Abstract
Samsoniella is a very important fungal resource, with some species in the genus having great medical, economic and ecological value. This study reports five new species of Samsoniella from Yunnan Province and Guizhou Province in Southwestern China and Dole Province in Vietnam, providing morphological descriptions, illustrations, phylogenetic placements, associated hosts and comparisons with allied taxa. Based on morphological observations and phylogenetic analyses of combined nrSSU, nrLSU, tef-1α, rpb1 and rpb2 sequence data, it was determined that these five new species were located in the clade of Samsoniella and different from other species of Samsoniella. The five novel species had morphologies similar to those of other species in the genus, with bright orange cylindrical to clavate stromata (gregarious). The fertile part lateral sides usually had a longitudinal ditch without producing perithecia, and superficial perithecia. The phialides had a swollen basal portion, tapering abruptly into a narrow neck and oval or fusiform one-celled conidia, often in chains. The morphological characteristics of 23 species in Samsoniella, including five novel species and 18 known taxa, were also compared in the present study.
Keywords: isaria-like fungi, micromorphology, phylogenetic analyses, taxonomy
1. Introduction
Samsoniella was established by Mongkolsamrit et al. (2018) based on morphological and molecular evidence to accommodate three isaria-like species: the type species S. inthanonensis and two other species, S. alboaurantia and S. aurantia [1]. Samsoniella species were characterized by the formation of bright orange, cylindrical to clavate stromata (gregarious). The fertile part lateral sides usually had a longitudinal ditch without producing perithecia, superficial perithecia. Or the formation of anamorphic synnemata, the phialides had a swollen basal portion, tapering abruptly into a narrow neck, conidia oval to fusiform, one-celled, often in chains [1,2]. In order to account for the phylogenetic diversity of isaria-like species and to segregate these isaria-like fungi from the Akanthomyces group, the genus Samsoniella was established [1]. Isaria Pers. was one of the oldest names for asexually typified genera in Cordycipitaceae; however, many entomogenous fungi morphologically similar to Isaria could be found distributed throughout Hypocreales [3]. In 2017, Kepler et al. revealed a polyphyletic distribution of Isaria species within Cordycipitaceae, proposed the rejection of Isaria and combined 11 species of Isaria into Cordyceps Fr., owing to the confusion surrounding the application of Isaria [4]. Two isolates of I. farinosa (CBS 240.32 and CBS 262.58) that remained genetically distant from CBS 111113 were renamed S. alboaurantium [1].
The species of Samsoniella have diverse biological characteristics. The genus currently contains 18 species (http://www.indexfungorum.org, accessed on 1 May 2022), among which S. hepiali is an essential medicinal fungus [1,2]. The chemical profile of S. hepiali is very similar to the profiles of Ophiocordyceps sinensis, and recent studies show that S. hepiali performs various biological pharmacological activities such as anti-cancer, analgesic and hypoglycaemic activity, and is a good substitute for O. sinensis [5]. The related species of S. hepiali may have similar pharmacological activities. However, no research on other members of the genus has yet been reported.
During surveys of entomopathogenic fungi from different regions in Yunnan Province, Guizhou Province of Southwestern China and Dole Province of Vietnam, five Samsoniella species were found and identified. Based on morphological evidence together with multigene (nrSSU, nrLSU, tef-1α, rpb1 and rpb2) sequence analyses, it was shown that these five new Samsoniella species were distinguished from other species of the genus. They were named S. coccinellidicola, S. farinospora, S. haniana, S. pseudotortricidae and S. sinensis. Furthermore, the morphological characteristics of 23 species in Samsoniella, comprising 5 novel species and 18 known taxa, were also compared.
2. Materials and Methods
2.1. Sample Collection and Isolation
The majority of the specimens used in this study were collected from Yunnan Province in China. Some specimens were collected from the Chu Yang Sin National Park of Dole Province in Vietnam. The specimens were noted and photographed in the fields. The sample was placed in an ice box and brought to the laboratory for preservation at 4 °C. To obtain axenic cultures, the stromata or synnemata were removed from the insect bodies and divided into 3–4 segments, each 2 mm long. The segments were immersed in 30% H2O2 for 30 s and then soaked in sterilized water for 1 minute. After drying on sterilized filter paper, the segments were inoculated onto potato dextrose agar (PDA: fresh potato 200 g/L, dextrose 20 g/L, and agar 18 g/L) plates. The conidia of cordycipitoid fungi at the conidial masses were picked using an inoculating loop and spread on PDA plates containing 0.1 g/L streptomycin and 0.05 g/L tetracycline [2]. Pure cultures were incubated at room temperature (about 25 °C). After isolation into pure cultures, they were transplanted to a PDA slant and stored at 4 °C. The specimens were deposited in the Yunnan Herbal Herbarium (YHH) at the Institute of Herb Biotic Resources, Yunnan University. The strain was deposited at the Yunnan Fungal Culture Collection (YFCC) of the Institute of Herb Biotic Resources, Yunnan University.
2.2. Morphological Observations
For descriptions of the sexual morph, fruiting bodies were photographed and measured using an Olympus SZ61 (Tokyo, Japan) stereomicroscope. Stromata were sectioned at a thickness of ca. 40 µm with a freezing microtome and mounted in water or lactic acid cotton blue on a slide for microscopic studies and photomicrography. The micro-morphological characteristics of the fungi, such as the perithecia, asci and ascospores, were examined using Olympus CX40 (Tokyo, Japan) and BX53 (Tokyo, Japan) microscopes. The circular agar blocks, circa 5 mm in diameter, from a colony were removed and placed on new PDA plates to observe the colony morphology. The colonies on PDA plates were cultured at 25 °C for 2 weeks, and the colony characteristics (size, texture and colour) were photographed with a Canon 700D camera. To observe the asexual morphological characteristics (e.g., conidiophores, phialides and conidia), Olympus CX40 and BX53 microscopes were employed.
2.3. DNA Extraction, PCR and Sequencing for Nuclear Genes
Total genomic DNA was extracted from axenic living cultures using the MiniBEST Plant Genomic DNA Extraction Kit (TaKaRa, Beijing, China), following the manufacturer’s instructions. The nuclear ribosomal small subunit (nrSSU) was amplified with the primer pair nrSSU-CoF and nrSSU-CoR [6]. The nuclear ribosomal large subunit (nrLSU) was amplified with the primer pair LR5 and LR0R [7,8]. The translation elongation factor 1α (tef-1α) was amplified with the primers EF1α-EF and EF1α-ER [9,10]. The largest and second largest subunits of RNA polymerase II (rpb1 and rpb2) were amplified with the primers RPB1-5′F and RPB1-5′R, RPB2-5′F and RPB2-5′R, respectively [9,10]. In this study, five nuclear gene loci of all the samples were amplified, and the primers used were shown in Table 1. The above five pairs were synthesized by Kunming Xiuqi Technology Co., Ltd. Each 50 µL PCR included 25 μL of 2 × Taq PCR Master Mix (Tiangen Biotech Co., Ltd., Beijing, China), 0.5 µL of each forward and reverse primer (10 μM), 1 μL of genomic DNA and 23 μL of sterilized distilled water. The polymerase chain reaction (PCR) assay was performed as described by Wang et al. [11]. The PCR products were separated by electrophoresis in 1.0% agarose gels, purified using a Gel Band Purification Kit (Bio Teke Co., Ltd., Beijing, China) and then sequenced on an automatic sequence analyser (BGI Co., Ltd., Shenzhen, China). When the PCR products could not be sequenced directly, cloning was performed using a TaKaRa PMDTM18-T vector system (TaKaRa Biotechnology Co., Ltd., Dalian, China).
Table 1.
PCR primers used in this study.
| Gene | Primer | 5′-Sequence-3′ | References |
|---|---|---|---|
| nrSSU | nrSSU-CoF | TCTCAAAGATTAAGCCATGC | [6] |
| nrSSU-CoR | TCACCAACGGAGACCTTG | ||
| nrLSU | LR5 | ATCCTGAGGGAAACTTC | [7,8] |
| LR0R | GTACCCGCTGAACTTAAGC | ||
| tef-1α | EF1α-EF | GCTCCYGGHCAYCGTGAYTTYAT | [9,10] |
| EF1α-ER | ATGACACCRACRGCRACRGTYTG | ||
| rpb1 | RPB1-5′F | CAYCCWGGYTTYATCAAGAA | [9,10] |
| RPB1-5′R | CCNGCDATNTCRTTRTCCATRTA | ||
| rpb2 | RPB2-5′F | CCCATRGCTTGTYYRCCCAT | [9,10] |
| RPB2-5′R | GAYGAYMGWGATCAYTTYGG |
2.4. Phylogenetic Analyses
Phylogenetic analyses were performed based on the nrSSU, nrLSU, tef-1α, rpb1 and rpb2 sequences. The DNA sequences generated in this study were submitted to GenBank. Reference sequences were downloaded from NCBI (http://www.ncbi.nlm.nih.gov/, accessed on 1 May 2022). The specimen information and GenBank accession numbers were provided in Table 2. The sequences were aligned using the Clustal X2.0 (developted by European Bioinformatics Institute, Cambridge, the United Kingdom) and MEGA v6.06 (developted by Tokyo Metropolitan University, Tokyo, Japan) software with manual adjustment [12,13]. The aligned sequences of five genes were concatenated after sequence alignment and specific processing according to Wang et al. [2]. Phylogenetic analyses were conducted using the Bayesian Inference (BI) and the Maximum Likelihood (ML) methods employing MrBayes v3.1.2 and RAxML 7.0.3 [14,15]. The BI analysis was run on MrBayes v3.1.2 for five million generations using a GTR + G + I model determined by the jModelTest version 2.1.4 (developted by The University of Vigo, Vigo, Spain) [16]. The GTR + I was selected as the optimal model for the ML analyses, with 1000 rapid bootstrap replicates performed on the five-gene datasets.
Table 2.
