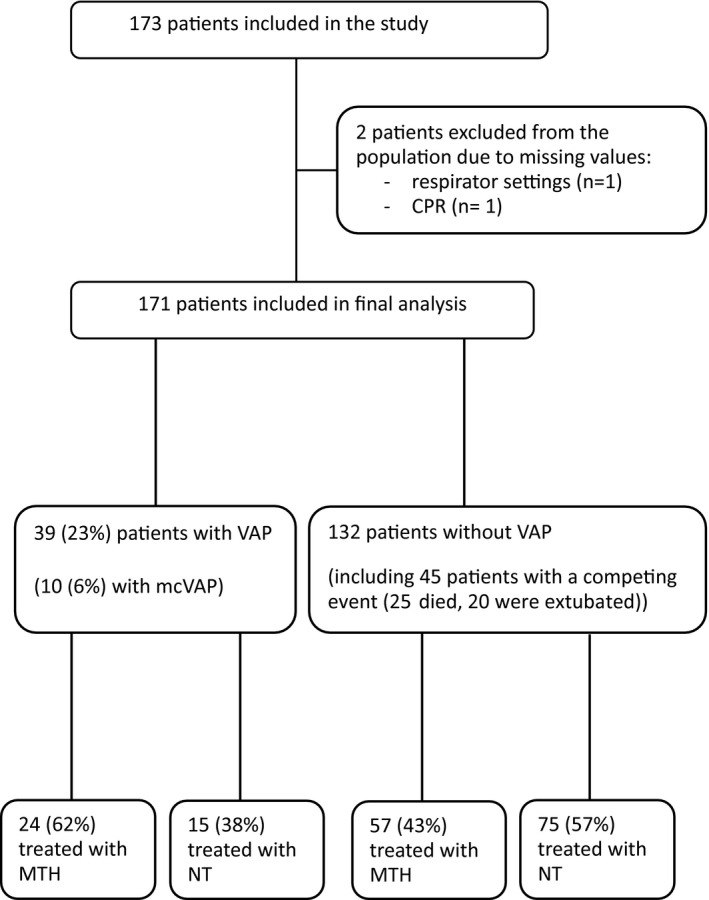
Flow chart. VAP, ventilator‐associated pneumonia; CPR, cardiopulmonary resuscitation; MTH, mild therapeutic hypothermia; NT, normothermia
The median age was 66 (IQR 53–75) years, 70% of the patients received bystander CPR, 61% had an initially shockable rhythm and 15% IHCA. Time to return of spontaneous circulation exceeded 20 min in 53% of the patients. At admission the median SOFA and APACHE II scores were 10 (IQR 9–12) and 26 (IQR 22–31), respectively and 82% were treated with vasopressors: 43 (25%) patients presented with an infiltrate in the chest X‐ray at admission and 47% were treated with MTH (Table 1).
TABLE 1.
Characteristics and outcome in all patients, patients with VAP versus patients without VAP
| All patients (n = 171) | VAP (n = 39) | No VAP (n = 132) | p‐value | |
|---|---|---|---|---|
| Age in years, median (IQR) | 66 (53–75) | 67 (53–78) | 65 (53–74) | .593 |
| Female, n (%) | 51 (30) | 7 (18) | 44 (33) | .066 |
| Bystander‐initiated CPR, n (%) | 120 (70) | 28 (72) | 91 (69) | .617 |
| Time to ROSC >20 min, n (%) | 91 (53) | 18 (46) | 69 (52) | .712 |
| Cardiac arrest out of hospital, n (%) | 146 (85) | 32 (82) | 114 (86) | .476 |
| Shockable first monitored rhythm, n (%) | 104 (61) | 25 (64) | 78 (59) | .589 |
| SOFA score on admission, median (IQR) | 10 (9–12) | 11 (9–12) | 10 (8–12) | .024 |
| APACHE II on admission, median (IQR) | 26 (22–31) | 29 (25–33) | 25 (22–30) | .002 |
| Lactate at admission [mg/dl], median (IQR) | 48 (29–85) | 50 (24–71) | 48 (30–89) | .280 |
| CRP max [mg/dl], median (IQR) | 14.8 (8.6–21.7) | 20.4 (14.5–27.2) | 12.3 (7.6–18.9) | .0001 |
| PCT max [µg/L], median (IQR) | 2.8 (0.6–14.1) | 3.3 (1.1–12.3) | 2.2 (0.5–14.4) | .188 |
| Leucocyte count max [G/L], median (IQR) | 17.2 (13.1–22.9) | 18.2 (14.2–23.9) | 16.5 (12.8–22.8) | .171 |
| Mild therapeutic hypothermia, n (%) | 81 (47) | 24 (62) | 57 (43) | .044 |
| Poor outcome at hospital discharge, n (%) | 92 (54) | 25 (64) | 67 (51) | .833 |
| Mortality at hospital discharge, n (%) | 83 (49) | 18 (46) | 65 (49) | .735 |
Abbreviations: VAP, ventilator‐associated pneumonia; IQR, interquartile range; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; SOFA, sequential organ failure assessment; APACHE II, acute physiology and chronic health evaluation II; CRP max, C‐reactive protein (maximum within 192 h); PCT max, procalcitonin (maximum within 192 h); Leukocyte count max, leukocyte count (maximum within 192 h).
