Abstract
UV inactivation, photoreactivation, and dark repair of Escherichia coli and Cryptosporidium parvum were investigated with the endonuclease sensitive site (ESS) assay, which can determine UV-induced pyrimidine dimers in the genomic DNA of microorganisms. In a 99.9% inactivation of E. coli, high correlation was observed between the dose of UV irradiation and the number of pyrimidine dimers induced in the DNA of E. coli. The colony-forming ability of E. coli also correlated highly with the number of pyrimidine dimers in the DNA, indicating that the ESS assay is comparable to the method conventionally used to measure colony-forming ability. When E. coli were exposed to fluorescent light after a 99.9% inactivation by UV irradiation, UV-induced pyrimidine dimers in the DNA were continuously repaired and the colony-forming ability recovered gradually. When kept in darkness after the UV inactivation, however, E. coli showed neither repair of pyrimidine dimers nor recovery of colony-forming ability. When C. parvum were exposed to fluorescent light after UV inactivation, UV-induced pyrimidine dimers in the DNA were continuously repaired, while no recovery of animal infectivity was observed. When kept in darkness after UV inactivation, C. parvum also showed no recovery of infectivity in spite of the repair of pyrimidine dimers. It was suggested, therefore, that the infectivity of C. parvum would not recover either by photoreactivation or by dark repair even after the repair of pyrimidine dimers in the genomic DNA.
UV light irradiation is one of the effective disinfection methods for bacteria, viruses, and parasites in water and wastewater. UV disinfection systems are easy to maintain and need no additional chemical inputs. Moreover, UV irradiation produces no hazardous by-products like many of the conventional chlorination processes (22). Therefore, water treatment plants that utilize UV disinfection processes have increased in number in recent decades (14).
Inactivation of microorganisms by UV-B and UV-C (220 to 320 nm) radiation is effected through the formation of lesions in the genomic DNA of the organisms (10, 13). The major lesions induced by the germicidal UV light (254 nm) are cis-syn cyclobutane pyrimidine dimers (CPD), while (6–4) photoproducts are also formed at about 10% of CPD and other kinds of lesion are also produced at lower ratios (13). The presence of UV-induced lesions would inhibit the normal replication of DNA and therefore result in inactivation of the microorganisms.
Some organisms, however, are known to possess the ability to repair their DNA by mechanisms such as photoreactivation and dark repair (10, 13). Photoreactivation is the phenomenon by which UV-inactivated microorganisms recover activity through the repair of pyrimidine dimers in the DNA under near-UV light (310 to 480 nm) with the enzyme photolyase. In this study, the repair of pyrimidine dimers in the DNA would be named photo repair, while the recovery of the activity of the organism would be referred to photoreactivation. DNA repair mechanisms other than photo repair, such as excision repair, are named dark repair because they can repair the damaged DNA without light. Photoreactivation and dark repair enable UV-inactivated microorganisms to recover and may reduce the efficacy of UV inactivation. Therefore, they disadvantage the UV disinfection methods. Much attention has been paid to photoreactivation especially because it can cause a great deal of recovery of microorganisms within a short time. Photoreactivation may occur in organisms, especially in UV-treated wastewater after its discharge to watersheds, because UV-inactivated microorganisms would normally be exposed to sunlight, including near-UV light.
The ability to perform photo repair depends on whether the organism has the enzyme photolyase. Most strains of Escherichia coli, one of the indicator bacteria for water quality control, are known to be able to perform photoreactivation. This ability has been shown to occur in bacteria, plants, and animals, but evolutionarily allied species need not necessarily show similar photoreactivation characteristics (10). The ability for dark repair is also known to differ greatly from species to species. It would be necessary, therefore, to investigate the ability of photoreactivation and dark repair in each organism individually. This is especially so for the target microorganisms of disinfection processes, such as indicator or pathogenic organisms.
The presence of Cryptosporidium in water resources and water supply systems has recently been identified as a public health risk (7, 12, 19). This parasite is pathogenic for both humans and animals and can be transmitted through the water supply. Outbreaks of Cryptosporidium infection have been reported in many countries, most often associated with the consumption of drinking or recreational water contaminated with Cryptosporidium oocysts.
Cryptosporidium oocysts show high resistance to conventional disinfectants such as chlorine. Therefore, effective techniques for removing or disinfecting oocysts, other than chlorination, have been explored (23, 27). Many researchers have reported that UV irradiation is an effective method to disinfect Cryptosporidium oocysts (1, 6, 8, 16). However, the ability of Cryptosporidium to perform photoreactivation and dark repair has not been clarified yet in spite of the importance of these phenomena. In order to evaluate the efficiency and effectiveness of UV irradiation to Cryptosporidium, it is essential to investigate its photoreactivation and dark repair abilities.
