Abstract
Background
A few studies compared the characteristics and outcomes of COVID-19 patients during the first and second surges of the disease. We aimed to describe the clinical features and outcomes of COVID-19 patients across the first, second, and third surges of the disease in Tehran, Iran.
Method
We conducted a retrospective cohort study of patients with COVID-19 admitted to Sina hospital in Tehran, Iran, during three surges of COVID-19 from February 16 to October 28, 2020.
Result
Surge 1 patients were younger with more prevalence of hypertension. They also presented with significantly higher oxygen saturation, systolic blood pressure, and respiratory rate on admission. Patients had higher levels of neutrophil to lymphocyte ratio, Urea, CRP, and ESR, in surge 2. The incidence of dyspnea, chest pain, and neurological manifestations followed a significant increasing trend from surge 1 to surge 3. There was no difference in severity and in-hospital mortality between the surges. However, the length of hospital stays and acute cardiac injury (ACI) was less in surge 1 and acute respiratory distress syndrome (ARDS) in surge 2 than in other surges.
Conclusion
Patients did not significantly differ in disease severity, ICU admission, and mortality between surges; however, length of hospital stay and ACI increased during surges, and the number of patients developing ARDS was significantly less in surge 2 compared to other peaks.
Keywords: ARDS, COVID-19, Comorbidities, Mortality
Introduction
Iran has been one of the most affected countries during the COVID-19 pandemic in the middle east, experiencing the COVID-19 outbreak with 6,019,947 confirmed cases and 127,809 deceased cases till November 12, 2021 [1]. During some periods of COVID-19 outbreaks, Iran was ranked the third and second country worldwide regarding the total number of patients and mortality, respectively [2]. Up to now, this country has faced different surges of COVID-19 outbreak. Previous observations have reported COVID-19 mortality association with several risk factors and comorbidities such as diabetes, hypertension, cancer, cardiovascular disease, chronic kidney disease, and other chronic diseases [3, 4]. Thus, evaluating the epidemiological characteristics of COVID-19 and specifying the underlying comorbidities of SARS-CoV2 infected patients could be of great importance to help public health officials, decision-makers, and clinicians to make pragmatic strides toward reducing the burden of COVID-19 and the subsequent control of the pandemic during upcoming surges.
This study aims to describe the demographic features, comprehensive clinical and laboratory parameters, and in-hospital outcome differences across the first, second, and third surges of COVID-19 hospitalized patients during a large cohort study in Iran.
Methods
All participants provided informed written consent before enrolling in the study, and the ethics committee of the Tehran University of Medical Sciences was approved for the study (IR.TUMS.VCR.REC.1399.018.). Our study has been performed in accordance with the declaration of Helsinki.
This retrospective cohort study was performed in the Sina Hospital, a COVID-19 referral center affiliated with Tehran University of Medical Sciences (TUMS) in Tehran, Iran, From February 16 to October 28, 2020. We included patients over 18 years of age with the diagnosis of COVID-19 who fulfilled one of the two following criteria: (i) PCR -confirmed Covid-19 oropharyngeal or endotracheal swab specimens. (ii) Highly suspected COVID-19 patients according to the World Health Organization's interim guidance [5] and Iranian national committee of COVID-19 [6], including patients with the signs of COVID-19 involvement in their chest computed tomography scan (presence of ground-glass opacity, either isolated or with consolidation), which cannot be fully defined by volume overload, lobar or lung collapse, or nodules along with the history compatible with COVID-19. Demographic data, comorbidities, clinical presentations, vital signs on admission, and laboratory data were extracted from patients' electronic medical records. All patients were followed up for in-hospital complications, severe COVID-19 manifestations, and in-hospital mortality across their admission period. We classified patients into three surges based on admission time: surge 1 (February 15 to April 30, 2020), surge 2 (May 1 to August 30, 2020), and surge 3 (September 1 to October 30, 2020). Disease severity, comorbidities, and in-hospital outcomes were defined based on previous studies [7, 8].
Categorical variables were presented as numbers (%) and compared using the chi-square and chi-square post hoc test, in which we assumed P ≤ 0.00833 as statistically significant according to the Bonferroni correction. The normality of distribution for numerical variables was evaluated by Kolmogorov–Smirnov and Shapiro–Wilk tests. Numerical variables with normal distribution were presented as mean ± standard deviation and compared using the one-way ANOVA test. In contrast, variables with skewed distribution were presented as median [interquartile range] and compared using the Kruskal–Wallis test. All statistical analyses were performed using SPSS Statistics for Windows, Version 21, and P < 0.05 was considered statistically significant.