Of 171 patients, 162 received antibiotic treatment at admission (for one patient the information was not available). Initial antibiotic treatment was in most of the cases amoxicillin/clavulanic acid or ampicillin/sulbactam (n = 117), piperacillin/tazobactam (n = 26), levofloxacin (n = 6) or piperacillin/tazobactam combined with levofloxacin (n = 4). Twelve patients received another initial therapy (n = 4 cephalosporin, n = 4 carbapenem, n = 2 clindamycin, and n = 2 metronidazol). Median duration of antibiotic treatment on ICU was 6 (IQR 3–10) days.
Out of 171 patients, 127 received a sedation regime with midazolam combined with fentanyl, morphine, or ketamine, 12 a combination of propofol and remifentanyl, and 9 an alternative regime. Twenty patients received no initial sedation and in 3 patients the information was not available: 45% of the patients had need of subsequent sedation after initial discontinuation at a median of 2 (IQR 2–2) days.
Fifty‐four percent of the patients had poor neurological outcome and mortality rate at hospital discharge was 49%. Patients had a median ICU – LOS of 7 (IQR 3–11) days and a median of 4 (IQR 2–7) ventilator‐dependent days (Table 1).
3.2. Ventilator‐associated pneumonia
Thirty‐nine patients (23%) developed a VAP and 10 patients (6%) a mcVAP (Figure 1). No significant differences in baseline characteristics such as age, sex, rate of bystander‐initiated CPR or IHCA, time to return of spontaneous circulation, and initial rhythm could be detected between patients with VAP vs. no VAP. However, we observed a significant difference in severity of illness scores at admission (Table 1).
With an infiltrate in the initial chest X‐ray at admission, patients had an increased risk for VAP development (OR 4.28 (CI 95%: 1.98–9.22); p < .01). Among these patients with an infiltrate at admission (n = 43), 12 developed VAP in the later course. There was no significant difference in the number of patients with initial antibiotic treatment between the VAP/no VAP group: 39 (100%) vs. 124 (95%); p = .35. Duration of antibiotic treatment on ICU was significantly longer in patients developing VAP with a median of 9 (IQR 5–13) days compared with patients without VAP (median of 5 days (IQR 2–9); p < .01).
Concerning inflammatory parameters, patients with VAP showed significantly higher CRP and PCT levels at several time intervals after CPR. Maximum CRP levels within 192 h were also significantly higher compared with to patients without VAP (p < .01). Leukocyte count and lactate levels were not significantly different at any time interval determined within 192 h.
Among the 10 patients developing a microbiologically confirmed VAP, bacteriological culture was obtained with endotracheal aspirate or bronchoalveolar lavage between day 3 and 5 after CPR. Isolated microorganisms in confirmed cases of pneumonia are listed in Table 2.
TABLE 2.