Names, voucher information, host and corresponding GenBank accession numbers of the taxa used in this study.
| Taxon | Voucher Information | Host | GenBank Accession Number | References | ||||
|---|---|---|---|---|---|---|---|---|
| nrSSU | nrLSU | tef-1α | rpb1 | rpb2 | ||||
| Akanthomyces attenuatus | CBS 402.78 | Leaf litter | AF339614 | AF339565 | EF468782 | EF468888 | EF468935 | [10] |
| coccidioperitheciatus | NHJ 6709 | Araneae | EU369110 | EU369042 | EU369025 | EU369067 | EU369086 | [17] |
| A. dipterigenus | CBS 126.27 | Hemiptera: Monophlebidae | AF339605 | AF339556 | KM283820 | KR064300 | KR064303 | Unpublished |
| A. lecanii | CBS 101247 | Hemiptera: Coccidae | AF339604 | AF339555 | DQ522359 | DQ522407 | DQ522466 | [18] |
| A. muscarius | CBS 143.62 | Hemiptera: Aleyrodidae | KM283774 | KM283798 | KM283821 | KM283841 | KM283863 | Unpublished |
| A. sabanensis | ANDES-F 1024 | Hemiptera: Coccidae | KC633251 | KC875225 | KC633266 | KC633249 | [19] | |
| A. sulphureus | TBRC 7248 | Araneae | MF140722 | MF140843 | MF140787 | MF140812 | [1] | |
| A. waltergamsii | TBRC 7252 | Araneae | MF140714 | MF140834 | MF140782 | MF140806 | [1] | |
| Amphichorda felina | YFCC 850 | Bird droppings | MW181774 | MW173986 | MW168227 | MW168193 | MW168210 | [20] |
| A. felina | YFCC 851 | Bird droppings | MW181775 | MW173987 | MW168228 | MW168194 | MW168211 | [20] |
| A. guana | CGMCC 3.17908 | Bat guano | KY883262 | KU746711 | KX855211 | KY883202 | KY883228 | [21] |
| A. guana | CGMCC 3.17909 | Bat guano | KY883263 | KU746712 | KX855212 | KY883203 | [21] | |
| Beauveria acridophila | HUA 179219 | Orthoptera: Acrididae | JQ895541 | JQ958613 | JX003857 | JX003841 | [22] | |
| B. acridophila | HUA 179220 | Orthoptera: Acrididae | JQ895527 | JQ895536 | JQ958614 | JX003852 | JX003842 | [22] |
| B. araneola | GZAC 150317 | Araneae | KT961699 | KT961701 | [23] | |||
| B. asiatica | ARSEF 4850 | Coleoptera: Cerambycidae | AY531937 | HQ880859 | HQ880931 | [24] | ||
| B. australis | ARSEF 4598 | Soil | HQ880995 | HQ880861 | HQ880933 | [24] | ||
| B. bassiana | YFCC 3369 | Coleoptera: Scarabaeidae | MN576768 | MN576824 | MN576994 | MN576884 | MN576938 | [2] |
| B. baoshanensis | CCTCC AF 2018011 | Coleoptera: Chrysomelidae | MG642882 | MG642840 | MG642897 | MG642854 | MG642867 | [25] |
| B. caledonica | YFCC 7025 | MN576771 | MN576827 | MN576997 | MN576887 | MN576941 | [2] | |
| B. kipukae | ARSEF 7032 | Homoptera: Delphacidae | HQ881005 | HQ880875 | HQ880947 | [24] | ||
| B. majiangensis | YFCC 852 | Hemiptera: Pentatomidae | MW181776 | MW173988 | MW168229 | MW168195 | MW168212 | [20] |
| B. polyrhachicola | YFCC 859 | Hymenoptera: Formicidae | MW181783 | MW173995 | MW168236 | MW168202 | MW168219 | [20] |
| B.scarabaeidicola | ARSEF 5689 | Coleoptera: Scarabaeidae | AF339574 | AF339524 | DQ522335 | DQ522380 | DQ522431 | [20] |
| B. varroae | ARSEF 8257 | Coleoptera: Curculionidae | HQ881002 | HQ880872 | HQ880944 | [24] | ||
| Blackwellomyces cardinalis | OSC 93609 | Lepidoptera: Tineidae | AY184973 | AY184962 | DQ522325 | DQ522370 | DQ522422 | [18] |
| B. cardinalis | OSC 93610 | Lepidoptera: Tineidae | AY184974 | AY184963 | EF469059 | EF469088 | EF469106 | [18] |
| B. pseudomilitaris | BCC 1919 | Lepidoptera (Larva) | MF416588 | MF416534 | MF416478 | MF416440 | [4] | |
| B. pseudomilitaris | BCC 2091 | Lepidoptera (Larva) | MF416589 | MF416535 | MF416479 | MF416441 | [4] | |
| Cordyceps amoene-rosea | CBS 107.73 | Coleoptera (Pupa) | AY526464 | MF416550 | MF416494 | MF416651 | MF416445 | [26] |
| C. amoene-rosea | CBS 729.73 | Coleoptera: Nitidulidae | MF416604 | MF416551 | MF416495 | MF416652 | MF416446 | [26] |
| C. bifusispora | EFCC 5690 | Lepidoptera (Pupa) | EF468952 | EF468806 | EF468746 | EF468854 | EF468909 | [10] |
| C. bifusispora | spat 08-133.1 | Lepidoptera (Pupa) | MF416577 | MF416524 | MF416469 | MF416631 | MF416434 | [4] |
| C. cateniobliqua | YFCC 3367 | Coleoptera adult | MN576765 | MN576821 | MN576991 | MN576881 | MN576935 | [2] |
| C. exasperata | MCA 2288 | Lepidoptera (Larva) | MF416592 | MF416538 | MF416482 | MF416639 | [4] | |
| C. militaris | YFCC 6587 | Lepidoptera (Pupa) | MN576762 | MN576818 | MN576988 | MN576878 | MN576932 | [2] |
| C. militaris | YFCC 5840 | Lepidoptera (Pupa) | MN576763 | MN576819 | MN576989 | MN576879 | MN576933 | [2] |
| C. ninchukispora | EGS 38.166 | Plant (Beilschmiedia erythrophloia) | EF468992 | EF468847 | EF468794 | EF468901 | [10] | |
| C. polyarthra | MCA 996 | Lepidoptera | MF416597 | MF416543 | MF416487 | MF416644 | [4] | |
| C. pruinosa | ARSEF 5413 | Lepidoptera: Limacodidae | AY184979 | AY184968 | DQ522351 | DQ522397 | DQ522451 | [18] |
| C. pseudotenuipes | YFCC 8404 | Lepidoptera | OL468559 | OL468579 | OL473527 | OL739573 | OL473538 | [27] |
| C. tenuipes | TBRC 7265 | Lepidoptera (Pupa) | MF140707 | MF140827 | MF140776 | MF140800 | [1] | |
| Samsoniella alboaurantium | CBS 240.32 | Lepidoptera (Pupa) | JF415958 | JF415979 | JF416019 | JN049895 | JF415999 | [1] |
| S. alboaurantium | CBS 262.58 | Soil | MF416497 | MF416654 | MF416448 | [1] | ||
| S. alpina | YFCC 5818 |
Hepialidae (Hepialus baimaensis) |
MN576753 | MN576809 | MN576979 | MN576869 | MN576923 | [2] |
| S. alpina | YFCC 5831 |
Hepialidae (Hepialus baimaensis) |
MN576754 | MN576810 | MN576980 | MN576870 | MN576924 | [2] |
| S. antleroides | YFCC 6016 | Noctuidae (Larvae) | MN576747 | MN576803 | MN576973 | MN576863 | MN576917 | [2] |
| S. antleroides | YFCC 6113 | Noctuidae (Larvae) | MN576748 | MN576804 | MN576974 | MN576864 | MN576918 | [2] |
| S. aurantia | TBRC 7271 | Lepidoptera | MF140728 | MF140846 | MF140791 | MF140818 | [1] | |
| S. aurantia | TBRC 7272 | Lepidoptera | MF140727 | MF140845 | MF140817 | [1] | ||
| S. cardinalis | YFCC 5830 | Limacodidae (Pupa) | MN576732 | MN576788 | MN576958 | MN576848 | MN576902 | [2] |
| S. cardinalis | YFCC 6144 | Limacodidae (Pupa) | MN576730 | MN576786 | MN576956 | MN576846 | MN576900 | [2] |
| S. coccinellidicola | YFCC 8772 | Coccinellidae | ON563166 | ON621670 | ON676514 | ON676502 | ON568685 | This study |
| S. coccinellidicola | YFCC 8773 | Coccinellidae | ON563167 | ON621671 | ON676515 | ON676503 | ON568686 | This study |
| S. coleopterorum | A19501 | Curculionidae (Snout beetle) | MN101586 | MT642600 | MN101585 | [28] | ||
| S. cristata | YFCC 6021 | Saturniidae (Pupa) | MN576735 | MN576791 | MN576961 | MN576851 | MN576905 | [2] |
| S. cristata | YFCC 7004 | Saturniidae (Pupa) | MN576737 | MN576793 | MN576963 | MN576853 | MN576907 | [2] |
| S. farinospora | YFCC 8774 | Araneae (Spider) | ON563168 | ON621672 | ON676516 | ON676504 | ON568687 | This study |
| S. farinospora | YFCC 9051 | Lepidoptera : Hepialus | ON563169 | ON621673 | ON676517 | ON676505 | ON568688 | This study |
| S. haniana | YFCC 8769 | Lepidoptera (pupa) | ON563170 | ON621674 | ON676518 | ON676506 | ON568689 | This study |
| S. haniana | YFCC 8770 | Lepidoptera (pupa) | ON563171 | ON621675 | ON676519 | ON676507 | ON568690 | This study |
| S. haniana | YFCC 8771 | Lepidoptera (pupa) | ON563172 | ON621676 | ON676520 | ON676508 | ON568691 | This study |
| S. hepiali | ICMM 82-2 | Fungi (O. sinensis) | MN576738 | MN576794 | MN576964 | MN576854 | MN576908 | [2] |
| S. hepiali | YFCC 661 | Fungi (O. sinensis) | MN576739 | MN576795 | MN576965 | MN576855 | MN576909 | [2] |
| S. hepiali | Cor-4 | Fungi (O. sinensis) | MN576743 | MN576799 | MN576969 | MN576859 | MN576913 | [2] |
| S. hymenopterorum | A19521 | Vespidae (Bee) | MN101588 | MT642603 | MT642604 | [28] | ||
| S. hymenopterorum | A19522 | Vespidae (Bee) | MN101591 | MN101589 | MN101590 | [28] | ||
| S. inthanonensis | TBRC 7915 | Lepidoptera (Pupa) | MF140725 | MF140849 | MF140790 | MF140815 | [1] | |
| S. kunmingensis | YHH 16002 | Lepidoptera (Pupa) | MN576746 | MN576802 | MN576972 | MN576862 | MN576916 | [2] |
| S. lanmaoa | YFCC 6148 | Lepidoptera (Pupa) | MN576733 | MN576789 | MN576959 | MN576849 | MN576903 | [2] |
| S. lanmaoa | YFCC 6193 | Lepidoptera (Pupa) | MN576734 | MN576790 | MN576960 | MN576850 | MN576904 | [2] |
| S. pseudogunii | GY407201 | Lepidoptera (Larvae) | MZ827010 | MZ855233 | MZ855239 | [29] | ||
| S. pseudogunii | GY407202 | Lepidoptera (Larvae) | MZ831865 | MZ855234 | MZ855240 | [29] | ||
| S. pseudotortricidae | YFCC 9052 | Lepidoptera (pupa) | ON563173 | ON621677 | ON676521 | ON676509 | ON568692 | This study |
| S. pseudotortricidae | YFCC 9053 | Lepidoptera (pupa) | ON563174 | ON621678 | ON676522 | ON676510 | ON568693 | This study |
| S. pupicola | DY101681 | Lepidoptera (Pupa) | MZ827009 | MZ855231 | MZ855237 | [29] | ||
| S. pupicola | DY101682 | Lepidoptera (Pupa) | MZ827635 | MZ855232 | MZ855238 | [29] | ||
| S. ramosa | YFCC 6020 | Limacodidae (Pupa) | MN576749 | MN576805 | MN576975 | MN576865 | MN576919 | [2] |
| S. sinensis | YFCC 8766 | Lepidoptera (Larvae) | ON563175 | ON621679 | ON676523 | ON676511 | ON568694 | This study |
| S. sinensis | YFCC 8767 | Dermaptera | ON563176 | ON621680 | ON676524 | ON676512 | ON568695 | This study |
| S. sinensis | YFCC 8768 | Dermaptera | ON563177 | ON621681 | ON676525 | ON676513 | ON568696 | This study |
| S. tortricidae | YFCC 6013 | Tortricidae (Pupa) | MN576751 | MN576807 | MN576977 | MN576867 | MN576921 | [2] |
| S. tortricidae | YFCC 6131 | Tortricidae (Pupa) | MN576750 | MN576806 | MN576976 | MN576866 | MN576920 | [2] |
| S. yunnanensis | YFCC 1527 | Fungi (Cordyceps cicadae) | MN576756 | MN576812 | MN576982 | MN576872 | MN576926 | [2] |
| S. yunnanensis | YFCC 1824 | Fungi (Cordyceps cicadae) | MN576757 | MN576813 | MN576983 | MN576873 | MN576927 | [2] |
| Simplicillium formicae | MFLUCC 18-1379 | Hymenoptera: Formicidae | MK765046 | MK766512 | MK926451 | MK882623 | [30] | |
| S. lamellicola | CBS 116.25 | Fungi (Agaricus bisporus) | AF339601 | AF339552 | DQ522356 | DQ522404 | DQ522462 | [18] |
| S. lanosoniveum | CBS 704.86 | Fungi (Hemileia vastatrix) | AF339602 | AF339553 | DQ522358 | DQ522406 | DQ522464 | [18] |
| S. lanosoniveum | CBS 101267 | Fungi (Hemileia vastatrix) | AF339603 | AF339554 | DQ522357 | DQ522405 | DQ522463 | [18] |
| S. obclavatum | CBS 311.74 | Air above sugarcane field | AF339567 | AF339517 | EF468798 | [10] | ||
| S. yunnanense | YFCC 7133 | Fungi (A. waltergamsii) | MN576728 | MN576784 | MN576954 | MN576844 | [2] | |
| Trichoderma deliquescens | ATCC 208838 | On decorticated conifer wood | AF543768 | AF543791 | AF543781 | AY489662 | DQ522446 | [31] |
| T. stercorarium | ATCC 62321 | Cow dung | AF543769 | AF543792 | AF543782 | AY489633 | EF469103 | [31] |
Boldface: data generated in this study.
3. Results
3.1. Sequencing and Phylogenetic Analyses
The 92 taxa of eight genera—Akanthomyces, Amphichorda, Beauveria, Blackwellomyces, Cordyceps, Samsoniella, Simplicillium and Trichoderma—were used for the ML and BI phylogenetic analyses. Two Trichoderma strains (Trichoderma deliquescens ATCC 208838 and Trichoderma stercorarium ATCC 62321) were designated as the outgroup. The concatenated sequence dataset of the five genes consisted of 4642 bp of sequence data (1055 bp for nrSSU, 897 bp for nrLSU, 969 bp for tef-1α, 756 bp for rpb1 and 965 bp for rpb2). Both phylogenetic trees from the BI and ML analyses exhibited similar topologies that had seven recognized, statistically well-supported clades in Cordycipitaceae, designated as Akanthomyces, Amphichorda, Beauveria, Blackwellomyces, Cordyceps, Samsoniella and Simplicillium (Figure 1). Most of the well-resolved genera and lineages in Cordycipitaceae shared similar relationships with previous analyses [1,4,10]. The 12 samples of five undescribed species also clustered in the genus Samsoniella clade based on the phylogenetic analyses of the combined dataset and were clearly distinct from S. hepiali and 16 described species, viz., S. alboaurantia, S. alpina, S. antleroides, S. aurantia, S. cardinalis, S. coleopterorum, S. cristata, S. hymenopterorum, S. inthanonensis, S. kunmingensis, S. lanmaoa, S. pseudogunii, S. pupicola, S. ramosa, S. tortricidae and S. yunnanensis (Figure 1). Similarly, phylogenetic relationships between the genus Samsoniella and closely related species, based on multigene dataset (nrLSU, nrSSU, tef-1α, rpb1 and rpb2) (see Figure 2). Both phylogenetic trees from the BI and ML analyses exhibited similar topologies and the five undescribed species also clustered in the genus Samsoniella clade that were clearly distinct from S. hepiali and 16 described species.