Results
Demographic and baseline characteristics
As of February 16 to October 28, 2020, 19,722 patients were screened, and 3309 patients were admitted with COVID-19 diagnosis. A total of 649 (19.6%) patients were deceased during this cohort, of whom 387 (59.6%), 149 (23.0%), and 113 (17.4%) patients were admitted to the intensive care unit (ICU), emergency department, and wards respectively. The majority of deceased patients (160 out of 649 (24.6%)) were in the 70–80 age group. In this study, we excluded patients with a lack of key information in their medical records. Finally, 1323 patients were included representing 667, 489, and 167 patients during surges 1, 2, and 3. Baseline characteristics of patients during the three surges are presented in Table. 1. During the first, second, and third surges, the mean ages of hospitalized patients were 57.6 ± 16.3, 61.0 ± 16.6, and 60.7 ± 16.6, respectively (P: 0.001). Comparing the three waves, men constituted the majority of COVID-19 cases (61.1%) during our study period; however, patients did not differ between the three surges with similar gender and body mass index (BMI). Hypertension was the most common comorbidity presenting in 595 patients (45.0%) in all three surges, followed by diabetes mellitus (29.7%) and a history of cardiac disease (23.3%). The prevalence of comorbidities did not differ significantly between the surges except for hypertension (P: 0.015), and the proportion of patients with hypertension was significantly lower in surge 1 in comparison with surges 2 and 3 (P < 0.0083).
Table 1.
Baseline characteristics and in-hospital outcomes of COVID-19 during different surges
| Characteristic† | Total (N = 1323) | Surge 1 (N = 667) | Surge 2 (N = 489) | Surge 3 (N = 167) | P* | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (year) | 59.2 ± 16.5 | 57.6 ± 16.3 | 61.0 ± 16.6 | 60.7 ± 16.6 | 0.001 | |
| Sex | ||||||
| Female | 515(38.9%) | 249(37.3%) | 197(40.3%) | 69(41.3%) | 0.473 | |
| Male | 808(61.1%) | 418(62.7%) | 292(59.7%) | 98(58.7%) | ||
| BMI (kg/m2) | 27.4 ± 4.7 | 27.5 ± 4.9 | 27.3 ± 4.6 | 27.4 ± 4.6 | 0.917 | |
| Comorbidities | ||||||
| Hypertension | 595(45.0%) | 274(41.1%)** | 237(48.5%) | 84(50.3%) | 0.015 | |
| Diabetes mellitus | 393(29.7%) | 197(29.5%) | 145(29.7%) | 51(30.5%) | 0.968 | |
| Cardiac disease | 308(23.3%) | 147(22.0%) | 122(24.9%) | 39(23.4%) | 0.512 | |
| Cerebrovascular disease | 58(4.4%) | 22(3.3%) | 28(5.7%) | 8(4.8%) | 0.133 | |
| Chronic lung disease | 82(6.2%) | 50(7.5%) | 22(4.5%) | 10(6.0%) | 0.112 | |
| Malignancy | 59(4.5%) | 28(4.2%) | 25(5.1%) | 6(3.6%) | 0.641 | |
| Chronic kidney disease | 63(4.8%) | 33(4.9%) | 23(4.7%) | 7(4.2%) | 0.971 | |
| Symptoms | ||||||
| Fever | 721(54.5%) | 354(53.1%) | 271(55.4%) | 96(57.5%) | 0.518 | |
| Cough | 825(62.4%) | 447(67.0%)** | 273(55.8%)** | 105(62.9%) | 0.001 | |
| Dyspnea | 799(60.4%) | 361(54.1%)** | 325(66.5%)** | 113(67.7%) | < 0.001 | |