Some detection methods for oocysts of Cryptosporidium have been established. Excystation and vital dye staining have commonly been used for the determination of the viability of oocysts (4). However, these methods only determine their viability and do not address their ability to be infectious. The animal infectivity assay is said to be the gold standard for the determination of infection potential (16), but this method requires a number of animal hosts, is highly expensive, needs skillful technicians, and also involves a long testing time. Upton et al. pointed out that cultured cells such as HCT-8 could be used as a host for Cryptosporidium instead of animal hosts (33), and many researchers have modified this cell culture method by combining it with other enzymatic or genetic techniques (11, 24, 30, 36). Such cell culture methods have become attractive alternatives to the animal infectivity assay because they were reported to be equivalent to the animal method in determining the infectivity of Cryptosporidium parvum (16). On the other hand, it has also been reported that the methods based on reverse transcription-PCR detection of the specific mRNA in Cryptosporidium are effective to investigate their infectivity (20, 25, 31). Accurate and simple detection methods for Cryptosporidium are still being explored.
In this study, the UV inactivation, photo repair, and dark repair of E. coli and C. parvum were investigated by an endonuclease sensitive site (ESS) assay, which can determine the number of UV-induced pyrimidine dimers in the genomic DNA as the number of ESS. The ESS assay was initially proposed by Achey et al. (2) for detecting pyrimidine dimers in nonradioactive DNA and developed by Sutherland and Shih (32) for quantifying the number of dimers. This assay proved to be effective not only for the comparative study of the UV sensitivity of different kinds of organisms but also for the quantitative investigation of DNA repair mechanisms. ESS assay, therefore, has commonly been used in the field of radiobiology (9, 18, 21), but only a few studies have investigated the relationships between the number of pyrimidine dimers in the DNA and the activity of the organism. Moreover, ESS assay has never been applied for the investigation of UV disinfection of water.
The purpose of this study is to investigate the mechanisms of UV inactivation, photo repair, and dark repair of E. coli and C. parvum with the ESS assay. In addition, a colony-forming ability assay was applied to E. coli in order to clarify the relationship between the number of pyrimidine dimers in the genomic DNA and the colony-forming ability. For C. parvum, an animal infectivity assay was applied in parallel with the ESS assay in order to investigate the relationship between the number of pyrimidine dimers and animal infectivity. It was further aimed to compare the UV sensitivity and the photo repair ability of E. coli with those of C. parvum quantitatively.
MATERIALS AND METHODS
Microorganisms.
As test microorganisms, E. coli IFO 3301 and C. parvum HNJ-1 strain were used. The E. coli were picked up from a few purified colonies formed on a broth medium (10 g of polypeptone, 5 g of yeast extract, 1.5 g of glucose, 5 g of NaCl, 0.2 g of MgSO4 · 7H2O, 0.05 g of MnSO4 · 4H2O, and 11 g of agar powder for 1 liter of purified water) after 24 h of incubation at 37°C and suspended in a sterilized phosphate buffer solution (pH 7.6) to be at an initial concentration of 2.5 × 10 to 4.0 × 107 CFU ml−1). An E. coli preparation (40 ml) was placed into a sterilized petri dish (100-mm diameter) and used for the light irradiation procedures.
The strain of C. parvum, HNJ-1, which belongs to the group of genotype 2 (bovine), was originally isolated from feces of a naturally infected human by M. Iseki at Osaka Medical School, Osaka, Japan. The C. parvum were passaged in SCID mice at the Research Institute of Biosciences, Azabu University, and the oocysts were purified from the fresh feces of several infected mice by sucrose flotation. The purified oocysts were stored at 4°C in phosphate-buffered saline (pH 7.4) till used. It was previously confirmed that the animal infectivity of the oocysts would not change within a month in the stock suspension. The stock suspension of purified oocysts was diluted by a sterilized phosphate buffer solution (pH 7.6) to produce an initial concentration of 1.0 × 106 oocysts ml−1. Then 40 ml of the preparation was placed into a sterilized petri dish (100-mm diameter) and used for the light irradiation procedures. The age of the oocysts was 5 to 15 days at the time of use. In the animal infectivity assay, the initial concentration of the oocysts was 2.0 × 106 oocysts ml−1, and 10 ml of the preparation was used for the light irradiation in a sterilized petri dish of 60-mm diameter.
UV and fluorescent light irradiation.
Two low-pressure UV lamps (Stanley germicidal lamp, 20 W; Toshiba) were used for the UV irradiation procedures. The intensity of the UV at a wavelength of 254 nm was 0.24 mW cm−2, which was measured by a UV radiometer (UVR-2 UD-25; Topcon). The dose of UV was altered by controlling the exposure time.
