Abstract
Knowledge about the relationship between microbial community structure and hydrogeochemistry (e.g., pollution, redox and degradation processes) in landfill leachate-polluted aquifers is required to develop tools for predicting and monitoring natural attenuation. In this study analyses of pollutant and redox chemistry were conducted in parallel with culture-independent profiling of microbial communities present in a well-defined aquifer (Banisveld, The Netherlands). Degradation of organic contaminants occurred under iron-reducing conditions in the plume of pollution, while upstream of the landfill and above the plume denitrification was the dominant redox process. Beneath the plume iron reduction occurred. Numerical comparison of 16S ribosomal DNA (rDNA)-based denaturing gradient gel electrophoresis (DGGE) profiles of Bacteria and Archaea in 29 groundwater samples revealed a clear difference between the microbial community structures inside and outside the contaminant plume. A similar relationship was not evident in sediment samples. DGGE data were supported by sequencing cloned 16S rDNA. Upstream of the landfill members of the β subclass of the class Proteobacteria (β-proteobacteria) dominated. This group was not encountered beneath the landfill, where gram-positive bacteria dominated. Further downstream the contribution of gram-positive bacteria to the clone library decreased, while the contribution of δ-proteobacteria strongly increased and β-proteobacteria reappeared. The β-proteobacteria (Acidovorax, Rhodoferax) differed considerably from those found upstream (Gallionella, Azoarcus). Direct comparisons of cloned 16S rDNA with bands in DGGE profiles revealed that the data from each analysis were comparable. A relationship was observed between the dominant redox processes and the bacteria identified. In the iron-reducing plume members of the family Geobacteraceae made a strong contribution to the microbial communities. Because the only known aromatic hydrocarbon-degrading, iron-reducing bacteria are Geobacter spp., their occurrence in landfill leachate-contaminated aquifers deserves more detailed consideration.
Contamination of groundwater is a serious environmental problem throughout the world as it affects drinking water resources and has an impact on oligotrophic environments. In The Netherlands, an important source of contamination is landfill leachate. In the past, landfilling was performed without the presence of appropriate liners to prevent percolation of leachate into underlying aquifers. Although many old landfills are closed now, the cessation of landfill operations does not stop chemical release into the environment. Organic compounds originating from household and industrial waste are found in most municipal landfills. Dramatic changes in aquifer geochemistry and microbiology downstream of landfills occur as a result of the high organic load of leachate (11). A sequence of redox zones develops in time and space, as the organic matter is microbiologically degraded and electron acceptors are depleted (11, 29).
Iron reduction and manganese reduction are important redox processes in polluted aquifers (2, 11, 21, 27, 28). Solid iron oxyhydroxides and manganese oxides are reduced, which releases soluble metal species into the groundwater. These metals, together with other reduced species, such as methane, ammonium, and hydrogen sulfide, can pose a threat to drinking water and oligotropic nature reserves (11, 28). Also, pathogenic bacteria might be present in the leachate (11). However, of particular concern is contamination of groundwater by aromatic compounds (especially benzene, toluene, ethylbenzene, and xylene [BTEX]). These compounds are often encountered in landfills (11). Although they account for at most a few percent of the organic matter in leachate, concern about them is related to their toxicity and relatively high solubility. BTEX components are readily degraded under aerobic conditions but are far more persistent under anaerobic conditions (29), which are typical within and downgradient of landfills (11).
It is often difficult and expensive to remediate a subsurface environment. However, despite unfavorable conditions, appreciable anaerobic microbial degradation of BTEX has been observed in landfill leachate-polluted aquifers (1, 34, 44). The ability to predict the potential for natural attenuation and the ability to monitor on-going degradation processes should help limit the number of landfills and aquifers that have to be actively remediated. Thorough knowledge of microbial community structure in polluted aquifers, the capabilities of the microbial populations present, and how these populations affect their environment and vice versa should aid in the development of tools for predicting and monitoring natural degradation. Here, we describe the relationship between hydrogeochemistry and microbial community structure in a landfill leachate-polluted aquifer close to the town of Boxtel, The Netherlands. From this aquifer 29 groundwater samples and five sediment samples were obtained. Chemical analyses were conducted to determine the level of pollution and deduce the principal redox processes. The community structures for members of the Archaea and Bacteria were determined by denaturing gradient gel electrophoresis (DGGE) (35), and the profiles were numerically compared (41). For three groundwater samples clone libraries were constructed to obtain more detailed information about the composition of the microbial communities.
MATERIALS AND METHODS
Site description and installation of piezometers.
Banisveld landfill is located 5 km southwest of Boxtel, The Netherlands. Unlined landfilling of primarily household refuse occurred in a 6-m-deep sand pit between 1965 and 1977. The aquifer consists of an 11-m-thick layer of fine to coarse unconsolidated sand located on less permeable clay and peat deposits alternating with sandy layers. The direction of the groundwater flow (approximately 10 m/year) is northeast to north towards a nature reserve, which is a habitat for a rare oligotrophic ecosystem. An electromagnetic survey and cone penetration tests revealed the horizontal and vertical location of the leachate (48). In June 1998, this information was used to install a transect consisting of 11 bailer drillings along the direction of groundwater flow (Fig. 1). Two or three polyvinyl chloride piezometers with an inside diameter of 52 mm were installed per bore hole (inside diameter, 22 cm); the piezometers usually had one screen above the leachate plume (Fig. 1, positions a), one screen inside the leachate plume (positions b), and one screen below the leachate plume (positions c). The screens were 20 cm long. Samples from piezometer screens were designated by using the distance downstream of the landfill and the position of the screen; e.g., samples −200a and 0a were samples from screens above the leachate plume in a piezometer 200 m upstream and in a piezometer in the landfill (19 m from the downstream border), respectively.
FIG. 1.
Cross section of Banisveld landfill (shaded area) and the plume of leachate (cross-hatched area) downstream of the landfill, showing the locations of the 11 bore holes. Each bore hole is indicated by a number corresponding to the distance (in meters) from the downstream border of the landfill. Two or three screens were placed in each bore hole, as indicated by a, b, and c. Symbols: •, screen from which in September 1998 a groundwater sample with a nitrate concentration of >0.5 mg/liter was obtained; ▪, screen from which a sample containing no nitrate was obtained; ▿, sediment (S) sampled in October 1998.
Sampling
In September 1998, anaerobic groundwater samples were collected in sterile glass bottles by letting the bottles overflow, after 3 volumes of standing water in each piezometer was removed with a peristaltic pump. The bottles were capped with as small a headspace as possible. In October 1998, sediment cores were taken anaerobically with a core pushing device (Delft Geotechnics, Delft, The Netherlands) (7) at five locations (one upstream and four downstream) in the plume of leachate (Fig. 1). After retrieval, the ends of the stainless steel cores (length, 20 cm; inside diameter, 30 mm) were immediately capped, and the cores were stored in a container which was made anaerobic by flushing with nitrogen gas. Sediment cores and groundwater were transferred to the laboratory and stored for less than 24 h at 4°C. Next, 100 ml of groundwater was vacuum filtered with 45-mm-diameter, 0.2-μm-pore-size filters (Sartorius). Cores were sampled under a nitrogen atmosphere in an anaerobic glove box (Mecaplex). Several centimeters at the ends of the cores were not used. For molecular analysis, sediment and filters were frozen at −80°C until DNA isolation.
Chemical analysis.