Microbiology
| Pathogen count | n | |
|---|---|---|
| Gram positive pathogens | ||
| Streptococcus pneumoniae | Heavy | 2 |
| Staphylococcus aureus | Moderate–heavy | 4 |
| Gram negative pathogens | ||
| Enterobacter asburiae | Heavy | 1 |
| Enterobacter cloacae | Moderate | 2 |
| Enterobacter aerogenes | Moderate–heavy | 1 |
| Klebsiella oxytoca | Moderate | 2 |
| Morganella morganii | Moderate | 1 |
| Pseudomonas aeruginosa | Heavy | 1 |
| Serratia marcescens | Moderate | 1 |
| Stenotrophomonas maltophilia | Heavy | 1 |
Abbreviation: n, number of patients with a positive culture for each pathogen.
Patients with VAP had a median of 6 (IQR 4–9) ventilator‐dependent days, which is significantly longer than in patients without VAP (4 (IQR 2–7) days; p < .01). However, there was no significant correlation between ventilator‐dependent days and development of VAP (r = .355; p = .27). In accordance to ventilator‐dependent days, also ICU‐LOS was significantly longer in patients with VAP vs. no VAP (9 (IQR 5–14) vs. 6 (IQR 3–9) days; p < .01). Other groups showed similar rates of poor neurological outcome (25 (64%) vs. 67 (51%); p = .83) and mortality (18 (46%) vs. 65 (49%); p = .74) (Table 1).
In univariate logistic regression analysis, occurrence of VAP was not significantly associated with poor neurological outcome (OR 1.73 (CI 95%: 0.83–3.62); p = .14) or mortality (OR 0.88 (CI 95%: 0.43–1.81); p = .74). Furthermore, no significant correlation between the development of VAP and the CPC score at discharge (p = .35) could be detected. An infiltrate in the initial chest X‐ray was also not significantly accompanied with poor neurological outcome or mortality (OR 1.87 (CI 95%: 0.91–3.82); p = .09).
3.3. Mild therapeutic hypothermia and VAP
In general, we observed significant differences in age, rate of bystander‐initiated CPR and OHCA, shockable first monitored rhythm, APACHE II score at admission, ventilator‐dependent days, neurological outcome and mortality in patients treated with MTH (vs. NT). The leucocyte count was not significantly different at any time interval within 192 h (Table 3).
TABLE 3.
Comparison of patients treated with mild therapeutic hypothermia versus normothermia
| MTH (n = 81) | NT (n = 0) | p‐value | |
|---|---|---|---|
| Age in years, median (IQR) | 62 (51–70) | 69 (56–78) | .004 |
| Female, n (%) | 21 (26) | 30 (33) | .292 |
| Bystander‐initiated CPR, n (%) | 63 (78) | 57 (63) | .040 |
| Time to ROSC >20 min, n (%) | 44 (54) | 47 (52) | .844 |
| Cardiac arrest out of hospital, n (%) | 77 (95) | 69 (77) | .001 |
| Shockable first monitored rhythm, n (%) | 70 (86) | 34 (38) | .0001 |
| SOFA score on admission, median (IQR) | 10 (8–11) | 10 (9–12) | .214 |
| APACHE II on admission, median (IQR) | 25 (21–29) | 27 (23–31) | .001 |
| Lactate at admission [mg/dl], median (IQR) | 42 (30–75) | 52 (24–102) | .459 |
| CRP max [mg/dl], median (IQR) | 16.1 (11.3–22.4) | 11.2 (7.0–20.7) | .012 |
| PCT max [µg/L], median (IQR) | 1.7 (0.5–8.3) | 5.0 (0.9–19.5) | .063 |
| Leucocyte count max [G/L], median (IQR) | 17.7 (13.9–23.6) | 16.4 (12.9–22.0) | .251 |
| VAP, n (%) | 24 (30) | 15 (7) | .040 |
| McVAP, n (%) | 8 (10) | 2 (7) | .034 |
| Ventilator‐dependent days, median (IQR) | 6 (3–8) | 3 (1–6) | .001 |
| Poor outcome at hospital discharge, n (%) | 36 (44) | 56 (62) | .020 |
| Mortality, n (%) | 31 (38) | 52 (57) | .011 |
Abbreviations: MTH, mild therapeutic hypothermia; NT, normothermia; VAP, ventilator‐associated pneumonia; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; SOFA, sequential organ failure assessment; APACHE II, acute physiology and chronic health evaluation II; CRP max, C‐reactive protein (maximum within 192 h); PCT max, procalcitonin (maximum within 192 h); Leukocyte count max, leukocyte count (maximum within 192 h).