Figure 1.
Phylogenetic tree of Cordycipitaceae inferred from multigene dataset (nrLSU, nrSSU, tef-1α, rpb1 and rpb2) based on maximum likelihood (ML) and Bayesian inference (BI) analyses. Statistical support values greater than 50% are shown at the nodes for BI posterior probabilities/ML bootstrap proportions. Isolates in bold type are those analysed in this study.
Figure 2.
Phylogenetic relationships between the genus Samsoniella and closely related species, based on multigene dataset (nrLSU, nrSSU, tef-1α, rpb1 and rpb2). Statistical support values greater than 50% are shown at the nodes for BI posterior probabilities/ML bootstrap proportions. Isolates in bold type are those analysed in this study.
SYNOPTIC KEYS
Samsoniella
Samsoniella alboaurantium
Samsoniella alpina
Samsoniella antleroides
Samsoniella aurantia
Samsoniella cardinalis
Samsoniella coccinellidicola
Samsoniella coleopterorum
Samsoniella cristata
Samsoniella farinospora
Samsoniella haniana
Samsoniella hepiali
Samsoniella hymenopterorum
Samsoniella inthanonensis
Samsoniella kunmingensis
Samsoniella lanmaoa
Samsoniella lepidopterorum
Samsoniella pseudogunii
Samsoniella pseudotortricidae
Samsoniella pupicola
Samsoniella ramosa
Samsoniella sinensis
Samsoniella tortricidae
Samsoniella yunnanensis
| Teleomorph characters |
| Insect host |
| 1. Lepidoptera (pupa,larva) .................................................................................................3, 5, 8, 13, 14, 15, 18, 20, 22 |
| Stromata |
| 1. Number |
| a. Fasciculate ................................................................................................................................................3, 13, 20, 22 b. Several ...................................................................................................................................................................5 c. Solitary or two ........................................................................................................................................................8 d. Solitary or Several ...................................................................................................................................................18 e. Solitary ....................................................................................................................................................................14 f. Two to five ................................................................................................................................................................15 |
| 2. Size (long) |
| a. 10~20 mm ..................................................................................................................................................................... 5 b. 15~40 mm .......................................................................................................................................................8, 14, 20 c. 20~70 mm ..............................................................................................................................................3, 13, 15, 18, 22 |
| 3. Shape |
| a. Cylindrical to clavate, branches .........................................................................................................................3, 13 b. Cylindrical, unbranches .............................................................................................................................................5 c. Cylindrical, unbranches or dichotomous ..............................................................................................................18 d. Crista-like, much branched .......................................................................................................................................8 e. Cylindrical to clavate, bifurcated ...........................................................................................................................14 f. Palmately branched ..................................................................................................................................................15 g. Fascicular, multi-branched, often confluent at the base .....................................................................................20 h. Unbranched or dichotomous ...................................................................................................................................22 |
| Fertile parts |
| 1. Shape |
| a. Clavate to fake-like ....................................................................................................................................................3 b. Clavate ...............................................................................................................................................................5, 14, 15 c. Clavate to subulate ....................................................................................................................................................18 d. Crista-like or subulate .........................................................................................................................................8, 22 e. Having no obvious boundary with stipes, with a tapering sterile part ...........................................................20 |
| 2. Colour |
| a. Orange red ................................................................................................................................................................13 b. Orange to orange red ................................................................................................................................................3 c. Scarlet ..........................................................................................................................................................................5 d. Reddish orange ........................................................................................................................................8, 14, 15, 18 e. White to pale brown ..........................................................................................................................................20, 22 |
| Perithecia |
| 1. shape |
| a. Superficial, ovoid ........................................................................................................................................................13 b. Superficial, fusiform ................................................................................................................................................3 c. Superficial, oblong-ovate to fusiform ...................................................................................................................5 d. Superficial, narrowly ovoid ..................................................................................................................................8 e. Superficial, narrowly ovoid to fusiform ....................................................................................14, 15, 18, 20, 22 |
| 2. Length |
| a. 280~450 µm ................................................................................................................................................3, 14, 18, 20 b. 330~470 µm ..........................................................................................................................................................15, 22 c. 370~490 µm ........................................................................................................................................................5, 8, 13 |
| 3. Width |
| a. 110~210 µm ......................................................................................................................................... 14, 15, 18, 20 b. 130~250 µm ................................................................................................................................................3, 5, 8, 22 c. 200~260 µm ..............................................................................................................................................................13 |
| Asci |
| 1. Shape |
| a. Cylindrical, eight-spored, hyaline ...........................................................................................3, 5, 8, 13, 14, 15, 22 |
| 2. Length |
| a. 150~300 µm ............................................................................................................................................3, 13, 14, 22 b. 160~360 µm .....................................................................................................................................................5, 8, 15 |
| 3. Width |
| a. 2~3 µm ................................................................................................................................................................3, 13 b. 2.5~4 µm .................................................................................................................................................................22 c. 3~5 µm .......................................................................................................................................................5, 8, 14, 15 |
| Ascospores |
| 1. Shape |
| a. Bola-shaped, septate, central part fliform, terminal part narrowly fusiform .....................3, 5, 8, 13, 14, 15, 22 |
| 2. Length |
| a. 110~185 µm ................................................................................................................................................................3 b. 120~300 µm .....................................................................................................................................5, 8, 13, 14, 15, 22 |
| 3. Width |
| a. 0.5~1.0 µm ......................................................................................................................................................... 5, 13 b. 0.8~1.5 µm ............................................................................................................................................3, 8, 14, 15, 22 |
| Anamorph characters |
| Insect host |
| 1. Lepidoptera (pupa, larva) .................................................................................1, 2, 4, 9, 10, 11, 16, 17, 19, 21, 23 2. Dermaptera ................................................................................................................................................................21 3. Coleoptera (Snout beetle) .....................................................................................................................................6, 7 4. Hymenoptera (bee) ....................................................................................................................................................12 5. Araneae .......................................................................................................................................................................9 |
| Synnemata |