Oxygen content, pH, and electrical conductivity were measured in the field with electrodes placed in flow cells. Hydrochemical parameters (alkalinity; benzene, toluene, ethylbenzene, xylene, naphthalene, Mn, Fe, Si, Al, Mg, NH4, Ca, K, Na, Cl, SO4, H2S, NO2, NO3, CH4, and dissolved organic carbon contents) and sedimentological parameters (lime, humus, sand, clay, silt, carbon, and nitrogen contents) were determined by using Dutch NEN standards and laboratory procedures. Samples were grouped based on chemical characteristics by using principal-component analysis and cluster analysis (Systat 7).
DGGE profiling.
DNA extraction was performed as described previously (41). A Bacteria-specific PCR was performed in a 25-μl (total volume) mixture containing 0.4 μM primer F341-GC (35), 0.4 μM primer R518 (35), each deoxynucleoside triphosphate at a concentration of 0.4 mM, 10 μg of bovine serum albumin, Expand buffer (Boehringer, Mannheim, Germany), 2.6 U of Expand enzyme, and 1 μl of undiluted DNA template. Amplification was performed with a Perkin-Elmer DNA Thermo Cycler as follows: 94°C for 4 min, followed by 35 cycles of 94°C for 0.5 min, 54°C for 1 min, and 72°C for 1 min, and a final elongation at 72°C for 5 min. For profiling of Archaea, a nested approach was used. Primers pRA46f (37) and univ907r (6) were used to produce a 0.9-kb fragment, which after a 100-fold dilution was used as a template in an amplification reaction with primers pARCH340f and pARCH519r (37). Amplification was performed with the same settings as those used for Bacteria-specific amplification.
DGGE was performed with the Bio-Rad DCode system. The PCR product was loaded onto 1-mm-thick 8% (wt/vol) polyacrylamide (ratio of acrylamide to bisacrylamide, 37.5:1) gels containing a 40 to 60% or 40 to 70% linear denaturing gradient for Bacteria and a 45 to 70% linear denaturing gradient for Archaea; 100% denaturant was defined as 7 M urea and 40% (vol/vol) formamide. The gels were electrophoresed in 1× TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM Na-EDTA; pH 8.0) at 70 V and 60°C for 16 h. The gels were stained in 1× TAE buffer containing 1 μg of ethidium bromide ml−1 and were recorded with a charge-coupled device camera system (The imager; Appligen, Illkirch, France). Gel images were converted, normalized, and analyzed with the GelCompar 4.0 software package (Applied Maths, Kortrijk, Belgium), using the Pearson product moment correlation coefficient and the unweighted pair group clustering method with arithmetic averages (UPGMA). To aid in conversion and normalization of gels, a marker consisting of 11 clones was added on the outsides of the gels, as well as after every four samples. The outer two lanes of each gel were not used. In all analyses the markers clustered over 95% similarity.
Cloning and sequencing of 16S rDNA.
PCR primers 8f and 1512r (17) were used to amplify almost complete 16S ribosomal DNA (rDNA). Products (cleaned with a Qiaquick Rep purification kit [Qiagen, Hilden, Germany]) were cloned into Escherichia coli JM109 by using the pGEM-T vector system (Promega, Madison, Wis.). Transformants were checked for inserts of the correct size by performing a PCR with pGEM-T-specific primers T7 and Sp6. Products of the correct size were used as templates in a PCR with primers F341-GC and R518 to compare the band position in DGGE gels to that of the environmental sample from which the clone was derived. Sequencing PCR was carried out with an ABI PRISM dye terminator cycle sequencing core kit (Perkin-Elmer), and the purified products were electrophoresed on a SEQUAGEL-6 sequence gel (National Diagnostics, Atlanta, Ga.) with a 373A DNA sequencer (PE Biosystems, Applied Biosystems, Foster City, Calif.). At least the V3 region (E. coli positions 341 to 518) was sequenced, and a number of clones were sequenced completely. Both strands of the 16S rRNA gene were sequenced. Sequences were compared to sequences deposited in the GenBank DNA database by using the BLAST algorithm (5).
MPN-PCR.
Serial twofold dilutions of DNA extracts were made in sterile water and used as templates for PCR. Most-probable-number PCR (MPN-PCR) of members of the family Geobacteraceae was performed with primers 8f and Geo825 (46). MPN-PCR numbers of members of the Bacteria were determined with primers 8f and R518. To account for variations in the efficiency of DNA extraction and recovery, the numbers of members of the Geobacteraceae were expressed relative to the numbers of members of the Bacteria.
Nucleotide sequence accession numbers.
Nucleotide sequences have been deposited in the GenBank database under accession numbers AY013585 to AY013658 and AY013660 to AY013698.
RESULTS
Hydrogeochemistry of the plume of landfill leachate.
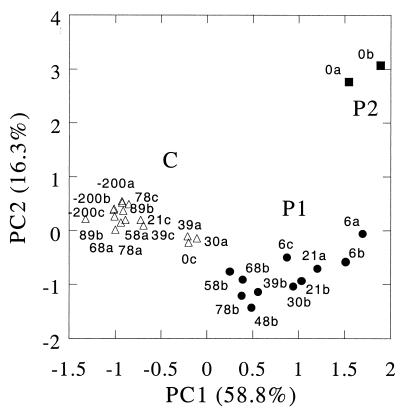
Groundwater samples for hydrochemical and microbiological analyses were retrieved in September 1998 from 29 piezometers (Fig. 1). An ordination plot constructed on the basis of the measured hydrogeochemical parameters (Fig. 2) revealed clustering of the sampling points into three groups, two large clusters (clusters C and P1) and one small cluster (cluster P2). The two large clusters were mainly separated along the principal component 1 (PC1) axis, which explained 58.8% of the total variance. PC1 correlated strongly with the following parameters indicative of pollution by landfill leachate (correlation coefficients are given in parentheses): electrical conductivity (0.985), alkalinity (0.978), total inorganic carbon (0.977), magnesium (0.970), dissolved organic carbon (0.957), calcium (0.934), ammonium (0.929), potassium (0.894), chloride (0.891), and sodium (0.856). Cluster C (Fig. 2) contained groundwater samples having low values for these parameters (slightly polluted or clean), while clusters P1 and P2 contained samples that had high values for these parameters and therefore were obviously polluted. The grouping of the samples (Fig. 2) corresponded exactly with the delineation of the plume by vertical continuous profiles of bulk conductivity obtained by cone penetration tests performed in May 1998 (48).
FIG. 2.
Ordination plot produced from principal-component analysis of hydrochemical parameters of groundwater samples from the aquifer surrounding Banisveld landfill. Three clusters of clean (C [▵]) and polluted (P1 [•] and P2 [▪]) groundwater samples are shown. The numbers and lowercase letters indicate the samples examined (Fig. 1).
Clusters P1 and P2 were separated along the PC2 axis. This axis (which explains 16.3% of the variance) positively correlated with silica (0.860), ethylbenzene (0.781), xylene (0.759), and naphthalene (0.563) and correlated negatively with the reduced redox species Fe(II) (−0.733) and Mn(IV) (−0.617). Only cluster P2 samples (piezometer screens 0a and 0b) contained obvious concentrations of ethylbenzene (53 μg/liter) and xylene (120 μg/liter). These aromatic compounds were not present 6 m downstream of the landfill, while naphthalene had disappeared 21 m downstream. Benzene (maximum concentration, 28 μg/liter) was more persistent, and its concentration decreased along the flow path, to 6 μg/liter at 78 m from the landfill. The concentration of chloride (used as a conservative tracer, with a background concentration of 12 to 70 mg/liter upstream of the landfill) was constant (mean value in the plume of pollution, 270 mg/liter), indicating that the decreases in the concentrations of organic contaminants were not due to dilution. As the organic content of the sediment was low (<0.1%), sorption alone cannot explain the decreases (48).