When patients presented with an infiltrate in the initial chest X‐ray, there was no significant difference in allocation to further treatment (MTH vs. NT: (18 (42%) vs. 25 (58%); p = .40)).
In patients treated with MTH (vs. NT), we could observe a significantly higher VAP rate (24 (30%) vs. 15 (17%) patients, p = .04) and mcVAP rate (8 (11%) vs. 2 (2%); p = .03) (Table 3). Treatment with MTH was significantly associated with the development of a VAP in univariable (OR 2.11 (CI 95%: 1.01–4.37); p < .05) and SOFA score adjusted multivariable logistic regression analysis (OR 2.67 (CI 95%: 1.22–5.86); p = .01).
Duration of sedation was significantly longer in patients treated with MTH vs NT (median of 2 days (IQR 2–3) vs. median of 2 days (IQR 1–2); p < .01).
3.4. Landmark analysis at 48 h and 7 days
Fourty‐five (26%) patients had a competing event, of which 25 died and 20 were extubated within 48 h after CPR (Figure 1). Within the remaining population of 126 patients at 48 h eligible for VAP diagnosis, the VAP rate was 31% and the mcVAP rate 8%.
In order to consider a likely bias, we performed logistic regression analysis with MTH as covariate at two landmark times. In the 126 patients eligible for analysis at 48 h after CPR, the OR for developing VAP was 1.49 (CI 95%: 0.69–3.23); p = .31. After >7 days, the observed risk for VAP development in the remaining population (n = 40) was still elevated (OR 2.54 (CI 95%: 0.63–10.17); p = .19).
4. DISCUSSION
In our study, we demonstrated that VAP is a common complication in ventilated ICU patients after cardiac arrest. The development of a VAP did not impact neurological outcome or mortality, but increased duration of antibiotic treatment on ICU, ventilator‐dependent days and ICU‐LOS. Treatment with MTH is significantly associated with the development of VAP in both, univariable and SOFA‐score adjusted multivariable logistic regression analysis.
In our population of 171 comatose resuscitated patients, 23% developed a VAP and 6% a mcVAP. When respecting the issue, that 45 patients had a competing event (death, extubation) before 48 h and were therefore not eligible for VAP diagnosis, the incidence increased to 31% for VAP and 8% for mcVAP. Reported rates of VAP in OHCA patients show a huge variation. 4 , 7 In a recent randomized multicenter trial 4 including 194 patients after OHCA treated with MTH, the definition of VAP was comparable to our study. Here, the VAP rate was 31% and 41% when including cases without pathogen documentation. In the TTM trial, 15 50% of the patients developed a pneumonia, half of them with a positive culture. The authors used a minimum number of criteria for pneumonia diagnosis and did not specify patients with VAP. Applying a similar definition of pneumonia in the TTM2 12 compared with the TTM trial, 15 a rate of 35% was reported. In the study of Perbet et al 7 with more than 600 patients included, 65% presented with early‐onset pneumonia and 52% with bacteriological confirmation. The incidence of VAP was reported to be 14%, but the definition was not specified in the methods section. In a general ICU population of mechanically ventilated patients, VAP is estimated to occur in 9%–27%. 2 , 3 The lower rates of VAP in our study might be explained by different definitions of pneumonia or our strict adherence to recommendations for VAP prevention. 26
Development of VAP was accompanied with a significantly longer duration of antibiotic treatment on ICU, ICU‐LOS and more ventilator‐dependent days, but not with poor neurological outcome or mortality. Equally, early‐onset pneumonia was also associated with prolonged ventilator support and ICU–LOS, but not with ICU mortality. 7 This is in accordance with the study by Francois et al. who could not find increased death rates attributable to VAP or sepsis in hypothermic CA patients. 4 Also, in the TTM trial, 15 patients developing pneumonia did not show a significantly higher mortality compared with patients without pneumonia.
In patients treated with MTH, we found a significantly higher rate of VAP and mcVAP. Furthermore, there was a significant association of MTH treatment and the development of VAP in uni‐ and multivariable analysis.