| Myalgia and arthralgia | 637(48.1%) | 273(40.9%)** | 261(53.4%)** | 103(61.7%)** | < 0.001 | |
| Headache | 223(16.9%) | 100(15.0%) | 89(18.2%) | 34(20.4%) | 0.154 | |
| Nausea and vomiting | 354(26.8%) | 133(19.9%)** | 151(30.9%)** | 70(41.9%)** | < 0.001 | |
| Sore throat | 57(4.3%) | 31(4.6%) | 16(3.3%) | 10(6.0%) | 0.272 | |
| Chest pain | 167(12.6%) | 62(9.3%)** | 70(14.3%) | 35(21.0%)** | < 0.001 | |
| Abdominal pain | 227(17.2%) | 160(24.0%)** | 44(9.0%)** | 23(13.8%) | < 0.001 | |
| Neurological manifestations | 110(8.3%) | 38(5.7%)** | 42(8.6%) | 30(18.0%)** | < 0.001 | |
| LOC | 83(6.3%) | 35(5.2%) | 35(7.2%) | 13(7.8%) | 0.288 | |
| Vital signs on admission | ||||||
| Heart rate | 91.2 ± 24.0 | 88.6 ± 16.4 | 93.7 ± 32.5 | 93.4 ± 15.5 | < 0.001 | |
| SBP (mmHg) | 126.3 ± 21.2 | 123.8 ± 20.5** | 128.3 ± 21.8 | 128.7 ± 21.4 | < 0.001 | |
| DBP (mmHg) | 76.6 ± 12.9 | 75.8 ± 11.7 | 76.9 ± 13.4 | 78.2 ± 15.0 | 0.131 | |
| Respiratory rate | 21.0 ± 5.7 | 19.8 ± 5.6** | 22.3 ± 5.9 | 21.2 ± 4.6 | < 0.001 | |
| Temperature (oC) | 37.2 ± 0.9 | 37.2 ± 0.9 | 37.2 ± 0.9 | 37.1 ± 0.9 | 0.706 | |
| Oxygen saturation (%) | 89.2 ± 8.1 | 90.7 ± 7.4** | 87.7 ± 8.8 | 88.1 ± 7.7 | < 0.001 | |
| Laboratory data on admission | ||||||
| WBC (× 109/L) | 6.9[5.2–9.5] | 6.6[5.2–9.5] | 7.4[5.3–9.5] | 7.2[5.1–9.6] | 0.338 | |
| Neutrophil (× 109/L) | 5.3[3.7–7.7] | 4.8[3.6–7.3] | 5.9[3.9–8.1] | 5.6[3.7–7.5] | 0.003 | |
| Lymphocyte (× 109/L) | 1.1[0.8–1.6] | 1.2[0.9–1.7] | 1.0 [0.7–1.4] | 1.1[0.8–1.5] | < 0.001 | |
| Platelets (× 109/L) | 194.0[151.0–265.5] | 191.0[150.0–258.0] | 203.0[157.0–282.7] | 192.5[152.0–262.2] | 0.094 | |
| Neutrophil-to-lymphocyte ratio | 4.5[2.7–8.1] | 3.8[2.5–6.5] | 5.9[3.4–10.1] | 4.7[3.0–7.0] | < 0.001 | |
| Platelet-to-lymphocyte ratio | 173.1[119.7–259.3] | 154.4[114.0–216.1] | 206.8[138.3–336.1]** | 178.1[121.7–259.9] | < 0.001 | |
| SII | 889.6[498.5–1745.4] | 762.1[445.8–1380.9] | 1221.4[610.2–2607.7]** | 913.2[518.1–1714.6] | < 0.001 | |
| Hemoglobin (g/dL) | 13.5[12.1–14.9] | 13.7[12.4–15.0] | 13.3[11.8–14.6] | 13.5[12.0–14.8] | 0.008 | |
| Urea (mg/dL) | 34.0[24.0–53.0] | 32.0[23.0–49.0] | 39.0[27.0–60.0]** | 34.0[25.7–48.0] | < 0.001 | |
| BUN/creatinine ratio | 14.7[11.2–19.5] | 13.9[10.7–18.3] | 15.9[12.2–20.7] | 14.7[11.3–18.6] | < 0.001 | |
| Creatinine (mg/dL) | 1.1[0.9–1.3] | 1.1[0.9–1.3] | 1.1[0.9–1.3] | 1.1[0.9–1.3] | 0.157 | |
| Sodium (mmol/L) | 136.3[133.1–139.4] | 136.0[132.8–139.4] | 137.0[133.8–139.9] | 135.9[132.7–138.2] | 0.009 | |
| Potassium (mmol/L) | 4.3[4.0–4.7] | 4.3[3.4–4.6] | 4.4[4.0–4.9] | 4.4[3.9–4.7] | < 0.001 | |
| Calcium (mmol/L) | 8.8[8.3–9.2] | 8.7[8.2–9.1]** | 8.8[8.4–9.2] | 8.8[8.4–9.2] | < 0.001 | |
| Phosphorous (mmol/L) | 3.3[2.8–3.9] | 3.4[2.9–4.0] | 3.3[2.7–4.0] | 3.0[2.5–3.6] | < 0.001 | |
| Magnesium (mmol/L) | 2.2[2.0–2.5] | 2.2[2.0–2.5] | 2.1[1.9–2.4] | 2.2[1.9–2.6] | < 0.001 | |