Attenuation of organic contaminants in the plume appeared to occur under iron-reducing conditions. Oxygen (<0.1 mg/liter) was not detected in any of the samples. Nitrate (>0.5 mg/liter; maximum concentration, 76 mg/liter) was encountered only upstream of the landfill and above the plume (Fig. 1), indicating that denitrification is probably a dominant redox process at the top fringes of the plume. In the plume, Fe(II) concentrations in general increased along the transect, while the presence of a pool of Fe(III) oxyhydroxides and hydrogen concentrations (van Breukelen et al., unpublished data) also indicated that iron reduction was a dominant redox process. Also, below the plume the absence of nitrate and the measured concentrations of hydrogen indicated that iron reduction was the dominant redox process.
Microbial community structure of groundwater inside and outside the plume of leachate.
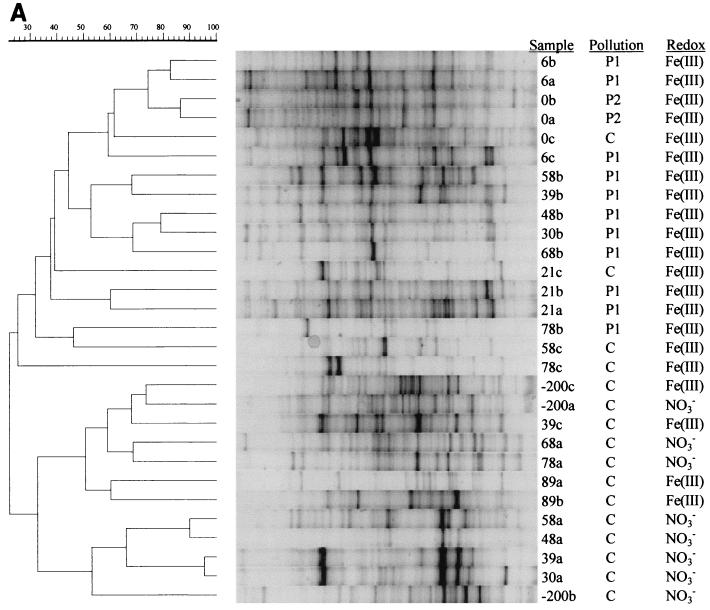
Microbial communities in groundwater were profiled by DGGE of amplified 16S rDNA fragments. The profiles of the bacterial communities were complex, and the data revealed that there was a high degree of variation between samples (Fig. 3A). To establish relationships between samples, the entire densitometric curves for the tracks were numerically compared by using the Pearson product moment correlation coefficient (40, 41). In general, cluster analysis with UPGMA grouped samples of polluted groundwater in one large cluster at a level of similarity of 35%, while clean samples clustered separately (Fig. 3A). Only three DGGE profiles (those for samples 21c, 0c, and 78b) from the 29 groundwater samples examined did not cluster in accordance with the degree of pollution. There were clearly differences in microbial composition and thus community heterogeneity within the plume because samples from the plume clustered at a level of only 35%. Samples from within and just beneath the landfill (samples 0a and 0b; cluster P2 in Fig. 2) and from 6 m downstream (samples 6a, 6b, and 6c; cluster P1 in Fig. 2) produced the most similar profiles. The bacterial communities in groundwater obtained from outside the plume showed more variation than those from within the plume. The nitrate-containing groundwater samples from above the plume (samples 30a, 39a, 48a, and 58a) clustered together, while samples from further downstream that also contained nitrate (samples 68a and 78a) clustered separately.
FIG. 3.
UPGMA cluster analysis of DGGE profiles of Bacteria (40 to 60% denaturant gradient) (A) and Archaea (45 to 70% denaturant gradient) (B) in groundwater after Pearson product moment correlation. For each lane the sample designation (Fig. 1), pollution level (P1, P2, and C refer to groups in Fig. 2), and proposed dominant redox process [NO3−, denitrification; Fe(III), iron reduction] are indicated.
A more distinctive difference between community structures within and outside the plume was observed for archaeal communities (Fig. 3B). The DGGE profiles were less complex than those observed for Bacteria. The profiles of samples from the plume contained a few strong dominant bands, resulting a strong correlation at >70% for most of the samples from the plume. Two of the dominant bands were clearly visible only in the profiles of polluted groundwater samples; interestingly, one of these bands was not present in the profiles of samples obtained furthest downstream from the landfill (samples 58b, 68b, and 78b). Archaeal PCR products were not obtained from any of the samples from below the plume.
Composition of microbial communities in groundwater.
Analysis of clone libraries was used as a second method to characterize the microbial communities in groundwater, and this analysis allowed more detailed phylogenetic information on the microorganisms present in groundwater samples. It also generated more specific data on how community structure was affected by landfill leachate. The libraries were prepared from three groundwater samples, each representing one of the three clusters (Fig. 2), and the samples were obtained from approximately the same depth, as follows: sample −200b from upstream (clean, cluster C), sample 0b from beneath the landfill (polluted, cluster P2), and sample 6b from downstream of the landfill (polluted, cluster P1).
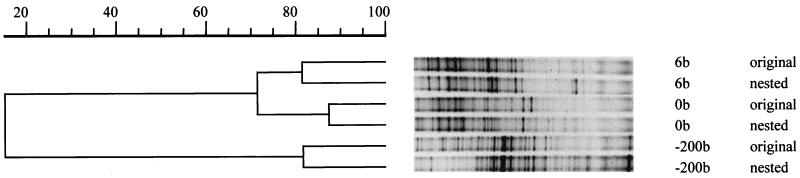
Nearly complete 16S rDNA sequences of members of the Bacteria were amplified and cloned. Between 95 and 105 clones were screened per clone library. Clones, as well as the PCR fragments used for cloning, were reamplified with primers F341-GC and R518, and their DGGE profiles were compared to that of the original sample (Fig. 4 and 5). The similarity between the results for directly amplified groundwater DNA samples and nested PCR data (amplification with the 1.5-kb PCR fragment used for cloning as template) was more than 80% (Fig. 4). This indicates that the PCR required for cloning did not lead to an obvious cloning bias; the data for 74% (sample −200b) to 85% (sample 6b) of the clones matched bands in the community DGGE profiles.
FIG. 4.
UPGMA cluster analysis of DGGE profiles (40 to 70% denaturant gradient) of groundwater samples −200b, 0b, and 6b used for constructing clone libraries. For each sample, primers F341-GC and R518 were used directly with isolated groundwater DNA (original) or with the PCR fragment obtained with primers 8f and 1512r and used for cloning (nested).
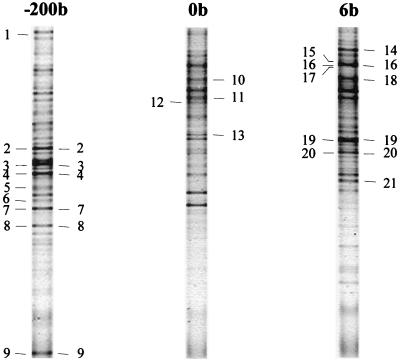
FIG. 5.
Linking of bacterial clone identities to DGGE profiles (40 to 70% denaturant gradient) of groundwater samples taken upstream (sample −200b), beneath (sample 0b), and downstream (sample 6b) of Banisveld landfill. The band positions for clones that showed DGGE migration similar to that of a dominant band in the groundwater community DGGE profile are indicated to the right of each track. The band positions for clones with identities indicating an ability to perform redox reactions are shown to the left of each track. The identities of the numbered bands are given in Table 2.