For the photoreactivation procedures, samples were irradiated by three fluorescent lamps (white light fluorescent lamp, 18 W; Hitachi) within 5 min after the 99.9% inactivation by UV irradiation. A 6.0-mJ cm−2 dose of UV was used for E. coli to achieve 99.9% reduction in the colony-forming ability, which was determined via colony-forming ability assay (detailed below). A 2.2-mJ cm−2 dose of UV was used for C. parvum to achieve 99.9% reduction in infectivity, which was determined via animal infectivity assay (detailed below). The intensity of the photoreactivating light at 360 nm was 0.1 mW cm−2, as measured by a UV radiometer (UVR-2 UD-36; Topcon). Sample collection was intermittently performed during the exposure to fluorescent light for 120 min. For the investigation of dark repair, samples were kept in darkness for 24 h after the UV irradiation. All samples were constantly stirred magnetically throughout the experiment and kept in darkness except at the times for the UV and fluorescent light irradiation procedures. Sample temperatures were kept constant at 20°C by circulating cooling water around the petri dishes.
In the experiment with the animal infectivity assay of C. parvum, a low-pressure UV lamp (QCGL 5W-14 97D, 5 W; Iwasaki) and a fluorescent lamp (FL15N, 15 W; Toshiba) were used for the UV inactivation and photoreactivation procedures, respectively. The conditions of UV and fluorescent light irradiation were the same as mentioned above, except that the UV dose was set to be either 0.72 or 1.44 mJ cm−2 and the intensity of the photoreactivating light at 360 nm was 0.05 mW cm−2.
Colony-forming ability assay for E. coli
The colony-forming ability of E. coli was investigated using desoxycholate-acid medium (Eiken) in the dark room following standard methods (35). All the plated samples were covered with aluminum foil and incubated at 37°C for 18 h. The number of colonies formed after the incubation were counted, and the ratio of the colony-forming ability was calculated by equation 1,
 |
1 |
where St is the ratio of the colony-forming ability at light irradiation time t, Nt is the number of colonies at light irradiation time t, and N0 is the number of colonies before UV irradiation.
Animal infectivity assay for C. parvum
Samples of C. parvum for the infectivity tests were successively diluted by a fixed dilution factor of five. Six-week-old SCID mice were inoculated orally with aliquots of selected series of dilutions. After a 4-week incubation, fresh feces from each mouse were collected, purified, stained by indirect immunofluorescent antibodies (Hydrofluoro combo kit; Strategic Diagnostics) and then observed by epifluorescent microscopy to determine the presence or absence of the oocysts. The infective doses, i.e., the number of oocysts for 1 most-probable-number (MPN) of infection, were calculated from the data set of the numbers of oocyst-positive mice, using the MPN program proposed by Hurley and Roscoe (17). The relative infectivity of each sample was calculated by equation 2,
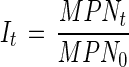 |
2 |
where It is the relative infectivity at light irradiation time t, MPNt is the most probable number of infective oocysts at light irradiation time t, and MPN0 is the most probable number of infective oocysts before UV irradiation.
ESS assay. (i) Principle of ESS assay.
DNA samples are incubated with UV endonuclease, which incises a phosphodiester bond specifically at the site of a pyrimidine dimer. This enables the recognition of a pyrimidine dimer in the DNA as an ESS. After the incision of nicks, the DNA strands are separated according to their molecular lengths by electrophoresis on alkaline agarose gels. The double-stranded DNA is denatured by the alkalinity and therefore fragmented according to the number of nicks incised by the UV endonuclease. By analyzing the migration patterns of DNA fragments relative to the molecular length of standard markers, the median molecular length of the DNA can be determined graphically and the number of ESS per DNA molecule can be calculated theoretically (32).
(ii) DNA extraction from microorganisms.
One milliliter of E. coli sample was centrifuged at 5,000 × g for 7 min, and the pellet was used for the Genomic-tip DNA extraction kit (Qiagen) following the manufacturer's protocol. A 40-ml sample of C. parvum was centrifuged at 1,050 × g for 10 min, and the pellet was resuspended in 2 ml of sterilized phosphate buffer (pH 7.6). This suspension of C. parvum was frozen in liquid nitrogen for 3 min and thawed at 95°C for 5 min in order to break the oocyst walls, following the method of Rochelle et al. (24). This freezing-thawing treatment was repeated three times. After the breakage of the oocyst walls, the DNA was extracted from the sporozoites using the Genomic-tip DNA extraction kit (Qiagen) following the manufacturer's protocol. All the DNA solutions of E. coli and C. parvum were then concentrated by extraction with 2-butanol (Wako). After concentration, the samples were dialyzed overnight at 4°C against 30 mM Tris (pH 8.0)–40 mM NaCl–1 mM EDTA in order to remove any excess 2-butanol completely.
(iii) Conditions for ESS assay.