| CRP (mg/L) | 62.1[27.1–99.5] | 56.0[19.8–98.3] | 81.4[37.4–99.5] | 49.5[28.8–111.8] | 0.001 | |
| ESR (mm/h) | 50.0[28.0–81.0] | 44.0[26.0–75.0]** | 56.0[36.0–87.0] | 55.5[35.0–87.2] | < 0.001 | |
| LDH (U/L) | 603.5[472.7–793.0] | 536.0[435.0–700.0]** | 648.0[513.0–822.0] | 713.5[532.5–925.0] | < 0.001 | |
| hs-cTnI (pg/mL) | 5.3[1.5–17.8] | 6.0[1.5–19.8] | 5.2[1.5–16.1] | 4.4[1.5–16.4] | 0.650 | |
| AST (U/L) | 53.0[39.7–71.0] | 50.0[38.0–67.7] | 56.0[42.0–71.2] | 55.5[41.0–76.7] | 0.010 | |
| ALT (U/L) | 39.0[29.0–56.0] | 36.0[27.0–51.0] | 43.0[31.0–61.2] | 39.0[28.0–57.0] | < 0.001 | |
| ALP (U/L) | 175.0[137.0–232.0] | 171.0[131.0–224.0] | 175.5[142.7–237.0] | 180.5[137.2–425.5] | 0.108 | |
| In-hospital outcomes | ||||||
| Hospital length of stay (day) | 5.0[2.2–8.0] | 4.0[2.0–7.0]** | 6.0[3.0–9.0] | 6.0[4.0–9.0] | < 0.001 | |
| ICU admission | 217(16.4%) | 111(16.6%) | 81(16.6%) | 25(15.0%) | 0.866 | |
| Severity | 896(67.7%) | 437(65.5%) | 349(71.4%) | 110(65.9%) | 0.094 | |
| Mortality | 235(17.8%) | 114(17.1%) | 91(18.6%) | 30(18.0%) | 0.798 | |
| ARDS | 373(28.2%) | 205(30.7%) | 46(23.7%)** | 52(31.1%) | 0.022 | |
| Invasive ventilation | 169(12.8%) | 97(14.5%) | 53(10.8%) | 19(11.4%) | 0.149 | |
| ACI | 303(22.9%) | 133(19.9%) | 121(24.7%) | 49(29.3%) | 0.017 | |
| AKI | 173(13.1%) | 83(12.4%) | 65(13.3%) | 25(15.0%) | 0.676 | |
| ALI | 147(11.1%) | 64(9.6%) | 62(12.7%) | 21(12.6%) | 0.209 | |
| Multi-organ damage | 260(19.7%) | 127(19.0%) | 95(19.4%) | 38(22.8%) | 0.551 | |
ACI acute cardiac injury, AKI acute kidney injury, ALI acute liver injury, ALP alkaline phosphatase, ALT alanine transaminase, ARDS acute respiratory distress syndrome, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, CRP C-reactive protein, DBP diastolic blood pressure, ESR erythrocyte sedimentation rate, hs-cTnI high sensitive cardiac troponin I, ICU intensive care unit, LDH lactate dehydrogenase, LOC loss of consciousness, SBP systolic blood pressure, SII systemic immune-inflammation index, WBC white blood cells
†Data are presented as mean ± standard deviation, number (%), or median [interquartile range]
*Statistically significant P-values are bolded
**Statistically significant post hoc Bonferroni's P-value (P-value < 0.0083)
Clinical presentation
Clinical presentation symptoms including cough, dyspnea, fever, myalgia, and arthralgia were the most common among hospitalized patients with COVID-19. Apart from fever, headache, sore throat, and loss of consciousness, the prevalence of all symptoms differed significantly between the surges (P < 0.001 except for cough P: 0.001). After Bonferroni's post hoc analysis, we found out that the incidence of dyspnea, myalgia and arthralgia, nausea and vomiting, chest pain, and neurological manifestations followed a significant increasing trend from surge 1 to surge 3 (P < 0.008). Cough and abdominal pain were more frequent in surge 1 and then significantly decreased during surge 2.