Ninety-six clones were randomly selected, and the part of the cloned 16S rDNA that was also profiled by DGGE (corresponding to E. coli positions 341 to 518, including the V3 region) was sequenced. Later, 17 of the partially sequenced clones and seven additional clones, mainly clones with DGGE bands corresponding to dominant bands in the original profiles, were nearly completely sequenced. Sequencing nearly complete 16S rDNA did not result in assignment to phylogenetic groups that differed from those based on the V3 region. The majority of the clones resembled (facultatively) anaerobic and microaerophilic microorganisms. Sequences related to facultatively anaerobic and microaerophilic microorganisms were especially observed with the upstream sample. No pathogens were encountered. The distribution of the 96 randomly sequenced clones in phylogenetic groups is shown in Table 1; 16 to 25% of the sequences showed less than 90% similarity to sequences deposited in GenBank and were described as unclassified. It is obvious that the microbial composition of each groundwater sample was different. Upstream of the landfill there was strong dominance by bacteria belonging to the β subclass of the class Proteobacteria (β-proteobacteria) (48.6%), which mainly resembled Gallionella ferruginea (four clones, 93 to 95% similarity) and Azoarcus sp. strain BS5.8 (five clones, 93 to 95% similarity). Linking of the clone identities to band positions in DGGE gels (Fig. 5 and Table 2) indicated that these sequences also were related to dominant bands in the DGGE profile of the microbial community. Several sequences related to genera capable of denitrification (Azoarcus, members of the Actinobacteria) were found in this groundwater sample obtained from a denitrifying environment and also in the dominant bands (bands 2, 7, and 8 in Fig. 5). Furthermore, two sequences related to sulfate reducers were encountered, and one of these sequences corresponded to a dominant band in the DGGE profile (band 9).
TABLE 1.
Relative levels of bacterial clones related to various phylogenetic groups in clone libraries from aquifer groundwater samples obtained upstream (sample −200b), beneath (sample 0b), and downstream (sample 6b) of the Banisveld landfill
| Phylogenetic group | % in the following groundwater samples:
|
||
|---|---|---|---|
| −200ba | 0bb | 6bc | |
| Low-G+C-content gram-positive group | 2.9 | 37.5 | 11.4 |
| High-G+C-content gram-positive group | 8.6 | 12.5 | 5.7 |
| α-Proteobacteria | 5.7 | 0.0 | 0.0 |
| β-Proteobacteria | 48.6 | 0.0 | 20.0 |
| γ-Proteobacteria | 0.0 | 8.3 | 0.0 |
| δ-Proteobacteria | 8.6 | 4.2 | 25.7 |
| Green nonsulfur bacteria | 2.9 | 4.2 | 5.7 |
| Spirochaetales | 0.0 | 8.3 | 0.0 |
| Cytophaga-Flexibacter-Bacteroides group | 2.9 | 0.0 | 5.8 |
| Holophaga | 0.0 | 0.0 | 2.9 |
| Verrucomicrobia | 2.9 | 0.0 | 0.0 |
| WS5 division | 0.0 | 0.0 | 2.9 |
| Unclassified (<90% similarity) | 16.9 | 25.0 | 20.0 |
TABLE 2.
Identities of clones related to numbered bands in Fig. 5, as determined by partial or nearly complete 16S rDNA sequencing
| Band | Accession no. | Closest relative in GenBank database (accession no.) | % Similarity | Phylogenetic group |
|---|---|---|---|---|
| 1 | AY013676a | Desulfosporosinus sp. strain S10 (AF07527) | 96 | Low-G+C-content gram-positive bacteria |
| 2 | AY013696ab | Gallionella ferruginea (L07897) | 94 | β-Proteobacteria |
| AY013693b | Uncultured Duganella sp. strain CTHB-18 (AF067655) | 93 | β-Proteobacteria | |
| 3 | AY013670 | Unidentified β-proteobacterium cda-1 (Y17060) | 96 | β-Proteobacteria |
| AY013688 | Unidentified β-proteobacterium cda-1 (Y17060) | 96 | β-Proteobacteria | |
| AY013694ab | Gallionella ferruginea (L07897) | 95 | β-Proteobacteria | |
| AY013698ab | Gallionella ferruginea (L07897) | 93 | β-Proteobacteria | |
| AY013691b | Actinomyces sp. (X92701) | 96 | High-G+C-content gram-positive bacteria | |
| AY013663 | Uncultured bacterium RB25 (Z95718) | 88 | Unclassified | |
| 4 | AY013697ab | Gallionella ferruginea (L07897) | 92 | β-Proteobacteria |
| AY013695b | Unidentified bacterium BD4-9 (AB015559) | 88 | Unclassified | |
| 5 | AY013690a | Azoarcus sp. strain BS5–8 (AF011350) | 93 | β-Proteobacteria |
| 6 | AY013666a | Azoarcus sp. strain BS5–8 (AF011350) | 93 | β-Proteobacteria |
| 7 | AY013669a | Azoarcus sp. strain BS5–8 (AF011350) | 94 | β-Proteobacteria |
| AY013681a | Azoarcus sp. strain BS5–8 (AF011350) | 94 | β-Proteobacteria | |
| 8 | AY013674 | Unidentified bacterium DGGE band 10 (AJ009652) | 98 | High-G+C-content gram-positive bacteria |
| AY013684a | Azoarcus sp. strain BS5–8 (AF011350) | 98 | High-G+C-content gram-positive bacteria | |
| AY013664a | Denitrifying bacterium 72Chol (Y09967) | 95 | β-Proteobacteria | |
| AY013675a | Azoarcus sp. strain BS5–8 (AF011350) | 93 | β-Proteobacteria | |
| AY013689 | Uncultured bacterium t0.6.f (AF005745) | 91 | Green nonsulfur bacteria | |
| AY013682 | Candidate division OP11 clone OPd29 (AF047561) | 90 | Unclassified | |
| 9 | AY013665a | Desulfovibrio aminophilus (AF067964) | 93 | δ-Proteobacteria |
| 10 | AY013593 | Acetobacterium carbonolicum (X96956) | 98 | Low-G+C-content gram-positive bacteria |
| AY013613 | Acetobacterium wieringae (X96955) | 97 | Low-G+C-content gram-positive bacteria | |
| AY013610b | Acetobacterium malicum (X96957) | 97 | Low-G+C-content gram-positive bacteria | |
| AY013607b | Acetobacterium malicum (X96957) | 95 | Low-G+C-content gram-positive bacteria | |
| 11 | AY013591 | Acetobacterium wieringae (X96955) | 98 | Low-G+C-content gram-positive bacteria |
| 12 | AY013609ab | Geobacter akaganeitreducens (U96918) | 94 | δ-Proteobacteria |
| 13 | AF013603 | Uncultured eubacterium WCHB1–21 (AF505080) | 96 | Low-G+C-content gram-positive bacteria |
| 14 | AY013658 | Uncultured freshwater bacterium (AF109142) | 98 | Unclassified |
| 15 | AY013644ab | Geobacter sp. strain CdA-2 (Y19190) | 96 | δ-Proteobacteria |
| 16 | AY013634a | Metal-contaminated soil clone K20-06 (AF145810) | 98 | δ-Proteobacteria |
| AY013642a | Metal-contaminated soil clone K20-06 (AF145810) | 95 | δ-Proteobacteria | |
| AY013647ab | Geobacter sp. strain CdA-2 (Y19190) | 96 | δ-Proteobacteria | |
| AY013648ab | Geobacter sp. strain CdA-2 (Y19190) | 94 | δ-Proteobacteria | |
| AY013651a | Geobacter sp. strain CdA-3 (Y13131) | 93 | δ-Proteobacteria | |
| AY013641a | Azoarcus sp. PCR strain (X85434) | 93 | δ-Proteobacteria | |
| AY013649b | Uncultured bacterium WCHB1–60 (AF050598) | 91 | Candidate division WS5 | |
| 17 | AY013652/3ac | Geobacter sp. strain (GSPY19190) | 96 | δ-Proteobacteria |
| 18 | AY013646b | Acidovorax sp. strain UFZ-B517 (AF235010) | 96 | δ-Proteobacteria |
| AY013643b | Rhodoferax fermentans (D16211) | 96 | δ-Proteobacteria | |
| AY013650b | Acidovorax devluvii (Y18616) | 91 | δ-Proteobacteria | |
| 19 | AY013645ab | Geobacter sp. strain CdA-2 (Y19190) | 95 | δ-Proteobacteria |
| AY013633 | Eubacterium limosum (M59120) | 94 | Low-G+C-content gram-positive bacteria | |
| AY013638 | Uncultured bacterium clone H1.4.f (AF005748) | 93 | Green nonsulfur bacteria | |
| 20 | AY013620a | Uncultured sulfate-reducing bacterium 368 (AJ389629) | 91 | δ-Proteobacteria |
| 21 | AY013625 | Uncultured clone CRE-FL35 (AF141457) | 97 | β-Proteobacteria |
The identity of the closest relative in the GenBank database gives an indication of the ability to perform redox reactions (microaerophilic, denitrification, iron reduction, or sulfate reduction).