In our cohort, patients treated with MTH presented with a significantly better pre‐arrest profile (age, bystander‐initiated CPR, shockable first monitored rhythm) and showed better outcomes at hospital discharge. Accordingly, APACHE II scores at admission were significantly lower in the MTH group. However, SOFA scores at admission and rates of patients presenting with an infiltrate in the initial chest X‐ray were similar in the MTH and NT group.
Due to the more favorable pre‐arrest factors including a higher rate of shockable rhythms and bystander‐initiated CPR, patients with better predicted prognosis were probably more likely conferred to MTH treatment. Therefore, one would expect that patients treated with NT would have an increased risk for complications such as development of VAP. Contrarily, MTH treatment was significantly associated with VAP development, even in multivariate analysis, when corrected for the SOFA score at admission. Obviously the risk of developing VAP increased over time. We speculated that this was related to a longer exposure to mechanical ventilation of patients treated with MTH, being itself a risk factor for VAP development. However, there was no significant correlation between ventilator‐dependent days and VAP development, making the duration of mechanical ventilation a less important factor under these conditions.
Development of VAP is driven by an interplay of the endotracheal tube and the presence of risk factors, virulence of involved pathogens, and the competence of the host´s immune system, 16 which is generally compromised in critical ill patients. 27 They often suffer from profound neutrophil dysfunction, also occurring in the inflamed lung, 28 resulting in a higher risk for the acquisition of nosocomial infection. 29 Treatment with MTH may additionally attenuate the general inflammatory response occurring after return of spontaneous circulation also leading to a higher rate of infectious complications 10 , 11 and consecutively to VAP.
Our results are in accordance to a previous study, which identified hypothermia to be a single independent risk factor for the development of early‐onset pneumonia after OHCA in multivariate analysis. This association was even more pronounced in patients with a bacteriologically documented pneumonia. 7 In contrast, no difference in the incidence of infectious complications including pneumonia between hypothermia vs. NT 12 and targeted temperature management at 33°C vs. 36°C 15 could be established. When comparing the incidence of pneumonia between patients treated with targeted temperature management for 48 vs. 24 h, there was no significant difference detected (49% vs. 43%; p = .24). 30
For patients treated with MTH, sedation was mandated, whereas in the NT group, sedation was used if necessary. Therefore we cannot rule out that mandatory sedation in the MTH group could have caused prolonged need for mechanical ventilation. However, duration of sedation was only slightly different between the two groups, albeit statistical significant.
Reporting of VAP remains a difficult topic, because definitions are very heterogeneous, which also complicates the comparability of studies. Especially in our population of CA patients treated with hypothermia, diagnostic criteria might be confounded. Inflammatory response after achieving return of spontaneous circulation might mimic a real infection 31 and MTH influences body temperature, inflammatory parameters, and leukocyte count. 32 However, in our population the leucocyte count integrated in the diagnostic process was not significantly different between MTH/NT. We defined our diagnostic criteria in accordance to the recently published work of Francois et al 4 relying on criteria from the Food and Drug Administration guidance for diagnosis and confirmation of VAP in 2010. 33 Criteria are also consistent with the ATS/IDSA guidelines from 2005 2 and 2016 17 and the European guidelines 34 that recommend obtaining quantitative or qualitative lower respiratory tract samples for culture and microbiology. Recent data are challenging the concept of integrating PaO2/FiO2 < 240 mm Hg (32 kPa) in the diagnostic criteria of VAP, as independently associated with less microbiological confirmation. 35 During the study period, VAP definitions were not amended. 17 Of note, we did not integrate the Clinical Pulmonary infection Score (CPIS) 36 in our approach. As body temperature and WBC are affected by treatment with MTH 32 and both parameters are considered, the score is probably not applicable in our cohort of hypothermic CA patients.
Isolated microorganisms in case of mcVAP were within the usual spectrum of causative pathogen agents. 9 By trend, more gram‐negative bacteria were isolated.
4.1. Strengths and Limitations
This is the first study investigating the incidence of VAP and the association of MTH with VAP in a mixed population of CA patients treated with MTH or NT.