The UV endonuclease was prepared from Micrococcus luteus following the method of Carrier and Setlow (5). DNA solutions were incubated with the UV endonuclease (1 μl of endonuclease solution per μg of DNA) at 37°C for 45 min in 30 mM Tris (pH 8.0)–40 mM NaCl–1 mM EDTA in order to incise the nicks at the site of pyrimidine dimers. The reaction was stopped by the addition of an alkaline loading dye at a final concentration of 100 mM NaOH, 1 mM EDTA, 2.5% Ficoll and 0.05% bromocresol green. The treated samples were subjected to electrophoresis at 0.5 V/cm for 16 h on 0.5% alkaline agarose gels in an alkaline buffer containing 30 mM NaOH and 1 mM EDTA. Two molecular length markers, 7GT (T4dC+T4dC/BglI digest mixture; Wako) and 8GT (T4dC+T4dC/BglII digest mixture; Wako) were used as molecular length standards. After electrophoresis, the gel was dipped in ethidium bromide solution (0.5 μg/ml) overnight to stain the DNA. The images of the stained gels were photographed (Gel Doc 1000; BioRad) and analyzed (Molecular Analyst software; BioRad). With this analysis system, the fluorescence of the DNA was detected as a set of pixels, and a distribution pattern of the fluorescence intensity in relation to the migration distance was obtained. The total count of the pixels, that is, the integrated area of the fluorescence intensity, corresponded to the total amount of DNA in the sample because the fluorescence intensity was previously confirmed to correlate linearly with the DNA concentration in the conditions used in this study. The midpoint of the DNA mass, that is, the median migration distance of each sample, was graphically determined to be the representative migration distance of the sample. The median value of migration distance was converted into the median molecular length (Lmed) of the DNA by means of the quadratic regression curve obtained from the analysis of the molecular standards. The average molecular length (Ln) of the DNA was obtained by equation 3 (Veatch and Okada [34]):
 |
3 |
The number of ESS per base was obtained by equation 4 (Freeman et al. [9]):
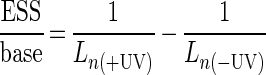 |
4 |
where Ln(+UV) and Ln(−UV) are the Ln of UV-irradiated and nonirradiated samples, respectively.
For the discussion of photo repair, the ESS remaining ratio, defined as the ratio of the number of ESS during fluorescent light irradiation to the number of ESS before fluorescent light irradiation, was calculated by equation 5:
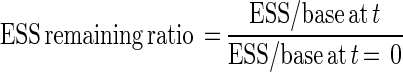 |
5 |
where t is the time of exposure to fluorescent light.
RESULTS AND DISCUSSION
UV inactivation of E. coli
Figure 1 shows an example of the result of the ESS assay during UV irradiation of E. coli. UV doses of 2, 4, and 6 mJ cm−2 corresponded to 90, 99, and 99.9% inactivation of colony-forming ability, respectively. The photographic image of the gel (Fig. 1A) was analyzed, and the distribution patterns of the fluorescence intensity were determined as depicted in Fig. 1B, in which the horizontal axis indicates the migration distance or the corresponding molecular length and the vertical axis shows the fluorescent intensity of the DNA fragments. As shown in Fig. 1B, the median molecular length of each of the samples became shorter and shorter with an increase of the UV dose, indicating that the DNA was fragmented into smaller and smaller pieces with an increase of the dose of UV irradiation.
FIG. 1.
Example of ESS assay during UV irradiation of E. coli. (A) Photographic image of the alkaline agarose gel after electrophoresis. (B) Distribution patterns of DNA in relation to molecular length standard markers. Arrows indicate the median point of each distribution pattern. UV doses of 2, 4, and 6 mJ cm−2 corresponded to 90, 99, and 99.9% inactivation of the colony-forming ability of E. coli, respectively.
Figure 2 shows that the number of ESS during UV irradiation increased linearly with an increase of the UV dose. A linear regression line between the UV dose and the number of ESS was determined by the least-squares method. The coefficient of determination (r2) was as high as 0.987 for eight data, indicating that the number of ESS correlated highly with the UV dose. The yield of ESS formation was calculated to be 4.0 × 10−5 per base per 1 mJ cm−2 of UV dose, which is approximately the same as the result of Howard-Flanders (15), who reported that the yield of pyrimidine dimers after irradiation by a low-pressure mercury germicidal lamp was 6.4 × 10−5 per base per 1 mJ cm−2 of UV for E. coli K-12 (wild type).
FIG. 2.
Relationship between UV dose and number of ESS during UV irradiation of E. coli. The regression on a straight line which passes through the origin of the coordinates was determined by the least-squares method for two independent series of experiments. Coefficient of determination (r2) was 0.987 for eight data.