The 16S rDNA was almost completely sequenced.
E. coli positions 8 to 518 and 1002 to 1512 were sequenced.
None of the clones from the groundwater beneath the landfill (sample 0b) showed affiliation to β-proteobacteria (Table 1). Here a strong dominance by gram-positive bacteria was observed; 12.5% of the clones belonged to the high-G+C-content gram-positive bacteria, and 37.5% belonged to the low-G+C-content gram-positive bacteria. The sequences of five clones (21%) closely resembled Acetobacterium sequences (95 to 98% similarity). These clones could be linked to dominant bands in the DGGE profile of the groundwater beneath the landfill (bands 10 and 11 in Fig. 5). Another clone falling in the low-G+C-content gram-positive group also had a mobility similar to that of a dominant band in the DGGE profile (band 13 in Fig. 5), further demonstrating the apparent dominance of low-G+C-content gram-positive bacteria beneath the landfill. Only one sequence related to known iron reducers (Geobacter-like sequence) was encountered; this sequence was related to a subdominant band in the DGGE profile of the microbial community (band 12 in Fig. 5).
Downstream of the landfill the relative number of low-G+C-content gram-positive clones decreased, and β-proteobacteria reappeared (Table 1). The β-proteobacteria present were quite different from those encountered upstream of the landfill. Sequences related to Acidovorax (two clones, 93 to 96% similarity), Rhodoferax, and several uncultured β-proteobacteria were most frequently encountered in this clone library. Also, δ-proteobacteria, especially sequences related to the family Geobacteraceae (eight clones, 93 to 98% similarity), strongly contributed to the clone library (25.7% of the clones analyzed). Two clones, which based on sequencing of the V3 region were related to clone K20-06 (GenBank accession number AF145810), were also identified as Geobacter spp. Initially, four clones with similar migration in DGGE gels (band 16 in Fig. 5) showed this affiliation after sequencing of the V3 region. Sequencing of nearly complete 16S rDNA of two of these clones showed that both were closely related to Geobacter sp. strain CdA2. Dominant bands in the DGGE profiles for groundwater samples obtained downstream of the plume also appeared to be contributed by members of the δ-proteobacteria (Geobacteraceae; bands 16 and 19 in Fig. 5) and β-proteobacteria (bands 18 and 21 in Fig. 5). The strong dominance by iron-reducing members of the Geobacteraceae is in agreement with iron reduction being the major redox process. One sequence related to a potential denitrifier (Azoarcus related) and another sequence related to a sulfate reducer were also encountered. The potential denitrifier showed comigration with five Geobacter clones (band 16) and corresponded to a dominant component of the DGGE profiles. As Fig. 5 and Table 2 show, clones with different phylogenetic associations often exhibited similar migration patterns in DGGE gels.
Confirmation that members of the Geobacteraceae were an important group of bacteria in the iron-reducing aquifer was obtained by an MPN-PCR analysis by using Geobacteraceae-specific primers and expressing the number relative to the MPN obtained with general bacterial primers. Upstream the percentage was less then 0.5%, underneath the landfill the percentage was 6%, and downstream the percentage was 25%. Performing DGGE after a nested PCR with primers F341-GC and R518 on the Geobacter-specific PCR product revealed a dominant band corresponding to band 16 in Fig. 5 for all iron-reducing samples (groundwater from the plume and below the plume). This band was not present in any of the denitrifying samples (data not shown).
As the clustering of DGGE profiles of Archaea appeared to be due to the presence or absence of two dominant bands (Fig. 3B), only these bands were sequenced after excision from the gel. The sequence of the upper band was 100% similar to the sequence of methanogenic endosymbionts of the anaerobic protozoans Trimyema compressa (accession number Z16412) and Metopus contortus (accession number Z13957); the sequence of the lower dominant band was 96% similar to the sequence of an unidentified archeaon (accession number AF050617).
Geochemistry and microbial community structure of sediment.
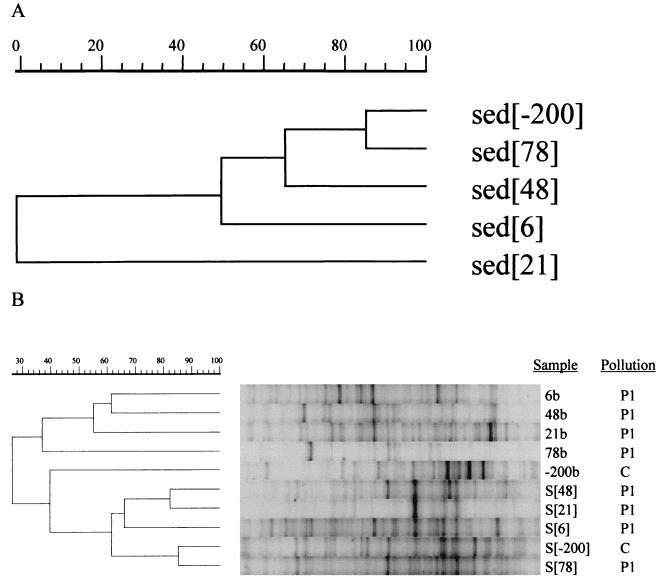
In October 1998 sediment samples were retrieved from five locations, one upstream and four in the plume of leachate (Fig. 1). Analysis of the chemical composition of the sediment porewater and subsequent cluster analysis clearly revealed that the sediment samples from the plume were polluted and that the upstream sample was clean and did not cluster with the four sediment samples (data not shown). When parameters not affected by pollution (percentages of lime, humus, clay, silt, carbon, and nitrogen in sediment) were used for cluster analysis, a low-level relationship was observed (Fig. 6A), indicating that the aquifer had a heterogeneous sediment composition. Sediment samples S[-200] and S[78] were most similar in terms of chemistry.