Clinical and laboratory findings
The vital signs and laboratory parameters on admission are presented in Table.1. The heart rate, systolic blood pressure (SBP), respiratory rate, and oxygen saturation of patients were significantly different across the surges (P < 0.001). Application of the Bonferroni post hoc test showed that patients in surge 1 presented with significantly lower SBP, lower respiratory rate, and higher oxygen saturation levels on admission compared to surges 2 and 3 (P < 0.008). Regarding laboratory parameters, a significant difference was observed in neutrophil (P: 0.003) and lymphocyte count (P < 0.001), neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, systemic immune-inflammation index (SII), urea, blood urea nitrogen/creatinine ratio, serum potassium, calcium, phosphorus, and magnesium levels of patients between the surges (P < 0.001). Additionally, serum erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alkaline phosphatase (ALP) (P < 0.001), hemoglobin (P: 0.008), sodium (P: 0.009), and aspartate transaminase levels (P: 0.010) differed significantly in patients hospitalized across the three surges. Bonferroni post hoc analysis demonstrated that patients in surge 2 were found to have significantly higher platelet to lymphocyte ratio, SII, and urea than the two other surges (P < 0.008). Patients from the first wave differed remarkably from those of the two subsequent surges of disease in that they had lower calcium, ESR, and lactate dehydrogenase (LDH) serum levels (P < 0.008).
In-hospital complications and outcomes
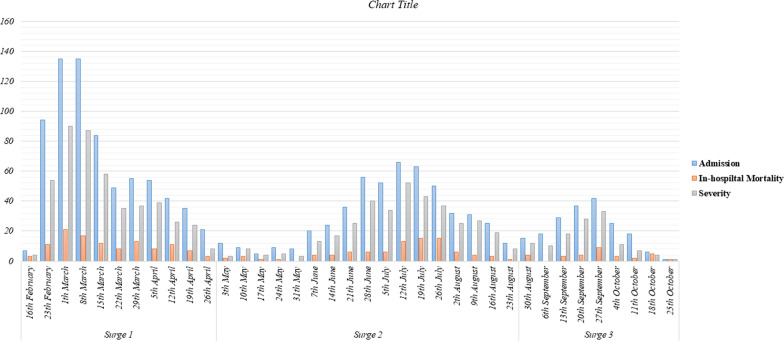
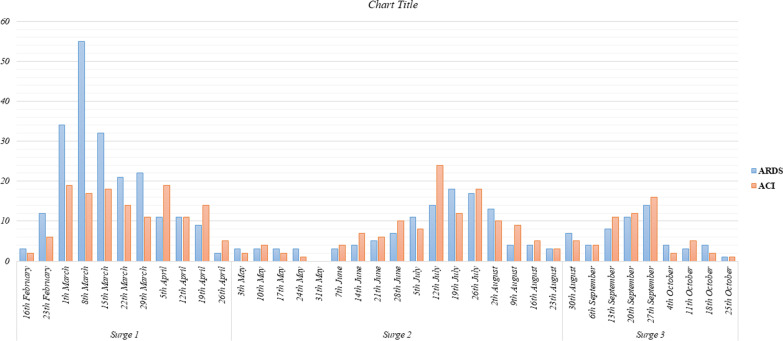
The median length of hospital stay during surges 1, 2, and 3 was 4 days (interquartile range (IQR): 2–7), 6 days (IQR: 3.0–9.0), and 6 days (IQR: 4.0–9.0), respectively (P < 0.001). The length of hospital stay was shorter during surge 1 than two other surges (P < 0.008). The number of patients requiring invasive ventilation did not differ between the three waves (P: 0.149). We presented the number of admitted, severe, and deceased patients based on 1-week intervals in Fig. 1. The mortality rate during surges 1 to 3 was not significantly different, and the number of deceased cases was 114 (17.1%), 91 (18.6%), and 30(18%), respectively (P < 0.798). There was no difference in the percentage of patients developing severe COVID-19 with 896 (67.7%) of patients in surge 1, 437 (65.5%) of patients in surge 2, and 349 (71.4%) of hospitalized patients during surge 3 (P = 0.094). There were statistically significant differences regarding acute respiratory distress syndrome (ARDS) (P < 0.022) and acute cardiac injury (ACI) development (P: 0.017) between patients during three surges, which presented based on 1-week intervals in Fig. 2. The number of patients developing acute kidney injury (AKI), acute liver injury (ALI), and multi-organ damage were not significantly different between the surges. After Bonferroni's post hoc analysis application, we observed that during surge 2, patients were less likely to incur ARDS than patients hospitalized during surge 1 and 3 (P < 0.008).
Fig. 1.