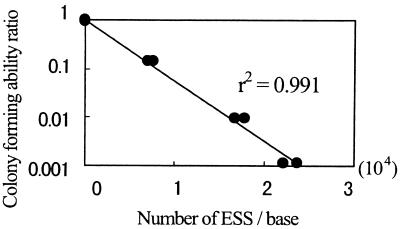
Figure 3 shows the relationship between the number of ESS and the ratio of colony-forming ability during UV irradiation. A linear regression line between the number of ESS and the logarithmic value of the ratio of colony-forming ability was determined by the least-squares method. The coefficient of determination (r2) was 0.991 for eight data, indicating that the ESS assay is comparable to the conventional colony-forming ability assay. Considering that the total genome size of E. coli is approximately 4.6 × 106 bp in a steady-state cell, the number of ESS necessary for 1 log inactivation of E. coli was calculated to be roughly 736.
FIG. 3.
Relationship between number of ESS and ratio of colony-forming ability during UV irradiation of E. coli. The regression on a straight line whose intercept was fixed to be 1 on the vertical axis was determined by the least-squares method for two independent series of experiments. Coefficient of determination (r2) was 0.991 for eight data.
Photoreactivation and dark repair of E. coli
Figure 4 shows an example of the result of the ESS assay during the exposure of E. coli to fluorescent light after 99.9% inactivation by UV irradiation. The photographic image (Fig. 4A) was analyzed and the distribution patterns of the fluorescence intensity were determined (Fig. 4B). The figure shows that the median molecular length of the DNA became gradually longer as the exposure time to fluorescent light increased. Figure 5 shows the profiles of the number of ESS in the DNA and the ratio of colony-forming ability during fluorescent light irradiation after UV inactivation. This figure indicates that, during the exposure to fluorescent light, pyrimidine dimers were continuously repaired and the colony-forming ability gradually recovered. When kept in darkness after UV inactivation, however, neither the repair of pyrimidine dimers nor the recovery of colony-forming ability was observed, as shown in Fig. 6. This indicates that E. coli did not perform dark repair under the conditions of this study.
FIG. 4.
Example of ESS assay during UV and fluorescent light irradiation of E. coli. (A) Photographic image of the alkaline agarose gel after electrophoresis. (B) Distribution patterns of DNA in relation to molecular length standard markers. Arrows indicate the median point of each distribution pattern. Dose of UV was 6 mJ cm−2 for all samples, which corresponded to 99.9% inactivation of the colony-forming ability of E. coli. FL indicates exposure to fluorescent light irradiation.
FIG. 5.
Profiles of number of ESS and ratio of colony-forming ability during fluorescent light irradiation of E. coli after UV inactivation. ●, number of ESS/base; ◊, ratio of colony-forming ability. Dose of UV for inactivation was 6 mJ cm−2. All symbols indicate the result of two independent series of experiments. Solid and dotted lines connect the arithmetic mean values of the number of ESS and the geometric mean values of the colony-forming ability ratio, respectively.
FIG. 6.
Profiles of number of ESS and ratio of colony-forming ability in darkness after UV inactivation of E. coli. ●, number of ESS/base; ◊, ratio of colony-forming ability. Dose of UV for inactivation was 6 mJ cm−2. All symbols indicate the result of two independent series of experiments. Solid and dotted lines connect the arithmetic mean values of the number of ESS and the geometric mean values of the colony-forming ability ratio, respectively.
Figure 7 shows the relationship between the ESS remaining ratio and the colony-forming ability ratio of E. coli during the exposure to fluorescent light. The coefficient of determination (r2) was 0.799 for the linear regression line determined by the least-squares method between the ESS remaining ratio and the logarithmic value of the colony-forming ability (number of data was 10). The intercept of the regression line was fixed to be 1 on the vertical axis, assuming that the ratio of colony-forming ability would be 1 when the photo repair of ESS was completely performed. The high correlation between the ESS remaining ratio and the colony-forming ability ratio indicated that, during the exposure of E. coli to fluorescent light irradiation, the repair of pyrimidine dimers in the genomic DNA did contribute to the recovery of colony-forming ability.
FIG. 7.
Relationship between ESS remaining ratio and colony-forming ability ratio during fluorescent light irradiation of E. coli. The regression on a straight line whose intercept was fixed to be 1 on the vertical axis was determined by the least-squares method. Coefficient of determination (r2) was 0.799 for 10 data.
UV inactivation of C. parvum
In the inactivation of C. parvum by UV irradiation, high correlation was observed between the number of ESS and the dose of UV (r2 was 0.978 for seven data) as shown in Fig. 8. The yield of ESS formation was calculated to be 7.5 × 10−5 per base per 1 mJ cm−2 of UV dose, which is in the same order as that calculated for E. coli (4.0 × 10−5 per base per 1 mJ cm−2 of UV dose). This result suggests that the oocyst wall of C. parvum is not more protective against UV light than the cell wall of E. coli. This might be the reason why UV irradiation is relatively effective at disinfecting C. parvum compared to chlorination or other chemical disinfectants that cannot penetrate the oocyst wall.
FIG. 8.
Relationship between UV dose and number of ESS during UV irradiation to C. parvum. The regression on a straight line which passes through the origin of the coordinates was determined by the least-squares method for three independent series of experiments. Coefficient of determination (r2) was 0.978 for seven data.