However, the external validity of the study may be limited by its design as a single‐center study. Patients were not randomized to the MTH or NT group. Allocation of treatment was based on the inclusion criteria described in the methods section. Therefore the MTH and NT group exhibit different characteristics. Among these are a higher rate of patients resuscitated from OHCA. Originally, the planning of this study together with the power calculation was targeted on the neuroprognostication by biomarkers. 37 , 38 The incidence of VAP was considered as secondary end‐point in the original protocol. According to the VAP definition, patients had to receive mechanical ventilation and survive for at least 48 h. Therefore, it remains unknown, if patients with a competing event within 48 h would have developed a VAP. In order to consider this potential bias, we performed logistic regression analysis at two landmark times after CPR showing that the OR was still elevated at 48 h and >7 days suggesting an independent risk for VAP development.
Furthermore, patient characteristics and inflammatory parameters at admission could have influenced a later development of VAP. However, in multivariate analysis when correcting for the SOFA score, MTH remained a significant covariate.
Finally, due to the small number of patients with VAP, the study may have limited power to detect significant differences in neurological outcome or mortality.
4.2. Conclusions
VAP seems to be a common complication in patients after CA, which is significantly associated with MTH treatment. VAP development is accompanied by more ventilator‐dependent days, prolonged antibiotic treatment, and ICU‐LOS, but not associated with outcome. Results have to be confirmed in larger prospective trials.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
JH was involved in the conceptualization and patient data collection, analysed and interpreted data and wrote the manuscript. FS performed the analysis of the chest X‐rays. HU performed the statistical analysis. GL, SK and TM were involved in the data collection and reviewed the manuscript. MJ performed the conceptualization, methodology and supervision of the study and validated, reviewed and edited the manuscript.
ACKNOWLEDGMENTS
Departmental funding only.
Hasslacher J, Steinkohl F, Ulmer H, et al. Increased risk of ventilator‐associated pneumonia in patients after cardiac arrest treated with mild therapeutic hypothermia. Acta Anaesthesiol Scand. 2022;66:704–712. doi: 10.1111/aas.14063
REFERENCES
- 1. Mongardon N, Perbet S, Lemiale V, et al. Infectious complications in out‐of‐hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med. 2011;39:1359‐1364. [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society; Infectious Diseases Society of America . Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171:388‐416. [DOI] [PubMed] [Google Scholar]
- 3. Chastre J, Fagon JY. Ventilator‐associated pneumonia. Am J Respir Crit Care Med. 2002;165:867‐903. [DOI] [PubMed] [Google Scholar]
- 4. François B, Cariou A, Clere‐Jehl R, et al. Prevention of early ventilator‐associated pneumonia after cardiac arrest. N Engl J Med. 2019;381:1831‐1842. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926‐934. [DOI] [PubMed] [Google Scholar]
- 6. Pabst D, Romer S, Samol A, Kumpers P, Waltenberger J, Lebiedz P. Predictors and outcome of early‐onset pneumonia after out‐of‐hospital cardiac arrest. Respir Care. 2013;58:1514‐1520. [DOI] [PubMed] [Google Scholar]
- 7. Perbet S, Mongardon N, Dumas F, et al. Early‐onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med. 2011;184:1048‐1054. [DOI] [PubMed] [Google Scholar]
- 8. Kakavas S, Mongardon N, Cariou A, Gulati A, Xanthos T. Early‐onset pneumonia after out‐of‐hospital cardiac arrest. J Infect. 2015;70:553‐562. [DOI] [PubMed] [Google Scholar]
- 9. Kalanuria AA, Ziai W, Mirski M. Ventilator‐associated pneumonia in the ICU. Crit Care. 2014;18:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186‐S202. [DOI] [PubMed] [Google Scholar]
- 11. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101‐1120. [DOI] [PubMed] [Google Scholar]
- 12. Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus normothermia after out‐of‐hospital cardiac arrest. N Engl J Med. 2021;384:2283‐2294. [DOI] [PubMed] [Google Scholar]
- 13. Hypothermia after Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549‐556. [DOI] [PubMed] [Google Scholar]