| 1. Present ......................................................................................................................................2, 4, 6, 10, 11, 21, 23 |
| Irregularly branched ................................................................................................................................2, 4, 6, 10, 21 b. Branched or unbranched ........................................................................................................................................11 c. Gregarious ................................................................................................................................................................23 |
| 2. Not observed ..............................................................................................................................1, 7, 9, 12, 16, 17, 19 |
| Cultural characteristics on PDA |
| 1. Growth rate on PDA at 25 °C at 2 wk |
| a. Relatively rapid (>60 mm diam) ..........................................................................................................................12 b. Moderate (30–60 mm diam) ...........................................................................................2, 6, 7, 9, 11, 16, 17, 21, 23 c. Slow (<30 mm diam) ......................................................................................................................................4, 10, 19 |
| 2. Conidiophores |
| a. Biverticillate ............................................................................................................................................................2 b. Solitary .............................................................................................................................................................11, 19 c. Solitary or verticillate .........................................................................................................6, 7, 9, 10, 12, 16, 21, 23 d. Verticillate .......................................................................................................................................................1, 4, 17 |
| 3. Phialides number in a whorls |
| a. 2–4 ..............................................................................................................................................................1, 4, 7, 9, 16 b. 2–5 ...........................................................................................................................................................6, 10, 11, 21 c. 2–7 .......................................................................................................................................................................2, 23 b. 2–9 .....................................................................................................................................................................17, 19 e. 3–4 .............................................................................................................................................................................12 |
| 4. Shape of conidia |
| a. fusiform or oval ............................................................................................................................2, 4, 6, 10, 11, 12, 23 b. fusiform, ellipsoidal or subglobose ..........................................................................................................................7 c. fusiform to subglobose ............................................................................................................................................16 d. fusiform ..............................................................................................................................................................17, 19 e. ellipsoidal to fusiform, sometimes lemon-shaped ...................................................................................................1 f. oblong to cylindrical ..................................................................................................................................................9 g. spherical, elliptical or fusiform .................................................................................................................................21 |
| Key to Samsoniella species |
| Sexual state present .........................................................................................................................................................1 |
| Sexual state not observed ................................................................................................................................................5 |
| 1a. Stromata fasciculate ...................................................................................................................................................2 1b. Stromata not fasciculate ...........................................................................................................................................4 2a. Stromata cylindrical to clavate, branches ..........................................................................................................................3 2b. Stromata fascicular, multi-branched, often confluent at the base ..................................................................S. ramosa 2c. Stromata unbranched or dichotomous .........................................................................................................S. tortricidae 3a. Fertile parts clavate to fake-like, orange to orange red; Perithecia superficial, fusiform .......................S. antleroides 3b. Fertile parts clavate, orange red; Perithecia superficial, ovoid ..............................................................S. inthanonensis 4a. Stromata several, cylindrical, unbranches; Fertile parts clavate, scarlet .....................................................S. cardinalis 4b. Stromata solitary or two, crista-like, much branched; Fertile parts crista-like or subulate, reddish orange; Ascospores 155–290 × 1.0–1.3 μm ...................................................................................................................................S. cristata 4c. Stromata solitary or several, cylindrical, unbranches or dichotomous; Fertile parts clavate to subulate, reddish orange; No mature ascospores were observed ........................................................................................S. pseudotortricidae 4d. Stromata solitary, cylindrical to clavate, bifurcated; Fertile parts clavate, reddish orange; Ascospores 127–190 × 0.5–1.5 μm ..........................................................................................................................................................S. kunmingensis 4e. Stromata two to five, palmately, branched; Fertile parts clavate, reddish orange; Ascospores 135–260 × 0.9–1.4 μm .................................................................................................................................................................................S. lanmaoa 5a. Synnemata Present .................................................................................................................................................................6 5b. Synnemata not observed ......................................................................................................................................................11 6a. Synnemata irregularly branched ..........................................................................................................................................7 6b. Synnemata branched or unbranched; Conidiophores solitary, with phialides in whorls of two to five ...................................................................................................................................................................................S. hepiali 6c. Synnemata gregarious; Conidiophores solitary or verticillate, with phialides in whorls of two to seven .....................................................................................................................................................................S. yunnanensis 7a. Colonies on PDA at 25 °C at 2 wk growing moderate (30–60 mm diam) ......................................................................8 7b. Colonies on PDA at 25 °C at 2 wk growing slow (<30 mm diam) ................................................................................10 8a. Conidiophores biverticillate....................................................................................................................................S. alpina 8b. Conidiophores solitary or verticillate ................................................................................................................................9 9a. Conidia fusiform or oval, 1.8–3.0 × 1.3–2.0 μm .....................................................................................S. coccinellidicola 9b. Conidia spherical, elliptical or fusiform, 2.0–3.1 × 1.3–1.9 μm .........................................................................S. sinensis 10a. Conidiophores solitary or verticillate, with phialides in whorls of two to five; Conidia fusiform or oval, 2.3–3.7 × 1.2–2.8 μm ...................................................................................................................................................................S. haniana 10b. Conidiophores verticillate, with phialides in whorls of two to four; Conidia fusiform or oval, 2.5–3.5 × 1.5 μm .................................................................................................................................................................................S. aurantia 11a. Colonies on PDA at 25 °C at 2 wk growing relatively rapid (>60 mm diam) ..............................S. hymenopterorum 11b. Colonies on PDA at 25 °C at 2 wk growing moderate (30–60 mm diam) ..................................................................12 11c. Colonies on PDA at 25 °C at 2 wk growing slow (<30 mm diam) ...............................................................S. pupicola 12a. Conidiophores verticillate ...............................................................................................................................................13 12b. Conidiophores solitary or verticillate ............................................................................................................................14 13a. Have phialides in whorls of two to four; Conidia ellipsoidal to fusiform, sometimes lemon-shaped, 2.0–3.0 × 1.0–1.8 μm ..........................................................................................................................................................S. alboaurantium 13b. Have phialides in whorls of two to nine; Conidia fusiform, 2.8–3.2 × 1.7–2.1 μm .............................S. pseudogunii 14a. Conidia fusiform, ellipsoidal or subglobose, 1.7–2.5 × 1.2–1.8 μm ...................................................S. coleopterorum 14b. Conidia oblong to cylindrical, 1.6–2.8 × 0.6–1.2 μm ..................................................................................S. farinospora 14c. Conidia fusiform to subglobose, 2.0–2.5 × 1.2–2.0 μm .......................................................................S. lepidopterorum |
3.2. Taxonomy
The key morphological characteristics that distinguish the current Samsoniella species were summarized in the literature (Table 3 and Table 4). Including the five new species, there were 23 species of Samsoniella involved in the current study, among which we compared 9 species of the sexual morphs in Samsoniella (Table 3) and 22 species of the asexual morphs in Samsoniella (Table 4).
Table 3.
Comparison between the sexual morphs in Samsoniella.