Photoreactivation and dark repair of C. parvum
Figure 9 shows an example of the result of the ESS assay during the exposure of C. parvum to fluorescent light after UV inactivation. The dose of UV was 2.2 mJ cm−2, which corresponded to 99.9% inactivation of animal infectivity. The photographic image of the gel (Fig. 9A) was analyzed to obtain the distribution patterns of the fluorescence intensity (Fig. 9B). The 99.9% inactivation of the infectivity of C. parvum was detected as a smear image of the DNA, and the median molecular length of each of the samples became gradually longer with increasing duration of exposure to fluorescent light after UV irradiation. As shown in Fig. 10, a gradual repair of pyrimidine dimers was observed during the exposure to fluorescent light irradiation. The result indicated that C. parvum had the ability to repair the pyrimidine dimers in genomic DNA during the exposure to fluorescent light.
FIG. 9.
Example of ESS assay during UV and fluorescent light irradiation to C. parvum. (A) Photographic image of the alkaline agarose gel after electrophoresis. (B) Distribution patterns of DNA in relation to molecular length standard markers. Arrows indicate the median point of each distribution patterns. Dose of UV was 2.2 mJ cm−2 for all samples, which corresponded to 99.9% inactivation of the infectivity of C. parvum. FL indicates exposure to fluorescent light irradiation
FIG. 10.
Profile of number of ESS during fluorescent light irradiation to C. parvum after UV inactivation. Dose of UV for inactivation was 2.2 mJ cm−2, which corresponded to 99.9% inactivation of the infectivity of C. parvum. All symbols indicate the result of two independent series of experiments. Solid line connects the arithmetic mean values of the data at each irradiation time.
The ESS remaining ratio, defined as the ratio of the number of ESS during fluorescent light irradiation to the total number of ESS induced by UV irradiation, was calculated for E. coli and C. parvum in order to compare these microorganisms from the viewpoint of photo repair. As shown in Fig. 11, the ratio of ESS remaining in C. parvum after 120 min of exposure to fluorescent light irradiation was almost the same as that in E. coli. This suggests that the photo repair ability of C. parvum is almost the same as that of E. coli, which clearly demonstrates the recovery of colony-forming ability during photoreactivation.
FIG. 11.
Profiles of ESS remaining ratio for E. coli and C. parvum during fluorescent light irradiation after 99.9% inactivation by UV. ◊, E. coli; ●, C. parvum. All symbols indicate the result of two independent series of experiments. Solid and dotted lines connect the arithmetic mean values for E. coli and C. parvum, respectively.
A further investigation was performed with the ESS assay and animal infectivity assay to determine the photoreactivation and dark repair ability of C. parvum. Two doses of UV, 0.72 mJ cm−2 and 1.44 mJ cm−2, were adopted for the inactivation procedures. Figure 12 shows the profiles of the number of ESS and the relative infectivity during the exposure to fluorescent light after UV irradiation, while Fig. 13 shows the profiles in darkness after UV irradiation. As shown in Fig. 12, recovery of the infectivity of C. parvum was not clearly observed during fluorescent light irradiation in spite of the gradual but apparent repair of pyrimidine dimers. The discrepancy between the recovery of animal infectivity and the repair of pyrimidine dimers was also observed in the case of dark repair, as shown in Fig. 13, where the infectivity did not recover clearly even after the repair of pyrimidine dimers. It was also reported by Shin et al. (28) that there was no phenotypic evidence of either photoreactivation or dark repair of the infectivity of C. parvum after UV inactivation.
FIG. 12.
Profiles of number of ESS and relative infectivity of C. parvum during fluorescent light irradiation after UV irradiation at 0.72 (A) or 1.44 (B) mJ cm−2. Solid circles, number of ESS/base; open diamonds, relative infectivity. All symbols indicate the data from one series of experiments. The 60-min time point in panel B was not tested.
FIG. 13.
Profiles of number of ESS and relative infectivity of C. parvum in darkness after UV irradiation at 0.72 (A) or 1.44 (B) mJ cm−2. Solid Circles, number of ESS/base; open diamonds, relative infectivity. All symbols indicate the data from one series of experiments.
Many researchers have pointed out that the infectivity of Cryptosporidium oocysts is lower than the viability of the oocysts, as determined by 4′,6′-diamidino-2-phenylindole-propidium iodide staining test or the excystation test (1, 3, 29). This suggests that the vitality of the oocysts would not always reflect their infectivity, because some of the viable oocysts could not infect the hosts. This further suggests that the oocysts would not always be infective even if their DNA were in a normal condition.