FIG. 6.
UPGMA cluster analysis of pollution-independent sediment parameters (A) and Bacteria DGGE profiles for sediment and corresponding groundwater samples (40 to 60% denaturant gradient) (B). For each lane the sample designation (Fig. 1; S[48], S[21], S[6], S[−200], and S[78] refer to sediment samples) and level of pollution (Fig. 2) are indicated.
After numerical comparison of the DGGE profiles of Bacteria, the five sediment samples clustered together at the 60% level, and S[-200] and S[78] were most similar to each other (Fig. 6B). Groundwater samples showed much less similarity. The profiles of sediment were quite different from the profiles of groundwater extracted from the same position and depth.
DISCUSSION
In this study we attempted to relate microbial community structure to hydrochemistry in a landfill leachate-polluted aquifer. Microbial community structures were determined by cultivation-independent, molecular methods. The different steps (DNA extraction, PCR, and profiling) in such a molecular approach have their pitfalls (49). However, since all samples were treated similarly, these pitfalls can be considered to be the same for all samples, allowing between-sample comparisons. The comparisons between samples were accomplished by numerical analysis of DGGE profiles, using the Pearson product moment correlation coefficient. This coefficient is robust and objective, since whole curves are compared and subjective band scoring is omitted (40). Difficulties with band assignment are especially likely to occur with highly complex and varying profiles, as in our study. Furthermore, the Pearson coefficient does not suffer from mismatches between peaks and shoulders, a problem often found when band scoring is used (40), and is much less laborious.
Comparison between microbial community structures of groundwater and sediment.
In contrast to the groundwater results, no relationship to pollution was apparent from the analysis of the microbial community structure of sediment. The number of particle-bound microorganisms per gram of sediment is usually 1 order of magnitude higher than the number of free-living microorganisms per milliliter in landfill leachate-polluted aquifers (4, 22). Since 1 cm3 of sediment weights 2.65 g and contains about 30% water, the number of sediment-associated microorganisms is about 2 orders of magnitude higher than the number present in water. Given that on a geological scale a relatively short time has elapsed since landfilling started (1965), leachate may have had little impact on the microorganisms closely associated with the 10,000- to 100,000-year-old sediments. A large portion of the sediment-bound microorganisms could be physically (e.g., in pores) or biologically (e.g., in biofilms) protected from the influence of leachate. Furthermore, the pollutant-independent heterogeneity of sediment composition (Fig. 6A) may have contributed to variability in microbial community structure (33) and hampered observation of changes related to pollution. The differences in community structure between sediment and nearby groundwater are in agreement with previous observations made at landfill leachate-polluted aquifers (41) and other environments for which communities of particle-bound and free-living bacteria were determined (15, 24).
Groundwater community structure in relation to pollution and redox processes.
In the leachate plume examined in this study, iron reduction is a dominant redox process, and in the zone of iron reduction BTEX compounds appear to be degraded. Similar observations have been made for other landfill leachate-affected aquifers (2, 21, 34, 44). Both DGGE and clone library data indicate that the microbial community structure of the iron-reducing leachate plume differs considerably from the microbial community structure of the unpolluted groundwater upstream, above, and below the plume of pollution. Clustering of DGGE profiles of Bacteria showed that 90% of the samples were correctly separated based on the level of pollution. Two clean samples (samples 0c and 21c) were identified as polluted, and one polluted sample (sample 78b) was identified as clean. The latter sample was from the piezometer in the plume that was farthest from the landfill and thus was influenced by landfill leachate for the shortest time. The values for some hydrochemical parameters of sample 0c, such as chloride concentration, were remarkably high for a clean sample (data not shown). Sample 21c was also the only sample wrongly assigned when culture-dependent anaerobic community-level physiological profiling was used (42). All DGGE profiles of Archaea were assigned to the correct cluster, based on the level of pollution. Thus, groundwater sampling was shown to be suitable for determining differences in microbial community structure associated with pollution. Microbial degradation can also be determined by using only groundwater samples, although the degradation rates are lower and groundwater sometimes exhibits lower degradation potential than aquifer sediment (3, 22).
Analysis of DGGE profiles showed that while communities of Archaea and Bacteria in the plume clustered together, more variation was observed outside the plume. Outside the plume more variation in dominant redox processes was found; denitrification occurred upstream and above the plume, and iron reduction occurred below the plume. Clustering of DGGE profiles of Bacteria correlated partially with these differences in redox processes. Communities of Archaea were clearly different, in the sense that all samples from iron-reducing, nonplume locations failed to yield a PCR product in the Archaea-specific PCR, while samples from locations characterized by denitrification did give rise to a PCR product. Cluster analysis of DGGE profiles of the latter samples showed that the profiles grouped together and were different from those of the communities of Archaea in the leachate plume.
The results for the clone libraries linking particular organisms to bands in DGGE profiles were consistent with the observed redox conditions. Upstream, where denitrifiying conditions prevailed, sequences related to potential denitrifiers (Azoarcus [51], members of he Actinobacteria [45]), as well as the microaerophilic iron-oxidizing organism G. ferruginea (19), were encountered. Sequences related to aerobic and denitrifying bacteria were seldom encountered beneath and downstream of the landfill. Beneath the landfill strictly anaerobic, fermentative microorganisms, especially members of the Clostridiaceae, dominated. Also, one sequence related to the Geobacteraceae was encountered. Downstream, where iron-reducing conditions dominated, a high percentage of the sequences (22%) was closely related to this family. Iron reduction is a general trait of cultivated members of the Geobacteraceae (26). Downstream one sequence related to a potential denitrifier (Azoarcus) and one sequence related to a sulfate reducer were obtained, while upstream two sequences related to sulfate reducers were also obtained. Culture-dependent studies of a Danish landfill leachate plume also showed that usually several types of redox reaction-performing microorganisms are present at the same location, even when redox conditions are unfavorable (33). The occurrence of specific phospholipid fatty acid (PLFA) biomarkers paralleled the occurrence of sulfate and iron reduction in the Danish aquifer (33).
Community structure and degradation in the leachate plume.