Census of patients admitted with coronavirus disease 2019 (COVID-19) with those who developed severe COVID-19 or were deceased across the first three surges of COVID-19 in Tehran, Iran
Fig. 2.
The number of patients who developed acute respiratory distress syndrome (ARDS) or acute cardiac injury (ACI) during the first three surges of coronavirus disease 2019 (COVID-19) in Tehran, Iran
Discussion
The most prominent finding in this study was the difference in age, clinical presentations, length of hospital stay, developing ARDS, and ACI in three different surges of COVID-19 infection. On the other hand, there was no difference regarding disease severity and in-hospital mortality among three different surges. Alpha variant of COVID-19 was active during the first surge in our study. Then, in the end of the second surge from June 22, we faced the Delta variant of the virus resulting in a large number of new cases in July [9].
Older people have been speculated to be more vulnerable to COVID-19 infection and its further complications [10, 11]. The mean age of hospitalized patients in our study was 59.2 ± 16.5. Mean age was lower in our study during the first wave, which is in contrast with the findings of Fluck et al., reporting lower mean age of patients during the second wave [12]. We believe this may be attributed to different study populations and smaller sample sizes in our study, while both studies have included only admitted patients.
The median BMI in our study was 27.4 ± 4.7 kg/m2 and was lower than that reported in New York (30 kg/m2) [13]. It has been reported that obesity is associated with worse outcomes among COVID-19 patients [14], and a lower BMI in the current study might have been protective. We demonstrated that hypertension, diabetes mellitus, and cardiac disease were the most common comorbidities among patients. Underlying diseases are associated with a higher risk of severe COVID-19 and its complications [15, 16]. In a study comparing two surges of COVID-19 pandemic showed that more than 80% of patients had at least one comorbidity with hypertension being the most common comorbidity similar to our results [17]. Vahidy et al. reported that the number of COVID-19 hospitalized patients with hypertension was significantly higher during surge 1 of the COVID-19 pandemic in Houston, which is in alignment with our results [18].
The prevalence of dyspnea, myalgia and arthralgia, nausea and vomiting, chest pain, and neurological manifestations significantly increased from surge 1 to 3. Gastrointestinal symptoms with more incidence in the second wave were reported in previous observations [19]. Patients admitted during surge 1 had higher oxygen saturation with lower SBP and respiratory rate than surges 2 and 3. The results demonstrate that the non-respiratory presentations of COVID-19 are increasing over time. In contrast, patients admitted during the first surge presented with lower oxygen saturation and higher temperature compared to surge 2 on admission according to Buttenshon et al. study. However, similar to our reports they presented with lower SBP during surge one [17]. The SARS-CoV-2 virus can infect various organs using angiotensin-converting enzyme (ACE2) receptors to enter cells [20]. Therefore, during this pandemic era, extra-pulmonary symptoms should be given more attention by physicians.
Lower than normal absolute lymphocyte count, higher neutrophil to lymphocyte ratio, and elevated than normal CRP, ESR levels are significantly associated with a higher mortality rate in hospitalized COVID-19 patients [21]. Besides, elevated serum levels of creatinine and urea were correlated with hospitalized COVID-19 non-survivors [22]. Our patients differed significantly in neutrophil to lymphocyte ratio, Urea, CRP, and ESR with higher levels of these laboratory markers in surge 2. In contrast, several studies found higher CRP and creatinine levels in patients admitted during surge 1 compared to surge two which were associated with more critical diseases [17, 23].
Patients who developed ACI were reported to be older with more comorbidities such as HTN and DM, lower lymphocyte counts, and higher ALT levels, leukocyte count, and hs-CRP on admission compared to those who did not develop ACI [24]. Similarly, we found that as the number of patients with hypertension rose from surge 1 to surge 3, more patients developed ACI and had lower lymphocyte count than surges 1 and 2. Furthermore, developing ARDS is also associated with elevated cardiac troponin levels and worsened clinical outcomes [25]. We similarly found that the more patients developed ARDS from surge 1 to 3, the more they developed ACI. However, disease severity, in-hospital mortality, and the number of patients receiving invasive mechanical ventilation in our study did not differ across the surges, and the overall in-hospital mortality rate was 17.8% during our study period.
Some previous studies demonstrated a lower disease severity, need for invasive mechanical ventilation, and mortality during the second wave in Europe and the United States [12, 26, 27]. The Netherlands achieved the most considerable reduction in mortality among European countries [26]. While in by Taboada et al. among critically ill patients in Spain, the ICU admission duration and mortality were similar in the three waves [28]. The rate of ICU admission was 16.4% in our study, and patients did not differ in terms of disease severity, ICU admission rate, or mortality among the three surges. This discrepancy may be attributed to different virus strains, vaccination rates, and isolation policies in different countries [12, 26–28].