The result of this study indicated that the repair of pyrimidine dimers in the genomic DNA did not contribute to the recovery of infectivity of C. parvum. This suggests that UV irradiation produces not only pyrimidine dimers in the DNA of C. parvum but also other kinds of damage in the DNA or other parts of the cell. The damage other than pyrimidine dimers would not be repaired by either photoreactivation or dark repair and should play an important role in infection. Further investigation of such damage would lead to clarification of the mechanisms by which UV irradiation inactivates C. parvum.
Conclusions.
The ESS assay was conducted to determine UV-induced pyrimidine dimers in the genomic DNA of E. coli and C. parvum. The following conclusions were obtained.
(i) The ESS assay was a useful method for the quantitative investigation of the UV inactivation, photo repair, and dark repair of E. coli and C. parvum.
(ii) In UV inactivation of E. coli, the number of pyrimidine dimers induced in the DNA was highly correlated with the dose of UV used as well as with colony-forming ability.
(iii) In E. coli, pyrimidine dimers induced in the DNA by UV irradiation were continuously repaired during the exposure to fluorescent light irradiation, while they were not repaired in darkness. The number of pyrimidine dimers remaining in the DNA during fluorescent light irradiation was highly correlated with colony-forming ability.
(iv) In UV inactivation of C. parvum, the number of pyrimidine dimers induced in the DNA was highly correlated with the dose of UV used.
(v) In C. parvum, pyrimidine dimers induced in the DNA by UV irradiation were continuously repaired by exposure to fluorescent light irradiation. The repair of pyrimidine dimers was observed even in darkness. The ability of C. parvum to repair pyrimidine dimers during exposure to fluorescent light was almost the same as that of E. coli. The animal infectivity of C. parvum, however, did not recover after either exposure to fluorescent light or storage in darkness, indicating that C. parvum would not recover infectivity either by photoreactivation or by dark repair even after the repair of pyrimidine dimers.
REFERENCES
- 1.Abbaszadegan M, Hasan M N, Gerba C P, Roessler P F, Wilson B R, Kuennen R, Dellen E V. The disinfection efficacy of a point-of-use water treatment system against bacterial, viral and protozoan waterborne pathogens. Water Res. 1997;31:574–582. [Google Scholar]
- 2.Achey P M, Woodhead A D, Setlow R B. Photoreactivation of pyrimidine dimers in DNA from thyroid cells of the teleost Poecilia formosa. Photochem Photobiol. 1979;29:305–310. doi: 10.1111/j.1751-1097.1979.tb07053.x. [DOI] [PubMed] [Google Scholar]
- 3.Bukhari Z, Marshall M M, Korich D G, Fricker C R, Smith H V, Rosen J, Clancy J L. Comparison of Cryptosporidium parvum viability and infectivity assay following ozone treatment of oocysts. Appl Environ Microbiol. 2000;66:2972–2980. doi: 10.1128/aem.66.7.2972-2980.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with the inclusion/exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrier W L, Setlow R B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970;102:178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy J L, Hargy T M, Marshall M M, Dyksen J E. UV light inactivation of Cryptosporidium oocysts. J Am Water Works Assoc. 1998;90:92–102. doi: 10.1002/j.1551-8833.2004.tb10576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craun G F, Hubbs S A, Frost F, Calderon R L, Via S H. Waterborne outbreaks of Cryptosporidiosis. J AWWA. 1998;90:81–91. [Google Scholar]
- 8.Dyksen J E, Marshall MM, Gera A, Clancy J L. Cost of advanced UV for inactivating Crypto. J AWWA. 1998;90:103–111. [Google Scholar]
- 9.Freeman S E, Blackett A D, Monteleone D C, Setlow R B, Sutherland B M, Sutherland J C. Quantitation of radiation-, chemical-, or enzyme-induced single strand breaks in nonradioactive DNA by alkaline gel electrophoresis: application of pyrimidine dimers. Anal Biochem. 1986;158:119–129. doi: 10.1016/0003-2697(86)90599-3. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg E R, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM Press; 1995. pp. 92–107. [Google Scholar]
- 11.Gargala G, Delaunay A, Favennec L, Brasseur P, Ballet J J. Enzyme immunoassay detection of Cryptosporidium parvum inhibition by sinefungin in sporozoite infected HCT-8 enterocytic cells. Int J Parasitol. 1999;29:703–709. doi: 10.1016/s0020-7519(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 12.Hancock C M, Rose J B, Callahan M. Crypto and Giardia in US groundwater. J AWWA. 1998;90:58–61. [Google Scholar]
- 13.Harm W. Biological effects of ultraviolet radiation. New York, N.Y: Cambridge University Press; 1980. pp. 31–39. [Google Scholar]
- 14.Ho C-F H, Pitt P, Mamais D, Chiu C, Jolis D. Evaluation of UV disinfection systems for large-scale secondary effluent. Water Environ Res. 1998;70:1142–1150. [Google Scholar]