| Species | Stromata (mm) | Fertile Part (mm) | Perithecia (μm) | Asci (μm) | Ascospores (μm) | Reference |
|---|---|---|---|---|---|---|
| Samsoniella antleroides | fasciculate, antler-like, cylindrical to clavate, long 22.3–57.8, oblate terminal branches, long 4.6–26.2 | clavate to fake-like, lateral sides have a longitudinal ditch without producing perithecia, 6.3–9.5 × 0.6–2.3 | superficial, fusiform, 294–442 × 131–216 |
cylindrical, 8-spored, 160–248 × 2.1–2.7 |
bola-shaped, septate, 110–184 × 0.8–1.3 | [2] |
| S. cardinalis | several, cylindrical, long 11.5–18.6 | clavate, lateral sides have a longitudinal ditch without producing perithecia, 2.5–6.8 × 0.5–2.6 | superficial, oblong-ovate to fusiform, 370–485 × 140–238 | cylindrical, 8-spored, 163–320 × 3.2–4.3 | bola-shaped, septate, 165–230 × 0.5–0.9 | [2] |
| S. cristata | solitary or two, crista-like, long 25–40, much branched | crista-like or subulate, 3.1–18.5 × 0.9–8.0 | superficial, narrowly ovoid, 370–485 × 150–245 | cylindrical, 8-spored, 180–356 × 3.0–4.8 | bola-shaped, septate, 155–290 × 1.0–1.3 | [2] |
| S. inthanonensis | gregarious, cylindrical to clavate, long 20–50, 1–1.5 broad | 8–15 long, 1.5–2 broad | superficial, ovoid, (380–)417.5–474.5(–500) × (150–)205–260(–265) | cylindrical, 8-spored, 300 × 2–2.5 | bola-shaped, 3 or 4septate, 221.5–267 × 0.5–1 |
[1] |
| S. kunmingensis | solitary, cylindrical to clavate, long 23, bifurcated, | clavate, lateral sides usually have a longitudinal ditch without producing perithecia, 3.3–4.2 × 0.8–1.2 | superficial, narrowly ovoid to fusiform, 330–395 × 110–185 |
cylindrical, 8-spored, 150–297 × 3.0–4.6 |
bola-shaped, septate, 127–190 × 0.8–1.5 | [2] |
| S. pseudotortricidae | solitary to several, long 20–65, unbranched or dichotomous | clavate to subulate, lateral side usually have a longitudinal section without producing perithecia, 10–17 × 1.5–4.2 | superficial, narrowly ovoid to fusiform, 285.7–313.2 × 149.2–154.9 | This study | ||
| S. lanmaoa | two to five, orange, long 38–69, palmately branched | clavate, lateral sides usually have a longitudinal ditch without producing perithecia, 8.5–11.2 × 0.6–2.3 | superficial, narrowly ovoid to fusiform, 360–467 × 124–210 |
cylindrical, 8-spored, 160–325 × 3.3–4.8 |
bola-shaped, septate, 135–260 × 0.9–1.4 | [2] |
| S. ramosa | fascicular, 15–32 × 0.8–1.5, multi-branched, often confluent at the base | having no obvious boundary with stipes | superficial, narrowly ovoid to fusiform, 340–435 × 130–197 | [2] | ||
| S. tortricidae | gregarious, long 25–60, unbranched or dichotomous | clavate to subulate, lateral side usually has a longitudinal section without producing perithecia, 5–15 × 1.2–2.3 | superficial, narrowly ovoid to fusiform, 350–468 × 140–225 | cylindrical, 8-spored, 170–285 × 2.8–4.0 | bola-shaped, septate, 120–235 × 0.8–1.3 | [2] |
Boldface: data generated in this study.
Table 4.
Comparison between the asexual morphs in Samsoniella.
| Species | Synnemata (mm) | Conidiophores (μm) | Phialides | Phialides Size (μm) | Conidia (μm) | References |
|---|---|---|---|---|---|---|
| Samsoniella alboaurantium | 30–400 × 2–2.5 |
5–8 × 2, tapering fairly abruptly at the tip | ovate to lemon-shaped, 2.3–2.5(–3) × 1.5–1.8 | [32] | ||
| S. alpina | irregularly branched, 3–20 long, cylindrical or clavate stipes with white powdery heads | 3.1–6.5 × 1.6–2.8 | verticillate on conidiophores, solitary or verticillate on hyphae | 4.7–9.5 × 1.9–3.1, wide (apex) 0.5–1.1, basal portion cylindrical to narrowly lageniform, tapering abruptly toward the apex | fusiform or oval, 2.0–3.1 × 1.3–2.1 |
[2] |
| S. antleroides | 3.5–9.7 × 1.3–3.2 | verticillate, in whorls of 2 to 9, sometimes solitary on hyphae | 3.5–16.3 × 1.7–2.9, wide (apex) 0.5–1.0, basal portion cylindrical to narrowly lageniform, tapering abruptly toward the apex | fusiform or oval, 2.3–3.5 × 1.6–2.5 |
[2] | |
| S. aurantia | irregularly branched starting 15–40 above the ground and continuously to the apex, 25–75 × 1–1.5 | verticillate, in whorls of 2 to 4 | (5–)5.5–8.5(–13) × 2–3, basal portion cylindrical to ellipsoidal, neck 2–4 × 1 | fusiform, (2–)2.5–3.5(–4) × (1–)1.5(–2) |
[1] | |
| S. cardinalis | 3.1–9.5 × 1.3–2.0 | verticillate, in whorls of 2 to 5, sometimes solitary on hyphae | 4.1–43.5 × 1.3–2.4, wide (apex) 0.6–1.2, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 2.4–3.2 × 1.4–2.2 |
[2] | |
| S. coccinellidicola | I rregularly branched, starting 2–2.5 above the cocoons of insect host, 15–25 × 0.8–1.2 | 4.8–15 × 1.0–1.9 | verticillate, usually in whorls of 2 to 5, or solitary on hyphae | 6.0–14.1 × 1.0–2.0 wide (apex) 0.3–0.8, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 1.8–3.0 × 1.3–2.0 | This study |
| S. coleopterorum | verticillate, in whorls of 2 to 4 | 5.4–9.7 × 1.2–1.8, a cylindrical to ellipsoidal basal portion, tapering into a short distinct neck | fusiform, ellipsoidal or subglobose, 1.7–2.5 × 1.2–1.8 |
[28] | ||
| S. cristata | 3.6–11.5 × 1.7–2.5 | verticillate, in whorls of 2 to 5, usually solitary on hyphae | 4.5–23.2 × 1.6–2.7, wide (apex) 0.5–1.1, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 2.4–3.2 × 1.6–2.3 | [2] | |
| S. farinospora | 2.4–14.0 × 0.9–1.8 | verticillate, usually in whorls of 2 to 4, or solitary on hyphae | 3.0–13.5 × 0.6–1.6, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | oblong to cylindrical, 1.6–2.8 × 0.6–1.2 | This study | |
| S. haniana | usually unbranched or irregularly branched at the apex, 20–40 × 1–1.8 | 3.8–10.2 × 1.1–2.9 | verticillate, usually in whorls of 2 to 5, or solitary on hyphae | 5.4–12.1 × 1.2–2.9, wide (apex) 0.3–1.1 basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval , 2.3–3.7 × 1.2–2.8 | This study |
| S. hepiali | branched or unbranched, 5–41 long | 4.0–7.6 × 1.4–2.2 | verticillate, in whorls of 2 to 5, solitary or opposite on hyphae | 3.5–13.6 × 1.3–2.1, wide (apex) 0.5–1.0, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 1.8–3.3 × 1.4–2.2 | [2] |
| S. hymenopterorum | Verticillate, in whorls of 3 to 4 | 6.5–10.6 × 1.2–2.0, a cylindrical basal portion, tapering to a distinct neck | fusiform to ovoid, 1.9–2.5 × 1.5–2.1 |
[28] | ||
| S. inthanonensis | verticillate in whorls of 2 to 5, sometimes solitary on hyphae | (4–)6.5–10(–12) × (1–)1.5–2(–3), cylindrical basal portion, tapering into a long neck, (1–)2.5(–4) × 0.5–1 | short fusiform, (2–)3(–3.5) × 1.5–2 |
[1] | ||
| S. lanmaoa | 3.8–13.3 × 1.5–2.1 | verticillate, in whorls of 2 to 6, usually solitary on hyphae | 3.5–20.7 × 1.7–2.6, wide (apex) 0.5–1.1, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 1.9–2.7 × 1.4–2.0 | [2] | |
| S. lepidopterorum | Verticillate, in whorls of 2 to 4 | 5.2–8.5 (–13.1) × 1.1–1.7, ellipsoidal basal portion, tapering into a distinct neck | fusiform to subglobose, 2.0–2.5 × 1.2–2.0 |
[28] | ||
| S. pseudotortricidae | 6.6–26.5 × 1.1–2.5 | verticillate, in whorls of 2 to 5, usually solitary on hyphae | 5.4–6.9 × 1.0–1.6, wide (apex) 0.5–0.8 basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 0.9–1.5 × 0.8–1.3 | This study | |
| S. pseudogunnii | solitary or in whorls of 2 to 9 | 6.8–11.0 × 2.2–2.4, cylindrical basal portion, tapering into a short distinct neck | fusiform 2.8–3.2 × 1.7–2.1 |
[29] | ||
| S. pupicola | solitary or in whorls of 2 to 9 | 7.0–9.2 × 2.5–3.3, a cylindrical basal portion, tapering into a short distinct neck | fusiform, 2.5–3.3 × 2.2–2.6 |
[29] | ||
| S. ramosa | 4.3–10.5 × 1.3–2.4 |
verticillate, in whorls of 2 to 6, usually solitary on hyphae | 5.3–14.6 × 1.3–2.8, wide (apex) 0.6–1.2, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 2.0–3.6 × 1.5–2.2 |
[2] | |
| S. sinensis | 3.5 – 5 long , branched, conidia in abundance at the apex. | 6.4–10.5 × 1.7–2.1 | verticillate, in whorls of 2 to 5, sometimes solitary on hyphae | 5.6–9.3 × 1.5–2.1, wide (apex) 0.6–1.0 basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | spherical, elliptical or fusiform, 2.0 – 3.1 × 1.3–1.9 | This study |
| S. tortricidae | 4.2–12.5 × 1.4–2.4 | verticillate, in whorls of 2 to 5, usually solitary on hyphae | 3.6–42.4 × 1.1–2.6, wide (apex) 0.4–0.9, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex |
fusiform or oval, 2.1–3.0 × 1.3–1.7 | [2] | |
| S. yunnanensis | gregarious, flexuous, fleshy, 4–18 long, with terminal branches of 3–7 × 1.0–2.0 | 4.2–23.5 × 1.4–2.3 | verticillate, in whorls of 2 to 7, usually solitary on hyphae | 4.5–11.6 × 1.2–2.4, wide (apex) 0.6–1.0, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex | fusiform or oval, 2.0–3.3 × 1.1–2.2 | [2] |
Boldface: data generated in this study.
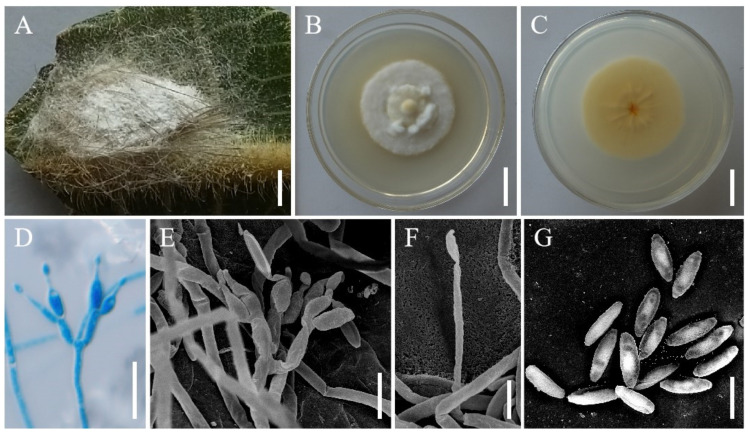
Samsoniella coccinellidicola H. Yu, Y. Wang & Z.Q. Wang, sp. nov. (Figure 3).