The strength of the current study is the comparison between three surges of COVID-19 patient's characteristics and outcomes for the first time in Iran as a country with a high burden of COVID-19 pandemic. Several limitations to the current study need to be addressed. First, the present study is an observational study with possible inherent biases. Second, it is a single-center study on the Iranian population, and future multicenter studies with larger sample sizes and different ethnicities are needed.
Acknowledgements
Not applicable
Abbreviations
- ACE2
Angiotensin-converting enzyme
- ACI
Acute cardiac injury
- AKI
Acute kidney injury
- ALI
Acute liver injury
- ALP
Alkaline phosphatase
- ALT
Alanine transaminase
- ARDS
Acute respiratory distress syndrome
- AST
Aspartate aminotransferase
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CRP
C-reactive protein
- DBP
Diastolic blood pressure
- ESR
Serum erythrocyte sedimentation rate
- hs-cTnI
High sensitive cardiac troponin I
- ICU
Intensive care unit
- IQR
Interquartile range
- LDH
Lactate dehydrogenase
- SBP
Systolic blood pressure
- SII
Systemic immune-inflammation index
Author contributions
AH: data acquisition. MP: drafted the manuscript. SK: design of the work, data analysis, revised the manuscript. HF: design of the work, revised the manuscript. ME: data acquisition. SKS: data acquisition. MT: data acquisition. All authors read and approved the final manuscript.
Funding
Tehran University of Medical Sciences.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants provided informed written consent before enrolling in the study, and the ethics committee of the Tehran University of Medical Sciences was approved for the study (IR.TUMS.VCR.REC.1399.018.).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Azar Hadadi and Marzieh Pirzadeh contributed equally to this work and shared co-first authorship
References
- 1.World health organization (WHO). WHO coronavirus (COVID-19) Dashboard [Internet]. Available from: https://covid19.who.int/. Accessed 12 Nov 2021.
- 2.Abdi M. Coronavirus disease 2019 (COVID-19) outbreak in Iran: actions and problems. Infect Control Hosp Epidemiol. 2020;41:754–755. doi: 10.1017/ice.2020.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikpouraghdam M, Jalali Farahani A, Alishiri G, Heydari S, Ebrahimnia M, Samadinia H, Sepandi M, Jafari NJ, Izadi M, Qazvini A, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, et al. Baseline characteristics and outcomes of 1591 Patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region Italy. Jama. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH: Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. World Health Organization, 2020. Available from: https://apps.who.int/iris/handle/10665/331446
- 6.Ministry of health and medical education. Epidemiology committee of COVID-19 [Internet]. Islam. Repub. Iran. 2021. Available from: http://corona.behdasht.gov.ir/
- 7.Soleimani A, Kazemian S, Karbalai Saleh S, Aminorroaya A, Shajari Z, Hadadi A, Talebpour M, Sadeghian H, Payandemehr P, Sotoodehnia M, et al. Effects of angiotensin receptor blockers (ARBs) on in-hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID-19. Am J Hypertens. 2020;33:1102–1111. doi: 10.1093/ajh/hpaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazoki M, Keykhaei M, Kafan S, Montazeri M, Mirabdolhagh Hazaveh M, Sotoodehnia M, Kazemian S, Talebpour M, Ashraf H, Shariat Moharari R, et al. Risk indicators associated with in-hospital mortality and severity in patients with diabetes mellitus and confirmed or clinically suspected COVID-19. J Diabetes Metab Disord. 2021;20:1–11. doi: 10.1007/s40200-020-00539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikhi F, Yousefian N, Tehranipoor P, Kowsari Z. Estimation of the basic reproduction number of alpha and delta variants of COVID-19 pandemic in Iran. PLoS ONE. 2022;17:e0265489. doi: 10.1371/journal.pone.0265489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbalai Saleh S, Oraii A, Soleimani A, Hadadi A, Shajari Z, Montazeri M, Moradi H, Talebpour M, Sadat Naseri A, Balali P, et al. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020;15:1415–1424. doi: 10.1007/s11739-020-02466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazoki M, Chichagi F, Hadadi A, Kafan S, Montazeri M, Kazemian S, Aminorroaya A, Ebrahimi M, Ashraf H, Hazaveh MM, et al. Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study. J Diabetes Metab Disord. 2021 doi: 10.1007/s40200-021-00901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fluck D, Rankin S, Lewis A, Robin J, Rees J, Finch J, Jones Y, Jones G, Kelly K, Murray P, et al. Comparison of characteristics and outcomes of patients admitted to hospital with COVID-19 during wave 1 and wave 2 of the current pandemic. Intern Emerg Med. 2021;17:1–10. doi: 10.1007/s11739-021-02842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, Mathieu D, Subtil F, Frobert E, Alligier M, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. doi: 10.1136/bmj.n436. [DOI] [PubMed] [Google Scholar]
- 16.Rahimzadeh H, Kazemian S, Rahbar M, Farrokhpour H, Montazeri M, Kafan S, Salimzadeh A, Talebpour M, Majidi F, Jannatalipour A, Razeghi E. The risk factors and clinical outcomes associated with acute kidney injury in patients with COVID-19: data from a large Cohort in Iran. Kidney Blood Press Res. 2021;46:620–628. doi: 10.1159/000517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buttenschøn HN, Lynggaard V, Sandbøl SG, Glassou EN, Haagerup A. Comparison of the clinical presentation across two waves of COVID-19: a retrospective cohort study. BMC Infect Dis. 2022;22:423. doi: 10.1186/s12879-022-07413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vahidy FS, Drews AL, Masud FN, Schwartz RL, Askary BB, Boom ML, Phillips RA. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston metropolitan area. JAMA. 2020;324:998–1000. doi: 10.1001/jama.2020.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalali SF, Ghassemzadeh M, Mouodi S, Javanian M, Akbari Kani M, Ghadimi R, Bijani A. Epidemiologic comparison of the first and second waves of coronavirus disease in Babol, North of Iran. Caspian J Intern Med. 2020;11:544–550. doi: 10.22088/cjim.11.0.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus disease (COVID-19): comprehensive review of clinical presentation. Front Public Health. 2020;8:582932. doi: 10.3389/fpubh.2020.582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousavi SA, Rad S, Rostami T, Rostami M, Mousavi SA, Mirhoseini SA, Kiumarsi A. Hematologic predictors of mortality in hospitalized patients with COVID-19: a comparative study. Hematology. 2020;25:383–388. doi: 10.1080/16078454.2020.1833435. [DOI] [PubMed] [Google Scholar]
- 22.Morell-Garcia D, Ramos-Chavarino D, Bauça JM, Argente Del Castillo P, Ballesteros-Vizoso MA, García de Guadiana-Romualdo L, Gómez-Cobo C, Pou JA, Amezaga-Menéndez R, Alonso-Fernández A, et al. Urine biomarkers for the prediction of mortality in COVID-19 hospitalized patients. Sci Rep. 2021;11:11134. doi: 10.1038/s41598-021-90610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollinedo-Gajate I, Villar-Álvarez F, Zambrano-Chacón M, Núñez-García L, de la Dueña-Muñoz L, López-Chang C, Górgolas M, Cabello A, Sánchez-Pernaute O, Romero-Bueno F, et al. First and second waves of coronavirus disease 2019 in Madrid, Spain: clinical characteristics and hematological risk factors associated with critical/fatal illness. Crit Care Explor. 2021;3:e0346. doi: 10.1097/CCE.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Wang L, Wang H, Xie Y, Yu Y, Sun J, Yan J, Du Y, Shen Y, Zeng H. Factors associated with acute cardiac injury and their effects on mortality in patients with COVID-19. Sci Rep. 2020;10:20452. doi: 10.1038/s41598-020-77172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivara MB, Bajwa EK, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Prognostic significance of elevated cardiac troponin-T levels in acute respiratory distress syndrome patients. PLoS ONE. 2012;7:e40515. doi: 10.1371/journal.pone.0040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James N, Menzies M, Radchenko P. COVID-19 second wave mortality in Europe and the United States. Chaos. 2021;31:031105. doi: 10.1063/5.0041569. [DOI] [PubMed] [Google Scholar]
- 27.Soriano V, de Mendoza C, Gómez-Gallego F, Corral O, Barreiro P. Third wave of COVID-19 in Madrid Spain. Int J Infect Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taboada M, González M, Alvarez A, Eiras M, Costa J, Álvarez J, Seoane-Pillado T. First, second and third wave of COVID-19. What have we changed in the ICU management of these patients? J Infect. 2021;82(6):e14–e15. doi: 10.1016/j.jinf.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.