- 15.Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- 16.Huffman D E, Slifko T R, Salisbury K, Rose J B. Inactivation of bacteria, virus and Cryptosporidium by a point-of-use device using pulsed broad spectrum white light. Water Res. 2000;34:2491–2498. [Google Scholar]
- 17.Hurley M, Roscoe M E. Automated statistical analysis of microbial enumeration by dilution series. J Appl Bacteriol. 1983;55:159–164. [Google Scholar]
- 18.Komura J, Mitani H, Nemoto N, Ishikawa T, Shima A. Preferential excision repair and nonpreferential photoreactivation of pyrimidine dimers in the c-ras sequence of cultured goldfish cells. Mutat Res DNA Repair. 1991;254:191–198. doi: 10.1016/0921-8777(91)90056-u. [DOI] [PubMed] [Google Scholar]
- 19.Lisle J T, Rose J B. Cryptosporidium contamination of water in the USA and UK: a mini-review. J Water Supply Res Technol AQUA. 1995;44:103–117. [Google Scholar]
- 20.Mead J R, Bonafonte M-T, Arrowood M J, Schinazi R F. In vitro expression of mRNA coding for a Cryptosporidium parvum oocyst wall protein. J Eukaryot Mcrobiol. 1996;43,5:84S. doi: 10.1111/j.1550-7408.1996.tb05011.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitani H, Shima A. Induction of cyclobutane pyrimidine dimer photolyase in cultured fish cells by fluorescent light and oxygen stress. Photochem Photobiol. 1995;6:373–377. doi: 10.1111/j.1751-1097.1995.tb08625.x. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheimer J A, Jacangelo J G, Laine J-M, Hoagland J E. Testing the equivalency of ultraviolet light and chlorine for disinfection of wastewater to reclamation standards. Water Environ Res. 1997;69:14–24. [Google Scholar]
- 23.Rennecker J L, Marinas B J, Owens J H, Rice E W. Inactivation of Cryptosporidium parvum oocysts with ozone. Water Res. 1999;33:2481–2488. [Google Scholar]
- 24.Rochelle P A, Ferguson D M, Handojo T J, Leon R D, Stewart M H, Wolfe R L. Development of a rapid detection procedure for Cryptosporidium, using in vitro cell culture combined with PCR. J Eukaryot Mcrobiol. 1996;43,5:72S. doi: 10.1111/j.1550-7408.1996.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 25.Rochelle PA, Ferguson D M, Handojo T J, Leon R D, Stewart M H, Wolfe R L. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of water-borne Cryptosporidium parvum. Appl Environ Microbiol. 1997;63:2029–2037. doi: 10.1128/aem.63.5.2029-2037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochelle P A, Leon R D, Stewart M H, Wolfe R L. Comparison of primers and optimization of PCR conditions for detection of Cryptosporidium parvum and Giardia lamblia in water. Appl Environ Microbiol. 1997;63:106–114. doi: 10.1128/aem.63.1.106-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruffell K M, Rennecker J L, Marinas B J. Inactivation of Cryptosporidium parvum oocysts with chlorine dioxide. Water Res. 2000;34:868–876. doi: 10.1016/s0043-1354(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 28.Shin G-A, Linden K G, Arrowood M J, Sobsey M D. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 2001;67:3029–3032. doi: 10.1128/AEM.67.7.3029-3032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slifko T R, Huffman D E, Rose J B. A most-probable-number assay for enumeration of infectious Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1999;65:3936–3941. doi: 10.1128/aem.65.9.3936-3941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slifko T R, Friedman D, Rose J B, Jakubowski W. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl Environ Microbiol. 1997;63:3669–3675. doi: 10.1128/aem.63.9.3669-3675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stinear T, Matusan A, Hines K, Sandery M. Detection of a single viable Cryptosporidium parvum oocyst in environmental water concentrates by reverse transcription-PCR. Appl Environ Microbiol. 1996;62:3385–3390. doi: 10.1128/aem.62.9.3385-3390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland B M, Shi A G. Quantitation of pyrimidine dimer contents of nonradioactive deoxyribonucleic acid by electrophoresis in alkaline agarose gels. Biochemistry. 1983;22:745–749. doi: 10.1021/bi00273a006. [DOI] [PubMed] [Google Scholar]
- 33.Upton S J, Tilley M, Brillhart D B. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 34.Veatch W, Okada S. Radiation-induced breaks of DNA in cultured mammalian cells. Biophys J. 1969;9:330–346. doi: 10.1016/S0006-3495(69)86390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Water Works Association. Standard methods for the examination of water. Tokyo, Japan: Japan Water Works Association; 1993. [Google Scholar]
- 36.Woods K M, Nesterenko M V, Upton S J. Development of a microtitre ELISA to quantify development of Cryptosporidium parvum in vitro. FEMS Microbiol Lett. 1995;128:89–94. doi: 10.1111/j.1574-6968.1995.tb07505.x. [DOI] [PubMed] [Google Scholar]