Figure 3.
Morphology of Samsoniella coccinellidicola. (A) Coccinellidae infected by S. coccinellidicola. (B) Culture character on PDA medium. (C–E) Conidiogenous cells (conidiophores, phialides) and conidia on PDA. (F) Conidia on PDA. Scale A: 5 mm; B: 10 mm; C–E: 10 µm; F: 5 µm.
MycoBank: MB 844383.
Etymology: “coccinellidicola” refers to the host (Coleoptera: Coccinellidae).
Holotype: China, Yunnan Province, Kunming City, Xishan Forest Park. On the Coccinellidae buried in soil, 12 August 2017, Yao Wang, (YHH 20178, holotype; YFCC 8772, ex-holotype living culture).
Sexual morph: Undetermined.
Asexual morph: Two synnemata arising from oval cocoons of insect host. Synnemata erect, irregularly branched, starting 2–2.5 mm above the oval cocoons of insect host, 15–25 × 0.8–1.2 mm, pale yellow, isaria-like morph producing a mass of conidia along the synnemata, powdery and floccose. Colonies on PDA fast-growing, 49–52 mm diameter in 14 days at 25 °C, white, cottony, sporulating abundantly, reverse white to pale yellow. Hyphae smooth-walled, branched, septate, hyaline, 0.7–2.1 µm wide. Conidiophores smooth-walled, cylindrical, solitary or verticillate, 4.8–15 × 1.0–1.9 µm. Phialides verticillate, usually in whorls of two to five, or solitary on hyphae, 6.0–14.1 µm long, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex, from 1.0–2.0 µm wide (base) to 0.3–0.8 µm wide (apex). Conidia smooth and hyaline, fusiform or oval, one-celled, 1.8–3.0 × 1.3–2.0 µm, often in chains. Size and shape of phialides and conidia similar in culture and on natural substratum.
Host: Coccinellidae.
Habitat: On the adults of Coccinellidae sp. buried in soil.
Distribution: Currently only known in Kunming City, Yunnan Province, China.
Other material examined: China, Yunnan Province, Kunming City, Xishan Forest Park. On the Coccinellidae buried in soil, 12 August 2017, Yao Wang (YHH 20179; YFCC 8773, living culture).
Notes: The phylogenetic analysis of five genes showed that S. coccinellidicola was closely related to S. pupicola. Morphologically, the new species S. coccinellidicola was distinctly different from S. pupicola due to its longer phialides (6.0–14.1 µm), smaller conidia (1.8–3.0 × 1.3–2.0 µm) and conidia shape. Moreover, S. coccinellidicola was found to occur on an adult beetle (Coleoptera: Coccinellidae), while S. pupicola was found on a Lepidopteran pupa. Based on the previous studies of cordycipitaceous isaria-like fungi as well as our study, there were two species of parasitic Samsoniella in the order Coleoptera, i.e., S. coccinellidicola and S. coleopterorum. However, S. coccinellidicola was easily distinguished from S. coleopterorum by its longer phialides (6.0–14.1 µm).
Samsoniella farinospora H. Yu, Y. Wang & Z.Q. Wang, sp. nov. (Figure 4).
Figure 4.
Morphology of Samsoniella farinospora. (A) Spider infected by S. farinospora. (B,C) Culture character on PDA medium. (D–F) Conidiogenous cells (conidiophores, phialides) and conidia on PDA. (G) Conidia on PDA. Scale A: 2 mm; B,C: 20 mm; D,E: 5 µm; F,G: 3 µm.
MycoBank: MB 844384.
Etymology: The species name refers to the farinose conidia covering the host.
Holotype: Vietnam, Dole Province, Chu Yang Sin National Park. On a spider on the back of fresh leaves, 22 October 2017, Hong Yu (YHH 20180, holotype; YFCC 8774, ex-holotype living culture).
Sexual morph: Undetermined.
Asexual morph: Mycosed hosts covered by dense white to lavender mycelia, produces numerous white, powdery conidia. Colonies on PDA fast-growing, 47–50 mm in diameter after 14 days at 25 °C, villiform, light yellow in the middle with a white edge, middle hyphae thickening, reverse light yellow. Hypha smooth-walled, hyaline, septate, 0.7–1.8 µm wide. Conidiophores smooth-walled, cylindrical, solitary or verticillate, 2.4–14.0 × 0.9–1.8 µm. Phialides verticillate, usually in whorls of two to four or solitary on hyphae, 3.0–13.5 µm long, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex, from 0.6–1.6 µm wide (base). Conidia smooth and hyaline, oblong to cylindrical, one-celled, 1.6–2.8 × 0.6–1.2 µm, often in chains. Size and shape of phialides and conidia similar in culture and on natural substratum.
Host: Spider, larva of Hepialus.
Habitat: On a spider on the back of fresh leaves, with a larva of Hepialus clinging to fallen leaves.
Distribution: Currently only known in Chu Yang Sin National Park, Dole Province, Vietnam.
Other material examined: Vietnam, Dole Province, Chu Yang Sin National Park. On a larva of Hepialus clinging to fallen leaves, 26 October 2017, Hong Yu (YHH 20188; YFCC 9051, living culture).
Notes: Morphologically, S. farinospora resembled the phylogenetically sister species S. hepiali. They had the same host, the Hepialid larva, and isaria-like asexual conidiogenous structures, producing synnemata with powdery conidia at the apex. However, S. farinospora was also found to occur on a spider. Parasitic Samsoniella species on spiders had rarely been reported. In addition, our morphological observation revealed a significant difference in conidia sizes between S. farinospora (1.6–2.8 × 0.6–1.2 µm) and S. hepiali (1.8–3.3 × 1.4–2.2 µm). Both the morphological study and phylogenetic analyses of combined nrSSU, nrLSU, tef-1α, rpb1 and rpb2 sequence data supported the idea that this fungus was a distinctive species in the genus of Samsoniella.
Samsoniella haniana H. Yu, Y. Wang & Z.Q. Wang, sp. nov. (Figure 5).
Figure 5.
Morphology of Samsoniella haniana. (A,B) Pupa of Lepidoptera infected by S. haniana. (C) Culture character on PDA medium. (D–G) Conidiogenous cells (conidiophores, phialides) and conidia on PDA. Scale A,B: 10 mm; C: 20 mm; D,E: 10 µm; F,G: 5 µm.
MycoBank: MB 844385.
Etymology: The haniana was named after the Hani nationality, living in Yunnan.
Holotype: China, Yunnan Province, Yuanyang County, Xinjie Town, Duoyishuxia Village. On a pupa of Lepidoptera in cocoons buried in soil, 15 December 2021, Yao Wang (YHH 20175, holotype; YFCC 8769, ex-holotype living culture).
Sexual morph: Undetermined.
Asexual morph: Synnemata arising from every part of the body of the insect host. Synnemata erect, usually irregularly branched at the apex, 20–40 × 1–1.8 mm, pale orange. isaria-like morph producing a mass of conidia at the branch apex, powdery and floccose. Colonies derived from germinating conidia. Colonies on PDA growing well, 24–29 mm diameter in 14 days at 25 °C, white, cottony, sporulating abundantly, reverse light orange. Hyphae smooth-walled, branched, septate, hyaline, 0.8–2.8 µm wide. Conidiophores smooth-walled, cylindrical, solitary or verticillate, 3.8–10.2 × 1.1–2.9 µm. Phialides verticillate, usually in whorls of two to five, or solitary on hyphae, 5.4–12.1 µm long, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex, from 1.2–2.9 µm wide (base) to 0.3–1.1 µm wide (apex). Conidia smooth and hyaline, fusiform or oval, one-celled, 2.3–3.7 × 1.2–2.8 µm, often in chains. Size and shape of phialides and conidia similar in culture and on natural substratum.
Host: Pupae of Lepidoptera.
Habitat: On the pupae of Lepidoptera in cocoons buried in soil.
Distribution: Currently only known in Yuanyang County, Yunnan Province, China; Puer City, Yunnan province, China.
Other material examined: China, Yunnan Province, Yuanyang County, Xinjie Town, Duoyishuxia Village. On a pupa of Lepidoptera in cocoons buried in soil, 15 December 2021, Yao Wang (YHH 20176; YFCC 8770, living culture); China, Yunnan province, Puer City, Simao District, Simao Gang Town, Dajiu Village. On a pupa of Lepidoptera in a cocoon buried in soil, 23 August 2021, Zhi-Qin Wang (YHH 20177; YFCC 8771, living culture).
Notes: Phylogenetically, S. haniana was identified as a Samsoniella species based on the phylogenetic analyses and was closely related to S. pseudogunii and S. coleopterorum (Figure 1). However, three samples of S. haniana were clustered together with strong statistical support and formed a separate clade. Morphologically, S. haniana differed from S. pseudogunii due to its several synnemata (usually irregularly branched at the apex) and oval conidia. Samsoniella haniana was distinguished from S. coleopterorum with several synnemata (irregularly branched at the apex), longer phialides (5.4–12.1 µm) and larger conidia (2.3–3.7 × 1.2–2.8 µm).
Samsoniella pseudotortricidae H. Yu, Y. Wang & Z.Q. Wang, sp. nov. (Figure 6).
Figure 6.
Morphology of Samsoniella pseudotortricidae. (A,B) Stromata of fungus arising from lepidopteran pupa. (C) Fertile part. (D) Perithecia. (E) Culture character on PDA medium. (F–H) Conidiogenous cells (conidiophores, phialides) and conidia on PDA. Scale A,B: 10 mm; C: 1 mm; D: 300 µm; E: 1 cm; F–H: 10 µm.
MycoBank: MB 844386.
Etymology: Referring to macromorphological resemblance of S. tortricidae and S. pseudotortricidae but phylogenetically distinct.