- 14. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557‐563. [DOI] [PubMed] [Google Scholar]
- 15. Dankiewicz J, Nielsen N, Linder A, et al. Infectious complications after out‐of‐hospital cardiac arrest‐a comparison between two target temperatures. Resuscitation. 2017;113:70‐76. [DOI] [PubMed] [Google Scholar]
- 16. Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82:172‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61‐e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out‐of‐Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132:1286‐1300. [DOI] [PubMed] [Google Scholar]
- 19. Neumar RW, Nolan JP, Adrie C, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452‐2483. [DOI] [PubMed] [Google Scholar]
- 20. Deakin CD, Nolan JP, Soar J, et al. European resuscitation council guidelines for resuscitation 2010 section 4. Adult advanced life support. Resuscitation. 2010;81:1305‐1352. [DOI] [PubMed] [Google Scholar]
- 21. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post‐cardiac arrest care: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S768‐S786. [DOI] [PubMed] [Google Scholar]
- 22. Broessner G, Beer R, Lackner P, et al. Prophylactic, endovascularly based, long‐term normothermia in ICU patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke. 2009;40:e657‐e665. [DOI] [PubMed] [Google Scholar]
- 23. Edgren E, Hedstrand U, Kelsey S, Sutton‐Tyrrell K, Safar P; BRCTI Study Group . Assessment of neurological prognosis in comatose survivors of cardiac arrest. Lancet. 1994;343:1055‐1059. [DOI] [PubMed] [Google Scholar]
- 24. Stiell IG, Nesbitt LP, Nichol G, et al. Comparison of the cerebral performance category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med. 2009;53:241‐248. [DOI] [PubMed] [Google Scholar]
- 25. Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol. 2019;26:391‐393. [DOI] [PubMed] [Google Scholar]
- 26. Sandiumenge A, Diaz E, Bodi M, Rello J. Therapy of ventilator‐associated pneumonia. A patient‐based approach based on the ten rules of "The Tarragona Strategy". Intensive Care Med. 2003;29:876‐883. [DOI] [PubMed] [Google Scholar]
- 27. Surbatovic M, Vojvodic D, Khan W. Immune response in critically ill patients. Mediators Inflamm. 2018;2018:9524315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conway Morris A, Kefala K, Wilkinson TS, et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am J Respir Crit Care Med. 2009;180:19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris AC, Brittan M, Wilkinson TS, et al. C5a‐mediated neutrophil dysfunction is RhoA‐dependent and predicts infection in critically ill patients. Blood. 2011;117:5178‐5188. [DOI] [PubMed] [Google Scholar]
- 30. Kirkegaard H, Søreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out‐of‐hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318:341‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis‐like syndrome? Curr Opin Crit Care. 2004;10:208‐212. [DOI] [PubMed] [Google Scholar]
- 32. Dufner MC, Andre F, Stiepak J, et al. Therapeutic hypothermia impacts leukocyte kinetics after cardiac arrest. Cardiovasc Diagn Ther. 2016;6:199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Infectious Diseases Society of America (IDSA), American College of Chest Physicians (ACCP), American Thoracic Society (ATS), Society of Critical Care Medicine (SCCM) , Spellberg B, Talbot G. Recommended design features of future clinical trials of antibacterial agents for hospital‐acquired bacterial pneumonia and ventilator‐associated bacterial pneumonia. Clin Infect Dis. 2010;51(S1):S150‐S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torres A, Ewig S, Lode H, Carlet J; European HAP working group . Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35:9‐29. [DOI] [PubMed] [Google Scholar]
- 35. Ferrer M, Sequeira T, Cilloniz C, et al. Ventilator‐associated pneumonia and PaO2/FIO2 diagnostic accuracy: changing the paradigm? J Clin Med. 2019;8:1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121‐1129. [DOI] [PubMed] [Google Scholar]
- 37. Hasslacher J, Lehner GF, Harler U, et al. Secretoneurin as a marker for hypoxic brain injury after cardiopulmonary resuscitation. Intensive Care Med. 2014;40:1518‐1527. [DOI] [PubMed] [Google Scholar]
- 38. Hasslacher J, Ulmer H, Lehner G, et al. Postresuscitation care and prognostication after cardiac arrest—Does sex matter? Wien Klin Wochenschr. 2022, doi: 10.1007/s00508-022-02026-x. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