While cluster analysis of DGGE profiles obtained with general bacterial and archaeal primers was able to separate communities from polluted groundwater and clean groundwater, it was not able to clearly distinguish samples within the plume and to relate them to hydrochemistry or processes. In part, this might have been due to the fact that iron reduction is the dominant redox process throughout the plume. Clustering of the DGGE profiles of members of the Bacteria revealed separation of samples close to the landfill (sampling wells 0 and 6) from samples farther away, but based on hydrochemistry the samples obtained near the landfill were members of cluster P1 (hardly any BTEX compounds) and P2 (containing BTEX compounds) (Fig. 2) and thus could not be clearly related to degradation. The lack of a relationship between microbial community structure and degradation is not surprising since (i) xenobiotic compounds (primarily BTEX [<204 μg/liter]) contribute less than 1% of the dissolved organic carbon (57 to 98 mg/liter) in the plume and thus microorganisms metabolizing BTEX make only a minor contribution to the total microbial community and (ii) in addition to organic carbon, microorganisms leach from the landfill and strongly contribute to the rDNA-based microbial community structure, although they are not active. Leaching of Bacteria is indicated by the fact that the DGGE profile of the groundwater sample from just below the landfill (sample 0b) is very similar to the DGGE profile of the sample taken from within the landfill (sample 0a). Also, the clone libraries from groundwater beneath and downstream of the landfill revealed a large number of sequences related to complex-compound-degrading fermentative bacteria and acetogens (the genera Acetobacterium, Clostridium, Cytophaga, Spirochaeta, and Bacteroides). In landfills, high numbers (>107 cells per g [dry weight]) of acetogenic, xylanolytic, and cellulolytic bacteria are present, while only simple organic compounds leach out (7). A large number of Clostridium- and Cytophaga-like sequences were also detected in a molecular study of a Canadian landfill (25). Microorganisms can persist in groundwater over long distances; anaerobic microorganisms from livestock wastewater constituted a major part of the microbial community at an aerobic sampling well 400 m from the point of pollution (9). Although molecular analysis of rRNA instead of rDNA is thought to be more useful as it should favor the detection of the active microbial community (17), it is unlikely to be of much benefit for studying environments such as those examined in this study. Starved bacteria can maintain high numbers of ribosomes, up to 30% of the maximum (18). Furthermore, if one assumes that indeed there is a universal relationship between RNA/DNA ratio and growth rate (μ) and that this relationship can be described by RNA/DNA = 1.65 + 6.01 μ0.73 (23), then even if microorganisms were growing in their natural environment at the unrealistically high rate of 0.5 h−1 (generation time, 80 min), their RNA/DNA ratio (with the RNA mainly being rRNA) is only three times higher than the ratio under zero-growth conditions. In the subsurface, growth rates can be assumed to be much lower (50). Therefore, like rDNA-based analysis, rRNA-based analysis indicates merely presence and not activity.
High methane concentrations in the groundwater indicated that there were methanogenic conditions in the landfill; thus, leaching of archaeal cells from the landfill might be expected. Remarkably, one of the dominant bands in the archaeal profiles was clearly related to a methanogenic endosymbiont of an anaerobic protozoan. This suggests the presence of anaerobic protozoans. Pollution usually increases protozoan numbers (36), although no protozoans could be detected in a Danish landfill leachate-polluted aquifer (33). Predation by protozoans and variations in hydrochemical composition in the plume could explain why despite the clustering considerable variation (profiles clustered only at the 35% level) was found in microbial community structure in the leachate plume.
Multivariate analysis of the relationship between PLFA profiles and microbial redox processes revealed that PLFA profiles also had limited value for identifying more specific microbial communities in a landfill leachate plume (32). It is well known that some numerically minor groups of microorganisms are essential for major environmental processes; e.g., nitrifiers are essential in the N cycle (38). In contrast to PLFA, specific functional groups of microorganisms can be more adequately investigated by molecular methods, such as those used in this study. Our limited knowledge concerning genes involved in anaerobic BTEX degradation (20) eliminates any possibility of direct measurement of degradation-related gene expression. However, molecular techniques linking community structure to function have recently been developed. Use of stable-isotope probing (39) or bromodeoxyuridine labeling (47) in carefully designed microcosm assays that mimic the natural situation as closely as possible should help establish a clearer relationship between microbial community structure and degradation processes. Also, for this aquifer, in which iron reduction is a major redox process and degradation occurs under these redox conditions, a logical choice for future research is to focus on iron-reducing bacteria. While iron-reducing bacteria are phylogenetically very diverse (8, 13, 16, 26, 28), only sequences related to the Geobacteraceae were encountered. Clone libraries linking identities to DGGE profiles of whole microbial communities and MPN-PCR revealed the considerable contribution of Geobacteraceae to the microbial community. The results presented here underline the finding that members of the Geobacteraceae are widely distributed and dominant in diverse iron-reducing environments (14, 46). Interestingly, until now only members of the genus Geobacter have been found to be capable of toluene oxidation under iron-reducing conditions (14, 30), while there are strong indications that members of the Geobacteraceae are also involved in anaerobic benzene degradation (43). Members of the Geobacteraceae are also important humic acid reducers (12) and are capable of using humic acids as electron shuttles to facilitate iron reduction (31). Humic acids account for about 10% of the dissolved organic carbon in landfill leachate (10). Consequently, members of the Geobacteraceae are a good first choice for more detailed community studies in relation to natural attenuation in landfill leachate-polluted aquifers.
ACKNOWLEDGMENTS
This investigation was financially supported by the Dutch research program Biotechnological In-situ Remediation (NOBIS) and the Dutch provinces of Zuid-Holland, Noord-Brabant, and Utrecht.
We thank Ian Head, Fossil Fuels and Environmental Geochemistry Post-Graduate Institute, University of Newcastle upon Tyne, Newcastle upon Tyne, United Kingdom, for correcting the English grammar and style.
REFERENCES
- 1.Acton D W, Barker J F. In situ biodegradation potential of aromatic hydrocarbons in anaerobic groundwaters. J Contam Hydrol. 1992;9:325–352. [Google Scholar]
- 2.Albrechtsen H J, Bjerg P L, Ludvigsen L, Rügge K, Christensen T H. An anaerobic field injection experiment in a landfill leachate plume, Grindsted, Denmark 2. Deduction of anaerobic (methanogenic, sulfate-, and Fe(III)-reducing) redox conditions. Water Resour Res. 1999;35:1247–1256. [Google Scholar]
- 3.Albrechtsen H J, Smith P M, Nielsen P, Christensen T H. Significance of biomass support particles in laboratory studies on microbial degradation of organic chemicals in aquifers. Water Res. 1996;30:2977–2984. [Google Scholar]
- 4.Albrechtsen H J, Winding A. Microbial biomass and activity in subsurface sediments from Vejen, Denmark. Microb Ecol. 1992;23:303–317. doi: 10.1007/BF00164102. [DOI] [PubMed] [Google Scholar]
- 5.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 6.Amann R I, Stromley J, Devereux R, Key R, Stahl D A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992;58:614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlaz M A, Schaefer D M, Ham R K. Bacterial population development and chemical characteristics of refuse decomposition in a simulated sanitary landfill. Appl Environ Microbiol. 1989;55:55–65. doi: 10.1128/aem.55.1.55-65.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caccavo F, Coates J D, Rossello-Mora R A, Ludwig W, Schleifer K H, Lovley D R, McInerney M J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol. 1996;165:370–376. doi: 10.1007/s002030050340. [DOI] [PubMed] [Google Scholar]