Holotype: China, Yunnan Province, Kunming City, Wild Duck Lake Forest Park. On a pupa of Lepidoptera in cocoons buried in soil, 12 August 2017, Hong Yu (YHH 20174, holotype; YFCC 9052, ex-holotype living culture).
Sexual morph: Stromata arising from insect cocoon, solitary to several, up to 20–65 mm long, unbranched or dichotomous. Stipes fleshly, flexuous, orange, cylindrical to clavate, 10–43 × 1.1–3.3 mm. Fertile parts reddish orange, clavate to subulate, lateral side usually have a longitudinal section without producing perithecia, 10–17 × 1.5–4.2 mm. Perithecia crowded, superficial, narrowly ovoid to fusiform, 285.7–313.2 × 149.2–154.9 µm. No mature asci or ascospores were observed.
Asexual morph: isaria-like. Colonies on PDA grow well, 30–36 mm diameter in 14 days at 25 °C, white, cottony, sporulating abundantly, reverse light orange. Hyphae smooth-walled, branched, septate, hyaline, 1.1–1.5 µm wide. Conidiophores smooth-walled, cylindrical, solitary or verticillate, 6.6–26.5 × 1.1–2.5 µm. Phialides verticillate, in whorls of two to five, usually solitary on hyphae, 5.4–6.9 µm long, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex, from 1.0–1.6 µm wide (base) to 0.5–0.8 µm wide (apex). Conidia smooth and hyaline, oblong, fusiform or oval, one-celled, 0.9–1.5 × 0.8–1.3 µm, often in chains.
Host: Pupae of Lepidoptera.
Habitat: On pupae of Lepidoptera in cocoons buried in soil.
Distribution: Currently only known in Kunming City, Yunnan Province, China.
Other material examined: China, Yunnan Province, Kunming City, Wild Duck Lake Forest Park. On a pupa of Lepidoptera in cocoons buried in soil, 12 August 2017, Hong Yu (YHH 20189, holotype; YFCC 9053, ex-holotype living culture).
Notes:Samsoniella pseudotortricidae was similar to its phylogenetically closely related species S. tortricidae in macromorphology. The stromata were both unbranched or dichotomous, both fertile parts were clavate to subulate, and reddish orange, and the lateral side usually had a longitudinal section without producing perithecia. However, S. pseudotortricidae was easily distinguished by its smaller ascus (285.7–313.2 × 149.2–154.9 µm), smaller phialides (5.4–6.9 × 1.0–1.6 µm) and smaller conidia (0.9–1.5 × 0.8–1.3 µm). It could be easily distinguished phylogenetically from S. tortricidae.
Samsoniella sinensis H. Yu, Y. Wang & Z.Q. Wang, sp. nov. (Figure 7).
Figure 7.
Morphology of Samsoniella sinensis. (A,B) Larva of Lepidoptera infected by S. sinensis. (C) Dermaptera infected by S. sinensis. (D) Culture character on PDA medium. (E–H) Conidiogenous cells (conidiophores, phialides) and conidia on PDA. Scale A,B: 5 mm; C: 3 mm; D: 10 mm; E–H: 10 µm.
MycoBank: MB 844387.
Etymology: Named after China (Yunnan and Guizhou provinces), where the species is distributed.
Holotype: China, Yunnan Province, Kunming City, Xishan Forest Park. On a larva of Lepidoptera clinging to fallen leaves, 12 August 2018, Hong Yu (YHH 20170, holotype; YFCC 8766, ex-holotype living culture).
Sexual morph: Undetermined.
Asexual morph: Synnemata arising from the host, 3.5–5 mm long, irregularly branched, conidia in abundance at the apex. Colonies fast-growing on PDA, 35–40 mm in 14 days at 25 °C, floccose, crater-shaped, white to pale pink, sporulating abundantly at the centrum, forming a white concentric ring. Reverse pale brown. Hyphae smooth-walled, branched, septate, hyaline, 1.3–3.1 µm wide. Conidiophores cylindrical, solitary or verticillate, 6.4–10.5 × 1.7–2.1 µm. Phialides verticillate, in whorls of two to five, sometimes solitary on hyphae, 5.6–9.3 µm long, basal portion cylindrical to narrowly lageniform, tapering gradually or abruptly toward the apex, from 1.5–2.1 µm wide (base) to 0.6–1.0 µm wide (apex). Conidia smooth and hyaline, spherical, elliptical or fusiform, one-celled, 2.0–3.1 × 1.3–1.9 µm, often in chains. Size and shape of phialides and conidia similar in culture and on natural substratum.
Host: Larvae of Lepidoptera, Dermaptera.
Habitat: On the larvae of Lepidoptera clinging to fallen leaves or on Dermaptera clinging to fallen leaves.
Distribution: Currently only known in Kunming City and Chuxiong City, Yunnan Province, China, and Guiyang City, Guizhou Province, China.
Other material examined: China, Yunnan Province, Kunming City, Kunming Wild Duck Lake Forest Park. On a pupa of Dermaptera clinging to fallen leaves, 13 August 2017, Yao Wang (YHH 20171; YFCC 8767, living culture); China, Yunnan Province, Chuxiong City, Zixi Mountain. On Dermaptera clinging to fallen leaves, 13 August 2016, Yao Wang (YHH 20172; YFCC 8768, living culture); China, Guizhou Province, Guiyang City. On a larva of Lepidoptera, 13 August 2017, Yao Wang (YHH 20173).
Notes: Regarding phylogenetic relationships, S. sinensis formed a distinct lineage and was closely related to S. hymenopterorum. Morphologically, synnemata were observed in S. sinensis, and synnemata were not observed in S. hymenopterorum. The phialides of S. sinensis (5.6–9.3 µm) were shorter than those of S. hymenopterorum (6.5–10.6 µm). The conidia of S. hymenopterorum were fusiform to ovoid, 1.9–2.5 × 1.5–2.1 µm, but those of S. sinensis were spherical, elliptical or fusiform, 2.0–3.1 × 1.3–1.9 µm. Samsoniella sinensis was also easily distinguished from S. hymenopterorum by its host. Samsoniella sinensis was found to occur on Lepidoptera and Dermaptera, while S. hymenopterorum was only found to occur on Hymenopterous insects.
4. Discussion
The macromorphology and micromorphology of some Samsoniella species were very similar, and thus, the species were not easy to distinguish using only morphological characteristics [1,2]. In addition, Samsoniella, Beauveria and Cordyceps shared many similar morphological characteristics of sexual morphs, viz., fleshy stromata, red to orange colours, superficial perithecia, cylindrical asci with thickened ascus apex and usually cylindrical and multiseptate ascospores. Samsoniella, Akanthomyces and Cordyceps species produced similar isaria-like asexual conidiogenous structures, such as flask-shaped phialides produced in whorls and conidia with divergent chains [2]. It was more difficult to identify individual Samsoniella species. In the present study, a comprehensive morphological and phylogenetic investigation was conducted in most of the lineages of Samsoniella. The microscopic observations were compared with those for other known species in the genus, revealing some obvious differences, although the morphological features generally overlapped (Table 3 and Table 4). In comparison with other known species, parasitic S. coccinellidicola on adult beetles possessed relatively long phialides, S. farinospora had oblong to cylindrical conidia, S. haniana produced larger conidia, S. pseudotortricidae had smaller conidia and S. sinensis produced a variety of shapes of conidia (viz., spherical, elliptical or fusiform). Based on the five-gene (nrSSU, nrLSU, tef-1α, rpb1 and rpb2) dataset, molecular phylogenetic analyses also supported the existence of the five distinct species in the genus, emphasizing the importance of micromorphology and molecular identification (Figure 1).
Samsoniella hepiali has a great medical value due to its therapeutic effects in cardiovascular, respiratory, immunomodulatory, hyposexuality, hyperglycaemia and renal disorder conditions as well as its antitumor properties [2,33,34,35,36,37,38,39]. The Ministry of Health of the People’s Republic of China issued File No. 84 on 23 March 2001 and approved S. hepiali mycelia for use as a standalone preparation or a component of health foods (equivalent to dietary supplements in other countries) [40]. Thus, over 260 healthcare products have been developed with S. hepiali as a raw material in the global market, especially the Jinshuibao capsule [2]. To date, S. hepiali has been widely used as an edible and medicinal fungus, generating an impressive economic value of approximately RMB 10 billion a year in China [2]. It seemed to us that the related species of S. hepiali with similar genetic traits should have similar pharmacological activities. In this study, the S. farinospora strain YFCC 9051, isolated from a larva of Hepialus, and the other isolate, such as YFCC 8774, formed an independent clade apart from their allied species of Samsoniella and were further grouped with S. hepiali (see Figure 1). It was suggested that the two species should have a close genetic relationship. Morphologically, the strain YFCC 9051 was very similar to S. hepiali. They shared the same host of the hepialid larva, and both possessed an isaria-like asexual conidiogenous structure, producing synnemata with powdery conidia at the apex. Moreover, the main components in the mycelium of S. farinospora were similar to those in the mycelium of S. hepiali, involving adenosine, alkaloids, amino acids, ergosterol, mannitol, organic acids and polysaccharides (unpublished data). The strains of S. farinospora will be further determined to develop a raw material for healthcare products in future.
Previous studies of cordycipitaceous isaria-like fungi showed that species of Samsoniella were globally distributed generalist entomopathogen that were soilborne and had relatively complicated hosts, including Lepidoptera (Hepialidae, Noctuidae, Limacodidae, Saturniidae and Tortricidae), Coleoptera (Curculionidae), Hymenoptera (Formicidae and Vespidae) and two fungi (O. sinensis and C. cicadae) [1,2,41]. Here, an extension of the host range was identified, also including Araneae, Dermaptera and Coccinellidae of Coleoptera, as shown in Figure 1 and Table 2. Among the hosts of Samsoniella species, Lepidoptera was the major order (Table 2). Because of their broad host range and wide geographical distribution, some species of Samsoniella may have high potential for the interspecific transmission and biological control of pest insects. Additional research is needed to determine the effectiveness of isolates in the field.
Author Contributions
Conceptualization, Z.W., Y.W. and H.Y.; methodology, Y.W.; software, Z.W.; validation, Z.W., Y.W. and H.Y.; formal analysis, Q.D.; investigation, Z.W., Y.W., Q.D., Q.F., V.-M.D. and H.Y.; resources, Y.W., Q.D., Q.F., V.-M.D. and H.Y.; data curation, Y.W.; writing—original draft preparation, Z.W.; writing—review and editing, Y.W. and H.Y.; visualization, H.Y.; supervision, H.Y.; project administration, H.Y.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://www.ncbi.nlm.nih.gov, accessed on 1 May 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31870017).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mongkolsamrit S., Noisripoom W., Thanakitpipattana D., Wutikhun T., Spatafora J.W., Luangsa-ard J. Disentangling cryptic species with Isaria-like morphs in Cordycipitaceae. Mycologia. 2018;110:230–257. doi: 10.1080/00275514.2018.1446651. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y.B., Wang Y., Fan Q., Duan D.E., Zhang G.D., Dai R.Q., Dai Y.D., Zeng W.B., Chen Z.H., Li D.D., et al. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020;103:1–46. doi: 10.1007/s13225-020-00457-3. [DOI] [Google Scholar]
- 3.Luangsa-ard J.J., Hywel-Jones N.L., Samson R.A. The order level polyphyletic nature of Paecilomyces sensu lato as revealed through 18S-generated rRNA phylogeny. Mycologia. 2004;96:773–780. doi: 10.1080/15572536.2005.11832925. [DOI] [PubMed] [Google Scholar]
- 4.Kepler R.M., Luangsa-ard J.J., Hywel-Jones N.L., Quandt C.A., Sung G.H., Rehner S.A., Aime M.C., Henkel T.W., Sanjuan T., Zare R., et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales) IMA Fungus. 2017;8:335–353. doi: 10.5598/imafungus.2017.08.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L.P., Xu J.Y., Li H.C., Song L.P., Feng C.Q. The complete mitochondrial genome of Paecilomyces hepiali (Ascomycota, Eurotiomycetes) Mitochondrial DNA J. Dna Mapp. Seq. Anal. 2014;27:916–917. doi: 10.3109/19401736.2014.926484. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y.B., Yu H., Dai Y.D., Wu C.K., Zeng W.B., Yuan F., Liang Z.Q. Polycephalomyces agaricus, a new hyperparasite of Ophiocordyceps sp. infecting melolonthid larvae in southwestern China. Mycol. Prog. 2015;14:70. doi: 10.1007/s11557-015-1090-7. [DOI] [Google Scholar]
- 7.Vilgalys R., Hester M. Rapid genetic identifcation and mapping of enzymatically amplifed ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994;98:625–634. doi: 10.1016/S0953-7562(09)80409-7. [DOI] [Google Scholar]
- 9.Bischof J.F., Rehner S.A., Humber R.A. Metarhizium frigidum sp. nov.: A cryptic species of M. anisopliae and a member of the M. favoviride Complex. Mycologia. 2006;98:737–745. doi: 10.1080/15572536.2006.11832645. [DOI] [PubMed] [Google Scholar]
- 10.Sung G.H., Hywel-Jones N.L., Sung J.M., Luangsa-Ard J.J., Shrestha B., Spatafora J.W. Phylogenetic classifcation of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y.B., Yu H., Dai Y.D., Chen Z.H., Zeng W.B., Yuan F., Liang Z.Q. Polycephalomyces yunnanensis (Hypocreales), a new species of Polycephalomyces parasitizing Ophiocordyceps nutans and stink bugs (Hemipteran adults) Phytotaxa. 2015;208:34–44. doi: 10.11646/phytotaxa.208.1.3. [DOI] [Google Scholar]
- 12.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 15.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 16.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: Moremodels, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D., Sung G.H., Hywel-Jones N.L., Luangsa-Ard J.J., Bischoff J.F., Kepler R.M., Spatafora J.W. Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota) Mycol. Res. 2009;113:279–289. doi: 10.1016/j.mycres.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Spatafora J.W., Sung G.H., Hywel-Jones N.L., White J.F. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 2007;16:1701–1711. doi: 10.1111/j.1365-294X.2007.03225.x. [DOI] [PubMed] [Google Scholar]
- 19.Chiriví-Salomón J.S., Danies G., Restrepo S., Sanjuan T. Lecanicillium sabanense sp. nov. (Cordycipitaceae) a new fungal entomopathogen of coccids. Phytotaxa. 2015;234:63–74. doi: 10.11646/phytotaxa.234.1.4. [DOI] [Google Scholar]
- 20.Wang Y., Fan Q., Wang D., Zou W.Q., Tang D.X., Hongthong P., Yu H. Species Diversity and Virulence Potential of the Beauveria bassiana Complex and Beauveria scarabaeidicola Complex. Front. Microbiol. 2022;13:841604. doi: 10.3389/fmicb.2022.841604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z.F., Liu F., Zhou X., Liu X.Z., Liu S.J., Cai L. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Pers. Mol. Phylogeny Evol. Fungi. 2017;39:1–31. doi: 10.3767/persoonia.2017.39.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanjuan T., Tabima J., Restrepo S., Læssøe T., Spatafora J.W., FrancoMolano A.E. Entomopathogens of Amazonian stick insects and locusts are members of the Beauveria species complex (Cordyceps sensu stricto) Mycologia. 2014;106:260–275. doi: 10.3852/13-020. [DOI] [PubMed] [Google Scholar]
- 23.Chen W.H., Han Y.F., Liang Z.Q., Jin D.C. A new araneogenous fungus in the genus Beauveria from Guizhou, China. Phytotaxa. 2017;302:57–64. doi: 10.11646/phytotaxa.302.1.5. [DOI] [Google Scholar]
- 24.Rehner S.A., Minnis A.M., Sung G.H., Luangsa-ard J.J., Devotto L., Humber R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia. 2011;103:1055–1073. doi: 10.3852/10-302. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z.H., Chen K., Dai Y.D., Zheng Y., Xu L. Beauveria species diversity in the Gaoligong Mountains of China. Mycol. Prog. 2019;18:933–943. doi: 10.1007/s11557-019-01497-z. [DOI] [Google Scholar]
- 26.Luangsa-ard J.J., Hywel-Jones N.L., Manoch L., Samson R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005;109:581–589. doi: 10.1017/S0953756205002741. [DOI] [PubMed] [Google Scholar]
- 27.Dong Q.Y., Wang Y., Wang Z.Q., Tang D.X., Zhao Z.Y., Wu H.J., Yu H. Morphology and phylogeny reveal five novel species in the genus Cordyceps (Cordycipitaceae, Hypocreales) from Yunnan, China. Front. Microbiol. 2022;13:846909. doi: 10.3389/fmicb.2022.846909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W.H., Han Y.F., Liang J.D., Tian W.Y., Liang Z.Q. Morphological and phylogenetic characterisations reveal three new species of Samsoniella (Cordycipitaceae, Hypocreales) from Guizhou, China. MycoKeys. 2020;74:1–15. doi: 10.3897/mycokeys.74.56655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W.H., Liang J.D., Ren X.X., Zhao J.H., Han Y.F., Liang Z.Q. Cryptic diversity of isaria-like species in Guizhou, China. Life. 2021;11:1093. doi: 10.3390/life11101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei D.P., Wanasinghe D.N., Hyde K.D., Mortimer P.E., To-Anun C. The genus Simplicillium. MycoKeys. 2019;60:69–92. doi: 10.3897/mycokeys.60.38040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currie C.R., Wong B., Stuart A.E., Schultz T.R., Rehner S.A., Mueller U.G., Sung G.H., Spatafora J.W., Straus N.A. Ancient Tripartite Coevolution in the Attine Ant-Microbe Symbiosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155. [DOI] [PubMed] [Google Scholar]
- 32.Smith G. Some new and interesting species of micro-fungi. Trans. Br. Mycol. Soc. 1957;40:481–488. doi: 10.1016/S0007-1536(57)80054-0. [DOI] [Google Scholar]
- 33.Lou Y.Q., Liao X.M., Lu Y.C. Cardiovascular pharmacological studies of ethanol extracts of Cordyceps mycelia and Cordyceps fermentation solution. Chin. Tradit. Herb. Drugs. 1986;17:17–21. [Google Scholar]
- 34.Huang M.M., Zhang J.F., Pang L., Jiang Z., Wang D.W. Studies on immunopharmacology of Cordyceps (Fr.) Link IV. Observations on the immunosuppressive activity of artifcial substance of Paecilomyces hapiali Chen. Acta Univ. Med. Tongji. 1988;5:329–331. [Google Scholar]
- 35.Wang D.W., Huang M.M. Studies on immunopharmacology of Cordyceps (Fr.) Link V. Infuence of artifcial fermentative substance of Paecilomyces hapiali Chen on the function of T cell and its subgroup in mice. Acta Univ. Med. Tongji. 1988;5:332–334. [Google Scholar]
- 36.Dai R.Q., Lan J.L., Chen W.H., Li X.M., Chen Q.T., Shen C.Y. Research on Paecilomyces hepiali Chen et Dai, sp. nov. Acta Agric. Univ. Pekin. 1989;15:221–224. [Google Scholar]
- 37.Zou W.P., Huang M.M. Primary studies on the mechanism of Paecilomyces hepiali Chen against the rejection reaction. Acta Univ. Med. Tongji. 1993;22:282–284. [Google Scholar]
- 38.Xiang M., Tang J., Chu T., Zhang C.L., Zou X.L. Hypoglycemic effect and mechanism study on streptozocin induced diabetes in mice by Paecilomyces hepiali Chen mycelium. Chin. J. Hosp. Pharm. 2006;26:556–559. [Google Scholar]
- 39.Jiang L., Bao H.Y., Yang M. Antitumor activity of a petroleum ether extract from Paecilomyces hepiali mycelium. Acta Edulis Fungi. 2010;17:58–60. [Google Scholar]
- 40.Dai R.Q., Shen C.Y., Li X.M., Lan J.L., Lin S.F., Shao A.J. Response to neotypification of Paecilomyces hepiali (Hypocreales) (Wang & al., 2015) Taxon. 2018;67:784–786. doi: 10.12705/674.7. [DOI] [Google Scholar]
- 41.Samson R.A. Paecilomyces and some allied hyphomycetes. Stud. Mycol. 1974;6:1–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://www.ncbi.nlm.nih.gov, accessed on 1 May 2022.