- 9.Cho J-C, Kim S-J. Increase in bacterial community diversity in subsurface aquifers receiving livestock wastewater input. Appl Environ Microbiol. 2000;66:956–965. doi: 10.1128/aem.66.3.956-965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen J B, Jensen D L, Gron C, Filip Z, Christensen T H. Characterization of the dissolved organic carbon in landfill leachate-polluted groundwater. Water Res. 1998;32:125–135. [Google Scholar]
- 11.Christensen T H, Kjeldsen P, Albrechtsen H J, Heron G, Nielsen P H, Bjerg P L, Holm P E. Attenuation of landfill leachate pollutants in aquifers. Crit Rev Environ Sci Technol. 1994;24:119–202. [Google Scholar]
- 12.Coates J D, Ellis D J, Blunt-Harris E L, Gaw C V, Roden E E, Lovley D R. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates J D, Ellis D J, Gaw C V, Lovley D R. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int J Syst Bacteriol. 1999;49:1615–1622. doi: 10.1099/00207713-49-4-1615. [DOI] [PubMed] [Google Scholar]
- 14.Coates J D, Phillips E J P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump B C, Armbrust E V, Baross J A. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl Environ Microbiol. 1999;65:3192–3204. doi: 10.1128/aem.65.7.3192-3204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings D E, Caccavo F, Spring S, Rosenzweig R F. Ferribacterium limneticum, gen. nov., sp. nov., an Fe(III)-reducing microorganism isolated from mining-impacted freshwater lake sediments. Arch Microbiol. 1999;171:183–188. [Google Scholar]
- 17.Felske A, Wolterink A, VanLis R, Akkermans A D L. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands) Appl Environ Microbiol. 1998;64:871–879. doi: 10.1128/aem.64.3.871-879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukui M, Suwa Y, Urushigawa Y. High survival efficiency and ribosomal RNA decaying pattern of Desulfobacter latus, a highly specific acetate-utilizing organism, during starvation. FEMS Microbiol Ecol. 1996;19:17–25. [Google Scholar]
- 19.Hanert H H. The genus Gallionella. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. Vol. 4. New York, N.Y: Springer-Verlag; 1991. pp. 4082–4088. [Google Scholar]
- 20.Heider J, Spormann A M, Beller H R, Widdel F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol Rev. 1998;22:459–473. [Google Scholar]
- 21.Heron G, Christensen T H, Tjell J C. Oxidation capacity of aquifer sediments. Environ Sci Technol. 1994;28:153–158. doi: 10.1021/es00050a021. [DOI] [PubMed] [Google Scholar]
- 22.Holm P E, Nielsen P H, Albrechtsen H J, Christensen T H. Importance of unattached bacteria and bacteria attached to sediment in determining potentials for degradation of xenobiotic organic contaminants in an aerobic aquifer. Appl Environ Microbiol. 1992;58:3020–3026. doi: 10.1128/aem.58.9.3020-3026.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp P F. Can we estimate bacterial growth rates from ribosomal RNA content? In: Point I, editor. Molecular ecology of aquatic microbes. Berlin, Germany: Springer-Verlag; 1995. pp. 279–302. [Google Scholar]
- 24.Kilb B, Kuhlmann B, Eschweiler B, Preuss G, Ziemann E, Schottler U. Community structures of different groundwater habitats investigated using methods of molecular biology. Acta Hydrochim Hydrobiol. 1998;26:349–354. [Google Scholar]
- 25.Lloyd-Jones G, Lau P C K. A molecular view of microbial diversity in a dynamic landfill in Quebec. FEMS Microbiol Lett. 1998;162:219–226. doi: 10.1111/j.1574-6968.1998.tb13002.x. [DOI] [PubMed] [Google Scholar]
- 26.Lonergan D J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D R. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J Ind Microbiol. 1995;14:85–93. doi: 10.1007/BF01569889. [DOI] [PubMed] [Google Scholar]
- 28.Lovley D R. Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol Rev. 1997;20:305–313. [Google Scholar]
- 29.Lovley D R. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. J Ind Microbiol Biotechnol. 1997;18:75–81. [Google Scholar]
- 30.Lovley D R, Baedecker M J, Lonergan D J, Cozzarelli I M, Phillips E J P, Siegel D I. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature. 1989;339:297–299. [Google Scholar]
- 31.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 32.Ludvigsen L, Albrechtsen H J, Holst H, Christensen T H. Correlating phospholipid fatty acids (PLFA) in a landfill leachate polluted aquifer with biogeochemical factors by multivariate statistical methods. FEMS Microbiol Rev. 1997;20:447–460. [Google Scholar]
- 33.Ludvigsen L, Albrechtsen H J, Ringelberg D B, Ekelund F, Christensen T H. Distribution and composition of microbial populations in landfill leachate contaminated aquifer (Grindsted, Denmark) Microb Ecol. 1999;37:197–207. doi: 10.1007/s002489900143. [DOI] [PubMed] [Google Scholar]
- 34.Lyngkilde J, Christensen T H. Fate of organic contaminants in the redox zones of a landfill leachate pollution plume (Vejen, Denmark) J Contam Hydrol. 1992;10:291–307. [Google Scholar]
- 35.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novarino G, Warren A, Butler H, Lambourne G, Boxshall A, Bateman J, Kinner N E, Harvey R W, Mosse R A, Teltsch B. Protistan communities in aquifers: a review. FEMS Microbiol Rev. 1997;20:261–275. doi: 10.1111/j.1574-6976.1997.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 37.Øvreås L, Forney L, Daae F L, Torsvik V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1997;63:3367–3373. doi: 10.1128/aem.63.9.3367-3373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips C J, Harris D, Dollhopf S L, Gross K L, Prosser J I, Paul E A. Effects of agronomic treatments on structure and function of ammonia-oxidizing communities. Appl Environ Microbiol. 2000;66:5410–5418. doi: 10.1128/aem.66.12.5410-5418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radajewski S, Ineson P, Parekh N R, Murrell J C. Stable-isotope probing as a tool in microbial ecology. Nature. 2000;403:646–649. doi: 10.1038/35001054. [DOI] [PubMed] [Google Scholar]
- 40.Rademaker J L W, Louws F J, Rossbach U, Vinuesa P, de Bruijn F J. Computer-assisted pattern analysis of molecular fingerprints and database construction. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 7.1.3/1–7.1.3/33. [Google Scholar]
- 41.Röling W F M, Van Breukelen B M, Braster M, Goeltom M T, Groen J, Van Verseveld H W. Analysis of microbial communities in a landfill leachate polluted aquifer using a new method for anaerobic physiological profiling and 16S rDNA based fingerprinting. Microb Ecol. 2000;40:177–188. doi: 10.1007/s002480000033. [DOI] [PubMed] [Google Scholar]
- 42.Röling W F M, Van Breukelen B M, Braster M, Van Verseveld H W. Linking microbial community structure to pollution: Biolog-substrate utilization in and near a landfill leachate plume. Water Sci Technol. 2000;41:47–53. [Google Scholar]
- 43.Rooney-Varga J N, Anderson R T, Fraga J L, Ringelberg D, Lovley D R. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl Environ Microbiol. 1999;65:3056–3063. doi: 10.1128/aem.65.7.3056-3063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rügge K, Bjerg P L, Christensen T H. Distribution of organic compounds from municipal solid waste in the groundwater downgradient of a landfill (Grindsted, Denmark) Environ Sci Technol. 1995;29:1395–1400. doi: 10.1021/es00005a036. [DOI] [PubMed] [Google Scholar]
- 45.Schaal K P. The genera Actinomyces, Arcanobacterium, and Rothia. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. Vol. 1. New York, N.Y: Springer-Verlag; 1991. pp. 850–905. [Google Scholar]
- 46.Snoeyenbos-West O L, Nevin K P, Anderson R T, Lovley D R. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb Ecol. 2000;39:153–167. doi: 10.1007/s002480000018. [DOI] [PubMed] [Google Scholar]
- 47.Urbach E, Vergin K L, Giovannoni S J. Immunochemical detection and isolation of DNA from metabolically active bacteria. Appl Environ Microbiol. 1999;65:1207–1213. doi: 10.1128/aem.65.3.1207-1213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Verseveld H W, Van Breukelen B M, Röling W F M, Braster M, Soeteman S, Groen J. In situ bioremediation at old Dutch landfills. Plume delineation, intrinsic degradation capacity and determination of microbial community structure. In: Johnston C D, editor. Contaminated site remediation: challenges posed by urban and industrial contaminants. Wembley, Western Australia, Australia: Center for Groundwater Studies; 1999. pp. 415–424. [Google Scholar]
- 49.von Wintzingerode F, Gobel U B, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 50.Whitman W B, Coleman D C, Wiebe W J. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J Z, Fries M R, Cheesanford J C, Tiedje J M. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol. 1995;45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]